Abstract
Overactive bladder (OAB) syndrome is a common condition characterised by urinary urgency, with or without urgency incontinence, frequency and nocturia, in the absence of any other pathology. Clinical diagnosis is based upon patient self-reported symptomology. Currently there is a plethora of treatments available for the management of OAB. Clinical guidelines suggest treatment via a multidisciplinary pathway including behavioural therapy and pharmacotherapy, which can be commenced in primary care, with referral to specialist services in those patients refractory to these treatments. Intradetrusor botulinum A and sacral neuromodulation provide safe and efficacious management of refractory OAB. Percutaneous tibial nerve stimulation and augmentation cystoplasty remain available and efficacious in a select group of patients. Unfortunately, there remains a high rate of patient dissatisfaction and discontinuation in all treatments and thus there remains a need for emerging therapies in the management of OAB.
Keywords: antimuscarinic, beta-agonist, BOTOX, overactive bladder, sacral neuromodulation, urinary incontinence
Introduction
Overactive bladder (OAB) syndrome is defined by the International Continence Society as urinary urgency, with or without urgency urinary incontinence, usually accompanied by frequency and/or nocturia, in the absence of urinary tract infection (UTI) or other obvious pathology.1 OAB is a highly prevalent condition affecting 16.6% of the European population. Its incidence increases with age and has a known adverse effect on quality of life.2 Women are more commonly affected, and there is increased incidence with age; US studies suggest a prevalence of up to 43% in women and 27% in men older than 40 years of age. There are significant differences by racial/ethnic group with OAB being highest in African Americans.2–4
In addition, OAB has a substantial economic burden. It is estimated to cost US$267 per person per year (comparable with gynaecological and breast cancer) and approximately US$26 billion annually.5
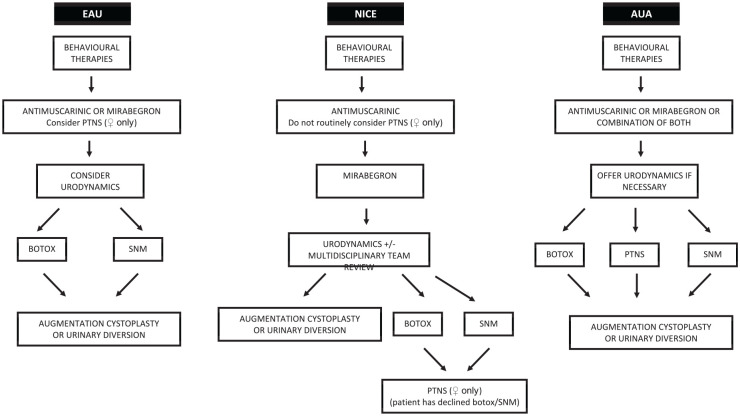
There is a general consensus between guidelines (Figure 1), with a stepwise approach taken to diagnosis and management, although it is not necessary for every patient to go through each step in order. Initial conservative management strategies (behavioural therapy and/or pharmacotherapy) should ideally be instituted in primary care. Although there is no standard definition, those who fail this initial management are generally deemed to have refractory OAB and should be referred to specialist services.6–8
Figure 1.
Comparison of NICE, EAU and AUA guidelines for the management of OAB.6–8
AUA, American Urological Association; EAU, European Association of Urology; NICE, National Institute for Health and Care Excellence; OAB, overactive bladder; PTNS, posterior tibial nerve stimulation; SNM, sacral neuromodulation.
Diagnosis
OAB is a clinical diagnosis based on patient symptomology of daytime frequency and urgency, with or without urgency incontinence (UUI). Patients may often have suffered with these symptoms for long periods of time before self-presenting when it becomes bothersome to themselves (or their carers).6
A focused history is paramount in diagnosing OAB. It is critical to assess onset of symptoms as well as aggravating and alleviating factors and 24-h pad use. Physical examination should include the genitourinary system, as well as digital rectal examination and assessment of prostate in men and vaginal examination in women. Urinalysis, by dipstick initially, should be performed to rule out haematuria and infection.9
Validated questionnaires are available to assess effects on quality of life as well as symptoms. Bladder diaries or frequency–volume charts provide an accurate and reliable measure of voiding patterns.10
Imaging of the urinary tract is not required for diagnosis, but may be used as an adjunct in those patients with suspected bladder outflow obstruction. The European Association of Urology (EAU) guidelines do not recommend routinely performing imaging of the upper or lower urinary tract as part of the assessment of OAB. Cystoscopy is also not beneficial, unless malignancy is suspected.7
The use of urodynamics in the diagnosis of OAB remains controversial. Although the gold standard diagnostic test for detrusor overactivity, it is an invasive procedure and therefore should be limited to those with refractory OAB. However, treatment should be based on patient symptoms as normal urodynamics do not rule out OAB. Urodynamic proven detrusor overactivity is more common in men (69% men dry OAB versus 44% women), and those with wet OAB (90% men versus 58% women).10 The National Institute for Health and Care Excellence (NICE) advises urodynamics prior to third-line therapy, EAU only if findings may change management and the American Urological Association (AUA) for patients with complicated OAB (such as those with concurrent urethral dysfunction or in those in whom the diagnosis is not clear.6–8
In the UK, a nationally funded National Institute for Health Research superiority trial has just finished recruitment looking at the usefulness of urodynamics prior to treatment for refractory OAB syndrome.11
Behavioural therapies
Behavioural therapies aim to increase voiding time interval, reduce episodes of urgency and nocturia, and prevent incontinence, by directing patients to interrupt or inhibit detrusor contractions via pelvic floor muscle training.9,12 In motivated patients, this can prove to be very efficacious reducing leakage by 50–80% and up to 30% becoming dry.13
Limiting fluid intake to 1–1.5 L a day is recommended. Patients can significantly improve OAB symptoms by reducing fluid intake by 25%.14,15 Evidence for this remains weak however, with no significant improvement in symptoms when discontinuing caffeinated beverages.16,17
Diuretics are also known be a cause of incontinence and should be avoided where possible, particularly in the elderly.18
Pharmacotherapy
There are a number of antimuscarinic agents available in both transdermal and oral preparations (Table 1), and these remain the mainstay of treatment in OAB with an efficacy of 65–70% in reducing major symptoms.12 Side effects such as dry mouth and constipation may prove bothersome to some patients in spite of efficacy. In addition, as these agents have the ability to bind and block muscarinic receptors in the whole body, including those in the brain, there is concern regarding the anticholinergic burden in elderly patients contributing to adverse events such as falls, constipation, cognitive impairment and development of delirium.12,19,20 Anticholinergic scales attempt to quantify the risk versus benefits of prescribing anticholinergic medication, however there remains no consensus between the medications assessed on these scales and the degree of effect.21
Table 1.
| Drug | Dose | Uroselective?* | Number needed to treat to achieve cure of urinary incontinence22 | Relative risk of discontinuation (95% CI)22 | Adverse events12,23 | |
|---|---|---|---|---|---|---|
| Oxybutynin | Oral | 5–15 mg/day | No | 9 (6–16) | 1.7 (1.1–2.5) | Dry mouth (68%) Constipation (10%) |
| Transdermal | 3.9 mg twice weekly | Dry mouth (7%) Constipation (2.1%) Erythaema at site (8%) |
||||
| Solifenacin | 5–10 mg/day | Yes | 9 (6–17) | 1.3 (1.1–1.7) | Dry mouth (26%) Constipation (12%) Blurred vision (5%) |
|
| Darifenacin | 7.5–15 mg/day | Yes | 1.2 (0.8–1.8) | Dry mouth (35%) Constipation (21%) |
||
| Tolterodine | 2 mg twice daily | No | 12 (8–25) | 1.0 (0.6–1.7) | Dry mouth (23%) Constipation (6%) Dry eyes (4%) |
|
| Trospium | 20 mg twice daily | No | 9 (7–12) | 1.5 (1.1–1.9) | Dry mouth (22.8%) Constipation (9.5%) Abdominal pain (3.1%) |
|
| Fesoterodine | 4–8 mg once daily | No | 8 (5–17) | 2.0 (1.3–3.1) | Dry mouth (87%) Constipation (87%) |
|
| Mirabegron | 25–50 mg/day | NA | 1.22 (0.84–1.76)24,$ | Hypertension (6.9%) | ||
Antimuscarinic more selective for bladder muscarinic receptor M3.
Odds ratio.
CI, confidence interval; NA, not available; OAB, overactive bladder.
Patient compliance with antimuscarinic therapy remains poor due to intolerable side effects, with discontinuation rates up to 85% over 12 months.25 In comparison, persistence and adherence with mirabegron is statistically superior to those with other antimuscarinics in a large UK primary care population.26
Mirabegron is a beta-agonist that acts to facilitate bladder detrusor relaxation. Mirabegron has demonstrated sustained improvements in number of micturitions and incontinence. Intolerable side effects, such as dry mouth, are statistically less compared with antimuscarinic therapy. In addition, although there are concerns regarding blood pressure rises, this remains small and mirabegron is efficacious and safe, with no difference in treatment-emergent hypertension compared with placebo.27 The Medicine and Healthcare products Regulatory Agency recommends the use of mirabegron with caution in those patients with stage 2 hypertension (systolic blood pressure ⩾160 mmHg and/or diastolic ⩾100 mmHg). It is contraindicated in patients with severe uncontrolled hypertension (systolic blood pressure ⩾180 mmHg and/or diastolic 100 mmHg).28
Combination therapy (antimuscarinic and beta3-agonist) may be considered in patients refractory to monotherapy. Co-administration appears to improve efficacy with minimal increase in side-effect profile. Solifenacin and mirabegron combination therapy (in doses of 5 mg and 25 mg or 5 mg and 50 mg, respectively) is reported to have a statistically significant decrease in number of incontinence episodes and micturition compared with solifenacin or mirabegron alone.29 Combination therapy with 10 mg solifenacin greatly increased its side-effect profile with only marginal benefit in efficacy.30 Although EAU guidelines recognise there may be more benefit from addition of mirabegron to solifenacin 5 mg, rather than increasing solifenacin to 10 mg, currently only the AUA recommends combination therapy in patients who are refractory to either, in their treatment algorithms.6,7
Virabegron is a novel selective beta3-agonist that has demonstrated significant improvement in symptoms compared with placebo, as well as fewer adverse events compared with imidafenacin (7.6% 50 mg, 5.4% 100 mg versus 10.3%).31 At present, virabegron has not been approved by either the European Medicines Agency or the US Food and Drug Administration (FDA). Other beta3-agonists, such as solabegron and ritobegron are currently undergoing randomised clinical trials.32
Intradetrusor botulinum toxin A
The effectiveness of botulinum toxin A has been demonstrated in a number of randomised placebo-controlled trials, with a 60% statistically significant improvement in symptoms for median duration 373 days.33 Currently 100 units onabotulinum toxin A (onabotA; BOTOX®) dissolved in 10 ml of saline and injected into 20 points of the bladder wall above the trigone is the only licensed formulation in Europe to treat wet OAB, and as third-line therapy in those who have failed behavioural therapy and pharmacotherapy in the USA. The requirement for repeat injections every 6–9 months, risk of UTIs, as well as increased post-void residuals requiring clean intermittent catheterization (CIC), may lead to patient discontinuation, thus appropriate patient selection (i.e. those willing to engage in post-void residual evaluation and CIC) is imperative.6,7,34 Other formulations of botulinum toxin, such as Dysport and Xeomin, although used to treat refractory OAB, are not licensed for that use. For neurogenic detrusor overactivity, BOTOX is licensed at 200 units dissolved in 30 ml of saline and given in 30 injections.
Sacral neuromodulation
Sacral neuromodulation (SNM) requires a two-stage approach in which a percutaneous electrode is placed under fluoroscopic guidance into the sacral foramen to stimulate the S3 or S4 nerve roots. Subsequently patients undergo a test phase, and a permanent device is implanted if there is >50% improvement in symptoms.7 Therapeutic success rates are reported at 69.3% over a 23-year follow up, with no life threatening or irreversible adverse events (implant site pain and undesirable change in stimulation being the most reported).35–37 Lower success rates are seen in men. SNM and onabotA are comparable in terms of efficacy and safety, with no difference in reduction of UUI episodes over 24 months.38
The need for removal of SNM devices in those patients requiring body magnetic resonance imaging (MRI) remains a concern in those who had implants prior to 2020. The standard Medtronic (Dublin, Ireland) SNM device, prior to 2020, was both non-rechargeable and only head MRI compatible (1.5T). Since 2020, Medtronic’s Interstim II recharge-free system is full body MRI compatible up to 3T. Axonics® (Irvine, CA, USA) SNM System and Medtronic InterStim™ Micro are new devices with FDA approval for the treatment of urinary incontinence and are both rechargeable and full body MRI compatible (up to 3T). Rechargeable devices are usually smaller, with a battery life expectancy of 15 years compared with 3–5 years with standard SNM devices. However, the additional need for weekly recharging by the patient, requiring dexterity and good cognition, may limit its use and compliance.39
Posterior tibial nerve stimulation
Posterior tibial nerve stimulation (PTNS) delivers electrical stimulation to the sacral micturition centres via a fine needle placed just above the medial aspect of the ankle. Treatment consists of 12 consecutive outpatient sessions, lasting 30 min, usually once a week but can be up to three times a week. Effects may be sustained with maintenance therapy every 2–3 weeks, for up to 3 years.7,40 PTNS has shown a 71–79.5% patient-reported response to treatment; however, there is no statistically significant benefit to PTNS compared with tolterodine.41,42 Newer implantable PTNS devices, allowing continuous tibial nerve stimulation, appear to be well tolerated, with preliminary results showing a significant improvement in UUI and a similar efficacy to SNM. However, only short-term (6 months) data are currently available.43,44
There is currently no consensus regarding the use of PTNS between guidelines. NICE appears to advise against the use of PTNS, compared with the EAU, which suggests second-line use instead of pharmacotherapy where the side effects of antimuscarinics are deemed intolerable. The AUA recommends PTNS as a third-line treatment. These recommendations are a reflection of both limited evidence for the efficacy of PTNS, particularly as placebo effect is up to 21%, and secondary to the need for regular visits and weekly attention by healthcare professionals to patient’s symptoms, as well as cost effectiveness when compared with antimuscarinics.42,45,46 In addition, it must be noted that there is no evidence for the use of PTNS in men.6–8
New neuromodulation targets including pudendal nerve and dorsal genital nerve (DGN) stimulation have shown promise. Pudendal nerve stimulation has a 42.8–63% overall reduction in voiding symptoms, including those patients who may have previously failed SNM.47 Although DGN has been shown to have a 33–47% dry rate, trial numbers remain small and therefore further review is required.47,48
Augmentation cystoplasty
Augmentation cystoplasty remains a ‘last resort’ option for those patients with OAB refractory to both medication and minimally invasive treatment options. Advancements in surgical technique have seen laparoscopic and robotic augmentation cystoplasty being performed with minimal morbidity.49 Patients should be counselled for the need to perform CIC post procedure; however, it must be noted urinary retention and the need for CIC is preferable for some patients when compared with severe intractable frequency, urgency and urgency incontinence. In those unable or unwilling to perform CIC, urinary diversion, in the form of an ileal conduit/stoma, may be an option.50 Outcomes remain excellent with a continence rate of 93% in those with OAB (compared with 78% in neuropathic bladders). Long-term complications of augmentation, including recurrent UTIs, bladder stone and possible malignancy are well documented.51 Long-term surveillance cystoscopy is controversial. It has been suggested that in asymptomatic patients, annual surveillance cystoscopy is not required, with no evidence of malignancy in at least the first 10 years after augmentation cystoplasty.52,53 It remains relatively cost effective with an average insurance re-imbursement estimated at approximately US$25,041 over 5 years. In comparison SNM is estimated to cost US$64,111 over 15 years (US$36,990 for rechargeable devices).54 However, BOTOX is more cost effective but only over the first 5 years, costing US$2946.83 per injection, as long as effects are sustained to at least 5.1 months between injections.54,55
Emerging therapies
Phosphodiesterase (PDE) 5 inhibitors are currently approved for the management of men with lower urinary tract symptoms and erectile dysfunction, but not OAB.7 In addition, daily low-dose tadalafil has proved to be effective in women with OAB, with a significant improvement in symptoms scores and incontinence.56 More recently, both PDE1 and PDE5 inhibitors have demonstrated improvement in urodynamic parameters, particularly bladder volume at first desire and maximum detrusor pressure.57
Percutaneous radiofrequency neurotomy has been demonstrated as feasible in patients with neurogenic detrusor overactivity secondary to spinal cord injury, with significant improvement in patient symptoms scores.58 However, there is currently no evidence that transurethral radiofrequency improves patient-reported symptoms of incontinence.59
Conclusion
There is currently a plethora of treatment options available for OAB. It is important for both clinicians and patients to recognise OAB as a syndrome with no cure and not a disease, therefore the importance of patient education on therapeutic options, including no treatment, is critical. The vast majority of patients with OAB will have persistence of symptoms, with variable remission rates (3–40%). Those with most severe UUI, high body mass index and low physical activity are more likely to progress.60 OAB is a chronic condition and available treatment options may improve, but not cure symptoms, leading to dissatisfaction and high rates of discontinuation of all available therapies.32 When given the choice, most patients appear to opt for minimally invasive surgery (i.e. onabotA or SNM) with preoperative preparation associated with improved patient outcome.61–63
Footnotes
Author contributions: CF: main author; EP and JP: reviewers; HH: chief reviewer.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Christina Fontaine  https://orcid.org/0000-0002-6669-467X
https://orcid.org/0000-0002-6669-467X
John Pascoe  https://orcid.org/0000-0003-4996-4428
https://orcid.org/0000-0003-4996-4428
Hashim Hashim  https://orcid.org/0000-0003-2467-407X
https://orcid.org/0000-0003-2467-407X
Contributor Information
Christina Fontaine, Specialist Registrar in Urology, University Hospitals Plymouth, Derriford Road, Devon, PL6 8AU, UK.
Emma Papworth, Bristol Urological Institute, Southmead Hospital, Bristol, Somerset, UK.
John Pascoe, University Hospitals Plymouth, Devon, UK.
Hashim Hashim, Bristol Urological Institute, Southmead Hospital, Bristol, Somerset, UK.
References
- 1.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 2010; 21: 5–26. [DOI] [PubMed] [Google Scholar]
- 2.Milsom I, Abrams P, Cardozo L, et al. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 2001; 87: 760–766. [DOI] [PubMed] [Google Scholar]
- 3.Coyne KS, Margolis MK, Kopp ZS, et al. Racial differences in the prevalence of overactive bladder in the United States from the epidemiology of LUTS (EpiLUTS) study. Urology 2012; 79: 95–101. [DOI] [PubMed] [Google Scholar]
- 4.Coyne KS, Sexton CC, Bell JA, et al. The prevalence of lower urinary tract symptoms (LUTS) and overactive bladder (OAB) by racial/ethnic group and age: results from OAB-POLL. Neurourol Urodyn 2013; 32: 230–237. [DOI] [PubMed] [Google Scholar]
- 5.Hu TW, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology 2003; 61: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 6.American Urological Association. Overactive Bladder (OAB) guideline, https://www.auanet.org/guidelines/overactive-bladder-(oab)-guideline (2020, accessed 29 December 2020).
- 7.European Association of Urology. EAU Guidelines: urinary incontinence, https://uroweb.org/guideline/urinary-incontinence/ (2020, accessed 29 December 2020).
- 8.NICE Pathways. Managing overactive bladder in women, https://pathways.nice.org.uk/pathways/urinary-incontinence-and-pelvic-organ-prolapse-in-women/managing-overactive-bladder-in-women#content=view-node%3Anodes-augmentation-cystoplasty-for-idiopathic-detrusor-overactivity&path=view%3A/pathways/urinary-incontinence-and-pelvic-organ-prolapse-in-women/invasive-procedures-for-overactive-bladder-in-women.xml (2020, accessed 29 December 2020).
- 9.Truzzi JC, Gomes CM, Bezerra CA, et al. Overactive bladder – 18 years – Part I. Int Braz J Urol 2016; 42: 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashim H, Abrams P. Is the bladder a reliable witness for predicting detrusor overactivity?. J Urol 2006; 175: 191–194. [DOI] [PubMed] [Google Scholar]
- 11.NIHR Funding and Awards Search Website [Internet]. Fundingawards.nihr.ac.uk, https://www.fundingawards.nihr.ac.uk/award/15/150/05 (2021, accessed 23 January 2021).
- 12.Arnold J, McLeod N, Thani-Gasalam R, et al. Overactive bladder syndrome: management and treatment options. Aust J Gen Pract 2012; 41: 878–883. [PubMed] [Google Scholar]
- 13.Markland AD, Vaughan CP, Johnson TM, II, et al. Incontinence. Med Clin North Am 2011; 95: 539–554. [DOI] [PubMed] [Google Scholar]
- 14.Hashim H, Abrams P. How should patients with an overactive bladder manipulate their fluid intake?. BJU Int 2008; 102: 62–66. [DOI] [PubMed] [Google Scholar]
- 15.Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol 2005; 174: 187–189. [DOI] [PubMed] [Google Scholar]
- 16.Wells MJ, Jamieson K, Markham TC, et al. The effect of caffeinated versus decaffeinated drinks on overactive bladder: a double-blind, randomized, crossover study. J Wound Ostomy Continence Nurs 2014; 41: 371–378. [DOI] [PubMed] [Google Scholar]
- 17.Srikrishna S, Robinson D, Cardozo L, et al. Management of overactive bladder syndrome. Postgrad Med J 2007; 83: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier EM, Vats V, Clemens JQ. Pharmacotherapy adherence and costs versus nonpharmacologic management in overactive bladder. Am J Manag Care 2009; 15(Suppl. 4): S108–S114. [PubMed] [Google Scholar]
- 19.Araklitis G, Robinson D, Cardozo L. Cognitive effects of anticholinergic load in women with overactive bladder. Clin Interv Aging 2020; 15: 1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lozano-Ortega G, Johnston KM, Cheung A, et al. A review of published anticholinergic scales and measures and their applicability in database analyses. Arch Gerontol Geriatr 2020; 87: 103885. [DOI] [PubMed] [Google Scholar]
- 21.Wagg A, Compion G, Fahey A, et al. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int 2012; 110: 1767–1774. [DOI] [PubMed] [Google Scholar]
- 22.Epstein BJ, Gums JG, Molina E. Newer agents for the management of overactive bladder. Am Fam Physician 2006; 74: 2061–2068. [PubMed] [Google Scholar]
- 23.Cui Y, Zong H, Yang C, et al. The efficacy and safety of mirabegron in treating OAB: a systematic review and meta-analysis of phase III trials. Int Urol Nephrol 2014; 46: 275–284. [DOI] [PubMed] [Google Scholar]
- 24.Shamliyan T, Wyman J, Kane RL. Nonsurgical treatments for urinary incontinence in adult women: diagnosis and comparative effectiveness. Comparative Effectiveness Reviews, No. 36. Rockville, MD: Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
- 25.Chapple CR, Nazir J, Hakimi Z, et al. Persistence and adherence with mirabegron versus antimuscarinic agents in patients with overactive bladder: a retrospective observational study in UK clinical practice. Eur Urol 2017; 72: 389–399. [DOI] [PubMed] [Google Scholar]
- 26.Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β3-adrenoceptor agonist, in overactive bladder. Eur Urol 2013; 63: 296–305. [DOI] [PubMed] [Google Scholar]
- 27.Mirabegron (Betmiga▼): risk of severe hypertension and associated cerebrovascular and cardiac events [Internet]. GOV.UK., https://www.gov.uk/drug-safety-update/mirabegron-betmiga-risk-of-severe-hypertension-and-associated-cerebrovascular-and-cardiac-events (2021, accessed 23 January 2021).
- 28.Herschorn S, Chapple CR, Abrams P, et al. Efficacy and safety of combinations of mirabegron and solifenacin compared with monotherapy and placebo in patients with overactive bladder (SYNERGY study). BJU Int 2017; 120: 562–575. [DOI] [PubMed] [Google Scholar]
- 29.Drake MJ, Chapple C, Esen AA, et al. Efficacy and safety of mirabegron add-on therapy to solifenacin in incontinent overactive bladder patients with an inadequate response to initial 4-week solifenacin monotherapy: a randomised double-blind multicentre phase 3B study (BESIDE). Eur Urol 2016; 70: 136–145. [DOI] [PubMed] [Google Scholar]
- 30.Abrams P, Kelleher C, Staskin D, et al. Combination treatment with mirabegron and solifenacin in patients with overactive bladder: efficacy and safety results from a randomised, double-blind, dose-ranging, phase 2 study (symphony). Eur Urol 2015; 67: 577–588. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida M, Takeda M, Gotoh M, et al. Vibegron, a novel potent and selective β3-adrenoreceptor agonist, for the treatment of patients with overactive bladder: a randomized, double-blind, placebo-controlled phase 3 study. Eur Urol 2018; 73: 783–790. [DOI] [PubMed] [Google Scholar]
- 32.Bientinesi R, Sacco E. Managing urinary incontinence in women – a review of new and emerging pharmacotherapy. Expert Opin Pharmacother 2018; 19: 1989–1997. [DOI] [PubMed] [Google Scholar]
- 33.Brubaker L, Richter HE, Visco A, et al. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol 2008; 180: 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahai A, Khan MS, Dasgupta P; GKT Botulinum Study Group. Efficacy of botulinum toxin-A for treating idiopathic detrusor overactivity: results from a single center, randomized, double-blind, placebo controlled trial. J Urol 2007; 177: 2231–2236. [DOI] [PubMed] [Google Scholar]
- 35.Siegel S, Noblett K, Mangel J, et al. Five-year followup results of a prospective, multicenter study of patients with overactive bladder treated with sacral neuromodulation. J Urol 2018; 199: 229–236. [DOI] [PubMed] [Google Scholar]
- 36.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 2007; 178: 2029–2034. [DOI] [PubMed] [Google Scholar]
- 37.Gandhi S, Gajewski JB, Koziarz A, et al. Long-term outcomes of sacral neuromodulation for lower urinary tract dysfunction: a 23-year experience. Neurourol Urodyn 2021; 40: 461–469. [DOI] [PubMed] [Google Scholar]
- 38.Amundsen CL, Komesu YM, Chermansky C, et al. Two-year outcomes of sacral neuromodulation versus onabotulinumtoxinA for refractory urgency urinary incontinence: a randomized trial. Eur Urol 2018; 74: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghoniem G. Use of new rechargeable battery and MRI-Friendly technologies in sacral neuromodulation systems to treat fecal incontinence. Japanese J Gstro Hepato 2020; 5: 1–5. [Google Scholar]
- 40.Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 2009; 182: 1055–1061. [DOI] [PubMed] [Google Scholar]
- 41.Finazzi-Agrò E, Petta F, Sciobica F, et al. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trial. J Urol 2010; 184: 2001–2006. [DOI] [PubMed] [Google Scholar]
- 42.de Wall LL, Heesakkers JP. Effectiveness of percutaneous tibial nerve stimulation in the treatment of overactive bladder syndrome. Res Rep Urol 2017; 9: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groen J, Amiel C, Bosch JR. Chronic pudendal nerve neuromodulation in women with idiopathic refractory detrusor overactivity incontinence: results of a pilot study with a novel minimally invasive implantable mini-stimulator. Neurourol Urodyn 2005; 24: 226–230. [DOI] [PubMed] [Google Scholar]
- 44.Vollstedt A, Gilleran J. Update on implantable PTNS devices. Curr Urol Rep 2020; 21: 28. [DOI] [PubMed] [Google Scholar]
- 45.Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 2010; 183: 1438–1443. [DOI] [PubMed] [Google Scholar]
- 46.Peters KM, Carrico DJ, Wooldridge LS, et al. Percutaneous tibial nerve stimulation for the long-term treatment of overactive bladder: 3-year results of the STEP study. J Urol 2013; 189: 2194–2201. [DOI] [PubMed] [Google Scholar]
- 47.Marinkovic SP. New technologies in the management of overactive bladder: current research and future prospects. Ther Adv Urol 2019; 11: 1756287219844669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farag FF, Martens FM, Rijkhoff NJ, et al. Dorsal genital nerve stimulation in patients with detrusor overactivity: a systematic review. Curr Urol Rep 2012; 13: 385–388. [DOI] [PubMed] [Google Scholar]
- 49.Veeratterapillay R, Thorpe AC, Harding C. Augmentation cystoplasty: contemporary indications, techniques and complications. Indian J Urol 2013; 29: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyblat P, Ginsberg DA. Augmentation enterocystoplasty in overactive bladder: is there still a role? Curr Urol Rep 2010; 11: 432–439. [DOI] [PubMed] [Google Scholar]
- 51.Venn SN, Mundy AR. Long-term results of augmentation cystoplasty. Eur Urol 1998; 34(Suppl. 1): 40–42. [DOI] [PubMed] [Google Scholar]
- 52.Biardeau X, Chartier-Kastler E, Rouprêt M, et al. Risk of malignancy after augmentation cystoplasty: a systematic review. Neurourol Urodyn 2016; 35: 675–682. [DOI] [PubMed] [Google Scholar]
- 53.Hamid R, Greenwell TJ, Nethercliffe JM, et al. Routine surveillance cystoscopy for patients with augmentation and substitution cystoplasty for benign urological conditions: is it necessary?. BJU Int 2009; 104: 392–395. [DOI] [PubMed] [Google Scholar]
- 54.Padmanabhan P, Scarpero HM, Milam DF, et al. Five-year cost analysis of intra-detrusor injection of botulinum toxin type A and augmentation cystoplasty for refractory neurogenic detrusor overactivity. World J Urol 2011; 29: 51–57. [DOI] [PubMed] [Google Scholar]
- 55.Noblett KL, Dmochowski RR, Vasavada SP, et al. Cost profiles and budget impact of rechargeable versus non-rechargeable sacral neuromodulation devices in the treatment of overactive bladder syndrome. Neurourol Urodyn 2017; 36: 727–733. [DOI] [PubMed] [Google Scholar]
- 56.Chen H, Wang F, Yu Z, et al. Efficacy of daily low-dose tadalafil for treating overactive bladder: results of a randomized, double-blind, placebo-controlled trial. Urology 2017; 100: 59–64. [DOI] [PubMed] [Google Scholar]
- 57.Ückert S, Oelke M. Phosphodiesterase (PDE) inhibitors in the treatment of lower urinary tract dysfunction. Br J Clin Pharmacol 2011; 72: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jo HM, Kim HS, Cho YW, et al. Two-year outcome of percutaneous bipolar radiofrequency neurotomy of sacral nerves S2 and S3 in spinal cord injured patients with neurogenic detrusor overactivity: a randomized controlled feasibility study. Pain Physician 2016; 19: 373–380. [PubMed] [Google Scholar]
- 59.Kang D, Han J, Neuberger MM, et al. Transurethral radiofrequency collagen denaturation for the treatment of women with urinary incontinence. Cochrane Database Syst Rev 2015; 3: CD010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hagan KA, Erekson E, Austin A, et al. A prospective study of the natural history of urinary incontinence in women. Am J Obstet Gynecol 2018; 218: 502-e1–502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fontaine CL, Rudd I, Pakzad M, et al. Patient treatment preferences for symptomatic refractory urodynamic idiopathic detrusor overactivity. Urol Ann 2017; 9: 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malde S, Dowson C, Fraser O, et al. Patient experience and satisfaction with Onabotulinumtoxin A for refractory overactive bladder. BJU Int 2015; 116: 443–449. [DOI] [PubMed] [Google Scholar]
- 63.Firoozi F, Gill B, Ingber MS, et al. Increasing patient preparedness for sacral neuromodulation improves patient reported outcomes despite leaving objective measures of success unchanged. J Urol 2013; 190: 594–597. [DOI] [PubMed] [Google Scholar]