Abstract
Background:
Liver fibrosis has been identified as an outcome predictor in cardiovascular disease and has been associated with hematoma expansion and mortality in patients with primary intracerebral hemorrhage. We aimed to explore whether clinically inapparent liver fibrosis is related to neurological outcome, mortality, and intracranial hemorrhage risk in ischemic stroke patients after mechanical thrombectomy.
Methods:
We included consecutive patients with anterior circulation large vessel occlusion stroke treated at our center with mechanical thrombectomy between January 2011 and April 2019. Clinical data had been collected prospectively; laboratory data were extracted from our electronic hospital information system. We calculated the Fibrosis-4 index (FIB-4), an established non-invasive liver fibrosis test. The main outcomes were postinterventional intracranial hemorrhage, unfavorable functional status (modified Rankin scale scores of 3–6), and mortality three months post-stroke.
Results:
In the 460 patients (mean age 69 years, 49.3% female) analyzed, FIB-4 indicated advanced liver fibrosis in 22.6%. Positive FIB-4 was associated with unfavorable neurological outcomes and mortality three months post-stroke, even after correction for co-factors [Odds Ratio (OR) 2.15 for unfavorable outcome in patients with positive FIB-4, 95% confidence interval (CI) 1.21–3.83, p = 0.009, and 2.16 for mortality, 95% CI 1.16–4.03, p = 0.01]. However, FIB-4 was neither related to hemorrhagic transformation nor symptomatic intracranial hemorrhage. Moreover, atrial fibrillation was more frequent in patients with liver fibrosis (p < 0.001). Two further commonly-used liver fibrosis indices (Forns index and the Easy Liver Fibrosis Test) yielded comparable results regarding outcome and atrial fibrillation.
Conclusions:
Clinically inapparent liver fibrosis (based on simple clinical and laboratory parameters) represents an independent risk factor for unfavorable outcomes, including mortality, at three months after stroke thrombectomy. Elevated liver fibrosis indices warrant further hepatological work-up and thorough screening for atrial fibrillation in stroke patients.
Keywords: atrial fibrillation, ischemic stroke, liver fibrosis, outcome, thrombectomy
Background
Non-alcoholic fatty liver disease (NAFLD) is estimated to affect up to 25% of the global adult population.1 NAFLD is an independent risk factor for cardiovascular disease, type 2 diabetes mellitus, and chronic kidney disease.2 In patients with NAFLD, simple steatosis may progress to non-alcoholic steatohepatitis (NASH), with liver fibrosis, cirrhosis, and hepatocellular carcinoma. The stage of fibrosis is considered to be the most important determinant of cardiovascular disease and mortality in patients with NAFLD.3,4 Several non-invasive scores, including clinical information and routine blood serum parameters, have been developed to estimate liver fibrosis with high sensitivity and specificity.4
NAFLD and liver fibrosis have also been found to be specifically related to the risk of ischemic stroke.5–7 However, there is limited data on the clinical outcomes of stroke patients with liver fibrosis, aside from one single-center retrospective study indicating increased long-term mortality in stroke patients with significant liver fibrosis determined by transient elastography.8
In addition, a recent study in intracerebral hemorrhage reported positive liver fibrosis indices, i.e. the Fibrosis-4 index (FIB-4) and the Aspartate Aminotransferase (AST) to Platelet Ratio Index (APRI), to be associated with the size of hematoma at admission and its subsequent expansion.9 Hemorrhagic transformation after recanalization (intravenous thrombolysis and/or mechanical thrombectomy) is a frequent complication and an independent predictor of poor outcome.10 Patients with liver fibrosis may be at a higher risk of both hemorrhagic transformation and symptomatic intracerebral hemorrhage; however, this has not yet been investigated in patients with large vessel occlusion stroke.
Aims
Our goal was to investigate the effect of liver fibrosis (determined by non-invasive fibrosis tests based on simple clinical and laboratory parameters) on the risk of intracranial hemorrhage, neurological outcomes, and mortality at three-months poststroke in a cohort of consecutive patients with large vessel occlusion stroke treated with mechanical thrombectomy.
Methods
Study cohort
We identified all consecutive patients with large vessel occlusion stroke in the anterior cerebral circulation (internal carotid artery and/or proximal middle cerebral artery) treated at our center with mechanical thrombectomy between January 2011 and April 2019. Mechanical thrombectomy was performed by interventional radiologists using stent retrievers and/or aspiration techniques. A clinical follow-up examination at our stroke outpatient department three months post-stroke was scheduled for all patients; if a physical consultation was not possible, we performed a telephone interview. Three patients, for whom no follow-up data was available, were excluded from the study.
We excluded patients with severe liver disease (n = 3, all apparent liver cirrhosis). We did not exclude patients with chronic alcohol abuse to assure a coherent ‘real-world’ large vessel occlusion stroke cohort. Clinical stroke-related data had been collected prospectively via our prospective thrombectomy registry.11 Laboratory data and information regarding liver disease were extracted from our electronic hospital information system.
Data assessment
Laboratory values were obtained on day 1 after mechanical thrombectomy, when a standardized laboratory assessment is performed at our center. For assessment of liver fibrosis, we primarily analysed the FIB-4 index, a well-validated and clinically established liver fibrosis index.12,13 The cut-off value for prediction of advanced liver fibrosis (i.e., bridging fibrosis or cirrhosis) was used as established in current literature (⩾2.67). In addition, we analyzed the lower cut-off value of FIB-4 (<1.30), which excludes advanced liver fibrosis with a high probability.14,15
To broader analyze our data and confirm potential associations, we chose to investigate two additional liver fibrosis scores. These were the Forns index (cut-off > 6.9) and the Easy Liver Fibrosis Test (eLIFT; cut-off ⩾8).16,17 Respective formulas of these three scores are shown in Figure 1.
Figure 1.
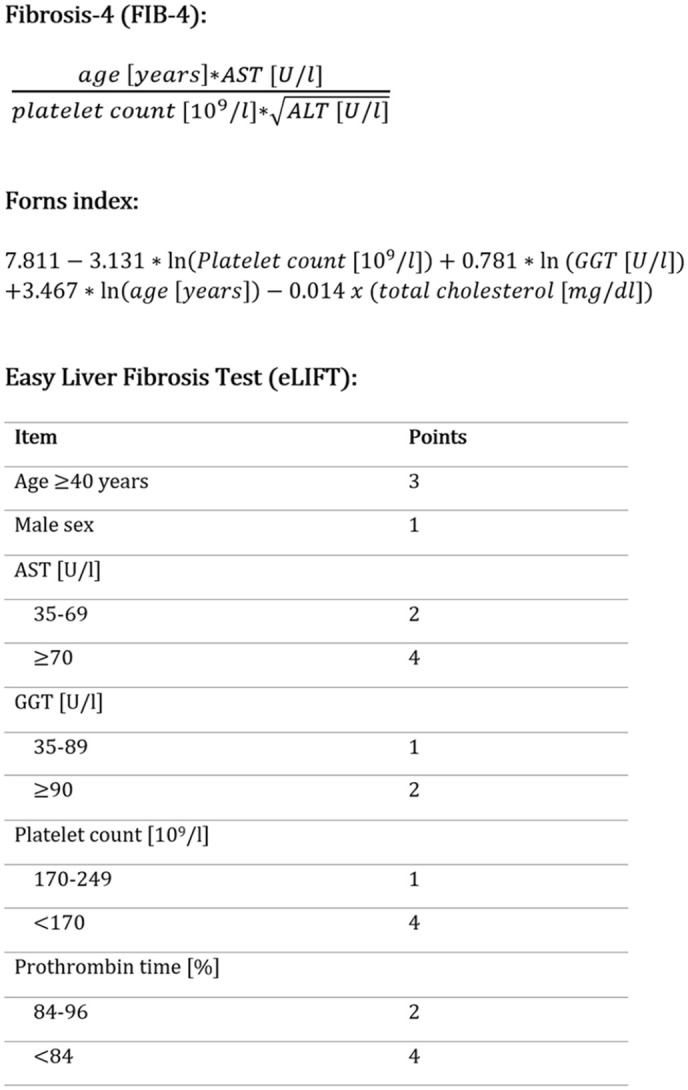
Formulas of the liver fibrosis tests used in this study.
Outcome parameters
Functional outcomes were assessed by an experienced stroke neurologist using the modified Rankin Scale (mRS). We dichotomized outcomes into favorable (mRS scores 0–2) or unfavorable (mRS scores 3–6). We also separately analyzed mortality at three months post-stroke. Postinterventional parenchymal hematoma and hemorrhagic infarction were defined according to the ECASS (European Cooperative Acute Stroke Study) criteria.18 According to our institutional protocol, all patients underwent cerebral magnetic resonance imaging (MRI) or computed tomography (CT) 24 hours after the endovascular procedure.
Statistical analysis
We performed statistical analysis using IBM SPSS Statistics for Windows, version 25 (IBM Corp, Armonk, NY, USA). The distribution of continuous variables was evaluated using the Kolmogorov–Smirnov test and histograms. Normally distributed continuous variables were compared using the unpaired Student’s t-test; for other distributions, non-parametric tests such as the Mann-Whitney U-test were used. Categorical variables were investigated using Pearson’s chi-square test. For the analyses of functional neurological outcomes and mortality, we used logistic regression to correct for all covariates which showed significant associations (p < 0.05) in univariable analysis. We did not correct for age or sex, as these factors are included in the liver fibrosis indices used in this study. p-values of less than 0.05 were considered statistically significant.
Ethical approval
The study was approved by the ethics committee of the Medical University of Graz (approval number 27-045 ex 14/15); as a retrospective cohort study, the need for of informed consent was waived.
Results
Patient characteristics
The study cohort comprised 460 consecutive patients with a mean age of 69 years (50.7% male). The median NIHSS at admission was 15 [interquartile range (IQR) 11–18] and 58% of patients were co-treated with intravenous thrombolysis. Successful recanalization (defined as thrombolysis in cerebral infarction scores of 2b-3) was achieved in 87.6% of cases. See Table 1 for clinical details including stroke risk factors, treatment-related aspects, and laboratory results. Chronic alcohol abuse (defined as ⩾2 drinks or ⩾20 g of ethanol at least 5 days per week)19 was documented in 5.7% of patients.
Table 1.
Clinical characteristics of study participants categorized by FIB-4 status.
| Study cohort n = 460 | FIB-4 positive n = 104 (22.6%) | FIB-4 negative n = 356 (77.4%) | p-value | |
|---|---|---|---|---|
| Clinical data | ||||
| Age (years) | 69.0 ± 13.4 | 77.0 ± 9.2 | 66.7 ± 13.5 | <0.001 |
| Male sex | 233 (50.7%) | 52 (50.0%) | 181 (50.8%) | 0.88 |
| Arterial hypertension | 322 (70.0%) | 81 (77.9%) | 241 (67.7%) | 0.046 |
| Dyslipidemia | 104 (22.6%) | 23 (22.1%) | 81 (22.8%) | 0.89 |
| Chronic heart disease | 92 (20.0%) | 24 (23.1%) | 68 (19.1%) | 0.37 |
| Diabetes mellitus | 77 (16.7%) | 17 (16.3%) | 60 (16.9%) | 0.90 |
| Atrial fibrillation | 192 (41.7%) | 64 (61.5%) | 128 (36.0%) | <0.001 |
| Body mass index | 27.2 ± 5.0 | 26.4 ± 4.7 | 27.4 ± 5.1 | 0.07 |
| Alcohol abuse | 26 (5.7%) | 3 (2.9%) | 23 (6.5%) | 0.17 |
| NIHSS at admission (median, IQR) | 15 (11–18) | 16 (12–19) | 14 (11–17) | 0.02 |
| MCA/M1-occlusion | 296 (64.3%) | 63 (60.6%) | 233 (65.4%) | 0.36 |
| MCA/M2-occlusion | 59 (12.8%) | 11 (10.6%) | 48 (13.5%) | 0.44 |
| Intracranial ICA occlusion | 95 (20.7%) | 28 (26.9%) | 67 (18.8%) | 0.07 |
| Acute stroke treatment | ||||
| Intravenous thrombolysis | 257 (58.3%) | 52 (52.5%) | 205 (59.9%) | 0.19 |
| Symptom onset to groin puncture (minutes) (median, IQR) | 200 (159–247) | 189 (151–227) | 195 (160–250) | 0.03 |
| Successful recanalization | 403 (87.6%) | 88 (84.6%) | 315 (88.5%) | 0.29 |
| Laboratory parameters at admission | ||||
| Platelet count (109/l) | 205 ± 67 | 155 ± 41 | 220 ± 65 | <0.001 |
| International normalized ratio | 1.17 ± 0.28 | 1.31 ± 0.43 | 1.13 ± 0.21 | <0.001 |
| Activated partial thromboplastin time (sec) | 33.6 ± 12.4 | 36.5 ± 13.1 | 32.7 ± 12.0 | <0.001 |
| Aspartate transaminase (U/l) | 26.3 ± 20.7 | 38.4 ± 36.7 | 22.8 ± 10.3 | <0.001 |
| Alanine transaminase (U/l) | 23.1 ± 21.4 | 23.2 ± 22.9 | 23.1 ± 20.9 | 0.96 |
| Gamma glutamyl transferase (U/l) | 46.2 ± 53.7 | 53.4 ± 55.4 | 44.1 ± 53.1 | 0.13 |
| Bilirubin (mg/dl) | 0.65 ± 0.37 | 0.76 ± 0.41 | 0.62 ± 0.35 | <0.001 |
| Low-density lipoprotein (mg/dl) | 87.9 ± 32.0 | 84.7 ± 31.9 | 88.8 ± 32.1 | 0.29 |
| C-reactive protein (mg/l) | 17.9 ± 21.6 | 20.5 ± 22.0 | 17.1 ± 21.5 | 0.17 |
FIB-4, Fibrosis-4; ICA, Internal carotid artery IQR, interquartile range; MCA, Middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
The combined rate of postinterventional hemorrhagic infarction or parenchymal hematoma was 20.9%; symptomatic intracranial hemorrhage occurred in 3.0% of patients. At three months post-stroke, 57.6% of patients had an unfavorable outcome (mRS scores of 3–6), and 19.8% had died (Table 2).
Table 2.
Outcome data categorized by liver fibrosis indices.
| Study cohort n = 460 | FIB-4 positive n = 104 (22.6%) | p-value# | Forns positive n = 175 (38.0%) | p-value# | eLIFT positive n = 271 (58.9%) | p-value# | |
|---|---|---|---|---|---|---|---|
| Intracerebral hemorrhage | |||||||
| Symptomatic intracranial hemorrhage | 14 (3.0%) | 3 (2.9%) | 0.92 | 5 (2.9%) | 0.83 | 11 (4.1%) | 0.13 |
| Hemorrhagic infarction | 69 (15.0%) | 16 (15.4%) | 0.90 | 25 (14.3%) | 0.74 | 39 (14.4%) | 0.74 |
| Parenchymal hemorrhage | 27 (5.9%) | 7 (6.7%) | 0.67 | 10 (5.7%) | 0.87 | 18 (6.6%) | 0.41 |
| Clinical outcomes | |||||||
| mRS 0–2 three months post-stroke | 195 (42.4%) | 28 (26.9%) | <0.001 | 56 (32.0%) | <0.001 | 93 (34.3%) | <0.001 |
| mRS 3–6 three months post-stroke | 265 (57.6%) | 76 (73.1%) | <0.001 | 119 (68.0%) | <0.001 | 178 (65.7%) | <0.001 |
| Mortality three months post-stroke | 91 (19.8%) | 34 (32.7%) | <0.001 | 51 (29.1%) | <0.001 | 67 (24.7%) | 0.002 |
| Clinical outcomes corrected for covariates* | OR (95% CI) | p-value# | OR (95% CI) | p-value# | OR (95% CI) | p-value# | |
| mRS 0–2 three months post-stroke | 0.46 (0.26–0.83) | 0.009 | 0.60 (0.37–0.99) | 0.04 | 0.56 (0.35–0.89) | 0.01 | |
| mRS 3–6 three months post-stroke | 2.15 (1.21–3.83) | 0.009 | 1.66 (1.01–2.73) | 0.04 | 1.80 (1.12–2.88) | 0.01 | |
| Mortality three months post-stroke | 2.16 (1.16–4.03) | 0.01 | 2.11 (1.15–3.88) | 0.01 | 1.46 (0.78–2.75) | 0.24 | |
Corrected for arterial hypertension, chronic heart disease, diabetes, atrial fibrillation, body mass index, alcohol abuse, NIHSS at admission, occlusion site and successful recanalization.
Compared to patients with respective negative liver fibrosis indices.
CI, confidence interval; eLIFT, easy liver fibrosis test; FIB-4, Fibrosis-4; mRS, modified Rankin Scale; OR, odd’s ratio.
Liver fibrosis indices
The FIB-4 index indicated advanced liver fibrosis in 104 (22.6%) of patients (Table 1). Patients with a positive FIB-4 index were older and more frequently had arterial hypertension. Notably, atrial fibrillation was more frequent in patients with positive liver fibrosis indices (p < 0.001). The rate of admitted alcohol abuse was not higher in patients with liver fibrosis. NIHSS at admission was slightly higher and symptom onset to groin puncture time slightly shorter in patients with a positive FIB-4 index. In contrast, vessel occlusion site, rates of thrombolysis, and successful recanalization were not different in patients with or without positive liver fibrosis indices (Table 1).
Outcome parameters
There was no difference in risk for hemorrhagic infarction, parenchymal hematoma, or symptomatic intracranial hemorrhage between patients with or without FIB-4 scores indicating advanced liver fibrosis (Table 2). However, patients with a positive FIB-4 score had a significantly higher mortality and higher rate of unfavorable outcomes (mRS 3–6) at three months. This relationship remained significant after correction for covariates which showed significant associations with outcome in univariable analysis (Table 3). FIB-4 index score was independently associated with unfavorable outcome [odds ratio (OR) 2.15, 95% confidence interval (CI) 1.21–3.83, p = 0.009] and mortality (OR 2.16, CI 1.16–4.03, p = 0.01) three months post-stroke (Table 2). The association between outcomes at three months post-stroke and FIB-4 scores is visualized in Figure 2.
Table 3.
Baseline clinical data categorized by neurological outcome three months poststroke.
| Study cohort n = 460 | mRS 0–2 n = 195 (42.4%) | mRS 3–6 n = 265 (57.6%) | p-value | |
|---|---|---|---|---|
| Clinical data | ||||
| Age (years) | 69.0 ± 13.4 | 72.4 ± 12.2 | 64.4 ± 13.5 | <0.001 |
| Male sex | 233 (50.7%) | 104 (53.3%) | 129 (48.7%) | 0.32 |
| Arterial hypertension | 322 (70.0%) | 111 (56.9%) | 211 (79.6%) | <0.001 |
| Dyslipidemia | 104 (22.6%) | 41 (21.0%) | 63 (23.8%) | 0.49 |
| Chronic heart disease | 92 (20.0%) | 29 (14.9%) | 63 (23.8%) | 0.02 |
| Diabetes mellitus | 77 (16.7%) | 21 (10.8%) | 56 (21.1%) | 0.003 |
| Atrial fibrillation | 192 (41.7%) | 64 (32.8%) | 128 (48.3%) | 0.001 |
| Body mass index | 27.2 ± 5.0 | 26.4 ± 4.5 | 27.8 ± 5.3 | 0.007 |
| Alcohol abuse | 26 (5.7%) | 16 (8.2%) | 10 (3.8%) | 0.04 |
| NIHSS at admission (median, IQR) | 15 (11–18) | 13 (10–16) | 16 (14–19) | <0.001 |
| MCA/M1-occlusion | 296 (64.3%) | 127 (65.1%) | 169 (63.8%) | 0.76 |
| MCA/M2-occlusion | 59 (12.8%) | 33 (16.9%) | 26 (9.8%) | 0.02 |
| Intracranial ICA occlusion | 95 (20.7%) | 31 (15.9%) | 64 (24.2%) | 0.03 |
| Acute stroke treatment | ||||
| Intravenous thrombolysis | 257 (58.3%) | 119 (63.0%) | 138 (54.8%) | 0.08 |
| Symptom onset to groin puncture (minutes, median, IQR) | 200 (159–247) | 200 (159–244) | 200 (160–254) | 0.89 |
| Successful recanalization | 403 (87.6%) | 189 (96.9%) | 214 (80.8%) | <0.001 |
ICA, Internal carotid artery; IQR, interquartile range; MCA, Middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
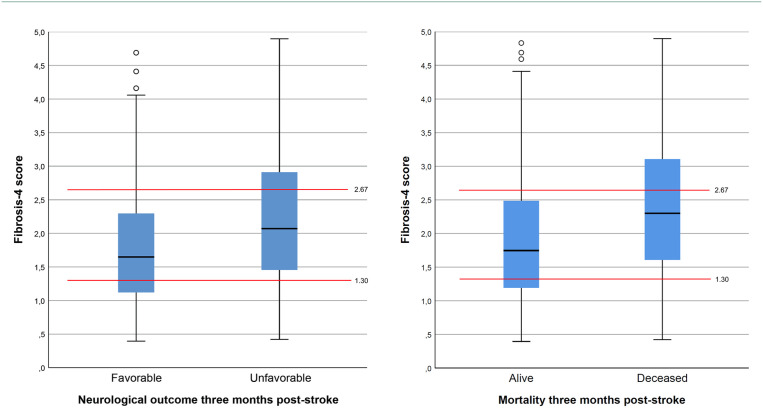
Figure 2.
Boxplot of Fibrosis-4 index and clinical outcomes at three months post-stroke.
Red lines depicting the established upper (2.67) and lower (1.30) cut-off values for inclusion and exclusion of liver fibrosis, respectively. Black line depicting the median value, blue boxes showing the 25th and 75th percentiles.
When analyzing patients below the lower cut-off value of the FIB-4 index (⩽1.30) in order to exclude significant liver fibrosis with high probability, we found that those 122 patients (26.5%) more frequently had favorable neurological outcomes three months post-stroke (mRS scores of 0–2 in 57.4% versus 37.0%, p < 0.001) and a decreased mortality rate (10.7% versus 23.1%, p < 0.003). However, after correction for all outcome-relevant co-factors (Table 3), the lower cut-off of the FIB-4 index was no longer significantly associated with favorable outcomes (p = 0.15) or a decreased mortality rate (p = 0.13).
Additional liver fibrosis indices
When also investigating the Forns index and the eLIFT scores, we found that, while indicating larger proportions of patients with liver fibrosis in our study cohort (38.0% and 58.9%, respectively, compared to 22.6% using the FIB-4 index), they yielded similar associations with outcomes and atrial fibrillation. The Forns index and eLIFT scores were significantly associated with unfavorable neurological outcomes and mortality three months post-stroke, both in univariable analysis and after correction of important co-factors (Table 2). Similar to the FIB-4, no associations regarding hemorrhagic infarction or parenchymal hematoma were observed. Both the Forns index and the Elift scores were also associated with atrial fibrillation (p < 0.001). The three indices were strongly intercorrelated (p < 0.001 for each two indices compared).
Discussion
Our study demonstrates that clinically inapparent advanced liver fibrosis, determined by simple non-invasive indices, predicts poor functional neurological outcomes and mortality at three months post-stroke in a cohort of consecutive patients with anterior circulation large vessel occlusion stroke. Here, we primarily analyzed the FIB-4 index, a clinically well-established, non-invasive liver fibrosis score, which was recently recommended by the American Association for the Study of Liver Diseases as a screening tool in patients with type 2 diabetes mellitus.13 The FIB-4 index is easy to use, and, as a combination of widely available routine clinical and laboratory parameters, causes no additional costs. It should be noted that two other applied scores in our study have also shown good predictive values for advanced liver fibrosis.16,17
To date, clinical outcomes in stroke patients regarding NAFLD have only been investigated scarcely. A Korean single-center study found that among patients with ischemic stroke or transient ischemic attack, long-term mortality was increased in patients with significant liver fibrosis evaluated by transient elastography.8 Another recent study from the same center found an increased risk of recurrent stroke and mortality in a large cohort of ischemic stroke and transient ischemic attack patients evaluated by the FIB-4 index.20 Although direct comparison with our results is challenging due to differences in the severity of stroke, risk factors, ethnicity, and different indicators of liver fibrosis used, these two studies support our finding of an increased mortality post-stroke.
It should be noted that patients with positive liver fibrosis indices had an increased rate of atrial fibrillation, which in turn could explain the higher stroke recurrence risk found in the previously described Korean studies. In line with our results, prior studies have identified higher rates of atrial fibrillation among patients with NAFLD;21 however, this relationship has not yet been shown in patients with stroke. Therefore, screening for atrial fibrillation is of even higher importance in patients with NAFLD, and especially in those with positive liver fibrosis indices both in terms of primary and secondary prevention of ischemic stroke.
Apart from the higher rate of atrial fibrillation, a number of other factors may be responsible for the worse outcome of patients with liver fibrosis in our study. These could include increased systemic inflammation,22 hypercoagulable state,23 and endothelial dysfunction,24 all of which are associated with liver fibrosis.25 In addition, NAFLD has been associated with increased carotid intimal medial thickness and an increased prevalence of carotid plaques.26 Remodeling of the left ventricle27 with thromboembolic potential is another known potential consequence of liver fibrosis. All those factors may contribute to an increased risk of recurrent stroke, other cardiovascular events, and vascular death. A large, community-based cohort study also found a higher risk for ischemic stroke in participants with elevated liver enzymes.28 It is important to recognise that the predictive value of positive fibrosis indices for post-stroke outcomes was independent from the presence of arterial hypertension, diabetes mellitus, or body mass index (i.e., components of the metabolic syndrome).
We found no difference regarding postinterventional intracerebral hemorrhage between patients with and without positive liver fibrosis indices. Such a potential association has not yet been investigated in previous studies in ischemic stroke patients; however, a recent study found that positive liver fibrosis indices, including the FIB-4 index, were associated with hematoma volume, hematoma expansion, and mortality in patients with primary intracerebral hemorrhage.9 While functional outcomes were worse in our patients with positive liver fibrosis indices, it is important to note that the most serious thrombectomy-related complication was not more frequent in these patients.
Here we primarily evaluated the well-established FIB-4 index as our main liver fibrosis index, as it is easily calculated and already broadly established. When confirming the associations found using the FIB-4 index with two other liver fibrosis indices (the Forns index and eLIFT), we found comparable results with regards to the association with clinical outcomes and atrial fibrillation. As the FIB-4 index is the most established liver fibrosis index in clinical practice, we would recommend its primary usage, although further research regarding the predictive value of different liver fibrosis indices in the setting of cerebrovascular disease would be of interest. We chose not to investigate the rather crude AST/alanine aminotransferase (ALT) ratio or the APRI as the sensitivity and specificity of those fibrosis indices is lower compared to the indices applied in our study.4,17
An important limitation of our work is the single-center design and the specific stroke population studied (i.e., large vessel occlusion stroke). This makes generalization of our results less reliable. In addition, we were not able to investigate other liver fibrosis indices such as the NAFLD Fibrosis Score because albumin levels were not routinely available in our study cohort. However, given the robust results of all three applied liver fibrosis tests, it seems unlikely that the analysis of additional scores would have yielded diverging results. Unfortunately, transient elastography was not available in this retrospective study.
To summarize, we showed that patients with positive indices of advanced liver fibrosis had worse clinical outcomes three months poststroke and more frequently atrial fibrillation, while their risk of postinterventional intracerebral hemorrhage was not increased. Further studies should investigate long-term outcomes in stroke patients, including the recurrence of cerebrovascular events, using both liver fibrosis indices and transient elastography in a prospective and multi-center setting.
Acknowledgments
None.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Simon Fandler-Höfler  https://orcid.org/0000-0001-9043-0378
https://orcid.org/0000-0001-9043-0378
Markus Kneihsl  https://orcid.org/0000-0002-6334-9432
https://orcid.org/0000-0002-6334-9432
Contributor Information
Simon Fandler-Höfler, Department of Neurology, Medical University of Graz, Graz, Austria.
Rudolf E. Stauber, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Medical University of Graz, Graz, Austria
Markus Kneihsl, Department of Neurology, Medical University of Graz, Graz, Austria.
Gerit Wünsch, Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz, Graz, Austria.
Melanie Haidegger, Department of Neurology, Medical University of Graz, Graz, Austria.
Birgit Poltrum, Department of Neurology, Medical University of Graz, Graz, Austria.
Alexander Pichler, Department of Neurology, Medical University of Graz, Graz, Austria.
Hannes Deutschmann, Division of Neuroradiology, Vascular and Interventional Radiology, Department of Radiology, Medical University of Graz, Graz, Austria.
Christian Enzinger, Department of Neurology, Medical University of Graz, Graz, Austria.
Peter Fickert, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Medical University of Graz, Graz, Austria.
Thomas Gattringer, Department of Neurology, Medical University of Graz, Auenbruggerplatz 22, Graz, 8036, Austria Division of Neuroradiology, Vascular and Interventional Radiology, Department of Radiology, Medical University of Graz, Austria.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017; 66: 1138–1153. [DOI] [PubMed] [Google Scholar]
- 3.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015; 61: 1547–1554. [DOI] [PubMed] [Google Scholar]
- 4.Stefan N, Häring H-U, Cusi K.Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019; 7: 313–324. [DOI] [PubMed] [Google Scholar]
- 5.Kim SU, Song D, Heo JH, et al. Liver fibrosis assessed with transient elastography is an independent risk factor for ischemic stroke. Atherosclerosis 2017; 260: 156–162. [DOI] [PubMed] [Google Scholar]
- 6.Hagström H, Nasr P, Ekstedt M, et al. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int 2019; 39: 197–204. [DOI] [PubMed] [Google Scholar]
- 7.Parikh NS, VanWagner LB, Elkind MSV, et al. Association between nonalcoholic fatty liver disease with advanced fibrosis and stroke. J Neurol Sci 2019; 407: 116524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baik M, Kim SU, Kang S, et al. Liver fibrosis, not steatosis, associates with long-term outcomes in ischaemic stroke patients. Cerebrovasc Dis 2019; 47: 32–39. [DOI] [PubMed] [Google Scholar]
- 9.Parikh NS, Kamel H, Navi BB, et al. Liver fibrosis indices and outcomes after primary intracerebral hemorrhage. Stroke 2020; 51: 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaesmacher J, Kaesmacher M, Maegerlein C, et al. Hemorrhagic transformations after thrombectomy: risk factors and clinical relevance. Cerebrovasc Dis 2017; 43: 294–304. [DOI] [PubMed] [Google Scholar]
- 11.Fandler-Höfler S, Heschl S, Kneihsl M, et al. Ventilation time and prognosis after stroke thrombectomy: the shorter, the better! Eur J Neurol 2020; 27: 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67: 328–357. [DOI] [PubMed] [Google Scholar]
- 14.Shah AG, Lydecker A, Murray K, et al. Use of the FIB4 index for non-invasive evaluation of fibrosis in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staufer K, Halilbasic E, Spindelboeck W, et al. Evaluation and comparison of six noninvasive tests for prediction of significant or advanced fibrosis in nonalcoholic fatty liver disease. United European Gastroenterol J 2019; 7: 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forns X, Ampurdanès S, Llovet JM, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002; 36: 986–992. [DOI] [PubMed] [Google Scholar]
- 17.Boursier J, de Ledinghen V, Leroy V, et al. A stepwise algorithm using an at-a-glance first-line test for the non-invasive diagnosis of advanced liver fibrosis and cirrhosis. J Hepatol 2017; 66: 1158–1165. [DOI] [PubMed] [Google Scholar]
- 18.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274: 1017–1025. [PubMed] [Google Scholar]
- 19.Gattringer T, Enzinger C, Fischer R, et al. IV thrombolysis in patients with ischemic stroke and alcohol abuse. Neurology 2015; 85: 1592–1597. [DOI] [PubMed] [Google Scholar]
- 20.Baik M, Nam HS, Heo JH, et al. Advanced liver fibrosis predicts unfavorable long-term prognosis in first-ever ischemic stroke or transient ischemic attack. Cerebrovasc Dis 2020; 49: 474–480. [DOI] [PubMed] [Google Scholar]
- 21.Wijarnpreecha K, Boonpheng B, Thongprayoon C, et al. The association between non-alcoholic fatty liver disease and atrial fibrillation: a meta-analysis. Clin Res Hepatol Gastroenterol 2017; 41: 525–532. [DOI] [PubMed] [Google Scholar]
- 22.du Plessis J, van Pelt J, Korf H, et al. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology 2015; 149: 635–648.e14. [DOI] [PubMed] [Google Scholar]
- 23.Verrijken A, Francque S, Mertens I, et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2014; 59: 121–129. [DOI] [PubMed] [Google Scholar]
- 24.Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005; 42: 473–480. [DOI] [PubMed] [Google Scholar]
- 25.Francque SM, van der Graaff D, Kwanten WJ.Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol 2016; 65: 425–443. [DOI] [PubMed] [Google Scholar]
- 26.Madan SA, John F, Pyrsopoulos N, et al. Nonalcoholic fatty liver disease and carotid artery atherosclerosis in children and adults: a meta-analysis. Eur J Gastroenterol Hepatol 2015; 27: 1237–1248. [DOI] [PubMed] [Google Scholar]
- 27.VanWagner LB, Wilcox JE, Colangelo LA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: a population-based study. Hepatology 2015; 62: 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruban A, Daya N, Schneider ALC, et al. Liver enzymes and risk of stroke: the Atherosclerosis Risk in Communities (ARIC) study. J Stroke 2020; 22: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]