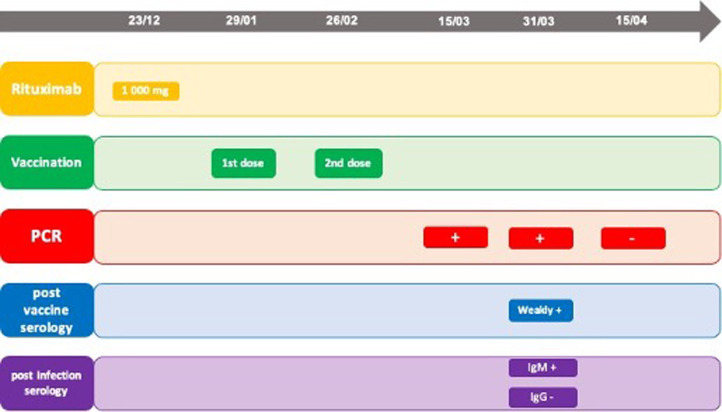
A 65 years old woman, known for seronegative rheumatoid arthritis associated with Sjögren's syndrome, presented in December 2020 a flare of her rheumatoid arthritis with multiple synovitis. Rheumatoid arthritis was diagnosed in 2011 and she was successfully treated with rituximab, every 6 months, since 2013. The last rituximab course (2 × 1000 mg infusions) was realized in June 2020 with a good efficacy and tolerance. Due to the epidemic context, we performed a single infusion of 1000 mg of rituximab on 23.12.2020. The total IgG level was 6.92 g/l in December 2020. Vaccination against the SARS-CoV2 was quickly available and the patient received her first RNA vaccine (Moderna) on January 29th, 2021. The second vaccination occurred on February 26th, 2021. Due to the onset of respiratory symptoms, a PCR was performed and showed the presence of SARS-CoV2 RNA on March 15th (Fig. 1 ). 2 weeks after her infection, a new PCR was performed on April 31st and showed the persistence of SARS-CoV2 RNA. We analyzed the different serologies of the patient on the 15th day of her infection. The post-infection serology showed the presence of IgM at a significant level (21.56 index (S/CO) while IgG were always negative. We evaluated the post-vaccination serology (anti protein S antibody) and this last one showed the presence of IgG at the limit of the signicativity. Two weeks later, on April 15th, 2021, a new PCR test was performed and showed the absence of viral RNA. The patient was then asymptomatic.
Fig. 1.
Chronology of the different findings. Chronology of events. PCR: polymerase chain reaction.
This is the first report of SARS-CoV2 infection after a well conducted vaccination in a patient treated with rituximab. Impairment of immunogenicity mRNA vaccine was also showed but this is the first case report of SARS-CoV2 infection after an mRNA vaccine [1]. Rituximab is known to be associated with more severe SARS CoV-2 [2], [3]. Moreover, recents findings suggested that the seroconversion is delayed by the treatment with rituximab secondary to the inhibition of the humoral response to SARS-CoV-2 infection by decreasing CD19+IgG+ [4] [5]. This delay in the seroconversion is present in our case and this is in line with hypothesis of Schulze-Koops et al. Than, Benucci et al. recommended that rituximab retreatment regimen at B cell recovery rather than fixed retreatment schedule might be safer. In our case, the total IgG level was at a satisfactory level for a new infusion. Finally, the serological response to the vaccination was poor. This element is of particular interest because it is the first case report showing a poor response to the SARS-CoV-2 ARN vaccine in a patient treated with rituximab. The lower vaccine response in rituximab-treated patients was known for pneumococcal and influenza virus vaccine [6]. Moreover, these elements can serve as a basis for reflection on the vaccination strategy in these patients. A third dose seems to be essential and the delay after the last treatment must be taken into account. It could be discussed to propose vaccination preferably at least 4 months after the last course of rituximab to optimize the vaccination strategy in these patients. The results of large cohorts will provide more answers to these questions.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Deepak P., Kim W., Paley M.A., et al. Glucocorticoids and B Cell Depleting Agents Substantially Impair Immunogenicity of mRNA Vaccines to SARS-CoV-2. medRxiv [Preprint] 2021 doi: 10.1101/2021.04.05.21254656. [DOI] [Google Scholar]
- 2.FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. 2020;80:527–538. doi: 10.1136/annrheumdis-2020-218310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avouac J., Drumez E., Hachulla E., et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. 2021;3(6):e419–e426. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulze-Koops H., Krueger K., Vallbracht I., et al. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218075. [annrheumdis-2020-218075] [DOI] [PubMed] [Google Scholar]
- 5.Benucci M., Quartuccio L., Li Gobbi F., et al. Persistence of rT-PCR-SARS-CoV-2 infection and delayed serological response, as a possible effect of rituximab according to the hypothesis of Schulze-Koops et al. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218590. [annrheumdis-2020-218590] [DOI] [PubMed] [Google Scholar]
- 6.Hua C., Barnetche T., Combe B., et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66:1016–1022. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]