Abstract
We believe the uterosacral ligaments (USLs) are an essential key to resolving the dilemma of diagnosing endometriosis non‐invasively. This editorial will utilise laparoscopic and ultrasonographic figures and videos, along with written descriptive techniques, to educate clinicians, sonographers, sonologists and radiologists on normal and abnormal USLs to improve knowledge and skill in scanning patients with possible endometriosis.
Keywords: endometriosis, non‐invasive diagnosis, pelvic pain, superficial endometriosis, ultrasonography, uterosacral ligament
Introduction
Patients with endometriosis face a significant delay in diagnosis between onset of symptoms and confirmation of disease presence.1, 2, 3 The underlying mechanism for this problem is multifactorial, but the most likely contributing factor is the sustained belief that surgery is required to diagnose endometriosis.4 Neither patients nor clinicians want to rely on invasive and risky investigations to simply diagnose a problem.5, 6
Non‐invasive ultrasonography for endometriosis is growing in utility and knowledge of this tool is spreading.7, 8, 9 It is becoming more widely understood that transvaginal ultrasound (TVS) can reliably diagnose deep endometriosis (DE),9 while it is already common knowledge that ovarian endometriomas (OEs) are accurately diagnosed on TVS. Unfortunately, it has long been thought, including by these authors, that the USLs were not visible on TVS7, 10 unless there was abnormality or fluid in the pouch of Douglas (POD).11 We believe this mentality has led to a lower diagnostic accuracy for USL DE than other areas of DE; a meta‐analysis demonstrated for USL DE on TVS, the mean sensitivity and specificity were 0.64 (95% CI 0.50–0.79) and 0.97 (95% CI 0.93–1.00), respectively.9 Moreover, it may be more difficult to visualise this structure compared to others; Guerriero et al.12 have demonstrated that trainees learning to interpret still images of the posterior compartment take the longest to become proficient in identifying USL disease compared to other areas.
In a large, observational study by Chapron et al.,13 DE of the USLs has been reported to be the most common site of DE in the pelvis, with 69.2% of patients with DE exhibiting USL DE. Our local data suggest that, of those who ultimately undergo laparoscopy, 35% have isolated superficial endometriosis (SE) and 33% have DE.14 A study that excluded all patients with DE reported that USL SE was present in 54% of patients.15 Though these studies with surgical findings harbour selection bias that impacts our understanding of disease prevalence, they still provide value in emphasising the importance of the USLs in endometriosis.
Beyond the ability to diagnose USL DE non‐invasively, there is significant value in mapping disease. A nodule within the USL may grow and exert extrinsic force on the ipsilateral ureter, leading to stricture and proximal hydroureter or hydronephrosis.16, 17 It has been proposed that USL nodules measuring 17 mm or greater should raise suspicion for ureteral involvement,16 which generally means infiltration of the disease into the parametrium has occurred. Parametrium is defined as the fibrous and fatty connective tissue that surrounds the uterus. Parametrial infiltration, even in the absence of ureteral involvement, increases the complexity of surgical resection.18 Unfortunately, there is a paucity of literature on evaluation of the lateral compartment and parametrium, from a preoperative diagnostic perspective,19 despite advancements in TVS for the diagnosis of DE.7 Exacoustos et al.20 describe sensitivities of identifying right and left parametrial DE of 67.9% and 78.8%, respectively. The ability to diagnose parametrial DE does yet not appear to have been evaluated systematically elsewhere, nor has there been anything on reproducibility been published. This may be related to the overall mediocre sensitivity of diagnosing nearby and related DE of the USL.9
Objective
In this paper, we aim to improve the understanding of the USLs and their involvement in endometriosis, provide technical tips on how to visualise normal and abnormal USLs on TVS and begin to appreciate the extremes of USL endometriosis – subtle findings suggestive of SE and severe DE that infiltrates the parametrium.
Normal anatomy
It is important to note that the distal insertion of the USL is the posterior cervix and vaginal dome. Sonographically, this is roughly at the level of the internal cervical os. The ligament then fans out caudally, where it merges with the lateral rectal ligaments and proximally to the sacrum.21 Buller et al.22 describe the distance between ureter and the cervical portion of the USL as 0.9 ± 0.4 cm. Figures 1 and 2 depict normal anatomy of the USLs (and surrounding tissue) on laparoscopy and 2‐dimensional (2D) TVS, respectively.
Figure 1.
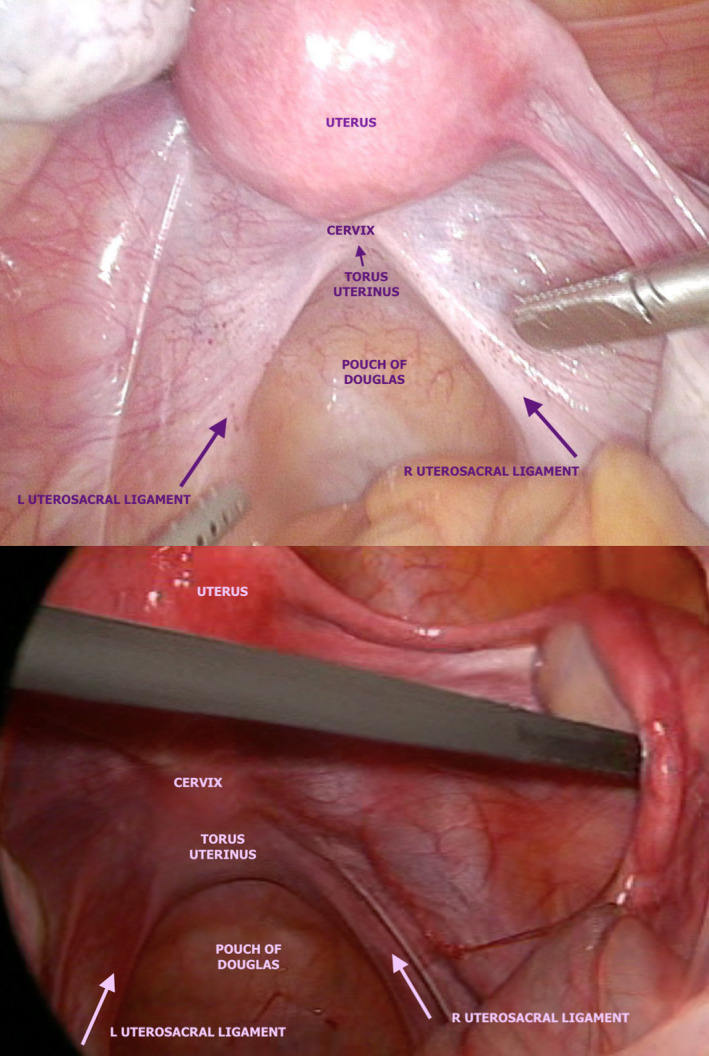
Laparoscopic Photographs Depicting Normal Pelvic Anatomy with Emphasis on the Normal Structure of the USL. L, Left; R, Right; USL, Uterosacral Ligament.
Figure 2.
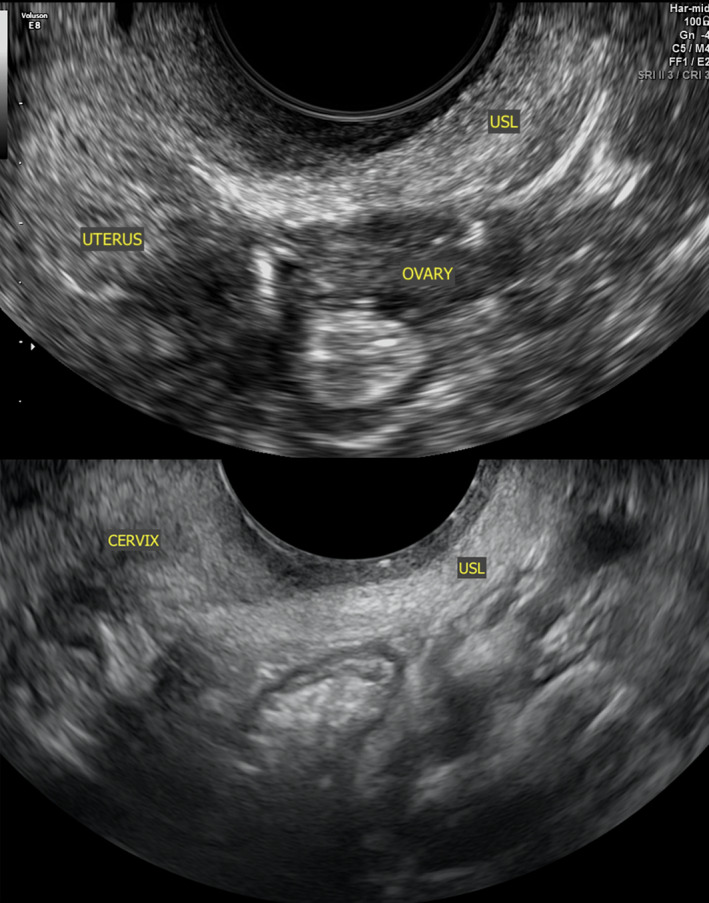
Sonographic Images Depicting Normal Anatomy of a USL, Represented by the Hyperechoic Band of Tissue Close in Proximity to the TVS Probe in an Oblique Sagittal Orientation. TVS, Transvaginal Ultrasound; USL, Uterosacral Ligament.
Technique to visualise normal USLs
Insert the TVS probe in the posterior vaginal fornix with the cervix and uterus anterior.
Decrease the penetration depth of field to have a small depth of field as the region of interest is now very near to the probe. Position the focal point nearest to the TVS probe.
- Begin with the probe in midsagittal position, angled toward the rectum. Attempt to visualise the hypoechoic vaginal mucosa, nearest to the probe, and hyperechoic the posterior pouch of Douglas (POD) peritoneum, the next layer after the vagina (Figure 3). It is this hyperechoic line that must be followed closely in the next step.
Figure 3.
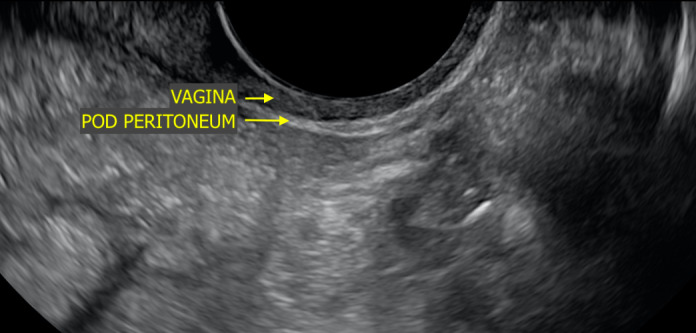 Sonographic Image Depicting Normal Anatomy of a Vagina and POD Peritoneum in Midsagittal Orientation. POD, Pouch of Douglas.
Sonographic Image Depicting Normal Anatomy of a Vagina and POD Peritoneum in Midsagittal Orientation. POD, Pouch of Douglas. For the left USL, slowly sweep the TVS probe to the right leg and rotate counter‐clockwise (usually not more than 45°); the hyperechoic line (peritoneum) should begin to thicken (Video S1). When you identify this region, stop when the hyperechoic line is thickest. This is the USL in sagittal section. If the probe bypasses the USL laterally, the heterogenous contents of the pelvic sidewall will be reached. Slowly reverse in the opposite direction.
The same can be repeated for the right USL, with the rotation in this case being clockwise.
Abnormal anatomy
As stated above, the USLs are the most common site of DE and they are involved in the majority of cases of isolated SE.13, 15 When there is severe disease in the posterior compartment and the USL is involved, it is sometimes not visible laparoscopically because of POD obliteration (Figure 4). This is partly why it is so important to recognise USL disease preoperatively using imaging; otherwise, the surgeons may not recognise the disease in the setting of significant anatomical distortion.
Figure 4.
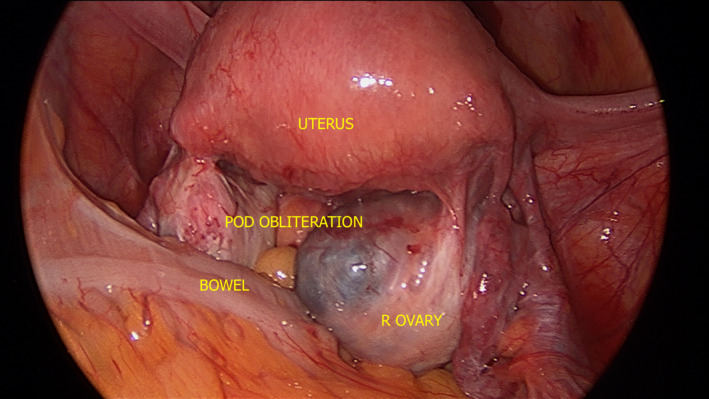
Laparoscopic Photograph Depicting ASRM Stage 4 Endometriosis with Complete POD Obliteration and R Ovarian Endometrioma. ASRM, American Society of Reproductive Medicine; POD, Pouch of Douglas; R, Right.
When a USL DE nodule is noted in our clinical practice, we evaluate whether the nodule is contained within the USL or extends into the parametrium and/or torus uterinus (defined as the thickening between the insertion of the USLs behind the posterior cervix; this area may also be known as the retrocervix). If there is infiltration into either of these structures, we document the proportion of the lesion that is within the USL versus the parametrium/torus uterinus, as per the recently published proposed USL DE classification system (Table 1),23 which was inspired by the classification system of submucosal leiomyomas.24 This system has not yet been validated and does not have demonstrated reproducibility beyond our unit, so we suggest use with caution for now. Figures 5 and 6 depict graphic representation of this USL DE Classification System.
Table 1.
Summary table of proposed nomenclature system for USL DE that is either confined to the USL or infiltrates the parametrium or torus uterinus
| USL DE Classification System | |
|---|---|
| USL‐parametrium | USL‐torus uterinus |
| Type 0: DE nodule is confined to the USL; No infiltration into parametrium or torus uterinus | |
| Type 1P: DE nodule partially infiltrating parametrium; >50% of DE nodule within USL | Type 1T: DE nodule partially infiltrating torus uterinus; >50% of DE nodule within USL |
| Type 2P: DE nodule significantly infiltrating parametrium; <50% of DE nodule within USL | Type 2T: DE nodule significantly infiltrating torus uterinus; <50% of DE nodule within USL |
DE, deep endometriosis; P, parametrium; T, torus uterinus; USL, uterosacral ligament.
Reprinted with permission from Wiley Publishers.23
Figure 5.
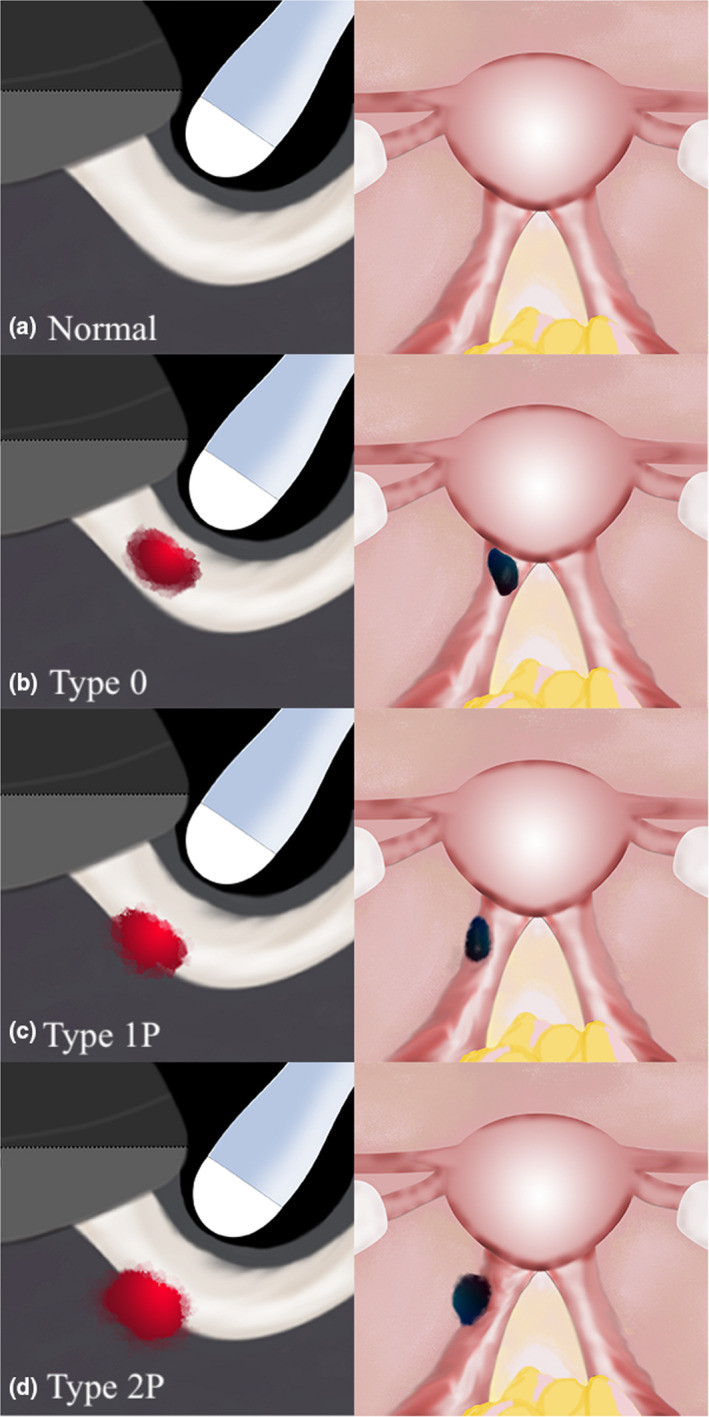
Sonographic (Oblique Sagittal Orientation) and Laparoscopic Graphic Depictions of USL DE with Varying Degrees of Infiltration into the Parametrium. (a) Represents a Normal Pelvis; (b) Represents a Type 0 USL DE Nodule; (c) Represents a Type 1P USL DE Nodule with >50% of the Nodule Within the USL; (d) Represents a Type 2P USL DE Nodule with <50% of the Nodule Within the USL. DE, Deep Endometriosis; USL, Uterosacral Ligament. Reprinted with Permission from Wiley Publishers.23
Figure 6.
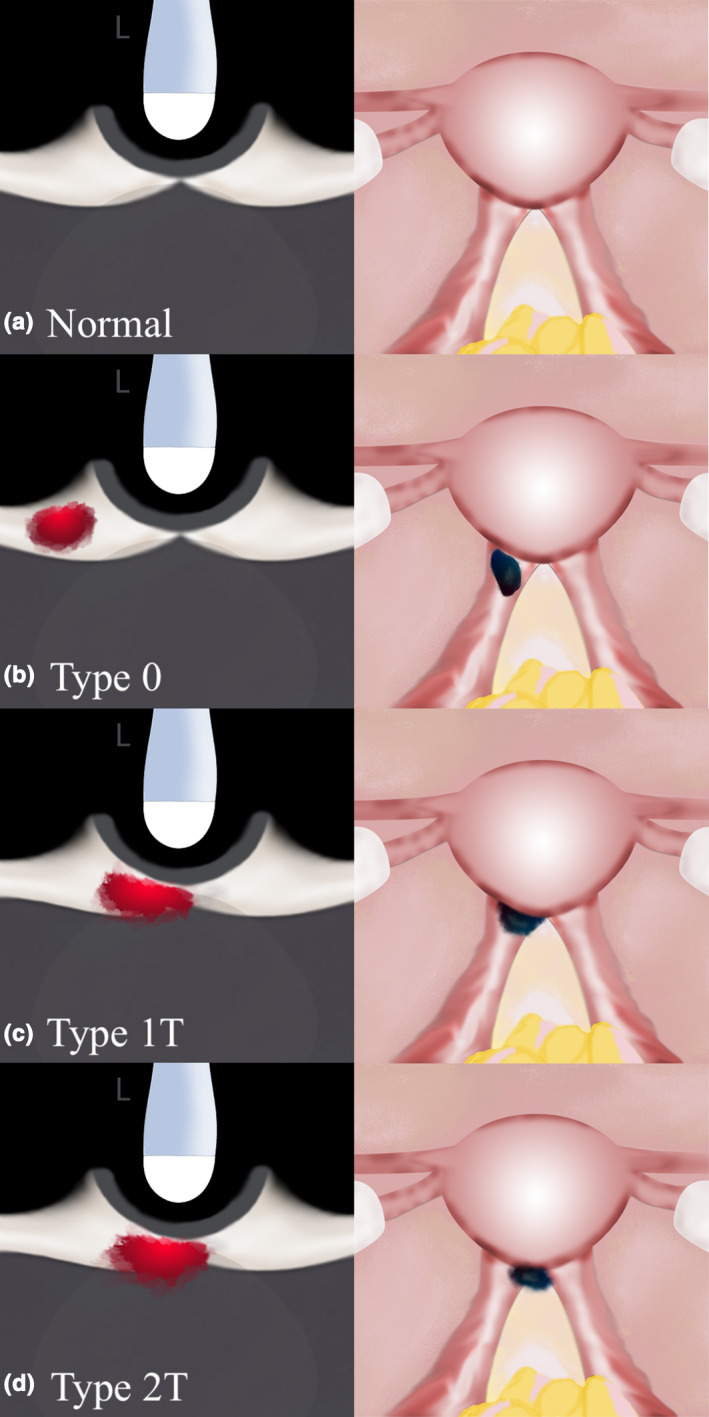
Sonographic (Transverse Orientation) and Laparoscopic Graphic Depictions of USL DE with Varying Degrees of Infiltration into the Torus Uterinus. (a) Represents a Normal Pelvis; (b) Represents a Type 0 USL DE nodule; (c) Represents a Type 1T USL DE Nodule with >50% of the Nodule Within the USL; (d) Represents a Type 2T USL DE Nodule with < 50% of the Nodule Within the USL. DE, Deep Endometriosis; USL, Uterosacral Ligament. Reprinted with Permission from Wiley Publishers.23
Figure 7 provides two laparoscopic views of USL DE. Figure 8 provides six 2D TVS views of USL DE, including Types 1P and Type 2P. Video S2 depicts a Type 1 USL DE nodule in the context of POD obliteration and bowel DE. In our experience, when USL DE lesions infiltrate the torus uterinus, there is generally POD obliteration and/or bowel DE present. Therefore, we cannot portray the laparoscopic view yet. However, Figure 9 presents the 2D TVS view of USL DE that infiltrates the torus uterinus.
Figure 7.
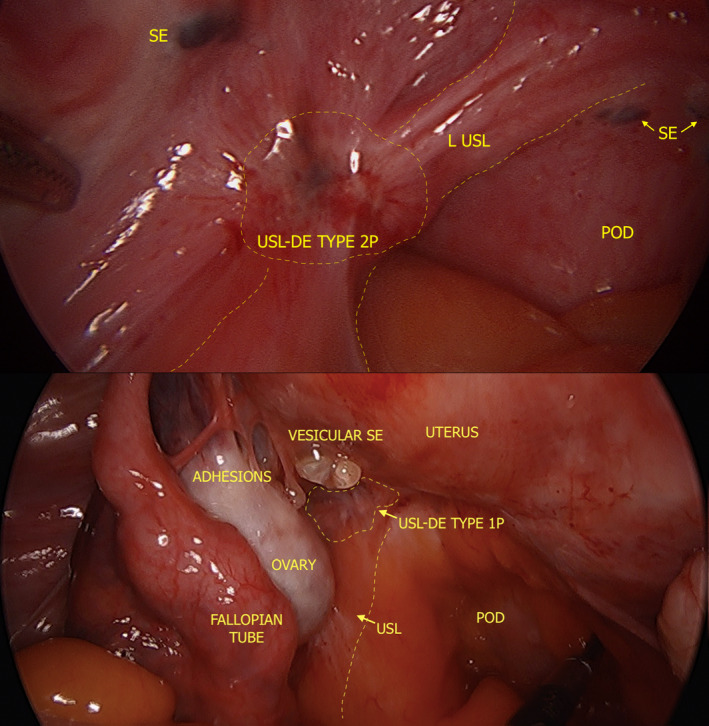
Laparoscopic Photographs Depicting USL DE [Type 2P (above), Type 1P (below)] and Adjacent SE. DE, Deep Endometriosis; POD, Pouch of Douglas; SE, Superficial Endometriosis; USL, Uterosacral Ligament.
Figure 8.
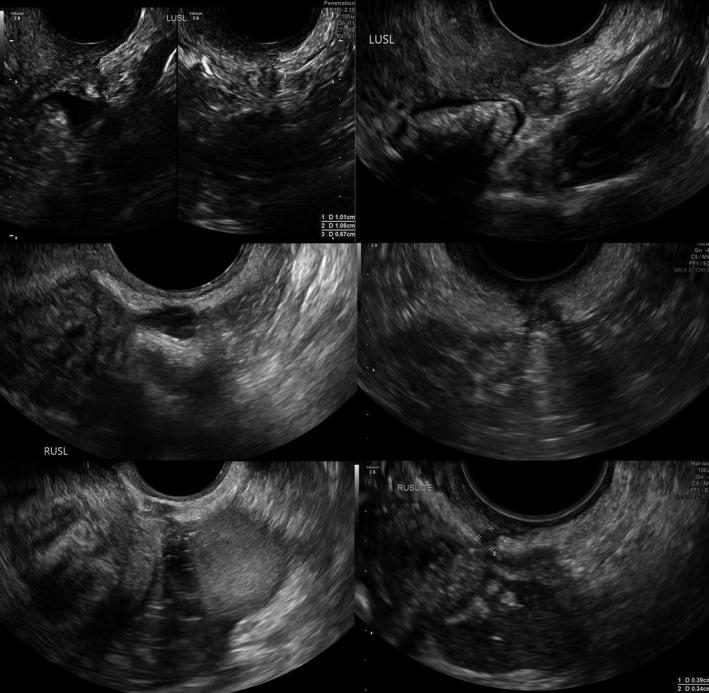
Sonographic Images Depicting Various Forms of USL DE. USL, Uterosacral Ligament; DE, Deep Endometriosis.
Figure 9.

Sonographic Images Depicting Various Forms of USL DE Infiltrating the Torus Uterinus [Type 2T (above) and Type 1T (below)]. DE, Deep Endometriosis; L, Left; R, Right; USL, Uterosacral Ligament.
Technique to describe abnormal USL
Follow above steps to visualise USL.
In the event of abnormality such as a hypoechoic lesion within the hyperechoic USL, this should be measured in three orthogonal planes as per the IDEA statement.7 In many cases, the DE nodules occur in the proximal aspect of the USL (i.e. closer to the insertion at the torus uterinus). Site‐specific tenderness is commonly encountered at the USLs and should be documented.7
Describe the USL DE lesion as per the USL DE Classification System.
- Evaluation of the ureters should be done for all patients undergoing a DE TVS. In women with noted USL/parametrial nodules, the presence of hydroureter should be especially assessed (by comparing both ureters and by assessing distal and proximal ureters, using the area adjacent to the nodule as a possible transitional reference point). Carfagna et al.25 describe ureteric diameter of ≥6 mm in all cases of ureteric dilation visualised surgically. Figure 10 demonstrates how close the ureter is to the USL and USL DE, when present, at laparoscopy.
Figure 10.
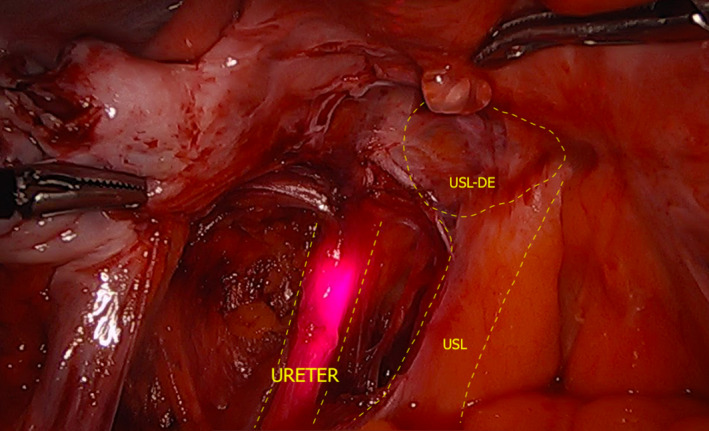 Laparoscopic Photograph Depicting the Close Proximity of the Left Ureter to a USL DE Nodule. The Ureter is Being Lit Up by Infrared‐Lighted Ureteral Stents. DE, Deep Endometriosis; USL, Uterosacral Ligament.
Laparoscopic Photograph Depicting the Close Proximity of the Left Ureter to a USL DE Nodule. The Ureter is Being Lit Up by Infrared‐Lighted Ureteral Stents. DE, Deep Endometriosis; USL, Uterosacral Ligament. If hydroureter is identified or the operator has a high degree of ureteral involvement, a transabdominal ultrasound should also be performed to assess for hydronephrosis.
Superficial USL endometriosis
This phenotype of disease has remained evasive from a non‐invasive imaging perspective.9 These lesions are very small, generally only a few millimetres in diameter (Figure 11). Though they may be visible on TVS, they are very difficult to appreciate (Video S3). Sometimes they are flush with the peritoneum on which the lesion is attached, making it nearly impossible to visualise on TVS. However, we propose that SE could be possibly visualised if there was fluid in the area of the lesion based on our experience with a feasibility study on a novel technique called saline‐infusion sonoPODography (SPG).26 In the case of SPG, we artificially introduce fluid into the POD via the uterus and patent fallopian tubes. This fluid provides a separation of tissues that are in or settle into the POD (i.e. small and large bowel). By filling this potential space with a clear fluid (anechoic on TVS), the contours of the pelvis are visible on TVS. Firstly, structures such as the USLs are more easily appreciated (Video S4). This is an important step in visualising disease and localising it using anatomical landmarks. Superficial lesions may appear as small hypoechoic areas, cystic lesions or hyperechoic foci overlying the peritoneum (Figure 12). Thin adhesions or peritoneal pockets may also be identified, and often these are in close proximity to the USLs.
Figure 11.
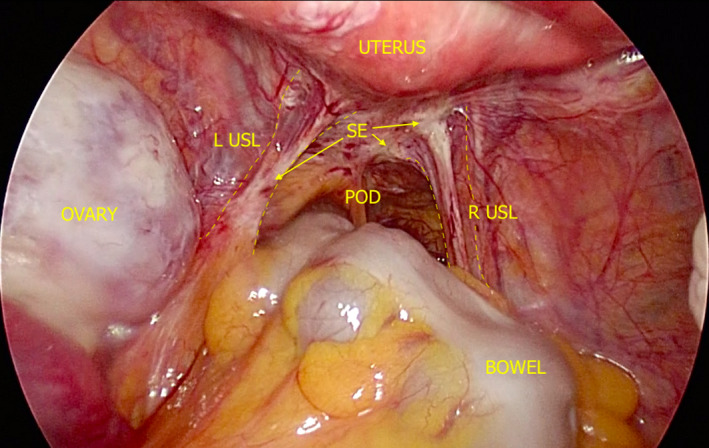
Laparoscopic Photograph Depicting SE of the USLs and POD. L, Left; POD, Pouch of Douglas; R, Right; SE, Superficial Endometriosis; USL, Uterosacral Ligament.
Figure 12.
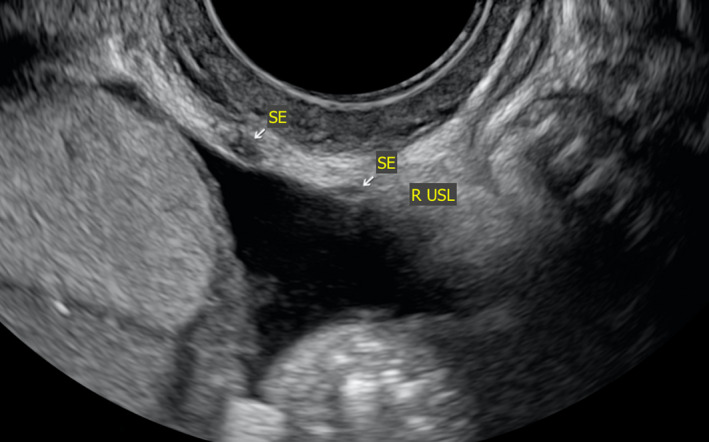
Sonographic Image Depicting USL SE Represented by Small Hypoechoic Areas Overlying the Peritoneum of the USL. R, Right; SE, Superficial Endometriosis; USL, Uterosacral Ligament.
Conclusion
We believe improving the understanding of the USLs is a central piece to reaching the goal of a non‐invasive diagnosis for endometriosis. Knowledge of USL endometriosis also has relevance to clinical and surgical practice. We have provided background on anatomy and endometriosis in this editorial as a foundation followed by technical tips on how to visualise the USLs and disease on TVS. Much prospective research still needs to be completed to demonstrate the diagnostic potential and reproducibility of some concepts discussed here. However, we recommend that all individuals performing and interpreting pelvic ultrasound make an effort to better understand the anatomy of the USLs. It is only when one begins to look and question what they are seeing when learning can happen.
Funding
None.
Conflict of interest
None.
Supporting information
Video S1. This clip depicts the lateral and oblique (clockwise) rotation of the transvaginal probe from midsagittal orientation to the right uterosacral ligament. At the beginning of the clip, one can appreciate the thin hyperechoic structure close in proximity to the transvaginal probe tip (just beyond the hypoechoic vagina). This represents the pouch of Douglas peritoneum. As the probe moves from midline laterally, the thickening of that peritoneum represents the uterosacral ligaments coming into view. Not surprisingly, uterosacral ligaments are not all in the same orientation, so the distance of lateral movement and degree of rotation varies from patient to patient.
Video S2. This clip depicts a uterosacral ligament deep endometriosis Type 0 nodule. This is represented by the circular‐shaped hypoechoic lesion in the hyperechoic band (i.e. uterosacral ligament) close in proximity to the transvaginal probe. Also in this clip is demonstration of an obliterated pouch of Douglas and bowel deep endometriosis.
Video S3. This clip depicts superficial endometriosis of the uterosacral ligament as represented by the small and irregular hypoechoic area sitting superficially on the uterosacral ligament. As the transvaginal probe moves from side‐to‐side, one can appreciate this area, which is up against the adjacent bowel.
Video S4. This clip depicts a normal uterosacral ligament with fluid in the pouch of Douglas. This fluid was introduced in an artificial way by a novel procedure called saline‐infusion sonoPODography. The presence of fluid in the pouch of Douglas does make it easier to identify the uterosacral ligaments and appreciate superficial endometriosis lesions, which would otherwise be potentially hidden by the overlying bowel. Fluid may be present in the luteal phase of the menstrual cycle, at which point, one could take advantage and attempt easier visualisation of the uterosacral ligaments.
References
- 1.Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril 2006; 86(5): 1296–301. [DOI] [PubMed] [Google Scholar]
- 2.Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand 2003; 82(7): 649–53. [DOI] [PubMed] [Google Scholar]
- 3.Hudelist G, Fritzer N, Thomas A, Niehues C, Oppelt P, Haas D, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod 2012; 27(12): 3412–6. [DOI] [PubMed] [Google Scholar]
- 4.Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. Br J Obstet Gynaecol 2004; 111(11): 1204–12. [DOI] [PubMed] [Google Scholar]
- 5.Singh SS, Suen MWH. Surgery for endometriosis: beyond medical therapies. Fertil Steril 2017; 107(3): 549–54. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, et al. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol 2019; 220(4): 354.e1–12. [DOI] [PubMed] [Google Scholar]
- 7.Guerriero S, Condous G, van den Bosch T, Valentin L, Leone FPG, Van Schoubroeck D, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol 2016; 48(3): 318–32. [DOI] [PubMed] [Google Scholar]
- 8.Piessens S. Is it time to include assessment of the most common gynaecological condition in the routine ultrasound evaluation of the pelvis? Australas J Ultrasound Med 2019; 22(2): 83–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nisenblat V, Bossuyt PMM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non‐invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016; (2): CD009591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonardi M, Condous G. How to perform an ultrasound to diagnose endometriosis. Australas J Ultrasound Med 2018; 21(2): 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazot M, Thomassin‐Naggara I, Nassar‐Slaba J, Cortez A, Darai E. Endometriose pelvienne et infertilite. J Radiol 2005; 86(10): 1375. [Google Scholar]
- 12.Guerriero S, Pascual MA, Ajossa S, Rodriguez I, Zajicek M, Rolla M, et al. Learning curve for the ultrasonographic diagnosis of deep endometriosis using a structured off‐line training program. Ultrasound Obstet Gynecol 2018. 10.1002/uog.20176 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Chapron C, Fauconnier A, Vieira M, Barakat H, Dousset B, Pansini V, et al. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod 2003; 18(1): 157–61. [DOI] [PubMed] [Google Scholar]
- 14.Reid S, Lu C, Casikar I, Reid G, Abbott J, Cario G, et al. Prediction of pouch of Douglas obliteration in women with suspected endometriosis using a new real‐time dynamic transvaginal ultrasound technique: the sliding sign. Ultrasound Obstet Gynecol 2013; 41(6): 685–91. [DOI] [PubMed] [Google Scholar]
- 15.Robinson AJ, Rombauts L, Ades A, Leong K, Paul E, Piessens S. Poor sensitivity of transvaginal ultrasound markers in diagnosis of superficial endometriosis of the uterosacral ligaments. J Endometr Pelvic Pain Disord 2018; 10(1): 10–7. [Google Scholar]
- 16.Lima R, Abdalla‐Ribeiro H, Nicola AL, Eras A, Lobao A, Ribeiro PA. Endometriosis on the uterosacral ligament: a marker of ureteral involvement. Fertil Steril 2017; 107(6): 1348–54. [DOI] [PubMed] [Google Scholar]
- 17.Ceccaroni M, Ceccarello M, Caleffi G, Clarizia R, Scarperi S, Pastorello M, et al. Total laparoscopic ureteroneocystostomy for ureteral endometriosis: a single‐center experience of 160 consecutive patients. J Minim Invasive Gynecol 2018; 26: 78–86. [DOI] [PubMed] [Google Scholar]
- 18.Mabrouk M, Raimondo D, Arena A, Iodice R, Altieri M, Sutherland N, et al. Parametrial endometriosis: the occult condition that makes the hard harder. J Minim Invasive Gynecol 2018; 26(5): 871–6. [DOI] [PubMed] [Google Scholar]
- 19.Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril 2017; 108(6): 886–94. [DOI] [PubMed] [Google Scholar]
- 20.Exacoustos C, De Felice G, Pizzo A, Morosetti G, Lazzeri L, Centini G, et al. Isolated ovarian endometrioma: a history between myth and reality. J Minim Invasive Gynecol 2018; 25(5): 884–91. [DOI] [PubMed] [Google Scholar]
- 21.Vu D, Haylen BT, Tse K, Farnsworth A. Surgical anatomy of the uterosacral ligament. Int Urogynecol J 2010; 21(9): 1123–8. [DOI] [PubMed] [Google Scholar]
- 22.Buller J. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol 2001; 97(6): 873–9. [DOI] [PubMed] [Google Scholar]
- 23.Leonardi M, Martins WP, Espada M, Arianayagam M, Condous G. A proposed technique to visualize and classify uterosacral ligament deep endometriosis with and without infiltration into the parametrium or torus uterinus. Ultrasound Obstet Gynecol 2019 10.1002/uog.20300. [DOI] [PubMed] [Google Scholar]
- 24.Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol 1993; 82(5): 736–40. [PubMed] [Google Scholar]
- 25.Carfagna P, De Cicco Nardone C, De Cicco Nardone A, Testa AC, Scambia G, Marana R, et al. Role of transvaginal ultrasound in evaluation of ureteral involvement in deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2018; 51(4): 550–5. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi M, Espada M, Lu C, Stamatopoulos N, Condous G. A novel ultrasound technique called saline infusion SonoPODography to visualize and understand the pouch of douglas and posterior compartment contents: a feasibility study. J Ultrasound Med 2019. 10.1002/jum.15022 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. This clip depicts the lateral and oblique (clockwise) rotation of the transvaginal probe from midsagittal orientation to the right uterosacral ligament. At the beginning of the clip, one can appreciate the thin hyperechoic structure close in proximity to the transvaginal probe tip (just beyond the hypoechoic vagina). This represents the pouch of Douglas peritoneum. As the probe moves from midline laterally, the thickening of that peritoneum represents the uterosacral ligaments coming into view. Not surprisingly, uterosacral ligaments are not all in the same orientation, so the distance of lateral movement and degree of rotation varies from patient to patient.
Video S2. This clip depicts a uterosacral ligament deep endometriosis Type 0 nodule. This is represented by the circular‐shaped hypoechoic lesion in the hyperechoic band (i.e. uterosacral ligament) close in proximity to the transvaginal probe. Also in this clip is demonstration of an obliterated pouch of Douglas and bowel deep endometriosis.
Video S3. This clip depicts superficial endometriosis of the uterosacral ligament as represented by the small and irregular hypoechoic area sitting superficially on the uterosacral ligament. As the transvaginal probe moves from side‐to‐side, one can appreciate this area, which is up against the adjacent bowel.
Video S4. This clip depicts a normal uterosacral ligament with fluid in the pouch of Douglas. This fluid was introduced in an artificial way by a novel procedure called saline‐infusion sonoPODography. The presence of fluid in the pouch of Douglas does make it easier to identify the uterosacral ligaments and appreciate superficial endometriosis lesions, which would otherwise be potentially hidden by the overlying bowel. Fluid may be present in the luteal phase of the menstrual cycle, at which point, one could take advantage and attempt easier visualisation of the uterosacral ligaments.


