Abstract
The adoption of point‐of‐care lung ultrasound for both suspected and confirmed COVID‐19 patients highlights the issues of accessibility to ultrasound training and equipment. Lung ultrasound is more sensitive than chest radiography in detecting viral pneumonitis and preferred over computed tomography for reasons including its portability, reduced healthcare worker exposure and repeatability. The main lung ultrasound findings in COVID‐19 patients are interstitial syndrome, irregular pleural line and subpleural consolidations. Consolidations are most likely found in critical patients in need of ventilatory support. Hence, lung ultrasound may be used to timely triage patients who may have evolving pneumonitis. Other respiratory pathology that may be detected by lung ultrasound includes pulmonary oedema, pneumothorax, consolidation and large effusion. A key barrier to incorporate lung ultrasound in the assessment of COVID‐19 patients is adequate decontamination of ultrasound equipment to avoid viral spread. This tutorial provides a practical method to learn lung ultrasound and a cost‐effective method of preventing contamination of ultrasound equipment and a practical method for performing and interpreting lung ultrasound.
Keywords: coronavirus infections, COVID‐19, pandemics, point‐of‐care systems, ultrasonography
Introduction
The adoption of point‐of‐care lung ultrasound (LUS) for initial assessment, review assessment, and clinical decision‐making in both suspected and confirmed COVID‐19 patients highlights the issues of accessibility to training and equipment without facilitating viral spread.1, 2 The key barriers to widespread use are training and contamination of ultrasound equipment. The traditional method of face‐to‐face supervised training of learners on patients raises the concern of increased disease transmission risk. Contamination and subsequent intensive decontamination of equipment can lead to significant delay in‐between use, resulting in less frequent LUS performed in settings where its use may be beneficial.
The earliest findings of lung involvement resemble interstitial syndrome (B‐lines). These could be used to timely triage and identify patients who may have evolving pneumonitis. Advanced lung findings of subpleural consolidation, interlobular and lobar consolidations should prompt practitioners to consider escalation of care. Consideration should be given to managing patients with any of these LUS findings as potential COVID‐19 pneumonitis in evolution with inpatient observation, repeat LUS and possible early novel interventional therapies. Those with no LUS pathology may need to undergo outpatient observation depending on institutional and local policies. Abnormal LUS findings should be documented and followed up with repeat LUS examinations.
This tutorial provides practical methods for teaching and learning how to perform and interpret LUS and minimising contamination of ultrasound equipment in COVID‐19 patients, whereby only routine cleaning rather than high‐level decontamination would be required, thus preventing restriction of the use of this very valuable diagnostic tool.
Review of lung ultrasound
Lung ultrasound is very sensitive and will detect small areas of lung consolidation (pneumonia), collapse, pleural effusion, interstitial syndrome (including pulmonary oedema) and pneumothorax. Using either a phased array or curvilinear probe, we recommend scanning three zones of each lung that approximates the upper and lower lobes (Figure 1). All parts of the lung should be examined to avoid missing lung pathology, although as the predominant site of COVID‐19 lung pathology is the posterior basal segments, it is suggested to scan these areas first (Figure 1).
Figure 1.
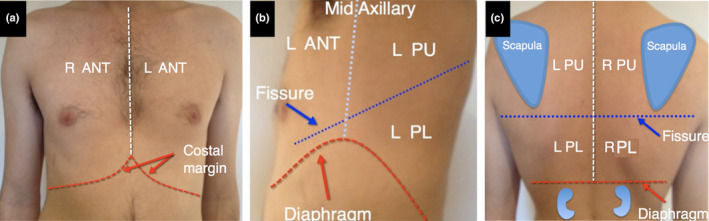
Lung zones. We recommend scanning only three zones on each side. The anterior zone corresponds to the anterior surface of the upper lobe (a). The middle lobes are small and may not be visible with ultrasound and hence are ignored. The posterior lower zone corresponds to the lateral and posterior surface of the lower lobe (b and c). The posterior upper zone corresponds to the lateral (b) and posterior (c) surface of the upper lobe. ANT, anterior; L, left; R, right; PU, posterior upper, PL, posterior lower.
Apart from identifying COVID‐19 pneumonitis, LUS is also effective at diagnosing other common causes of symptomatic respiratory disease that could be related to COVID‐19 such as consolidation in pneumonia, or perhaps unrelated like lung collapse (atelectasis), pleural effusion, pneumothorax and cardiogenic pulmonary oedema. During the initial stages of patient assessment in emergency departments, when lung ultrasound is used as an additional examination tool, the triage process is enhanced narrowing the differential diagnosis and more accurate patient disposition can be determined earlier.
Normal
Normal lung ultrasound findings appear as a bright (hyperechoic) pleural line immediately beneath the ribs that appears to move with respiration, which is due to sliding of the parietal and visceral pleura – lung sliding. A‐lines are artefactual copies (reverberation artefact) of the pleural line that appear as motionless horizontal white lines equally spaced below the pleural line (Figure 2, Video S1).
Figure 2.
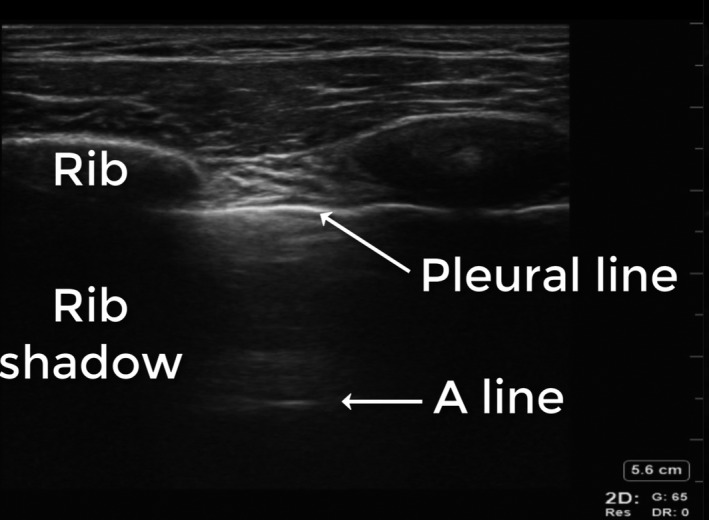
Normal. Normal lung ultrasound findings appear as a bright (hyperechoic) pleural line immediately beneath the ribs which is due to the highly ultrasound reflective parietal and visceral pleura. A‐lines are artefactual copies (reverberation artefact) of the pleural line that appear as motionless horizontal white lines equally spaced below the pleural line.
Lung collapse (atelectasis) and effusion
The key finding is of a grey tissue appearance. In contrast to pneumonia, the airways are collapsed and not fluid‐filled and hence the air bronchograms are immobile and there is usually substantial volume loss, seen as either effusion or displacement of the diaphragm or heart (Figure 3, Video S2).
Figure 3.
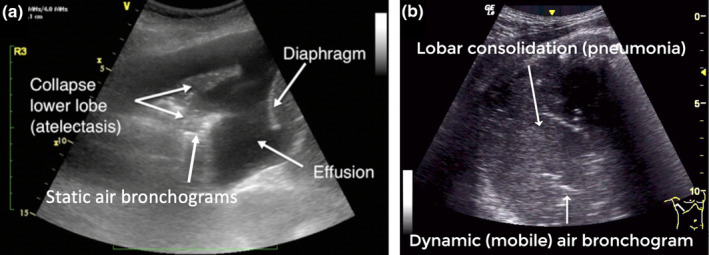
Atelectasis and consolidation (pneumonia). Lung ultrasound image of atelectasis (collapse) showing a grey tissue appearance, volume loss (effusion) and static air bronchograms (a). Lung ultrasound image of consolidation (lobar pneumonia) showing a grey tissue appearance, lack of volume loss and dynamic (mobile) air bronchogram (b). The movement of the dynamic air bronchogram is more apparent in the corresponding video.
Consolidation
The key finding is of a grey tissue appearance with minimal volume loss.3 Consolidation may represent multiple pathologies including pneumonia, contusion, neoplasia and pulmonary infarction such as in pulmonary embolism. In pneumonia, the lung is fluid‐filled, which results in mobile air bubbles (dynamic air bronchograms) and minimal loss of lung volume. Air bronchograms appear as bright white hyperechoic dots or linear pattern (Figure 3, Video S3).
Pleural effusion
The key finding is a black (anechoic) space between the lung, diaphragm and/or chest wall. The presence of ultrasound reflective material within the fluid is indicative of fibrinous and other debris within the fluid, which usually is suggestive of an inflammatory process. The volume can be estimated by multiplying the distance (in cm) between the diaphragm and the junction between the collapsed and inflated lung, which is essentially the distance between the parietal and visceral pleura at the end of expiration on a spontaneously breathing patient in supine position, by 200 to yield a volume in mL that is typically within 10–15% of the true volume (Figure 4, Video S2).4, 5
Figure 4.
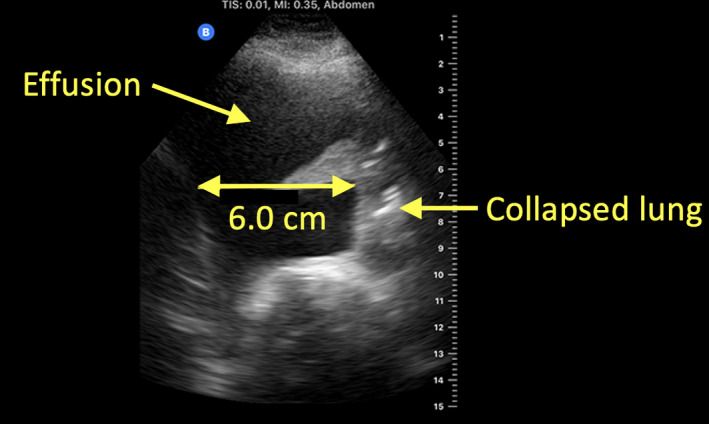
Pleural effusion. Simple pleural fluid conducts ultrasound well and is seen as a black space on ultrasound. Here, the estimated volume is 6.0 cm × 200 = 1200 mL.
Pneumothorax
Air prevents ultrasound transmission, which means that pneumothorax cannot be directly seen in a LUS, but the diagnosis is made based on the absence of lung sliding and presence of lung point. The lung (or contact) point seen in pneumothorax is the visible junction between the pleura separated by air and the pleura not separated by air. Lung sliding is the visible movement at the pleural line beneath the ribs that represents the apposed parietal and visceral pleura that rub against each other during respiration (Figure 5, Video S4). In a metanalysis, Ding et al.6 showed that absence of lung sliding and presence of lung point are highly specific for pneumothorax. On the other hand, false negatives may occur in patients with, for instance, loculation preventing air from rising to highest point of the chest.7 In other words, presence of lung sliding sign rules out only pneumothorax underneath the probe.
Figure 5.
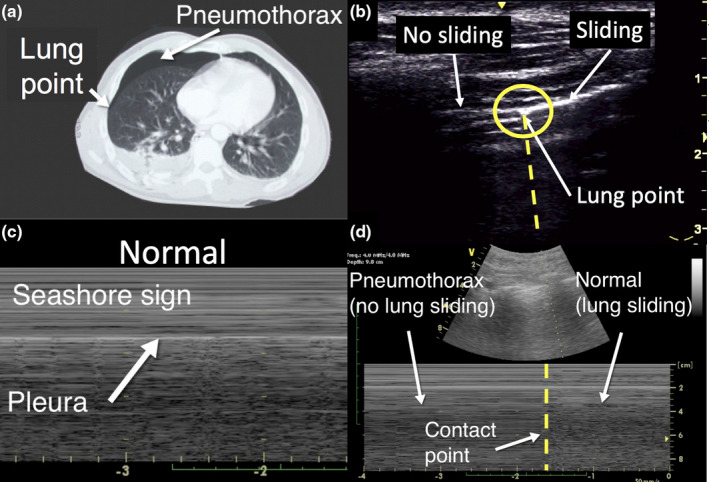
Pneumothorax. The computed tomography figure (a) of a pneumothorax shows the lung (contact) point of separation of the parietal and visceral pleura. The lung ultrasound image shows the lung point at the intersection of lung sliding on the right and no lung sliding on the left (b). The corresponding lung ultrasound M‐mode image demonstrates lung sliding on the right and no lung sliding on the left of the lung point, a vertical transition zone (d). the lung ultrasound image (c) shows normal lung sliding (seashore sign).
Interstitial syndrome
This group of respiratory disorders is characterised by increased number and spread (bilateral) of mobile long laser‐like B‐lines that travel from the pleural line away from the ultrasound transducer all the way to the bottom of the screen without fading. Respiratory disorders that commonly cause generalised (bilateral) B‐lines include pulmonary oedema, pulmonary fibrosis, pneumonitis and acute respiratory distress syndrome (ARDS). COVID‐19 has the typical appearance of viral pneumonitis that may develop into ARDS and is cited in more detail below. B‐lines may also appear adjacent to focal disorders such as lung collapse, consolidation, neoplasia, pleural disease, pulmonary infarction and contusion (Figure 6, Video S5).
Figure 6.
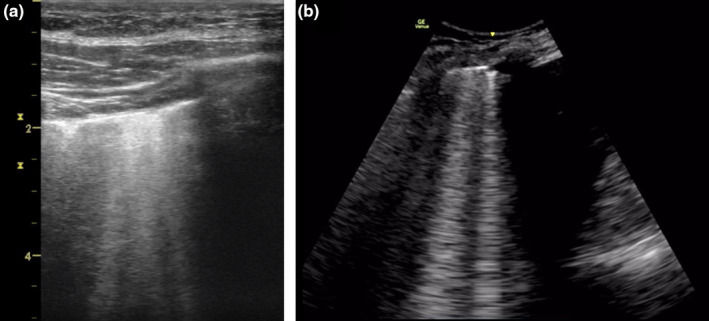
Interstitial syndrome. Lung ultrasound findings in interstitial syndrome shows multiple laser‐like hyperechoic (white) B‐lines originating from the pleural line traversing the sector at the anterior lung zone (a) and posterior lower lung zone (b) where there is also an effusion.
Utility of lung ultrasound
The key role of LUS may be to identify patients with early interstitial pneumonitis, which may reflect pneumonitis in evolution by the detection of even subtle or minor abnormalities. This could allow patients to be isolated, admitted for observation or return as outpatients for review ultrasound scan the following day, even when asymptomatic. It may also be the logical time to consider novel interventions or treatments. Repeated scans may detect progression during evolution or improvements during recovery. In severe cases, consolidations predominantly posterior locality may prompt a decision for prone positioning (Figure 7).8
Figure 7.
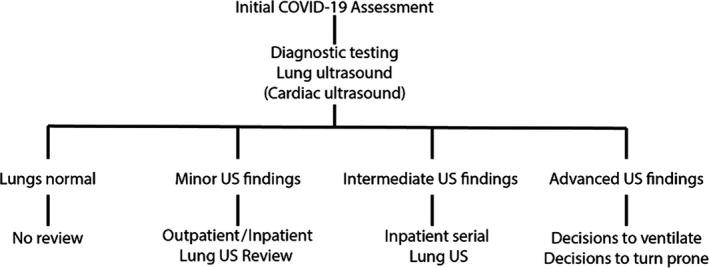
Considerations for use of lung ultrasound in clinical practice. The use of lung ultrasound may be of assistance in triage of patients with suspected or confirmed COVID‐19 respiratory infection.
Benefits of lung ultrasound
Portability and low cost.
No ionising radiation exposure
High diagnostic accuracy [strong correlation with computed tomography (CT)].
Able to accurately identify other pathology that can be linked to respiratory symptoms.
Earlier detection than chest X‐ray.
Lower risk of contamination compared to CT and X‐ray.
Repeatable and can be used for monitoring.
Limitations of point‐of‐care lung ultrasound
Cannot detect pathological features limited to deep within the lung (where air is present between the lesion and the chest wall).
Accuracy strongly influenced by training and experience of the operator.
Potential for human transmission of COVID‐19.
The non‐specific nature of the signs makes the modality very dependent on base rate prevalence of COVID‐19.
Ultrasound in COVID‐19 infection
A characteristic of COVID‐19 is a common progression in the LUS signs, which may be used to predict the stage of disease and even likelihood of clinical deterioration. Diverse ultrasound patterns can be found in COVID‐19 patients. However, in most cases, findings are similar to other viral interstitial pneumonia that may progress to ARDS.9 A summary of the key features is presented below.
Lung ultrasound findings in COVID‐19 infection
Ultrasound is more sensitive than plain chest X‐ray at detecting abnormality. Any abnormality of LUS is likely to reflect some degree of lung inflammation and could represent an early finding in a patient that may later deteriorate. It is suggested that a regimen of close follow‐up with serial ultrasounds may provide an accurate monitor and provide more insights into the sequelae of disease COVID 19. More advanced findings reflect greater lung involvement and may be of assistance in admission and early intervention approaches. Late findings may also be of benefit in decisions regarding the institution of invasive support or rescue therapies in ventilated patients, such as prone positioning (indicated by consolidation).10, 11, 12, 13
Early ultrasound findings
The earliest findings are isolated patchy areas of B‐lines (interstitial syndrome) due to inflammatory lung oedema. B‐lines are also seen in cardiogenic pulmonary oedema. In COVID‐19, unlike pulmonary oedema, there are ‘skipped’ areas of normal lung without B‐lines and there is also irregular thickening of the outer lung lining (pleural line) (Figure 8). Although COVID‐19 pneumonitis is typically bilateral, localised interstitial syndrome could suggest an early phase of the disease. These ultrasound findings often precede chest X‐ray alterations and clinical deterioration.14, 15
Figure 8.
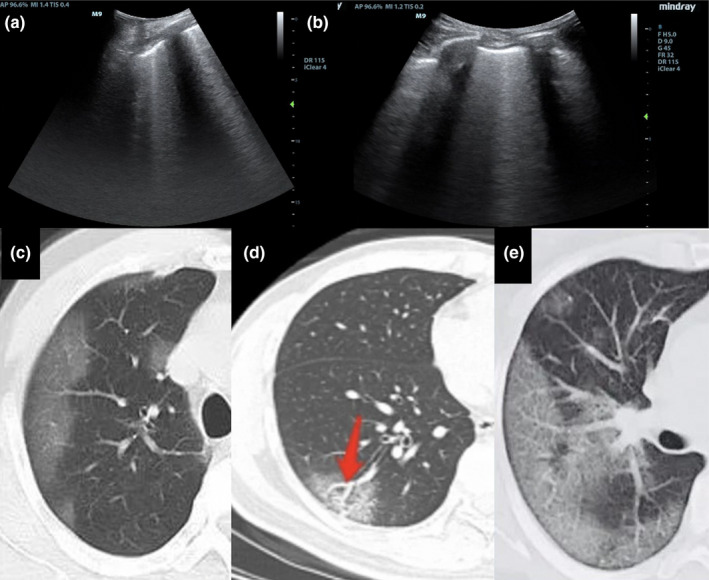
Lung ultrasound and CT findings in early stage COVID‐19 pneumonia. Lung ultrasound shows B‐lines and thickening of the pleural line (a and b). Computed tomography shows ground‐glass opacification in mainly a peripheral distribution (c), bronchovascular thickening (d) and ‘crazy paving’ (e).
Intermediate ultrasound findings
Thickened and irregular appearance of the pleural line and more prominent and confluent B‐lines (Figure 9).15
Figure 9.
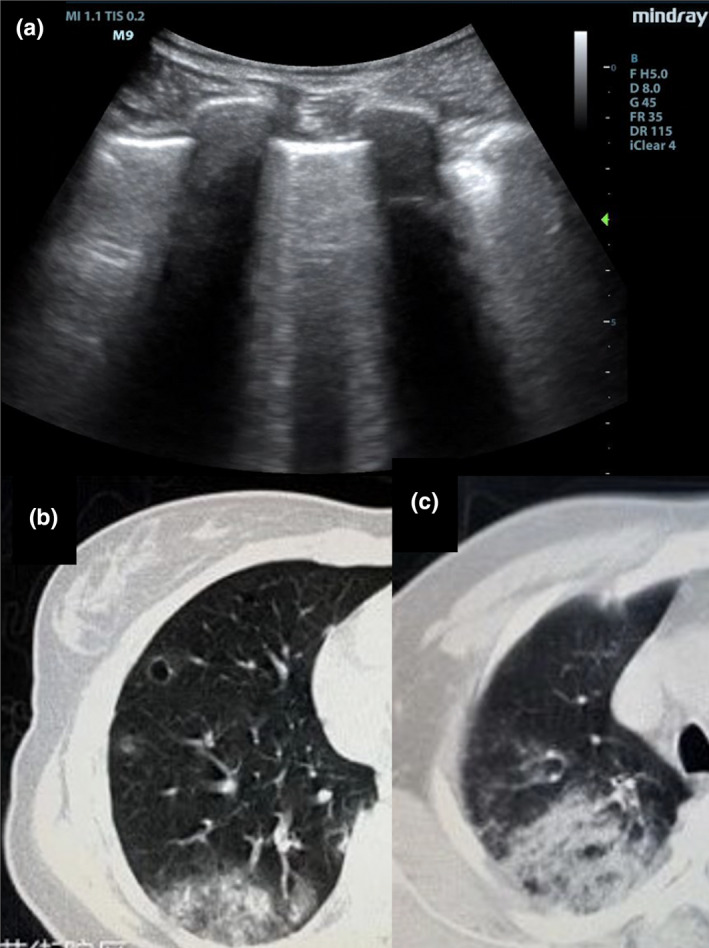
Lung ultrasound findings in intermediate stage COVID‐19 pneumonia shows thickened and irregular pleura and confluent B‐lines(A). Computed tomography shows ground‐glass opacification in increased quantity, density, and area of distribution (B + C).
Late ultrasound findings
The presence of small areas of consolidation adjacent to the pleural line (subpleural consolidation), which appear like solid tissue. These are usually no larger than 1 cm in diameter and often have B‐lines adjacent to the consolidated areas (Figure 10), which separates them from other types of pneumonia that are usually deeper or involve whole segments or lobes.16 Infarcted subpleural consolidations demonstrated by lack of colour flow Doppler pulsatility have been reported.17 This could be due to microangiopathy, as described recently by autopsies series from the United States and a potential association of COVID‐19 with thrombotic complications such as pulmonary embolism.18, 19 However, this finding is not specific of COVID‐19. Pleural effusion and isolated lobar consolidation are uncommon in COVID‐19 respiratory infection and should prompt a search for an alternative diagnosis.15
Figure 10.
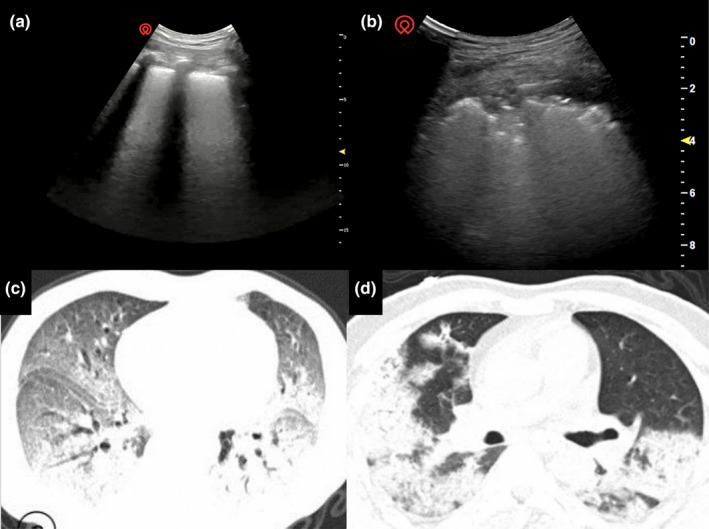
Computed tomography demonstrates bilateral lung infiltrate with two‐thirds of the lung field affected, consolidations with evidence of air bronchogram and fibrous strips. The patient exhibited evidence of ARDS clinically (C + D)
Recovery
The severity of the lung involvement reaches a peak during the second week since symptoms onset.20 In critical cases, recovery may involve several weeks. A‐lines, reverberation artefacts of the pleural line that are a normal finding, will emerge in the transition to a normal lung pattern (Figure 2).21 During the assessment of these patients, storing the ultrasound images is highly recommended to be used for later comparison.15
Infectious control measures
Ultrasound procedures on the chest surface are typically considered a low infectious risk procedure due to there being no contact with broken skin, mucous membranes, blood or bodily fluid. However, given COVID‐19 is caused by a highly contagious virus where aerial droplets, aerosols and contact transmission are thought to be possible, rigorous infectious control measures are imperative following each suspected and confirmed COVID‐19 patient interaction to avoid contamination of the ultrasound device and onward transmission of the virus. The issues of infection precaution vary between the different sizes of ultrasound machine.22, 23
Handheld ultrasound machines
Handheld ultrasound devices are the ideal option to assess COVID‐19 patients as the entire device can be introduced into a single‐use plastic sheath.
Larger ultrasound machines
Clinicians may choose to utilise other forms of portable ultrasound devices for a number of reasons that include image quality, device availability and familiarity. The main challenge with the larger ultrasound machines is that they are difficult to be fully covered and operated with plastic covers. When exposed to COVID‐19, they are also time‐consuming to decontaminate. These machines may also encounter electrical issues with liquid decontamination as they are not usually waterproof. Lastly, the cooling fans commonly seen in these larger machines could increase the likelihood of COVID‐19 transmission by aerosol or droplets.
Infectious precautions should follow the policy of your local healthcare institution and are influenced by resources and contagious risk of the patient. The list below can be downloaded and laminated for bedside use (Table S1).
Before the ultrasound examination
Familiarise with and adhere to the ‘5 Moments for Hand hygiene’ before, during and after performing ultrasound.
Remove all unnecessary accessories from the ultrasound machine.
The console and display should be cleaned prior using low‐level instrument grade cleaning agents or wipes.
Cover the machine with a plastic sheet.
Wear personal protective equipment in accordance with local policy.
Preferably, arrange an assistant who remains clean to operate the machine controls. The probe, placed in a sheath, is only used by the person performing the scan.
Use ultrasound gel from single‐use gel sachets, for example 1 large or 2 small sachets, rather than multidose containers. Alternatively, a large volume of gel can be dispensed from a multidose into a disposable container, which can be discarded in the room.
Bring wipes for the patient and the machine if not easily accessible at patient bedside.
In emergency departments, allocate dedicated ultrasound machines to the suspected COVID‐19 area (Hot Zone) where possible. Depending on the cohort and prevalence of disease, certain emergency department have dedicated areas for suspected and confirmed patients with COVID‐19 patients.
During the ultrasound examination
Limit the length of the examination to reduce exposure to the healthcare workers.
Limit the use of ultrasound to answering pertinent clinical questions.
Delay performing measurements to after the examination.
Allow the most experienced operator available to perform the ultrasound. If performed by a non‐expert, real‐time remote supervision is recommended.
Avoid touching any objects in the patient area.
After the ultrasound examination
For larger machines, the entire machine must be cleaned twice using low‐level instrument grade cleaning agents or wipes. First, immediately after completing the scan at the patient’s bedside. Second, away from the patient’s bedside wearing clean personal protective equipment. If no assistant is available to move the machine, consider pushing it with your feet to avoid direct hand contact.
For small portable devices that fit entirely into a protective sheath, extraction from the sheath without contamination is paramount.
Adhere to the wait time advised by the disinfectant and device manufacture instructions to ensure proper disinfection.
If contact of the device with non‐intact skin, blood, body fluid or mucous membrane is suspected or confirmed, high‐level disinfection should be undertaken according to local guidelines. The process can be time‐consuming.
How to use an entire ultrasound machine in a sterile sheath
All non‐portable ultrasound machines offer the facility to place an ultrasound probe into a conventional sterile sheath as is routinely used for ultrasound‐guided procedures. However, the console and display unit may not be adequately protected by a plastic barrier.
All portable ultrasound machines have the advantage of only requiring one operator to perform the scan, to operate the controls, and to acquire images or videos. However, very few are small enough to fit within a conventional sterile sheath and even fewer can adequately acquire images and videos for archiving to a PACS.
The need to change frequency settings or probes is fortunately mitigated for COVID‐19 patients as both the lung and cardiac ultrasound examinations can be performed with the same probe and similar frequencies. However, the pre‐sets for lung and cardiac examinations vary significantly and alterations are required in order to acquire images of adequate quality.
Smartphone in a plastic sheath
The only control unit currently available that allows both good image visualisation as well as ease of control when wearing gloves is the touch sensitive smartphone such as an iPhone or Android device. Also, all smartphones currently in use will fit into the conventional plastic sheath used for sterile procedures. Thereby the entire ultrasound machine can be protected by a physical barrier (Figure 11).
Figure 11.
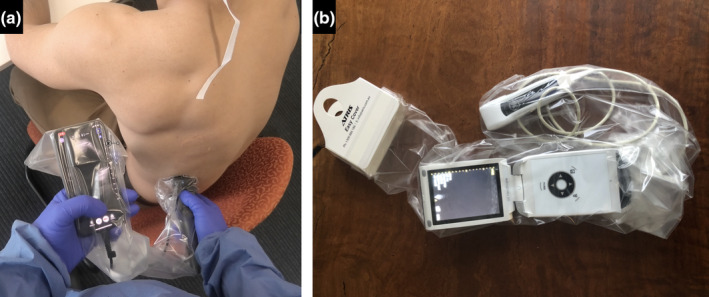
Protective barrier for handheld ultrasound devices. Portable ultrasound machines capable of fitting into a conventional sterile plastic sheath. Butterfly IQ (a) (Butterfly Network, Inc., Guilford, Connecticut, United States) and V‐scan (b) (GE Healthcare, Chicago, Illinois, United States).
The smartphone can be connected to a Butterfly iQ device (Butterfly Network, Inc., Guilford, Connecticut, United States), which has novel features of advantage for this purpose:
The probe is multipurpose, able to cater for all purposes by alteration of frequency and pre‐sets.
Connected to conventional smartphones.
High‐resolution images on smartphone able to be clearly seen through plastic sheath.
Control of the full functionality of the device possible through the plastic sheath when wearing gloves.
Ability to archive images and videos to PACS or cloud for later reference.
Full control of the ultrasound machine by one operator only.
Preparations prior to the examination
The operator prepares the equipment in an adjacent room prior to entering the room containing the COVID‐19 patient. For this examination, no specific sterile procedure technique is necessary although a similar but sterile technique could be used for sterile procedures such as vascular access (Video S6).
The cord of the Butterfly iQ device should be mostly wrapped around the device leaving a short length of cord to separate the smartphone and the device. This facilitates placement within the sterile sheath, whereas a full length of cord makes insertion into the sheath difficult. The smartphone is then attached such that it lies in close proximity to the Butterfly iQ device. Open the sterile plastic sheath, place half the sterile gel into the sheath, then place the probe into the sheath and grasp the probe through the plastic. Grasp the paper border of the plastic sheath and extend the sheath to incorporate the smartphone entirely for some distance. Optionally, tie the plastic sheath near the cardboard sheath holder, after evacuating most of the air (Video S7).
Afterwards, the operator dresses in barrier gown, gloves, mask and eye protection. Prepare the trolley to be used to remove the ultrasound machine following use and take it into the room with the known or potential COVID‐19 patient. The trolley should be covered in a soft layer (e.g. sterile theatre drape) and so as to cushion the Butterfly and smartphone should they fall a few centimetres from the sterile sheath onto the trolley, so as not to damage them.
Conducting the examination
If this study forms part of a screening assessment, and then in addition, nasopharyngeal swabs should be taken prior to the ultrasound examination.
Conduct the lung and cardiac ultrasound examination according to the protocols previously outlined. If time does not permit, concentrate imaging on the posterior basal segments of the lungs since the majority of findings are present in this location. Adjust the settings, acquire images and archive as necessary. It is strongly recommended that images and video with full patient details are acquired and stored on the local hospital PACS, cloud storage or both.
Extraction of the ultrasound device without contamination after the examination
Retrieval of the Butterfly iQ probe and smartphone from the plastic sheath in a manner that does not contaminate either is essential in order to preserve the ultrasound machine for immediate use on the next patient. Without contamination, the device may be cleaned using the approved wipes available at the hospital.
A disposable set of sterile scissors is required in order to cut the plastic sheath near to the smartphone. The smartphone and Butterfly iQ are then gently lowered out of the plastic sheath without the sheath touching the trolley and its drapes. There is a risk that the smartphone or Butterfly iQ may ‘fall out’ of the plastic sheath onto the trolley and hence it is wise to include some padding during preparation of the trolley. The plastic sheath is then disposed of with the protective gown and gloves in the prescribed manner. The Butterfly iQ and smartphone are then wiped clean with the recommended wipes (Video S8).
Conclusion
Point‐of‐care application of LUS for initial assessment, review assessment and clinical decision‐making in both suspected and confirmed COVID‐19 patients is currently being adopted into clinical practice. However, adequate training and decontamination of ultrasound equipment are some of the key barriers to a more ubiquitous adoption. This tutorial outlines the main LUS findings and a technique for the avoidance of contamination of ultrasound equipment in COVID‐19 patients.
Author contributions
Ximena Cid: Conceptualization (equal); Methodology (lead); Writing‐review & editing (lead). Andrew Wang: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Johan Heiberg: Conceptualization (supporting); Methodology (lead); Writing‐original draft (lead). David Canty: Conceptualization (equal); Data curation (lead); Methodology (lead); Writing‐review & editing (lead). Colin Royse: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Xiaoqiang Li: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Doa El‐Ansary: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Yang Yang: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). kavi haji: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Darsim Haji: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Andre Denault: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Lynda Tivendale: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Kyle Brooks: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Alistair Royse: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal).
Authorship statement
The authorship listing conforms to the journal’s authorship policy, and all authors are in agreement with the content of the submitted manuscript.
Funding
There are no funding disclosures.
Conflict of interest
Colin Royse, Alistair Royse and David Canty are employees of the University of Melbourne. Educational content is available for sale in Australia.
Supporting information
Video S1 Normal. Normal lung ultrasound findings appear as a bright (hyperechoic) pleural line immediately beneath the ribs that appears to move with respiration, which is due to sliding of the parietal and visceral pleura – lung sliding. A‐lines are artefactual copies (reverberation artefact) of the pleural line that appear as motionless horizontal white lines equally spaced below the pleural line.
Video S2 Atelectasis. Lung ultrasound of atelectasis demonstrates grey tissue appearance with volume loss (effusion) and static (motionless) air bronchograms (white dots).
Video S3 Consolidation (pneumonia). Lung ultrasound of consolidation demonstrates grey tissue appearance without volume loss (no effusion). The presence of dynamic (mobile) air bronchograms (white dots) reflects pneumonia.
Video S4 Pneumothorax. Lung ultrasound demonstrating pneumothorax. There is initially no lung sliding seen but then during inspiration a lung point is seen to slide along the pleural line from right to left with lung sliding following behind the lung point, which then slides away to the right with expiration.
Video S5 Interstitial syndrome. Interstitial syndrome is characterised as more than two B‐lines in an interspace, which are hyperechoic (bright white) laser‐like lines originating from the pleural line and extending to the bottom of the display that move with respiration.
Video S6 Setup prior to entering patient room. Insertion of probe and smartphone entirely within the plastic sheath.
Video S7 Entering room and conduct examination. Entering the room with the COVID‐19 patient and conduct the examination.
Video S8 Removal of the ultrasound device and smartphone without contamination. The plastic sheath is cut with sterile scissors and the device is then removed without the sheath coming into contact with the recipient trolley or drapes.
Table S1 Infectious control measures. Infectious control measures before, during, and after a lung ultrasound examination in suspected and confirmed COVID‐19 patients.
Acknowledgements
We thank Dr. Hongli Bai (Department of Radiology, West China Hospital, Sichuan University) and Dr. Wentao Bao (Department of Respiratory Intensive Care Unit, Tai'an City Central Hospital) for sharing their valuable insight and COVID‐19‐related clinical images. We acknowledge the significant contributions to performing this manuscript from the administrative staff of the Ultrasound Education Group, the University of Melbourne.
References
- 1.Canty D, Haji K, Denault A, Royse A. Lung ultrasound in anaesthesia and critical care medicine. In: Stuart‐Smith K, ed. Perioperative medicine – current controversies. New York, US: Springer; 2016. 345–389. [Google Scholar]
- 2.Ford JW, Heiberg J, Brennan AP, Royse CF, Canty DJ, El‐Ansary D, et al. A pilot assessment of 3 point‐of‐care strategies for diagnosis of perioperative lung pathology. Anesth Analg 2016; 124: 734–42. [DOI] [PubMed] [Google Scholar]
- 3.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012; 38: 577–91. [DOI] [PubMed] [Google Scholar]
- 4.Balik M, Plasil P, Waldauf P, Pazout J, Fric M, Otahal M, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med 2006; 32: 318. [DOI] [PubMed] [Google Scholar]
- 5.Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta‐analysis. Chest 2011; 140: 859–866. [DOI] [PubMed] [Google Scholar]
- 6.Gillman LM, Alkadi A, Kirkpatrick AW. The "pseudo‐lung point" sign: all focal respiratory coupled alternating pleural patterns are not diagnostic of a pneumothorax. J Trauma 2009; 67: 672–3. [DOI] [PubMed] [Google Scholar]
- 7.Ibitoye BO, Idowu BM, Ogunrombi AB, Afolabi BI. Ultrasonographic quantification of pleural effusion: comparison of four formulae. Ultrasonography 2018; 37: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mongodi S, Pozzi M, Orlando A, Bouhemad B, Stella A, Tavazzi G, et al. Lung ultrasound for daily monitoring of ARDS patients on extracorporeal membrane oxygenation: preliminary experience. Intensive Care Med 2018; 44: 123–124. [DOI] [PubMed] [Google Scholar]
- 9.Peng QY, Wang XT, Zhang LN. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med 2020;46 :849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonsenso D, Pata D, Chiaretti A. COVID‐19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med 2020; 8: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buonsenso D, Piano A, Raffaelli F, Bonadia N, de Gaetano Donati K, Franceschi F. Point‐of‐Care Lung Ultrasound findings in novel coronavirus disease‐19 pnemoniae: a case report and potential applications during COVID‐19 outbreak. Eur Rev Med Pharmacol Sci 2020; 24: 2776–2780. [DOI] [PubMed] [Google Scholar]
- 12.Poggiali E, Dacrema A, Bastoni D, Tinelli V, Demichele E, Mateo Ramos P, et al. Can lung us help critical care clinicians in the early diagnosis of novel coronavirus (COVID‐19) pneumonia? Radiology 2020; 295: 200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF, et al. Proposal for international standardization of the use of lung ultrasound for patients With COVID‐19: a simple, quantitative, reproducible method. J Ultrasound Med 2020; 39: 1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardinale L, Priola AM, Moretti F, Volpicelli G. Effectiveness of chest radiography, lung ultrasound and thoracic computed tomography in the diagnosis of congestive heart failure. World J Radiol 2014; 6: 230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID‐19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol 2019; 2020: 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Smith MJ, Hayward SA, Innes SM, Miller ASC. Point‐of‐care lung ultrasound in patients with COVID‐19 – a narrative review. Anaesthesia 2020; 75: 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, Wang S, Liu Y, Zhang Y, Zheng C, Zheng Y, Zhang C, Min W, Yu M, Hu M. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non‐critical novel coronavirus pneumonia (COVID‐19). Available at SSRN: https://ssrncom/abstract=3544750.
- 18.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in Covid‐19: the first autopsy series from New Orleans. medRxiv 2020; 2020:2020.04.06.20050575. [Google Scholar]
- 19.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 2020; 191: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis 2020. ePub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, et al. Temporal changes of CT findings in 90 patients with COVID‐19 pneumonia: a longitudinal study. Radiology 2020; 200843.286: E55–E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abramowicz JS, Basseal JM. World Federation for Ultrasound in medicine and biology position statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID‐19. Ultrasound Med Biol 2020; 46: 1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costello C, Basseal JM, Yang Y, Anstey J, Yastrebov K. Prevention of pathogen transmission during ultrasound use in the Intensive Care Unit: recommendations from the College of Intensive Care Medicine Ultrasound Special Interest Group (USIG). Australas J Ultrasound Med 2012; 15: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Normal. Normal lung ultrasound findings appear as a bright (hyperechoic) pleural line immediately beneath the ribs that appears to move with respiration, which is due to sliding of the parietal and visceral pleura – lung sliding. A‐lines are artefactual copies (reverberation artefact) of the pleural line that appear as motionless horizontal white lines equally spaced below the pleural line.
Video S2 Atelectasis. Lung ultrasound of atelectasis demonstrates grey tissue appearance with volume loss (effusion) and static (motionless) air bronchograms (white dots).
Video S3 Consolidation (pneumonia). Lung ultrasound of consolidation demonstrates grey tissue appearance without volume loss (no effusion). The presence of dynamic (mobile) air bronchograms (white dots) reflects pneumonia.
Video S4 Pneumothorax. Lung ultrasound demonstrating pneumothorax. There is initially no lung sliding seen but then during inspiration a lung point is seen to slide along the pleural line from right to left with lung sliding following behind the lung point, which then slides away to the right with expiration.
Video S5 Interstitial syndrome. Interstitial syndrome is characterised as more than two B‐lines in an interspace, which are hyperechoic (bright white) laser‐like lines originating from the pleural line and extending to the bottom of the display that move with respiration.
Video S6 Setup prior to entering patient room. Insertion of probe and smartphone entirely within the plastic sheath.
Video S7 Entering room and conduct examination. Entering the room with the COVID‐19 patient and conduct the examination.
Video S8 Removal of the ultrasound device and smartphone without contamination. The plastic sheath is cut with sterile scissors and the device is then removed without the sheath coming into contact with the recipient trolley or drapes.
Table S1 Infectious control measures. Infectious control measures before, during, and after a lung ultrasound examination in suspected and confirmed COVID‐19 patients.


