Abstract
Point‐of‐care ultrasound (POCUS) use is widespread amongst emergency physicians (EPs). Many sonographic modalities have proven useful in the emergency department (ED), including basic echocardiography. Progressing to more advanced echocardiography allows for improved accuracy when making time‐critical diagnoses and management decisions, particularly among the sickest patients. Acquisition of this skill set by EPs is feasible and enhances patient care.
Keywords: echocardiography, emergency department, emergency medicine, point‐of‐care ultrasound, trans‐oesophageal echocardiography, trans‐thoracic echocardiography, ultrasound
Introduction
EPs have been enthusiastic adopters of point‐of‐care ultrasound. This is unsurprising. Extending the physical examination beyond inspection, palpation, percussion and auscultation to include the fifth pillar of insonation increases speed and accuracy in diagnosis.1, 2 However, because few clinicians will ever gain the equivalent experience of a full‐time sonographer, disagreement exists regarding what degree of expertise clinicians can, or should, aspire to. We will argue that the benefits of progressing to advanced sonographic practice outweigh the feared harms, using the example of echocardiography.
EPs interpret X‐ray radiographs or computed tomography (CT) images dozens of times per shift, immediately after they are performed. Our aim is to identify anything important which would affect management or disposition in the hours or days before a radiologist finalises their formal report. EPs train to improve our radiology skills, with no ceiling placed on what can be reasonably achieved, knowing we will never achieve the skill level of a radiologist. A common‐sense approach is taken – if an interpretation is uncertain, a second opinion from a colleague can be sought before making a management decision. Taking the role of ‘bedside radiologist’ is natural and necessary for EPs when making time‐critical decisions.
Bedside sonographic training begins with simple binary questions. For example, is there an aortic aneurysm, or is there free peritoneal fluid? Once EPs’ visuospatial skills progress and sonography becomes intuitive, naturally many have pursued a more advanced level of training and practice in their various sonographic areas of interest. We bring the same ethos to this field as when interpreting X‐rays and CTs, aligning the aggressiveness of our management with the certainty of our interpretation. If suitable systems of training and support are present – in the case of echocardiography with the expert guidance of our cardiology colleagues – our experience is that incorporating more advanced echocardiography into early clinical assessment increases accuracy and reduces the miss‐rate of life‐threatening pathology.
Definitions
Numerous definitions exist in the literature to describe abbreviated cardiac ultrasound protocols. Comprehensive echo refers to a complete scan, often performed in an echocardiography laboratory. A limited echo is a scan omitting some elements of a comprehensive ultrasound, performed by an operator with the skills to perform comprehensive echo, on an advanced machine typically used to perform comprehensive echo, and will often include advanced imaging techniques.
A focused cardiac ultrasound (FoCUS), as defined in 2014 by the International Liaison Committee on Focused Cardiac Ultrasound (ILC‐FoCUS),3 is a universal term for a transthoracic scan performed typically by clinicians at point‐of‐care, across a wide range of specialties including emergency medicine. It is goal and problem‐oriented, limited in scope, simplified, time‐sensitive, repeatable, and qualitative or semi‐quantitative. Suggested targets of FoCUS include LV dimension and systolic function, RV systolic function, volume status, pericardial effusion, tamponade physiology, gross signs of chronic heart disease, gross valvular abnormalities, and large intracardiac masses. FoCUS is a good description of the majority of echocardiography performed in modern‐day emergency departments.
The Australasian College of Emergency Medicine (ACEM) state they encourage all EPs to be competent in Basic Echocardiography in Life Support, and encourage continued research in the area of any other known or evolving imaging techniques and modalities.4
Different bodies draw different boundaries around which elements of cardiac ultrasound are appropriate or necessary to be performed at point‐of‐care in ED. We believe many elements traditionally reserved for comprehensive echocardiography can enhance emergency medical care and would like to share our experience of progressing beyond the boundaries of FoCUS.
ED physicians can learn advanced echo
Aspiring advanced POCUS users must start by answering the question – is learning advanced echo even feasible for EPs? The answer is yes. Studies have shown that with adequate training EPs can learn advanced echocardiography modalities, including complex and subjective assessments such as diastology5, 6, 7, 8 and regional wall motion abnormalities (RWMA's).9, 10, 11 When EPs’ images are reviewed by a cardiologist or a comprehensive echo is subsequently performed, a high level of concordance is obtained for severe or life‐threatening clinical findings. However, as anticipated, EP's are less accurate at grading severity, for example differentiating mild or moderate LV mitral regurgitation.
Four examples
The next question is then raised – in emergency medicine what does advanced echocardiography bring to patient care, beyond what is provided by basic echocardiography? Four key examples are listed below.
Pulmonary embolism
Echo has high utility in patients with atraumatic, undifferentiated hypotension. In particular, often the history, examination and basic investigations do not provide enough certainty to rule in or rule out pulmonary embolism (PE). Thrombolytics are associated with considerable risk and are reserved for patients with high probability of venous thromboembolism. Certainty can be achieved with a positive Computed Tomography Pulmonary Angiogram (CTPA), but transferring an unstable patient for CT is difficult for multiple reasons. Often a patient is too short of breath to lie flat, and if they arrest in the radiology department, their resuscitation will be delayed and suboptimal.
In the hypotensive patient, basic echocardiography allows identification of obvious RV dilation or new tricuspid regurgitation, indicating PE as the cause for hypotension. More advanced echo allows measurement of the RV free wall thickness to determine chronicity of right heart impairment, recognition of McConnell's sign, quantification of pulmonary artery pressures, and visualisation of thrombus in the pulmonary artery (Figure 1).
Figure 1.
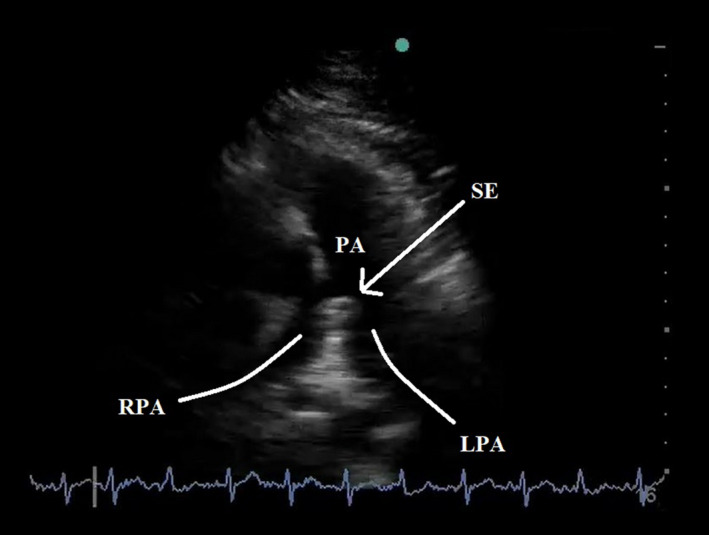
Parasternal Short‐Axis View of a Saddle Embolus, Straddling the Pulmonary Artery Bifurcation Into Left and Right Pulmonary Arteries.
Multiple studies have assessed the optimal management for patients with submassive PE,12, 13, 14, 15, 16 yet uncertainty remains. In a normotensive patient with sub‐massive PE confirmed on CTPA, the use of advanced echo to evaluate the Tricuspid Annular Plane Systolic Excursion (TAPSE) allows prognostication of clinical course and can influence the difficult decision regarding use of thrombolysis.17, 18, 19, 20, 21, 22 In addition, a finding of clot‐in‐transit is associated with increased mortality, and endovascular techniques or thrombolysis should be carefully considered in a normotensive patient with this finding. However, further research is needed to clarify more precisely how echocardiography can guide management in submassive PE.
Valvulopathies
An increasing proportion of ED patients in the developed world are elderly. Undiagnosed aortic stenosis can lead to catastrophic operative consequences if a patient requires induction for theatre or other reasons – these patients require invasive blood pressure monitoring, avoidance of the peripheral vasodilation caused by neuraxial techniques, and careful selection of pharmacological agents.23 2D findings including calcification and restricted cusp movement, turbulence of flow on colour Doppler, and secondary signs such as left ventricular hypertrophy (LVH) can provide a large amount of information regarding aortic valve anatomy. However, it is very unlikely EP's have the time to thoroughly quantify aortic stenosis to the standard of published guidelines. If aortic stenosis is suspected, an urgent comprehensive echocardiogram can be performed to confirm and quantify the degree of stenosis. This is a good example of potential collaboration between EP's and cardiology, where a suspected finding could trigger a discussion and review of an EP's images, and lead to appropriately triaged comprehensive imaging.
Another important valvulopathy is infectious endocarditis. This is a time‐critical diagnosis which is frequently delayed, causing significant morbidity and mortality (Figures 2 and 3). Undoubtedly, TTE is inferior to TOE for identifying small vegetations. However, with good windows and modern equipment, the majority of vegetations requiring urgent operative intervention will be identifiable.
Figure 2.
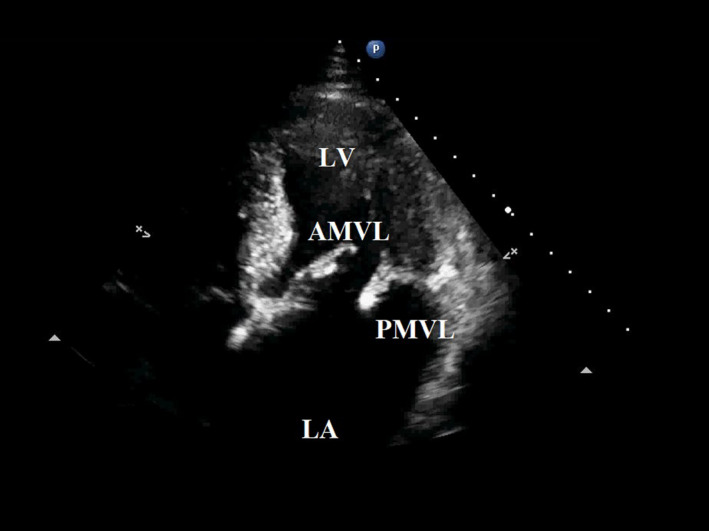
Apical Four‐Chamber View of Bulky Vegetations on a Flail Posterior Mitral Valve Leaflet and Anterior Mitral Valve Leaflet, in a Young Adult with Infectious Endocarditis. Left Atrium and Left Ventricle Chambers Visible.
Figure 3.

Parasternal Long‐Axis View of Bulky Vegetations on a Flail Posterior Mitral Valve Leaflet and Anterior Mitral Valve Leaflet, in a Young Adult with Infectious Endocarditis. Left Atrium and Left Ventricle Chambers Visible.
Chest pain
Chest pain is a frequent reason for ED presentation. Inaccuracies at initial diagnosis can arise for multiple reasons – imprecision in the obtained history, non‐contributory examination findings and a high rate of indeterminate initial investigations such as electrocardiography, troponin laboratory tests and chest X‐ray. Pulmonary embolism is a cause of chest pain which has been discussed already.
The European Association of Echocardiography guidelines state that TTE may be used as the initial imaging modality when aortic dissection is clinically suspected in the ED.24 Suspicion for aortic dissection is raised with basic echo by identifying new aortic regurgitation, a pericardial effusion or a dilated aortic root. This examination can be extended to examine a large proportion of the thoracic aorta in many patients by incorporating information from the right parasternal, suprasternal, left parasternal and subcostal windows. When used with contrast and modern machines, diagnosis of acute aortic syndromes with TTE is becoming increasingly sensitive.25
If noted, a new RWMA in a patient with recent or ongoing chest pain increases the likelihood of an acute coronary syndrome (ACS),9, 10, 26, 27, 28 although this highly advanced skill is a subjective assessment. Judicious integration of echocardiographic data is required here, rather than a binary approach. The more obvious the RWMA, the more confident the EP can be regarding administering antiplatelet agents and advocating for emergent coronary angiography. With software advances, automated detection of RWMA's may make this a more reliable part of the ED risk stratification process.
Future directions in cardiac arrest
The use of transthoracic echocardiography (TTE) in cardiac arrest is widespread, with a recent timely reminder for users to maintain situational awareness and minimise associated pauses in chest compressions.29 This is not the time to use advanced transthoracic echo. Basic echo should be performed for PEA patients, aiming to identify gross signs of reversible pathology such as pericardial tamponade or pulmonary embolism, and to prognosticate in patients with the absence of cardiac activity.
Progression to the use of transoesophageal echo (TOE) in ED management of cardiac arrest is being explored, with the American College of Emergency Physicians (ACEP) publishing a guideline in 2017.30 When using TOE, pauses for echocardiographic assessment need only be transient, as a window can be established while compressions are ongoing. In addition, dynamic optimisation of hand‐positioning during chest compressions can occur. Human anatomy has wide variability, and the location of thoracic contents correlates poorly with external landmarks. CT analysis and observational data reveal that using recommended compression landmarks leads to a degree of LVOT or aortic valve compression in the majority of cases,31, 32 and LV stroke volume is rarely optimised using the sternum or inter‐nipple line as landmarks.33
The evidence base for TOE in cardiac arrest is sparse, and its use is not yet widespread. A case series of 54 TOE examinations in ED demonstrated diagnostic influence in 78% of cases and therapeutic impact in 67%,34 while another series of 55 TOEs undertaken during cardiac arrest demonstrated feasibility in the intensive care unit (ICU).35 Although seventy years of unguided compressions have provided a net benefit across the total population of arrest victims, further research should be encouraged to investigate whether the technique can be evolved any further in the modern era.
ED's considering establishing a TOE program today need to consider whether the current evidence base justifies the considerable expense, although trends towards cheaper technology are expected to continue. Further barriers include the strict guidelines for reprocessing of reusable medical devices, as detailed in the recent Australian National Safety and Quality Health Service Standards Guide.36 Extensive education, quality control, maintenance and staffing would need to be invested in a procedure occurring only rarely in a highly selected population.
More examples
The potential utility of advanced echocardiography in emergency medicine is vast, with many examples beyond the core topics discussed already. In patients with pericardial effusion where the presence of tamponade is indeterminate, RV mid‐diastolic collapse can be sought using M‐mode alongside an electrocardiographic trace, and LV/RV inflow velocity variability may be assessed with spectral Doppler. Echocardiographic assessment of fluid responsiveness in shock has been embraced of late by the critical care community.37 Identification of left ventricular outflow tract obstruction in a hypotensive patient should lead to caution with inotrope use prior to correcting hypovolaemia.38 Diagnosing left ventricular thrombus in the right clinical setting will lead to timely anticoagulation. The relevance of diastology in emergency medicine is less clear, but could lead to early diagnosis of ischaemic heart disease, guide fluid management and support the diagnosis of heart failure with preserved ejection fraction (HFpEF) in patients with dyspnoea of unclear origin.5, 6, 7, 8
Training, collaboration, documentation and image storage
The logistics of progressing some or all EPs to more advanced degrees of echocardiographic practice is not straightforward. Training and credentialing, quality control and review/referral triggers, skills maintenance and feedback mechanisms, and documentation standards and image storage are all indispensable elements of any system aiming to enhance patient care with a high degree of safety.
Ideally training and quality control should be a collaborative effort across the cardiology and acute care specialties, providing a common set of standards and expectations. EPs within an ED with an advanced ultrasound or echocardiography skill set must assume the mantle of quality control and cardiology liaison. All imaging and reports by uncredentialed clinicians require review and critique, with several layers of review being established and available, starting with the EP ultrasound lead and finishing with a cardiologist's review or a comprehensive echocardiogram being requested. It is vital to have regular multi‐specialty POCUS and echocardiography forums where areas needing improvement are highlighted, and cases are discussed.
Standards of documentation and image storing should be stringent, with dual aims of both patient safety and medicolegal compliance. Reporting should be structured and be adapted from established guidelines to the ED environment, with the degree of detail correlated to the degree to which advanced modalities were utilised. Reports and images should be available for immediate review through the local Picture Archiving and Communication System (PACS) by any clinician involved in a patient's care. This allows immediate discussion with inpatient colleagues regarding patient management with images available for review in unstable patients or where findings are indeterminate; serial imaging can be compared to initial imaging; and on case review, clinicians’ integration of available imaging data can be assessed accurately.
The aforementioned would appear to be a large investment of time and energy by hospital and inpatient services into ED echocardiography. We would advocate that the money and time invested in training EPs will be offset by reductions in unnecessary ICU and ward admissions, and reduced hospital length of stay for patients due to early diagnosis and intervention. Also we would anticipate a reduction in unnecessary comprehensive echocardiography requests, and a more accurate degree of triage acuity for the comprehensive echocardiography requests that are made.
Conclusion
POCUS is analogous to the Uber taxi company – a disruptive movement altering traditional medical imaging pathways. EPs (Uber drivers) are using ultrasonography in increasing numbers each year and are gaining experience with more advanced echocardiography. But some established users (taxi drivers) object that not all POCUS users are trained with enough rigor, they do not pay their dues to the relevant authorities, and they are not held to strict enough standards.
The landscape of echocardiography is evolving. Former conventions regarding the who, what, where and when of echocardiography are changing. In this time of shifting goalposts, a point of agreement must persist – that optimal care of our patients (passengers) remains our common goal. We would advocate that a collaborative approach at this exciting intersection of cardiology, critical care, and emergency medicine is mutually beneficial to upskill motivated acute care clinicians in their care of unwell patients.
Authorship declaration
The authorship listing conforms with the journal's authorship policy, and all authors are in agreement with the contents of the submitted manuscript.
References
- 1.Narula J, Chandrashekhar Y, Braunwald E. Time to Add a Fifth Pillar to Bedside Physical Examination. JAMA Cardiology 2018; 3(4): 346–50. [DOI] [PubMed] [Google Scholar]
- 2.Jones AE, Tayal VS, Sullivan DM, Kline JA. Randomized, controlled trial of immediate versus delayed goal‐directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med 2004; 32(8): 1703–8. [DOI] [PubMed] [Google Scholar]
- 3.Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble V, et al. International Evidence‐Based Recommendations for Focused Cardiac Ultrasound. J Am Soc Echocardiogr 2014; 27: 683.e1–683.e33. [DOI] [PubMed] [Google Scholar]
- 4.ACEM . Policy on the use of Focussed Ultrasound in Emergency Medicine, 2016. https://acem.org.au/getmedia/000b84ee-378f-4b65-a9a7-c174651c2542/Feb_16_P21_Use_of_Focussed_US_in_EM.aspx (accessed 30/11/18).
- 5.Ünlüer EE, Bayata S, Postaci N, Yeşil M, Yavaşi Ö, Kara PH, et al. Limited bedside echocardiography by emergency physicians for diagnosis of diastolic heart failure. Emerg Med J 2012; 29: 280–3. [DOI] [PubMed] [Google Scholar]
- 6.Nazerian P, Vanni S, Zanobetti M, Polidori G, Pepe G, Federico R, et al. Diagnostic accuracy of emergency doppler echocardiography for identification of acute left ventricular heart failure in patients with Acute Dyspnea: comparison with Boston criteria and N‐terminal prohormone brain natriuretic peptide. Acad Emerg Med 2009; 17: 18–26. [DOI] [PubMed] [Google Scholar]
- 7.Ehrman R, Russell FM, Ansari AH, Margeta B, Clary JM, Christian E, et al. Can emergency physicians diagnose and correctly classify diastolic dysfunction using bedside echocardiography? Am J Emerg Med 2015; 33: 1178–83. [DOI] [PubMed] [Google Scholar]
- 8.Adhikari S, Fiorello A, Stolz L, Jones T, Amini R, O'Brien K, et al. Ability of emergency physicians with advanced echocardiographic experience at a single center to identify complex echocardiographic abnormalities. Am J Emerg Med 2014; 32(4): 363–6. [DOI] [PubMed] [Google Scholar]
- 9.Frenkel O, Riguzzi C, Nagdev A. Identification of high‐risk patients with acute coronary syndrome using point‐of‐care echocardiography in the ED. Am J Emerg Med 2014; 32: 670–2. [DOI] [PubMed] [Google Scholar]
- 10.Sobczyk D, Nycz K, Andruszkiewicz P. Validity of a 5‐minute focused echocardiography with A‐F mnemonic performed by non‐echocardiographers in the management of patients with acute chest pain. Cardiovasc Ultrasound 2015; 13: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerwin C, Tommaso L, Kulstad E. A brief training module improves recognition of echocardiographic wall‐motion abnormalities by emergency medicine physicians. Emerg Med Int 2011; 2011: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, et al. A prospective, single‐arm, multicenter trial of ultrasound‐facilitated, catheter‐directed, low‐dose fibrinolysis for acute massive and submassive pulmonary embolism. JACC: Cardiovasc Interv 2015;8(10):1382–92. [DOI] [PubMed] [Google Scholar]
- 13.Riera‐Mestre A, Jiménez D, Muriel A, Lobo JL, Moores L, Yusen RD, et al. Thrombolytic therapy and outcome of patients with an acute symptomatic pulmonary embolism. J Thromb Haemos 2012; 10(5): 751–9. [DOI] [PubMed] [Google Scholar]
- 14.Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002; 347(15): 1143–50. [DOI] [PubMed] [Google Scholar]
- 15.Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111(2): 273–7. [DOI] [PubMed] [Google Scholar]
- 16.Meyer G, Vicaut E, Danays T, Giancarlo A, Becattini C, Beyer‐Westendorf J, et al. Fibrinolysis for patients with intermediate‐risk pulmonary embolism. N Engl J Med 2014; 370(15): 1402–11. [DOI] [PubMed] [Google Scholar]
- 17.Lobo JL, Holley A, Tapson V, Moores L, Oribe M, Barrón M, et al. Prognostic significance of tricuspid annular displacement in normotensive patients with acute symptomatic pulmonary embolism. J Thromb Haemost 2014; 12: 1020–7. [DOI] [PubMed] [Google Scholar]
- 18.Pruszczyk P, Goliszek S, Lichodziejewska B, Kostrubiec M, Ciurzyński M, Kurnicka K, et al. Prognostic value of echocardiography in normotensive patients with acute pulmonary embolism. JACC Cardiovasc Imaging 2014; 7: 553–60. [DOI] [PubMed] [Google Scholar]
- 19.Zanobetti M, Converti C, Conti A, Viviani G, Guerrini E, Boni V, et al. Prognostic value of emergency physician performed echocardiography in patients with acute pulmonary thromboembolism. West J Emerg Med 2013; 14: 509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paczyńska M, Sobieraj P, Burzyński Ł, Kostrubiec M, Wiśniewska M, Bienias P, et al. Tricuspid annulus plane systolic excursion (TAPSE) has superior predictive value compared to right ventricular to left ventricular ratio in normotensive patients with acute pulmonary embolism. Arch Med Sci 2016; 12: 1008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmid E, Hilberath JN, Blumenstock G, Shekar PS, Kling S, Shernan SK, et al. Tricuspid annular plane systolic excursion (TAPSE) predicts poor outcome in patients undergoing acute pulmonary embolectomy. Heart Lung Vessel 2015; 7: 151–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Khemasuwan D, Yingchoncharoen T, Tunsupon P, Kusunose K, Moghekar A, Klein A, et al. Right ventricular echocardiographic parameters are associated with mortality after acute pulmonary embolism. J Am Soc Echocardiogr 2015; 28: 355–62. [DOI] [PubMed] [Google Scholar]
- 23.Brown J, Morgan‐Hughes NJ. Aortic stenosis and non‐cardiac surgery. Cont Educat Anaesth Crit Care Pain 2015; 5: 1–4. [Google Scholar]
- 24.Evangelista A, Flachskampf FA, Erbel R, Antonini‐Canterin F, Vlachopoulos C, Rocchi G, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr 2010; 11: 645–58. [DOI] [PubMed] [Google Scholar]
- 25.Evangelista A, Avegliano G, Aguilar R, Cuellar H, Igual A, González‐Alujas T, et al. Impact of contrast‐enhanced echocardiography on the diagnostic algorithm of acute aortic dissection. Eur Heart J 2010; 31: 472–9. [DOI] [PubMed] [Google Scholar]
- 26.Peels CH, Visser CA, Kupper AJ, Visser FC, Roos JP. Usefulness of two‐dimensional echocardiography for immediate detection of myocardial ischemia in the emergency room. Am J Cardiol 1990; 65: 687–91. [DOI] [PubMed] [Google Scholar]
- 27.Sabia P, Afrookteh A, Touchstone DA, Keller MW, Esquivel L, Kaul S. Value of regional wall motion abnormality in the emergency room diagnosis of acute myocardial infarction. A prospective study using two‐dimensional echocardiography. Circulation 1991;84:I85–92. [PubMed] [Google Scholar]
- 28.Kontos MC, Arrowood JA, Paulsen WH, Nixon JV. Early echocardiography can predict cardiac events in emergency department patients with chest pain. Ann Emerg Med 1998; 31: 550–7. [DOI] [PubMed] [Google Scholar]
- 29.Huis In't Veld MA, Allison MG, Bostick DS, Fisher KR, Goloubeva OG, Witting MD, et al. Ultrasound use during cardiopulmonary resuscitation is associated with delays in chest compressions. Resuscitation 2017;119:95–8. [DOI] [PubMed] [Google Scholar]
- 30.Nestaas S, Stensæth KH, Rosseland V, Kramer‐Johansen J. Radiological assessment of chest compression point and achievable compression depth in cardiac patients. Scand J Trauma Resusc Emerg Med 2016; 24: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ACEP Board of Directors . Guidelines for the use of transesophageal echocardiography (TEE) in the ED for cardiac arrest. Ann Emerg Med 2017; 70: 442–5. [DOI] [PubMed] [Google Scholar]
- 32.Hwang SO, Zhao PG, Choi HJ, Park KH, Cha KC, Park SM, et al. Compression of the left ventricular outflow tract during cardiopulmonary resuscitation. Acad Emerg Med 2009; 16: 928–33. [DOI] [PubMed] [Google Scholar]
- 33.Shin J, Rhee JE, Kim K. Is the inter‐nipple line the correct hand position for effective chest compression in adult cardiopulmonary resuscitation? Resuscitation 2007; 75: 305–10. [DOI] [PubMed] [Google Scholar]
- 34.Arntfield R, Pace J, Hewak M, Thompson D. Focused transesophageal echocardiography by emergency physicians is feasible and clinically influential: observational results from a novel ultrasound program. J Emerg Med 2016; 50(2): 286–94. [DOI] [PubMed] [Google Scholar]
- 35.Arntfield R, Lau V, Landry Y, Priestap F, Ball I. Impact of critical care transesophageal echocardiography in medical‐surgical ICU patients: characteristics and results from 274 consecutive examinations. J Intensive Care Med 2018; [Epub ahead of print]. 10.1177/0885066618797271 [DOI] [PubMed] [Google Scholar]
- 36.Australian Commission on Safety and Quality in Health Care . National Safety and Quality Health Service Standards. Sydney: ACSQHC; 2017. [Google Scholar]
- 37.Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care 2016; 20: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chauvet JL, El‐Dash S, Delastre O, Bouffandeau B, Jusserand D, Michot JB, et al. Early dynamic left intraventricular obstruction is associated with hypovolemia and high mortality in septic shock patients. Crit Care 2015; 19: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]


