Abstract
Outline
Lung ultrasound can detect B‐lines in both disease states and normal patients. B‐lines are sensitive indicators for interstitial oedema, but research is limited in terms of what is a ‘normal’ amount in healthy adults. Current belief is that 3 B‐lines in laterobasal areas can be normal. We aimed to determine what is normal in healthy patients of different ages. We hypothesised that older patients and patients with the previous history of lung disease or smoking would have more B‐lines.
Methods
We performed a cross‐sectional study on a convenience sample of 200 volunteers: 100 aged 18–49 (median age 33.5) and 100 aged 50–91 (median age 70.5). Volunteers were recruited in 2017 from two participating sites. All participants were scanned by a single researcher using a standardised lung protocol. Multivariate regression was conducted to determine independent predictors of B‐line presence.
Results
B‐lines were found in 12.5% (95%CI: 8.4–17.6) of all volunteers (n = 25/200), with 20% (95%CI: 13.3–28.9) prevalence in the younger group and 5% (95%CI: 1.9–10.7) in the older group (P < 0.0001). A total of 84% (95%CI: 65.3–93.6) had only 1 B‐line (n = 21/25). 31.3% (95%CI: 20.0–45.6) of young females had B‐lines. Only one volunteer had ≥3 B‐lines in one scanned area. Participants with chronic lung disease had more B‐lines (P = 0.03). Smokers (n = 13) also had more B‐lines (31% vs. 11% of non‐smokers). Smoking and younger age were independent predictors of B‐line presence multivariate logistic regression models, but only for females.
Conclusion
≥2 B‐lines are uncommon in healthy, ambulatory adults. Further research is needed to investigate the higher prevalence found in young females.
Keywords: age comparison, B‐lines, healthy patients, lung ultrasound, normal
Introduction
Lung ultrasound is a useful diagnostic modality in the critical care setting. It can detect multiple lung surface abnormalities, as well as interstitial abnormalities which are visible as ‘B‐lines’.1 The utility of B‐lines is contentious as they are sensitive but non‐specific.2 There are few studies describing their diagnostic utility (Box 1).
Box 1. Summary of diagnostic utility of B‐lines for lung conditions.
| Author, year | Condition | Sample characteristics | Findings |
|---|---|---|---|
| Volpicelli et al. 20083 | Alveolar consolidation | 217 consecutive patients admitted to EMU with no diagnosis (Turin, Italy) | 65.3% of patients with alveolar consolidation had ≥3 B‐lines in the surrounding area |
| Baldi et al. 20134 | Interstitial oedema | 20 consecutive in‐patients admitted to ICU who also had chest CT (Pisa, Italy) | >6 B‐lines corresponded with interstitial oedema (94% sensitivity) |
| Barskova et al. 20135 | Systemic sclerosis | 58 consecutive out‐patients with systemic sclerosis seen in Rheumatology Clinic (Florence, Italy) |
Average 40 B‐lines each overall Average 57 B‐lines in patients with ILD Average 9 B‐lines without ILD |
| Miglioranza et al. 20136 | Heart failure | 97 consecutive out‐patients with heart failure seen in the Heart Failure Clinic (Rio Grande do Sul, Brazil) |
≥15 B‐lines in a single patient correlated with decompensation Patients with higher BNP had more B‐lines than patients with lower BNP (54 vs. 17 B‐lines per patient) |
EMU, emergency medicine unit; ILD, interstitial lung disease; BNP, B‐type natriuretic peptide (higher values = worse heart disease).
Protocols have been developed to identify disease by counting the frequency and distribution of B‐lines.7, 8, 9 Most studies have focused on acutely unwell or intensive care patients4, 8, 10, 11, 12, 13 or on resolution of B‐lines with treatment14, 15, 16 (Box 2).
Box 2. Summary of resolution of B‐lines in certain conditions.
| Author, year | Condition | Sample characteristics | Findings |
|---|---|---|---|
| Noble et al. 200914 | Renal failure | 40 renal failure patients undergoing haemodialysis (Massachusetts, USA) | Decrease of 2.7 B‐lines per 500 ml fluid removed with haemodialysis |
| Vitturi et al. 201415 | Renal failure | 71 consecutive patients undergoing haemodialysis (Treviso, Italy) | Significant decrease in B‐lines with haemodialysis (no exact numbers given) |
| Volpicelli et al. 200816 | Decompensated heart failure | 81 consecutive patients admitted to EMU with established symptomatic ADHF (Turin, Italy) | 76.3% reduction in positive scans after treatment |
ADHF, acute decompensated heart failure; EMU, emergency medicine unit.
There is limited research on healthy participants. A USA study by Chiesa et al. 201417 looked at the incidence of B‐lines in 50 younger (age 6–56) and 100 older (>65 years) participants over a 3‐year period (2009–2012). They found 10% of the younger group and 37% of the older group had ≥1 B‐lines, with 28% (95%CI: 21.4–35.7) of their total population having B‐lines. In the older group, 10% (95%CI: 6.2–15.8) had ≥3 B‐lines (with eight participants having >3 in a single region). Smokers and patients with pre‐existing lung disease were excluded from the study.
An earlier Italian study (Sperandeo et al. 201218) found an average of 1.93 (95%CI: 1.89–1.97) B‐lines (across all lung fields) in 193 healthy non‐smoking hospital employees. This was significantly lower than 3.11 B‐lines found in patients with acute dyspnoea. The proportion of participants who had no B‐lines was not reported.
Another Italian study (Volpicelli et al. 20083) performed chest X‐ray and ultrasound on 217 consecutive emergency department patients. They defined a pathological ‘B+ pattern’ as ≥3 B‐lines in a single lung area. The B+ pattern was found in 34.4% of 145 patients with a normal chest X‐ray (76% of these were in the laterobasal area). However, 26 of these 145 patients were later found to have respiratory disease.
Current literature is therefore speculative in terms of what is ‘normal’ when it comes to incidence of lung surface abnormalities. We found no studies that investigated a healthy population and tried to determine what factors may contribute to the presence of B‐lines.
In this study, we aimed to determine what is normal for B‐lines in the lungs of well Australian participants, including current and past smokers, and those who have past history of illness that are thought to contribute to B‐lines and other lung surface abnormalities. We aimed to describe variation in B‐line presence in healthy volunteers and to find predictors of B‐line presence.
Methods
Study design
This was a prospective, cross‐sectional observational study of a convenience sample of healthy, ambulatory volunteers.
Setting
Data collection occurred at two sites between April and December 2017: the Gold Coast University Hospital Emergency Department (GCUH – Southport, QLD, 4215) and the Australian Institute of Ultrasound (AIU – Broadbeach Waters, QLD, 4218). The AIU is a private ultrasound teaching facility with a large cohort of volunteers who act as teaching models.
Participants
There were 200 participants in the study. Eligible participants were over 18 years of age with no symptoms of respiratory illness, not pregnant and able to provide written consent.
Staff from GCUH and AIU as well as volunteers and students from AIU were approached to participate. Ambulatory GCUH emergency department patients with minor injuries not affecting their respiratory system (limb sprains, simple lacerations, etc.) were also approached.
After participating in the study on the day, there was no further follow‐up of participants, except where findings raised concerns. In those cases, the participant's general practitioner was notified of the findings (a small number of participants had a finding of irregular pleura or 3+ B‐lines in one area, and they were referred to GP for chest X‐ray).
There was minimal literature available to support an expected correlation coefficient and suggested sample size for our study. With a sample size of 200, a correlation coefficient of 0.2 or above between the variables (B‐lines and age) would achieve a statistically significant difference from zero (P < 0.05 with power of 80%).
Chiesa et al.17 found 10% B‐lines in their younger group and 35% in their older group. A sample size of 200 would also allow us to identify a difference of 25% in the prevalence of B‐lines between the older and younger age group, with 99% power at the 95% confidence interval.
Questionnaire
Participants completed a short questionnaire prior to their lung scan. The questionnaire included screening for potential exposures or conditions where B‐lines are known to occur. Specifically, history of smoking, asthma, pneumonia, pulmonary embolism, radiotherapy of chest wall/lung, lung operation, bronchiectasis, heart failure, emphysema, pleurodesis, lupus and rheumatoid arthritis. Participants also indicated the side of the affected lung if known.
The investigator performing the scan was not blinded to the results of the questionnaire.
Scan technique
All participants were scanned with a Fujifilm Sonosite X‐Porte machine (manufactured in Bothell, USA). A curvilinear probe and the same settings were used for all participants: frequency 5–2 MHz, depth 9.9 cm, MI 1.1, TIS 0.3, G 50, frequency −3, Pen, tissue harmonics and compound imaging off.
The Volpicelli protocol was followed, but using longitudinal probe orientation as recommended in the 2012 consensus guidelines19; each scan consisted of four anterior sites (R1, R2, L1 and L2), four lateral sites (R3, R4, L3 and L4) and with the addition of four posterior sites (R5, R6, L5 and L6; Figure 1). Areas 1–4 were scanned with the subject supine with head of bed elevated to ~20°. Areas 5–6 were scanned with subject sitting forward.
Figure 1.
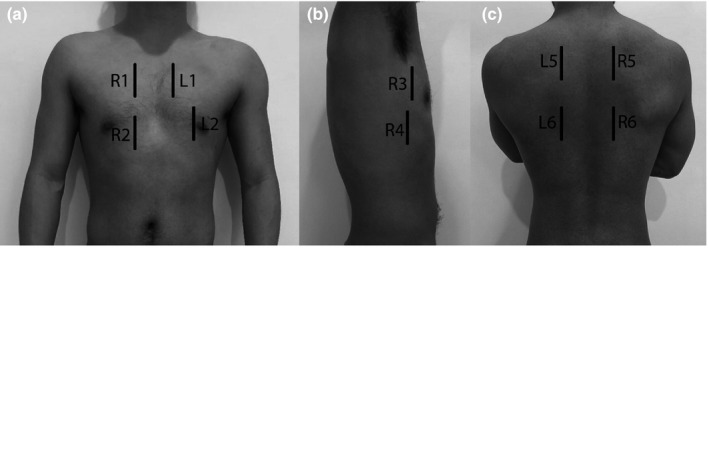
Lung areas scanned. (a) anterior chest. (b) lateral chest. (c) posterior chest.
A B‐line was defined as a discrete vertical hyperechoic reverberation artefact that arises from the pleural line and extends the full depth of the image without fading, obliterates any A‐lines (normal horizontal repetition artefacts arising from the pleural line12) and moves synchronously with lung sliding12, 19 (Figure 2).
Figure 2.
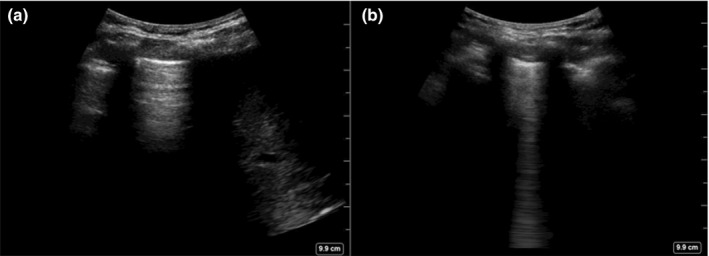
Both images from the same participant, different areas of lung. (a) Normal lung with no B‐line, liver visible on right of image. (b) Lung with single B‐line.
The two outcomes measured were the number and location of any B‐lines. Incidental findings of irregular pleura, consolidation and pleural effusion were noted as text entries. These were recorded on de‐identified hard‐copy templates during scanning.
All participants were live‐scanned by a single investigator. De‐identified still images of the 12 lung areas were saved and labelled. Images were then reviewed by an expert clinician sonologist who was blinded to patient demographics, questionnaire answers and outcomes measured on live scanning. The expert sonologist also recorded outcomes (number and location of B‐lines) per area reviewed as well as incidental findings (as above). Measured outcomes were compared to determine inter‐rater reliability.
Ethics
Ethics approval was granted by the Gold Coast Hospital and Health Service Ethics Committee (HREC/16/QGC/295).
Statistical methods
Data were analysed in SPSS v24.0. Means and proportions were reported to describe the number of B‐lines and characteristics of participants in each age group. To determine an association between B‐lines and outcome variables, we conducted two‐tailed t‐tests for continuous normally distributed variables and chi‐square tests for binary and categorical variables, using Fisher's exact tests for cells with less than five cases. Differences were considered statistically significant if corresponding P‐values were less than 0.05. 95% confidence intervals around proportions were calculated using the Wilson score method.
Multivariate analysis in the form of regression modelling was undertaken. Generalised linear models using a robust estimator were built using a binary logit outcome (B‐lines none vs. any) and a linear outcome (number of B‐lines). Interactions between age and sex were considered. Independent variables tested for their effect on the B‐line dependent variable were as follows: age, sex, smoking status, any lung disease, asthma and pneumonia.
Results
Participants
The 200 participants were aged between 20 and 91 years. In total, 56% were female. All were active and ambulant.
Participants were divided into two groups: 100 in the <50 age group and 100 in the ≥50 age group. Most of the older volunteers were recruited through AIU and most of the younger volunteers from GCUH (Table 1). The majority of AIU participants were regular volunteers of AIU, and most of the GCUH participants were staff (nursing and medical).
Table 1.
Participant recruitment from research sites
| AIU | GCUH | |
|---|---|---|
| Number of participants recruited | 92 | 108 |
| Percentage of participants in <50 age group | 12% | 96% |
| Percentage of participants in ≥50 age group | 88% | 4% |
Thirteen (6.5%) participants reported current smoking. Twenty‐three (11.5%) reported a history of pneumonia, and 36 (18%) had a history of asthma. All other conditions were rare; four had bronchiectasis, and two had heart failure and none or one for other conditions of interest (Table 2).
Table 2.
Characteristics of the sample: all volunteers and by age group
| All volunteers (n = 200) | <50 years (n = 100) | 50+ years (n = 100) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Sex | ||||||
| Female | 111 | 55.5 | 48 | 48.0 | 63 | 63.0 |
| Male | 89 | 44.5 | 52 | 52.0 | 37 | 37.0 |
| History of medical condition | ||||||
| Any lung disease | 53 | 26.5 | 27 | 27.0 | 26 | 26.0 |
| Asthma | 36 | 18.0 | 23 | 23.0 | 13 | 13.0 |
| Pneumonia | 23 | 11.5 | 9 | 9.0 | 14 | 14.0 |
| Smoking status | ||||||
| Current smoker | 13 | 6.5 | 8 | 8.0 | 5 | 5.0 |
| Ex‐smoker | 65 | 32.5 | 17 | 17.0 | 48 | 48.0 |
| Never smoker | 122 | 61.0 | 75 | 75.0 | 47 | 47.0 |
Statistically significant differences were identified by age group for sex (with more females in the older group) and in smoking status (with larger proportions of non‐smokers in the younger group and ex‐smokers in the older group). Current smoking did not vary significantly by age group (Table 2).
B‐lines and other lung findings
B‐lines were found in 25 of the 200 participants (12.5%, 95%CI: 8.6–17.8). Twenty‐one (84%) had only one B‐line. One participant had 2 B‐lines in one area (L4). Three participants had ≥3 B‐lines (1.5%, 95%CI: 0.5–4.3). In regard to these three participants, one had 1 B‐line in areas R3, R4 and L6 (total of 3), one had 2 B‐lines in L3 and 1 in R6 (total of 3), and one had 3 B‐lines in L1 and 1 in R6 (total of 4, which was the most B‐lines found in a single individual). A total of 33 B‐lines were found in the 200 participants (13 in males, 20 in females; Figure 3).
Figure 3.
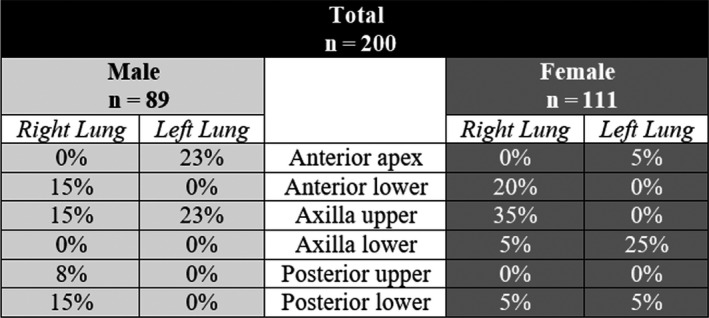
B‐line distribution differences between males and females.
Pleural irregularities were identified in seven volunteers (3.5%); five of these also had B‐lines. Fifteen of the 23 patients with past pneumonia could not recall which side the pneumonia was on. Of the 8 that could, only one had a B‐line on the same side and six had no B‐lines. B‐line presence was associated with younger age (P < 0.001; Figure 4) and a history of any lung disease (P < 0.05). In total, 31% of current smokers had B‐lines (compared with 11% of non‐ and ex‐smokers, P = 0.062). Twenty‐two per cent of asthmatics had B‐lines compared to 10.4% of non‐asthmatics (P = 0.051; Table 3).
Figure 4.
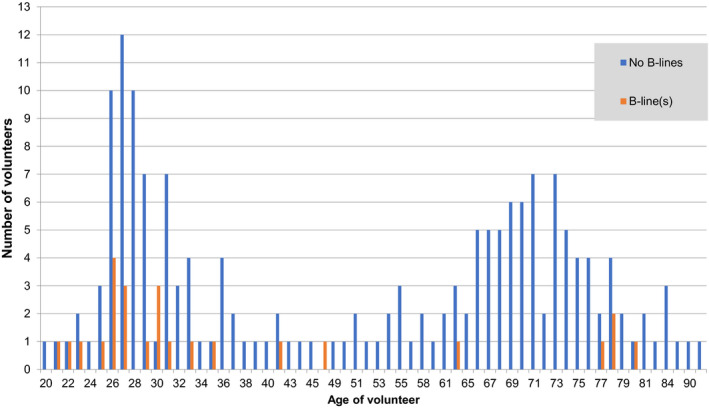
Histogram of age for 200 volunteers in study by B‐line vs. no B‐line.
Table 3.
Presence of B‐lines by volunteer characteristics
| All volunteers | B‐LINES | P‐value for none vs. any | ||||
|---|---|---|---|---|---|---|
| Absent | Present | |||||
| Total N | n | % | n | % | ||
| 200 | 175 | 87.5 | 25 | 12.5 | ||
| Sex | ||||||
| Male | 89 | 81 | 91.0 | 8 | 9.0 | 0.179 |
| Female | 111 | 94 | 84.7 | 17 | 15.3 | |
| Age group | ||||||
| <50 | 100 | 80 | 80.0 | 20 | 20.0 | 0.001 |
| 50+ | 100 | 95 | 95.0 | 5 | 5.0 | |
| Smoking status | ||||||
| Current smokers | 13 | 9 | 69.2 | 4 | 30.8 | 0.062 |
| vs. All others | 187 | 166 | 88.8 | 21 | 11.2 | |
| Ex‐smokers | 65 | 59 | 90.8 | 6 | 9.2 | 0.527 |
| vs. Never smokers | 122 | 107 | 87.7 | 15 | 12.3 | |
| Any smoking | 78 | 68 | 87.2 | 10 | 12.8 | 0.913 |
| vs. Never smokers | 122 | 107 | 87.7 | 15 | 12.3 | |
| Lung disease history | ||||||
| Any lung disease history | 53 | 42 | 79.2 | 11 | 20.8 | 0.034 |
| No lung disease history | 147 | 133 | 90.5 | 14 | 9.5 | |
| Asthma | 36 | 28 | 77.8 | 8 | 22.2 | 0.051 |
| No asthma | 164 | 147 | 89.6 | 17 | 10.4 | |
| History of pneumonia | 23 | 19 | 82.6 | 4 | 17.4 | 0.500 |
| No history pneumonia | 177 | 156 | 88.1 | 21 | 11.9 | |
We identified an interaction between age group and gender on the presence of B‐lines (Figure 5). B‐lines were more prevalent in the younger group of females. In this group, nearly one‐third of volunteers had B‐lines [n = 15 of 48 (31.3%, 95%CI: 20.0–45.6)]. Seven of these 48 had B‐lines in the R3 area. Comparatively, only two females in the older group had B‐lines (3.2%). In males, both groups had similar proportions of cases with B‐lines (9.6% younger vs. 8.1% older). No consistent correlation was found between the number of B‐lines and increasing age.
Figure 5.
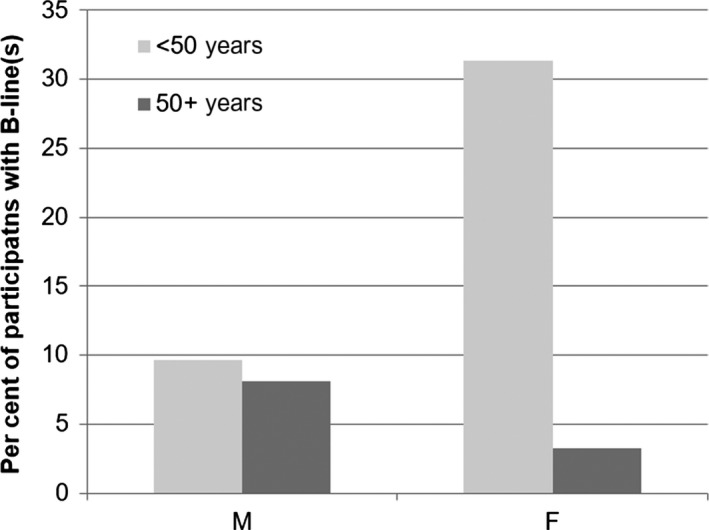
Differences in B‐line presence by sex and age group.
Other analyses
Multivariate findings
Because of the significant discrepancy between the sexes, separate logistic regression models were built for males and females.
For males, none of the variables tested in the model were significant predictors of B‐lines, including age, current smoking and any medical history. For females, in the final model, only age remained a significant predictor, whether entered as a continuous variable, or as age category (P < 0.001 for either). Current smoking almost reached significant association with the presence of B‐lines with P = 0.05 and remained in the binary outcome regression model after adjusting for age (P = 0.067); however, there were only four current smokers amongst the 111 females.
In the continuous generalised linear model, age was negatively associated with the number of B‐lines (β = −0.003, 95%CI: −0.006−0.001), but not statistically significant (P = 0.216). In multivariate linear modelling, none of the variables examined were significantly correlated with the number of B‐lines.
Comparisons of live scans to blinded review
Twenty of 200 cases (10%) had one or more scan regions missing for the second review and were excluded for the inter‐rater accuracy analysis. Of the remaining 180 cases, reviewers agreed on the presence or absence of B‐lines for 166 cases (92.2%). However, although the match rate and specificity were high (specificity = 96.9%), the sensitivity of identification of B‐lines with image review was only 55% (Figure 6). Interobserver variability was only fair, with a kappa of 0.57 (95%CI: 0.35–0.79).
Figure 6.

Comparison of findings for 180 scans: live scanning vs expert review.
Discussion
Current belief is that three or more B‐lines in any single lung region suggest pathology1 and previous research have indicated that 34% of hospitalised patients have ≥3 B‐lines in their laterobasal areas.3 Overall, however, there is a paucity of research in healthy patients to support this. Any condition that can cause interstitial oedema (heart failure,10, 16 renal failure,14, 15, 20 acute respiratory distress syndrome11) will increase the number of B‐lines found. There is some correlational evidence demonstrating that the presence of B‐lines increases in diseases including systemic sclerosis,5, 21 rheumatoid arthritis22 and connective tissue disorders,23 but this is secondary to interstitial oedema as a result of the systemic disease.
An understanding of the distribution of B‐lines in a normal population, however, is not well established. Chiesa et al.17 were the only study that looked at healthy participants, and they found 10% met the criteria for pathology (3+ B‐lines in one area). In our study, only one participant (0.5%) had 3 B‐lines in one area (this same participant had 4 total B‐lines). Two other participants (1%) had 3 B‐lines across multiple lung fields.
Based on the literature, we expected that B‐lines would increase with age in a healthy adult population and that a history of lung disease/smoking would also affect the number of B‐lines found. We were only partially correct.
Unexpectedly, older age was not a predictor of B‐line prevalence in this study. Only 5% of our older population had B‐lines, compared to 37% reported by Chiesa et al.17 Whilst Chiesa found a 10% prevalence of B‐lines in their younger group, our study reports 20% prevalence in the <50 group.
The highest prevalence of B‐lines was found in young females (31% had B‐lines). In this group, seven (46.7%) had B‐lines in the R3 area. It is possible that this B‐line is actually a reflection of the right horizontal lung fissure. Lichtenstein24 has previously suggested that interlobar septa can appear as B‐lines on ultrasound. It is unclear why this is present in young females and not males, but may be a result of the area being scanned more posteriorly to avoid breast tissue. Further research is needed to explore this anomaly.
Although not statistically significant, current smoking appeared to increase the presence of B‐lines (31% vs. 11% in non‐smokers). In our study, only 13 (6.5%) of the volunteers were current smokers (compared with 16% of Australians aged ≥1825). It is likely that our study was not adequately powered to find a difference between current smokers and non‐smokers. Further research into this area could be of benefit accounting for past or present smoking, including magnitude of smoking habit.
Patients with a history of asthma had an increase in B‐lines (22.4% vs. 10.4%). This finding is hypothesis generating, and further research would be needed to verify this finding and explore possible causation.
Our interobserver variability was only fair, and in future studies, this could be improved by the capturing of clips rather than still images of each lung area.
Overall, our study found a much lower incidence of B‐lines than was previously thought to be present in a normal population, with the exception of young females. We also found that in participants who did have B‐lines, the majority (84%) had only one. The usual belief that ≥3 B‐lines means pathology may need revision, and based on the results of our study, detecting ≥2 B‐lines in a single patient is abnormal and should prompt consideration for further investigation depending on the clinical context.
Limitations
Since participant recruitment was based on convenience samples, our study likely suffers from a degree of selection bias. The external validity of this study may also be suboptimal, and the participants may not be full representative of the general population. The majority of participants in the <50 age group worked in health care, and the majority of the ≥50 age group were regular AIU volunteers. This may be an explanation for why our study had such a low percentage of smokers.
A limitation was found in distinguishing B‐lines between live scanning and looking at still images. Ultrasound is known to be operator dependent, and therefore, real‐time imaging and post‐exam film review are known to generate some differences.26 B‐lines are more obvious when they move across the screen with breathing, and artefacts that appear in still pictures may not be evident in live scanning. Having two researchers scanning every participant and comparing results would increase inter‐rater reliability but at the expense of being more time consuming and impractical.
This study used a Sonosite X‐Porte – a newer machine which uses multiple beam focusing technology, preventing the beam divergence beyond a single focus. It is not possible to optimise image parameters for a high focus and lower depth. This may cause some lack of clarity in B‐line rendition and reduce the number counted. Given that all participants were scanned with the same model machine, there is consistency in this study; however, it may not reflect the ‘true’ number of B‐lines. This is at least useful in Australia as the Sonosite X‐Porte is a commonly used machine in Australian emergency departments.
Conclusion
This is the first study to target healthy patients with a variety of past medical conditions and smoking to see if B‐lines are truly an ‘abnormal finding’. We can say with confidence, that very few B‐lines are present in the self‐reported ‘healthy’ population. A previously well patient newly arrived in the emergency department should not be expected to have any B‐lines. Finding ≥2 B‐lines in a patient indicates a likely abnormality; however, the implications of practice cannot be determined from this study, though it does appear that B‐lines are more common in younger patients and females. We would recommend that clinicians consider the need for further investigation in any dyspnoeic patient in which ≥2 B‐lines are found, depending on the clinical context.
Conflicts of Interest
There were no conflicts of interest in this study.
Authorship Declaration
The above authorship listing conforms with AJUM's authorship policy. All authors are in agreement with the content of the submitted manuscript.
Acknowledgements
Participants were not offered any incentives to participate in the study. Investigators donated their time. There were no monetary contributions or gratuities received by the researchers. The researchers would like to thank AIU and Sonosite for their help and support during this study.
Disclosure statement
There were no financial arrangements between the investigators, Gold Coast University Hospital, the Australian Institute of Ultrasound or Sonosite. There were no conflicts of interest.
References
- 1.Lichtenstein D, Meziere G, Biderman P, Gepner A, Barre O. The comet‐tail artifact. An ultrasound sign of alveolar‐interstitial syndrome. Am J Respir Crit Care Med 1997; 156: 1640–6. [DOI] [PubMed] [Google Scholar]
- 2.Hew M, Ren Tay T. The efficacy of bedside chest ultrasound: from accuracy to outcomes. Eur Respir Rev 2016; 25: 230–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF. Detection of sonographic B‐lines in patients with normal lung or radiographic alveolar consolidation. Med Sci Monit 2008a; 14: 122–8. [PubMed] [Google Scholar]
- 4.Baldi G, Gargani L, Abramo A, D'Errico L, Caramella D, Picano E, et al. Lung water assessment by lung ultrasonography in intensive care: a pilot study. Intensive Care Med 2013; 39: 74–84. [DOI] [PubMed] [Google Scholar]
- 5.Barskova T, Gargani L, Guiducci S, Randone SB, Bruni C, Carnesecchi G, et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann Rheum Dis 2013; 72: 390–5. [DOI] [PubMed] [Google Scholar]
- 6.Miglioranza MH, Gargani L, Sant'Anna RT, Rover MM, Martins VM, Mantovani A, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 2013;6:1141–51. [DOI] [PubMed] [Google Scholar]
- 7.Bedetti G, Gargani L, Corbisiero A, Frassi F, Poggianti E, Motolla G. Evaluation of ultrasound lung comets by hand‐held echocardiography. Cardiovasc Ultrasound 2006; 4: 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liteplo AS, Marill KA, Villen T, Miller RM,Murray AF,Croft PE, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B‐lines and N‐terminal pro‐brain‐type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med 2009; 16: 201–10. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the blue protocol. Chest 2008; 134: 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med 2013; 31: 1208–14. [DOI] [PubMed] [Google Scholar]
- 11.Corradi F, Brusasco C, Pelosi P. Chest ultrasound in acute respiratory distress syndrome. Curr Opin Crit Care 2014; 20: 98–103. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein DA, Meziere GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A‐lines and B‐lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 2009; 136: 1014–20. [DOI] [PubMed] [Google Scholar]
- 13.Volpicelli G, Caramello V, Cardinale L, Cravino M. Diagnosis of radio‐occult pulmonary conditions by real‐time chest ultrasonography in patients with pleuritic pain. Ultrasound Med Biol 2008b; 34: 1717–23. [DOI] [PubMed] [Google Scholar]
- 14.Noble VE, Murray AF, Capp R, Sylvia‐Reardon MH, Steele DJR, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing haemodialysis: time course for resolution. Chest 2009; 135: 1433–9. [DOI] [PubMed] [Google Scholar]
- 15.Vitturi N, Dugo M, Soattin M, Simoni F, Maresca L, Zagatti R, et al. Lunga ultrasound during haemodialysis: the role in the assessment of volume status. Int Urol Nephrol 2014; 46: 169–74. [DOI] [PubMed] [Google Scholar]
- 16.Volpicelli G, Caramello V, Cardinale L, Musso A, Bar F, Frascisco MF. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med 2008c; 26: 585–91. [DOI] [PubMed] [Google Scholar]
- 17.Chiesa AM, Ciccarese F, Gardelli G, Regina UM, Feletti F, Bacchi Reggiani ML, et al. Sonography of the normal lung: comparison between young and elderly subjects. J Clin Ultrasound 2014; 43: 230–4. [DOI] [PubMed] [Google Scholar]
- 18.Sperandeo M, Varriale A, Sperandeo G, Polverino E, Feragalli B, Piattelli ML, et al. Assessment of ultrasound acoustic artifacts in patients with acute dyspnea: a multicenter study. Acta Radiol 2012; 53: 885–92. [DOI] [PubMed] [Google Scholar]
- 19.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012; 38: 577–91. [DOI] [PubMed] [Google Scholar]
- 20.Trezzi M, Torzillo D, Ceriani E, Costantino G, Caruso S, Damavandi PT. Lung ultrasonography for the assessment of rapid extravascular water variation: evidence from haemodialysis patients. Intern Emerg Med 2013; 8: 409–15. [DOI] [PubMed] [Google Scholar]
- 21.Moazedi‐Fuerst FC, Zechner PM, Tripolt NJ, Kielhauser SM, Brickmann K, Scheidl S, et al. Pulmonary echocardiography in systemic sclerosis. Clin Rheumatol 2012; 31: 1621–5. [DOI] [PubMed] [Google Scholar]
- 22.Cogliati C, Antivalle M, Torzillo D, Birocchi S, Norsa A, Bianco R, et al. Standard and pocket‐size lung ultrasound devices can detect interstitial lung disease in rheumatoid arthritis patients. Rheumatology 2014; 53: 1497–503. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez M, Salaffi F, Carotti M, Tardella M, Pineda C, Bartolazzi C. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders – preliminary results. Arthritis Res Ther 2011; 13: 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtenstein DA. Lung and interstitial syndrome. In: Lichtenstein DA, editor. Whole body ultrasonography in the critically Ill. Berlin: Springer‐Verlag; 2010. 154. [Google Scholar]
- 25.Greenhaigh EM, Bayly M, Winstanley MH. Prevalence of smoking – adults. In: Scollo MM, Winstanley MH, editors. Tobacco in Australia: facts and issues. Melbourne: Cancer Council Victoria; 2015. [Google Scholar]
- 26.Pinto A, Pinto F, Faggian A, Rubini G, Caranci F, Macarini L, et al. Sources of error in emergency ultrasonography. Critical Ultrasound Journal 2013; 5: S1. [DOI] [PMC free article] [PubMed] [Google Scholar]


