Abstract
Introduction
Antenatal diagnosis of isolated infracardiac total anomalous pulmonary venous connection (TAPVC) is rare. Making the diagnosis antenatally is critical as delayed management could result in neonatal deterioration and poor outcome after surgery.
Method
A multipara at 29 weeks of gestation was referred to our tertiary unit for ultrasound review. The fetal growth and biophysical profile were normal. A fetal echocardiogram revealed normal cardiac position with atrioventricular and ventriculoarterial concordance. There was a mild discrepancy in size of the right and left chambers of the heart. A connection between the pulmonary veins and the left atrium could not be established. A pulmonary venous confluence was noted posterior to the left atrium, from which a descending vertical vein emerged traversing the diaphragm and draining into the left portal vein into the liver.
Results
A diagnosis of infracardiac infradiaphragmatic total anomalous pulmonary venous connection was made. The pregnancy was delivered at 39 weeks by lower segment caesarean section. The antenatal findings were confirmed by postnatal echocardiogram. Successful sutureless repair of the pulmonary veins was performed.
Conclusion
Isolated infracardiac total anomalous pulmonary venous connection can be diagnosed antenatally. This ensures early postnatal evaluation and successful repair.
Keywords: antenatal ultrasound, congenital heart disease, total anomalous pulmonary venous connection
Introduction
Total anomalous pulmonary venous connection (TAPVC) constitutes 0.2–2% 1, 2 of congenital heart disease (CHD). In TAPVC, all four pulmonary veins drain into the systemic venous system instead of the left atrium. This condition can be isolated or associated with other CHDs, most commonly as part of a heterotaxy syndrome. Antenatal diagnosis of isolated TAPVC is very challenging. Antenatal detection along with appropriate stabilisation of the neonate and early surgical repair results in a good long‐term prognosis.3
Methods and patients
A 32‐year‐old multiparous woman was referred to our tertiary unit at fourteen‐week gestation in view of returning a high‐risk result for Trisomy 21 (1:22) in a combined first‐trimester screen. The nuchal translucency thickness was increased at 5.8 mm. Amniocentesis was performed, and the result was normal (46XY). A routine fetal morphology scan was reported as normal. She was again referred at 29‐week gestation to our tertiary centre for an ultrasound review. The growth and liquor were normal. Written consent from the patient and ethics approval was obtained to publish this case.
Results
On cardiac examination, the left atrium (LA) appeared to be smaller than the right atrium. There was mild asymmetry of the right and left heart ventricles. The mitral valve measured 4.75 mm (z‐score: −2.82), and the tricuspid valve measured 8.1 mm (z‐score: −1.16). The foramen ovale showed unrestricted flow, and the outflow tracts were unobstructed. The normal connection between the pulmonary veins to the LA could not be established. The LA to descending aorta (DAo) distance was noted to be increased. A pulmonary venous confluence was noted posterior to the left atrium (Figure 1a and b). On tracing the confluent vessel, a descending vertical vein (VV) was noted coursing towards the abdomen and draining into the left portal vein (Figures 2a, b and 3). Pulse wave Doppler study showed an abnormal pulmonary venous pattern with biphasic continuous flow and no significant obstruction of the descending VV at the draining point (Figure 4b). The ductus venosus was normal. A diagnosis of infracardiac TAPVC to the portal vein was made and confirmed by a paediatric cardiologist using fetal echocardiography.
Figure 1.
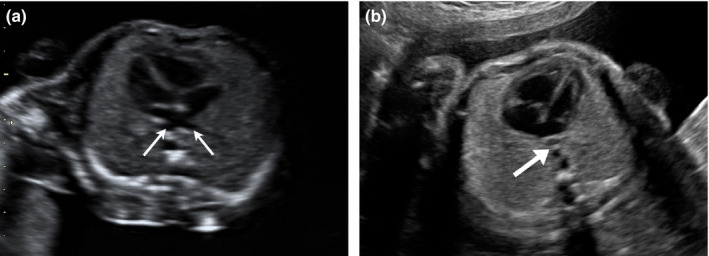
Four‐chamber View of the Fetal Heart. (a) Normal Pulmonary Venous Drainage. Two Pulmonary Veins are seen Draining into the Left Atrium (Thin Arrows). Note the Distance Between Left Atrium and the Descending Aorta. (b) Abnormal Venous Drainage in Total Anomalous Pulmonary Venous Connection. An Abnormal Vessel is Noted Posterior to the Left Atrium which Represents the Anomalous Pulmonary Venous Confluence (Thick Arrow). The Normal Pulmonary Venous Drainage into the Left Atrium is not Visualised. The Distance Between Left Atrium and Descending Aorta is Increased. The Size of the Left Atrium is Smaller than Usual.
Figure 2.
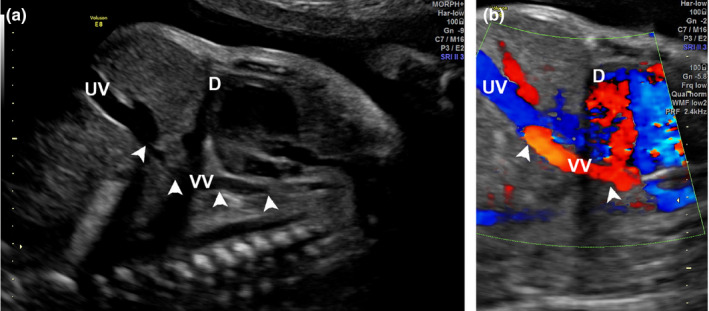
Sagittal Section of Thorax and Abdomen Demonstrating the Vertical Vein (VV). (a) A VV, Arrowhead) is seen Traversing the Diaphragm (D) and Coursing Towards the Umbilical Vein (UV). (b) Colour Doppler Image Depicting the VV Draining into the UV. D = Diaphragm.
Figure 3.
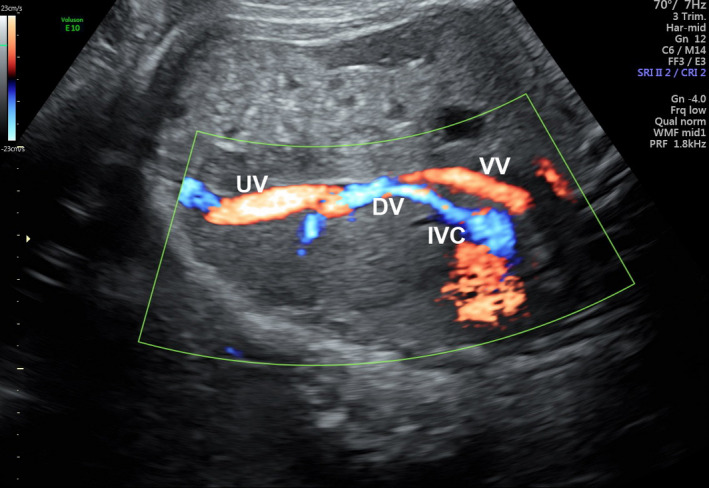
Colour Doppler Image of the Fetal Abdomen. There is Flow in the Vertical Vein Draining into the Umbilical Vein and the Ductus Venosus Near the Communication with the Inferior Vena Cavae.
Figure 4.
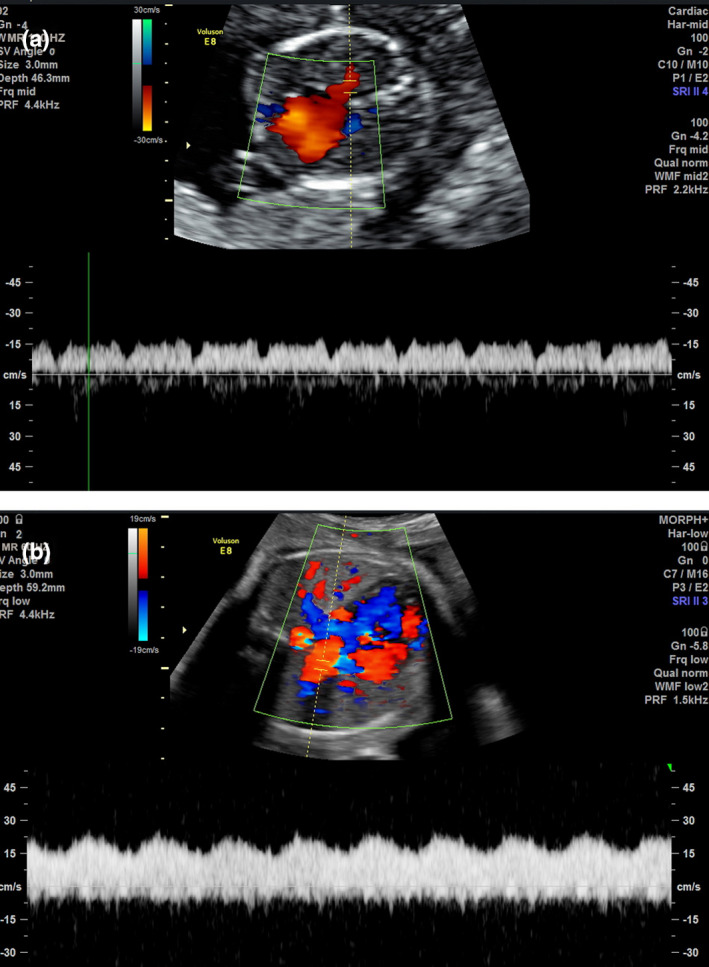
Pulmonary Vein Doppler Flow Pattern. (a) Normal Pulmonary Vein Showing Triphasic Pulsatile Flow Pattern. (b) Pulmonary Vein in Total Anomalous Pulmonary Venous Connection. Abnormal Continuous Monophasic Flow is Demonstrated.
The pregnancy was continued till term and electively delivered at 39 weeks by lower segment caesarean section, due to her history of having a previous caesarean section. Postnatal echocardiogram confirmed the diagnosis of infracardiac TAPVC as well as drainage of the pulmonary vein confluence into the left portal vein. There was mild obstruction at the site of entry into the liver. The baby underwent repair of the TAPVC at five hours of life. The pulmonary veins were re‐anastomosed to the LA by a sutureless technique. The post‐operative course was uncomplicated. The baby was discharged home 2 weeks after surgery.
Discussion
TAPVC is the fifth most common critical congenital heart disease.4 A delay in diagnosis of TAPVC and hence surgical repair leads to high rates of neonatal morbidity and mortality. Without surgical repair of an obstructed VV in TAPVC, the neonate will suffer from progressing pulmonary oedema, pulmonary artery hypertension and cardiac failure.5 The neonate usually presents with low oxygen saturation. Oxygen therapy may worsen the lung damage. Prostaglandin therapy to maintain a patent ductus arteriosus is contraindicated in TAPVC. Antenatal diagnosis of isolated TAPVC is challenging. There are no data available on the antenatal detection rates of TAPVC and the literature are limited to isolated case reports.
Anomalous pulmonary venous connection collectively refers to conditions where some or all of the pulmonary veins return blood to the systemic venous system and eventually to the right atrium, instead of the left atrium. This can be total (TAPVC) or partial (PAPVC). The latter is clinically less severe, and neonates are usually asymptomatic. The ultrasound features of TAPVC are listed in Table 1. The abnormal drainage may either be directly into the right atrium, or through a connecting venous structure, known as the vertical vein (VV), to the superior vena cava (SVC) or inferior vena cava (IVC). Based on the drainage pattern, TAPVC is anatomically classified as supracardiac, cardiac, infracardiac or mixed type (Figure 5).
Table 1.
Ultrasound features of total anomalous pulmonary venous connection
| Cardiac view | Ultrasound feature |
|---|---|
| Four‐chamber view |
Diagnostic feature: Lack of connection between LA and pulmonary veins 5 Additional vessel behind LA (representing the pulmonary venous confluence) Increased LA–DAo distance 5 Smooth wall LA (suggesting absence of PV ostia) 13 Dilated coronary sinus (cardiac type) 13 Chamber asymmetry (right larger than left) 5 Small left atrium |
| Three vessel view |
Dilated SVC (supracardiac) Additional vessel–ascending VV (supracardiac) |
| Abdomen view | Additional vessel–descending VV (infracardiac) |
LA, left atrium; DAo, descending aorta; SVC, superior vena cavae; PV, pulmonary veins; VV, vertical vein.
Figure 5.
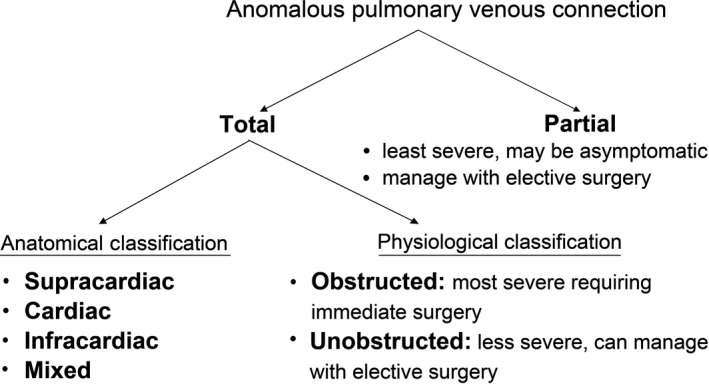
Classification of Cases of Anomalous Pulmonary Venous Connection. Patients with Anomalous Pulmonary Venous Connection may have a Total (All Four Pulmonary Veins Effected) or Partial (One to Three Pulmonary Veins Effected) Abnormality. The Cases with Total Anomalous Pulmonary Venous Connection may be Classified Based on their Anatomical Configuration, or their Physiology. This Classification Impacts Upon the Severity of the Condition and Subsequent Management Required.
Supracardiac TAPVC is the most common type of TAPVC, accounting for 45–55% of cases. In this type, the pulmonary venous confluence connects to an ascending VV that in turn connects to the innominate vein. This drains all pulmonary venous blood into the SVC and right atrium. The cardiac type of TAPVC accounts for 25–30% of cases. It is defined by drainage of the pulmonary venous confluence into the coronary sinus or directly to the right atrium. Infracardiac cases of TAPVC (13–25% of cases) have the pulmonary venous confluence connecting to a descending VV that courses through the oesophageal hiatus, and usually drains into the portal venous system. Mixed types of TAPVC are rare and account for less than 5% of cases.6
The pathophysiology of TAPVC depends mostly on the degree of obstruction to pulmonary venous flow. Infracardiac TAPVC is more likely to be obstructed, with the tortuous course of the pulmonary venous return through the liver. Patients with supracardiac TAPVC are less likely to have obstruction. Cardiac TAPVC, with connection of the pulmonary venous return to the coronary sinus, is least likely to be obstructed. Obstruction of the pulmonary venous return leads to severe pulmonary venous congestion, pulmonary oedema and pulmonary arterial hypertension which results in severe cyanosis and respiratory distress in the first few hours of life. Hence, significant obstruction requires immediate neonatal surgery.
The degree of functional obstruction is also influenced by the size of any interatrial communication, with a smaller communication associated with more severe symptoms. If the interatrial communication is large, however, it allows flow of blood into the left heart reducing pulmonary hypertension. Infants with TAPVC who are not obstructed, or who have an adequate atrial septal defect, may escape diagnosis in the neonatal period and present later in childhood.6
There are direct and indirect ultrasound markers which should raise the suspicion of TAPVC antenatally (Table 1). In a four‐chamber view, there may be an increase in the distance between the descending aorta (DAo) and LA (Figure 1b). The LA wall may appear smooth suggesting the absence of pulmonary vein ostia or there may be an additional vessel seen between the LA and descending aorta, representing the pulmonary vein confluence. Right left asymmetry may be more marked in third trimester as the pulmonary venous flow increases.
Ultrasound features can also help define the type of TAPVC. Supracardiac TAPVC will demonstrate a dilated SVC and innominate vein. Cardiac TAPVC will show a dilated coronary sinus, while an additional vessel seen in the sagittal section of the fetal thorax and abdomen may be seen in the infracardiac type.
In addition to two‐dimensional ultrasound, pulse wave Doppler is performed as part of a comprehensive fetal echocardiogram. This provides valuable physiological information which can be used to plan delivery and also counsel the parents appropriately. The pulse wave Doppler of the pulmonary veins is abnormal in fetuses with TAPVC. A normal pulmonary vein pulse Doppler shows a triphasic pulsatile pattern reflecting left atrial pressure changes (Figure 4a). In TAPVC, this pattern is lost. The abnormal waveform may be biphasic/pulsatile (Figure 4b), biphasic/continuous, monophasic/pulsatile or monophasic/pulsatile.7 The pattern is driven by the distant or dampened connection to a systemic vein.8 Doppler studies can also be used to investigate the degree of obstruction. High velocity and turbulent flow at the drainage site would suggest obstruction.
The ISUOG guidelines for routine second‐trimester evaluation suggest imaging the four‐chamber view, outflow tract view and three‐vessel‐trachea view as part of an extended cardiac scan, but do not mention about visualisation of the pulmonary veins.9 However, the guidelines on fetal heart examination recommend visualisation of at least two pulmonary veins when technically feasible during cardiac examination.10 The AIUM guidelines recommend imaging four‐chamber view, right and left ventricular outflow tracts in the second‐ and third‐trimester fetal examination.11 However, considering the technically difficulty, the ASUM guideline does not include visualisation of pulmonary veins in their ‘statement of Mid Trimester Obstetric Scan’ guidelines.12
During routine cardiac screening, failure to establish a connection between the pulmonary veins and left atrium should raise suspicion of TAPVC and hence referral for detailed fetal echocardiography. Asymmetry between right and left heart chambers may occur later in gestation and hence should not be used as primary feature to rule out TAPVC. Other indirect markers act as clues to diagnosis but are subjective. The visualisation of pulmonary veins during routine fetal screening ultrasound will aid in increasing antenatal diagnosis of this often missed cardiac anomaly. In our patient, the diagnosis was missed despite a comprehensive anomaly evaluation in a tertiary care centre. This highlights the challenges in establishing the diagnosis and the need for dedicated fetal echocardiography in high‐risk cases.
Conclusion
Antenatal diagnosis of TAPVC is challenging, but identification is crucial if optimal perinatal management is to be performed. Suspicion of TAPVC can be raised during a standard fetal cardiac ultrasound examination in the second or third trimester. Diagnosis of TAPVC in the fetus can lead to planning delivery in a tertiary centre with appropriate neonatal cardiac surgical capabilities.
References
- 1.Allan LD, Sharland GK. The echocardiographic diagnosis of totally anomalous pulmonary venous connection in the fetus. Heart 2001; 85(4): 433–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman JI. Incidence of congenital heart disease: I. Postnatal incidence. Pediatr Cardiol 1995; 16(3): 103–13. [DOI] [PubMed] [Google Scholar]
- 3.Shen I, Ungerleider R. Repair of supracardiac total anomalous pulmonary venous return. Oper Tech Thorac Cardiovasc Surg 2001; 6(1): 8–11. [Google Scholar]
- 4.Nasr VG, diNardo JA (eds). Total anomalous pulmonary venous return. The paediatric cardiac anaesthesia handbook, 1st edn. Hoboken, NJ: John Wiley & Sons Ltd; 2017; 107. [Google Scholar]
- 5.Chen YY, Hsu CY. Prenatal diagnosis and antenatal history of total anomalous pulmonary venous return. Taiwan J Obstet Gynecol 2006; 45(3): 283–5. [DOI] [PubMed] [Google Scholar]
- 6.Andropoulos DB, Gottlieb EA. Congenital Heart Disease. In: Fleisher LA, editor. Anesthesia and uncommon diseases, 6th edn. Philadelphia, PA: Saunders; 2012; 75–136. [Google Scholar]
- 7.Ganesan S, Brook MM, Silverman NH, Moon‐Grady AJ. Prenatal findings in total anomalous pulmonary venous return: a diagnostic road map starts with obstetric screening views. J Ultrasound Med 2014; 33(7): 1193–207. [DOI] [PubMed] [Google Scholar]
- 8.Boopathy Vijayaraghavan S, Rao AR, Padmashree G, Raman ML. Prenatal diagnosis of total anomalous pulmonary venous connection to the portal vein associated with right atrial isomerism. Ultrasound Obstet Gynecol 2003; 21(4): 393–6. [DOI] [PubMed] [Google Scholar]
- 9.Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Hernandez‐Andrade E, Johnsen SL, et al. Practice guidelines for performance of the routine mid‐trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2011; 37(1): 116–26. [DOI] [PubMed] [Google Scholar]
- 10.International Society of Ultrasound in Obstetrics and Gynecology , Carvalho JS, Allan LD, Chaoui R, Copel JA, DeVore GR, et al. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013; 41(3): 348–59. [DOI] [PubMed] [Google Scholar]
- 11.American Institute of Ultrasound in Medicine . AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med 2013; 32(6): 1083–101. [DOI] [PubMed] [Google Scholar]
- 12.ASUM Council . Statement on the Mid Trimester Obstetric Scan. ASUM Guidelines, Policies and Statements, 2014.
- 13.Laux D, Fermont L, Bajolle F, Boudjemline Y, Stirnemann J, Bonnet D. Prenatal diagnosis of isolated total anomalous pulmonary venous connection: a series of 10 cases. Ultrasound Obstet Gynecol 2013; 41(3): 291–7. [DOI] [PubMed] [Google Scholar]


