Abstract
Gaucher disease is a rare autosomal recessive disease caused by the beta-glicosidase activity deficiency, which will lead to substrate accumulation mainly in the liver, spleen or bone marrow. The main symptoms are liver and spleen enlargement, anemia and low platelet count, bone crisis and fatigue. Several treatment options are available, as enzyme replacement therapy, substrate reduction therapy, or chaperones treatment whose effect is still studied. There are 77 adult patients treated at this time in Romania, 54 with intravenous enzyme replacement ant 23 with oral substrate reduction therapy. No severe adverse effects have been reported by now. All patients had improved disease related symptoms after the receiving of the treatment.
Keywords: Gaucher disease, enzyme replacement therapy, substrate reduction therapy
Rare diseases are differently characterized among countries and institutions. While US regulations define a disease as “rare” with under 200.000 patients among the country (roughly 1 to 1.600 ppl prevalence in our days), in Europe the “under 5 patients to 10.000 people” rule is used. Overall, a “1 patient to 2,500 ppl” prevalence is accepted as a global average [1].
Among the rare diseases, lysosomal storage diseases are the most frequently diagnosed, with over 50 entities described, so consequently the most known and studied. The lysosome, with its phagocytosis role in the organism, uses over 60 enzymes inside its lumen to degrade complex cellular components into smaller parts, easier to eliminate [2]. Any disorder in the activity of such an enzyme would lead to the accumulation of the substrate in the lysosomes, which would lead to progressive symptomatology.
Gaucher disease (GD) was first described in 1882 by Phillipe Charles Ernest Gaucher in a patient with an enlarged spleen. Its prevalence varies from 1/50.000 to 1/850 (Ashkenazi Jewish population) [3]. It is a rare autosomal recessive genetic disorder characterized by a lysosomal glucocerebrosidase (beta glucosidase) reduced activity caused by GBA1 gene defect. This leads to a deficit of glucocerebrosidaze degradation, which will accumulate in the macrophages. The main organs involved are the liver, spleen, kidney, brain, lungs and bone marrow [4]. The clinical manifestations are correlated with substrate accumulation in the cells and in the involved organs. There are 3 main types of GD.
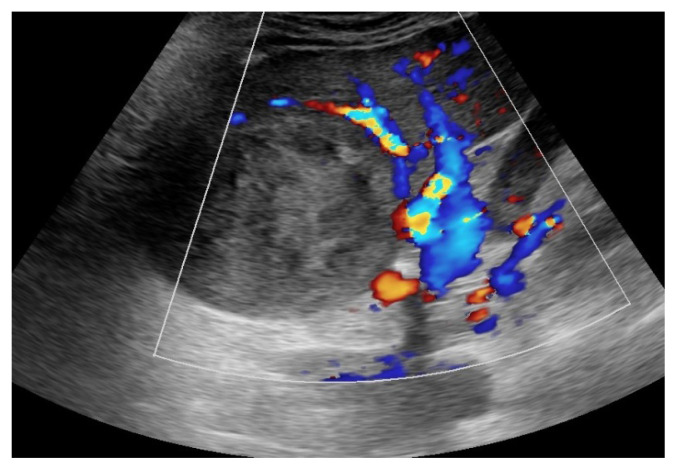
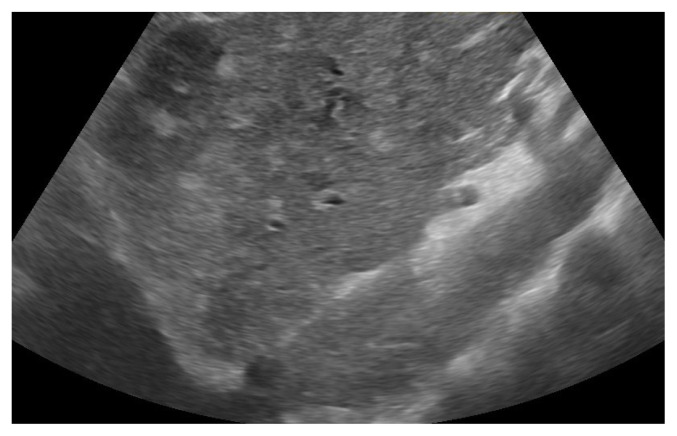
Type 1, also known as non-neuronopathic form, is the most frequent form, with up to 95% of the cases in Romania, with the liver, spleen and bone marrow as the main organs involved, without (or very low) neurological involvement. The main mutation is N370S/N370S. The glucocerebrosidase activity is still present, but very low, leading to mild (sometimes severe if not treated) symptomatology in the young adults. Its chronic evolution will lead to a later diagnosis, with a median age of 28 [5]. Splenomegaly (with gaucheromas - tumor like lesions, figure 1) and hepatomegaly (with gaucheromas - figure 2, but rarely cirrhosis), with abdominal pain or discomfort, are the main symptoms as the substrate accumulates in the organs, but easy bleeding or bruising, with low platelets count and anemia can also develop when bone marrow is involved. Erlenmeyer flask sign can be found by radiologists at the distal femur as the disease evolve, or even gaucheromas. Bone pains (or bone crisis) are also frequently described by patients, especially on the lower extremities joints area, and even necrosis areas can be found. Osteoporosis tends to develop at a younger age in GD patients [6]. Fatigue is frequently encountered but in different stages.
Figure 1.
Spleen Gaucheroma.
Figure 2.
Liver Gaucheromas.
Type 2, or acute neuronopathic GD with severe neurological involvement (with L444P involvement) when no or very low enzyme activity is detected, with very low life expectancy (under the age of 5).
Type 3, chronic neuronopathic GD, under 5 percent of the cases (with 2 cases known in Romania), also with L344P involvement, but with later and milder onset of the disease.
The diagnosis is based on the detection of low enzymatic activity. The enzyme activity can be determined in any nucleated cell, but peripheral blood is preferred as dry blood samples (DBS) are now widely available. B-glucosidase activity is measured in peripheral blood leucocytes. Prenatal screening is available, but lysosomal storage disease panel is not usually performed in general population. In selected groups (with identified high risk, as Ashkenazi Jewish population), where the mutations are known, general screening can be performed or even GBA1 entire gene sequencing. In the affected families, prenatal screening from amniotic fluid or chorionic villi should be performed, as the mutations are already known [7].
As newborn are diagnosed with GD, through any of the methods above, they do not necessary require treatment in all cases. Only 5% will develop hematological manifestations, up to 15% had bone manifestations, and only 1 in 5 growth issues. Splenomegaly and hepatomegaly were encountered at 12% and 74%, respectively, but no severe enlargement was found. As the enzymatic testing showed progressive disease and severe clinical features were only rarely found (with stable severity score), only 10% of the children required treatment [8]. If symptomatic, the children are recommended to receive treatment to avoid complications (bone necrosis, growth delay, specific organ damage) [9]. Romanian pediatric patients were referred to pediatricians and genetic departments after bone involvement (frequently diagnosed with arthritis) or anemia with severe splenomegaly, unfortunately after spleen removal.
The adult GD patients are usually diagnosed after several years of unspecific manifestations (especially in type 1 GD). These may include hematological manifestations as anemia or thrombocytopenia, liver or spleen enlargement or bone involvement (femoral necrosis). Lately, with the general and better accessibility to health services, with increasing awareness of rare diseases, more and more patients are tested after years of unexplained anemia or after hepatosplenomegaly of unknown origin. Adult patients require treatment if they become symptomatic. As the majority of the patients were diagnosed after multiple symptoms and multiple diseases investigated, as no general screening is done, they often require treatment at the time of the diagnosis. In Romanian patients, malignancies were quite rare, with kidney and liver being the most affected.
The treatment for GD has been life changing. As no specific therapy was known, only symptomatic treatment was available. Splenectomy, blood transfusions or painful surgeries after bone necrosis have been the main choices for many years, with severe consequences on life quality, growth deficiencies [10]. The first treatment available worldwide was the enzyme replacement therapy (ERT) in 1991, as the GD was described as an enzyme deficiency disease [11]. Several ERT are now available, but GD remains one of the most expensive disease to treat. Imiglucerase, velaglucerase and taliglucerase are widely available, without any severe or irreversible long time adverse effects. The response for each patient is different, with different times to achieve the therapeutic goals. It can take up to 2 years of continuous treatment to reduce an enlarged spleen or liver (and reduce mechanical discomfort) or to increase the platelets count and decrease the risk of hemorrhage. The pediatric patients, if symptomatic and treated constantly and rapidly after the diagnosis, will achieve a normal growth rate after 2–3 years of treatment [12]. The ERT is administered intravenously in different rates, with different dosages, depending on the severity of the disease and the patient adjusted response to the treatment. There are 54 adult patients with ERT in Romania (imiglucerase and velaglucerase) with dosages of 30–60 UI/kgbw every other week.
Another option in the treatment of GD is now the substrate reduction therapy and it is based on the theory of adjusting de substrate to the personal capacity of GD patients. There are now 2 known options: miglustat and eliglustat. The second one is the SRT option in Romania. There are currently 23 patients treated with SRT. 21 pts were subject to treatment switch from ERT when oral treatment became available and 2 pts were firstly prescribed SRT as first choice treatment after diagnosis. It is assumed that SRT trough elilustat has the same long time results as SRT, as ENGAGE and ENCORE studied showed in naïve and previously treated patients with SRT, respectively. The results were similar to ERT, making the SRT a viable option to the patients who desire an oral treatment instead to intravenous option [13,14]. The usualy dosage used in Romanian patients is 84 mg twice daily, with no severe long time adverse reactions reported so far.
The chaperones therapy is widely studied. Off-label usage of ambroxol in several studies showed a good response in platelet count and in reducing spleen volume (although in slower rates), showing another path in the treatment of GD [15].
All the adult patients treated so far in Romania had generally good response to SRT and ERT. Spleen reduction, platelets count and hemoglobin levels were improved in all patients. No splenectomy or transfusions related to GD were necessary so far. Orthopedic surgeries were necessary only for the correction of bone complications before therapy.
References
- 1.Ferreira CR. The burden of rare diseases. Am J Med Genet A. 2019;179:885–892. doi: 10.1002/ajmg.a.61124. [DOI] [PubMed] [Google Scholar]
- 2.Saftig P. In: Physiology of the lysosome In Fabry Disease: Perspectives from 5 Years of FOS. Mehta A, Beck M, Sunder-Plassmann G, editors. Oxford: Oxford PharmaGenesis; 2006. pp. 21–31. [PubMed] [Google Scholar]
- 3.Morales LE. Gaucher’s disease: a review. Ann Pharmacother. 1996;30:381–388. doi: 10.1177/106002809603000411. [DOI] [PubMed] [Google Scholar]
- 4.Zimran A, Szer J, Revel-Vilk S. Impact of Gaucher disease on COVID-19. Intern Med J. 2020;50:894–895. doi: 10.1111/imj.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grabowski GA, Kolodny EH, Weinreb NJR, Rosenbloom BE, Prakash-Cheng A, Kaplan P, et al. Gaucher disease: phenotypic and genetic variation. In: Scriver CR, Beaudet A, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited diseases. 9th ed . New York: McGraw-Hill; 2006. [Google Scholar]
- 6.Mucci JM, Rozenfeld P. Pathogenesis of Bone Alterations in Gaucher Disease: The Role of Immune System. J Immunol Res. 2015;2015:192761. doi: 10.1155/2015/192761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gary SE, Ryan E, Steward AM, Sidransky E. Recent advances in the diagnosis and management of Gaucher disease. Expert Rev Endocrinol Metab. 2018;13:107–118. doi: 10.1080/17446651.2018.1445524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang AC, Bier L, Overbey JR, Cohen-Pfeffer J, Desai K, Desnick RJ, et al. Early manifestations of type 1 Gaucher disease in presymptomatic children diagnosed after parental carrier screening. Genet Med. 2017;19:652–658. doi: 10.1038/gim.2016.159. [DOI] [PubMed] [Google Scholar]
- 9.Baldellou A, Andria G, Campbell PE, Charrow J, Cohen IJ, Grabowski GA, et al. Paediatric non-neuronopathic Gaucher disease: recommendations for treatment and monitoring. Eur J Pediatr. 2004;163:67–75. doi: 10.1007/s00431-003-1363-z. [DOI] [PubMed] [Google Scholar]
- 10.Zimran A. How I treat Gaucher disease. Blood. 2011;118:1463–1471. doi: 10.1182/blood-2011-04-308890. [DOI] [PubMed] [Google Scholar]
- 11.Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, et al. Replacement therapy for inherited enzyme deficiency–macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- 12.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372:1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 13.Mistry PK, Lukina E, Ben Turkia H, Amato D, Baris H, Dasouki M, et al. Effect of oral eliglustat on splenomegaly in patients with Gaucher disease type 1: the ENGAGE randomized clinical trial. JAMA. 2015;313:695–706. doi: 10.1001/jama.2015.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox TM, Drelichman G, Cravo R, Balwani M, Burrow TA, Martins AM, et al. Eliglustat compared with imiglucerase in patients with Gaucher’s disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet. 2015;385:2355–2362. doi: 10.1016/S0140-6736(14)61841-9. [DOI] [PubMed] [Google Scholar]
- 15.Zimran A, Altarescu G, Elstein D. Pilot study using ambroxol as a pharmacological chaperone in type 1 Gaucher disease. Blood Cells Mol Dis. 2013;50:134–137. doi: 10.1016/j.bcmd.2012.09.006. [DOI] [PubMed] [Google Scholar]