Abstract
Despite the decades old knowledge that diabetes mellitus is a major risk factor for cardiovascular disease, the reasons for this association are only partially understood. While this association is true for both type 1 and type 2 diabetes, different pathophysiological processes may be responsible. Lipids and other risk factors are indeed important, whereas the role of glucose is less clear. This lack of clarity stems from clinical trials that do not unambiguously show that intensive glycemic control reduces cardiovascular events. Animal models have provided mechanisms that link diabetes to increased atherosclerosis, and evidence consistent with the importance of factors beyond hyperglycemia has emerged. We review clinical, pathological, and animal studies exploring the pathogenesis of atherosclerosis in humans living with diabetes and in mouse models of diabetes. An increased effort to identify risk factors beyond glucose is now needed to prevent the increased cardiovascular disease risk associated with diabetes.
eTOC
Diabetes increases cardiovascular disease risk, but the reasons for the association are not fully understood. Here, Eckel et al. review clinical and animal studies that explore the pathogenesis of atherosclerosis in the diabetes context. They discuss the need to identify and understand risk factors beyond glucose in order to prevent increased cardiovascular disease risk in diabetes.
Introduction
The association of cardiovascular disease (CVD) and diabetes has been known for decades. Despite this, the reasons for this relationship are incompletely understood. Because treatment of hyperglycemia alters a number of factors other than circulating glucose levels, clinical and experimental data support the hypothesis that factors other than hyperglycemia are the culprit. As shown in Figure 1, clinical trials have assessed whether glycemic control or reduction of other risk factors, such as LDL-cholesterol (LDL-C), triglycerides (TGs), blood pressure, and systemic inflammation prevent incident CVD in humans with diabetes. These trials have overwhelmingly shown a beneficial effect of LDL-C lowering, and some additional beneficial effect of targeting inflammation, while the roles of glucose reduction, TG lowering and blood pressure control are less clear. While an important clinical goal is reduction of hyperglycemia to reduce microvascular complications, primarily disease of the eyes and kidneys, the influence of glucose reduction in atherosclerotic CVD (ASCVD) prevention remains elusive in the majority of patients with type 2 diabetes mellitus (T2DM). In this review, we will integrate the clinical and experimental data from animal models of diabetes and atherosclerosis, focusing on how diabetes alters the steps involved in atherogenesis.
Figure 1. Cardiovascular outcome trials (CVOTs) and relative risk modification by changes in CVD risk factors in patients with T1DM or T2DM.
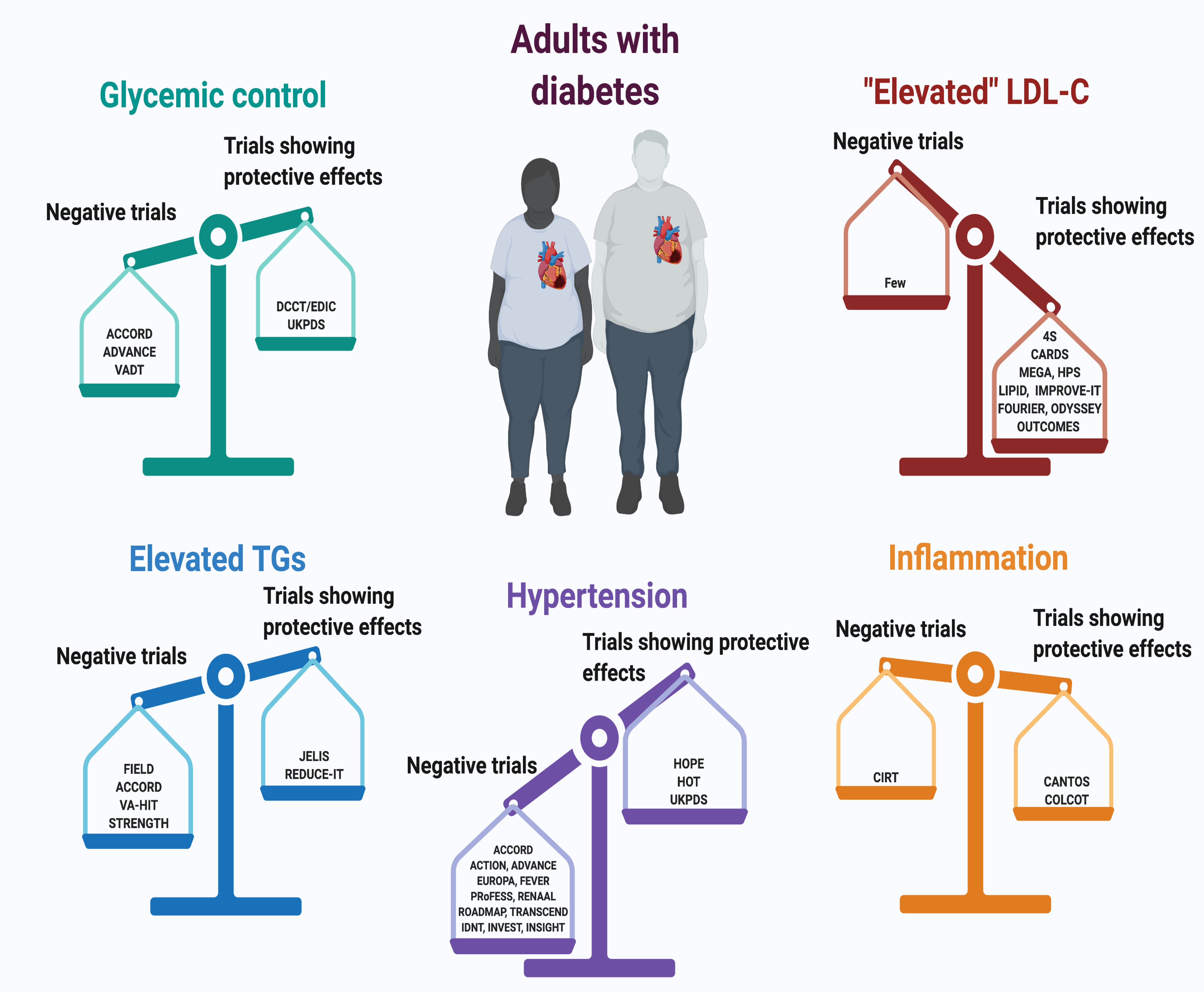
This review will consider in more depth four factors associated with diabetes and CVD risk: glucose reduction (upper left), LDL-cholesterol reduction (upper right), triglycerides (lower left) and inflammatory factors (lower right). Major clinical trials are indicated: Glycemic control: ACCORD (Gerstein et al., 2011); ADVANCE (Ninomiya et al., 2009); VADT (Duckworth et al., 2009); DCCT/EDIC; (Diabetes Control Complications Trial/Epidemiology of Diabetes, 2016)UKPDS, (Holman et al., 2008); “Elevated” LDL-C: 4S (Pyörälä et al., 2004); CARDS (Colhoun et al., 2004); MEGA (Tajima et al., 2008); HPS (Heart Protection Study Collaborative Group, 2002); LIPID (Keech et al., 2003); IMPROVE-IT (Giugliano et al., 2018); FOURIER (Sabatine et al., 2017); ODYSSEY OUTCOMES (Ray et al., 2019); Elevated TGs: FIELD (Scott et al., 2009); ACCORD (Ginsberg et al., 2010); VA-HIT, (Rubins et al., 1999); STRENGTH (Nicholls et al., 2020); JELIS (Yokoyama et al., 2007); REDUCE-IT (Bhatt et al., 2019); Inflammation: CIRT (Ridker et al., 2019); CANTOS (Everett et al., 2018); COLCOT (Nidorf et al., 2020). Hypertension: HOPE (Heart Outcomes Prevention Evaluation Study Investigators, 2000); HOT (Zanchetti et al., 2003); UKPDS (UK Prospective Diabetes Study Group, 1998b); ACCORD (Accord Study Group et al., 2010); ACTION (Elliott et al., 2011); ADVANCE (Patel et al., 2007); EUROPA (Daly et al., 2005); FEVER (Zhang et al., 2011); PRoFESS (Yusuf et al., 2008); RENAAL (Brenner et al., 2001); ROADMAP (Haller et al., 2011); TRANSCEND (Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease Investigators et al., 2008); IDNT (Lewis et al., 2001); INVEST (Bakris et al., 2004); INSIGHT (Mancia et al., 2003). Created with BioRender.com.
Lesion pathology:
Before considering the clinical trial and experimental data, it is important to review the vascular pathology in patients with type 1 diabetes mellitus (T1DM) and T2DM. ASCVD in patients with diabetes differs somewhat from that in patients without diabetes, but these differences are subtle. Although greater calcification in the arterial media is found in patients with diabetes, especially T2DM (Yahagi et al., 2017) (Figure 2), in asymptomatic T2DM and T1DM patients examined by multi-slice computerized tomography with similar coronary artery calcification scores, patients with T2DM had more extensive ASCVD and more non-calcified plaques than those with T1DM (Djaberi et al., 2009). In the CACTI (Coronary Artery Calcification in Type 1 Diabetes) study of subjects with T1DM, coronary calcification was associated with insulin resistance, a finding typically more characteristic of T2DM and poor glycemic control (Bjornstad et al., 2016). This suggests that insulin resistance, when present, contributes to vascular disease also in people with T1DM (Purnell et al., 2013). However, in general the lesions’ morphology appears similar, and the principal effect of diabetes is likely due to acceleration of several of the phases of atherogenesis. Unlike renal and retinal microvascular disease, there is no pathological fingerprint identifying a distinct atherosclerotic plaque in the setting of diabetes. Post-mortem coronary plaques from subjects with either T1DM or T2DM tend to have more macrophages and larger necrotic core areas, concomitant with increased plaque burden, as compared to plaques from subjects without diabetes (Yahagi et al., 2017). These studies also suggested that plaques from patients with T1DM or T2DM have more evidence of rupture and repair. Overall, these data indicate that the lesions from patients with diabetes might progress faster. Risk factors that likely drive this process will be discussed below.
Figure 2. Morphological features of lesions of atherosclerosis are similar in the presence and absence of diabetes, but diabetes enhances lesion progression and slows regression.
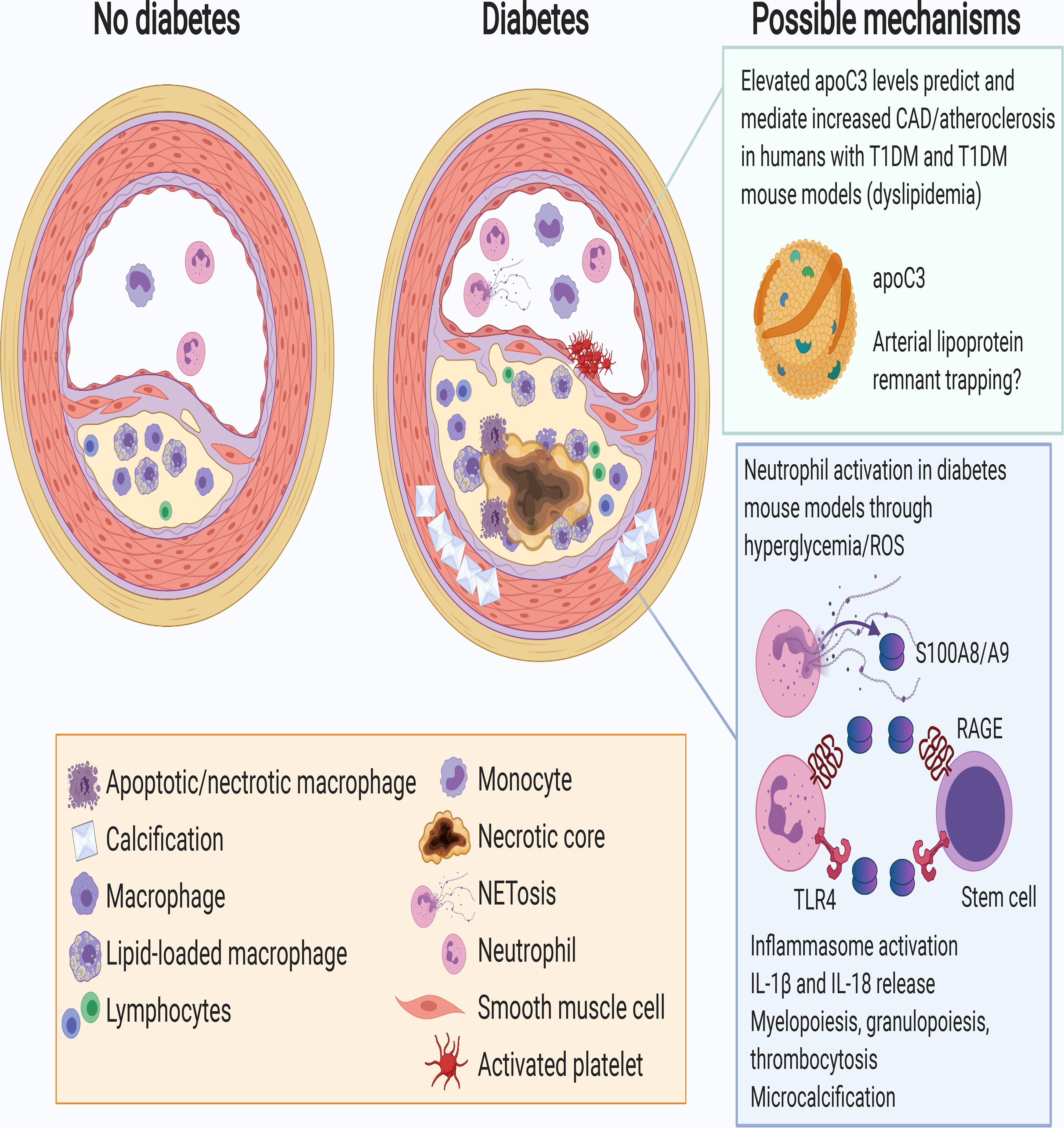
This schematic representation shows an intermediate lesion in the absence of diabetes (left) and a more advanced lesion in the presence of diabetes (right). The more advanced lesion in the setting of diabetes is characterized by an increased abundance of macrophages and lymphocytes (T cells), and an increased prevalence of necrotic cores, which can make the lesion more unstable and prone to rupturing or fissuring, and subsequent platelet activation and thrombus formation. The increased inflammatory state of the lesion in diabetes has been attributed, in part, to monocytosis and neutrophilia, leading to increased levels of monocytes and neutrophils in circulation and in the lesion, as well as activation of neutrophils and release of the damage-associated molecular pattern proteins S100A8 and S100A9 and neutrophil extracellular traps (NETs) through NETosis. Moreover, T2DM is often associated with increased medial calcification. The boxes on the right show mechanisms of worsened atherosclerosis and impaired lesion regression based on data from mouse models of diabetes. These mechanisms, which may include increased trapping of remnant lipoproteins in the artery wall (top) and increased neutrophil activation, toll-like receptor 4 and RAGE activation (bottom), are discussed in the text. Created with BioRender.com.
Animal models of diabetes often show accelerated atherogenesis. Most of these models, however, are confounded by diabetes-induced changes in circulating lipoproteins. In others, extraordinary levels of circulating cholesterol appear to “swamp out” effects of hyperglycemia or defective insulin actions. One approach to illustrate the effects of diabetes without severe hypercholesterolemia involves assessment of early lesions, assuming these represent the initiation of disease. In one model, insulin-deficient LDL receptor-deficient (Ldlr−/−) mice, a model of T1DM without marked hyperlipidemia, had more macrophage accumulation in early lesions in (Renard et al., 2004). In the same model, diabetes promoted the incidence of hemorrhage and necrotic core expansion in advanced lesions (Johansson et al., 2008; Kanter et al., 2019). Increased necrotic core formation, an indication of advanced plaques, also occurred in mice with loss of insulin receptors in macrophages (Han et al., 2006). Greater necrotic core could result from reduced efferocytosis (the clearance of dead cells by phagocytes), which was reported in ob/ob mice, a mouse model of T2DM (Li et al., 2009). Although rodent lesions are generally deficient in calcification, the combination of insulin resistance, dietary cholesterol, and saturated fat was reported to create calcification in the arterial wall (Nguyen et al., 2013). Thus, animal pathology indicates that diabetes leads to faster lesion initiation and progression to more advanced lesions.
Glycemic control and CVD
Human epidemiologic and clinical trial data clearly show that improved glycemic control is associated with fewer CVD events in patients with T1DM (Diabetes Control Complications Trial/Epidemiology of Diabetes, 2016; Nathan et al., 2005). The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study was and remains a clinical trial on improved glucose control by intensive insulin treatment vs. conventional treatment in subjects with T1DM (DCCT) and a subsequent observational period (EDIC) following most of the DCCT subjects. The DCCT reported a decrease in cardiovascular events (~40%) but this decrease was not statistically significant, likely due to the small number of events in this low-risk population. In the EDIC observational study the decrease in cardiovascular events became statistically significant as more events accrued. However, because more intensive insulin treatment affects many parameters, these data cannot provide information on the primary cause of greater ASCVD with diabetes. For instance, studies have shown that at the 15.8-year DCCT/EDIC follow-up period the development of hypertension was decreased in the intensive insulin group (de Boer et al., 2008). Moreover, early in the active phase of DCCT there were favorable changes in several inflammatory markers (soluble ICAM-1, TNF-R1) with intensive glucose control, and decreases in high-sensitivity C-reactive protein (hsCRP; a marker of systemic inflammation) in patients in the lower third of weight gain (Schaumberg et al., 2005). Epidemiologic data show that a number of risk factors other than glycemic control strongly associate with ASCVD risk in patients with diabetes, especially T2DM (Orchard et al., 2003). The failure of improved glycemia with insulin, sulfonylureas, and DPP4 inhibitors to reduce CVD events in patients with T2DM also implies that factors other than hyperglycemia are important, as reviewed (Schmidt, 2019). Moreover, the observation that patients with metabolic syndrome who do not yet have an overall increase in circulating glucose—levels of glycated hemoglobin (hemoglobin A1c; HbA1c; a marker of glycemic control) are not in the currently accepted diabetic range—also have greater CVD risk has been interpreted as evidence that hypertension, obesity, dyslipidemia, thrombosis, and inflammation and not glucose levels alone drive CVD risk in patients with T2DM (Haffner et al., 2000). This increased CVD might be especially profound in women (Haffner et al., 1997). As will be reviewed below in more detail, apart from LDL-C lowering, clinical trials have failed to provide a clear picture, either because of the many actions of drugs, the many causes of atherosclerosis, and/or differences in human response to a specific intervention.
Clinical trials of glucose reduction:
For many years, clinicians accepted that hyperglycemia explained the increased incidence of CVD in patients with diabetes. Recent epidemiological data from the Swedish National Diabetes Registry in a large cohort of patients with T1DM (n=33,170) support the importance of improved glycemic control in reducing acute myocardial infarction, CVD death and overall CVD risk (Matuleviciene-Anangen et al., 2017). However, even in patients with HbA1c ≤6.9% the relative risk was 2.53, suggesting that factors other than average glucose impart vascular toxicity. Further clinical trial support for the adverse effects of hyperglycemia in T1DM was obtained in the DCCT/EDIC population and the UKPDS population with recent onset of T2DM. The UK Prospective Diabetes Study (UKPDS) was a multicenter trial of glycemic and blood pressure lowering therapies in 5,102 patients with newly diagnosed T2DM. It ran for twenty years in 23 UK clinical sites and showed that improving blood glucose and/or blood pressure control reduced the microvascular complications of T2DM (UK Prospective Diabetes Study Group, 1998a). Subsequently, all surviving patients were entered into a 10-year, open label monitoring program. Subjects in the original intensive treatment group experienced a 15% reduction in cardiovascular events, which became statistically significant despite loss of within trial blood glucose and antihyperglycemic therapy differences, the so-called legacy effect (Holman et al., 2008). However, the predictive association of mean HbA1c on subsequent CVD events in both studies was partly dependent on standard risk factors (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group, 2016; Stevens et al., 2004). Thus, the importance of hyperglycemia per se on CVD risk remains unclear (Figure 1, upper left).
The argument that factors other than hyperglycemia fuel the greater incidence of CVD events and related mortality in patients with T1DM and T2DM is supported by several “failed” clinical trials (Laakso, 2001; Meigs, 2003) briefly summarized in Figure 1, upper left. The open label EDIC extension of the DCCT confirmed that better glycemic control in subjects with T1DM during the first seven years of intensive glycemic control reduced CVD events by over 50%; and this effect was evident despite similar levels of HbA1c in the intensive vs. control groups (Nathan et al., 2005). As discussed below, the importance of glucose control in this outcome has been challenged.
Of interest, in DCCT/EDIC there was an increased risk for any CVD event and major adverse cardiovascular events (MACE) in patients on higher insulin doses but when these data were adjusted for systolic blood pressure, pulse rate, HDL-C and LDL C, TG and HbA1c, there was no longer a relationship and the risk factor that explained most of this confounding was TG (Braffett et al., 2019). Moreover, urinary albumin excretion and sustained microalbuminuria or macroalbuminuria were associated with ~2-fold increased risk of any CVD event and rendered the effect of HbA1c no longer significant (Diabetes Control Complications Trial/Epidemiology of Diabetes, 2016). Moreover, with age, the association of mean HbA1c on subsequent CVD events was increasingly related to favorable modification of standard risk factors (Bebu et al., 2017). In another assessment of ASCVD in the DCCT/EDIC cohort using carotid intima-media thickness, not CVD events, fibrinogen was the best predictor for intima-media thickness progression (Hunt et al., 2015). Thus, the benefit of improved glycemic control in the DCCT/EDIC on ASCVD appears to be multifactorial, associates with a number of changes, and fails to prove cause and effect.
If glucose control were the primary driver of increased ASCVD in T2DM, then one would have expected that insulin, sulfonylureas, and the many other glucose-reducing agents would also have reduced CVD events. But several large studies of T2DM have failed to prove benefits of glucose reductions in these patients (Figure 1, upper left). These trials are difficult to interpret, and the results lend themselves to multiple interpretations. One view is that failure of these trials to show CVD benefit from glucose reduction supports the hypothesis that factors other than hyperglycemia are more important accelerators of ASCVD in these patients. Unlike UKPDS, previously outlined, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, tighter glycemic control actually led to more death (ACCORD Study Group., 2011). Although not a usual focus of discussion, the better-controlled subjects in ACCORD had fewer new myocardial infarctions, leading some to suggest that better glycemic control reduced atherogenesis while more hypoglycemia in the intensive treatment group with underlying CVD increased hypoglycemia, arrhythmic events and sudden death (Koska et al., 2018). Of interest, patients in ACCORD who died were those in whom reductions in glycemia by more intensive insulin management were less likely to be achieved. ADVANCE was a similar cardiovascular outcome trial (CVOT) that enrolled 11,140 T2DM patients in 215 collaborating centers in 20 countries from around the world. Sulfonylurea-based intensive glycemic therapy targeting a HbA1c ≤6.5% vs. standard of care was associated with a 10% reduction in combined microvascular and macrovascular events compared with standard therapy, an effect largely driven by a 23% reduction in the risk of microvascular events, principally nephropathy (Advance Collaborative Group et al., 2008). A subsequent publication by the ADVANCE group presented an additional 5.4 years of follow-up data. The group assigned to intensive glucose control still failed to demonstrate any macrovascular or mortality benefits over the standard therapy group (Zoungas et al., 2014). Another large glucose reduction study, VADT, showed an overall benefit but only during the prolonged period in which the glycated hemoglobin curves were separated (Reaven et al., 2019). In a separate analysis, patients in VADT with low coronary artery calcium had the greatest benefit (Koska et al., 2018). Could it be that glucose reduction prevents atherosclerosis in patients without severely damaged arteries, but does not prevent the final processes that lead to CVD events? Although a meta-analysis of these 4 trials did report a modest and statistically significant 9% decrease in death from cardiovascular disease, non-fatal myocardial infarction, and non-fatal stroke (Control Group et al., 2009), perhaps, with long-standing diabetes, the horse has left the barn and many years of hyperglycemic damage is mostly irreversible and not impeded. This could reflect arterial stiffness, a signature for vascular aging that in part is explained by glycation-induced accumulation of advanced glycation endproducts (AGEs) (Stehouwer et al., 2008).
Another issue with interpretation of these clinical trials are questions related to the method used to track glycemic control. HbA1c reflects average glucose control over a 3-month period, but this measurement does not reveal the variation in glucose levels during the day. It is argued that glucose variability and/or time in range and not average glucose leads to CVD risk. This conclusion is supported by clinical data correlating glucose variability with evidence of greater plaque vulnerability (more necrotic core) (Hirsch, 2015). A study of coronary calcium also associated higher score with glucose variability (Snell-Bergeon et al., 2010a). However, this hypothesis was not supported in PRANDIAL, a study in patients with T2DM following an acute myocardial infarction who were randomized to three premeal doses of a fast-acting insulin targeting 2-hour postprandial blood glucose to <7.5 mmol/L (135 mg/dL) vs. an intermediate-acting insulin twice daily or a long-acting insulin once daily targeting fasting/premeal blood glucose <6.7 mmol/L (120 mg/dL) (Raz et al., 2009). Although differences in fasting blood glucose were achieved, less-than-expected differences in postprandial blood glucose were noted and there was no difference in CVD events at a mean of 963 days after randomization. Overall, in patients with T1DM, both long term observational and one well done randomized controlled trial provide evidence that better control of glycemia, likely associated with improvement in other risk factors, is associated with less ASCVD events. However, in patients with T2DM, this relationship appears unconvincing.
Anti-diabetic drugs - SGLT-2 inhibitors, GLP-1 receptor agonists, PPARγ receptor agonists and metformin:
The requirement that glucose-lowering agents be assessed for CVD safety and efficacy has generated large clinical trial data using newer agents approved for the treatment of T2DM. These studies have been reviewed in detail elsewhere (Dardano et al., 2020; Lim et al., 2018; Rodriguez et al., 2017; Wilcox et al., 2020). Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) reduce cardiovascular events, the mechanism proposed is a reduction in atherosclerosis. Sodium glucose co-transporter 2 inhibitors (SGLT-2is) also are beneficial; however, their major benefits are to reduce hospitalization for heart failure and favorably modify the natural history of diabetic nephropathy. In contrast, although DPP4 inhibitors raise circulating levels of GLP-1 these agents do not reduce CVD events. It appears that the benefits of GLP-1 RAs and SGLT-2is are exclusive of their glucose-reducing actions (Table 1). This contention is supported by data that demonstrate similar heart failure benefits in patients without diabetes (McMurray et al., 2019; Packer et al., 2020).
Table 1.
SGLT-2 inhibitor and GLP-1 receptor agonist glucose lowering CVOTs in diabetes
| Trial | Primary outcome | CVD death | Myocardial infarction | Stroke | All-cause mortality | HHF | Mean reduction in HbA1c (%) | Effect of reduced glycemia on MACE |
|---|---|---|---|---|---|---|---|---|
| GLP-1 RAs | ||||||||
| LEADER | P=0.01 | P=0.007 | P=0.046 | NS | P=0.02 | NS | −0.40 | NA or NS |
| SUSTAIN-6 | P=0.02 | NS | NS | P=0.04 | NS | NS | −1.3 | NS |
| ELIXA | NS | NS | NS | NS | NS | NS | −0.27 | NS |
| EXSCEL | P=0.06 | NS | NS | NS | NS | NS | −0.53 | NA or NS |
| REWIND | P=0.02 | NS | NS | P=0.01 | NS | NS | −0.61 | NA or NS |
| SGLT2 Inhibitors | ||||||||
| EMPA-REG | P=0.04 | P<0.001 | NS | HS | P<0.001 | P<0.001 | −0.42 | NS |
| CANVAS | P=0.02 | NS | NS | NS | NS | P=0.002 | −0.58 | NS |
| CREDENCE | P=0.01 | NS | NS | NS | NS | P=0.001 | −0.25 | NA |
| DECLARE-58 | NS | NS | NS | NS | NS | P=0.005 | −0.42 | NA or NS |
| VERTIS | NS | NS | NS | NS | NS | P=0.006 | −0.50 | NA or NS |
HHF, hospitalization for heart failure; NS, not significant; NA, not assessed or published
LEADER (Marso et al., 2016b); SUSTAIN-6 (Marso et al., 2016a); ELIXA (Pfeffer et al., 2015); EXSCEL (Holman et al., 2017); REWIND (Gerstein et al., 2019); EMPA-REG (Inzucchi et al., 2018; Zinman et al., 2015); CANVAS (Neal et al., 2017); CREDENCE (Cannon et al., 2020a; Mahaffey et al., 2019; Perkovic et al., 2019); DECLARE-58 (Wiviott et al., 2019); VERTIS (Cannon et al., 2020b)
Another class of agents peroxisome proliferator-activated receptor (PPAR)γ agonists that includes pioglitazone,, led to fewer recurrent strokes in a pre-diabetes population (Kernan et al., 2016) and reduced CVD events by ~15% in subjects with T2DM and evidence of CVD in the PROactive trial (Dormandy et al., 2005). However, the significance of this benefit was lost when peripheral vascular disease events were included in the analysis. Of note, in PROactive, less than half of the patients were taking statins and average LDL-C was ~110 mg/dL (2.9 mmol/L). Pioglitazone, however, reduced plasma TG and increased HDL-C levels, and reduced blood pressure. In a lower risk population, the TOSCA-IT study use of pioglitazone more than sulfonylurea when added to metformin did not provide CVD benefit (Vaccaro et al., 2017). A meta-analysis of ten studies using pioglitazone demonstrated a reduced rate of recurrent MACE, myocardial infarction, or stroke (de Jong et al., 2017). However, the effect of pioglitazone was not mediated by reductions in glycemia and was associated with increases in heart failure; thus, this drug is contraindicated in patients with more advanced heart failure (Paneni and Lüscher, 2017).
PPARγ agonists do reduce atherosclerosis in mouse models (Collins et al., 2001; Li et al., 2000), suggesting a direct vascular or macrophage effect of these drugs. Several attempts were made to show similar effects in humans, all were comparisons with sulfonylureas. The CHICAGO Trial showed a decrease in carotid-medial thickness in subjects treated with pioglitazone rather than a sulfonylurea (Mazzone et al., 2006). The benefits of pioglitazone were most evident at the first analysis (24 weeks); the rate of thickening between 48 and 72 weeks in the treated and untreated groups was parallel. In PERISCOPE, intravascular ultrasound analysis of atherosclerosis progression showed a benefit of pioglitazone over glimepiride to halt disease progression over 18 months (Nissen et al., 2008). In the short-term 6-month Pioneer study, with a similar reduction in HbA1c, pioglitazone increased HDL-C, increased adiponectin, and reduced carotid intimal medial thickness, hsCRP and several other inflammatory markers (Pfutzner et al, 2005).
Another glucose reducing agent, metformin, appears to have some benefit to reduce CVD events. While studies with metformin have been small and not of the level of quality of many CVOTs, the results of these trials have demonstrated a beneficial effect of metformin on CVD (Hong et al., 2013; Kooy et al., 2009; UK Prospective Diabetes Study (UKPDS) Group, 1998). It is unknown if these effects are mediated by glucose lowering, weight loss, or other factors
A direct role of hyperglycemia in animal models of diabetes and atherosclerosis is likewise uncertain:
Diabetes and atherosclerosis have been studied in a variety of animal models (Table 2). The data here are also conflicted as some models have reported more atherosclerosis or more inflammatory plaques, while others have shown no effect or, in some cases, less disease in the presence of hyperglycemia and diabetes.
Table 2.
Animal models of diabetes and atherosclerosis
| Mouse models (Hsueh et al., 2007; Wu and Huan, 2007) |
| Insulin Deficiency |
| Streptozotocin |
| Akita |
| T cell-mediated destruction of islets |
| Insulin Resistance |
| Leptin deficiency |
| Leptin receptor deficiency |
| Tissue specific insulin receptor deficiency (esp. liver-specific insulin receptor-deficiency) |
| High fat diets |
| High fructose diets |
| Diet-induced obesity and insulin deficiency |
| Eliminating hyperglycemia |
| SGLT-2 inhibitors |
| Rat models (Islam and Wilson, 2012) |
| Partial pancreatectomy |
| High-fat diet combined with low dose streptozotocin (Azemi et al., 2020) |
| Sucrose diet leads to insulin resistance (Plazas Guerrero et al., 2019) |
| Goto-Kakizaki (GK) model (Movassat et al., 2007) |
| Rabbit models |
| Alloxan (Bacevic et al., 2020) |
| Swine models (Sturek et al., 2020) |
| Alloxan (Badin et al., 2018) |
| Streptozotocin (Shim et al., 2016) no effect on atherosclerosis (Al-Mashhadi et al., 2015) |
| Metabolic syndrome high-fat diet increased CAD but in only some strains (Neeb et al., 2010) |
| Non-human primate models (Clarkson et al., 1985; Pound et al., 2014) |
| Streptozotocin (Kim et al., 2016) |
| T2DM – Insulin resistance spontaneous in some male rhesus monkeys (Qian et al., 2015) |
| High-fat and sucrose and streptozotocin (Lu et al., 2015) |
Before reviewing the studies, we should note that many animal models of diabetes and CVD are extreme. Human ASCVD and diabetes complications occur over years, longer than the relative lifespan of most experimental animals. Thus, studies of insulin-deficient diabetes are often performed with levels of hyperglycemia in excess of those found in even poorly controlled human diabetes. Moreover, the levels of cholesterol in Western diet-fed or high cholesterol-fed LDL receptor-deficient or apoE-deficient mice (the most common models of atherosclerosis) exceed those found in humans except for rare dyslipidemic patients. Studies of models with lower cholesterol levels produce small lesions that some have argued are more akin to fatty streaks than true atherosclerosis. Nevertheless, the role of these models is to analyze effects in an experimentally tractable period allowing investigators to uncover pathological mechanisms, some of which are not valid for humans.
Mouse models reproduce many diabetes-mediated processes that are thought to accelerate atherosclerosis, as reviewed in (Hsueh et al., 2007). The most commonly used mouse diabetes models are those created by either genetic (Akita), T-cell mediated, or chemical destruction of pancreatic islet β cells (streptozotocin). In these models, the insulin deficiency is often severe and more akin to T1DM than T2DM. Moreover, with the usual atherosclerosis-prone models—LDL receptor-deficiency or apoE deficiency—circulating cholesterol levels are higher with diabetes when the mice are fed a fat-enriched diet. This increase is either due to changes in lipoprotein production and removal or to greater food intake that accompanies the diabetes (Hsueh et al., 2007; Jun et al., 2011). Mouse models in which circulating cholesterol levels are excessively elevated often fail to show the effects of diabetes on lesion development even in the presence of marked hyperglycemia (Hsueh et al., 2007; Reaven et al., 1997; Schmidt, 2019), perhaps because the extremely high cholesterol levels generated with these knockouts drive rapid atherogenesis and obliterate contributions of other physiologically meaningful processes. An example is the study by Biddinger et al., who reported that loss of insulin receptors in the liver led to more atherosclerosis, likely caused by the accompanying hyperlipidemia (Biddinger et al., 2008). Similarly, infusion of insulin into apoE-deficient mice reduces atherosclerosis, but this benefit is associated with reduced circulating lipoprotein levels (Park et al., 2018). Some interventions do increase lesion size in hypercholesterolemic models of diabetes. Expression of the enzyme aldose reductase, which leads to aberrant metabolism of glucose, increases lesions in both diabetic LDL receptor-deficient (Vikramadithyan et al., 2005) and apoE-deficient models (Vedantham et al., 2011). A recent study using repetitive peritoneal infusion of glucose to reproduce glucose variability showed accelerated atherosclerosis associated with increased myelopoiesis (Flynn et al., 2020). These effects were prevented by deletion of the glucose transporter GLUT1 in myeloid cells, an effect attributed to reduced glucose uptake in neutrophils (Flynn et al., 2020). These two studies support a role for glucose in promoting atherosclerosis in mouse models.
The obvious candidates responsible for greater CVD with diabetes are hyperglycemia and defective insulin action, leading for example to dyslipidemia or direct pro-atherogenic processes in vascular cells. The receptor for advanced glycation endproducts (RAGE) is activated in the presence of diabetes and other inflammatory diseases, and infusion of a soluble portion of RAGE prevents atherosclerosis in hypercholesterolemic mice with diabetes (Park et al., 1998). However, RAGE is activated by many ligands, including several damage-associated molecular pattern molecules, such as S100 calprotectins and high-mobility group box1 (HMGB1) proteins (Palanissami and Paul, 2018), which serve an important role even in the absence of hyperglycemia. An effect of RAGE deletion therefore does not necessarily suggest a direct effect of hyperglycemia.
Cellular processes mimicking those of increased glucose uptake in lesion cells were studied by cell type-selective overexpression of the glucose transporter GLUT1 in mice. Overexpression of GLUT1 in myeloid cells increased glucose uptake but did not increase atherosclerosis in LDL receptor-deficient mice fed a low-fat diet or a high-fat high-carbohydrate diet, which caused insulin resistance (Nishizawa et al., 2014; Wall et al., 2018). When GLUT1 was overexpressed in smooth muscle cells (SMCs) (Wall et al., 2018), a worsening of atherosclerosis was evident, but only in mice fed the high-fat high-carbohydrate diet, which was associated with myelopoiesis. In the absence of myelopoiesis, SMC overexpression of GLUT1 had no effect. Thus, forcing increased glucose uptake into myeloid cells or SMCs is not sufficient to promote atherosclerosis.
The role of insulin signaling in CVD was studied by deletion of the insulin receptor in various vascular cell types. Deletion of insulin receptors in endothelial cells increased atherosclerosis (Rask-Madsen et al., 2010), suggesting an anti-atherogenic effect of insulin in endothelial cells. Insulin signaling modulates endothelial cell function, both by altering vascular growth and vascular reactivity (King et al., 2016). Deletion of insulin receptors in hematopoietic cells or myeloid cells produced discrepant effects on atherosclerosis in two different studies (Baumgartl et al., 2006; Han et al., 2006). Loss of insulin receptors in SMCs reduced intimal thickening and SMC proliferation after vascular injury (Li et al., 2019). Increased SMC proliferation in lesions, mediated by insulin signaling, might be beneficial if it results in thickening of the fibrous cap.
Mouse models to replicate the biology of T2DM are limited. High-fat high-carbohydrate diets create insulin resistance, dyslipidemia, and increase atherosclerosis in Ldlr−/− mice (Subramanian et al., 2008; Wall et al., 2018). A combination of high-fat diets to create obesity along with a milder degree of islet destruction by streptozotocin has been used to study lipoprotein metabolism (Yu et al., 2017) (Table 2). In general, when corrected for circulating cholesterol levels, an independent effect of insulin resistance on vascular disease is not apparent. Another commonly used model of T2DM is that due to defects in leptin signaling (ob/ob and db/db), such mice have been used for atherosclerosis studies. Despite the obesity and hyperglycemia, leptin defective mice have reduced vascular disease attributed to less lymphocyte-driven inflammation (Taleb et al., 2007). Thus, definite evidence on whether hyperglycemia accelerates atherosclerosis in mouse models of T2DM in addition to the dyslipidemia and increased inflammation in these models awaits further studies and better models.
Another approach to assessing the effects of diabetes on arteries without the overwhelming effects of severe hypercholesterolemia involves assessment of lesion regression after transplantation of arteries into non-hypercholesterolemic mice or after genetic or pharmacologic reduction of circulating cholesterol (Fisher, 2016). Regression, defined as the reduction in macrophages and lipid but not lesion size, is defective in mice with streptozotocin-induced insulin deficiency (Parathath et al., 2011; Yuan et al., 2018) and is discussed in more detail below. With current clinical practice to markedly reduce circulating LDL-C levels in patients with established or high risk of ASCVD, there is a greater focus on the repair/regression of established lesions. With marked LDL-C reduction, vascular lesions in most patients either do not expand or regress and become more stable (Nicholls et al., 2011). Despite marked cholesterol reduction, patients with diabetes (mostly T2DM) still have more CVD events (Dash and Leiter, 2019). Limited data in humans also support the concept that diabetes prevents regression (Nicholls et al., 2008).
Together, these studies show that loss of insulin receptors in cells in lesions alters the atherogenic process in cell type-specific manners, reflecting beneficial effects of insulin in endothelial cells and possibly SMCs, whereas a clear direct role for hyperglycemia is lacking. It is possible that hyperglycemia has more direct effects in endothelial cells, as suggested by Brownlee and colleagues (Shah and Brownlee, 2016), and that these effects explain why microvascular complications are more easily prevented by improved glucose control than is ASCVD.
LDL and CVD in diabetes
Although diabetes leads to several alterations in lipoprotein metabolism (Figure 3), the relationship primarily of LDL to CVD events has been most studied. Adults with T1DM most often have normal levels of total cholesterol, TG, HDL-C and LDL-C and in some cohorts have more favorable lipid and lipoprotein profiles (Wadwa et al., 2005). However, when T1DM is poorly controlled, plasma TG levels are increased (Ginsberg, 1991). In the EURODIAB study, patients with T1DM and CVD had lower levels of HDL-C and higher TG levels than those without CVD (Koivisto et al., 1996). In the T1DM Pittsburgh Epidemiology of Diabetes Complications study, higher levels of TG and LDL-C and lower levels of HDL-C were associated with greater risk for CVD and mortality (Orchard et al., 2001). In DCCT/EDIC, despite relatively normal levels of plasma lipids and lipoproteins, there remained a relationship between TG and LDL-C and any CVD event (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group, 2016).
Figure 3. Effects of diabetes on lipoprotein metabolism.
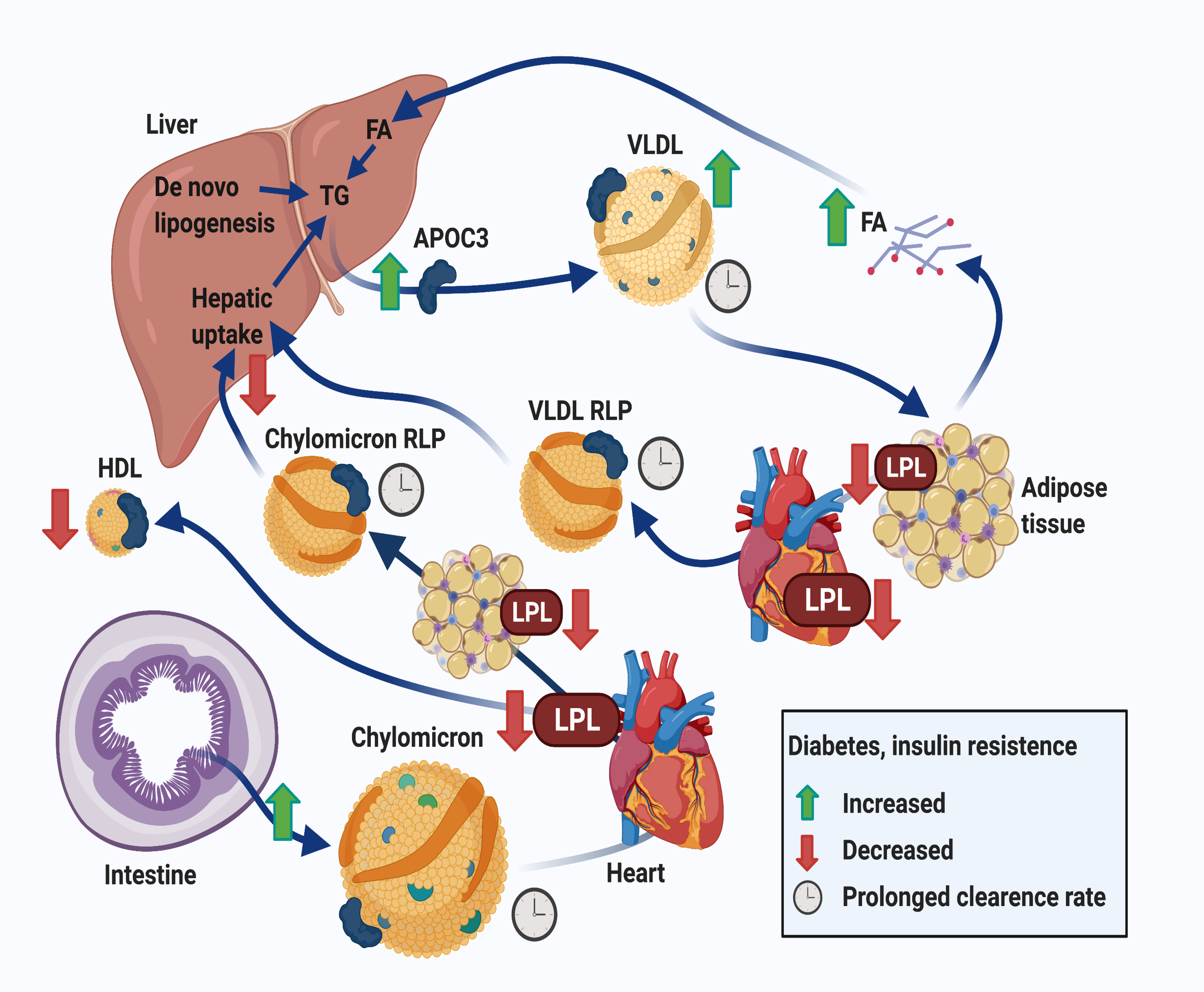
Insufficient insulin action in adipose tissue results in increased adipose tissue lipolysis and reduced fatty acid uptake, which in turn increases fatty acid delivery to the liver and hepatic VLDL production. Hepatic uptake of remnants as well as de novo lipogenesis also contribute to increased hepatic TG levels and VLDL production. Insufficient hepatic insulin action causes an elevation of APOC3 levels, which reduce lipolysis and hepatic clearance of VLDL and chylomicrons, derived from the intestine after a meal, and their remnant lipoprotein particles (RLPs). Together, these effects result in a reduced clearance rate of triglyceride-rich lipoproteins (VLDL and chylomicrons) and RLPs, including their cholesterol content (indicated by a clock). Furthermore, diabetes is associated with reduced lipoprotein lipase (LPL) activity, primarily in adipose tissue and heart, further contributing to the slowed clearance of triglyceride-rich lipoproteins. When LPL activity is severely inhibited, which is observed primarily in T2DM, HDL levels are reduced as a result. Moreover, human studies demonstrate that chylomicron secretion is increased in T2DM. Together, the overproduction and reduced clearance of triglyceride-rich lipoproteins and their remnants are features of both T2DM and poorly controlled T1DM, although the relative extent of these processes differ with glycemic control and type of diabetes. Created with BioRender.com.
Patients with T2DM do not typically have higher LDL-C levels than age and weight matched controls, but they do have more dyslipidemia. These patients are typically obese and have a higher prevalence of the metabolic syndrome, including elevated prevalence of plasma TG >150 mg/dL and reduced HDL-C (levels <40 for men and <50 mg/dL for women) (Alexander et al., 2003; Isomaa et al., 2001). The prevalence of dyslipidemia in patients with T2DM is in the range of 40–60% (Eriksson et al., 2011; Scott et al., 2011). The lipid and lipoprotein abnormalities correlate with increased CVD risk (Eckel, 2007; Eckel et al., 2005; Wadwa et al., 2005). While LDL-C levels are typically normal, LDL particles are frequently small and dense, which some have argued makes them more atherogenic (Lamarche et al., 1997). It is possible that small dense LDL penetrate the arterial wall more easily, stick to matrix with a greater affinity, or are more susceptible to oxidation. Others have questioned the independent contribution of small dense LDL to atherosclerotic risk (Sacks and Campos, 2003). Because small dense LDL have less cholesterol, greater atherogenicity could indicate that apoB rather than cholesterol drives disease, as larger LDL have a greater cholesterol content.
Numerous LDL-C lowering CVOTs have been implemented in patients with T2DM using statins and to a lesser extent ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9is). The benefits of statin-induced LDL-C reduction have been repeatedly seen and if anything, are greater in subjects with diabetes (Figure 1, upper right). Although post-hoc analysis of the 4S trial data demonstrated that CVD events, CVD mortality and all-cause mortality were similar in non-diabetic CVD patients with and without the metabolic syndrome, based on expected higher risk in patients with the metabolic syndrome a higher absolute risk reduction was seen in the metabolic syndrome patients (Pyörälä et al., 2004). In most of the LDL-C lowering CVOTs with statins, relative risk reduction in patients with T2DM was similar to that in patients without T2DM (Kearney et al., 2008). Of great interest, however, in the IMPROVE-IT trial where statin-treated patients were randomized to ezetimibe, a drug that reduces cholesterol absorption in the small intestine, vs placebo and then stratified by the TIMI (thrombolysis in myocardial infarction) risk score for a second CVD event, all patients with T2DM demonstrated benefit with ezetimibe/simvastatin regardless of risk. In contrast, among patients without T2DM, only those with a high-risk score experienced a significant (18%) relative reduction in the composite of CVD, myocardial infarction, and ischemic stroke; patients without T2DM at low or moderate risk demonstrated no benefit with the addition of ezetimibe to simvastatin (Giugliano et al., 2018).
LDL-C can also be reduced by inhibitors of PCSK9. PCSK9 inhibitors act in large part by increasing hepatocyte cell surface levels of LDL receptors, which leads to increased LDL removal. In FOURIER, the PCSK9 inhibitor evolocumab reduced CVD outcomes consistently in patients with and without T2DM (Sabatine et al., 2017). In ODYSSEY OUTCOMES, alirocumab produced similar relative reductions in the primary endpoint, a composite of death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization, over a gradient of glycemic categories, but a greater absolute reduction was experienced in patients with T2DM than in those with prediabetes or normoglycemia (Ray et al., 2019). Considering T2DM as a major ASCVD risk factor, analysis of CVOTs by absolute risk rather than relative risk reduction is best; thus, LDL-C lowering improves outcomes more in patients with T2DM than in patients without T2DM.
Although the contribution of plasma lipids to the increased risk of ASCVD in patients with T1DM is likely, this hypothesis remains unproven by RCTs (de Ferranti et al., 2014). Differences in CVD rates among countries, however, support a role for lifestyle and circulating lipids and CVD risk in T1DM. The modification of LDL-C with lifestyle and medication is consistent with many current standards of medical care for patients living with T1DM (American Diabetes Association, 2021).
LDL-lowering in animal models of diabetes consistently reduces atherosclerosis, but residual risk remains.
In the absence of increases in LDL, hyperglycemia is not sufficient to induce atherosclerosis, as is the case e.g., in C57BL/6 mice, which have very low LDL-C levels and carry most of their cholesterol in HDL. Also, in swine, which exhibit a human-like LDL-rich lipoprotein profile, diabetes does not enhance atherosclerosis in the absence of fat-feeding, which increases LDL-C levels (Gerrity et al., 2001).
Studies of atherosclerosis regression in rodents, all by reduced circulating LDL-C, permit investigators to assess effects of diabetes on arteries exclusive of effects on circulating lipoproteins. Defective regression due to diabetes, i.e., the reduction in vascular macrophage content after marked cholesterol reduction, is readily demonstrable in atherosclerotic arteries transplanted into mice with diabetes with low LDL-C levels (Nagareddy et al., 2013; Parathath et al., 2011), or by restoration of apoE in apoE-deficient mice (Gaudreault et al., 2013). Glucose reduction via treatment with an SGLT-2i improves lesion regression (Nagareddy et al., 2013), as does an increase in HDL-C (Barrett et al., 2019). In contrast, the diabetes-mediated defect in regression is exacerbated by increasing aberrant glucose metabolism via genetic expression of human aldose reductase (Vedantham et al., 2011; Vikramadithyan et al., 2005; Yuan et al., 2018). Analysis of lesion macrophages in these models shows that diabetes increases the population of inflammatory cells and decreases the number of less inflammatory/reparative macrophages within plaques (Barrett et al., 2019). Loss of RAGE signaling also improves regression (Senatus et al., 2020), whereas high-fat diet alone does not affect regression (Willecke et al., 2015). Whether this process mimics human lesion biology (postulated to involve vascular injury followed by repair), and whether arteries, like other wounds, do not heal as well under the condition of diabetes remain to be determined.
Together, these results indicate that diabetes does not accelerate atherosclerosis under conditions in which LDL-C levels are very low. This is consistent with the marked effectiveness of LDL-lowering drugs to prevent CVD in human subjects with diabetes (Figure 1, upper right). However, the lesion regression studies in mouse models also shows that diabetes impedes lesion regression induced by aggressive lipid lowering, perhaps mirroring the residual CVD risk in statin-treated human subjects with diabetes.
Triglycerides and CVD in diabetes
Limited human data support a role for reduction of circulating TG levels in CVD prevention in patients with T2DM (Figure 1, lower left). However, population studies strongly support a relationship between genes that regulate TG levels and the lipolysis reaction via lipoprotein lipase (LPL) and CVD risk (Chait et al., 2020; Ference et al., 2019). CVOTs in patients with T2DM have utilized fibrates (fibric acid derivatives), niacin and high doses of omega-3 fatty acids to lower plasma TGs. Fibric acid drugs lower plasma TG levels by activating PPARs (Staels et al., 1998). Although the overall effect of fibrates on CVD risk in T2DM has so far been largely negative, subgroup analysis of several fibric acid trials, including ACCORD (Elam et al., 2017), suggest benefit in patients with diabetes and increased TGs (>200 mg/dL) and reduced HDL-C (<35 mg/dL). Another trial to address this subgroup is PROMINENT, which has randomized hypertriglyceridemic patients with T2DM and reduced levels of HDL-C to the selective PPARα modulator (SPPARM-α), pemafibrate vs. placebo (Pradhan et al., 2018). This trial should report in 2022. After a number of studies using a combination of low doses of omega-3 fatty acids failed to show benefit of TG lowering, the REDUCE-IT study performed in a T2DM-enriched population with increased TG levels demonstrated that 4 grams daily of icosapent ethyl (eicosapentaenoic acid; EPA) reduced CVD events by ~25% (Bhatt et al., 2019). The reasons for this benefit are debated, however, larger doses of EPA only were utilized, and higher levels of plasma EPA were achieved (Jo et al., 2021) as compared to previous negative omega-3 fatty acid trials. Because reductions in clinical events did not track with TG reductions and plasma levels of TGs are not directly pro-atherogenic, it may be that the primary actions of this therapy were on inflammation or thrombosis (Mason and Eckel, 2021). Similarly, despite only a 9% reduction in TGs, the Japanese Eicosapentaenoic Acid (EPA) Lipid Intervention Trial (JELIS) also noted reduced CVD events in an EPA-treated Japanese population (Yokoyama et al., 2007). More recently the STRENGTH trial, which included 70% of patients with T2DM, showed that carboxylic omega-3 fatty acids (EPA + DHA; docosahexaenoic acid) failed to demonstrate any CVD benefit despite a 19% reduction in TGs (Nicholls et al., 2020). In analyzing omega-3-fatty acid CVOTs it is important to assess outcomes based on the dose of omega-3-fatty acid used. Most studies used low doses (1 gram or less) and this level has minimal or no effect on triglyceride levels. The REDUCE-IT and STRENGTH trials used high doses (4 grams). Another explanation for this discrepancy has been the placebo chosen in REDUCE-IT. In REDUCE-IT, LDL-C and hsCRP levels increased in the placebo group treated with mineral oil, however, evidence to support this effect appears somewhat weak (Olshansky et al., 2020). Therefore, either the trial designs, patient populations, the amount of EPA, or an adverse effect of combining EPA with DHA led to dissimilar outcomes in REDUCE-IT and STRENGTH (Jo et al., 2021). The disappointing results of STRENGTH are consistent with previous trials using TG-lowering fibric acid derivatives (fibrates) and niacin in statin treated patients (Ganda et al., 2018). Therefore, definitive CVOTs showing that TG reduction benefits patients with diabetes are required.
Fasting TG levels correlate with the elevation of TG after a meal - postprandial lipemia. Chylomicrons and their partially digested products termed remnants comprise much of this increase (Figure 3). Remnants, likely not chylomicrons, are atherogenic. Although increased non-fasting TG levels track with atherosclerosis risk in several studies (Jackson et al., 2012), assessment of postprandial lipemia does not always predict risk (Kats et al., 2017). In this regard, one study of postprandial lipemia did not correlate with CVD in patients with diabetes, all of whom had greater lipemia than the control group (Reyes-Soffer et al., 2009). The reason for greater postprandial lipemia in patients with diabetes appears to be defective lipolysis, leading to more non-TG-hydrolyzed chylomicrons and VLDL (Sondergaard et al., 2017). The cholesterol content of TG-rich lipoprotein remnants might explain why hypertriglyceridemia in patients with diabetes is associated with increased ASCVD (Tannock and Chait, 2004). If remnants do indeed contribute to the increased CVD risk in diabetes, this could also explain the lack of benefit of plasma TG lowering and CVD risk; because the much larger chylomicrons and VLDL contain more TG/particle than do remnants. Of interest, patients with dysbetalipoproteinemia, a condition due to increased circulating remnant lipoproteins, develop peripheral vascular disease (PVD) in addition to coronary artery disease (Feussner et al., 1993). PVD is markedly increased in patients with diabetes. Perhaps remnants are especially toxic to vessels other than the coronaries.
The composition of circulating lipoproteins may also define their atherogenicity. Following the observation that genetic defects in APOC3 were associated with lower TG levels and apparent CVD protection (Pollin et al., 2008), two recent studies explored the relationship of this protein to incident ASCVD in two different cohorts of humans with T1DM (Basu et al., 2019; Kanter et al., 2019). In both studies, even without overt hypertriglyceridemia, serum apoC3 levels predicted an increased risk of ASCVD. ApoC3 is a small apolipoprotein associated with TG-rich lipoproteins (chylomicrons, VLDL and their remnants), HDL and some forms of LDL. ApoC3 prevents clearance of TG-rich lipoproteins and their remnants. Importantly, apoC3 levels are suppressed by insulin (Chen et al., 1994), and increased serum apoC3 levels associate with insulin resistance in subjects with T1DM (Buckner et al., 2021). Apoc3 therefore appears to be particularly relevant as a CVD risk factor in diabetes and insulin resistant states.
Together, these studies suggest that increased TG-rich lipoproteins associate with CVD risk in people with diabetes, but the atherosclerotic lipoprotein particle is likely to be the lipoprotein remnant.
Animal models support an important role for lipoprotein remnants in diabetes-accelerated atherosclerosis, whereas large TG-rich lipoproteins are not overtly pro-atherogenic:
While more recent studies have focused on mice due to the development of genetic modification technology, early investigators utilized the cholesterol-fed rabbit as a model of atherosclerosis (Table 2). Alloxan treatment to create insulin deficiency in rabbits led to marked hyperlipidemia with increases in both TG and cholesterol. Surprisingly, atherosclerosis decreased in diabetic dyslipidemic rabbits, as compared to non-diabetic controls (Duff and McMillan, 1948). Rabbit dyslipidemia with diabetes appears to be due to an exaggeration of the reduction in LPL activity that occurs with diabetes (Nordestgaard and Zilversmit, 1988) and which prevents the conversion of nascent TG-rich chylomicrons and VLDL into remnants. These very large chylomicron-like particles are believed to be too large to enter the arterial wall (Nordestgaard et al., 1988). Thus, it is likely that both lipoprotein size and its apoprotein complement modulate the ability to interact with and eventually cross the endothelial wall barrier. Aside from illustrating the importance of lipoprotein size, this study showed that despite the greater inflammatory milieu due to diabetes, atherosclerosis does not develop without excess circulating levels of lipoproteins that have the potential to enter the artery and lead to lipid deposition.
Mouse models of marked hypertriglyceridemia have thus far failed to show major effects of hypertriglyceridemia on vascular lesions (Basu and Bornfeldt, 2020), consistent with human studies. Loss of LPL and its binding protein glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) lead to marked hypertriglyceridemia and small plaques in older mice (Weinstein et al., 2010; Zhang et al., 2008); however, atherosclerosis was far less severe than that seen with hypercholesterolemic models, supporting the hypothesis that large TG-rich lipoprotein particles, without generation of remnants, are not particularly atherogenic. More recently, hypertriglyceridemia induced by LPL-deficiency was shown to not affect lesion regression in non-diabetic mice. In these LPL-deficient mice, lesion regression proceeded normally despite the resultant hypertriglyceridemia and reduced HDL-C (Josefs et al., 2021). Importantly, in this model as well as in the GPIHBP1-deficient model, hypertriglyceridemia is due to loss of LPL activity, which results in increased circulating levels of nascent TG-rich lipoproteins, but not remnants. Conversely, increased clearance of TG-rich lipoproteins and their remnants results in improved atherosclerosis in mice with diabetes. In one study, treatment of mice with diabetes by overexpression of the VLDL receptor to lower plasma TGs reduced arterial hemorrhages (Johansson et al., 2008); a reduction in a pathology associated with disrupted plaques which might have resulted from TG reduction. Furthermore, the composition of lipoproteins appears to markedly affect their atherogenicity. ApoC3 silencing completely prevented the effects of diabetes on both early and more advanced lesions, despite the fact that the mice were severely hyperglycemic (Kanter et al., 2019), demonstrating that apoC3 is a causal mediator of diabetes-accelerated atherosclerosis, consistent with its role as a CVD predictor in humans with T1DM (Basu et al., 2019; Kanter et al., 2019). The mechanism whereby apoC3 increases atherosclerosis in mice with diabetes is likely related to the slower clearance of remnant lipoproteins and increased trapping of these particles in the arterial wall at sites susceptible to lesion formation (Kanter and Bornfeldt, 2020). Consistently, a transgenic mouse carrying human apoC3 show increased atherosclerosis concomitant with increased levels of VLDL and LDL (Masucci-Magoulas et al., 1997). Whether the apoC3 affects lipoprotein interaction with the vessel wall or other factors remains to be determined.
Together, the findings in animal models are consistent with human studies and suggest that remnants of TG-rich lipoprotein particles which are enriched in cholesterol, rather than plasma TGs, are likely to contribute to the increased CVD risk in diabetes. Therapies that target clearance of remnants, such as apoC3 inhibition might therefore be more effective than drugs that target TGs. Future CVOTs are needed to address this possibility.
HDL and CVD in diabetes
The failure of clinical interventions along with the lack of a direct correlation of HDL-regulating genes with CVD has led to a reassessment of the relationship of HDL and atherosclerosis. Thus, while HDL-C levels indicate CVD risk, whether HDL itself plays a biological role in changing atherogenesis remains uncertain. HDL-C levels are reduced by parameters such as hypertriglyceridemia, obesity, and insulin resistance that also associate with CVD risk. This literature is very extensive, and readers can consult several recent reviews for more detail (Madsen et al., 2021; Ouimet et al., 2019).
HDL levels and composition have been cataloged in patients with diabetes. HDL-C levels are often reduced in patients with T2DM and are a criteria for diagnosis of the metabolic syndrome (reviewed in (Bjornstad and Eckel, 2018)). This reduction might result from insulin resistance, as insulin treated T1DM patients tend to have greater HDL-C levels (Eckel et al., 1981). In contrast to the usual reduced CVD risk associated with increased HDL-C, dramatic increases in HDL-C (>80 mg/dL) in women with T1DM were associated with greater CVD (Costacou et al., 2011). Could this reflect a defect in HDL actions?
HDL function, rather than HDL-C levels, has been associated with better prediction of CVD risk in some but not all studies (reviewed in (Soria-Florido et al., 2020)). The most widely promoted benefit of HDL is that it mediates reverse cholesterol transport, which allows return of excess cholesterol from tissues, including arterial tissue, to the liver. Processes responsible for reverse cholesterol transport have all been studied in the setting of diabetes. The ability of HDL to promote transfer of intracellular cholesterol out of cultured macrophages, termed cholesterol efflux capacity (CEC), is used as a surrogate for in vivo reverse cholesterol transport. A number of studies suggest that both T2DM (He et al., 2020) and T1DM (Ganjali et al., 2017) lead to HDL with a reduced ability to mediated CEC. Defective CEC could also result from defective expression of the primary cholesterol transporters, ABCA1 and ABCG1. Indeed, diabetes reduces ABCA1 expression in macrophages from mice with diabetes (Tang et al., 2010). ABCG1 also is reduced in macrophages from patients with T2DM (Mauldin et al., 2008). Furthermore, HDL might affect immune regulation (Gaddis et al., 2018), bone marrow proliferation (Yvan-Charvet et al., 2010), macrophage inflammatory phenotypes (Fotakis et al., 2019), compensation for oxidized lipids (Rosenson et al., 2016), and response to infections (Guo et al., 2013). One study showed that T2DM impaired the anti-oxidant effects of HDL and its ability to prevent the stimulatory effect of LDL on monocyte migration (Morgantini et al., 2011). Whether diabetes alters some of these other HDL functions and whether such effects are responsible for greater vascular disease risk is unknown.
Mouse models have used overexpression and knockout of apoAI, the major protein of HDL, to investigate the role of HDL in atherosclerosis. Overexpression of apoAI reduces atherosclerosis in mice (Rubin et al., 1991). Ectopic expression of apoAI in transplanted bone marrow also reduced atherosclerosis in mice (Tavori et al., 2015). Moreover, apoAI and increased HDL levels improve lesion regression (Feig et al., 2011) and mitigate the defect in atherosclerosis regression in diabetic mice (Barrett et al., 2019). In contrast, low HDL fails to alter repair of vascular lesions (Josefs et al., 2021). Moreover, apoAI knockout effects on atherosclerosis were shown to be strain specific (Sontag et al., 2014). Overall, the role HDL contributes to atherosclerosis in diabetes remains uncertain.
Diabetes, CVD risk and inflammation
Inflammation is an unlisted component of the metabolic (insulin resistant) syndrome (Dandona et al., 2004; Grandl and Wolfrum, 2018) and its relationship to insulin resistance is considered central to T2DM pathophysiology. There may be a role in T1DM also (Buckner et al., 2021; Schram et al., 2005; Snell-Bergeon et al., 2010b). One study of obese subjects noted that the persistent elevation of circulating levels of hsCRP accompanied sustained T2DM after weight loss (McLaughlin et al., 2002). In T2DM, markers of inflammation have been linked to CVD (Jager et al., 2000) and CVD death (Sanchez et al., 2004; Soinio et al., 2006). Patients with T1DM in the intensive arm of the DCCT demonstrated increased hsCRP levels along with weight gain (Schaumberg et al., 2005). Thus, the benefits of intensive glycemic control may not be uniform. In addition, in adolescents with T1DM inflammation is related to the progression of arterial stiffness (Alman et al., 2018).
Extracellular matrix metalloproteinases (MMPs) are proteases that degrade extracellular matrix and are increased by diabetes and pro-inflammatory mediators, however their contribution to increased ASCVD in patients with diabetes remains uncertain. In a prospective study of patients with T1DM (n=337), increased plasma levels of MMP-1, MMP-2 and MMP-3 associated with CVD events and all-cause mortality (Peeters et al., 2017). But, in another study (EURODIAB) higher plasma levels of MMP-2, MMP-3 and MMP-10 were not associated with CVD (Peeters et al., 2015). Another line of evidence that links inflammation in T1DM to CVD are levels of HMGB1, released by necrotic or immune cells, that elicit a pro-inflammatory response. Over a median follow-up of 12.3 years in patients with T1DM, higher levels of HMGB1 independently associated with CVD and all-cause mortality (Nin et al., 2012a), but not in the EURODIAB population (Nin et al., 2012b).
As discussed above, hsCRP has been used an indicator of inflammatory risk exclusive of hyperlipidemia. Patients with obesity, metabolic syndrome and T2DM have elevated hsCRP. The JUPITER study (Ridker et al., 2008), which showed that rosuvastatin reduced CVD events in subjects with hsCRP >2 mg/L, was enriched in subjects with metabolic syndrome so that >40% of participants were classified as having metabolic syndrome. The benefits of statin therapy in these subjects were most dramatic when associated with hsCRP reduction. These findings raise some interesting questions. Did cholesterol reduction alone reduce inflammation, did the statin have anti-inflammatory effects, or did the hsCRP indicate reduced vascular disease? Of note, the more potent cholesterol reduction with PCSK9 inhibition has limited effects on hsCRP (Ruscica et al., 2019). Several reasons could account for this failure: 1) in most trials PCSK9 reduction was on top of statin therapy, 2) hsCRP reductions might primarily occur at higher LDL-C levels, 3) statins might have non-LDL reducing “pleiotropic” actions.
Two agents that reduce inflammation have been shown to reduce CVD events in subjects with a history of CVD (Figure 1, lower right). Colchicine, a drug used for decades to treat arthritic conditions, reduced recurrent CVD events in the Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease (LoDoCo) (Nidorf et al., 2020) and the Cardiovascular Outcomes Trial (COLCOT) (Tardif et al., 2019) trials. Both trials had a relatively low number of subjects with diabetes, <20%. A second successful method to reduce CVD events used canakinumab, a monoclonal antibody that blocks IL-1β from activating its receptors (Ridker et al., 2017). Trial inclusion in the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) required an increased hsCRP > 2 mg/L. Although ~40% of the subjects had T2DM and 49% had prediabetes, the benefits of treatment were similar to those in the normoglycemic group with increased hsCRP (Everett et al., 2018). One might conclude that CANTOS does not support a greater benefit for reducing inflammation in patients with diabetes and CVD. However, an explanation for the increased hsCRP in the CANTOS subjects without T2DM is unclear but suggests other etiologies. Before prescribing anti-inflammatory agents for all patients with diabetes and CVD, we should note that a number of studies using other presumed anti-inflammatory agents, including methotrexate (Ridker et al., 2019), inhibitors of lipoprotein-associated phospholipase A2 (O’Donoghue et al., 2014), and salsalate (Hauser et al., 2016) have not shown benefit. Moreover, in CANTOS, the downside of suppressing inflammation was an increase in infectious complications. We should also note that inflammation is a broad term, which encompasses many different inflammatory pathways. It is possible that if specific inflammatory mediators associated with CVD in diabetes could be identified, more effective strategies for CVD prevention could be developed.
Suppression of inflammatory processes is beneficial in animal models of diabetes-exacerbated atherosclerosis:
Many studies have investigated the role of different inflammatory pathways in mouse models of diabetes and atherosclerosis progression and regression (Figure 2). A particularly important mediator of inflammatory activation in diabetes appears to be the damage-associated molecular pattern protein S100A9 and its binding partner S100A8 (Averill et al., 2012). S100A9 levels are increased in myeloid cells in mouse models of diabetes (Johansson et al., 2008; Nagareddy et al., 2013) and has been shown to interact with both toll-like receptor 4 (TLR4) and RAGE to promote inflammatory effects in the setting of diabetes (Figure 2). Lesion macrophages in mice with diabetes are characterized by increased S100A9 immunoreactivity (Johansson et al., 2008), and in diabetic lesion regression models lesion macrophages express elevated levels of S100a8 mRNA as well as iNOS and IL-1 and CHOP, a marker of ER stress (Parathath et al., 2011). Interaction of S100A8/A9 with RAGE results in increased myelopoiesis and thrombocytosis associated with worsened atherosclerosis in mice with diabetes (Kraakman et al., 2017; Nagareddy et al., 2013) as well as increased microcalcification of lesions (Kawakami et al., 2020). S100A8/A9 interaction with TLR4 has been shown to contribute to granulopoiesis (Sreejit et al., 2020). Whereas elevated lipid levels and cholesterol accumulation in myeloid progenitor cells promote myelopoiesis (Tall et al., 2012), hyperglycemia also appears to play a role because myelopoiesis can be suppressed by an SGLT-2i (Nagareddy et al., 2013) and intermittent hyperglycemia increases S100A9 levels in neutrophils (Flynn et al., 2020). Other studies have implicated changes in bone marrow endothelial cells in mediating diabetes-induced myelopoiesis (Hoyer et al., 2020). Intriguingly, the effects of diabetes on myelopoiesis are prevented by elevating HDL levels, suggesting that the mechanism whereby diabetes increases myelopoiesis can be blunted by cholesterol efflux (Barrett et al., 2019) and therefore is dependent on lipid alterations. Other studies have placed NETosis upstream of release of S100A9 from neutrophils and subsequent granulopoiesis (Nagareddy et al., 2020) (Figure 2). NETosis is a form of neutrophil death characterized by release of decondensed chromatin and granular materials to aid in the trapping and killing of pathogens. Indeed, neutrophil extracellular traps are now known to promote macrophage inflammatory phenotypes and impair lesion regression in mice with diabetes (Josefs et al., 2020).
However, diabetes promotes atherosclerosis also in the absence of myelopoiesis (Kanter et al., 2019), indicating that whereas increased myelopoiesis can worsen atherosclerosis in the setting of diabetes in mouse models, it does not fully explain the increased atherosclerosis. In part, this might be explained by the ability of S100A8/A9 to induce TLR4-dependent NLRP3 inflammasome activation and IL-1β release by neutrophils (Sreejit et al., 2020). Systemic inhibition of the NLRP3 inflammasome was recently described to prevent increased atherosclerosis in mice with diabetes (Sharma et al., 2020). In this context, it is interesting that another modulator of diabetes-induced inflammatory processes in myeloid cells, long chain acyl-CoA synthetase 1 (ACSL1), has been shown to be required for diabetes-accelerated atherosclerosis (Kanter et al., 2012). ACSL1 is induced by TLR4 activation and acts to synthesize acyl-CoAs from free fatty acids in myeloid cells. Deletion of ACSL1 in myeloid cells also prevents NLRP3 inflammasome activation (Kalugotla et al., 2019), raising the possibility that NETosis, S100A8/A9, ACSL1 induction and NLRP3 inflammasome activation act along the same pathway to worsen atherosclerosis in diabetes, at least in mouse models.
The role of myelopoiesis in CVD risk associated with diabetes in humans is so far unclear, although CVD has been shown to associate with elevated levels of circulating neutrophils and plasma S100A9 levels in humans with T1DM (Nagareddy et al., 2013). Together with the positive effects of IL-1β blockade in humans with diabetes shown by CANTOS, these results suggest a treatable inflammatory component to CVD in both humans and animal models.
Other possible atherogenic associations of diabetes and ASCVD
Lifestyle:
A heart-healthy lifestyle including nutrition, physical activity and absence of tobacco products is associated with reduced CVD risk in individuals with and without diabetes (Van Horn et al., 2016; Young et al., 2016). There are several aspects to consider for both nutrition and physical activity. For nutrition, these aspects relate to dietary quality and quantity, for physical activity, to duration and intensity. Dietary patterns include very low fat, very low carbohydrate, Mediterranean-style, DASH (Dietary Approaches to Stop Hypertension), low glycemic index and many more. Data exist only showing effects of the Mediterranean-style diet on CVD outcomes in patients with T2DM. A meta-analysis including 41 reports (3 RCTs + 38 cohorts) showed a beneficial effect of the Mediterranean diet on total CVD incidence and total myocardial infarction incidence (Becerra-Tomás et al., 2020). Moreover, in those prospective studies adherences to the dietary pattern reduced CVD mortality, coronary artery disease (CAD) incidence and mortality, stroke incidence, stroke mortality and myocardial infarction incidence in patients with T2DM. Although to be addressed more thoroughly, the Look AHEAD study examined the importance of excess body fat in patients with T2DM by randomizing overweight and obese patients to an intensive lifestyle intervention that promoted weight loss or to obtaining diabetes support and education only. Although many biomarkers for CVD and quality of life were improved in the intervention group, weight loss failed to reduce the rate of CVD events (Wing et al., 2013). Thus, the composition of the diet and its effects on circulating lipoproteins may be more important than effects on overall body weight and insulin resistance.
Dietary patterns may also affect CVD risk in T1DM. In 136 youth with T1DM enrolled in an 18-month behavioral nutrition intervention trial, the Healthy Eating Index-2015 scale failed to associate with CVD markers but whole grain intake was inversely associated with total cholesterol and diastolic blood pressure, and a greater increase in whole fruit intake was associated with lower diastolic blood pressure (Sanjeevi et al., 2018). Without surprise, added sugar was associated with higher TG, and saturated fat with higher levels of HDL-C. Of important note, the DCCT/EDIC trial demonstrated that with more intensive glycemic management in patients with T1DM, weight gain was accompanied by a modest increase in CVD risk factors (Purnell et al., 2013). However, although CVD events over a median of 26 years were reduced in the patients randomized to intensive glycemic control (Sousa et al., 2019), total CVD events increased in the intensive group that gained the most weight, becoming equivalent to events in those in the conventional group (Purnell et al., 2017).
Randomized trials examining the impact of sedentary behavior and higher levels of physical activity on CVD events are lacking in T2DM. Unequivocally, sedentary behavior is a risk factor for new onset T2DM (Patterson et al., 2018), however, the benefit of increasing amounts of aerobic physical activity and cardiorespiratory fitness on CVD events in patients with T2DM is only suggested by changes in CVD risk factors including glycemia, lipids, endothelial function, oxidative stress, myocardial function and adiposity but not outcomes (Boulé et al., 2003; Miele and Headley, 2017).
Randomized trials are also lacking for physical activity and CVD in T1DM, but several lines of evidence indicate beneficial effects of activity in patients with T1DM. Greater exercise predicted fewer CVD events in adult patients with T1DM (n=4,306) (Vistisen et al., 2016). In T1DM patients from FinnDiane (n=2,180), a 10-year follow-up demonstrated that patients with higher levels of total and higher intensity physical activity had fewer CVD events, an effect that remained significant after adjusting for classic CVD risk factors (Tikkanen-Dolenc et al., 2017). In EURODIAB (n=1,880), a longitudinal analysis revealed a borderline inverse relationship between moderate or vigorous physical activity and all-cause mortality (men and women combined) and incident CVD (women only).
Overall, it stands to reason that a heart-healthy lifestyle is beneficial to reduce CVD risk in people living with T1DM or T2DM (as well as in those without diabetes).
Smoking:
It has been known for years that smoking is a risk factor for coronary heart disease in patients with diabetes (Fagard and Nilsson, 2009). However, the relative risk has not been shown to be greater in people with diabetes than in subjects without diabetes. Because of underlying added risk for CVD related events and mortality in patients with diabetes, the absolute risk of smoking is typically higher.
Obesity:
Excess body fat, and in particular its distribution, relates to risk for T2DM and CVD (Bjorntorp, 1992). Well-accepted is the added CVD risk of increased central/abdominal/visceral obesity with or without T2DM (Albu et al., 2010; Neeland et al., 2019). Sarcopenic obesity also predicts CVD in patients with T2DM (Fukuda et al., 2018). To some extent, this may relate to the protective effect of greater subcutaneous adipose tissue in the lower body on CVD risk relative to greater central or visceral adipose tissue distribution in patients with T2DM (Vasan et al., 2018). In an Asian T2DM cohort (n=13,278), when adjusting for BMI, patients with the highest quintiles of waist circumference experienced the highest all-cause and CVD mortality versus those with the lowest quintiles (Vasan et al., 2018).
Look AHEAD was the only major RCT to examine the impact of an intensive lifestyle approach on reducing CVD events in patients with T2DM (Look AHEAD Research Group et al., 2013). The trial included 5,145 overweight or obese patients with T2DM with a lifestyle intervention noted above. The primary outcome was a composite of death from cardiovascular causes, nonfatal myocardial infarction, non-fatal stroke, or hospitalization for angina. Although the maximum follow-up was 13.5 years, the trial was stopped at 9.6 years by the data and safety monitoring board based on futility. Importantly, the outcome was accompanied by many favorable results including more reductions in weight (6.9 vs. 3.6 %), glycated hemoglobin and most CVD risk factors, except for LDL-C. Thus, despite the metabolic benefit of this small amount of sustained weight loss, reduced CVD events were not seen. Other approaches wherein there is greater weight loss may result in CVD benefit.
Presently there are no RCTs that have demonstrated a benefit of bariatric/metabolic surgery on CVD events in patients with T2DM. However, a few observational studies have demonstrated an association between metabolic surgery and reduction in MACE (Sjöström et al., 2014). Recently, in an extensive experience in 287,438 patients with diabetes and obesity at the Cleveland Clinic between 1998 and 2017, 2,287 patients who underwent metabolic surgery were matched retrospectively 1:5 to nonsurgical patients with a similar history, diabetes, and obesity (BMI ≥30) (Aminian et al., 2019). Over a median follow-up of 3.9 years, 385 patients in the surgical group and 3,243 patients in the nonsurgical group experienced a primary end point defined as extended MACE (i.e., a rate of 30.8% in the surgical group and 47.7% in the nonsurgical group). Yet, because these data were observational, they remain hypothesis-generating only. A RCT is desperately needed to confirm this favorable outcome.
Chronic kidney disease:
Abnormalities such as renal failure and hypertension associate with diabetes CVD risk. Chronic kidney disease (CKD) defined here as Stage 3 or a GFR ≤60 mL/min per 1.73 m2 is present in ~25% of patients with T2DM and has been known for decades to contribute to CVD risk in patients with diabetes (Go et al., 2004; Kannel and McGee, 1979a; Noth et al., 1989). An uncertainty remains as to whether CVD risk factors, e.g. hypertension, dyslipidemia, inflammation and the pro-thrombotic state that are associated with CKD provide the link to CVD or whether there is more to this relationship than risk factors (Kosmas et al., 2018). And, in general, macroalbuminuria is a major contributor to these CVD risk factors. In the Action in Diabetes and Vascular disease, preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) study, T2DM patients with both urinary albumin >300 mg/g and eGFR <60 ml/min per 1.73 m2 at baseline had a 3.2-fold higher risk for CVD events compared with patients with neither of these risk factors (Ninomiya et al., 2009). However, increasingly appreciated is that non-albuminuric renal impairment is an important phenotype, particularly in women with T2DM (Pugliese et al., 2014). CKD in patients with T1DM is also changing in terms of clinical phenotype, i.e., progressive decline and in many patients in the absence of albuminuria (Krolewski, 2015; Penno et al., 2017). The Joslin 50-Year Medalist study of patients with longstanding T1DM (n=752) and a replication cohort (n=675) from FinnDiane was used to examine the association of CKD and proliferative retinopathy with CVD (Gordin et al., 2018). A strong association of CKD with CVD was noted; however, the absence of retinopathy in people with T1DM and CKD was associated with a decreased prevalence of CVD, suggesting that common protective factors for retinopathy and CVD may exist.
Blood pressure:
Hypertension and increases in pulse pressure are common in patients with T2DM and T1DM with increasing BMI, and central adiposity. In T2DM, the prevalence of hypertension is nearly 70% (Ferrannini and Cushman, 2012); in adults with T1DM, EURODIAB found 24% were hypertensive (Collado-Mesa et al., 1999) whereas the CACTI Study found a prevalence of 43% (Maahs et al., 2005). In T2DM patients studied in the UKPDS, the risk of CVD was strongly associated with increased systolic blood pressure (Adler et al., 2000). In T1DM, hypertension in the absence of nephropathy correlated with carotid artery plaque (Distiller et al., 2006) and was the only metabolic syndrome component associated with coronary artery calcification (Rodrigues et al., 2011). Moreover, in the Pittsburgh Epidemiology of Diabetes Complications study, hypertension was a major contributor to coronary heart disease mortality, morbidity, and lower extremity arterial disease (Forrest et al., 2000). Together, these studies show that hypertension contributes to CVD risk in the presence of diabetes, as it does in individuals without diabetes.
As of 2017, 38 blood pressure-lowering RCTs in 61,038 patients with T2DM had been reviewed by Thomopoulos et al. (Thomopoulos et al., 2017). Although blood pressure lowering is capable of reducing all types of cardiovascular outcomes in hypertensive patients with and without diabetes, relative risk reductions of coronary heart disease events and all-cause death are significantly greater in patients with than without diabetes and absolute risk reduction is about twice as large in patients with diabetes as in those without (Blood Pressure Lowering Treatment Trialists Collaboration, 2014; Thomopoulos et al., 2014). However, in this review, we chose to select 15 trials that each included >1,000 T2DM patients with hypertension, most of whom had ASCVD or were at high risk for CVD events at baseline, a strategy consistent with treatment of CVD risk factors in patients with diabetes only, without comparisons to patients without diabetes. Pharmaceuticals used for blood pressure lowering included diuretics, ACE inhibitors or angiotensin II receptor blockers, calcium channel blockers and/or beta blockers. Typically, one drug was tested, and results were compared with existing therapy with or without a targeted blood pressure reduction established. In general, the amount of blood pressure lowering by drug(s) related to the baseline extent of elevation in systolic blood pressure. For the most part the primary outcome was a standard 3-point MACE of myocardial infarction (fatal or non-fatal), stroke (fatal or non-fatal) and CVD death. A minority of studies included heart failure and/or progression of chronic kidney disease in the primary outcome.
As shown in Figure 1 (lower center), only 2 trials successfully achieved a positive outcome, Heart Outcomes Prevention Evaluation (HOPE) (Heart Outcomes Prevention Evaluation Study Investigators, 2000) and Hypertension Optimal Treatment (HOT) (Zanchetti et al., 2003) with UKPDS demonstrating a reduction in stroke but not myocardial infarction (UK Prospective Diabetes Study Group, 1998b). For all trials, the average reduction in systolic blood pressure in the intervention vs. control group was a difference of 7.7 mm with a high variance of 6.9 mm (SD).
Data analyzed from 25 RCTs wherein 174,235 individuals with T2DM were randomized to a variety of more recently approved glucose lowering medications including DPP-4 inhibitors, GLP-1 receptor agonists and SGLT2 inhibitors, showed a modest average systolic/diastolic blood pressure difference of −1.4/−0.4 mm and there was no difference in the CVD outcomes before and after adjustment for this very small difference in blood pressure (Thomopoulos et al., 2019).
Recently, the Antihypertensive Long-term Use Evaluation (VALUE) trial compared cardiovascular outcomes in hypertensive patients with T2DM to all high-risk hypertensive patients (Olsen et al., 2021). In the high-risk hypertensive patients more frequently achieving BP <140/90 mm, but not <130/80 mm, a beneficial effect on overall and cause-specific cardiovascular outcomes was experienced in T2DM patients; this was similar to that seen in the general high-risk hypertensive population. Intensive systolic blood pressure lowering compared with a systolic blood pressure control goal <120 vs. <140 mm was beneficial in patients without T2DM in SPRINT (Systolic Blood Pressure Intervention Trial), similar reduction did not benefit subjects with T2DM in ACCORD BP (Action to Control Cardio-vascular Risk in Diabetes Blood Pressure). Additional analyses revealed that intensive systolic blood pressure lowering decreased the risk of CVD events and all-cause mortality similarly in SPRINT and ACCORD BP but only in participants randomized to standard glycemic control. Why subjects in the intensive glycemic control did not benefit is unclear (Beddhu et al., 2018). These findings support the current ACC/AHA guidelines of a systolic blood pressure goal of <130 mm in patients both with and without T2DM.
Hypercoagulability:
Diabetes is a pro-thrombotic state (Biondi-Zoccai et al., 2003; Rollini et al., 2013; Sobel et al., 2003). Reviewing the many CVOTs addressing coagulation in patients with T2DM is beyond the scope of this review article, but we list recommendations resulting from these trials based on current guidelines. Current guidelines from the American Heart Association (Arnett et al., 2019) recommend aspirin therapy in patients with diabetes and existing ASCVD and for patients with diabetes who have multiple CVD risk factors (Lin et al., 2019; Majithia and Bhatt, 2018; Zhao et al., 2020). The American Diabetes Association (ADA) also states that dual antiplatelet therapy with low-dose aspirin and a P2Y12 inhibitor is reasonable for a year after an acute coronary syndrome with possible benefit beyond this period (American Diabetes Association, 2021). The ADA states that combination therapy with aspirin plus low dose rivaroxaban (Factor Xa inhibitor) should be considered for stable coronary and/or peripheral vascular disease and in patients with low bleeding risk.
Genetic markers:
It remains uncertain whether genetic markers for atherosclerosis predict ASCVD development in patients with diabetes. In an eight-year cohort study, subclinical atherosclerosis measured by coronary artery or abdominal aortic calcium score, common and internal carotid artery intima-media thickness, and ankle-brachial index failed to demonstrate any relationship between a 62 loci-based T2DM risk score and subclinical atherosclerosis (Younis et al., 2013). However, other lines of evidence have been more informative. Using a genetic predisposition risk score (GPS) based on 36 established independent diabetes-predisposing variants, each additional variant increased risk for CVD by 3% with a maximum odds ratio of 1.47 comparing the fourth with the first quartile of GPS (Qi et al., 2013). Genome-wide association study (GWAS) data were used to examine genetic correlations between a number of phenotypes and showed a modest correlation between T2DM and CAD (Qi et al., 2013). Of additional interest is that variation in the GLP-1 receptor influences CAD risk, supporting the use of GLP-1 receptor agonists for CAD prevention (Scott et al., 2016). Moreover, GPS data from the ACCORD trial demonstrated that the CC rs57922 variant predicted a 22% increase in plasma levels of active GLP-1 in the intensive glycemic management arm and was associated with significant reduction in CVD mortality (Shah et al., 2018). A similar effect of this variant was not seen in the standard treatment group. Using a 201-variant polygenic risk score for coronary artery disease in DPP participants, investigators found that although subjects in both lifestyle and metformin groups had greater improvement in the majority of recognized CVD risk factors compared with placebo, the benefit of the interventions was unrelated to the polygenic risk score. Thus, at the present time diabetes-related preventive interventions improve CVD risk regardless of genetic risk for CVD.
Lipoprotein(a) [Lp(a)] is a well-established genetic marker for ASCVD in the general population and in patients with diabetes (Wilson et al., 2019). Its relationship to ASCVD in patients with diabetes, however, appears to be confounded because lower Lp(a) levels associate with greater risk of diabetes (Schwartz et al., 2021; Ward et al., 2021).
Sex:
The relative risk of CVD in female patients with diabetes is higher because women without diabetes are the comparator. However, absolute CVD remains higher for men with diabetes versus men without diabetes. A series of studies document this higher relative risk for women with diabetes (predominantly T2DM) including Evans County, Framingham, and Rancho Bernardo (Heyden et al., 1980; Kannel and McGee, 1979b; Wingard et al., 1983). A more recent meta-analysis including data from 64 cohorts (n=858,507) and 28,203 incident CAD events demonstrated the relative risk for CVD in women with diabetes was 2.82 and in men with diabetes 2.16 (Peters et al., 2014). This approximate 40% increased relative CVD and related-death in women vs. men with T2DM remains unexplained, yet again, the absolute risk of CVD and related death was still higher in men with T2DM (Peters et al., 2014).
In T1DM the incident risk ratios for CVD events in women and men range from 3.0–7.7 and 2.3–3.6, respectively (Peters et al., 2014; Soedamah-Muthu et al., 2006). However, in general, these differences in CVD incidence and mortality between genders dissipate with age (Lee et al., 2015). Importantly, the increased relative risk in women vs. men with T1DM was associated with less favorable CVD risk factors in adolescent girls than boys with T1DM (Brown et al., 2016), an association also manifested in women with T1DM (Cree-Green et al., 2016; Millstein et al., 2018).
An interesting comparison of preclinical CVD (common carotid and femoral intimal media thickness) demonstrated that atherosclerotic burden was greater in women with T1DM with greater body fat and central distribution whereas biomarkers reflecting glucose and lipid metabolism were more associated with CVD in women with T2DM (Pithova et al., 2016). Overall, the sex-based differences in CVD risk in women with diabetes appear to be largely dependent on expressing data as relative risk versus absolute risk.
Race:
Race/ethnicity has a major influence on CVD risk in patients with diabetes. The increased prevalence of CVD in African Americans versus Caucasians relates to both conventional and non-traditional risk factors including disparities in health care (Conway et al., 2012). Blacks have more insulin resistance, which includes components of the metabolic syndrome - obesity, glucose intolerance, T2DM and hypertension, with hypertension alone contributing to more CVD risk, including stroke, heart failure and kidney failure (Osei and Gaillard, 2017). In addition, markers of inflammation, e.g., hsCRP, oxidized LDL, Lp(a) and plasminogen activator-1 are increased in Blacks (or African Americans).
Native Americans such as Alaskan Natives not only have a higher prevalence of T2DM, but T2DM is a leading cause of death in this population, mostly from CVD (Espey et al., 2014). In Hispanics, the prevalence of T2DM is also higher than the national average (Aguayo-Mazzucato et al., 2019). Although the prevalence of obesity and related CVD risk factors is higher in these populations, genetics, in addition to cultural factors and disparities in health care delivery, contribute to the higher CVD risk (Lee et al., 2020; Poudel et al., 2018; Rodriguez et al., 2014). T2DM associated with CVD risk is also common among Asian Indians with both genetic predisposition and modifiable risk factors such as diet, physical activity, obesity, and smoking (O’Keefe et al., 2016).
Inflammation appears to contribute to CVD mortality in Blacks with T1DM (Roy et al., 2016). Although T1DM is less prevalent in Blacks than in Caucasians, following hospitalization in one study Blacks experienced an 11.4% readmission rate for CVD events within 6 years, with older age, increased BMI, higher diastolic BP, proteinuria, retinopathy and depression as predictors (Roy et al., 2007). African Americans with T1DM also have reduced levels of adiponectin, which likely contribute to their increased insulin resistance (Osei et al., 2005). Little is known about the relationship between T1DM and CVD in other populations.
CONCLUSIONS AND FUTURE AREAS OF RESEARCH
Observations in human populations and the effects of treatments have guided experimental approaches to understanding the effects of diabetes on CVD. Diabetes accelerates progression of atherosclerotic lesions and leads to defects in remodeling of plaques after cholesterol reduction. Newer classes of drugs approved for glucose lowering in diabetes reduce CVD, but the mechanisms of this benefit do not appear to be explained by glucose lowering. Whereas the effects of glucose lowering on CVD outcomes is unclear, LDL-C lowering convincingly reduces CVD risk associated with diabetes. Both T1DM and T2DM associate with greater vascular inflammation. This could result from defective insulin action or other factors associated with the metabolic environment surrounding diabetes. It is still unclear if strategies to target inflammatory pathways will provide sufficient long-term cardioprotection without adverse effects in subjects with diabetes. Abnormalities in circulating lipoproteins, including an increase in VLDL and lipoprotein remnants during fasting and after a meal, and greater amounts of apoC3, which slows clearance of such lipoproteins, likely increase pathology.
Because diabetes, especially T2DM but increasingly also T1DM, associates with dyslipidemia, hypertension, and obesity, in the clinic the benefits of better glycemic control can be obscured by the continuing detrimental effects of CVD risk factors. Animal models provide a route to isolate the pathology and elucidate mechanisms. We can and should answer the following questions: 1) Is glycemic control more important in CVD risk associated with T1DM than T2DM, and if so, why? 2) What is the importance of obesity and insulin resistance and associated apolipoprotein and lipoprotein changes versus glycemic control? 3) Will inhibiting apoC3 prevent CVD risk associated with T1DM or T2DM in humans and if so, does the mechanism involve reduced remnant lipoproteins? 4) How important is inflammation and defective tissue repair in diabetic vascular disease in humans, and which specific inflammatory processes are most likely to contribute to CVD risk? 5) What are the effects of glycemic variability versus sustained less favorable glycemic control on CVD risk factors and incident CVD? 6) What are the roles of underlying genetic and environmental factors?
To answer these questions, we need more CVOTs approaching specific pathological pathways to segregate the effects of obesity, defective insulin actions, hyperglycemia, dyslipidemia, and inflammation. Unbiased analysis of human tissues and circulating proteins and metabolites could uncover novel pathological processes leading to greater CVD risk with diabetes. The natural history and pathophysiology of ASCVD in patients with T1DM needs more attention. Moreover, RCTs to examine the impact of CVD risk factor modification on ASCVD events are desperately needed in T1DM. Better models to isolate the major components of diabetes need to be developed that allow investigators to assess the importance of defective insulin actions in specific cells/tissues, poor glycemic control, and obesity in models of atherosclerosis and its repair. In particular, better animal models of atherosclerosis associated with T2DM are needed.
Because the goal is to predict and then treat diabetes-associated processes affecting the vasculature, approaches likely will differ in individual patients. This will require a method to identify the most relevant causes of disease in each person by looking for individual CVD risk beyond glucose.
Funding
Dr. Eckel’s funding includes NIH grants T32 HL116276; NIH R21NS102506; NIH R21AG061549. Dr. Bornfeldt’s research is funded in part by NIH grants R35HL150754, R01HL149685, P01HL151328 and P30DK017047. Dr. Goldberg’s funding includes NIH grants HL45095, HL073029, P01HL151328, and AHA SFRN35210245.
Footnotes
Disclosures
R.H.E. serves as a consultant for Kowa Corp. and Novo Nordisk. K.E.B. has received research support from Novo Nordisk A/S. I.J.G. has served as a consultant for Arrowhead, Akcea and Esperion and received research support for pre-clinical studies from Arrowhead Pharma. I.J.G has no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Accord Study Group, Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, et al. (2010). Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362, 1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, and Holman RR (2000). Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. Bmj 321, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advance Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, et al. (2008). Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358, 2560–2572. [DOI] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C, Diaque P, Hernandez S, Rosas S, Kostic A, and Caballero AE (2019). Understanding the growing epidemic of type 2 diabetes in the Hispanic population living in the United States. Diabetes Metab Res Rev 35, e3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mashhadi RH, Bjørklund MM, Mortensen MB, Christoffersen C, Larsen T, Falk E, and Bentzon JF (2015). Diabetes with poor glycaemic control does not promote atherosclerosis in genetically modified hypercholesterolaemic minipigs. Diabetologia 58, 1926–1936. [DOI] [PubMed] [Google Scholar]
- Albu JB, Lu J, Mooradian AD, Krone RJ, Nesto RW, Porter MH, Rana JS, Rogers WJ, Sobel BE, Gottlieb SH, et al. (2010). Relationships of obesity and fat distribution with atherothrombotic risk factors: baseline results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Obesity (Silver Spring) 18, 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National H, Nutrition Examination S, and National Cholesterol Education P (2003). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 52, 1210–1214. [DOI] [PubMed] [Google Scholar]
- Alman AC, Talton JW, Wadwa RP, Urbina EM, Dolan LM, Hamman RF, D’Agostino RB Jr., Marcovina SM, and Dabelea DM (2018). Inflammation, adiposity, and progression of arterial stiffness in adolescents with type 1 diabetes: The SEARCH CVD Study. J Diabetes Complications 32, 995–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (2021). 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes Care 44, S125–S150. [DOI] [PubMed] [Google Scholar]
- Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, Kattan MW, and Nissen SE (2019). Association of Metabolic Surgery With Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes and Obesity. Jama 322, 1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett DK, Khera A, and Blumenthal RS (2019). 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Part 1, Lifestyle and Behavioral Factors. JAMA Cardiol 4, 1043–1044. [DOI] [PubMed] [Google Scholar]
- Averill MM, Kerkhoff C, and Bornfeldt KE (2012). S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol 32, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azemi AK, Mokhtar SS, Hou LJ, Sharif SET, and Rasool AHG (2020). Model for type 2 diabetes exhibits changes in vascular function and structure due to vascular oxidative stress and inflammation. Biotech Histochem, 1–9. [DOI] [PubMed] [Google Scholar]
- Bacevic M, Rompen E, Radermecker R, Drion P, and Lambert F (2020). Practical considerations for reducing mortality rates in alloxan-induced diabetic rabbits. Heliyon 6, e04103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badin JK, Kole A, Stivers B, Progar V, Pareddy A, Alloosh M, and Sturek M (2018). Alloxan-induced diabetes exacerbates coronary atherosclerosis and calcification in Ossabaw miniature swine with metabolic syndrome. J Transl Med 16, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakris GL, Gaxiola E, Messerli FH, Mancia G, Erdine S, Cooper-DeHoff R, Pepine CJ, and Investigators I (2004). Clinical outcomes in the diabetes cohort of the INternational VErapamil SR-Trandolapril study. Hypertension 44, 637–642. [DOI] [PubMed] [Google Scholar]
- Barrett TJ, Distel E, Murphy AJ, Hu J, Garshick MS, Ogando Y, Liu J, Vaisar T, Heinecke JW, Berger JS, et al. (2019). Apolipoprotein AI) Promotes Atherosclerosis Regression in Diabetic Mice by Suppressing Myelopoiesis and Plaque Inflammation. Circulation 140, 1170–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Bebu I, Jenkins AJ, Stoner JA, Zhang Y, Klein RL, Lopes-Virella MF, Garvey WT, Budoff MJ, Alaupovic P, et al. (2019). Serum apolipoproteins and apolipoprotein-defined lipoprotein subclasses: a hypothesis-generating prospective study of cardiovascular events in T1D. J Lipid Res 60, 1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D, and Bornfeldt KE (2020). Hypertriglyceridemia and Atherosclerosis: Using Human Research to Guide Mechanistic Studies in Animal Models. Front Endocrinol (Lausanne) 11, 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Tobe K, Kadowaki T, Fazio S, et al. (2006). Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab 3, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebu I, Braffett BH, Pop-Busui R, Orchard TJ, Nathan DM, Lachin JM, and Group DER (2017). The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: the DCCT/EDIC study. Diabetologia 60, 2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra-Tomás N, Blanco Mejía S, Viguiliouk E, Khan T, Kendall CWC, Kahleova H, Rahelić D, Sievenpiper JL, and Salas-Salvadó J (2020). Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit Rev Food Sci Nutr 60, 1207–1227. [DOI] [PubMed] [Google Scholar]
- Beddhu S, Chertow GM, Greene T, Whelton PK, Ambrosius WT, Cheung AK, Cutler J, Fine L, Boucher R, Wei G, et al. (2018). Effects of Intensive Systolic Blood Pressure Lowering on Cardiovascular Events and Mortality in Patients With Type 2 Diabetes Mellitus on Standard Glycemic Control and in Those Without Diabetes Mellitus: Reconciling Results From ACCORD BP and SPRINT. J Am Heart Assoc 7, e009326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., Juliano RA, Jiao L, Granowitz C, et al. (2019). Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 380, 11–22. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, et al. (2008). Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 7, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi-Zoccai GG, Abbate A, Liuzzo G, and Biasucci LM (2003). Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 41, 1071–1077. [DOI] [PubMed] [Google Scholar]
- Bjornstad P, and Eckel RH (2018). Pathogenesis of Lipid Disorders in Insulin Resistance: a Brief Review. Curr Diab Rep 18, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornstad P, Maahs DM, Duca LM, Pyle L, Rewers M, Johnson RJ, and Snell-Bergeon JK (2016). Estimated insulin sensitivity predicts incident micro- and macrovascular complications in adults with type 1 diabetes over 6 years: the coronary artery calcification in type 1 diabetes study. J Diabetes Complications 30, 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P (1992). Abdominal fat distribution and disease: an overview of epidemiological data. Ann Med 24, 15–18. [DOI] [PubMed] [Google Scholar]
- Blood Pressure Lowering Treatment Trialists Collaboration (2014). Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet 384, 591–598. [DOI] [PubMed] [Google Scholar]
- Boulé NG, Kenny GP, Haddad E, Wells GA, and Sigal RJ (2003). Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia 46, 1071–1081. [DOI] [PubMed] [Google Scholar]
- Braffett BH, Dagogo-Jack S, Bebu I, Sivitz WI, Larkin M, Kolterman O, and Lachin JM (2019). Association of Insulin Dose, Cardiometabolic Risk Factors, and Cardiovascular Disease in Type 1 Diabetes During 30 Years of Follow-up in the DCCT/EDIC Study. Diabetes Care 42, 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, et al. (2001). Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345, 861–869. [DOI] [PubMed] [Google Scholar]
- Brown TL, Maahs DM, Bishop FK, Snell-Bergeon JK, and Wadwa RP (2016). Influences of gender on cardiovascular disease risk factors in adolescents with and without type 1 diabetes. Int J Pediatr Endocrinol 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner T, Shao B, Eckel RH, Heinecke JW, Bornfeldt KE, and Snell-Bergeon J (2021). Association of apolipoprotein C3 with insulin resistance and coronary artery calcium in patients with type 1 diabetes. J Clin Lipidol 15, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CP, Perkovic V, Agarwal R, Baldassarre J, Bakris G, Charytan DM, de Zeeuw D, Edwards R, Greene T, Heerspink HJL, et al. (2020a). Evaluating the Effects of Canagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease According to Baseline HbA1c, Including Those With HbA1c <7%: Results From the CREDENCE Trial. Circulation 141, 407–410. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, et al. (2020b). Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med 383, 1425–1435. [DOI] [PubMed] [Google Scholar]
- Chait A, Ginsberg HN, Vaisar T, Heinecke JW, Goldberg IJ, and Bornfeldt KE (2020). Remnants of the Triglyceride-Rich Lipoproteins, Diabetes, and Cardiovascular Disease. Diabetes 69, 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Breslow JL, Li W, and Leff T (1994). Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: correlation with changes in plasma triglyceride levels. J Lipid Res 35, 1918–1924. [PubMed] [Google Scholar]
- Clarkson TB, Koritnik DR, Weingand KW, and Miller LC (1985). Nonhuman primate models of atherosclerosis: potential for the study of diabetes mellitus and hyperinsulinemia. Metabolism 34, 51–59. [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, and Fuller JH (2004). Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364, 685–696. [DOI] [PubMed] [Google Scholar]
- Collado-Mesa F, Colhoun HM, Stevens LK, Boavida J, Ferriss JB, Karamanos B, Kempler P, Michel G, Roglic G, and Fuller JH (1999). Prevalence and management of hypertension in type 1 diabetes mellitus in Europe: the EURODIAB IDDM Complications Study. Diabet Med 16, 41–48. [DOI] [PubMed] [Google Scholar]
- Collins AR, Meehan WP, Kintscher U, Jackson S, Wakino S, Noh G, Palinski W, Hsueh WA, and Law RE (2001). Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 21, 365–371. [DOI] [PubMed] [Google Scholar]
- Control Group, Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, Evans GW, Gerstein HC, Holman RR, et al. (2009). Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 52, 2288–2298. [DOI] [PubMed] [Google Scholar]
- Conway BN, May ME, and Blot WJ (2012). Mortality among low-income African Americans and whites with diabetes. Diabetes Care 35, 2293–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costacou T, Evans RW, and Orchard TJ (2011). High-density lipoprotein cholesterol in diabetes: is higher always better? J Clin Lipidol 5, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree-Green M, Maahs DM, Ferland A, Hokanson JE, Wang H, Pyle L, Kinney GL, King M, Eckel RH, and Nadeau KJ (2016). Lipoprotein subfraction cholesterol distribution is more atherogenic in insulin resistant adolescents with type 1 diabetes. Pediatr Diabetes 17, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly CA, Fox KM, Remme WJ, Bertrand ME, Ferrari R, Simoons ML, and Investigators E (2005). The effect of perindopril on cardiovascular morbidity and mortality in patients with diabetes in the EUROPA study: results from the PERSUADE substudy. Eur Heart J 26, 1369–1378. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, and Bandyopadhyay A (2004). Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25, 4–7. [DOI] [PubMed] [Google Scholar]
- Dardano A, Miccoli R, Bianchi C, Daniele G, and Del Prato S (2020). Invited review. Series: Implications of the recent CVOTs in type 2 diabetes: Which patients for GLP-1RA or SGLT-2 inhibitor? Diabetes Res Clin Pract 162, 108112. [DOI] [PubMed] [Google Scholar]
- Dash S, and Leiter LA (2019). Residual cardiovascular risk among people with diabetes. Diabetes Obes Metab 21 Suppl 1, 28–38. [DOI] [PubMed] [Google Scholar]
- de Boer IH, Kestenbaum B, Rue TC, Steffes MW, Cleary PA, Molitch ME, Lachin JM, Weiss NS, Brunzell JD, Diabetes C, et al. (2008). Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med 168, 1867–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ, et al. (2014). Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation 130, 1110–1130. [DOI] [PubMed] [Google Scholar]
- de Jong M, van der Worp HB, van der Graaf Y, Visseren FLJ, and Westerink J (2017). Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials. Cardiovasc Diabetol 16, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group (2016). Risk Factors for Cardiovascular Disease in Type 1 Diabetes. Diabetes 65, 1370–1379.26895792 [Google Scholar]
- Diabetes Control Complications Trial/Epidemiology of Diabetes, I.C.S.R., Group (2016). Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care 39, 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distiller LA, Joffe BI, Melville V, Welman T, and Distiller GB (2006). Carotid artery intima-media complex thickening in patients with relatively long-surviving type 1 diabetes mellitus. J Diabetes Complications 20, 280–284. [DOI] [PubMed] [Google Scholar]
- Djaberi R, Schuijf JD, Boersma E, Kroft LJ, Pereira AM, Romijn JA, Scholte AJ, Jukema JW, and Bax JJ (2009). Differences in atherosclerotic plaque burden and morphology between type 1 and 2 diabetes as assessed by multislice computed tomography. Diabetes Care 32, 1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, et al. (2005). Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366, 1279–1289. [DOI] [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, et al. (2009). Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360, 129–139. [DOI] [PubMed] [Google Scholar]
- Duff GL, and McMillan GC (1948). The inhibition of experimental cholesterol atherosclerosis by alloxan diabetes in the rabbit. Am Heart J 36, 469. [DOI] [PubMed] [Google Scholar]
- Eckel RH (2007). Mechanisms of the components of the metabolic syndrome that predispose to diabetes and atherosclerotic CVD. Proc Nutr Soc 66, 82–95. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Albers JJ, Cheung MC, Wahl PW, Lindgren FT, and Bierman EL (1981). High density lipoprotein composition in insulin-dependent diabetes mellitus. Diabetes 30, 132–138. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, and Zimmet PZ (2005). The metabolic syndrome. Lancet 365, 1415–1428. [DOI] [PubMed] [Google Scholar]
- Elam MB, Ginsberg HN, Lovato LC, Corson M, Largay J, Leiter LA, Lopez C, O’Connor PJ, Sweeney ME, Weiss D, et al. (2017). Association of Fenofibrate Therapy With Long-term Cardiovascular Risk in Statin-Treated Patients With Type 2 Diabetes. JAMA Cardiol 2, 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott HL, Lloyd SM, Ford I, and Meredith PA (2011). Improving blood pressure control in patients with diabetes mellitus and high cardiovascular risk. Int J Hypertens 2010, 490769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Zethelius B, Eeg-Olofsson K, Nilsson PM, Gudbjornsdottir S, Cederholm J, and Eliasson B (2011). Blood lipids in 75,048 type 2 diabetic patients: a population-based survey from the Swedish National diabetes register. Eur J Cardiovasc Prev Rehabil 18, 97–105. [DOI] [PubMed] [Google Scholar]
- Espey DK, Jim MA, Cobb N, Bartholomew M, Becker T, Haverkamp D, and Plescia M (2014). Leading causes of death and all-cause mortality in American Indians and Alaska Natives. Am J Public Health 104 Suppl 3, S303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, Glynn RJ, Libby P, and Ridker PM (2018). Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J Am Coll Cardiol 71, 2392–2401. [DOI] [PubMed] [Google Scholar]
- Fagard RH, and Nilsson PM (2009). Smoking and diabetes--the double health hazard! Prim Care Diabetes 3, 205–209. [DOI] [PubMed] [Google Scholar]
- Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, and Fisher EA (2011). HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A 108, 7166–7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, Laufs U, Oliver-Williams C, Wood AM, Butterworth AS, et al. (2019). Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR Variants With Risk of Coronary Heart Disease. JAMA 321, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E, and Cushman WC (2012). Diabetes and hypertension: the bad companions. Lancet 380, 601–610. [DOI] [PubMed] [Google Scholar]
- Feussner G, Wagner A, and Ziegler R (1993). Relation of cardiovascular risk factors to atherosclerosis in type III hyperlipoproteinemia. Hum Genet 92, 122–126. [DOI] [PubMed] [Google Scholar]
- Fisher EA (2016). Regression of Atherosclerosis: The Journey From the Liver to the Plaque and Back. Arterioscler Thromb Vasc Biol 36, 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn MC, Kraakman MJ, Tikellis C, Lee MKS, Hanssen NMJ, Kammoun HL, Pickering RJ, Dragoljevic D, Al-Sharea A, Barrett TJ, et al. (2020). Transient Intermittent Hyperglycemia Accelerates Atherosclerosis by Promoting Myelopoiesis. Circ Res 127, 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest KY, Becker DJ, Kuller LH, Wolfson SK, and Orchard TJ (2000). Are predictors of coronary heart disease and lower-extremity arterial disease in type 1 diabetes the same? A prospective study. Atherosclerosis 148, 159–169. [DOI] [PubMed] [Google Scholar]
- Fotakis P, Kothari V, Thomas DG, Westerterp M, Molusky MM, Altin E, Abramowicz S, Wang N, He Y, Heinecke JW, et al. (2019). Anti-Inflammatory Effects of HDL (High-Density Lipoprotein) in Macrophages Predominate Over Proinflammatory Effects in Atherosclerotic Plaques. Arterioscler Thromb Vasc Biol 39, e253–e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Bouchi R, Takeuchi T, Tsujimoto K, Minami I, Yoshimoto T, and Ogawa Y (2018). Sarcopenic obesity assessed using dual energy X-ray absorptiometry (DXA) can predict cardiovascular disease in patients with type 2 diabetes: a retrospective observational study. Cardiovasc Diabetol 17, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, et al. (2018). Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun 9, 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganda OP, Bhatt DL, Mason RP, Miller M, and Boden WE (2018). Unmet Need for Adjunctive Dyslipidemia Therapy in Hypertriglyceridemia Management. J Am Coll Cardiol 72, 330–343. [DOI] [PubMed] [Google Scholar]
- Ganjali S, Dallinga-Thie GM, Simental-Mendia LE, Banach M, Pirro M, and Sahebkar A (2017). HDL functionality in type 1 diabetes. Atherosclerosis 267, 99–109. [DOI] [PubMed] [Google Scholar]
- Gaudreault N, Kumar N, Olivas VR, Eberle D, Stephens K, and Raffai RL (2013). Hyperglycemia impairs atherosclerosis regression in mice. Am J Pathol 183, 1981–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity RG, Natarajan R, Nadler JL, and Kimsey T (2001). Diabetes-induced accelerated atherosclerosis in swine. Diabetes 50, 1654–1665. [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Ryden L, et al. (2019). Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130. [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC Jr., Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, et al. (2011). Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 364, 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HN (1991). Lipoprotein physiology in nondiabetic and diabetic states. Relationship to atherogenesis. Diabetes Care 14, 839–855. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, et al. (2010). Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 362, 1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbálan R, Špinar J, Park JG, White JA, Bohula EA, and Braunwald E (2018). Benefit of Adding Ezetimibe to Statin Therapy on Cardiovascular Outcomes and Safety in Patients With Versus Without Diabetes Mellitus: Results From IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation 137, 1571–1582. [DOI] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, McCulloch CE, and Hsu CY (2004). Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351, 1296–1305. [DOI] [PubMed] [Google Scholar]
- Gordin D, Harjutsalo V, Tinsley L, Fickweiler W, Sun JK, Forsblom C, Amenta PS, Pober D, D’Eon S, Khatri M, et al. (2018). Differential Association of Microvascular Attributions With Cardiovascular Disease in Patients With Long Duration of Type 1 Diabetes. Diabetes Care 41, 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandl G, and Wolfrum C (2018). Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin Immunopathol 40, 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group AS, Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC Jr., Probstfield JL, Cushman WC, Ginsberg HN, et al. (2011). Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 364, 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Ai J, Zheng Z, Howatt DA, Daugherty A, Huang B, and Li XA (2013). High density lipoprotein protects against polymicrobe-induced sepsis in mice. J Biol Chem 288, 17947–17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner SM, Miettinen H, and Stern MP (1997). Relatively more atherogenic coronary heart disease risk factors in prediabetic women than in prediabetic men. Diabetologia 40, 711–717. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Mykkanen L, Festa A, Burke JP, and Stern MP (2000). Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation 101, 975–980. [DOI] [PubMed] [Google Scholar]
- Haller H, Ito S, Izzo JL Jr., Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM, et al. (2011). Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 364, 907–917. [DOI] [PubMed] [Google Scholar]
- Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, and Tall AR (2006). Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab 3, 257–266. [DOI] [PubMed] [Google Scholar]
- Hauser TH, Salastekar N, Schaefer EJ, Desai T, Goldfine HL, Fowler KM, Weber GM, Welty F, Clouse M, Shoelson SE, et al. (2016). Effect of Targeting Inflammation With Salsalate: The TINSAL-CVD Randomized Clinical Trial on Progression of Coronary Plaque in Overweight and Obese Patients Using Statins. JAMA Cardiol 1, 413–423. [DOI] [PubMed] [Google Scholar]
- He Y, Ronsein GE, Tang C, Jarvik GP, Davidson WS, Kothari V, Song HD, Segrest JP, Bornfeldt KE, and Heinecke JW (2020). Diabetes Impairs Cellular Cholesterol Efflux From ABCA1 to Small HDL Particles. Circ Res 127, 1198–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heart Outcomes Prevention Evaluation Study Investigators (2000). Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 355, 253–259. [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group (2002). MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyden S, Heiss G, Bartel AG, and Hames CG (1980). Sex differences in coronary mortality among diabetics in Evans County, Georgia. J Chronic Dis 33, 265–273. [DOI] [PubMed] [Google Scholar]
- Hirsch IB (2015). Glycemic Variability and Diabetes Complications: Does It Matter? Of Course It Does! Diabetes Care 38, 1610–1614. [DOI] [PubMed] [Google Scholar]
- Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, et al. (2017). Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 377, 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, and Neil HA (2008). 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359, 1577–1589. [DOI] [PubMed] [Google Scholar]
- Hoyer FF, Zhang X, Coppin E, Vasamsetti SB, Modugu G, Schloss MJ, Rohde D, McAlpine CS, Iwamoto Y, Libby P, et al. (2020). Bone Marrow Endothelial Cells Regulate Myelopoiesis in Diabetes Mellitus. Circulation 142, 244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, Dansky H, McClain DA, McIndoe R, Wassef MK, et al. (2007). Recipes for creating animal models of diabetic cardiovascular disease. Circ Res 100, 1415–1427. [DOI] [PubMed] [Google Scholar]
- Hunt KJ, Baker NL, Cleary PA, Klein R, Virella G, Lopes-Virella MF, and Investigators D.E.G.o. (2015). Longitudinal Association Between Endothelial Dysfunction, Inflammation, and Clotting Biomarkers With Subclinical Atherosclerosis in Type 1 Diabetes: An Evaluation of the DCCT/EDIC Cohort. Diabetes Care 38, 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzucchi SE, Kosiborod M, Fitchett D, Wanner C, Hehnke U, Kaspers S, George JT, and Zinman B (2018). Improvement in Cardiovascular Outcomes With Empagliflozin Is Independent of Glycemic Control. Circulation 138, 1904–1907. [DOI] [PubMed] [Google Scholar]
- Islam MS, and Wilson RD (2012). Experimentally induced rodent models of type 2 diabetes. Methods Mol Biol 933, 161–174. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, and Groop L (2001). Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24, 683–689. [DOI] [PubMed] [Google Scholar]
- Jackson KG, Poppitt SD, and Minihane AM (2012). Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 220, 22–33. [DOI] [PubMed] [Google Scholar]
- Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, and Stehouwer CD (2000). Increased levels of soluble vascular cell adhesion molecule 1 are associated with risk of cardiovascular mortality in type 2 diabetes: the Hoorn study. Diabetes 49, 485–491. [DOI] [PubMed] [Google Scholar]
- Jo S-H, Han SH, Kim S-H, Eckel RH, and Kon KK (2021). Cardiovascular effects of omega-3 fatty acids: Hope or Hype?. Atherosclerosis In Press. [DOI] [PubMed] [Google Scholar]
- Johansson F, Kramer F, Barnhart S, Kanter JE, Vaisar T, Merrill RD, Geng L, Oka K, Chan L, Chait A, et al. (2008). Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci U S A 105, 2082–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefs T, Barrett TJ, Brown EJ, Quezada A, Wu X, Voisin M, Amengual J, and Fisher EA (2020). Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefs T, Basu D, Vaisar T, Arets B, Kanter JE, Huggins LA, Hu Y, Liu J, Clouet-Foraison N, Heinecke JW, et al. (2021). Atherosclerosis Regression and Cholesterol Efflux in Hypertriglyceridemic Mice. Circ Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JY, Ma Z, and Segar L (2011). Spontaneously diabetic Ins2(+/Akita):apoE-deficient mice exhibit exaggerated hypercholesterolemia and atherosclerosis. Am J Physiol Endocrinol Metab 301, E145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalugotla G, He L, Weber KJ, Daemen S, Reller A, Razani B, and Schilling JD (2019). Frontline Science: Acyl-CoA synthetase 1 exacerbates lipotoxic inflammasome activation in primary macrophages. J Leukoc Biol 106, 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, and McGee DL (1979a). Diabetes and cardiovascular disease. The Framingham study. Jama 241, 2035–2038. [DOI] [PubMed] [Google Scholar]
- Kannel WB, and McGee DL (1979b). Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 2, 120–126. [DOI] [PubMed] [Google Scholar]
- Kanter JE, and Bornfeldt KE (2020). Apolipoprotein C3 and apolipoprotein B colocalize in proximity to macrophages in atherosclerotic lesions in diabetes. J Lipid Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter JE, Kramer F, Barnhart S, Averill MM, Vivekanandan-Giri A, Vickery T, Li LO, Becker L, Yuan W, Chait A, et al. (2012). Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A 109, E715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter JE, Shao B, Kramer F, Barnhart S, Shimizu-Albergine M, Vaisar T, Graham MJ, Crooke RM, Manuel CR, Haeusler RA, et al. (2019). Increased apolipoprotein C3 drives cardiovascular risk in type 1 diabetes. J Clin Invest 129, 4165–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kats D, Sharrett AR, Ginsberg HN, Nambi V, Ballantyne CM, Hoogeveen RC, and Heiss G (2017). Postprandial lipemia and the risk of coronary heart disease and stroke: the Atherosclerosis Risk in Communities (ARIC) Study. BMJ Open Diabetes Res Care 5, e000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami R, Katsuki S, Travers R, Romero DC, Becker-Greene D, Passos LSA, Higashi H, Blaser MC, Sukhova GK, Buttigieg J, et al. (2020). S100A9-RAGE Axis Accelerates Formation of Macrophage-Mediated Extracellular Vesicle Microcalcification in Diabetes Mellitus. Arterioscler Thromb Vasc Biol 40, 1838–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, and Baigent C (2008). Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 371, 117–125. [DOI] [PubMed] [Google Scholar]
- Keech A, Colquhoun D, Best J, Kirby A, Simes RJ, Hunt D, Hague W, Beller E, Arulchelvam M, Baker J, et al. (2003). Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial. Diabetes Care 26, 2713–2721. [DOI] [PubMed] [Google Scholar]
- Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, et al. (2016). Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med 374, 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Shin JS, Min BH, Kim HJ, Kim JS, Yoon IH, Jeong WY, Lee GE, Kim MS, Kim JE, et al. (2016). Induction, management, and complications of streptozotocin-induced diabetes mellitus in rhesus monkeys. Xenotransplantation 23, 472–478. [DOI] [PubMed] [Google Scholar]
- King GL, Park K, and Li Q (2016). Selective Insulin Resistance and the Development of Cardiovascular Diseases in Diabetes: The 2015 Edwin Bierman Award Lecture. Diabetes 65, 1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto VA, Stevens LK, Mattock M, Ebeling P, Muggeo M, Stephenson J, and Idzior-Walus B (1996). Cardiovascular disease and its risk factors in IDDM in Europe. EURODIAB IDDM Complications Study Group. Diabetes Care 19, 689–697. [DOI] [PubMed] [Google Scholar]
- Koska J, Saremi A, Howell S, Bahn G, De Courten B, Ginsberg H, Beisswenger PJ, Reaven PD, and Investigators V (2018). Advanced Glycation End Products, Oxidation Products, and Incident Cardiovascular Events in Patients With Type 2 Diabetes. Diabetes Care 41, 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmas CE, Silverio D, Tsomidou C, Salcedo MD, Montan PD, and Guzman E (2018). The Impact of Insulin Resistance and Chronic Kidney Disease on Inflammation and Cardiovascular Disease. Clin Med Insights Endocrinol Diabetes 11, 1179551418792257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraakman MJ, Lee MK, Al-Sharea A, Dragoljevic D, Barrett TJ, Montenont E, Basu D, Heywood S, Kammoun HL, Flynn M, et al. (2017). Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J Clin Invest 127, 2133–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski AS (2015). Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care 38, 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M (2001). Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med 249, 225–235. [DOI] [PubMed] [Google Scholar]
- Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, and Despres JP (1997). Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation 95, 69–75. [DOI] [PubMed] [Google Scholar]
- Lee SI, Patel M, Jones CM, and Narendran P (2015). Cardiovascular disease and type 1 diabetes: prevalence, prediction and management in an ageing population. Ther Adv Chronic Dis 6, 347–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Lloyd JT, Giuriceo K, Day T, Shrank W, and Rajkumar R (2020). Systematic review and meta-analysis of patient race/ethnicity, socioeconomics, and quality for adult type 2 diabetes. Health Serv Res 55, 741–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, and Glass CK (2000). Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest 106, 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Fu J, Xia Y, Qi W, Ishikado A, Park K, Yokomizo H, Huang Q, Cai W, Rask-Madsen C, et al. (2019). Homozygous receptors for insulin and not IGF-1 accelerate intimal hyperplasia in insulin resistance and diabetes. Nat Commun 10, 4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Sun Y, Liang CP, Thorp EB, Han S, Jehle AW, Saraswathi V, Pridgen B, Kanter JE, Li R, et al. (2009). Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res 105, 1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Eckel RH, and Koh KK (2018). Clinical implications of current cardiovascular outcome trials with sodium glucose cotransporter-2 (SGLT2) inhibitors. Atherosclerosis 272, 33–40. [DOI] [PubMed] [Google Scholar]
- Lin MH, Lee CH, Lin C, Zou YF, Lu CH, Hsieh CH, and Lee CH (2019). Low-Dose Aspirin for the Primary Prevention of Cardiovascular Disease in Diabetic Individuals: A Meta-Analysis of Randomized Control Trials and Trial Sequential Analysis. J Clin Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, et al. (2013). Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SY, Qi SD, Zhao Y, Li YY, Yang FM, Yu WH, Jin M, Chen LX, Wang JB, He ZL, et al. (2015). Type 2 diabetes mellitus non-genetic Rhesus monkey model induced by high fat and high sucrose diet. Exp Clin Endocrinol Diabetes 123, 19–26. [DOI] [PubMed] [Google Scholar]
- Maahs DM, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson J, Ehrlich J, Garg S, Eckel RH, and Rewers MJ (2005). Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes Care 28, 301–306. [DOI] [PubMed] [Google Scholar]
- Madsen CM, Varbo A, and Nordestgaard BG (2021). Novel Insights From Human Studies on the Role of High-Density Lipoprotein in Mortality and Noncardiovascular Disease. Arterioscler Thromb Vasc Biol 41, 128–140. [DOI] [PubMed] [Google Scholar]
- Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, et al. (2019). Canagliflozin and Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus and Chronic Kidney Disease in Primary and Secondary Cardiovascular Prevention Groups. Circulation 140, 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majithia A, and Bhatt DL (2018). Antiplatelet Therapy in Diabetes. Endocrinol Metab Clin North Am 47, 223–235. [DOI] [PubMed] [Google Scholar]
- Mancia G, Brown M, Castaigne A, de Leeuw P, Palmer CR, Rosenthal T, Wagener G, Ruilope LM, and Insight (2003). Outcomes with nifedipine GITS or Co-amilozide in hypertensive diabetics and nondiabetics in Intervention as a Goal in Hypertension (INSIGHT). Hypertension 41, 431–436. [DOI] [PubMed] [Google Scholar]
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. (2016a). Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 375, 1834–1844. [DOI] [PubMed] [Google Scholar]
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. (2016b). Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 375, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, and Eckel RH (2021). Mechanistic Insights from REDUCE-IT STRENGTHen the Case Against Triglyceride Lowering as a Strategy for Cardiovascular Disease Risk Reduction. Am J Med. [DOI] [PubMed] [Google Scholar]
- Masucci-Magoulas L, Goldberg IJ, Bisgaier CL, Serajuddin H, Francone OL, Breslow JL, and Tall AR (1997). A mouse model with features of familial combined hyperlipidemia. Science 275, 391–394. [DOI] [PubMed] [Google Scholar]
- Matuleviciene-Anangen V, Rosengren A, Svensson AM, Pivodic A, Gudbjornsdottir S, Wedel H, Kosiborod M, Haraldsson B, and Lind M (2017). Glycaemic control and excess risk of major coronary events in persons with type 1 diabetes. Heart 103, 1687–1695. [DOI] [PubMed] [Google Scholar]
- Mauldin JP, Nagelin MH, Wojcik AJ, Srinivasan S, Skaflen MD, Ayers CR, McNamara CA, and Hedrick CC (2008). Reduced expression of ATP-binding cassette transporter G1 increases cholesterol accumulation in macrophages of patients with type 2 diabetes mellitus. Circulation 117, 2785–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D’Agostino RB Sr., Perez A, Provost JC, and Haffner SM (2006). Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA 296, 2572–2581. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Abbasi F, Lamendola C, Liang L, Reaven G, Schaaf P, and Reaven P (2002). Differentiation between obesity and insulin resistance in the association with C-reactive protein. Circulation 106, 2908–2912. [DOI] [PubMed] [Google Scholar]
- McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlávek J, et al. (2019). Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 381, 1995–2008. [DOI] [PubMed] [Google Scholar]
- Meigs JB (2003). Epidemiology of cardiovascular complications in type 2 diabetes mellitus. Acta Diabetol 40 Suppl 2, S358–361. [DOI] [PubMed] [Google Scholar]
- Miele EM, and Headley SAE (2017). The Effects of Chronic Aerobic Exercise on Cardiovascular Risk Factors in Persons with Diabetes Mellitus. Curr Diab Rep 17, 97. [DOI] [PubMed] [Google Scholar]
- Millstein RJ, Pyle LL, Bergman BC, Eckel RH, Maahs DM, Rewers MJ, Schauer IE, and Snell-Bergeon JK (2018). Sex-specific differences in insulin resistance in type 1 diabetes: The CACTI cohort. J Diabetes Complications 32, 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, Ferrannini E, and Reddy ST (2011). Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 60, 2617–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movassat J, Calderari S, Fernández E, Martín MA, Escrivá F, Plachot C, Gangnerau MN, Serradas P, Alvarez C, and Portha B (2007). Type 2 diabetes - a matter of failing beta-cell neogenesis? Clues from the GK rat model. Diabetes Obes Metab 9 Suppl 2, 187–195. [DOI] [PubMed] [Google Scholar]
- Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, et al. (2013). Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 17, 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, Sreejit G, Abo-Aly M, Jaggers RM, Chelvarajan L, Johnson J, Pernes G, Athmanathan B, Abdel-Latif A, and Murphy AJ (2020). NETosis Is Required for S100A8/A9-Induced Granulopoiesis After Myocardial Infarction. Arterioscler Thromb Vasc Biol 40, 2805–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes C, Complications Trial/Epidemiology of Diabetes, I., et al. (2005). Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353, 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. (2017). Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 377, 644–657. [DOI] [PubMed] [Google Scholar]
- Neeb ZP, Edwards JM, Alloosh M, Long X, Mokelke EA, and Sturek M (2010). Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine. Comp Med 60, 300–315. [PMC free article] [PubMed] [Google Scholar]
- Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, et al. (2019). Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 7, 715–725. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Naik V, and Speer MY (2013). Diabetes mellitus accelerates cartilaginous metaplasia and calcification in atherosclerotic vessels of LDLr mutant mice. Cardiovasc Pathol 22, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M, Wolski K, et al. (2011). Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 365, 2078–2087. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, et al. (2020). Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. Jama 324, 2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls SJ, Tuzcu EM, Kalidindi S, Wolski K, Moon KW, Sipahi I, Schoenhagen P, and Nissen SE (2008). Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 52, 255–262. [DOI] [PubMed] [Google Scholar]
- Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, et al. (2020). Colchicine in Patients with Chronic Coronary Disease. N Engl J Med 383, 1838–1847. [DOI] [PubMed] [Google Scholar]
- Nin JW, Ferreira I, Schalkwijk CG, Jorsal A, Prins MH, Parving HH, Tarnow L, Rossing P, and Stehouwer CD (2012a). Higher plasma high-mobility group box 1 levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12 year follow-up study. Diabetologia 55, 2489–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin JW, Ferreira I, Schalkwijk CG, Prins MH, Chaturvedi N, Fuller JH, and Stehouwer CD (2012b). Serum high-mobility group box-1 levels are positively associated with micro- and macroalbuminuria but not with cardiovascular disease in type 1 diabetes: the EURODIAB Prospective Complications Study. Eur J Endocrinol 166, 325–332. [DOI] [PubMed] [Google Scholar]
- Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, et al. (2009). Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20, 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa T, Kanter JE, Kramer F, Barnhart S, Shen X, Vivekanandan-Giri A, Wall VZ, Kowitz J, Devaraj S, O’Brien KD, et al. (2014). Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Rep 7, 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochelliere R, Staniloae CS, Mavromatis K, et al. (2008). Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299, 1561–1573. [DOI] [PubMed] [Google Scholar]
- Nordestgaard BG, Stender S, and Kjeldsen K (1988). Reduced atherogenesis in cholesterol-fed diabetic rabbits. Giant lipoproteins do not enter the arterial wall. Arteriosclerosis 8, 421–428. [DOI] [PubMed] [Google Scholar]
- Nordestgaard BG, and Zilversmit DB (1988). Hyperglycemia in normotriglyceridemic, hypercholesterolemic insulin-treated diabetic rabbits does not accelerate atherogenesis. Atherosclerosis 72, 37–47. [DOI] [PubMed] [Google Scholar]
- Noth RH, Krolewski AS, Kaysen GA, Meyer TW, and Schambelan M (1989). Diabetic nephropathy: hemodynamic basis and implications for disease management. Ann Intern Med 110, 795–813. [DOI] [PubMed] [Google Scholar]
- O’Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im K, et al. (2014). Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. Jama 312, 1006–1015. [DOI] [PubMed] [Google Scholar]
- O’Keefe EL, DiNicolantonio JJ, Patil H, Helzberg JH, and Lavie CJ (2016). Lifestyle Choices Fuel Epidemics of Diabetes and Cardiovascular Disease Among Asian Indians. Prog Cardiovasc Dis 58, 505–513. [DOI] [PubMed] [Google Scholar]
- Olsen E, Holzhauer B, Julius S, Kjeldsen SE, Larstorp ACK, Mancia G, Mehlum MH, Mo R, Rostrup M, Soraas CL, et al. (2021). Cardiovascular outcomes at recommended blood pressure targets in middle-aged and elderly patients with type 2 diabetes mellitus compared to all middle-aged and elderly hypertensive study patients with high cardiovascular risk. Blood Press 30, 90–97. [DOI] [PubMed] [Google Scholar]
- Olshansky B, Chung MK, Budoff MJ, Philip S, Jiao L, Doyle RT Jr., Copland C, Giaquinto A, Juliano RA, and Bhatt DL (2020). Mineral oil: safety and use as placebo in REDUCE-IT and other clinical studies. Eur Heart J Suppl 22, J34–J48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard TJ, Forrest KY, Kuller LH, Becker DJ, and Pittsburgh Epidemiology of Diabetes Complications, S. (2001). Lipid and blood pressure treatment goals for type 1 diabetes: 10-year incidence data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 24, 1053–1059. [DOI] [PubMed] [Google Scholar]
- Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, and Becker DJ (2003). Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 26, 1374–1379. [DOI] [PubMed] [Google Scholar]
- Osei K, and Gaillard T (2017). Disparities in Cardiovascular Disease and Type 2 Diabetes Risk Factors in Blacks and Whites: Dissecting Racial Paradox of Metabolic Syndrome. Front Endocrinol (Lausanne) 8, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei K, Gaillard T, and Schuster D (2005). Plasma adiponectin levels in high risk African-Americans with normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes. Obes Res 13, 179–185. [DOI] [PubMed] [Google Scholar]
- Ouimet M, Barrett TJ, and Fisher EA (2019). HDL and Reverse Cholesterol Transport. Circ Res 124, 1505–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. (2020). Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 383, 1413–1424. [DOI] [PubMed] [Google Scholar]
- Palanissami G, and Paul SFD (2018). RAGE and Its Ligands: Molecular Interplay Between Glycation, Inflammation, and Hallmarks of Cancer-a Review. Horm Cancer 9, 295–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneni F, and Luscher TF (2017). Cardiovascular Protection in the Treatment of Type 2 Diabetes: A Review of Clinical Trial Results Across Drug Classes. Am J Cardiol 120, S17–s27. [DOI] [PubMed] [Google Scholar]
- Parathath S, Grauer L, Huang LS, Sanson M, Distel E, Goldberg IJ, and Fisher EA (2011). Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes 60, 1759–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Li Q, Evcimen ND, Rask-Madsen C, Maeda Y, Maddaloni E, Yokomizo H, Shinjo T, St-Louis R, Fu J, et al. (2018). Exogenous Insulin Infusion Can Decrease Atherosclerosis in Diabetic Rodents by Improving Lipids, Inflammation, and Endothelial Function. Arterioscler Thromb Vasc Biol 38, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr., Chow WS, Stern D, and Schmidt AM (1998). Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 4, 1025–1031. [DOI] [PubMed] [Google Scholar]
- Patel A, Group AC, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, et al. (2007). Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370, 829–840. [DOI] [PubMed] [Google Scholar]
- Patterson R, McNamara E, Tainio M, de Sa TH, Smith AD, Sharp SJ, Edwards P, Woodcock J, Brage S, and Wijndaele K (2018). Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol 33, 811–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters SA, Engelen L, Buijs J, Chaturvedi N, Fuller JH, Schalkwijk CG, and Stehouwer CD (2015). Plasma levels of matrix metalloproteinase-2, -3, -10, and tissue inhibitor of metalloproteinase-1 are associated with vascular complications in patients with type 1 diabetes: the EURODIAB Prospective Complications Study. Cardiovasc Diabetol 14, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters SA, Engelen L, Buijs J, Jorsal A, Parving HH, Tarnow L, Rossing P, Schalkwijk CG, and Stehouwer CDA (2017). Plasma matrix metalloproteinases are associated with incident cardiovascular disease and all-cause mortality in patients with type 1 diabetes: a 12-year follow-up study. Cardiovasc Diabetol 16, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penno G, Russo E, Garofolo M, Daniele G, Lucchesi D, Giusti L, Sancho Bornez V, Bianchi C, Dardano A, Miccoli R, et al. (2017). Evidence for two distinct phenotypes of chronic kidney disease in individuals with type 1 diabetes mellitus. Diabetologia 60, 1102–1113. [DOI] [PubMed] [Google Scholar]
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. (2019). Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 380, 2295–2306. [DOI] [PubMed] [Google Scholar]
- Peters SA, Huxley RR, and Woodward M (2014). Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 57, 1542–1551. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, Lawson FC, Ping L, Wei X, Lewis EF, et al. (2015). Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med 373, 2247–2257. [DOI] [PubMed] [Google Scholar]
- Pithova P, Stechova K, Pitha J, Lanska V, and Kvapil M (2016). Determinants of preclinical atherosclerosis are different in type 1 and type 2 diabetic women. Physiol Res 65, 219–228. [DOI] [PubMed] [Google Scholar]
- Plazas Guerrero CG, Acosta Cota SJ, Castro Sánchez FH, Vergara Jiménez MJ, Ríos Burgueño ER, Sarmiento Sánchez JI, Picos Corrales LA, and Osuna Martínez U (2019). Evaluation of sucrose-enriched diet consumption in the development of risk factors associated to type 2 diabetes, atherosclerosis and non-alcoholic fatty liver disease in a murine model. Int J Environ Health Res, 1–19. [DOI] [PubMed] [Google Scholar]
- Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, et al. (2008). A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 322, 1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel A, Zhou JY, Story D, and Li L (2018). Diabetes and Associated Cardiovascular Complications in American Indians/Alaskan Natives: A Review of Risks and Prevention Strategies. J Diabetes Res 2018, 2742565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound LD, Kievit P, and Grove KL (2014). The nonhuman primate as a model for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 21, 89–94. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Paynter NP, Everett BM, Glynn RJ, Amarenco P, Elam M, Ginsberg H, Hiatt WR, Ishibashi S, Koenig W, et al. (2018). Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am Heart J 206, 80–93. [DOI] [PubMed] [Google Scholar]
- Pugliese G, Solini A, Bonora E, Fondelli C, Orsi E, Nicolucci A, and Penno G (2014). Chronic kidney disease in type 2 diabetes: lessons from the Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicentre Study. Nutr Metab Cardiovasc Dis 24, 815–822. [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Braffett BH, Zinman B, Gubitosi-Klug RA, Sivitz W, Bantle JP, Ziegler G, Cleary PA, and Brunzell JD (2017). Impact of Excessive Weight Gain on Cardiovascular Outcomes in Type 1 Diabetes: Results From the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care 40, 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell JQ, Zinman B, and Brunzell JD (2013). The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation 127, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyörälä K, Ballantyne CM, Gumbiner B, Lee MW, Shah A, Davies MJ, Mitchel YB, Pedersen TR, and Kjekshus J (2004). Reduction of cardiovascular events by simvastatin in nondiabetic coronary heart disease patients with and without the metabolic syndrome: subgroup analyses of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 27, 1735–1740. [DOI] [PubMed] [Google Scholar]
- Qi Q, Meigs JB, Rexrode KM, Hu FB, and Qi L (2013). Diabetes genetic predisposition score and cardiovascular complications among patients with type 2 diabetes. Diabetes Care 36, 737–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian C, Gong L, Yang Z, Chen W, Chen Y, Xu Z, Wu B, Tang C, Gao F, and Zeng W (2015). Diastolic dysfunction in spontaneous type 2 diabetes rhesus monkeys: a study using echocardiography and magnetic resonance imaging. BMC Cardiovasc Disord 15, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, et al. (2010). Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab 11, 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray KK, Colhoun HM, Szarek M, Baccara-Dinet M, Bhatt DL, Bittner VA, Budaj AJ, Diaz R, Goodman SG, Hanotin C, et al. (2019). Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol 7, 618–628. [DOI] [PubMed] [Google Scholar]
- Raz I, Wilson PW, Strojek K, Kowalska I, Bozikov V, Gitt AK, Jermendy G, Campaigne BN, Kerr L, Milicevic Z, et al. (2009). Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 32, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven P, Merat S, Casanada F, Sutphin M, and Palinski W (1997). Effect of streptozotocin-induced hyperglycemia on lipid profiles, formation of advanced glycation endproducts in lesions, and extent of atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 17, 2250–2256. [DOI] [PubMed] [Google Scholar]
- Reaven PD, Emanuele NV, Wiitala WL, Bahn GD, Reda DJ, McCarren M, Duckworth WC, Hayward RA, and Investigators V (2019). Intensive Glucose Control in Patients with Type 2 Diabetes - 15-Year Follow-up. N Engl J Med 380, 2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, von Herrath MG, Chait A, and Bornfeldt KE (2004). Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest 114, 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Soffer G, Holleran S, Karmally W, Ngai CI, Chen NT, Torres M, Ramakrishnan R, Blaner WS, Berglund L, Ginsberg HN, et al. (2009). Measures of postprandial lipoproteins are not associated with coronary artery disease in patients with type 2 diabetes mellitus. J Lipid Res 50, 1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. (2008). Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359, 2195–2207. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, et al. (2019). Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med 380, 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. (2017). Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Rodrigues TC, Canani LH, Schvartzman P, and Gross JL (2011). Hypertension is the metabolic syndrome component most strongly associated with microvascular complications and coronary artery calcification in Type 1 diabetes. J Endocrinol Invest 34, e58–63. [DOI] [PubMed] [Google Scholar]
- Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Pina IL, Ramirez SM, Rodriguez B, et al. (2014). Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation 130, 593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez V, Weiss MC, Weintraub H, Goldberg IJ, and Schwartzbard A (2017). Cardiovascular disease leads to a new algorithm for diabetes treatment. J Clin Lipidol 11, 1126–1133. [DOI] [PubMed] [Google Scholar]
- Rollini F, Franchi F, Muñiz-Lozano A, and Angiolillo DJ (2013). Platelet function profiles in patients with diabetes mellitus. J Cardiovasc Transl Res 6, 329–345. [DOI] [PubMed] [Google Scholar]
- Rosenson RS, Brewer HB Jr., Ansell BJ, Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR, and Webb NR (2016). Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol 13, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MS, Janal MN, Crosby J, and Donnelly R (2016). Plasma markers of inflammation and prediction of cardiovascular disease and mortality in African Americans with type 1 diabetes. Diabetes Res Clin Pract 114, 117–125. [DOI] [PubMed] [Google Scholar]
- Roy MS, Peng B, and Roy A (2007). Risk factors for coronary disease and stroke in previously hospitalized African-Americans with Type 1 diabetes: a 6-year follow-up. Diabet Med 24, 1361–1368. [DOI] [PubMed] [Google Scholar]
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, and Clift SM (1991). Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 353, 265–267. [DOI] [PubMed] [Google Scholar]
- Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, et al. (1999). Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 341, 410–418. [DOI] [PubMed] [Google Scholar]
- Ruscica M, Tokgozoglu L, Corsini A, and Sirtori CR (2019). PCSK9 inhibition and inflammation: A narrative review. Atherosclerosis 288, 146–155. [DOI] [PubMed] [Google Scholar]
- Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, Murphy SA, Kuder JF, Gouni-Berthold I, Lewis BS, et al. (2017). Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol 5, 941–950. [DOI] [PubMed] [Google Scholar]
- Sacks FM, and Campos H (2003). Clinical review 163: Cardiovascular endocrinology: Low-density lipoprotein size and cardiovascular disease: a reappraisal. J Clin Endocrinol Metab 88, 4525–4532. [DOI] [PubMed] [Google Scholar]
- Sanchez PL, Morinigo JL, Pabon P, Martin F, Piedra I, Palacios IF, and Martin-Luengo C (2004). Prognostic relations between inflammatory markers and mortality in diabetic patients with non-ST elevation acute coronary syndrome. Heart 90, 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjeevi N, Lipsky LM, and Nansel TR (2018). Cardiovascular Biomarkers in Association with Dietary Intake in a Longitudinal Study of Youth with Type 1 Diabetes. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumberg DA, Glynn RJ, Jenkins AJ, Lyons TJ, Rifai N, Manson JE, Ridker PM, and Nathan DM (2005). Effect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the diabetes control and complications trial. Circulation 111, 2446–2453. [DOI] [PubMed] [Google Scholar]
- Schmidt AM (2019). Diabetes Mellitus and Cardiovascular Disease. Arterioscler Thromb Vasc Biol 39, 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, and Stehouwer CD (2005). Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes--the EURODIAB Prospective Complications Study. Diabetologia 48, 370–378. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Szarek M, Bittner VA, Bhatt DL, Diaz R, Goodman SG, Jukema JW, Loy M, Manvelian G, Pordy R, et al. (2021). Relation of Lipoprotein(a) Levels to Incident Type 2 Diabetes and Modification by Alirocumab Treatment. Diabetes Care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R, Donoghoe M, Watts GF, O’Brien R, Pardy C, Taskinen MR, Davis TM, Colman PG, Manning P, Fulcher G, et al. (2011). Impact of metabolic syndrome and its components on cardiovascular disease event rates in 4900 patients with type 2 diabetes assigned to placebo in the FIELD randomised trial. Cardiovasc Diabetol 10, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R, O’Brien R, Fulcher G, Pardy C, D’Emden M, Tse D, Taskinen MR, Ehnholm C, and Keech A (2009). Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care 32, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RA, Freitag DF, Li L, Chu AY, Surendran P, Young R, Grarup N, Stancakova A, Chen Y, Varga TV, et al. (2016). A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease. Sci Transl Med 8, 341ra376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatus L, Lopez-Diez R, Egana-Gorrono L, Liu J, Hu J, Daffu G, Li Q, Rahman K, Vengrenyuk Y, Barrett TJ, et al. (2020). RAGE impairs murine diabetic atherosclerosis regression and implicates IRF7 in macrophage inflammation and cholesterol metabolism. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah HS, Morieri ML, Marcovina SM, Sigal RJ, Gerstein HC, Wagner MJ, Motsinger-Reif AA, Buse JB, Kraft P, Mychaleckyj JC, et al. (2018). Modulation of GLP-1 Levels by a Genetic Variant That Regulates the Cardiovascular Effects of Intensive Glycemic Control in ACCORD. Diabetes Care 41, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MS, and Brownlee M (2016). Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ Res 118, 1808–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Choi JSY, Stefanovic N, Al-Sharea A, Simpson DS, Mukhamedova N, Jandeleit-Dahm K, Murphy AJ, Sviridov D, Vince JE, et al. (2020). Specific NLRP3 Inhibition Protects Against Diabetes-Associated Atherosclerosis. Diabetes. [DOI] [PubMed] [Google Scholar]
- Shim J, Al-Mashhadi RH, Sorensen CB, and Bentzon JF (2016). Large animal models of atherosclerosis--new tools for persistent problems in cardiovascular medicine. J Pathol 238, 257–266. [DOI] [PubMed] [Google Scholar]
- Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å, Bouchard C, Carlsson B, Karason K, Lönroth H, et al. (2014). Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. Jama 311, 2297–2304. [DOI] [PubMed] [Google Scholar]
- Snell-Bergeon JK, Roman R, Rodbard D, Garg S, Maahs DM, Schauer IE, Bergman BC, Kinney GL, and Rewers M (2010a). Glycaemic variability is associated with coronary artery calcium in men with Type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes study. Diabet Med 27, 1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell-Bergeon JK, West NA, Mayer-Davis EJ, Liese AD, Marcovina SM, D’Agostino RB Jr., Hamman RF, and Dabelea D (2010b). Inflammatory markers are increased in youth with type 1 diabetes: the SEARCH Case-Control study. J Clin Endocrinol Metab 95, 2868–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel BE, Taatjes DJ, and Schneider DJ (2003). Intramural plasminogen activator inhibitor type-1 and coronary atherosclerosis. Arterioscler Thromb Vasc Biol 23, 1979–1989. [DOI] [PubMed] [Google Scholar]
- Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, and Colhoun HM (2006). High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 29, 798–804. [DOI] [PubMed] [Google Scholar]
- Soinio M, Marniemi J, Laakso M, Lehto S, and Rönnemaa T (2006). High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care 29, 329–333. [DOI] [PubMed] [Google Scholar]
- Sondergaard E, Johansen RF, Jensen MD, and Nielsen S (2017). Postprandial VLDL-TG metabolism in type 2 diabetes. Metabolism 75, 25–35. [DOI] [PubMed] [Google Scholar]
- Sontag TJ, Krishack PA, Lukens JR, Bhanvadia CV, Getz GS, and Reardon CA (2014). Apolipoprotein A-I protection against atherosclerosis is dependent on genetic background. Arterioscler Thromb Vasc Biol 34, 262–269. [DOI] [PubMed] [Google Scholar]
- Soria-Florido MT, Schroder H, Grau M, Fito M, and Lassale C (2020). High density lipoprotein functionality and cardiovascular events and mortality: A systematic review and meta-analysis. Atherosclerosis 302, 36–42. [DOI] [PubMed] [Google Scholar]
- Sousa GR, Pober D, Galderisi A, Lv H, Yu L, Pereira AC, Doria A, Kosiborod M, and Lipes MA (2019). Glycemic Control, Cardiac Autoimmunity, and Long-Term Risk of Cardiovascular Disease in Type 1 Diabetes Mellitus. Circulation 139, 730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreejit G, Abdel-Latif A, Athmanathan B, Annabathula R, Dhyani A, Noothi SK, Quaife-Ryan GA, Al-Sharea A, Pernes G, Dragoljevic D, et al. (2020). Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction. Circulation 141, 1080–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, and Fruchart JC (1998). Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98, 2088–2093. [DOI] [PubMed] [Google Scholar]
- Stehouwer CD, Henry RM, and Ferreira I (2008). Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia 51, 527–539. [DOI] [PubMed] [Google Scholar]
- Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, and Holman RR (2004). Risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care 27, 201–207. [DOI] [PubMed] [Google Scholar]
- Sturek M, Alloosh M, and Sellke FW (2020). Swine Disease Models for Optimal Vascular Engineering. Annu Rev Biomed Eng 22, 25–49. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A 3rd, Kirk EA, O’Brien KD, and Chait A (2008). Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 28, 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima N, Kurata H, Nakaya N, Mizuno K, Ohashi Y, Kushiro T, Teramoto T, Uchiyama S, and Nakamura H (2008). Pravastatin reduces the risk for cardiovascular disease in Japanese hypercholesterolemic patients with impaired fasting glucose or diabetes: diabetes subanalysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) Study. Atherosclerosis 199, 455–462. [DOI] [PubMed] [Google Scholar]
- Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clement K, Holvoet P, et al. (2007). Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol 27, 2691–2698. [DOI] [PubMed] [Google Scholar]
- Tall AR, Yvan-Charvet L, Westerterp M, and Murphy AJ (2012). Cholesterol efflux: a novel regulator of myelopoiesis and atherogenesis. Arterioscler Thromb Vasc Biol 32, 2547–2552. [DOI] [PubMed] [Google Scholar]
- Tang C, Kanter JE, Bornfeldt KE, Leboeuf RC, and Oram JF (2010). Diabetes reduces the cholesterol exporter ABCA1 in mouse macrophages and kidneys. J Lipid Res 51, 1719–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock LR, and Chait A (2004). Lipoprotein-matrix interactions in macrovascular disease in diabetes. Front Biosci 9, 1728–1742. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. (2019). Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med 381, 2497–2505. [DOI] [PubMed] [Google Scholar]
- Tavori H, Su YR, Yancey PG, Giunzioni I, Wilhelm AJ, Blakemore JL, Zabalawi M, Linton MF, Sorci-Thomas MG, and Fazio S (2015). Macrophage apoAI protects against dyslipidemia-induced dermatitis and atherosclerosis without affecting HDL. J Lipid Res 56, 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease Investigators, Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, and Sleight P (2008). Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 372, 1174–1183. [DOI] [PubMed] [Google Scholar]
- Thomopoulos C, Bazoukis G, Ilias I, Tsioufis C, and Makris T (2019). Effects of glucose-lowering on outcome incidence in diabetes mellitus and the modulating role of blood pressure and other clinical variables: overview, meta-analysis of randomized trials. J Hypertens 37, 1939–1949. [DOI] [PubMed] [Google Scholar]
- Thomopoulos C, Parati G, and Zanchetti A (2014). Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk--overview and meta-analyses of randomized trials. J Hypertens 32, 2305–2314. [DOI] [PubMed] [Google Scholar]
- Thomopoulos C, Parati G, and Zanchetti A (2017). Effects of blood-pressure-lowering treatment on outcome incidence in hypertension: 10 - Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta-analyses of randomized trials. J Hypertens 35, 922–944. [DOI] [PubMed] [Google Scholar]
- Tikkanen-Dolenc H, Wadén J, Forsblom C, Harjutsalo V, Thorn LM, Saraheimo M, Elonen N, Rosengård-Bärlund M, Gordin D, Tikkanen HO, et al. (2017). Frequent and intensive physical activity reduces risk of cardiovascular events in type 1 diabetes. Diabetologia 60, 574–580. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group (1998a). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 837–853. [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group (1998b). Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317, 703–713. [PMC free article] [PubMed] [Google Scholar]
- Vaccaro O, Masulli M, Nicolucci A, Bonora E, Del Prato S, Maggioni AP, Rivellese AA, Squatrito S, Giorda CB, Sesti G, et al. (2017). Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol 5, 887–897. [DOI] [PubMed] [Google Scholar]
- Van Horn L, Carson JA, Appel LJ, Burke LE, Economos C, Karmally W, Lancaster K, Lichtenstein AH, Johnson RK, Thomas RJ, et al. (2016). Recommended Dietary Pattern to Achieve Adherence to the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines: A Scientific Statement From the American Heart Association. Circulation 134, e505–e529. [DOI] [PubMed] [Google Scholar]
- Vasan SK, Osmond C, Canoy D, Christodoulides C, Neville MJ, Di Gravio C, Fall CHD, and Karpe F (2018). Comparison of regional fat measurements by dual-energy X-ray absorptiometry and conventional anthropometry and their association with markers of diabetes and cardiovascular disease risk. Int J Obes (Lond) 42, 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantham S, Noh H, Ananthakrishnan R, Son N, Hallam K, Hu Y, Yu S, Shen X, Rosario R, Lu Y, et al. (2011). Human aldose reductase expression accelerates atherosclerosis in diabetic apolipoprotein E−/− mice. Arterioscler Thromb Vasc Biol 31, 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, and Goldberg IJ (2005). Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest 115, 2434–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vistisen D, Andersen GS, Hansen CS, Hulman A, Henriksen JE, Bech-Nielsen H, and Jorgensen ME (2016). Prediction of First Cardiovascular Disease Event in Type 1 Diabetes Mellitus: The Steno Type 1 Risk Engine. Circulation 133, 1058–1066. [DOI] [PubMed] [Google Scholar]
- Wadwa RP, Kinney GL, Maahs DM, Snell-Bergeon J, Hokanson JE, Garg SK, Eckel RH, and Rewers M (2005). Awareness and treatment of dyslipidemia in young adults with type 1 diabetes. Diabetes Care 28, 1051–1056. [DOI] [PubMed] [Google Scholar]
- Wall VZ, Barnhart S, Kanter JE, Kramer F, Shimizu-Albergine M, Adhikari N, Wight TN, Hall JL, and Bornfeldt KE (2018). Smooth muscle glucose metabolism promotes monocyte recruitment and atherosclerosis in a mouse model of metabolic syndrome. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NC, Vickneswaran S, and Watts GF (2021). Lipoprotein (a) and diabetes mellitus: causes and consequences. Curr Opin Endocrinol Diabetes Obes 28, 181–187. [DOI] [PubMed] [Google Scholar]
- Weinstein MM, Yin L, Tu Y, Wang X, Wu X, Castellani LW, Walzem RL, Lusis AJ, Fong LG, Beigneux AP, et al. (2010). Chylomicronemia elicits atherosclerosis in mice--brief report. Arterioscler Thromb Vasc Biol 30, 20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, De Block C, Schwartzbard AZ, and Newman JD (2020). Diabetic Agents, From Metformin to SGLT2 Inhibitors and GLP1 Receptor Agonists: JACC Focus Seminar. J Am Coll Cardiol 75, 1956–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke F, Yuan C, Oka K, Chan L, Hu Y, Barnhart S, Bornfeldt KE, Goldberg IJ, and Fisher EA (2015). Effects of High Fat Feeding and Diabetes on Regression of Atherosclerosis Induced by Low-Density Lipoprotein Receptor Gene Therapy in LDL Receptor-Deficient Mice. PLoS One 10, e0128996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, and Orringer CE (2019). Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol 13, 374–392. [DOI] [PubMed] [Google Scholar]
- Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, et al. (2013). Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard DL, Suarez L, and Barrett-Connor E (1983). The sex differential in mortality from all causes and ischemic heart disease. Am J Epidemiol 117, 165–172. [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. (2019). Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 380, 347–357. [DOI] [PubMed] [Google Scholar]
- Wu KK, and Huan Y (2007). Diabetic atherosclerosis mouse models. Atherosclerosis 191, 241–249. [DOI] [PubMed] [Google Scholar]
- Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, and Virmani R (2017). Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arterioscler Thromb Vasc Biol 37, 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. (2007). Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369, 1090–1098. [DOI] [PubMed] [Google Scholar]
- Young DR, Hivert MF, Alhassan S, Camhi SM, Ferguson JF, Katzmarzyk PT, Lewis CE, Owen N, Perry CK, Siddique J, et al. (2016). Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation 134, e262–279. [DOI] [PubMed] [Google Scholar]
- Younis NN, Soran H, Pemberton P, Charlton-Menys V, Elseweidy MM, and Durrington PN (2013). Small dense LDL is more susceptible to glycation than more buoyant LDL in Type 2 diabetes. Clin Sci (Lond) 124, 343–349. [DOI] [PubMed] [Google Scholar]
- Yu T, Sungelo MJ, Goldberg IJ, Wang H, and Eckel RH (2017). Streptozotocin-Treated High Fat Fed Mice: A New Type 2 Diabetes Model Used to Study Canagliflozin-Induced Alterations in Lipids and Lipoproteins. Horm Metab Res 49, 400–406. [DOI] [PubMed] [Google Scholar]
- Yuan C, Hu J, Parathath S, Grauer L, Cassella CB, Bagdasarov S, Goldberg IJ, Ramasamy R, and Fisher EA (2018). Human Aldose Reductase Expression Prevents Atherosclerosis Regression in Diabetic Mice. Diabetes 67, 1880–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, et al. (2008). Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 359, 1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, et al. (2010). ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328, 1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchetti A, Hansson L, Clement D, Elmfeldt D, Julius S, Rosenthal T, Waeber B, Wedel H, and Group HOTS (2003). Benefits and risks of more intensive blood pressure lowering in hypertensive patients of the HOT study with different risk profiles: does a J-shaped curve exist in smokers? J Hypertens 21, 797–804. [DOI] [PubMed] [Google Scholar]
- Zhang X, Qi R, Xian X, Yang F, Blackstein M, Deng X, Fan J, Ross C, Karasinska J, Hayden MR, et al. (2008). Spontaneous atherosclerosis in aged lipoprotein lipase-deficient mice with severe hypertriglyceridemia on a normal chow diet. Circ Res 102, 250–256. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Liu L, Zanchetti A, and Group FS (2011). Is a systolic blood pressure target <140 mmHg indicated in all hypertensives? Subgroup analyses of findings from the randomized FEVER trial. Eur Heart J 32, 1500–1508. [DOI] [PubMed] [Google Scholar]
- Zhao B, Wu Q, Wang L, Liao C, Dong Y, Xu J, Wei Y, and Zhang W (2020). Pros and Cons of Aspirin for the Primary Prevention of Cardiovascular Events: A Secondary Study of Trial Sequential Analysis. Front Pharmacol 11, 592116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. (2015). Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 373, 2117–2128. [DOI] [PubMed] [Google Scholar]
- Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, Arima H, Monaghan H, Joshi R, Colagiuri S, et al. (2014). Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 371, 1392–1406. [DOI] [PubMed] [Google Scholar]


