ABSTRACT
STUDY QUESTION
Are serum omega-3 and omega-6 essential fatty acid concentrations associated with the probability of conceiving?
SUMMARY ANSWER
There is no strong association between serum concentrations of omega-3 and omega-6 fatty acids and the probability of conceiving naturally.
WHAT IS KNOWN ALREADY
Omega-3 and omega-6 fatty acid serum concentrations have been shown to play an important role in reproduction in animal models, while conflicting results have been reported in human studies of infertile women. It is unknown to what extent omega fatty acid serum concentrations impact natural fertility.
STUDY DESIGN, SIZE, DURATION
A nested, case–control study was conducted consisting of 200 participants [fertile: conceived within 3 cycles of attempt (n = 50), subfertile: conceived within 4 and 12 cycles of attempt (n = 100) and infertile: did not conceive within 12 cycles of attempt (n = 50)] randomly selected from the Time to Conceive cohort, a prospective time-to-pregnancy study (2008 to 2015).
PARTICIPANTS/MATERIALS, SETTING, METHODS
In the Time to Conceive study, women aged 30–44 years who were trying to conceive for <3 months and had no history of infertility were recruited and followed until the end of their pregnancy or ~1 year of pregnancy attempt. For this study, serum collected early in the woman’s pregnancy attempt was analysed for anti-Müllerian hormone (AMH) and omega-3 and omega-6 fatty acid concentrations by liquid chromatography-mass spectrometry. The primary outcome was a positive home pregnancy test. The secondary outcomes were miscarriage and serum AMH level. A discrete-time Cox proportional hazards model was used to estimate the fecundability ratio. The odds ratios for miscarriage were calculated using logistic regression. The association between serum omega fatty acid concentrations and AMH level (natural log transformed) was analysed using Pearson’s Correlation.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 200 women provided 1321 cycles for analysis.
Mean omega-3, omega-6 and omega-6:omega-3 ratios did not significantly differ between the fertile, subfertile and infertile groups. There were no associations (all fecundability ratios ~1.0) between pregnancy and individual omega-3 fatty acid concentrations, including alpha-linolenic acid, eicosapentaenoic acid and docosahexaenoic acid, or omega-6 fatty acids, including linoleic acid (LA), dihommo-gamma linolenic acid and arachidonic acid. There was no significant association between any individual omega fatty acid serum concentration and the age-adjusted odds of miscarriage. No association was found between any serum omega fatty acid concentration and AMH.
LIMITATIONS, REASONS FOR CAUTION
This study is limited by the sample size. Omega-3 and omega-6 fatty acid concentrations were derived from serum provided at a single timepoint in the first cycle of enrollment. Serum concentrations may therefore not be representative of all critical timepoints in the menstrual cycle or throughout their attempts to conceive. Additionally, women enrolled in this study were 30 years of age and older, and therefore the findings may not apply to younger women.
WIDER IMPLICATIONS OF THE FINDINGS
These data would suggest that omega-3 and omega-6 serum levels are not associated with natural fertility or risk of miscarriage. However, due to the above-mentioned limitations, future investigation is still needed to determine whether omega-3 fatty acid supplementation may benefit women planning to conceive naturally.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the Division of Reproductive Endocrinology and Infertility at the University of North Carolina at Chapel Hill, by the NIH/NICHD (R21 HD060229-01 and R01 HD067683-01) and, in part, by the Intramural Research Program of the National Institute of Environmental Health Sciences (Z01ES103333). Dr. Jukic received vitamin D supplements for a research study from Theralogix, Inc. The authors have no other conflicts of interest to disclose.
TRIAL REGISTRATION NUMBER
N/A
Keywords: omega-3 fatty acids, fecundability, natural fertility, time to pregnancy, polyunsaturated fatty acids, fecundity, miscarriage, ovarian reserve
Introduction
Omega-3 and omega-6 fatty acids are polyunsaturated fatty acids (PUFA) that perform essential roles in human physiology. Because the human body cannot produce them, these omega fatty acids are referred to as ‘essential fats’ that must be consumed from the diet. The most common omega-3 fatty acids are alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and the most common omega-6 fatty acids are linoleic acid (LA), dihommo-gamma linolenic acid (DGLA) and arachidonic acid (AA). Both omega-3 and omega-6 fatty acids are essential; however, the western diet contains far more omega-6 fatty acids than necessary (Simopoulos et al., 2002). The recommended ratio of omega-6 to omega-3 fatty acids in the diet is 4:1 or less; however, the western diet has a ratio between 10:1 and 50:1 (Simopoulos et al., 2002). Studies have found that a lower ratio of omega-6 to omega-3 fatty acid intake improves health and reduces the risk of many chronic diseases (Simopoulos et al., 2002; Oscarsson et al., 2017). Omega-3 PUFAs compete for the enzymes involved in the omega-6 PUFA pathway, creating a system where increased dietary intake of omega-3 PUFAs may inhibit the omega-6 PUFA pathway and vice versa (Simopoulous et al., 2002).
Omega PUFAs have been shown to play an important role in reproductive function through myriad pathways (Wathes et al., 2007). PUFAs are sources of cholesterol, the precursor for all steroid hormones, and may therefore have direct and indirect effects on steroidogenesis (Wathes et al., 2007). PUFAs also serve as critical precursors for prostaglandins, which may impact a multitude of reproductive processes including, but not limited to, steroidogenesis, follicular maturation, rupture of the follicle in ovulation, fallopian tube motility, implantation and continued pregnancy (Clark and Myatt, 2008). In animal models, dietary supplementation with omega fatty acids not only impacts prostaglandin and ovarian steroid synthesis, but also improves folliculogenesis, oocyte maturation and ovulation, embryo quality and implantation, placentation and the female reproductive lifespan (Downs et al., 2009; Sturmey et al., 2009; Norwitz et al., 2001; Leghi et al., 2016; Meher et al., 2013; Nehra et al., 2012; Rodriguez et al., 2018; Gokuldas et al., 2018; Ponter et al., 2012; Elis et al., 2016).
Research in humans in natural fertility is inconclusive and currently limited to studies on dietary fatty acid intake alone (Mumford et al., 2016; Chavarro et al., 2007; Wise et al., 2018; Al-Safi et al., 2016; Chiu et al., 2018a). Data on the impact of omega fatty acid serum concentrations on natural fertility in women do not exist. Although there are studies that report on omega PUFA intake and serum concentration in the infertile population among women undergoing assisted reproductive technologies (ART), these studies have conflicting findings (Nouri et al., 2016; Vujkovic et al., 2010; Moran et al., 2016; Jungheim et al., 2011; and 2013; Chiu et al., 2018a). Given that the current literature investigating associations between serum omega fatty acid concentration and reproduction in humans is limited to the infertile population and currently inconclusive, the primary aim of our study was to determine the association between serum omega-3 and omega-6 fatty acid concentrations and natural fertility. The secondary aims of our study were to evaluate the relationship between serum omega fatty acid concentrations and risk of miscarriage, as well as ovarian reserve, as measured by anti-Müllerian hormone (AMH) level.
Materials and Methods
This was a case–control study nested in Time to Conceive (TTC), a prospective, time-to-pregnancy cohort study, which was conducted from 2008 to August 2015 (Steiner et al., 2017). TTC enrolled 820 women, between the ages of 30 and 44, who were trying to conceive for <3 months and had no history of or risk factors for infertility. At enrollment, participants completed a questionnaire on medical and reproductive history, habits and medication use. In the first or second menstrual cycle following enrollment, non-fasting women provided serum on cycle Day 2, 3 or 4, which was stored at −20°C. Serum samples were analysed for AMH using the Ansh assay. While trying to conceive, women kept a diary in which they recorded pregnancy test results. Women were provided free pregnancy tests and had standardized pregnancy testing instructions. Women, who reported a positive pregnancy test, were offered a first trimester ultrasound. Pregnancy outcomes, including miscarriage and livebirth, were determined. In the TTC study of 820 women enrolled, 770 women attended a study visit. Following this, 20 women were excluded due to being lost to follow-up (n = 13) or because the duration of the pregnancy attempt was unable to be determined (n = 7). Following participation, women were censored if they withdrew from the study (n = 37), started fertility treatment (n = 47) or were lost to follow-up (n = 61), totaling 145 women censored. Of the remaining 605 women, 264 conceived in the first 3 cycles of the pregnancy attempt, 258 conceived in cycles 4–12 of the pregnancy attempt and 83 did not conceive within 12 cycles of the pregnancy attempt.
For this nested, case–control study, 50 TTC participants who conceived in the first three cycles of pregnancy attempt were randomly selected from the TTC cohort of 605 women and defined as the fertile group. In addition, 100 TTC participants who conceived in cycles 4–12 of the pregnancy attempt were randomly selected from the TTC cohort and defined as the subfertile group. Finally, 50 TTC participants who did not conceive within 12 cycles of pregnancy attempt were randomly selected from TTC cohort and defined as the infertile group. Women were selected randomly from within their strata using Microsoft Excel’s random number generator function.
Early follicular phase serum samples from these 200 women were sent to the Metabolomics Facility at Washington University, where they were analysed for omega-3 fatty acids ALA, EPA and DHA, and omega-6 fatty acids LA, DGLA and AA using liquid chromatography-mass spectrometry (LC-MS) to obtain peak area ratio, as previously described (Jiang et al., 2011). The omega fatty acids were measured in the specific lipid fraction of total free fatty acids.
Statistical analysis
The primary outcome measure for this nested case–control study was a pregnancy, defined as a positive home pregnancy test. Secondary outcomes were miscarriage and serum AMH level, a biomarker of ovarian reserve. Bivariable analyses comparing participant characteristics and omega serum concentrations between fertile, subfertile and infertile women were performed using chi-squared test for categorical variables and analysis of variance (ANOVA) for continuous variables. We used a discrete-time Cox proportional hazards model to estimate the fecundability ratio (FR). The FR for exposures modeled as continuous variables is the relative probability of pregnancy for a unit change in the exposure. In such models, an FR of <1 suggests reduced fecundability as exposure levels increase. Time was menstrual cycles at risk of pregnancy (pregnancy attempt cycle). A woman’s current estimated pregnancy attempt cycle was determined from the time a woman started trying to conceive, not from the time of enrollment. The attempt cycle at enrollment was defined by the pregnancy attempt cycle (usually cycle 1, 2, or 3) in which the woman began participation in TTC. Women were censored at the time they withdrew, started fertility medications or were lost to follow-up. Thus, cycles from enrollment to censoring were included in the analysis.
We used inverse-probability weighting to account for the probability of being in the analytical sample. This weight was based on the following two probabilities: the probability of conceiving in a given number of cycles and the probability of being randomly sampled given the number of cycles until conception. For this analysis, women were divided into three strata of interest: conception in cycles 1–3, conception in cycles 4–12 or trying to conceive for >12 cycles. The probability of a woman being in each of these three strata (Pr(Stratum = i)) was calculated using life-table methods. For example, the probability of conception in cycles 1–3 (Pr(Stratum = 1)) was calculated as:
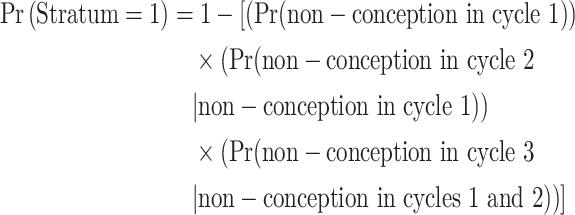 |
where these cycle-specific probabilities were from the entire TTC study population (see Table I of Steiner et al., 2017).
Table I.
Participant characteristics comparing fertile, subfertile and infertile women.
| Fertile pregnant 1–3 months (n = 50) | Subfertile pregnant 4–12 months (n = 100) | Infertile (n = 50) | P-value | |
|---|---|---|---|---|
| Mean ± SD or n (%) | ||||
| Age (years) | 32.42 ± 2.82 | 33.00 ± 2.92 | 34.64 ± 3.68 | 0.001 |
| 30–34 | 38 (76.00%) | 77 (77.00%) | 25 (50.00%) | |
| 35–37 | 9 (18.00%) | 14 (14.00%) | 15 (30.00%) | |
| 38–44 | 3 (6.00%) | 9 (9.00%) | 10 (20.00%) | |
| BMI (kg/m2) | 24.55 ± 4.88 | 24.71 ± 5.09 | 24.99 ± 5.55 | 0.909 |
| <18.50 | 2 (4.00%) | 0 (0%) | 1 (2.00%) | |
| 18.50–24.99 | 33 (66.00%) | 70 (70.00%) | 25 (50.00%) | |
| 25.00–29.99 | 9 (18.00%) | 15 (15.00%) | 11 (22.00%) | |
| ≥30 | 8 (16.00%) | 20 (20.00%) | 8 (16.00%) | |
| AMH (ng/ml) | 5.70 ± 5.04 | 3.93 ± 3.44 | 3.14 ± 2.84 | 0.003 |
| Previous Pregnancy | 22 (44.00%) | 45 (45.00%) | 20 (40.00%) | 0.841 |
| Regular periods | 40 (80.00%) | 84 (84.00%) | 45 (90.00%) | 0.378 |
| Menstrual cycle length (days) | 29.96 ± 4.36 | 29.29 ± 7.04 | 28.47 ± 2.68 | 0.412 |
| Race | 0.183 | |||
| White | 40 (80.00%) | 80 (80.00%) | 31 (62.00%) | |
| Non-White | 10 (20%) | 20 (20%) | 19 (38%) | |
| Education level | 0.205 | |||
| College or less | 11 (22.00%) | 25 (25.00%) | 17 (34.00%) | |
| Some graduate school or more | 39 (78.00%) | 75 (75.00%) | 33 (66.00%) | |
| Married | 49 (98.00%) | 95 (95.00%) | 39 (78.00%) | 0.002 |
| Smoking status | 0.117 | |||
| Never | 42 (84.00%) | 72 (72.00%) | 42 (84.00%) | |
| Past | 7 (14.00%) | 27 (27.00%) | 6 (12.00%) | |
| Current | 1 (2.00%) | 1 (1.00%) | 2 (4.00%) | |
| History of depression | 11 (22.00%) | 37 (37.00%) | 13 (26.00%) | 0.124 |
| Hormonal contraceptive use in previous 3 months | 10 (20.00%) | 29 (29.00%) | 11 (22.00%) | 0.415 |
| Hormonal contraceptive use in previous year | 23 (46.00%) | 45 (45.00%) | 17 (34.00%) | 0.371 |
| Intercourse frequency per week | 2.17 ± 1.22 | 2.08 ± 1.40 | 2.14 ± 1.28 | 0.914 |
| Intercourse frequency during fertile window (percentage of fertile days)* | 47.85% ± 23.07% | 43.26% ± 19.55% | 38.89% ± 25.00% | 0.468 |
| Hours of vigorous exercise in the past month* | 0.700 | |||
| 0 h | 5 (10.00%) | 13 (13.00%) | 5 (10.00%) | |
| <1 h | 7 (14.00%) | 23 (23.00%) | 15 (30.00%) | |
| 1–3 h | 12 (24.00%) | 36 (36.00%) | 17 (34.00%) | |
| 4–7 h | 12 (24.00%) | 21 (21.00%) | 8 (16.00%) | |
| >7 h | 5 (10.00%) | 6 (6.00%) | 4 (8.00%) | |
| Unsure | 9 (18.00%) | 1 (1.00%) | 1 (2.00%) | |
| Number of caffeinated beverages per day* | 1.27 ± 0.78 | 1.13 ± 0.99 | 1.14 ± 0.80 | 0.72 |
| Number of alcoholic beverages per month* | 8.00 ± 9.13) | 7.77 ± 9.49 | 5.06 ± 7.08 | 0.169 |
AMH, anti-Müllerian hormone; ANOVA, analysis of variance; BMI, body mass index; SD, standard deviation.
Chi-squared test for categorical variables, ANOVA for continuous variables.
*As reported in the first cycle of enrollment.
Probabilities for the other two strata were calculated analogously. The probability of being randomly sampled (Pr(Sampled|Stratum = i)) from within the first two strata was calculated as the number of women drawn from that stratum divided by the number of women who conceived in that stratum. The probability for selection from the third stratum was calculated as the number of women selected from that stratum divided by the number of women whose attempt lasted longer than 12 cycles. The final analysis weight was defined as:
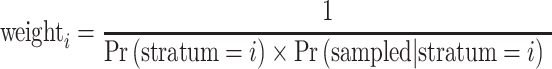 |
We initially evaluated the distribution of serum omega-3 and omega-6 fatty acids in both tertiles and quartiles but we did not see any evidence of non-linearity and so the linear parameterization was chosen. All omega fatty acid variables were multiplied by 100 except for LA, which is present at a relatively higher concentration than the other measured omega fatty acids. Other multiplications were explored and multiplying by 100 led to the most stable estimates; therefore for LA, the FRs represent a 1 μmol/ml increase in concentration. All other omega fatty acid variables were multiplied by 100 and therefore the FRs represent a 1/100 μmol/ml change in concentration.
Covariates found to be associated with omega levels in bivariate analyses were added to the model and maintained if they were clinically significant or significantly impacted the FR by at least 10%. The final model included only age, modeled categorically (<35 years, 35–37 years, ≥38 years).
Bivariate analyses of serum omega fatty acid concentrations by pregnancy outcome (live birth versus miscarriage) were performed using Student’s t-test. The odds of miscarriage by serum omega fatty acid concentrations were calculated using multiple logistic regression modeling adjusting for age, modeled as a categorical variable (<35, 35–37 and ≥38 years, respectively). The association between serum omega fatty acid concentrations and AMH level (natural log transformed) was analysed using Pearson’s Correlation. P < 0.05 was considered statistically significant. All statistical analyses were performed using Stata 15.0.
Results
A total of 200 women provided 1321 cycles for analysis. The mean age of participants was 33.27 ± 3.20 years. Most women were Caucasian, well-educated, married, non-smokers and of normal weight. Nearly half of the participants had conceived previously (Table I). Mean omega fatty acid serum concentrations by participant characteristics are presented in Supplemental Table SI.
Comparisons between the fertile (women who conceived within 3 cycles of attempt), subfertile (women who conceived within 4–12 cycles of attempt) and infertile (women who did not conceive after 12 cycles of attempt) groups are presented in Table I. Female age was lower and AMH was higher in the fertile group compared to the other two groups. Fertile and subfertile women were more likely to be married to their partners than infertile women.
In analyses comparing serum omega fatty acid concentrations between groups, mean omega-3 or omega-6 serum concentrations did not significantly differ between fertile, subfertile or infertile women (Table II). Age-adjusted fecundability ratios by serum essential fatty acid concentration are presented in Table II. As adjustment for AMH level did not substantially change these estimates, and it was not associated with the probability of pregnancy in the TTC cohort, it was not included in the model (Steiner et al., 2017). There was no strong nor statistically significant associations between serum concentrations of omega-3 or omega-6 fatty acids and the probability of conceiving in a given menstrual cycle.
Table II.
Omega fatty acid serum concentrations, presented with the mean ± SD, in fertile, subfertile and infertile women with age-adjusted fecundability ratios.
| Omega fatty acid | Fertile pregnant 1–3 months (n = 50) | Subfertile pregnant 4–12 months (n = 100) | Infertile (n = 50) | P-value* | Age-adjusted fecundability ratio** (95% CI) |
|---|---|---|---|---|---|
| Omega-3 | |||||
| ALA | 0.054 ± 0.045 | 0.043 ± 0.031 | 0.050 ± 0.043 | 0.201 | 0.98 (0.96, 1.01) |
| EPA | 0.004 ± 0.003 | 0.005 ± 0.037 | 0.004 ± 0.029 | 0.363 | 0.88 (0.75, 1.03) |
| DHA | 0.023 ± 0.014 | 0.025 ± 0.016 | 0.024 ± 0.012 | 0.829 | 0.95 (0.79, 1.14) |
| Omega-3 PUFAa | 0.082 ± 0.056 | 0.073 ± 0.043 | 0.080 ± 0.53 | 0.507 | 0.99 (0.96, 1.01) |
| Long-chain omega-3 PUFAb | 0.028 ± 0.017 | 0.030 ± 0.019 | 0.028 ± 0.015 | 0.737 | 0.97 (0.91, 1.04) |
| Omega-6 | |||||
| LA | 0.595 ± 0.468 | 0.522 ± 0.371 | 0.605 ± 0.472 | 0.423 | 0.85 (0.66, 1.09) |
| DGLA | 0.007 ± 0.004 | 0.007 ± 0.004 | 0.007 ± 0.003 | 0.958 | 1.01 (0.73, 1.40) |
| AA | 0.046 ± 0.018 | 0.048 ± 0.018 | 0.048 ± 0.015 | 0.798 | 0.97 (0.91, 1.03) |
| Omega-6 PUFAc | 0.648 ± 0.486 | 0.577 ± 0.386 | 0.671 ± 0.484 | 0.402 | 0.86 (0.69, 1.09) |
| Ratio of omega-6 to omega-3 | 8.075 ± 2.283 | 7.86 ± 2.068 | 8.500 ± 2.620 | 0.277 | 1.01 (0.97, 1.06) |
| Ratio of LA to ALA | 12.122 ± 3.246 | 13.125 ± 4.395 | 12.816 ± 3.812 | 0.348 | 1.04 (0.79, 1.36) |
| Total PUFA | 0.730 ± 0.538 | 0.650 ± 0.426 | 0.751 ± 0.533 | 0.411 | 0.87 (0.71, 1.08) |
*ANOVA.
**For LA, Omega-6 PUFA and Total PUFA the fecundability ratio is for a 1 μmol/ml increase in concentration. All other omega fatty acid variables were multiplied by 100 and therefore the fecundability ratio represents a 1/100 μmol/ml change in concentration.
aSum of ALA, EPA, DHA.
bSum of EPA, DHA.
cSum of LA, DGLA, AA.
AA, arachidonic acid; ALA, alpha linolenic acid; ANOVA, analysis of variance; CI, confidence interval; DGLA, dihommo-gamma linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; PUFA, polyunsaturated fatty acids.
Because of using inverse probability weighting, the 95% CIs were calculated using robust variance.
Bivariate analyses comparing serum omega fatty acid concentration and pregnancy outcome (live birth versus miscarriage) are presented in Table III. Women who miscarried had significantly higher EPA concentrations (0.06 ± 0.005 μmol/ml vs 0.04 ± 0.003 μmol/ml [mean ± standard deviation]); however, after adjusting for age and stratum, the risk of miscarriage did not differ by EPA concentration (Table III).
Table III.
Omega fatty acid serum concentrations, presented as mean ± SD, by pregnancy outcome, with age-adjusted OR for risk of miscarriage.
| Omega fatty acid | Miscarriage (n = 36) | Live birth (n = 114) | P-value* | Age-adjusted OR** (95% CI) |
|---|---|---|---|---|
| Omega-3 | ||||
| ALA | 0.047 ± 0.034 | 0.048 ± 0.038 | 0.930 | 1.01 (0.92, 1.12) |
| EPA | 0.006 ± 0.005 | 0.004 ± 0.003 | 0.030 | 1.32 (1.00, 1.47) |
| DHA | 0.026 ± 0.017 | 0.024 ± 0.015 | 0.502 | 1.11 (0.87, 1.41) |
| Omega-6 | ||||
| LA | 0.554 ± 0.347 | 0.560 ± 0.429 | 0.942 | 1.09 (0.42, 2.80) |
| DGLA | 0.007 ± 0.004 | 0.007 ± 0.003 | 0.715 | 1.42 (0.57, 3.5) |
| AA | 0.049 ± 0.016 | 0.047 ± 0.019 | 0.658 | 1.09 (0.89, 1.32) |
| Ratio of LA to ALA | 13.270 ± 5.137 | 12.710 ± 3.787 | 0.481 | 1.02 (0.93, 1.13) |
*Student’s t-test.
**For LA, the OR is for a 1 μmol/ml increase in concentration. All other omega fatty acid variables were multiplied by 100 and therefore the fecundability ratio represents a 1/100 μmol/ml change in concentration.
OR, odds ratio.
Due to using inverse probability weighting, the 95% CIs were calculated using robust variance.
There was no strong nor statistically significant correlation between any individual serum essential fatty acid concentration and AMH (Table IV).
Table IV.
Correlation between serum omega fatty acid concentrations and AMH level.
| Omega fatty acid | Pearson’s coefficient, r |
|---|---|
| Omega-3 | |
| ALA | 0.049 |
| EPA | −0.086 |
| DHA | −0.037 |
| Omega-3 PUFAa | 0.021 |
| Long chain omega-3 PUFAb | −0.048 |
| Omega-6 | |
| LA | 0.011 |
| DGLA | 0.004 |
| AA | −0.061 |
| Omega-6 PUFAc | 0.009 |
| Ratio of omega-6 to omega-3 | −0.085 |
| Ratio of LA to ALA | −0.005 |
| Total PUFA | 0.010 |
P non-significant for all (P > 0.05).
aSum of ALA, EPA and DHA.
bSum of EPA and DHA.
cSum of LA, DGLA and AA.
Discussion
In this nested case–control study from a prospective time to pregnancy cohort study, serum omega fatty acid concentrations did not significantly differ between fertile women who conceived in the first 3 months of trying, subfertile women who conceived within 4–12 months of trying and infertile women who did not conceive after 12 months of trying. There was no strong association between serum concentrations of omega-3 and omega-6 fatty acids and the probability of conceiving in a given menstrual cycle. There was no significant association between omega fatty acid serum concentrations and the risk of miscarriage. There was no correlation between any individual omega fatty acid concentration and ovarian reserve as measured by the biomarker, AMH.
The findings from our study suggest that omega-3 and omega-6 serum concentrations are not significantly associated with natural fertility. Omega fatty acid metabolism and physiologic downstream pathways are complex, and it is therefore unclear whether our findings suggest that increased omega-fatty acid intake does not significantly impact natural fertility as well. While our study is the first to evaluate omega fatty acid serum concentrations and natural fertility, several studies have evaluated omega fatty acid intake and natural fertility with conflicting findings. In the PRESTO study of women in North America, increased omega-3 fatty acid intake was associated with higher fecundability; however, no association was found in the Snart Foraeldre Denmark cohort (Wise et al., 2018). Although of note, the Snart Foraeldre Denmark cohort had a higher median intake of omega-3 fatty acids, and low intake of omega-3 fatty acids in this population was uncommon, possibly explaining the lack of association because of a threshold effect. The Biocycle study reported that increased omega-3 PUFA intake lowers the risk of anovulation; however, the NHS-II study found no association between omega-3 intake and ovulatory infertility (Mumford et al., 2016; Chavarro et al., 2007). Interestingly, Gaskins and colleagues in the LIFE study evaluated fish intake (the main source of marine omega-3 fatty acids in the United States population) and time to pregnancy and found that prospective seafood intake in cycles was associated with fecundity after multivariable adjustment; however, baseline seafood intake was not (Gaskins et al., 2018). Baseline seafood intake was defined as the monthly intake in the 12 months preceding enrollment in the study. In our study, omega fatty acid concentrations were assessed at baseline and likely also reflect prior intake of omega fatty acids. Thus, our findings are consistent with that of Gaskins et al.
While studies on omega fatty acid intake in natural fertility are conflicting, studies in the infertile population consistently report favorable associations between omega-3 fatty acid intake and reproductive endpoints in women undergoing ART (Nouri et al., 2016; Vujkovic et al., 2010). Our study’s primary outcome was a positive pregnancy test, which most closely corresponds to the outcome of implantation in the ART literature. Interestingly, the studies on serum omega fatty acid concentration and reproductive outcomes in ART have incongruous findings. Jungheim et al., 2011 and 2013 found that increasing fasting serum concentrations of the specific omega-3 fatty acid ALA was associated with decreased chances of pregnancy and, in a subsequent study, found that a higher omega-6 to omega-3 ratio (LA:ALA) in fasting serum was associated with higher implantation and pregnancy rates (Jungheim et al., 2011 and 2013). Conversely, in the EARTH cohort, serum omega-3 PUFA concentrations and intake were positively associated with the probability of pregnancy and live birth and no association was found after analysing omega-6 concentrations or the omega-6 to omega-3 ratio (Chiu et al., 2018b). In a recent review of the literature on omega fatty acids, Chiu et al. (2018a) balanced these conflicting studies in both the fertile and infertile populations and concluded that higher omega-3 fatty acids may enhance female fertility. However, our findings may challenge this conclusion.
No previous studies have evaluated omega PUFA serum concentration and the risk of miscarriage. However, omega-3 and omega-6 fatty acids are the direct precursors of prostaglandins. Suppression of uterine prostaglandin synthesis is important for maintenance of early pregnancy. Thus omegas could theoretically impact prostaglandin biosynthesis and metabolism and, in this way, impact early pregnancy (Clark et al., 2008). However, our study found no association with omega fatty acid serum concentrations and the risk of miscarriage.
Our study found no association between omega fatty acid concentrations and women’s AMH level. This finding in humans differs from an animal model wherein delayed ovarian aging was observed in murines with higher dietary omega-3 fatty acid intake (Nehra et al., 2012). Interestingly, in reproductive aged women with normal ovarian reserve (defined by normal AMH level with regular monthly menstrual cycles), decreased serum levels of FSH have been observed in normal weight but not obese women who received dietary administration of omega-3 fatty acids (Al-Safi et al., 2016). However, that study also found no significant impact on AMH level following increased dietary omega-3 fatty acid intake, supporting our current findings. Future studies may be indicated to analyse multiple biomarkers of ovarian reserve and more fully investigate omega fatty acids and the female reproductive lifespan.
Our study has several limitations. First, this study is limited by the sample size. Second, omega-3 and omega-6 fatty acid concentrations were derived from serum provided at a single timepoint in the first cycle of enrollment. Serum concentrations reported in our study may therefore not be representative of each subsequent attempt cycle or the cycle in which conception occurred. Third, the serum half-lives of omega-3 fatty acids range from as short as 1 h for ALA, to 20 h for DHA and as much as 67 h for EPA (Pawlosky et al., 2001). The serum concentrations found in our study may therefore not be representative of the levels of omega fatty acids present in the serum or the body at critical points in the menstrual cycle and early pregnancy. However, prospective epidemiologic studies generally rely on one baseline biologic sample from participants for measurement of prognostic biomarkers in an assumption that one measurement adequately represents participants’ typical levels. While not published on omega fatty acids in particular, studies on Vitamin D levels over time have revealed that participants are consistent in their daily habits and intake and that a single, baseline value is reasonably representative of their serum levels over time (Sonderman et al., 2012). Fourth, conception, not live birth, was the primary outcome. However, the concentrations were not associated with the age-adjusted odds of miscarriage, therefore, the findings most likely also relate to probability of live birth. Fifth, women enrolled in this study were older, aged 30–44 years. Therefore, our findings may not be generalizable to younger women trying to conceive. Sixth, a possible limitation is that the serum samples in our study were collected from non-fasting women. This may impact the results as serum free fatty acid levels are lower in non-fasting individuals, and higher in fasting individuals (Collins et al., 2019). Both studies by Jungheim et al., 2011 and 2013 evaluating omega fatty acid concentrations and ART outcomes in the infertile population analysed fasting serum, and it is not clear in the EARTH cohort by Chui and co-workers whether serum was drawn fasting or non-fasting women (Jungheim et al., 2011 and 2013; Chiu et al., 2018a).
This study has several strengths. First, it is a nested case–control study from a prospective TTC cohort. Its prospective design allowed for inclusion of participants with the full range of natural fertility. Second, most women included in the study enrolled during their first three menstrual cycles of their attempt to conceive. Enrolment of women later selects a less fertile cohort, since only 50% of women are likely to conceive in the first three cycles (Steiner et al., 2017). Third, the study protocol standardized the outcome measure. This was done by providing women with free pregnancy tests and instructing them on when to perform the pregnancy test. Thus, the sensitivity of the test was the same for all, and the pre-set timing of the testing minimized the potential for differential identification of pregnancies. Fourth, the omega PUFA were analysed using mass spectroscopy performed in the Metabolomics Facility at Washington University, which is the same laboratory and processes used in previous studies analszing serum PUFA in the infertile population (Jungheim et al., 2011 and 2013). Fifth, multiple omega-3 and omega-6 metabolites were analysed to account for complex physiologic omega fatty acid metabolism pathways.
In conclusion, high serum omega-3 concentrations do not appear to positively impact natural fertility and high omega-6 concentrations do not appear to negatively impact it. Omega-3 and omega-6 concentrations do not appear to affect the risk of miscarriage. They also do not appear to negatively or positively impact ovarian reserve. While we did not find an association with serum levels, future studies are needed to determine the extent to which supplementation with omega-3s may impact natural fertility.
Supplementary Material
Acknowledgements
We appreciate statistical input from Dr Shanshan Zhou.
Authors’ roles
J. Stanhiser, MD, MSCR: participation in study design, study execution, data analysis, manuscript drafting and editing, and critical discussion. A.M.Z. Jukic, PhD, MSPH: participation in study design, data analysis, manuscript drafting and editing, and critical discussion. A.Z. Steiner, MD, MPH: participation in study design, study execution, data analysis, manuscript drafting and editing, and critical discussion.
Funding
This study was supported by the Division of Reproductive Endocrinology and Infertility at the University of North Carolina at Chapel Hill, by the NIH/NICHD (R21 HD060229-01 and R01 HD067683-01) and, in part, by the Intramural Research Program of the National Institute of Environmental Health Sciences (Z01ES103333).
Conflict of interest
Dr Jukic received vitamin D supplements for a research study from Theralogix, Inc. The authors have no other conflict of interest to disclose.
References
- Al-Safi ZA, Liu H, Carlson NE, Chosich J, Harris M, Bradford AP, Robledo C, Eckel RH, Polotsky AJ.. Omega-3 fatty acid supplementation lowers serum FSH in normal weight but not obese women. J Clin Endocrinol Metabol 2016;101:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Dietary fatty acid intake and the risk of ovulatory infertility. Am J Clin Nutr 2007;85:231–237. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Karmon AE, Gaskins AJ, Arvizu M, Williams PL, Souter I, Rueda BR, Hauser R, Chavarro JE, for the EARTH Study Team.. Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum Reprod 2018a;33:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Chavarro JE, Souter I, Jorge E. Diet and female fertility: doctor, what should I eat? Fertil Steril 2018b;110:560–569. [DOI] [PubMed] [Google Scholar]
- Clark K, Myatt I. Prostaglandins and the reproductive cycle. Glob Libr Women’s Med 2008. doi: 10.3843/GLOWM.10314. [DOI] [Google Scholar]
- Collins SM, Broadney MM, Ghane H, Davis EK, Jarmaillo M, Shank LM, Brady SM, Yanovski JA. Free fatty acids as an indicator of the nonfasted state in children. Pediatric 2019;143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM, Mosey JL, Klinger J. Fatty acid oxidation and meiotic resumption in mouse oocytes. Mol Reprod Dev 2009;76:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elis S, Freret S, Desmarchais A, Maillard V, Cognié J, Briant E, Touzé JL, Dupont M, Faverdin P, Chajès Vet al. . Effect of a long chain n-3 PUFA-enriched diet on production and reproduction variables in Holstein dairy cows. Anim Reprod Sci 2016;164:121–132. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Sundaram R, Buck Louis GM, Chavarro JE. Seafood intake, sexual activity, and time to pregnancy. J Clin Endocrinol Metab 2018;103:2680–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokuldas PP, Singh SK, Tamuli MK, Vashi Y, Thomas R, Barman K, Pegu SR, Chethan SG< Agarwal SK. Dietary supplementation of n-3 polyunsaturated fatty acids alters endometrial expression of genes involved in prostaglandin biosynthetic pathway in breeding sows (sus scrofa). Theriogenology 2018; 110:201-208. [DOI] [PubMed] [Google Scholar]
- Jiang X, Sidhu R, Porter FD, Yanjanin NM, Speak AO, te Vruchte DT, Platt FM, Fujiwara H, Scherrer DE, Zhang Jet al. . A sensitive and specific LCMS/MS method for rapid diagnosis of NiemannPick C1 disease from human plasma. J Lipid Res 2011;52:1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Macones GA, Odem RR, Patterson BW, Moley KH. Eleveated serum alpha-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil Steril 2011;96:880–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Frolova AI, Jiang H, Riley JK. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J Clin Endocrinol Metab 2013;98:E1364–E1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leghi GE, Muhlausler BS. The effect of n-3 LCPUFA supplementation on oxidative stress and inflammation in the placenta and maternal plasma during pregnancy. Prostaglandins Leukot Essent Fatty Acids 2016;113:33–39. [DOI] [PubMed] [Google Scholar]
- Meher AP, Joshi AA, Joshi SR. Preconceptional omega-3 fatty acid supplementation on a micronutrient deficient diet improves the reproductive cycle in Wistar rats. Reprod Fertil Dev 2013;25:1085–1094. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Tsagareli V, Noakes M, Norman R. Altered preconception fatty acid intake is associated with improved pregnancy rates in overweight and obese women undertaking in vitro fertilization. Nutrients 2016;8:10. doi: 10.3390/nu8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford SL, Chavarro JE, Zhang C, Perkins NJ, Sjaarda LA, Pollack AZ, Schliep KC, Michels KA, Zarek SM, Plowden TCet al. . Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am J Clin Nutr 2016;103:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, Pan AH, Guo L, Rodig SJ, Tilly JLet al. . Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell 2012;11:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med 2001;345:1400–1408. [DOI] [PubMed] [Google Scholar]
- Nouri K, Walch K, Weghofer A, Imhof M, Egarter C, Ott J. The impact of a standardized oral multinutrient supplementation on embryo quality in in vitro fertilization/intracytoplasmic sperm injection: a prospective randomized trial. Gynecol Obstet Invest 2016. doi: 10.1159/000452662. [DOI] [PubMed] [Google Scholar]
- Oscarsson J, Hurt-Camejo E. Omega-3 fatty acids eicosapentaeonic acid and docosahexaenoic acid and their action on apoliporotein B-containing lipoproteins in humans: a review. Lipids Health Dis 2017;16:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N Jr. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res 2001;42:1257–1265. [PubMed] [Google Scholar]
- Ponter AA, Guyader-Joly C, Nuttinck E, Grimard B, Humblot P. Oocyte and embryo production and quality after OPU-IVF in dairy heifers given diets varying in their n-6/n-3 fatty acid ratio. Theriogenology 2012;78:632–645. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Arias-Alvarez M, Millán P, Febvrel N, Formoso-Rafferty N, López-Tello J, Lorenzo PL, Rebollar PG. Improvements in the conception rate, milk composition, and embryo quality of rabbit does after dietary enrichment with n3-polyunsaturated fatty acids. Animal 2018;12:2080–2088. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 2002;56:365–379. [DOI] [PubMed] [Google Scholar]
- Sonderman JS, Munro HM, Blot WJ, Signorello LB. Reproducibility of serum 25-hydroxyvitamin D and vitamin D-binding protein levels over time in a prospective cohort study of black and white adults. Am J Epidemiol 2012;176:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AZ, Pritchard D, Ztanczyk FZ. Association between biomarkers of ovarian reserve and infertility among women of reproductive age. JAMA 2017;318:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmey RG, Reis A, Leese HJ, McEvoy TG. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod Domest Anim 2009;44:50–58. [DOI] [PubMed] [Google Scholar]
- Vujkovic M, de Vries JH, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA, Steegers-Theunissen RP. The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertil Steril 2010;94:2096–2101. [DOI] [PubMed] [Google Scholar]
- Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod 2007;77:190–201. [DOI] [PubMed] [Google Scholar]
- Wise LA, Wesselink AK, Tucker KL, Saklani S, Mikkelsen EM, Cueto H, Riis AH, Trolle E, McKinnon CJ, Hahn KAet al. . Dietary fat intake and fecundability in 2 preconception cohort studies. Am J Epidemiol 2018;187:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


