We review the case for improving photosynthesis to increase yields in C4 crops, and highlight potential strategies to alleviate bottlenecks limiting C4 photosynthetic performance under optimal and suboptimal conditions.
Keywords: Agriculture, C4 pathway, crop yield, modelling, NADP-ME, photosynthesis, Rubisco, source–sink
Abstract
Although improving photosynthetic efficiency is widely recognized as an underutilized strategy to increase crop yields, research in this area is strongly biased towards species with C3 photosynthesis relative to C4 species. Here, we outline potential strategies for improving C4 photosynthesis to increase yields in crops by reviewing the major bottlenecks limiting the C4 NADP-malic enzyme pathway under optimal and suboptimal conditions. Recent experimental results demonstrate that steady-state C4 photosynthesis under non-stressed conditions can be enhanced by increasing Rubisco content or electron transport capacity, both of which may also stimulate CO2 assimilation at supraoptimal temperatures. Several additional putative bottlenecks for photosynthetic performance under drought, heat, or chilling stress or during photosynthetic induction await further experimental verification. Based on source–sink interactions in maize, sugarcane, and sorghum, alleviating these photosynthetic bottlenecks during establishment and growth of the harvestable parts are likely to improve yield. The expected benefits are also shown to be augmented by the increasing trend in planting density, which increases the impact of photosynthetic source limitation on crop yields.
Introduction
The global human population has seen a staggering increase over the last century, and is currently estimated to be 7.8 billion people (United Nations, 2019). This signifies a tripling of the population compared with 2.6 billion people in 1950 and, although this explosive growth is projected to gradually taper off, substantial further population growth is still predicted (UN Population Division projections). Current projections suggest that by 2050, the global population will have grown to 9.6 billion. Providing enough food for all these additional mouths will be challenging indeed. Historically, human population growth has often been a concern, as exemplified by Thomas Malthus’ two-century-old statement that ‘Population will always grow more rapidly than food supplies until numbers are reduced by war, disease or famine’ (Malthus, 1798). Malthus’ prediction has often been used to emphasize the power of science and technology, allowing human society to outpace his doomsday scenario. Indeed, most commodity crop yields have shown steady increases for many decades (e.g. Ray et al., 2013), and further increases may well be possible via improvements in farm management, plant breeding, as well as via utilization of transgenic (e.g. Pellegrino et al., 2018) and gene editing technologies. Improvements in crop yields can be achieved via development of new higher yielding varieties or via closure of the so-called ‘yield-gap’ between attainable and realized yield (Foley et al., 2011). With regards to the former, the plant breeding strategies of the green revolution have led to spectacular improvements in yield potential via increases in yield components such as harvest index and light capture efficiency, but less so for the conversion efficiency of captured solar energy to energy contained in plant biomass. Theoretical analyses of the maximum conversion efficiency (Zhu et al., 2010) have provided estimates for an upper limit of 4.6–6%. From the few existing measurements of this conversion efficiency in farmers’ fields (reviewed in Zhu et al., 2010), even the most productive crop canopies achieve less than one-third of the computed upper limit, mostly due to losses in photosynthetic efficiency. Thus, improving photosynthetic efficiency may have potential to increase crop yields, as demonstrated by a range of proof-of-concept studies for this strategy in C3 species (reviewed by Simkin et al., 2019).
Increasing crop productivity via improving photosynthetic efficiency: a C3 story?
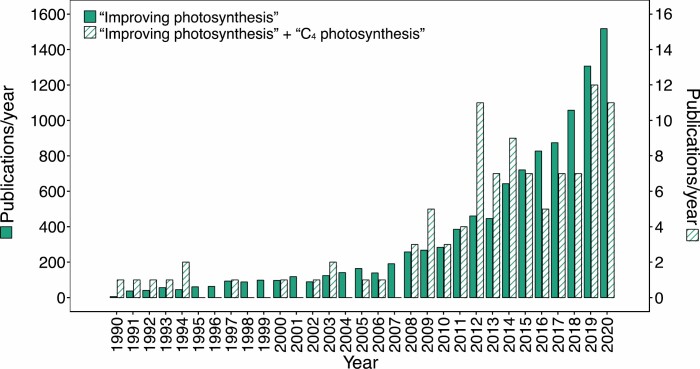
The vast majority of studies looking at photosynthetic efficiency gains to improve crop yield focus on species with C3 photosynthesis. In contrast to C3 photosynthesis, species with C4 photosynthesis drive a biochemical CO2-concentrating mechanism (CCM), which enhances the operating efficiency of Rubisco and competitively inhibits ribulose bisphosphate (RuBP) oxygenation and associated photorespiratory losses (Hatch, 1971; Dai et al., 1993). The CCM also allows plants to function with limited stomatal opening, which reduces water loss through transpiration and consequently increases photosynthetic water-use efficiency (WUE). In addition, less Rubisco is needed, which accounts for most of the nitrogen invested in leaves, and thus increases nitrogen-use efficiency (Ghannoum et al., 2005). Although only ~3% of plant species use the C4 pathway (Sage et al., 2012), C4 species are strongly over-represented in our agricultural crops, and their importance in supplying food, feed, and fuel is hard to overstate (USDA, 2020a; Fig. 1). As C4 photosynthetic species are topping the list of the most highly produced commodities (USDA, 2020a; Fig. 1), novel strategies to improve their productivity should be highly impactful. Despite this, analysis of the research output of the last three decades suggests that only ~1% of research on improving photosynthetic efficiency is focusing on C4 photosynthesis (Fig. 2); and even this might be an overestimate, considering that out of the 104 references found with this search, 14 studies are focusing on C4 photosynthesis as a means to improve photosynthetic efficiency in C3 species.
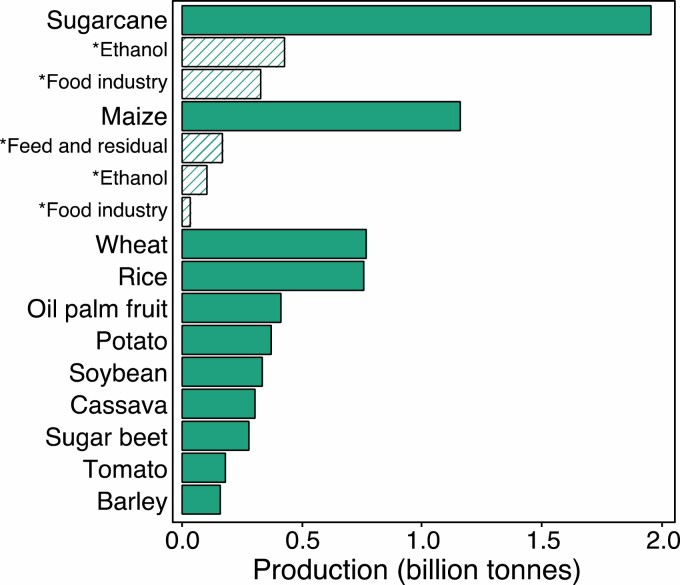
Fig. 1.
Annual global production of the 11 crops highest in production quantity. The C4 NADP-ME sugarcane and maize are at the top of the ranking. Data shown by the solid bars were extracted from the Food Agriculture Organization database (FAO, 2020). *Patterned bars show the approximate amount of different uses of the total production of sugarcane in Brazil (CONAB, 2018) and of maize in the USA (USDA, 2020a).
Fig. 2.
Number of publications per year in subject area ‘Plant Sciences’. The topic ‘Improving photosynthesis’ is shown by the solid green bars, and the number of publications per year ‘C4 photosynthesis’ found within this group is shown by the patterned bars. Search results were generated using Web of Science on 19 February 2021.
So why is there less focus on improving the efficiency of C4 photosynthesis? The attributes of C4 photosynthesis provide an advantage over C3 photosynthesis at high light and high temperatures (Sage and Zhu, 2011) and theoretically even at mildly chilling temperatures (Long and Spence, 2013). Does this mean that the conversion efficiency of solar energy to biomass is closer to its biological limit in C4 species? Based on the aforementioned theoretical analysis of potential conversion efficiency (Zhu et al., 2010), C4 species have an intrinsic advantage compared with C3 species but still fall short of the theoretical upper limit by a considerable margin. Thus, the case for improving photosynthetic efficiency to increase crop yield may also hold promise for C4 photosynthesis (see also von Caemmerer and Furbank, 2016). Here we therefore review potential strategies for improving C4 photosynthesis under optimal and suboptimal conditions.
How could C4 photosynthesis be improved under non-stressed conditions?
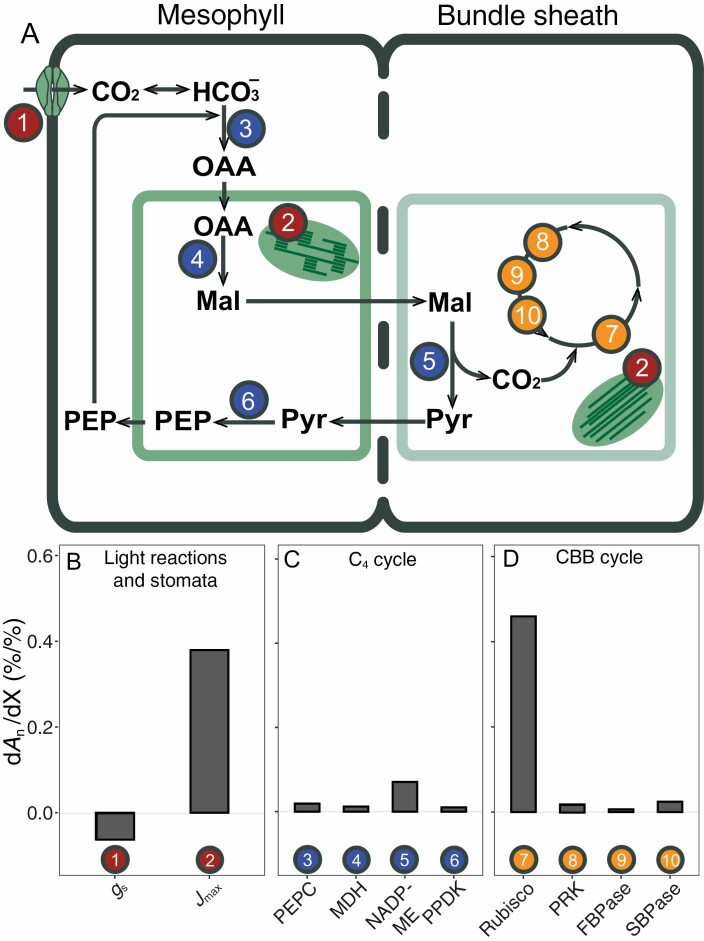
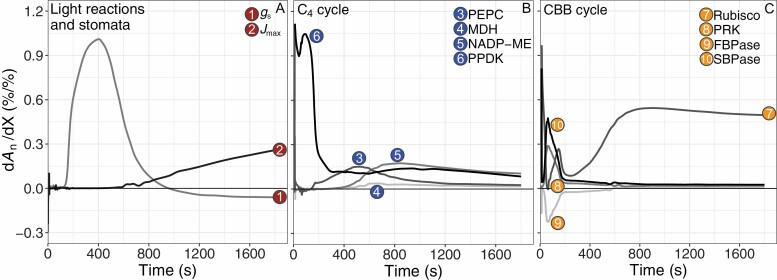
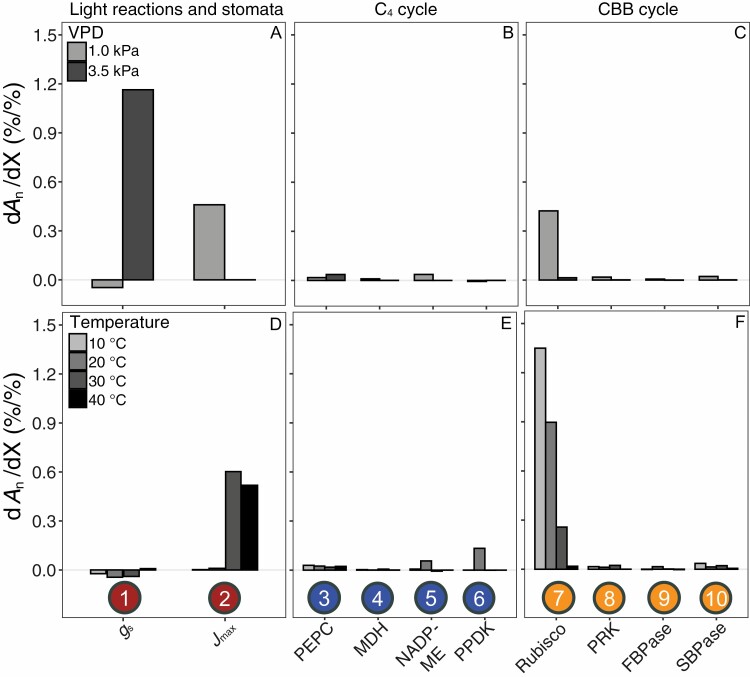
In the following paragraphs, we explore in which ways C4 photosynthesis could be improved. Focusing on the NADP-malic enzyme (ME) subtype (Box 1), in this section we will first look at factors that have control over the rate of CO2 assimilation under non-stressed conditions. The interdependence of the C4 acid shuttle and Calvin–Benson–Bassham (CBB) cycle across two different photosynthetic cell types (mesophyll, M; and bundle sheath, BS) makes it more difficult to pinpoint specific control factors in C4 photosynthesis, compared with C3 photosynthesis. In addition, the demands for ATP and NADPH in M and BS cells are distinct, and balancing the energy (Bellasio and Griffiths, 2014; Kromdijk et al., 2014) between both compartments is important for efficient functioning of C4 photosynthesis. In addition, there is significant carbon exchange between the CBB and C4 cycle (Arrivault et al., 2017), which probably helps to maintain flexibility to respond to variable environmental conditions. To account for this complexity, metabolic models which capture the kinetics of all the major reaction and diffusion steps in C4 photosynthesis can be used to identify the relative control exerted by any of the modelled factors over the rate of CO2 assimilation, by computing control coefficients, defined as the relative change in net CO2 assimilation rate (An), as a result of a relative change in the control factor. Using their model for NADP-ME photosynthesis (Fig. 3) to simulate the control of individual factors over the rate of assimilation, Wang et al. (2021) computed that under high light, control over steady-state An is shared between Rubisco in the CBB cycle (dAn/dRubisco=0.46) and Jmax, namely the capacity for chloroplastic electron transport (dAn/dJmax=0.38). Using the same model to simulate a step change in light intensity from darkness to 1800 μmol m−2 s−1 (Fig. 4), a strong transient control of pyruvate-orthophosphate di-kinase (PPDK) during the first minutes was predicted (Fig. 4B), to ramp up metabolic pools in the C4 cycle. Similarly, but less pronounced, sedoheptulose-1,7-bisphosphatase (SBPase) and phosphoribulokinase (PRK) share significant transient control, consistent with their role in the regeneration of CBB cycle substrate (Fig. 4C). The concomitant negative transient control of chloroplastic fructose-1.6-bisphosphatase (FBPase) can be explained by the role of fructose-6-phosphate in starch formation (Raines, 2003), which would compete with the availability of substrate in the CBB cycle. After An is induced far enough to significantly deplete intercellular CO2 concentrations, the control shifts transiently to stomatal conductance (gs) and phosphoenolpyruvate carboxylase (PEPC; Fig. 4A, B), before finally settling on the steady-state control by Rubisco and Jmax shown in Fig. 3.
Box 1. The NADP-ME C4 photosynthesis subtype.
Dual cell-type C4 photosynthesis can be classified into three biochemical subtypes, NADP-ME, NAD-ME, and PEPCK, based on the enzyme used to decarboxylate C4 acids (Hatch et al., 1975). Here, the main focus is on NADP-ME C4 grasses of the Andropogoneae clade which represent some of the most important cultivated plants with a large impact on food, feed, and fuel production (Christin et al., 2009; Fig. 1).
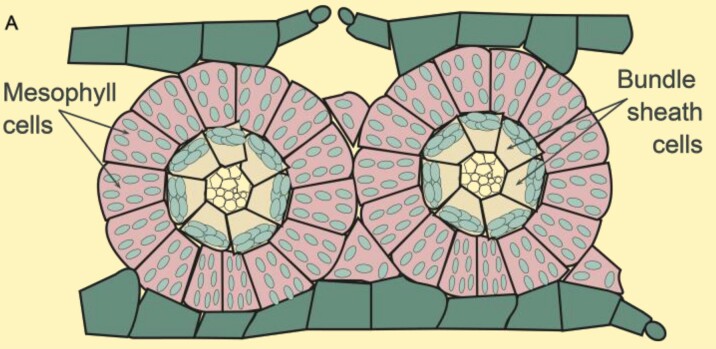
Chloroplast differentiation in NADP-ME C4
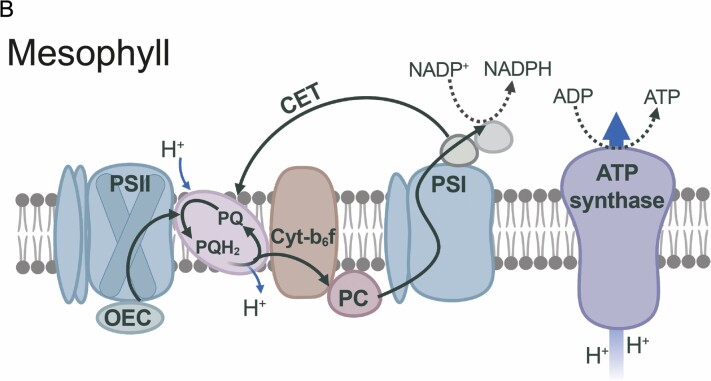
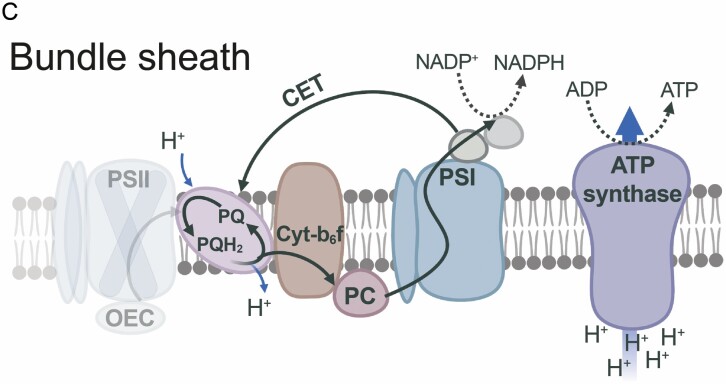
NADP-ME C4 grasses show classical ‘Kranz’ leaf anatomy (A). Low PSII abundance in BS thylakoids prevents high rates of whole-chain electron transfer. Instead, reductant is supplied via the malate shuttle, and ΔpH formation and ATP synthesis are predominantly driven by CET (Hatch, 1987; B and C). Chloroplasts in M cells retain the capacity to undergo aggregative movements in response to environmental stresses (Yamada et al., 2009), but in BS cells are more confined to their centrifugal position, which may facilitate metabolite transfer between M and BS (Maai et al., 2011).
Flexibility in the decarboxylation pathway
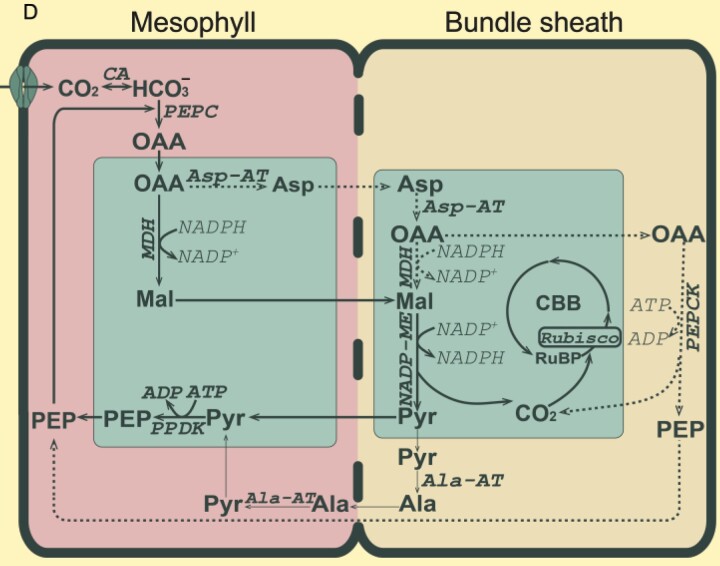
Carbon fixation starts in the M cytosol (D). CO2 is converted into HCO3− by CA and is used in the carboxylation of PEP (catalysed by PEPC) forming the C4 organic acid OAA. OAA is converted to malate by MDH in M chloroplasts and diffuses to the BS cells. Decarboxylation of malate by NADP-ME in BS chloroplasts elevates the CO2 concentration around Rubisco and provides NADPH.
Pyruvate diffuses back to the M cells, where PEP is regenerated by PPDK at the expense of 2 ATP/PEP (Kanai and Edwards, 1999).
Significant activity of PEPCK (dotted arrows) can supplement the NADP-ME route (Furbank, 2011; Yin and Struik, 2021); up to 25% in maize (Hatch, 1971); present in sugarcane (Calsa and Figueira, 2007; Sales et al., 2018; Cacefo et al., 2019); but undetectable in sorghum (Gutierrez et al., 1974).
Pyruvate can also be transaminated into alanine, before diffusing back to the M cell (Schlüter et al., 2019; faded arrows), which may help to balance nitrogen metabolism (Wang et al., 2014).
Abbreviations: Ala, alanine; Ala-AT, alanine aminotransferase; Asp, aspartate; Asp-AT, aspartate aminotransferase; BS, bundle sheath; CA, carbonic anhydrase; CBB, Calvin–Benson–Bassham cycle; CET, cyclic electron transfer; M, mesophyll; Mal, malate; MDH, malate dehydrogenase; NAD-ME, NAD-malic enzyme; NADP-ME, NADP-malic enzyme; OAA, oxaloacetate; OEC, oxygen-evolving complex; PC, plastocyanin; PEP, phosphoenolpyruvate; PEPC, phosphoenolpyruvate carboxylase; PEPCK, PEP carboxykinase; PPDK, pyruvate-orthophosphate-dikinase; PQ, oxidized plastoquinone; PQH2, reduced plastoquinone; Pyr, pyruvate; RuBP, ribulose 1,5 bisphosphate. Schemes B and C were created with BioRender (https://biorender.com/).
Fig. 3.
Simulated control coefficients of individual factors over net CO2 assimilation rate (An) during steady-state conditions. Light reactions and stomatal diffusion (B), C4 (C) and CBB cycle (D) enzymes, in maize. The individual factors are numbered and indicated in the diagrammatic representation in (A). Control coefficients were computed as the first derivative of An normalized to each respective control factor X (dAn/dX). Environmental settings for the simulation were air temperature of 28 °C, photosynthetically active radiation of 1800 μmol m−2 s−1, ambient CO2 concentration of 410 ppm, and air vapour pressure deficit (VPD) of 1.5 kPa. For a full description of the model and parameters, see Wang et al. (2021).
Fig. 4.
Simulated control coefficients of individual factors over An during a step increase in photosynthetically active radiation. Light reactions and stomatal diffusion (A), C4 (B), and CBB cycle (C) enzymes of An in maize. The individual factors are numbered and indicated in the diagrammatic representation in Fig. 3A. Control coefficients were computed as the first derivative of An normalized to each respective control factor X (dAn/dX). Photosynthetically active radiation was changed from 0 to 1800 μmol m−2 s−1 at time=0 s. Other environmental settings for the simulation were air temperature of 28 °C, ambient CO2 concentration of 410 ppm, and air vapour pressure deficit (VPD) of 1.5 kPa. For a full description of the model and parameters, see Wang et al. (2021).
CBB cycle control over C4 photosynthesis
It is well known that the Rubisco catalytic rate is relatively slow, and catalytic improvement may be constrained by a trade-off with its specificity for CO2 over O2 (Zhu et al., 2004b; Carmo-Silva et al., 2015). Lower abundance of Rubisco in C4 plants also affects the levels of CBB metabolites, in particular RuBP, which was significantly lower in four C4 species, relative to five C3 species (Arrivault et al., 2019). The high CO2 environment around Rubisco in C4 plants seems to have relaxed the selective pressure for high specificity, resulting in a higher catalytic turnover rate (kcat) of C4 Rubisco compared with C3, at the expense of affinity for CO2 (kc) (Kapralov et al., 2011). Substantial diversity in Rubisco activity and catalytic properties among C4 species is found (Hermida-Carrera et al., 2016; Orr et al., 2016; Sharwood et al., 2016a, b; Kolbe et al., 2018b), possibly reflecting differences in time since the evolution of the C4 pathway between different lineages, as well as systematic differences between different decarboxylation types (Ghannoum et al., 2005). Swapping the nuclear-encoded Rubisco small subunit for transgenic or chimeric versions can be used to alter Rubisco kinetic properties (Ishikawa et al., 2011; Atkinson et al., 2017) and may offer a tractable route to manipulating kcat.
A different strategy to increase Rubisco capacity is via enhancing its content. Space constraints in the BS chloroplasts were initially thought to limit Rubisco content in C4 species (Sage and McKown, 2006). However, recent work shows that C4 BS chloroplasts may be able to house Rubisco amounts sufficient to alleviate its control over C4 photosynthesis (Pignon et al., 2019). Indeed, using combined overexpression of Rubisco large and small subunits together with the Rubisco assembly chaperone RUBISCO ASSEMBLY FACTOR 1 (RAF1) in maize, Salesse-Smith et al. (2018) achieved a >30% increase in Rubisco content. The Rubisco-overexpressing maize plants showed significant increases in CO2 assimilation as well as plant growth. However, the gains were limited due to a decline in Rubisco activation state in the overexpression lines. Although Rubisco activase (Rca) content appears typically in excess in C4 species (von Caemmerer et al., 2005), it is possible that overexpression of Rca in parallel with increasing Rubisco content will further enhance the potential of this strategy (Salesse-Smith et al., 2018).
Electron transport capacity control over C4 photosynthesis
In C4 species, electron transport occurs in both M and BS cells, but the energy supply and demand vary considerably between both photosynthetic cell types. In NADP-ME C4 photosynthesis, which is simulated by the model, M cells perform whole-chain electron transport, whereas BS cells have low PSII activity. High rates of cyclic electron transport around PSI in BS chloroplasts drive ATP synthesis (see Box 1), whereas NADPH is instead supplied by the M cells via the malate shuttle. In an attempt to enhance cyclic electron transport in NADP-ME-type C4 plants, Tazoe et al. (2020) overexpressed PGR5 in Flaveria bidentis, an NADP-ME C4 dicot. Although this led to a higher electron sink downstream of PSI and alleviated acceptor-side limitation of PSI under fluctuating light, it did not impact CO2 assimilation.
Cyclic and linear electron flow are both subject to so-called ‘photosynthetic control’ via the cytochrome b6f (Cyt-b6f) complex, which upon acidification of the thylakoid lumen causes deceleration of the oxidation of plastoquinol and limits electron flow towards plastocyanin (Foyer et al., 2012). A decrease in Cyt-b6f content and proportional decline in electron transfer rates was observed in antisense Rieske FeS mutants of the C3 species tobacco (Price et al., 1995). More recently, the opposite strategy, overexpression of Rieske FeS, was shown to be sufficient to elevate Cyt-b6f content in the NADP-ME model C4 grass Setaria viridis (Ermakova et al., 2019). The resulting increase in Jmax also improved the rate of CO2 assimilation, via enhanced quantum yields of both photosystems and a decrease in loss of energy via non-photochemical quenching (NPQ). Slow temporal kinetics of NPQ have also been predicted to decrease CO2 fixation (Murchie and Niyogi, 2011), and faster NPQ in transgenic tobacco plants was shown to significantly boost photosynthetic efficiency and productivity (Kromdijk et al., 2016). Model simulations using maize PSII quantum yield recovery kinetics predict an even greater potential impact than found in tobacco (Zhu et al., 2004a), suggesting that the transgenic strategy by Kromdijk et al. (2016) may also have merit in C4 species.
C4 cycle control over C4 photosynthesis rates
None of the C4 cycle enzymes individually seems to have strong control (<0.07 dAn/dX in the model calculations) over the rate of photosynthesis under steady-state, highlight conditions (Fig. 3C), which is consistent with the notion that the CCM has to operate at slightly higher rates than the CBB cycle in order to achieve the CO2-concentrating effects while accounting for overcycling due to retrodiffusion of CO2 (termed leakiness, Farquhar, 1983; reviewed by Kromdijk et al., 2014). That is, when An is already saturated with CO2, any further increases in CO2 concentration should not affect the rate of photosynthesis and instead increase leakiness and concomitant energy loss. In addition, the C4 cycle rate also determines the rate of reductant shuttle from the M to BS chloroplasts. Although C4 acid transporters may also play a significant role (e.g. Weissmann et al., 2016), the C4 cycle rate is primarily controlled by the activity of NADP-malate dehydrogenase (NADP-MDH), the only thioredoxin-regulated enzyme in the C4 cycle (Leegood and Walker, 1999). However, activity of NADP-MDH under steady-state highlight conditions is typically in excess of the net assimilation rate (Usuda et al., 1984). Activity of NADP-MDH in mutant lines of F. bidentis could be reduced to <50% before CO2 fixation capacity was affected (Trevanion et al., 1997), which led the authors to suggest that instead of a direct regulatory role in photosynthesis, the covalent regulation of NADP-MDH activity may function to keep the chloroplastic NADP pool largely reduced and to limit reductant transfer from the chloroplast under darkness.
In contrast to steady-state, under transient conditions strong control coefficients are predicted for C4 cycle enzymes. During photosynthetic induction due to a change in light intensity from 0 to 1800 μmol m−2 s−1, PPDK exerts a strong control over An during the first minutes (Fig. 4). PPDK is an important enzyme in C4 photosynthesis, controlling the regeneration of PEP, the substrate for the primary carboxylation event (Chastain et al., 2011). The importance of PPDK for non-steady state photosynthesis is well known (Usuda et al., 1984). However, PPDK can be rapidly activated via dephosphorylation in response to changes in light intensity (Chen et al., 2014). Consequently, in response to a step increase in light, PPDK increases activity much faster than photosynthesis, which is consistent with the notion that the transient control coefficient of PPDK (Fig. 4B) is not associated with direct control over the carbon uptake flux, but rather with building up the large metabolic pools essential for C4 photosynthesis (Stitt and Zhu, 2014). PPDK corresponds to 7–10% of the protein content of M cells (Edwards et al., 1985), and its activity was found to exceed the rate of photosynthesis only slightly in maize (Usuda et al., 1984). In addition, small reductions in PPDK gene expression and amounts led to lower assimilation rates in F. bidentis (Trevanion et al., 1999). However, since a significant fraction of PPDK (up to one-third) remains phosphorylated under fully activated conditions (Edwards et al., 1985), some overcapacity seems to exist.
As mentioned above, after An is induced far enough to significantly deplete Ci, the control over An shifts strongly to stomatal conductance and to some extent to PEPC. Extractable activity of PEPC is typically several-fold higher than in vivo activity (Laisk and Edwards, 1997) due to strong post-translational control by metabolites as well as reversible phosphorylation. Reversible phosphorylation of a PEPC serine residue increases its activity, while at the same time it reduces the inhibitory effect of malate and aspartate and increases the sensitivity to activation by sugar-phosphates (and glycine in monocots) (Doncaster and Leegood, 1987; Leegood and Walker, 1999; Gowik and Westhoff, 2011). Structural analysis of the homotetrameric PEPC showed that each monomer contains separate binding sites for the substrate PEP and the allosteric inhibitors malate and aspartate (Schlieper et al., 2014). The evolutionary co-opting of PEPC in C4 photosynthesis has led to different kinetic properties in the C4 isoform (Gowik and Westhoff, 2011) and several residues responsible for these changes have been discovered. Comparative analysis of crystal structures of C3 and C4 PEPC identified an arginine to glycine mutation in the C4 variant, leading to decreased inhibition by malate/aspartate (Paulus et al., 2013). Another single serine to alanine substitution decreases PEPC affinity for bicarbonate in the C4 isoform (DiMario and Cousins, 2019). Based on the balance between in vivo PEPC and Rubisco capacity in 49 C4 species (Pignon and Long, 2020), it could be hypothesized that a strategy shifting leaf nitrogen (N) investment away from PEPC towards Jmax and Rubisco could be effective to achieve higher rates of photosynthesis. A similar hypothesis was proposed by Kromdijk and Long (2016) for C3 species, suggesting that leaf N investment for high An could be more effective by a shift from carboxylation towards regeneration capacity in the CBB cycle. However, considering the non-negligible control of PEPC over non-steady-state photosynthesis (Fig. 4), and the perceived importance of dynamic photosynthesis over total canopy CO2 fixation (Murchie et al., 2018; Slattery et al., 2018), there could be significant drawbacks to this strategy.
How can C4 photosynthesis under suboptimal conditions be improved?
Whereas the factors above are important for C4 performance under optimal non-stressed conditions, factors controlling photosynthetic rates under suboptimal, stressed conditions are arguably more important for crop productivity. We used the model flux analysis by Wang et al. (2021) to account for short-term environmental stresses (Fig. 5) simulating drought [vapour pressure deficit (VPD) changed from 1.5 kPa to 3.5 kPa], heat, and cold stress (air temperature changed between 10 °C and 40 °C). The simulated drought conditions induce a shift from enzymatic factors to diffusional control of An by stomatal conductance (Fig 5A–C), which suggests that under these dry conditions, An becomes limited by CO2 supply despite the concentrating action of the C4 cycle. The shifts in the control coefficients in response to air temperature (Fig. 5D–F) are reflecting the differential responses of enzymatic activities which are strongly impacted by temperature, and the photosynthetic light reactions, which are much less affected. As a result, low temperatures dramatically increase the control coefficient of Rubisco, whereas under high temperatures, An is primarily controlled by electron transfer capacity. Notably, the co-limitation between Rubisco and Jmax at 30 °C is replaced at 20 °C by co-limitation between Rubisco and C4 cycle activity via PEPC, NADP-ME, and PPDK activities.
Fig. 5.
Simulated control coefficients of individual factors over An during steady-state under environmental stress conditions. Light reactions and stomatal diffusion (A, D), C4 (B, E), and CBB cycle (C, F) enzymes, during steady-state An in maize under contrasting air vapour pressure deficit (VPD; A–C) and air temperature (D–F). The individual factors are numbered and indicated in the diagrammatic representation in Fig. 3A. Control coefficients were computed as the first derivative of An normalized to each respective control factor X (dAn/dX). Environmental settings for the simulation were air temperature of 10–40 °C, photosynthetically active radiation of 1800 μmol m−2 s−1, ambient CO2 concentration of 410 ppm, and VPD of 1 or 3.5 kPa. For a full description of the model and parameters, see Wang et al. (2021).
Chilling temperature effects on C4 photosynthesis
Due to their evolutionary origins from tropical and subtropical regions, most C4 species are maladapted to chilling temperatures, in particular in combination with exposure to light which gives rise to chilling-induced photoinhibition (Taylor and Craig, 1971; Long et al., 1983). The most extreme crop example is probably sugarcane, which is particularly chilling sensitive (Grantz, 1989; Głowacka et al., 2016), and severely limited in its latitudinal range. However, even though maize has better chilling tolerance, it is still the most susceptible crop to chilling-induced photoinhibition amongst those grown in temperate regions (Hetherington et al., 1989). Improving resilience to low-temperature conditions in C4 crops could have a strong economic impact by increasing latitudinal range, helping to reduce year by year yield variability and decreasing early season competition with weeds. The model predictions suggest that increasing Rubisco activity is key to maintaining photosynthetic capacity under chilling conditions (Fig. 5F). However, transgenic maize lines with increased Rubisco content did not show better performance during chilling conditions, which suggests that Rubisco is not the primary limiting factor that causes maize susceptibility to low temperatures, although Rubisco overexpression may help plants recover faster from chilling events (Salesse-Smith et al., 2020).
Protein lability under low temperatures could play an important role in chilling tolerance. Several important proteins, most notably PEPC (Kingston-Smith et al., 1997; Chinthapalli et al., 2003), PPDK (Du et al., 1999), as well as Rubisco (Kingston-Smith et al., 1997; Du et al., 1999; Pittermann and Sage, 2001b; Chinthapalli et al., 2003), show increased rates of breakdown under chilling conditions. It is worth noting that the model calculations shown in Fig. 5 do not consider any effects of protein breakdown. In other words, the enhanced control coefficient which the model predicts for Rubisco activity under chilling temperatures is based purely on the kinetic properties of the enzyme, but in reality it will be compounded by the loss of activity due to protein disintegration. The effects of protein breakdown enhance the control of the cold-labile proteins PEPC and especially PPDK over An under cool conditions. Indeed, comparisons between maize and its chilling-tolerant distant relative Miscanthus×giganteus demonstrate that the latter responds to chilling with a strong transcriptional up-regulation of gene expression networks involved in photosynthesis and carbon assimilation, as well as protein synthesis and degradation (Spence et al., 2014) to counteract the enhanced breakdown of proteins such as Rubisco, PEPC, and PPDK (Naidu et al., 2003; Wang et al., 2008; Serrano-Romero and Cousins, 2020), resulting in superior productivity under temperate climates (Dohleman and Long, 2009).
Experiments with chilling temperatures in the presence and absence of illumination show that light, although not a prerequisite for photoinhibition (Ortiz-Lopez et al., 1990), clearly has an exacerbating role in the extent of chilling damage in maize (Taylor and Craig 1971; Long et al., 1983). This is due to the fact that light-harvesting and electron transfer reactions are much less perturbed by low temperature than downstream electron sinks. The resulting imbalance increases the probability of formation of reactive oxygen species (ROS), which are the primary cause of photodamage. Photoprotective and ROS-scavenging mechanisms are therefore important to maintain efficient C4 photosynthesis under suboptimal temperatures. The differences between chloroplast populations in BS and M cells in NADP-ME C4 species (see Box 1) may need to be considered in this regard. In maize, BS and M cells have distinct antioxidant capacities (Doulis et al., 1997) relative to the availability of reducing power in each compartment (reviewed in Turkan et al., 2018), with BS cells often lacking antioxidant capacity. Under optimal conditions, intracellular transport of reduced and oxidized forms of antioxidants allows continued ROS scavenging in BS cells. However, under low temperatures, the transport between compartments may become impaired, exposing the BS cells to oxidative stress (Kingston-Smith and Foyer, 2000). In addition, although most work on chilling-induced photoinhibition has focused on PSII inhibition, PSI inhibition is particularly prominent under cool conditions combined with fluctuating light (Kono et al., 2014). PSI is especially sensitive to inhibition when PSI electron acceptors are limited and the lack of an efficient repair cycle leads to prolonged recovery times which can span several days (Sonoike, 2011). These characteristics are consistent with the hallmark signs of chilling-induced photoinhibition in maize and sugarcane (Pimentel et al., 2005; Głowacka et al., 2016). Indeed, PSI activity can be strongly reduced by chilling or photoinhibitory treatments (e.g. Baker and Nie, 1994; Savitch et al., 2011; Qiao et al., 2020), which would justify another look at a putative role for PSI in chilling-induced photoinhibition of C4 species.
Drought stress effects on C4 photosynthesis
Increasing temperatures and fewer predictable precipitation events caused by global climatic change, are expected to increase the frequency of high VPD conditions and reduced water availability during crop growth (Yuan et al., 2019). High WUE, defined as the amount of carbon fixed per unit of water lost, can help to conserve soil water content for critical moments during the growing season. Although C4 species typically have higher WUE than C3 plants, the differences diminish under drought conditions (Ripley et al., 2010; Taylor et al., 2010). Under mild drought, a decrease in Ci can significantly affect saturation levels of CO2 around Rubisco. Consequently, An becomes limited by stomatal diffusion (Fig. 5A) as well as by PEPC. In addition, and consistent with the CO2 limitation of An, work with Zea mays lines with strongly reduced carbonic anhydrase (CA) activity suggests that this may also become a minor limitation at low Ci (Studer et al., 2014; Kolbe et al., 2018). Similarly, the leaf internal conductance to CO2 from the intercellular airspaces to the sites of fixation (mesophyll conductance, gm) may also impact An and WUE at low Ci (e.g. Kolbe and Cousins, 2018), but the mechanism and role of gm in limiting An in C4 species under different environmental conditions are not very well understood. Native responses of PEPC in C4 species under water deficit do not show a clear picture, with some results indicating a decrease in activity (Becker and Fock, 1986; Du et al., 1996) and others showing little change or increased activities of PEPC (Saliendra et al., 1996; Foyer et al., 1998; Carmo-Silva et al., 2007; Ghannoum, 2009; Pissolato et al., 2020). Beneficial effects of overexpression of PEPC have been reported for maize plants grown under mild drought conditions, resulting in higher WUE and increased biomass (Jeanneau et al., 2002), but these effects were suggested to stem from a pleiotropic negative effect on stomatal density of the PEPC overexpression, rather than from direct enhancement of the C4 cycle. Indeed, transgenic maize with reduced stomatal density (Liu et al., 2015) or increased stomatal sensitivity to abscisic acid (Brugière et al., 2017) had lower gs and increased WUE when subjected to drought. In addition, reduced gs under high VPD was successfully used as a trait in breeding programmes to produce more drought-tolerant maize (Messina et al., 2020).
After prolonged exposure, drought-induced reductions in An can no longer be rescued by high CO2, suggesting that biochemical limitation replaces stomatal conductance as the dominant control factor (Ghannoum, 2009; Ripley et al., 2010; Bellasio et al., 2018). The exact biochemical bottlenecks are not easy to pin-point, but could be related to impaired activities of CBB or C4 cycle enzymes. Although effects of drought on different photosynthetic enzymes appear strongly species dependent, impairment of Rubisco under drought conditions is often found (Du et al., 1996; Saliendra et al., 1996; Carmo-Silva et al., 2007; Ghannoum, 2009; Perdomo et al., 2017). However, maize with increased Rubisco content did not have a higher assimilation rate or plant growth under drought stress, and the overexpression was beneficial only for the recovery of photosynthesis after rewatering (Doron et al., 2020). Prolonged drought stress and reduced leaf water content may also negatively affect the integrity of the chloroplastic ATPase, and the resulting decline in ATP synthesis can decrease regeneration of substrates in CBB and C4 cycles (reviewed by Ghannoum, 2009). These phenomena are compounded by the enhanced build-up of excess excitation energy under stress conditions, when the absorbed light exceeds the energy requirements to drive C4 and CBB cycle activities. Whereas this is true for C3 and C4 species alike, the capacity for photoprotection under stressed conditions may be impaired in C4 plants in a cell type-specific manner. BS chloroplasts appear to have only limited capacity to undergo photoprotective movements. In addition, photorespiration is an important alternative electron sink to dissipate excessive excitation energy in C3 plants (Takahashi et al., 2007) which may be impacted by the C4 pathway. Photorespiratory mutants are lethal in maize (Zelitch et al., 2009), demonstrating the required presence of the pathway; however, it seems plausible that the capacity of the photorespiratory pathway in C4 species may be less sufficient to offer photoprotection under drought and high light stress.
Heat stress effects on C4 photosynthesis
Consistent with the model simulation (Fig. 5), Jmax has been shown to limit C4 photosynthesis at superoptimal temperatures (Pittermann and Sage, 2001a; Kubien et al., 2003; Dwyer et al., 2007), but the differences in electron transport characteristics between M and BS chloroplasts (Box 1) make it difficult to discern more specific bottlenecks from these data. Additional limitations to C4 photosynthesis associated with high temperatures include reduction in Rubisco capacity, RuBP regeneration, and reductions in Rca activity (Crafts-Brandner and Salvucci, 2002; Kubien et al., 2003). Impairment of Rca activity can severely reduce photosynthesis at high temperature (Salvucci et al., 2001; Carmo-Silva and Salvucci, 2012; Perdomo et al., 2017; Scafaro et al., 2018; Degen et al., 2021). Although reduced transcript levels of Rca at high temperature in the C4 species F. bidentis appeared not to be directly related to Rca protein accumulation (Hendrickson et al., 2008), intrinsic heat sensitivity of Rca in maize did explain decreased Rubisco activation (Perdomo et al., 2017). Differential expression of Rca-α and Rca-β isoforms in response to temperature may contribute to heat tolerance. Rca-α expression in five C4 grasses was induced by high temperatures (Kim et al., 2021) and appeared to be involved in Rca hexamer stability. These results are different from what has been seen in the C3 grass wheat, in which Rca1β is more thermostable among the three Rca isoforms Rca1β, Rca2β, and Rca1α (Scafaro et al., 2019; Degen et al., 2021), but similar to heat-treated rice (Wang et al., 2010). Specific amino acid substitutions between thermosensitive and thermotolerant isoforms have been identified to act as thermal and regulatory switches in wheat Rca which strongly impact performance under high temperature in vitro (Scafaro et al., 2019; Degen et al., 2020). Transgenic expression of the thermostable Rca from Oryza australiensis improved yields in heat-stressed O. sativa (Scafaro et al., 2018), and a similar strategy based on overexpression of a transgenic or mutated thermally stable Rca isoform may also hold promise for improving photosynthetic heat tolerance in C4 species.
Substantial natural genetic variation in photosynthesis of C4 crop species
The C4 photosynthetic bottlenecks discussed in the previous paragraphs are summarized in Table 1, which provides a list of key attributes that could have potential to improve photosynthesis. To find out whether these attributes could be improved via breeding, the presence of existing genetic variation in a species germplasm is a prerequisite. Although some diversity has probably been lost during domestication (Doebley et al., 2006), there appears to be significant genetic variation for photosynthetic traits in the germplasm of several C3 crops (Driever et al., 2014; Gaju et al., 2016; Carmo-Silva et al., 2017; Pennacchi et al., 2018; Molero et al., 2019; Acevedo-Siaca et al., 2020, 2021), with varying degrees of heritability, in some cases presenting clear opportunities for marker-assisted breeding of future cultivars (e.g. Adachi et al., 2017). In this section, we review progress in using natural genetic variation to improve C4 photosynthesis.
Table 1.
Summary of strategies to improve C4 photosynthesis and presence/absence of evidence from simulation modelling (S) or experimental data (E) under non-stressed steady state conditions or photosynthetic induction, cool temperature, high temperature and drought stress
| Factor | Strategy for improvement |
Non-stressed | Stressed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Steady-state | Induction | Cool temperature | High temperature | Drought | |||||||
| S (Fig. 3) | E | S (Fig. 4) | E | S (Fig. 5) | E | S (Fig. 5) | E | S (Fig. 5) | E | ||
| g s | ✘ | ? | ✓ | ? | ✘ | ? | ✘ | ? | ✓ | ✓(1) ✘(2) | |
|
Electron
transport |
Enhance CET | ✘(3) | ✓ | ? | ✘ | ? | ✓ | ? | ✘ | ? | |
|
Increase
Rieske FeS |
✓ | ✓(4) | ? | ? | ? | ? | |||||
| Speed up NPQ | ? | ? | ? | ? | ? | ||||||
| C 4 cycle | PEPC | ✘ | ✘(5) | ✓ | ✓(5) | ✘ | ? | ✘ | ? | ✘ | ✓(6) |
| NADP-ME | ✘ | ? | ✘ | ? | ✓ | ? | ✘ | ? | ✘ | ? | |
| PPDK | ✘ | ? | ✓ | ? | ✓ | ? | ✘ | ? | ✘ | ? | |
| CBB cycle: Rubisco | Improve k cat | ? | ? | ? | ? | ? | |||||
| Change small subunit expression | ? | ? | ? | ? | ? | ||||||
| Increase content | ✓ | ✓(7) | ✓ | ? | ✓ | ✘(8) | ✓ | ? | ✘ | ✘(9) | |
|
Increase Rca
expression |
? | ? | ? | ? | ? |
Symbols denote no improvement of CO2 assimilation rate (✘), improvement of CO2 assimilation rate (✓) or unknown effect (?).
References: 1, Liu et al. (2015); 2, Brugière et al. (2017); 3, Tazoe et al. (2020); 4, Ermakova et al. (2019); 5, Laisk and Edwards, 1997; 6, Jeanneau et al. (2002); 7, Salesse-Smith et al. (2018); 8, Salesse-Smith et al. (2020); 9, Doron et al. (2020).
Chilling tolerance
Considerable variation in sensitivity to chilling-induced photoinhibition exists between accessions of different C4 species, as shown for example in maize (e.g. Aguilera et al., 1999; Fracheboud et al., 2004; Pimentel et al., 2005) and sorghum (Ortiz et al., 2017), but the mechanistic and genetic basis of this variation still remains largely undefined. The latter study with 304 sorghum accessions showed that there is significant natural variation in the photosynthetic response of sorghum lines to cold stress and their capacity to recover, and several putative genomic regions and candidate genes were identified. The understanding of allelic variants associated with these physiological traits could help identify key processes and genes to manipulate by breeding or engineering approaches (Ortiz et al., 2017). In maize, the replacement of landraces by hybrids has drastically reduced the allelic diversity utilized in elite germplasm. However, large-scale production of doubled-haploid lines from promising landrace accessions coupled with genotyping and broad phenotypic characterization should help to make the allelic diversity of landrace collections more readily available for breeding programmes. This approach was recently applied to European flint maize (Hölker et al., 2019). As an early sign of their potential to improve chilling tolerance in maize, doubled-haploid lines from these European landraces outperformed flint founder lines as well as commercial hybrids in early development across a range of 11 temperate environments.
An alternative strategy to improve chilling tolerance is via introgressions from closely related tolerant C4 species. The generation of intergeneric hybrids between chilling-sensitive Saccharum and the chilling-tolerant Miscanthus which show high levels of chilling tolerance in F1 ‘miscanes’ (Kar et al., 2019) can be seen as a first step in this strategy, which could allow production of sugarcane and energy cane cultivars for more temperate climes. The recent publication of the Miscanthus sinensis genome (Mitros et al., 2020) will help to accelerate the identification of genomic regions specifically relevant to its superior performance under low temperature.
Water-use efficiency
C4 plants are generally more efficient in water use than C3 plants, but might be improved further by leveraging natural variation in WUE traits within crop germplasm (reviewed by Leakey et al., 2019). Leaf-level WUE and its component traits An and gs show significant within-species variation in diversity or mapping populations of several C4 species, such as switchgrass (Taylor et al., 2016), sorghum (Ferguson et al., 2020a Preprint), and maize (Xie et al., 2020). Sugarcane genotypes with higher WUE, due to lower gs in combination with high photosynthetic capacity, were identified by Li et al. (2017), which may offer breeding potential for higher WUE. Similarly, Pignon et al. (2021) identified significant variation in leaf WUE traits across a collection of contrasting sorghum lines. However, detailed analysis of steady-state and dynamic WUE and its component traits An and gs across these accessions also identified inherent trade-offs between trait combinations which may severely limit the potential for improvement. Because An and gs are positively correlated, the range of variation in their ratio (WUE) is typically smaller than for the individual component traits. In most of the aforementioned studies, the major source of variation in leaf-level WUE is gs. Using hierarchical grouping by gs classes, Jackson et al. (2016) identified leaf intercellular [CO2] as a major correlate with leaf-level and whole-plant metrics for WUE in sugarcane. In C3 plants, the normalized Ci/Ca metric and related isotopic proxy (∆ 13C) have been successfully applied to develop wheat lines with higher WUE (Condon et al., 2004), which may also be possible in C4 species (Ellsworth and Cousins, 2016; Eggels et al., 2021). Δ 13C can be strongly genetically determined in C4 species such as maize (Gresset et al., 2014; Twohey et al., 2018). However, the theoretical slope of the correlation between ∆ 13C and Ci/Ca in C4 species can be negative or positive since the relationship is confounded by the impact of BS leakiness (i.e. the rate of retrodiffusion of CO2 from the BS cells relative to the rate of PEP carboxylation). Although BS leakiness is relatively constant under most conditions, it can increase considerably under drought or nutrient stress conditions (reviewed by Kromdijk et al., 2014) and in particular under low light intensity (Kromdijk et al., 2008, 2010). Despite these complications, co-localized quantitative trait loci (QTLs) for ∆ 13C and several WUE-related traits have been found in maize (Avramova et al., 2019) as well as in Setaria (Ellsworth et al., 2020).
Semi-automated pipelines to characterize leaf-level (e.g. Ferguson et al., 2020a, Xie et al., 2020) or whole-plant WUE (e.g. Feldman et al., 2018) can increase phenotyping throughput dramatically, which helps to alleviate the phenotyping bottleneck. Additional challenges associated with identification of genes or genomic loci underpinning differences in WUE are associated with the polygenic nature of the trait. To improve the reliability of genetic associations, several new approaches are being pioneered. Ferguson et al. (2020a) demonstrated that the integration of genome-wide and transcriptome-wide association studies (Kremling et al., 2019) can be used to identify candidate genes for WUE with enhanced confidence. Feldman et al. (2018) used their temporally rich dataset of whole-plant WUE in a Setaria italica×S. viridis recombinant inbred line population to develop function-valued QTL models based on the average log of the odds score across the time course of the experiment, yielding fewer, higher confidence QTL. Predictive models can also be used to speed up breeding efforts, by linking genomic variation with physiological trait variation, and simulate the impact across a wide range of environments (e.g. Inman-Bamber et al., 2016; Kadam et al., 2019; Wu et al., 2019).
Heat tolerance
Unfavourably high temperatures can severely impact crop yields. In sugarcane, high temperature induced significant decreases in net assimilation rate, maximal PSII efficiency, and activities of sucrose synthase and sucrose phosphate synthase, all of which were more strongly impacted in heat-sensitive compared with heat-tolerant genotypes (Kohila and Gomathi, 2018). In grain crops, the temperature during reproductive development is particularly critical, and temperatures >30 °C during flowering and seed set can negatively impact yield in maize and sorghum (reviewed by Kaushal et al., 2016). The C4 photosynthetic temperature response peaks at a higher temperature than the damage threshold temperature during reproductive development, hence crop breeding programmes have mostly focused on traits associated with reproductive success, such as pollen viability, stigma receptivity, and seed set percentage (e.g. Alam et al., 2017) to improve crop heat tolerance. Substantial variation in heat tolerance is present in germplasm of C4 crops such as sorghum (e.g. Chopra et al., 2017) and maize (Cairns et al., 2013; Naveed et al., 2016), but it is often unclear how much of this is underpinned by variation in photosynthetic, rather than reproductive, traits. Using different sowing dates to modulate exposure to moderate seasonal heat stress, Yadav et al. (2016) found a significant genotype-specific treatment response in photosynthesis rates across a panel of 21 maize inbred lines, which was correlated with biomass productivity. The genotypic differences in photosynthesis responses were not explained by dark-adapted PSII quantum yields, suggesting that enzymatic inhibition, rather than PSII inactivation, explained the observed differences. Metabolic profiling of two contrasting maize genotypes in response to sudden heat stress identified that the levels of nine key metabolites were strongly predictive for the difference in leaf photosynthetic recovery between the two lines (Qu et al., 2018). If this approach is more generally applicable, it may offer potential for high-throughput screening of photosynthetic heat tolerance. ‘Heat tents’ were used by Sunoj et al. (2017) to raise average temperature by 8 °C during growth of a collection of sorghum genotypes, achieving quite severe heat stress, with daytime maximum temperatures kept at 45 °C. The net assimilation rate was decreased in a genotype-dependent manner, and fluorescence-estimated thylakoid damage was weakly correlated with the heat stress inhibition, suggesting that this level of heat stress was sufficient to inactivate PSII. Considering that severe heat stress is particularly damaging to the oxygen-evolving complex of PSII (Murata et al., 2007), chlorophyll fluorescence measurements of PSII efficiency can be used to screen photosynthetic responses to heat stress (Murchie et al., 2018). Ferguson et al. (2020b) developed high-throughput screening for heat tolerance in rice by combining visual (stay-green) responses with dark-adapted PSII quantum yields to rapidly increasing temperatures in excised leaf material, which may be applicable to C4 species such as sorghum and maize.
Taken together, there appears to be substantial natural genetic variation in photosynthesis within germplasm of C4 crops, but physiological interpretation is often too minimal to assess how this variation is connected to the traits summarized in Table 1. Addressing this knowledge gap will require development of high-throughput proxies that specifically inform about the attributes in Table 1.
How could increased photosynthetic efficiency enhance yield in C4 crops?
Whereas the previous paragraphs have reviewed the range of strategies to alleviate photosynthetic bottlenecks in C4 crops and the potential to utilize natural genetic variation to make improvements, in this final section, the potential impacts of photosynthetic efficiency gains on yield are reviewed by looking at source–sink interactions in three main C4 crops: maize, sugarcane, and sorghum.
Photosynthetic source activity affects sink establishment and grain filling in major C4 crops
The constraints to growth and productivity can be formulated in terms of supply and demand, or source versus sink (for a detailed discussion of the source–sink concept, see White et al., 2016; Chang and Zhu, 2017). Plants need a supply of carbohydrates from photosynthesis, water, and mineral nutrients to provide the building blocks and energetic demands to produce new tissue. When supply is insufficient, the growth that is realized will fall short of the potential growth. If so, growth would be source limited. Alternatively, growth can be constrained by the capacity of the growing parts to accumulate biomass. In this situation, an increase in resource availability would not stimulate growth, which is termed as being sink limited. The relative simplicity of this concept is deceptive, and sink and source limitations are not mutually exclusive, but instead growth patterns continuously reflect a relative balance between source and sink constraints, termed the source–sink balance. The source–sink concept applied specifically to the plant’s carbon economy has been a popular framework for analysis of the interplay between photosynthetic activity and yield. For this purpose, the sink strength of the harvestable parts relative to the photosynthetic activity of the leaves reflects the source–sink balance. The plant parts that form the sink can, therefore, be markedly different between crop species.
For maize, the sink strength of the harvestable parts constitutes the developing ears, which can be seen as a collection of competing kernels. Sink strength at the plant level is largely controlled by the crop growth rate around silking, which strongly determines the number of kernels that set and fill (Tollenaar et al., 1992; Vega et al., 2001). The utilization of hybrid technology in maize has led to increased light capture and utilization via enhanced growing season length, and increased leaf area index and stay-green traits to maintain photosynthetic efficiency longer, jointly raising source capacity by an estimated 113% (Lee and Tollenaar, 2007). Since the harvest index in hybrids is maintained at ~50%, sink strength appears to have increased in proportion to source capacity, which can be explained by the strong relationship between dry matter accumulation around silking and establishment of sink size via kernel number (Echarte et al., 2004). Further evidence for the role of photosynthesis in seed set and grain filling comes from defoliation experiments. Defoliation in maize leads to a decrease in grain yield (Barnett and Pearce, 1983) and grain quality (Shekoofa et al., 2010), with the effect on kernel number and grain filling being dependent on the timing of defoliation. Leaf removal around silking has a strong impact on kernel number and yield, whereas leaf removal at later stages only impacts grain filling and has less impact on yield (Tollenaar and Daynard, 1978).
In sugarcane, the sink constitutes the sugar-accumulating culms, composed of elongated internodes. The accumulation of sugar to high concentrations (~500 mM sucrose in internode juice; Wu and Birch, 2006) is facilitated by several specialized features in the sucrose loading and translocation pathway (Wang et al., 2013). It includes high expression of SWEET13 sugar transporter genes in the photosynthetically mature leaf parts (Hu et al., 2018), a fine balance between soluble acid invertase and sucrose phosphate synthase to control the rate of sucrose formation in internodes, and expression of a specific sucrose transporter gene ShSUT1 to prevent sucrose backflow into the apoplast (Rae et al., 2005). Despite these specialized features, accumulation of sucrose in leaves of sugarcane can have a strong negative feedback on photosynthetic capacity. Partial shading or intermittent darkening of leaves alleviates feedback inhibition of photosynthesis (McCormick et al., 2006, 2008a; Ribeiro et al., 2017), whereas cold girdling or exogenous sucrose application promotes down-regulation of photosynthesis (McCormick et al., 2008b; Lobo et al., 2015). The inhibiting effect of sucrose accumulation seems to rely on signals derived from the concomitant accumulation of hexoses, trehalose-6-phosphate (T6P), and/or expression of hexokinase. The manipulation of these signals may allow decoupling of source activity from sink feedback and enable even greater sucrose accumulation (McCormick et al., 2009; Chandra et al., 2011).
In sorghum, the parts constituting the relevant sinks for yield are dependent on the variety, and can vary from the grains (grain sorghum) to the stem (sweet sorghum), or a combination of both in dual-purpose production systems. In sweet sorghum, the accumulation of sucrose in the stem internodes is facilitated by altered expression of several sucrose transporters (Milne et al., 2013) and vacuolar invertase isoforms (Chi et al., 2020), compared with grain varieties. In grain sorghum, grain sink strength is strongly determined by seed set in the panicle, which in turn depends on the crop growth rate around anthesis. As a result, photosynthetic activity around this period is especially important for sink formation and yield, similar to the situation in maize. Consistently, leaf removal at booting and anthesis stages has a strong negative impact on grain sorghum yield via reduction in seed number, as well as average seed weight (Stickler and Pauli, 1961; Legwaila et al., 2013). In dual-purpose production systems, both stem and grain are important sinks for yield. While the elongating internodes are potent sinks during vegetative growth, the grains develop later. Panicle pruning in a range of tropical sorghum genotypes did not affect stem sugar content (Gutjahr et al., 2013a, b), suggesting that competition between both sinks is largely prevented due to the temporal separation in development.
Crop management can have important implications for source–sink interactions. Strong source activity during the development and filling of sinks is needed for stable and high yields, but can be affected by the timing of planting. For example, late planting in sub-Saharan Sudan–Sahelian climates can expose sorghum to severe post-anthesis droughts, which negatively impacts grain filling (Tovignan et al., 2016). Longer maintenance of green leaf area during drought periods via stay-green traits can mitigate some of this yield loss in both grain and sweet sorghum (Borrell et al., 2000; Tovignan et al., 2016). Planting dates can also impact source–sink interactions at crucial developmental stages. For example, late plantings of summer maize to align crop growth with the timing of rainfall in rain-fed cropping systems of Argentina can push back the period of grain filling into weather with unfavourably low light levels, leading to low photosynthetic activity, decreased grain filling, and yield loss (Bonelli et al., 2016). A similar situation occurs in temperate monsoon climates in Northern China, where late plantings can confine maize grain filling to a period with suboptimal light levels, in this case caused instead by the onset of the rainy season (Gao et al., 2017).
Increasing plant density leads to a lower source–sink ratio
A major trend in crop production systems of grain crops is the steady increase in plant density. In species with only a few tillers such as sorghum, or with only a single stem such as maize, increasing plant density will increase the number of ears or panicles per unit land area. Taking maize as an example, planting density across nine US corn belt states has increased by 0.07±0.01 plants m−2 year−1 since the 1990s (Assefa et al., 2018) and is seen as an important factor underlying the steady increase in maize yield. How attainable these yield gains are depends on other crop management factors such as the fertilization level (Ruffo et al., 2016), as well as the weather conditions during the growing season. Despite breeding efforts to facilitate high planting density, for example steeper leaf angles, under stressful conditions such as drought, increased plant density may enhance year-by-year yield variability, via increased competition between neighbouring plants (Lobell et al., 2014).
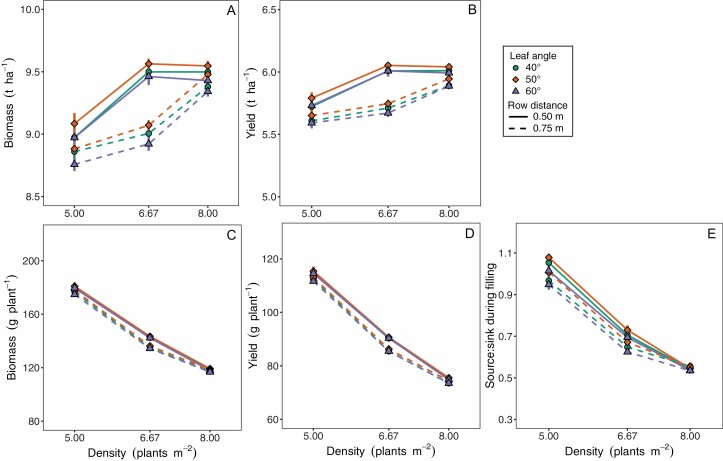
In addition, we reason that the increases in planting density have profound effects on source–sink balance by promoting sink strength, whereas photosynthetic activity is largely determined by the incident irradiance and much less impacted by plant density. To demonstrate the impact of planting density on source–sink balance further, we used a 3D functional–structural model (previously described by Evers and Bastiaans, 2016) to simulate a maize crop at different planting densities. The model simulations show that for the observed increase in average maize plant density from five to eight plants m−2 between 1987 and 2016 (Assefa et al., 2018), productivity and yield per unit area marginally increase (Fig. 6A, B), but productivity and yield per plant decline (Fig. 6C, D) while source–sink balance approximately halves (Fig. 6E). These results are only weakly affected by more upright leaf angles (different symbols, Fig. 6), which favours canopy photosynthetic activity in modern maize hybrids via more uniform vertical light distribution across the canopy (Ort et al., 2015). Thus, crop yield becomes more strongly source limited with increasing plant density, and the general trend of increasing plant density is likely to enhance the importance of photosynthetic efficiency for yield, especially in the grain crops sorghum and maize.
Fig. 6.
Simulated biomass productivity, yield and source-sink ratio of maize grown at different plant densities. Results are shown at field (A, B) and plant level (C, D) as means ±SD of 4–5 simulations. Source: sink ratio (E) was calculated during the grain-filling period. Input parameters were varied to simulate different leaf declination angles of 40°, 50°, and 60° (different symbols); and common row distances (Li et al., 2015; USDA, 2020b) of 0.50 m (solid line) and 0.75 m (dotted line). For a full description of the model and parameters, see Evers and Bastiaans (2016).
Conclusions
In this review we have explored the case for improvement of photosynthetic efficiency in C4 crops as a means to enhance productivity and yield. Despite the limited focus on improving photosynthetic efficiency in C4 compared with C3 species, there appears to be substantial evidence that this strategy may be achievable and beneficial for yield. Using model analysis and literature review, several tangible bottlenecks within the C4 pathway could be identified which exert strong control under relevant conditions for crop productivity, some of which can be alleviated via leveraging natural genetic variation in crop breeding programmes, whereas others may only be improved successfully via transgenic or gene editing methods. The decline in source–sink balance due to increases in planting density is likely to enhance the importance of photosynthetic efficiency for yield. Considering the predicted magnitude of the shortfall between food supply and demand, the timelines involved in crop breeding programmes, and the importance of C4 crops for global food, feed, and fuel production, implementing photosynthetic improvement as part of the C4 crop improvement toolbox is both urgent and timely.
Acknowledgements
The authors thank Julian Hibberd for valuable comments on earlier versions of the manuscript. This work was supported by the Biotechnology and Biological Sciences Research Council via grant BB/T007583/1 and the UK Research and Innovation - Future Leaders Fellowships scheme via an award to JK (MR/T042737/1).
References
- Acevedo-Siaca LG, Coe R, Quick WP, Long SP. 2021. Variation between rice accessions in photosynthetic induction in flag leaves and underlying mechanisms. Journal of Experimental Botany 72, 1282–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Siaca LG, Coe R, Wang Y, Kromdijk J, Quick WP, Long SP. 2020. Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytologist 227, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Yoshikawa K, Yamanouchi U, Tanabata T, Sun J, Ookawa T, Yamamoto T, Sage RF, Hirasawa T, Yonemaru J. 2017. Fine mapping of Carbon Assimilation Rate 8, a quantitative trait locus for flag leaf nitrogen content, stomatal conductance and photosynthesis in rice. Frontiers in Plant Science 8, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera C, Stirling CM, Long SP. 1999. Genotypic variation within Zea mays for susceptibility to and rate of recovery from chill-induced photoinhibition of photosynthesis. Physiologia Plantarum 106, 429–436. [Google Scholar]
- Alam MA, Seetharam K, Zaidi PH, Dinesh A, Vinayan T, Nath UK. 2017. Dissecting heat stress tolerance in tropical maize (Zea mays L.). Field Crops Research 204, 110–119. [Google Scholar]
- Arrivault S, Moraes TA, Obata T, et al. 2019. Metabolite profiles reveal interspecific variation in operation of the Calvin–Benson cycle in both C4 and C3 plants. Journal of Experimental Botany 70, 1843–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrivault S, Obata T, Szecówka M, Mengin V, Guenther M, Hoehne M, Fernie AR, Stitt M. 2017. Metabolite pools and carbon flow during C4 photosynthesis in maize: 13CO2 labeling kinetics and cell type fractionation. Journal of Experimental Botany 68, 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa Y, Carter P, Hinds M, Bhalla G, Schon R, Jeschke M, Paszkiewicz S, Smith S, Ciampitti IA. 2018. Analysis of long term study indicates both agronomic optimal plant density and increase maize yield per plant contributed to yield gain. Scientific Reports 8, 4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson N, Leitão N, Orr DJ, Meyer MT, Carmo-Silva E, Griffiths H, Smith AM, McCormick AJ. 2017. Rubisco small subunits from the unicellular green alga Chlamydomonas complement Rubisco-deficient mutants of Arabidopsis. New Phytologist 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramova V, Meziane A, Bauer E, et al. 2019. Carbon isotope composition, water use efficiency, and drought sensitivity are controlled by a common genomic segment in maize. Theoretical and Applied Genetics 132, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR, Nie GY. 1994. Chilling sensitivity of photosynthesis in maize. In: Bajaj YPS, ed. Biotechnology in agriculture and forestry: maize. Berlin: Springer-Verlag, 465–481. [Google Scholar]
- Barnett KH, Pearce RB. 1983. Source–sink ratio alteration and its effect on physiological parameters in maize. Crop Science 23, 294–299. [Google Scholar]
- Becker TW, Fock HP. 1986. Effects of water stress on the gas exchange, the activities of some enzymes of carbon and nitrogen metabolism, and on the pool sizes of some organic acids in maize leaves. Photosynthesis Research 8, 175–181. [DOI] [PubMed] [Google Scholar]
- Bellasio C, Griffiths H. 2014. The operation of two decarboxylases, transamination, and partitioning of C4 metabolic processes between mesophyll and bundle sheath cells allows light capture to be balanced for the maize C4 pathway. Plant Physiology 164, 466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellasio C, Quirk J, Beerling DJ. 2018. Stomatal and non-stomatal limitations in savanna trees and C4 grasses grown at low, ambient and high atmospheric CO2. Plant Science 274, 181–192. [DOI] [PubMed] [Google Scholar]
- Bonelli LE, Monzon JP, Carrudo A, Rizzalli RH, Andrade FH. 2016. Maize grain yield components and source–sink relationship as affected by the delay in sowing date. Field Crops Research 198, 215–225. [Google Scholar]
- Borrell AK, Hammer GL, Douglas ACL. 2000. Does maintaining green leaf area in sorghum improve yield under drought? I. Leaf growth and senescence. Crop Science 40, 1026–1037. [Google Scholar]
- Brugière N, Zhang W, Xu Q, et al. 2017. Overexpression of RING Domain E3 ligase ZmXerico1 confers drought tolerance through regulation of ABA homeostasis. Plant Physiology 175, 1350–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacefo V, Ribas AF, Zilliani RR, Neris DM, Domingues DS, Moro AL, Vieira LGE. 2019. Decarboxylation mechanisms of C4 photosynthesis in Saccharum spp.: increased PEPCK activity under water-limiting conditions. BMC Plant Biology 19, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns LE, Crossa J, Zaidi PH, et al. 2013. Identification of drought, heat, and combined drought and heat tolerant donors in maize. Crop Science 53, 1335–1346. [Google Scholar]
- Calsa T Jr, Figueira A. 2007. Serial analysis of gene expression in sugarcane (Saccharum spp.) leaves revealed alternative C4 metabolism and putative antisense transcripts. Plant Molecular Biology 63, 745–762. [DOI] [PubMed] [Google Scholar]
- Carmo-Silva AE, Salvucci ME. 2012. The temperature response of CO2 assimilation, photochemical activities and Rubisco activation in Camelina sativa, a potential bioenergy crop with limited capacity for acclimation to heat stress. Planta 236, 1433–1445. [DOI] [PubMed] [Google Scholar]
- Carmo-Silva AE, Soares AS, Marques da Silva J, Bernardes da Silva A, Keys AJ, Arrabaça MC. 2007. Photosynthetic response of three C4 grasses of metabolic subtypes to water deficit. Functional Plant Biology 34, 204–213. [DOI] [PubMed] [Google Scholar]
- Carmo-Silva E, Andralojc PJ, Scales JC, Driever SM, Mead A, Lawson T, Raines CA, Parry MAJ. 2017. Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. Journal of Experimental Botany 68, 3473–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Silva E, Scales JC, Madgwick PJ, Parry MA. 2015. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant, Cell & Environment 38, 1817–1832. [DOI] [PubMed] [Google Scholar]
- Chandra A, Jain R, Rai RK, Solomon S. 2011. Revisiting the source–sink paradigm in sugarcane. Current Science 100, 978–980. [Google Scholar]
- Chang TG, Zhu XG, Raines C. 2017. Source–sink interaction: a century old concept under the light of modern molecular systems biology. Journal of Experimental Botany 68, 4417–4431. [DOI] [PubMed] [Google Scholar]
- Chastain CJ, Failing CJ, Manandhar L, Zimmerman MA, Lakner MM, Nguyen TH. 2011. Functional evolution of C4 pyruvate, orthophosphate dikinase. Journal of Experimental Botany 62, 3083–3091. [DOI] [PubMed] [Google Scholar]
- Chen YB, Lu TC, Wang HX, Shen J, Bu TT, Chao Q, Gao ZF, Zhu XG, Wang YF, Wang BC. 2014. Posttranslational modification of maize chloroplast pyruvate orthophosphate dikinase reveals the precise regulatory mechanism of its enzymatic activity. Plant Physiology 165, 534–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Wilson K, Liu Z, Wu X, Shang L, Zhang L, Jing H, Hao H. 2020. Vacuolar invertase genes SbVIN1 and SbVIN2 are differently associated with stem and grain traits in sorghum (Sorghum bicolor). The Crop Journal 8, 299–312. [Google Scholar]
- Chinthapalli B, Murmu J, Raghavendra AS. 2003. Dramatic difference in the responses of phosphoenolpyruvate carboxylase to temperature in leaves of C3 and C4 plants. Journal of Experimental Botany 54, 707–714. [DOI] [PubMed] [Google Scholar]
- Chopra R, Burow G, Burke JJ, Gladman N, Xin Z. 2017. Genome-wide association analysis of seedling traits in diverse Sorghum germplasm under thermal stress. BMC Plant Biology 17, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Samaritani E, Petitpierre B, Salamin N, Besnard G. 2009. Evolutionary insights on C4 photosynthetic subtypes in grasses from genomics and phylogenetics. Genome Biology and Evolution 1, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONAB. 2018. Acompanhamento da safra brasileira de cana-de-açúcar. http://www.conab.gov.br. Accessed January 2021.
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. 2004. Breeding for high water-use efficiency. Journal of Experimental Botany 55, 2447–2460. [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME. 2002. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiology 129, 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Ku M, Edwards GE. 1993. C4 photosynthesis (the CO2-concentrating mechanism and photorespiration). Plant Physiology 103, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen GE, Orr DJ, Carmo-Silva E. 2021. Heat-induced changes in the abundance of wheat Rubisco activase isoforms. New Phytologist 229, 1298–1311. [DOI] [PubMed] [Google Scholar]
- Degen GE, Worrall D, Carmo-Silva E. 2020. An isoleucine residue acts as a thermal and regulatory switch in wheat Rubisco activase. The Plant Journal 103, 742–751. [DOI] [PubMed] [Google Scholar]
- DiMario RJ, Cousins AB. 2019. A single serine to alanine substitution decreases bicarbonate affinity of phosphoenolpyruvate carboxylase in C4Flaveria trinervia. Journal of Experimental Botany 70, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley JF, Gaut BS, Smith BD. 2006. The molecular genetics of crop domestication. Cell 127, 1309–1321. [DOI] [PubMed] [Google Scholar]
- Dohleman FG, Long SP. 2009. More productive than maize in the Midwest: how does Miscanthus do it? Plant Physiology 150, 2104–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncaster HD, Leegood RC. 1987. Regulation of phosphoenolpyruvate carboxylase activity in maize leaves. Plant Physiology 84, 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doron L, Xu L, Rachmilevitch S, Stern DB. 2020. Transgenic overexpression of rubisco subunits and the assembly factor RAF1 are beneficial to the recovery from drought stress in maize. Environmental and Experimental Botany 177, 104126. [Google Scholar]
- Doulis AG, Debian N, Kingston-Smith AH, Foyer CH. 1997. Differential localization of antioxidants in maize leaves. Plant Physiology 114, 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever SM, Lawson T, Andralojc PJ, Raines CA, Parry MA. 2014. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. Journal of Experimental Botany 65, 4959–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y-C, Kawamitsu Y, Nose A, Hiyane S, Murayama S, Muraya S, Wasabi K, Uchida Y. 1996. Effects of water stress in carbon exchange rate and activities of photosynthetic enzyme in leaves of sugarcane (Saccharum sp.). Functional Plant Biology 23, 719–726. [Google Scholar]
- Du Y-C, Nose A, Wasano K. 1999. Effects of chilling temperature on photosynthetic rates, photosynthetic enzyme activities and metabolite levels in leaves of three sugarcane species. Plant, Cell & Environment 22, 317–324. [Google Scholar]
- Dwyer SA, Ghannoum O, Nicotra A, von Caemmerer S. 2007. High temperature acclimation of C4 photosynthesis is linked to changes in photosynthetic biochemistry. Plant, Cell & Environment 30, 53–66. [DOI] [PubMed] [Google Scholar]
- Echarte L, Andrade FH, Vega CRC, Tollenaar M. 2004. Kernel number determination in Argentinean maize hybrids released between 1965 and 1993. Crop Science 44, 1654–1661. [Google Scholar]
- Edwards GE, Nakamoto H, Burnell JN, Hatch MD. 1985. Pyruvate,Pi dikinase and NADP-malate dehydrogenase in C4 photosynthesis: properties and mechanism of light/dark regulation. Annual Review of Plant Physiology 36, 255–286. [Google Scholar]
- Eggels S, Blankenagel S, Schön CC, Avramova V. 2021. The carbon isotopic signature of C4 crops and its applicability in breeding for climate resilience. Theoretical and Applied Genetics 134, 1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth PZ, Cousins AB. 2016. Carbon isotopes and water use efficiency in C4 plants. Current Opinion in Plant Biology 31, 155–161. [DOI] [PubMed] [Google Scholar]
- Ellsworth PZ, Feldman MJ, Baxter I, Cousins AB. 2020. A genetic link between leaf carbon isotope composition and whole-plant water use efficiency in the C4 grass Setaria. The Plant Journal 102, 1234–1248. [DOI] [PubMed] [Google Scholar]
- Ermakova M, Lopez-Calcagno PE, Raines CA, Furbank RT, von Caemmerer S. 2019. Overexpression of the Rieske FeS protein of the Cytochrome b6f complex increases C4 photosynthesis in Setaria viridis. Communications Biology 2, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers JB, Bastiaans L. 2016. Quantifying the effect of crop spatial arrangement on weed suppression using functional–structural plant modelling. Journal of Plant Research 129, 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO. 2020. FAOSTAT database. http://www.fao.org/faostat. Accessed January 2021.
- Farquhar GD. 1983On the nature of carbon isotope discrimination in C4 species. Australian Journal of Plant Physiology 10, 205–226. [Google Scholar]
- Feldman MJ, Ellsworth PZ, Fahlgren N, Gehan MA, Cousins AB, Baxter I. 2018. Components of water use efficiency have unique genetic signatures in the model C4 grass Setaria. Plant Physiology 178, 699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Fernandes SB, Monier B, et al. 2020a. Machine learning enabled phenotyping for GWAS and TWAS of WUE traits in 869 field-grown sorghum accessions. bioRxiv doi.org/ 10.1101/2020.11.02.365213. [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, McAusland L, Smith KE, Price AH, Wilson ZA, Murchie EH. 2020b. Rapid temperature responses of photosystem II efficiency forecast genotypic variation in rice vegetative heat tolerance. The Plant Journal 104, 839–855. [DOI] [PubMed] [Google Scholar]
- Foley JA, Ramankutty N, Brauman KA, et al. 2011. Solutions for a cultivated planet. Nature 478, 337–342. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J. 2012. Photosynthetic control of electron transport and the regulation of gene expression. Journal of Experimental Botany 63, 1637–1661. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Valadier MH, Migge A, Becker TW. 1998. Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiology 117, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracheboud Y, Jompuk C, Ribaut JM, Stamp P, Leipner J. 2004. Genetic analysis of cold-tolerance of photosynthesis in maize. Plant Molecular Biology 56, 241–253. [DOI] [PubMed] [Google Scholar]
- Furbank RT. 2011. Evolution of the C4 photosynthetic mechanism: are there really three C4 acid decarboxylation types? Journal of Experimental Botany 62, 3103–3108. [DOI] [PubMed] [Google Scholar]
- Gaju O, DeSilva J, Carvalho P, Hawkesford MJ, Griffiths S, Greenland A, Foulkes MJ. 2016. Leaf photosynthesis and associations with grain yield, biomass and nitrogen-use efficiency in landraces, synthetic-derived lines and cultivars in wheat. Field Crops Research 193, 1–15. [Google Scholar]
- Gao Z, Liang X-G, Lin S, Zhao X, Zhang L, Zhou L-L, Shen S, Zhou S-L. 2017. Supplemental irrigation at tasseling optimizes water and nitrogen distribution for high-yield production in spring maize. Field Crops Research 209, 120–128. [Google Scholar]
- Ghannoum O. 2009. C4 photosynthesis and water stress. Annals of Botany 103, 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, Evans JR, Chow WS, Andrews TJ, Conroy JP, von Caemmerer S. 2005. Faster Rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiology 137, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Głowacka K, Ahmed A, Sharma S, Abbott T, Comstock JC, Long SP, Sacks EJ. 2016. Can chilling tolerance of C4 photosynthesis in Miscanthus be transferred to sugarcane? Global Change Biology 8, 407–418. [Google Scholar]
- Gowik U, Westhoff P. 2011. The path from C3 to C4 photosynthesis. Plant Physiology 155, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantz DA. 1989. Effect of cool temperatures on photosynthesis and stomatal conductance in field-grown sugarcane in Hawaii. Field Crops Research 22, 143–155. [Google Scholar]
- Gresset S, Westermeier P, Rademacher S, Ouzunova M, Presterl T, Westhoff P, Schön CC. 2014. Stable carbon isotope discrimination is under genetic control in the C4 species maize with several genomic regions influencing trait expression. Plant Physiology 164, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M, Gracen VE, Edwards GE. 1974. Biochemical and cytological relationships in C4 plants. Planta 65, 3567–3578. [DOI] [PubMed] [Google Scholar]
- Gutjahr S, Vaksmann M, Dingkuhn ML, Thera K, Trouche G, Braconnier S, Luquet D. 2013a. Grain, sugar and biomass accumulation in tropical sorghums. I. Trade-offs and effects of phenological plasticity. Functional Plant Biology 40, 342–354. [DOI] [PubMed] [Google Scholar]
- Gutjahr S, Vaksmann M, Dingkuhn M, Thera K, Trouche G, Braconnier S, Luquet D. 2013b. Grain, sugar and biomass accumulation in tropical sorghums. II. Biochemical processes at internode level and interaction with phenology. Functional Plant Biology 40, 355–368. [DOI] [PubMed] [Google Scholar]
- Hatch MD. 1971. The C4-pathway of photosynthesis: evidence for an intermediate pool of carbon dioxide and the identity of the donor C4-dicarboxylic acid. The Biochemical Journal 125, 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895, 81–106. [Google Scholar]
- Hatch MD, Kagawa T, Craig S. 1975. Subdivision of C4-pathway species based on differing C4 decarboxylating system and ultrastructural features. Australian Journal of Plant Physiology 2, 111–128. [Google Scholar]
- Hendrickson L, Sharwood R, Ludwig M, Whitney SM, Badger MR, von Caemmerer S. 2008. The effects of Rubisco activase on C4 photosynthesis and metabolism at high temperature. Journal of Experimental Botany 59, 1789–1798. [DOI] [PubMed] [Google Scholar]
- Hermida-Carrera C, Kapralov MV, Galmés J. 2016. Rubisco catalytic properties and temperature response in crops. Plant Physiology 171, 2549–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington SE, He J, Smillie RM. 1989. Photoinhibition at low temperature in chilling-sensitive and -resistant plants. Plant Physiology 90, 1609–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölker AC, Mayer M, Presterl T, Bolduan T, Bauer E, Ordas B, Brauner PC, Ouzunova M, Melchinger AE, Schön CC. 2019. European maize landraces made accessible for plant breeding and genome-based studies. Theoretical and Applied Genetics 132, 3333–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Hua X, Zhang Q, et al. 2018. New insights into the evolution and functional divergence of the SWEET family in Saccharum based on comparative genomics. BMC Plant Biology 18, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman-Bamber NG, Jackson PA, Stokes CJ, Verrall S, Lakshmanan P, Basnayake J. 2016. Sugarcane for water-limited environments: enhanced capability of the APSIM sugarcane model for assessing traits for transpiration efficiency and root water supply. Field Crops Research 196, 112–123. [Google Scholar]
- Ishikawa C, Hatanaka T, Misoo S, Miyake C, Fukayama H. 2011. Functional incorporation of sorghum small subunit increases the catalytic turnover rate of Rubisco in transgenic rice. Plant Physiology 156, 1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P, Basnayake J, Inman-Bamber G, Lakshmanan P, Natarajan S, Stokes C. 2016. Genetic variation in transpiration efficiency and relationships between whole plant and leaf gas exchange measurements in Saccharum spp. and related germplasm. Journal of Experimental Botany 67, 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneau M, Gerentes D, Foueillassar X, Zivy M, Vidal J, Toppan A, Perez P. 2002. Improvement of drought tolerance in maize: towards the functional validation of the Zm-Asr1 gene and increase of water use efficiency by over-expressing C4-PEPC. Biochimie 84, 1127–1135. [DOI] [PubMed] [Google Scholar]
- Kadam NN, Jagadish SVK, Struik PC, van der Linden CG, Yin X. 2019. Incorporating genome-wide association into eco-physiological simulation to identify markers for improving rice yields. Journal of Experimental Botany 70, 2575–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Edwards GE. 1999. The biochemistry of C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 plant biology. San Diego: Academic Press, 49–87. [Google Scholar]
- Kapralov MV, Kubien DS, Andersson I, Filatov DA. 2011. Changes in Rubisco kinetics during the evolution of C4 photosynthesis in Flaveria (Asteraceae) are associated with positive selection on genes encoding the enzyme. Molecular Biology and Evolution 28, 1491–1503. [DOI] [PubMed] [Google Scholar]
- Kar S, Zhang N, Nakashima T, Villanueva-Morales A, Stewart JR, Sacks EJ, Terajima Y, Yamada T. 2019. Saccharum×Miscanthus intergeneric hybrids (miscanes) exhibit greater chilling tolerance of C4 photosynthesis and postchilling recovery than sugarcane (Saccharum spp. hybrids). Global Change Biology: Bioenergy 11, 1318–1333. [Google Scholar]
- Kaushal N, Bhandari K, Siddique KHM, Nayyar H. 2016. Food crops face rising temperatures: an overview of responses, adaptive mechanisms, and approaches to improve heat tolerance. Cogent Food & Agriculture 2, 1134380. [Google Scholar]
- Kim SY, Slattery RA, Ort DR. 2021. A role for differential Rubisco activase isoform expression in C4 bioenergy grasses at high temperature. Global Change Biology: Bioenergy 13, 211–223. [Google Scholar]
- Kingston-Smith AH, Foyer CH. 2000. Bundle sheath proteins are more sensitive to oxidative damage than those of the mesophyll in maize leaves exposed to paraquat or low temperatures. Journal of Experimental Botany 51, 123–130. [PubMed] [Google Scholar]
- Kingston-Smith AH, Harbinson J, Williams J, Foyer CH. 1997. Effect of chilling on carbon assimilation, enzyme activation, and photosynthetic electron transport in the absence of photoinhibition in maize leaves. Plant Physiology 114, 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohila S, Gomathi R. 2018. Adaptive physiological and biochemical response of sugarcane genotypes to high-temperature stress. Indian Journal of Plant Physiology 23, 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe AR, Brutnell TP, Cousins AB, Studer AJ. 2018a. Carbonic anhydrase mutants in Zea mays have altered stomatal responses to environmental signals. Plant Physiology 177, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe AR, Cousins AB. 2018. Mesophyll conductance in Zea mays responds transiently to CO2 availability: implications for transpiration efficiency in C4 crops. New Phytologist 217, 1463–1474. [DOI] [PubMed] [Google Scholar]
- Kolbe AR, Studer AJ, Cousins AB. 2018b. Biochemical and transcriptomic analysis of maize diversity to elucidate drivers of leaf carbon isotope composition. Functional Plant Biology 45, 489–500. [DOI] [PubMed] [Google Scholar]
- Kono M, Noguchi K, Terashima I. 2014. Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant & Cell Physiology 55, 990–1004. [DOI] [PubMed] [Google Scholar]
- Kremling KAG, Diepenbrock CH, Gore MA, Buckler ES, Bandillo NB. 2019. Transcriptome-wide association supplements genome-wide association in Zea mays. G3 9, 3023–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP. 2016. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861. [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Griffiths H, Schepers HE. 2010. Can the progressive increase of C4 bundle sheath leakiness at low PFD be explained by incomplete suppression of photorespiration? Plant, Cell & Environment 33, 1935–1948. [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Long SP. 2016. One crop breeding cycle from starvation? How engineering crop photosynthesis for rising CO2 and temperature could be one important route to alleviation. Proceedings of the Royal Society B: Biological Sciences 283, 20152578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromdijk J, Schepers HE, Albanito F, Fitton N, Carroll F, Jones MB, Finnan J, Lanigan GJ, Griffiths H. 2008. Bundle sheath leakiness and light limitation during C4 leaf and canopy CO2 uptake. Plant Physiology 148, 2144–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromdijk J, Ubierna N, Cousins AB, Griffiths H. 2014. Bundle-sheath leakiness in C4 photosynthesis: a careful balancing act between CO2 concentration and assimilation. Journal of Experimental Botany 65, 3443–3457. [DOI] [PubMed] [Google Scholar]
- Kubien DS, von Caemmerer S, Furbank RT, Sage RF. 2003. C4 photosynthesis at low temperature. A study using transgenic plants with reduced amounts of Rubisco. Plant Physiology 132, 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk A, Edwards GE. 1997. CO2 and temperature-dependent induction of C4 photosynthesis: an approach to the hierarchy of rate-limiting processes. Australian Journal of Plant Physiology 24, 163–171. [Google Scholar]
- Leakey ADB, Ferguson JN, Pignon CP, Wu A, Jin Z, Hammer GL, Lobell DB. 2019. water use efficiency as a constraint and target for improving the resilience and productivity of C3 and C4 crops. Annual Review of Plant Biology 70, 781–808. [DOI] [PubMed] [Google Scholar]
- Lee EA, Tollenaar M. 2007. Physiological basis of successful breeding strategies for maize grain yield. Crop Science 47, S-202–S-215. [Google Scholar]
- Leegood RC, Walker RP. 1999. Regulation of the C4 pathway. In: Sage RF, Monson RK, eds. C4 plant biology. San Diego: Academic Press, 89–131. [Google Scholar]
- Legwaila GM, Mathowa T, Jotia E. 2013. The effect of defoliation on growth and yield of sorghum (Sorghum bicolor (L) Moench) variety - segaolane. Agriculture and Biology Journal of North America 4, 594–599. [Google Scholar]
- Li C, Jackson P, Lu X, Xu C, Cai Q, Basnayake J, Lakshmanan P, Ghannoum O, Fan Y. 2017. Genotypic variation in transpiration efficiency due to differences in photosynthetic capacity among sugarcane-related clones. Journal of Experimental Botany 68, 2377–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xie RZ, Wang KR, Ming B, Guo YQ, Zhang GQ, Li SK. 2015. Variations in maize dry matter, harvest index, and grain yield with plant density. Agronomy Journal 107, 829–834. [Google Scholar]
- Liu Y, Qin L, Han L, Xiang Y, Zhao D. 2015. Overexpression of maize SDD1 (ZmSDD1) improves drought resistance in Zea mays L. by reducing stomatal density. Plant Cell, Tissue and Organ Culture 122, 147–159. [Google Scholar]
- Lobell DB, Roberts MJ, Schlenker W, Braun N, Little BB, Rejesus RM, Hammer GL. 2014. Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Science 344, 516–519. [DOI] [PubMed] [Google Scholar]
- Lobo AK, de Oliveira Martins M, Lima Neto MC, Machado EC, Ribeiro RV, Silveira JA. 2015. Exogenous sucrose supply changes sugar metabolism and reduces photosynthesis of sugarcane through the down-regulation of Rubisco abundance and activity. Journal of Plant Physiology 179, 113–121. [DOI] [PubMed] [Google Scholar]
- Long SP, East TM, Baker NR. 1983. Chilling damage to photosynthesis in young Zea mays. I. Effects of light and temperature variation on photosynthetic CO2 assimilation. Journal of Experimental Botany 34, 177–188. [Google Scholar]
- Long SP, Spence AK. 2013. Toward cool C4 crops. Annual Review of Plant Biology 64, 701–722. [DOI] [PubMed] [Google Scholar]
- Maai E, Shimada S, Yamada M, Sugiyama T, Miyake H, Taniguchi M. 2011. The avoidance and aggregative movements of mesophyll chloroplasts in C4 monocots in response to blue light and abscisic acid. Journal of Experimental Botany 62, 3213–3221. [DOI] [PubMed] [Google Scholar]
- Malthus TR. 1798. An essay on the principle of population, as it affects the future improvement of society. London: J. Johnson. [Google Scholar]
- McCormick AJ, Cramer MD, Watt DA. 2006. Sink strength regulates photosynthesis in sugarcane. New phytologist 171, 759–770. [DOI] [PubMed] [Google Scholar]
- McCormick AJ, Cramer MD, Watt DA. 2008a. Changes in photosynthetic rates and gene expression of leaves during a source–sink perturbation in sugarcane. Annals of Botany 101, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick AJ, Cramer MD, Watt DA. 2008b. Differential expression of genes in the leaves of sugarcane in response to sugar accumulation. Tropical Plant Biology 1, 142–158. [Google Scholar]
- McCormick AJ, Watt DA, Cramer MD. 2009. Supply and demand: sink regulation of sugar accumulation in sugarcane. Journal of Experimental Botany 60, 357–364. [DOI] [PubMed] [Google Scholar]
- Messina CD, Cooper M, Hammer GL, et al. 2020. Two decades of creating drought tolerant maize and underpinning prediction technologies in the US corn-belt: review and perspectives on the future of crop design. bioRxiv 10.1101/2020.10.29.361337 [Preprint]. [DOI] [Google Scholar]
- Milne RJ, Byrt CS, Patrick JW, Grof CP. 2013. Are sucrose transporter expression profiles linked with patterns of biomass partitioning in Sorghum phenotypes? Frontiers in Plant Science 4, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitros T, Session AM, James BT, et al. 2020. Genome biology of the paleotetraploid perennial biomass crop Miscanthus. Nature Communications 11, 5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero G, Joynson R, Pinera-Chavez FJ, Gardiner LJ, Rivera-Amado C, Hall A, Reynolds MP. 2019. Elucidating the genetic basis of biomass accumulation and radiation use efficiency in spring wheat and its role in yield potential. Plant Biotechnology Journal 17, 1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. 2007. Photoinhibition of photosystem II under environmental stress. Biochimica et Biophysica Acta 1767, 414–421. [DOI] [PubMed] [Google Scholar]
- Murchie EH, Kefauver S, Araus JL, Muller O, Rascher U, Flood PJ, Lawson T. 2018. Measuring the dynamic photosynthome. Annals of Botany 122, 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Niyogi KK. 2011. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiology 155, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu SL, Moose SP, AL-Shoaibi AK, Raines CA, Long SP. 2003. Cold tolerance of C4 photosynthesis in Miscanthus × giganteus: adaptation in amounts and sequence of C4 photosynthetic enzymes. Plant Physiology 132, 1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveed M, Ahsan M, Akram HM, Aslam M, Ahmed N. 2016. Genetic effects conferring heat tolerance in a cross of tolerant × susceptible maize (Zea mays L.) genotypes. Frontiers in Plant Science 7, 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr DJ, Alcântara A, Kapralov MV, Andralojc PJ, Carmo-Silva E, Parry MA. 2016. Surveying Rubisco diversity and temperature response to improve crop photosynthetic efficiency. Plant Physiology 172, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Merchant SS, Alric J, et al. 2015. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proceedings of the National Academy of Sciences, USA 112, 8529–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D, Hu J, Salas Fernandez MG. 2017. Genetic architecture of photosynthesis in Sorghum bicolor under non-stress and cold stress conditions. Journal of Experimental Botany 68, 4545–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Lopez A, Nie GY, Ort DR, Baker NR. 1990. The involvement of the photoinhibition of photosystem II and impaired membrane energization in the reduced quantum yield of carbon assimilation in chilled maize. Planta 181, 78–84. [DOI] [PubMed] [Google Scholar]
- Paulus JK, Schlieper D, Groth G. 2013. Greater efficiency of photosynthetic carbon fixation due to single amino-acid substitution. Nature Communications 4, 1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino E, Bedini S, Nuti M, Ercoli L. 2018. Impact of genetically engineered maize on agronomic, environmental and toxicological traits: a meta-analysis of 21 years of field data. Scientific Reports 8, 3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchi JP, Carmo-Silva E, Andralojc PJ, Feuerhelm D, Powers SJ, Parry MAJ. 2018. Dissecting wheat grain yield drivers in a mapping population in the UK. Agronomy 8, 94. [Google Scholar]
- Perdomo JA, Capó-Bauçà S, Carmo-Silva E, Galmés J. 2017. Rubisco and Rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Frontiers in Plant Science 8, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon CP, Leakey ADB, Long SP, Kromdijk J. 2021. Drivers of natural variation in water-use efficiency under fluctuating light are promising targets for improvement in Sorghum. Frontiers in Plant Science 12, 627432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon CP, Long SP. 2020. Retrospective analysis of biochemical limitations to photosynthesis in 49 species: C4 crops appear still adapted to pre-industrial atmospheric [CO2]. Plant, Cell & Environment 43, 2606–2622. [DOI] [PubMed] [Google Scholar]
- Pignon CP, Lundgren MR, Osborne CP, Long SP. 2019. Bundle sheath chloroplast volume can house sufficient Rubisco to avoid limiting C4 photosynthesis during chilling. Journal of Experimental Botany 70, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel C, Davey PA, Juvik JA, Long SP. 2005. Gene loci in maize influencing susceptibility to chilling dependent photoinhibition of photosynthesis. Photosynthesis Research 85, 319–326. [DOI] [PubMed] [Google Scholar]
- Pissolato MD, Silveira NM, Prataviera PJC, Machado EC, Seabra AB, Pelegrino MT, Sodek L, Ribeiro RV. 2020. Enhanced nitric oxide synthesis through nitrate supply improves drought tolerance of sugarcane plants. Frontiers in Plant Science 11, 970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittermann J, Sage RF. 2001a. The response of the high altitude C4 grass Muhlenbergia montana (Nutt.) A.S. Hitchc. to long- and short-term chilling. Journal of Experimental Botany 52, 829–838. [DOI] [PubMed] [Google Scholar]
- Pittermann J, Sage RF. 2001b. Photosynthetic performance at low temperature of Bouteloua gracilis Lag., a high-altitude C4 grass from the Rocky Mountains, USA. Plant, Cell & Environment 23, 811–823. [Google Scholar]
- Price GD, von Caemmerer S, Evans JR, Chow WS, Anderson JM, Hurry V, Badger MR. 1995. Chloroplastic cytocromo b6/f and ATP synthase complexes in tobacco: transformation with antisense RNA against nuclear encoded transcripts for the Rieske FeS and ATP polypeptides. Australian Journal of Plant Physiology 22, 285–297. [Google Scholar]
- Qiao M-Y, Zhang Y-J, Liu L-A, Shi L, Ma Q-H, Chow WS, Jiang C-D. 2020. Do rapid photosynthetic responses protect maize leaves against photoinhibition under fluctuating light? Photosynthesis Research doi: 10.1007/s11120-020-00780-5. [DOI] [PubMed] [Google Scholar]
- Qu M, Chen G, Bunce JA, Zhu X, Sicher RC. 2018. Systematic biology analysis on photosynthetic carbon metabolism of maize leaf following sudden heat shock under elevated CO2. Scientific Reports 8, 7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae AL, Grof CPL, Casu RE, Bonnet GD. 2005. Sucrose accumulation in the sugarcane stem: pathways and control points for transport and compartmentation. Field Crops Research 92, 159–168. [Google Scholar]
- Raines CA. 2003. The Calvin cycle revisited. Photosynthesis Research 75, 1–10. [DOI] [PubMed] [Google Scholar]
- Ray DK, Mueller ND, West PC, Foley JA. 2013. Yield trends are insufficient to double global crop production by 2050. PLoS One 8, e66428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RV, Machado EC, Magalhães Filho JR, Lobo AK, Martins MO, Silveira JA, Yin X, Struik PC. 2017. Increased sink strength offsets the inhibitory effect of sucrose on sugarcane photosynthesis. Journal of Plant Physiology 208, 61–69. [DOI] [PubMed] [Google Scholar]
- Ripley B, Frole K, Gilbert M. 2010. Differences in drought sensitivities and photosynthetic limitations between co-occurring C3 and C4 (NADP-ME) Panicoid grasses. Annals of Botany 105, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffo M, Olson R, Daverede I. 2016. Maize yield response to zinc sources and effectiveness of diagnostic indicators. Communications in Soil Science and Plant Analysis 47, 137–141. [Google Scholar]
- Sage RF, McKown AD. 2006. Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? Journal of Experimental Botany 57, 303–317. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47. [DOI] [PubMed] [Google Scholar]
- Sage RF, Zhu XG. 2011. Exploiting the engine of C4 photosynthesis. Journal of Experimental Botany 62, 2989–3000. [DOI] [PubMed] [Google Scholar]
- Sales CRG, Ribeiro RV, Hayashi AH, Marchiori PER, Silva KI, Martins MO, Silveira JAG, Silveira NM, Machado EC. 2018. Flexibility of C4 decarboxylation and photosynthetic plasticity in sugarcane plants under shading. Environmental and Experimental Botany 149, 34–42. [Google Scholar]
- Salesse-Smith CE, Sharwood RE, Busch FA, Kromdijk J, Bardal V, Stern DB. 2018. Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nature Plants 4, 802–810. [DOI] [PubMed] [Google Scholar]
- Salesse-Smith CE, Sharwood RE, Busch FA, Stern DB. 2020. Increased Rubisco content in maize mitigates chilling stress and speeds recovery. Plant Biotechnology Journal 18, 1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliendra NZ, Meinzer FC, Perry M, Thom M. 1996. Association between partitioning of carboxylase activity and bundle sheath leakiness to CO2, carbon isotope discrimination, photosynthesis, and growth in sugarcane. Journal of Experimental Botany 47, 907–914. [Google Scholar]
- Salvucci ME, Osteryoung KW, Crafts-Brandner SJ, Vierling E. 2001. Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiology 127, 1053–1064. [PMC free article] [PubMed] [Google Scholar]
- Savitch LV, Ivanov AG, Gudynaite-Savitch L, Huner NPA, Simmonds J. 2011. Cold stress effects on PSI photochemistry in Zea mays: differential increase pf FQR-dependent cyclic electron flow and functional implications. Plant & Cell Physiology 52, 1042–1054. [DOI] [PubMed] [Google Scholar]
- Scafaro AP, Atwell BJ, Muylaert S, Reusel BV, Ruiz GA, Rie JV, Gallé A. 2018. A Thermotolerant variant of Rubisco activase from a wild relative improves growth and seed yield in rice under heat stress. Frontiers in Plant Science 9, 1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafaro AP, Bautsoens N, den Boer B, Van Rie J, Gallé A. 2019. A conserved sequence from heat-adapted species improves Rubisco activase thermostability in wheat. Plant Physiology 181, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlieper D, Förster K, Paulus JK, Groth G. 2014. Resolving the activation site of positive regulators in plant phosphoenolpyruvate carboxylase. Molecular Plant 7, 437–440. [DOI] [PubMed] [Google Scholar]
- Schlüter U, Bräutigam A, DrozJ-M, Schwender J, WeberAPM. 2019. The role of alanine and aspartate aminotransferases in C4 photosynthesis. Plant Biology 21, 64–76. [DOI] [PubMed] [Google Scholar]
- Serrano-Romero EA, Cousins AB. 2020. Cold acclimation of mesophyll conductance, bundle-sheath conductance and leakiness in Miscanthus × giganteus. New Phytologist 226, 1594–1606. [DOI] [PubMed] [Google Scholar]
- Sharwood RE, Ghannoum O, Kapralov MV, Gunn LH, Whitney SM. 2016a. Temperature responses of Rubisco from Paniceae grasses provide opportunities for improving C3 photosynthesis. Nature Plants 2, 16186. [DOI] [PubMed] [Google Scholar]
- Sharwood RE, Ghannoum O, Whitney SM. 2016b. Prospects for improving CO2 fixation in C3-crops through understanding C4-Rubisco biogenesis and catalytic diversity. Current Opinion in Plant Biology 31, 135–142. [DOI] [PubMed] [Google Scholar]
- Shekoofa A, Emam Y, Pessaraki M. 2010. Effect of partial defoliation after silking stage on yield components of three grain maize hybrids under semi-arid conditions. Journal of Agronomy and Soil Science 58, 777–788. [Google Scholar]
- Simkin AJ, López-Calcagno PE, Raines CA. 2019. Feeding the world: improving photosynthetic efficiency for sustainable crop production. Journal of Experimental Botany 70, 1119–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery RA, Walker BJ, Weber APM, Ort DR. 2018. the impacts of fluctuating light on crop performance. Plant Physiology 176, 990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoike K. 2011. Photoinhibition of photosystem I. Physiologia Plantarum 142, 56–64. [DOI] [PubMed] [Google Scholar]
- Spence AK, Boddu J, Wang D, James B, Swaminathan K, Moose SP, Long SP. 2014. Transcriptional responses indicate maintenance of photosynthetic proteins as key to the exceptional chilling tolerance of C4 photosynthesis in Miscanthus × giganteus. Journal of Experimental Botany 65, 3737–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickler FC, Pauli AW. 1961. Leaf removal in grain sorghum. I. Effects of certain defoliation treatments on yield and components of yield. Agronomy Journal 53, 99–102. [Google Scholar]
- Stitt M, Zhu XG. 2014. The large pools of metabolites involved in intercellular metabolite shuttles in C4 photosynthesis provide enormous flexibility and robustness in a fluctuating light environment. Plant, Cell & Environment 37, 1985–1988. [DOI] [PubMed] [Google Scholar]
- Studer AJ, Gandin A, Kolbe AR, Wang L, Cousins AB, Brutnell TP. 2014. A limited role for carbonic anhydrase in C4 photosynthesis as revealed by a ca1ca2 double mutant in maize. Plant Physiology 165, 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunoj VSJ, Somayanda IM, Chiluwal A, Perumal R, Prasad PVV, Jagadish SVK. 2017. Resilience of pollen and post-flowering response in diverse sorghum genotypes exposed to heat stress under field conditions. Crop Science 57, 1658–1669. [Google Scholar]
- Takahashi S, Bauwe H, Badger M. 2007. Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiology 144, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AO, Craig AS. 1971. Plants under climatic stress: II. Low temperature, high light effects on chloroplast ultrastructure. Plant Physiology 47, 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SH, Hulme SP, Rees M, Ripley BS, Woodward FI, Osborne CP. 2010. Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment. New Phytologist 185, 780–791. [DOI] [PubMed] [Google Scholar]
- Taylor SH, Lowry DB, Aspinwall MJ, Bonnette JE, Fay PA, Juenger TE. 2016. QTL and drought effects on leaf physiology in lowland Panicum virgatum. Bioenergy Research 9, 1241–1259. [Google Scholar]
- Tazoe Y, Ishikawa N, Shikanai T, Ishiyama K, Takagi D, Makino A, Sato F, Endo T. 2020. Overproduction of PGR5 enhances the electron sink downstream of photosystem I in a C4 plant, Flaveria bidentis. The Plant Journal 103, 814–823. [DOI] [PubMed] [Google Scholar]
- Tollenaar M, Daynard TB. 1978. Effect of defoliation on kernel development in maize. Canadian Journal of Plant Science 58, 207–212. [Google Scholar]
- Tollenaar M, Dwyer LM, Stewart DW. 1992. Ear and kernel formation in maize hybrids representing three decades of grain yield improvement in Ontario. Crop Science 32, 432–438. [Google Scholar]
- Tovignan TK, Fonceka D, Ndoye I, Cisse N, Luquet D. 2016. The sowing date and post-flowering water status affect the sugar and grain production of photoperiodic, sweet sorghum through the regulation of sink size and leaf area dynamics. Field Crops Research 192, 67–77. [Google Scholar]
- Trevanion SJ, Ashton AR, Furbank RT. 1999. Antisense RNA inhibition of pyruvate, orthophosphate dikinase and NADP malate dehydrogenase in the C4 plant Flaveria bidentis: analysis of plants with a mosaic phenotype. Australian Journal of Plant Physiology 26, 537–547. [Google Scholar]
- Trevanion SJ, Furbank RT, Ashton AR. 1997. NADP-malate dehydrogenase in the C4 plant Flaveria bidentis (cosense suppression of activity in mesophyll and bundle-sheath cells and consequences for photosynthesis). Plant Physiology 113, 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkan I, Uzilday B, Dietz KJ, Bräutigam A, Ozgur R. 2018. Reactive oxygen species and redox regulation in mesophyll and bundle sheath cells of C4 plants. Journal of Experimental Botany 69, 3321–3331. [DOI] [PubMed] [Google Scholar]
- Twohey RJ 3rd, Roberts LM, Studer AJ. 2018. Leaf stable carbon isotope composition reflects transpiration efficiency in Zea mays. The Plant Journal 97, 475–484. [DOI] [PubMed] [Google Scholar]
- United Nations. 2019. World population prospects: the 2019 revision. https://population.un.org/wpp/DataQuery/ Accessed April 2021.
- USDA. 2020a. National Agriculture Economic Research Service, feed outlook Jan 2020. https://www.nass.usda.gov/Publications/. Accessed January 2021.
- USDA . 2020b. National Agriculture Statistics Service, crop production 2019 summary. https://www.nass.usda.gov/Publications/. Accessed December 2020.
- Usuda H, Ku MS, Edwards GE. 1984. Activation of NADP-malate dehydrogenase, pyruvate,Pi dikinase, and fructose 1,6-bisphosphatase in relation to photosynthetic rate in maize. Plant Physiology 76, 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega CRC, Andrade FH, Sadras VO. 2001. Reproductive partitioning and seed set efficiency in soybean, sunflower and maize. Field Crops Research 72, 163–175 [Google Scholar]
- von Caemmerer S, Furbank RT. 2016. Strategies for improving C4 photosynthesis. Current Opinion in Plant Biology 31, 125–134. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Hendrickson L, Quinn V, Vella N, Millgate AG, Furbank RT. 2005. Reductions of Rubisco activase by antisense RNA in the C4 plant Flaveria bidentis reduces Rubisco carbamylation and leaf photosynthesis. Plant Physiology 137, 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Li XF, Zhou ZJ, Feng XP, Yang WJ, Jiang DA. 2010. Two Rubisco activase isoforms may play different roles in photosynthetic heat acclimation in the rice plant. Physiologia Plantarum 139, 55–67. [DOI] [PubMed] [Google Scholar]
- Wang D, Portis AR Jr, Moose SP, Long SP. 2008. Cool C4 photosynthesis: pyruvate Pi dikinase expression and activity corresponds to the exceptional cold tolerance of carbon assimilation in Miscanthus × giganteus. Plant Physiology 148, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Nayak S, Koch K, Ming R. 2013. Carbon partitioning in sugarcane (Saccharum species). Frontiers in Plant Science 4, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bräutigam A, Weber AP, Zhu XG. 2014. Three distinct biochemical subtypes of C4 photosynthesis? A modelling analysis. Journal of Experimental Botany 65, 3567–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chan KX, Long SP. 2021. Toward a dynamic photosynthetic model to guide the yield improvement in C4 crops. The Plant Journal doi: 10.1111/tpj.15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann S, Ma F, Furuyama K, Gierse J, Berg H, Shao Y, Taniguchi M, Allen DK, Brutnell TP. 2016. Interactions of C4 subtype metabolic activities and transport in maize are revealed through the characterization of DCT2 mutants. The Plant Cell 28, 466–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AC, Rogers A, Rees M, Osborne CP. 2016. How can we make plants grow faster? A source–sink perspective on growth rate. Journal of Experimental Botany 67, 31–45. [DOI] [PubMed] [Google Scholar]
- Wu A, Hammer GL, Doherty A, von Caemmerer S, Farquhar GD. 2019. Quantifying impacts of enhancing photosynthesis on crop yield. Nature Plants 5, 380–388. [DOI] [PubMed] [Google Scholar]
- Wu L, Birch RG. 2006. Doubled sugar content in sugarcane plants modified to produce a sucrose isomer. Plant Biotechnology Journal 5, 109–117. [DOI] [PubMed] [Google Scholar]
- Xie J, Mayfield-Jones D, Erice G, Choi M, Leakey ADB. 2020. Optical topometry and machine learning to rapidly phenotype stomatal patterning traits for QTL mapping in maize. Plant Physiology doi: 10.1093/plphys/kiab299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav SK, Tiwari YK, Kumar DP, Shanker AK, Lakshmi NJ, Vanaja M, Maheswari M. 2016. Genotypic variation in physiological traits under high temperature stress in maize. Agricultural Research 5, 119–126. [Google Scholar]
- Yamada M, Kawasaki M, Sugiyama T, Miyake H, Taniguchi M. 2009. Differential positioning of C4 mesophyll and bundle sheath chloroplasts: aggregative movement of C4 mesophyll chloroplasts in response to environmental stresses. Plant & Cell Physiology 50, 1736–1749. [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC. 2021. Exploiting differences in the energy budget among C4 subtypes to improve crop productivity. New Phytologist 229, 2400–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Zheng Y, Piao S, et al. 2019. Increased atmospheric vapor pressure deficit reduces global vegetation growth. Science Advances 5, eaax1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I, Schultes NP, Peterson RB, Brown P, Brutnell TP. 2009. High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiology 149, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. 2010. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology 61, 235–261. [DOI] [PubMed] [Google Scholar]
- Zhu XG, Ort DR, Whitmarsh J, Long SP. 2004a. The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. Journal of Experimental Botany 55, 1167–1175. [DOI] [PubMed] [Google Scholar]
- Zhu X-G, Portis AR, Jr Long SP. 2004b. Would transformation of C3 crop plants with foreign Rubisco increase productivity? A computational analysis extrapolating from kinetic properties to canopy photosynthesis. Plant, Cell & Environment 27, 155–165. [Google Scholar]