Abstract
Purpose
To model juvenile-onset myopia progression as a function of race/ethnicity, age, sex, parental history of myopia, and time spent reading or in outdoor/sports activity.
Methods
Subjects were 594 children in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study with at least three study visits: one visit with a spherical equivalent (SPHEQ) less myopic/more hyperopic than −0.75 diopter (D), the first visit with a SPHEQ of −0.75 D or more myopia (onset visit), and another after myopia onset. Myopia progression from the time of onset was modeled using cubic models as a function of age, race/ethnicity, and other covariates.
Results
Younger children had faster progression of myopia; for example, the model-estimated 3-year progression in an Asian American child was −1.93 D when onset was at age 7 years compared with −1.43 D when onset was at age 10 years. Annual progression for girls was 0.093 D faster than for boys. Asian American children experienced statistically significantly faster myopia progression compared with Hispanic (estimated 3-year difference of −0.46 D), Black children (−0.88 D), and Native American children (−0.48 D), but with similar progression compared with White children (−0.19 D). Parental history of myopia, time spent reading, and time spent in outdoor/sports activity were not statistically significant factors in multivariate models.
Conclusions
Younger age, female sex, and racial/ethnic group were the factors associated with faster myopic progression. This multivariate model can facilitate the planning of clinical trials for myopia control interventions by informing the prediction of myopia progression rates.
Keywords: myopia, juvenile, progression, observational study
The prevalence of myopia varies around the world. A report from the United States indicated that roughly one-third of those 20 years and older were myopic.1 Studies from Asia report a higher prevalence of myopia compared with the United States. For example, in Korea, 96.5% of male military enlistees were reported to be myopic.2 He et al.3 found that 78% of Chinese 15-year-olds were myopic, whereas 62.5% of 12-year-old children in Hong Kong were myopic.4 Given that there is no accepted method to prevent the onset of myopia, identifying those children at greatest risk of myopia progression is a logical goal for clinicians and researchers.
Many studies have reported rates of myopia progression, most frequently based on data for the single-vision spectacle or placebo group in clinical trials evaluating interventions for myopia control. Reports of myopia progression from Singapore and Hong Kong found similar rates of progression, roughly −0.60 diopter (D)/yr.5–8 Several other studies found greater average yearly progression rates, between −0.80 D and −1.20 D/yr,9–11 although Hasebe et al.9 found that older children's myopia progressed more slowly than younger children (−1.03 D/yr vs. −1.36 D/yr). A cohort from Singapore further illustrates the declining rate of myopic progression with increasing age. During the first year, there was a progression of −0.88 D in a group of 7- to 9-year-old children, with the change slowing to −0.67 D during the second year. By the third year, the change was only −0.48 D.12 A study in China found that both age and sex differentially affected progression, that is, younger children progressed faster, as did females.13
In comparison to children in Asian countries, myopia progression rates reported for children in the United States tend to be slightly slower. In the placebo group in a US-based study of pirenzepine in a predominantly White sample,14 the average myopia progression was −0.53 D per year. Gwiazda et al.15 had a slightly more racially mixed sample aged 6 to 11 years and reported a 3-year myopia progression of −1.48 D in the single-vision lens group, with progression of −0.60 D in the first year. Age, sex, and race/ethnicity all played roles in the amount of myopic progression.16
A greater number of myopic parents has also been associated with a faster rate of progression. Kurtz et al.17 reported that progression over 5 years was −1.81 D among children in the single-vision lens group with no myopic parents compared with −2.59 D in children with two myopic parents. Saw et al.7 reported that having two myopic parents added −0.43 D every 6 months to the rate of myopia progression. In general, the annual myopia progression rates published to date range from −0.50 D to −0.90 D per year.
The data investigating patterns for progression in subgroups beyond sex and age are limited. Specifically, there are limited data on a variety of racial/ethnic groups in conjunction with age of myopia onset and other potential predictors of progression rate. With the exception of a subset of children from the Singapore study,18 the children typically presented in the literature are prevalent myopes, having been diagnosed some time prior to study entry. Known age of onset, as opposed to depending on parental recall or clinic records, allows for analysis of the effect of the age of onset on the rate of progression, for example, does the rate of myopia progression of an 11-year-old child differ depending on whether the myopia onset was at age 7, 9, or 10 years? Identifying the factors associated with myopia progression could also help target those groups that might benefit the most from early treatment to prevent or slow myopia progression. Detailed quantitative models relating risk factors to rates of progression are necessary for such identification. The success of several therapies to slow myopia progression, such as low-dose atropine and soft multifocal contact lenses, makes randomization of children to placebo or standard groups in future clinical trials less likely due to ethical concerns.19–22 Judging efficacy against an untreated control group may depend on comparison to historical, untreated control groups. Using data from the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study, myopia progression models by race/ethnicity, age at first myopic visit, sex, parental myopia, and environmental exposures are presented to address these questions.
Methods
The CLEERE Study began in 1989 as the Orinda Longitudinal Study of Myopia, a longitudinal, observational study designed to evaluate risk factors for the development of juvenile-onset myopia. The racial/ethnic composition of Orinda, California is predominantly White. The expansion to include other sites allowed for the recruitment of other racial/ethnic groups to improve generalizability. The study consisted of a volunteer sample, in which each clinic site focused recruitment on a specific racial/ethnic group: Eutaw, Alabama (Black children); Houston, Texas (Hispanic children); Tucson, Arizona (Native American children); Irvine, California (Asian American children); and Orinda, California (White children). Informed consent and assent were provided by the parents and children, respectively, according to the tenets of the Declaration of Helsinki. Informed consent procedures and study protocols were approved by each university's affiliated institutional review board.
Measurements were collected from the right eye only. Cycloplegic autorefraction was conducted by certified study personnel with the Canon R-1 autorefractor (Canon USA, Lake Success, NY, USA; no longer manufactured) from 1989–2000 and with the Grand Seiko WR-5100K autorefractor (Grand Seiko Co., Hiroshima, Japan) from 2001–2010. For cycloplegic autorefraction, subjects fixated on a reduced Snellen target through a +4.00 D-Badal lens in primary gaze. For subjects with grade 1 or 2 iris color (in general, a blue or a gray iris or with a green iris with a lesser amount of brown pigment),23 testing was performed 30 minutes after one drop of proparacaine 0.5% and two drops of tropicamide 1.0%. In subjects with dark iris color greater than grade 2, testing was performed 30 minutes after one drop of proparacaine 0.5% and one drop each of tropicamide 1.0% and cyclopentolate 1.0%.24 Ten autorefractor measurements were made and averaged according to a standard protocol.25
Racial/ethnic group for the child was supplied by a parent on the CLEERE medical history form at study enrollment by choosing among six categories (based on the National Institutes Health categories in 1997 when ethnic data were first gathered): American Indian or Alaskan Native; Asian or Pacific Islander; Black not of Hispanic origin; Hispanic; White not of Hispanic origin; other or unknown. Children who were identified as being “other” race/ethnicity were not included in this analysis.
Parents provided information on their own refractive error status through a survey. Typically, one parent provided both parents’ years of birth, whether they wore spectacles or contact lenses, if worn, the age when they were first prescribed spectacles, and how they primarily used the spectacles at the time of the survey (for viewing at distance, at near, or both). A parent was considered myopic if they used the spectacles primarily for distance or for both distance and near if the spectacles had been first been prescribed before the age of 17 years. This cutoff had a relatively high sensitivity and specificity (76% and 74%, respectively) in a previous validation study.26
Visual activity data were provided annually by a parent using a questionnaire that asked, “During the school year, how many hours per week (outside of regular school hours) would you estimate this child: (1) studies or reads for school assignments; (2) reads for fun (pleasure); (3) watches TV; (4) uses a computer/plays video games; and (5) engages in outdoor/sports activities?” Reported hours per week across all five activities that exceeded 82 were set to missing in 12 subjects. This exclusion assumed that 82 hours outside of school were not reasonably available to a child during a week. Diopter-hours were also calculated as a comprehensive near work exposure: 3 x hours of reading + 3 x hours of studying + 2 x video/computer hours + hours watching television.27
The aim of this particular analysis was to model spherical equivalent refractive error (SPHEQ: defined as sphere + one-half of the cylinder from cycloplegic autorefraction of the right eye) as a function of time from the observed myopia onset within the study. Myopia onset in previously nonmyopic children was identified in 594 subjects based on a SPHEQ of −0.75 D or more myopia. To be included in this analysis a child had to have at least three visits: one visit with a SPHEQ showing less myopia than −0.75 D, one visit with a SPHEQ equal to or more myopic than −0.75 D, and another visit after myopia onset. The first visit at which the SPHEQ was −0.75 D or more myopic was defined as the child's myopia-onset visit. SPHEQ values collected at all available visits at or after myopia onset were used in the analysis. Predictors of interest were age at the myopia-onset visit, baseline SPHEQ, sex, race/ethnicity, parental history of myopia, hours of reading, and hours of outdoor/sports activities per week. The final model was determined using only those subjects with complete data. After the final model was fitted, the model was applied to a test set of myopic children whose myopia-onset visit was not observed (i.e., myopic at study entry) (n = 461), and the performance of the model was evaluated. This was accomplished by determining the prediction error, defined as the SPHEQ predicted by the model−the observed SPHEQ (predicted–observed, negative value = average bias toward more myopia).
Statistical Methods
All analyses were done using SAS version 9.4 (SAS Institute, Cary, NC, USA) for Windows. The MIXED procedure was used for modeling. Fitting SPHEQ as a cubic in time provided enough flexibility to adequately fit myopia progression after onset. The base model had the following form:
In the model, i indexes the child. The four γ terms in the model (γ0 through γ3) are constants providing the mean intercept, slope, quadratic, and cubic coefficients, respectively. The four u terms (u0i through u3i) adjust the mean parameters (intercept, slope, quadratic, and cubic coefficients) for between-child variation in SPHEQ. The ε term accounts for the scatter of an individual child's data about his cubic fit (i.e., the amount of error between the actual data for a given child and the cubic fit across all visits). Because of variation in model convergence, the random effects included in a model depended on which model was being fit. For the model with no predictors, random effects were included in the intercept, linear, quadratic, and cubic model terms. For the univariate models in the backward selection process, random effects were included in the intercept and linear model terms for all predictors but baseline SPHEQ. For the univariate model with baseline SPHEQ, random effects were included in the linear, quadratic, and cubic model terms. For the multivariate model, random effects were included in the linear, quadratic, and cubic model terms.
A predictor (P) was added to the base model in the following way:
Predictor terms were sequentially removed to produce more parsimonious univariate models. The removal began with the cubic term. If the cubic coefficient for the predictor, P, was statistically significant (p < 0.05), there were no changes made to the model. If it was not statistically significant, the cubic P term was removed, and the reduced model was fit. In the reduced model, if the quadratic P term was statistically significant, no additional changes were made to the model. If it was not, the quadratic P term was removed, and the reduced model was fit (i.e., a cubic base model with P terms limited to the intercept and slope). If necessary, the process was repeated for the linear P term and, finally, the intercept P term. If the intercept P term was not statistically significant, we concluded that the predictor had no effect on the progression of myopia. In multivariate modeling, all predictors were included in all the polynomial terms, even if their univariate models had simpler final configurations. Again, to achieve a parsimonious model, predictor terms that were not statistically significant were sequentially removed, using backward stepwise selection with the term with the biggest P value selected for removal. The cubic predictor terms were evaluated first, followed by the quadratic, linear, and intercept terms. There was no pruning of lower order predictor terms if a higher order term was statistically significant.
Results
Tables 1 (continuous variables) and 2 (categorical variables) summarize characteristics of the data, including the distributions of the predictors of interest. The overall means and frequencies for the entire sample meeting all of the eligibility criteria for this analysis (all data), the means and frequencies of those with complete data for all of the predictors used to determine the model (complete data), and the characteristics of the subjects without an observed myopia-onset visit used to test the model (test set) are presented. The average number of visits was between three and four, and the average age at the myopia-onset visit was between 10 and 11 years old. By the myopia-onset visit, children had an average SPHEQ of −1.11 D. Fifty-eight percent of the sample was female. Approximately 30% were Hispanic, and 24% were White. Asian Americans made up 21%; Blacks represented 16.3%; and the remaining 8.8% were Native Americans. The percentage of children with no myopic parents was 42%, whereas 36% had one myopic parent, and the remaining 22% had two myopic parents.
Table 1.
Means and Standard Deviations for the Continuous Variables of Age, SPHEQ, Number of Hours per Week Spent Reading, and Number of Hours per Week Spent in Outdoors/Sports Activity at the Myopia-Onset Visit for the Overall Dataset, the Subset of Those With Complete Data, and the Test Set
| All Data (n = 594) | Complete Data (n = 457) | Test Set (n = 461) | |
|---|---|---|---|
| Number of visits overall | 3.58 ± 1.40 | 3.65 ± 1.43 | 4.15 ± 1.88 |
| Age at first myopic visit (yrs) | 10.50 ± 1.70 | 10.39 ± 1.67 | 10.14 ± 2.10 |
| SPHEQ (D) | −1.11 ± 0.32 | −1.12 ± 0.33 | −2.01 ± 1.25 |
| Hours of reading per week | 4.48 ± 4.36 | 4.56 ± 4.28 | 4.21 ± 5.23 |
| Hours of outdoors/sports activities per week | 6.69 ± 5.84 | 6.88 ± 5.91 | 6.80 ± 6.92 |
Table 2.
Distribution of Sex, Race/Ethnicity, and Parental History of Myopia (n = 594)
| Datasets | |||
|---|---|---|---|
| All Data (n = 594) | Complete Data (n = 457) | Test Set (n = 461) | |
| Sex n (%) | |||
| Female | 343 (57.7) | 255 (55.8) | 246 (53.4) |
| Male | 251 (42.3) | 202 (44.2) | 215 (46.6) |
| Race/ethnicity n (%) | |||
| Asian American | 125 (21.0) | 102 (22.3) | 109 (23.6) |
| Black | 97 (16.3) | 53 (11.6) | 55 (11.9) |
| Hispanic | 178 (30.0) | 149 (32.6) | 135 (29.3) |
| Native American | 52 (8.8) | 29 (6.3) | 78 (16.9) |
| White | 142 (23.9) | 124 (27.1) | 84 (18.2) |
| Number of myopic parents n (%) | |||
| 0 | 217 (42.0) | 182 (39.8) | 151 (39.9) |
| 1 | 186 (36.0) | 170 (37.2) | 135 (35.7) |
| 2 | 114 (22.1) | 105 (23.0) | 92 (24.3) |
Univariate Predictor Model Results
Building from the base cubic model, each of the predictor variables was evaluated. Univariate models were statistically significant for sex, race/ethnicity, parental history of myopia, hours of reading spent per week, baseline SPHEQ, and age at myopia-onset visit. Hours of outdoor/sports spent per week and diopter-hours did not contribute significantly to the model.
Multiple Predictors Model
After model selection was completed, the predictors in the final model were sex, race/ethnicity, age at myopia-onset visit, and baseline SPHEQ. Despite being statistically significant in the univariate models, parental history of myopia and hours of reading spent per week were not significant in the multivariate analysis. As with the univariate modeling, neither terms involving hours of sports nor those involving diopter hours were statistically significant in the multivariate model. For the model fitting, baseline SPHEQ was centered at −0.75 D, and age at the myopia-onset visit at 7 years old.
Table 3 provides a summary of the final multivariate model with the equation that provides the necessary information to calculate the estimated SPHEQ for any given sex, race/ethnicity, baseline SPHEQ, and age at myopia-onset visit referenced in the Table footnote. Age at the myopia-onset visit affected the model up through the cubic term, that is, it influenced not only the slope of myopia progression but also altered the curvature of the progression lines. Race/ethnicity and sex affected the slope of myopia progression. Differences in race/ethnicity were independent of the age at myopia-onset visit.
Table 3.
Final Multivariate Model Parameter Estimates for a Cubic Model of SPHEQ. Age at First Myopic Visit Centered at 7 Years Old and Baseline SPHEQ (SPHEQ0) Centered at −0.75 D
| Racial/Ethnic Group | Intercept Adjustment α1 (95% CI) | Linear Adjustment β1 (95% CI) |
|---|---|---|
| White | 0 | 0 |
| Asian American | −0.010 (−0.051, 0.031) | −0.062 (−0.139, 0.014) |
| African American | 0.002 (−0.042, 0.045) | 0.231 (0.150, 0.311) |
| Hispanic | 0.009 (−0.028, 0.046) | 0.092 (0.026, 0.159) |
| Native American | 0.001 (−0.053, 0.055) | 0.097 (0, 0.195) |
Race/ethnicity adjustments
Male = 0; Female = 1
[−0.739 – 0.002 * (age of first myopic visit - 7) + 0.998 * (SPHEQ0 + 0.75) −0.008 * sex + α1] + [−0.629 + 0.09 * (age of first myopic visit - 7) + 0.18 * (SPHEQ0 + 0.75) −0.093 * sex +β1]T + [0.041– 0.024 * (age of first myopic visit - 7) – 0.024 * (SPHEQ0 + 0.75)]T2 + [−0.003 + (0.004 * (age of first myopic visit - 7)]T3
CI, confidence interval; T, time since onset of myopia.
Figure 1 shows the projections for SPHEQ by age at myopia onset from 7 to 12 years. Beyond age 12 years there were fewer than 5 subjects aged 13 or 14 years. These curves are generated based on the following assumptions: 50% female; a SPHEQ of −0.75 D at baseline; and a race/ethnicity mix based on 2019 population values for the United States of 5.5% Asian, 14.2% Black, 26.8% Hispanic, 0.8% Native American, and 52.7% White (https://www.childstats.gov/americaschildren/tables/pop3.asp). Annual myopia progression slowed with increasing age across all ages of onset; however, the annual progression rate for a given age, sex, and race/ethnicity was independent of the age of onset. In other words, the rate of progression between age 11 and 12 years was essentially the same regardless of whether the age of onset was 7 or 11 years. Annual progression rates for a given age, sex, and race/ethnicity were always within ±0.065 D of this parallel pattern for progression.
Figure 1.

SPHEQ refractive error growth based on age at myopia-onset visit. These estimates assume an equal proportion of females and males, a baseline SPHEQ of −0.75 D, of race/ethnicity: 5.5% Asian American; 14.2% African American; 26.8% Hispanic; 0.8% Native American; and 52.7% White. Models are built only for ages with data available.
The average myopic progression (95% confidence interval) in the first year following onset is shown by age of myopia onset in Table 4. If age at the myopia-onset visit is 7 years, the average progression over the next year is −0.58 D. For each increasing year of onset age, progression decreases by 0.07 D. Table 5 shows 3-year progression estimates for the different racial/ethnic groups based on the age of the myopia-onset visit being 7 or 10 years. The 3-year progression estimates differed significantly among the Asian American and Black, Native American, and Hispanic groups. The differences in progression do not differ with respect to the age the myopia-onset visit, that is, regardless of whether onset occurred at age 7 or 10 years, the difference in progression between racial/ethnic groups was the same.
Table 4.
First Year Progression as a Function of Age at Myopia-Onset Visit
| Comparison | Estimate With 95% CI |
|---|---|
| First Year Progression for 7-year onset (D) | −0.58 (−0.69, −0.48) |
| First Year Progression for 8-year onset (D) | −0.51 (−0.60, −0.42) |
| First Year Progression for 9-year onset (D) | −0.44 (−0.51, −0.37) |
| First Year Progression for 10-year onset (D) | −0.37 (−0.43, −0.31) |
| First Year Progression for 11-year onset (D) | −0.30 (−0.36, −0.24) |
| First Year Progression for 12-year onset (D) | −0.23 (−0.30, −0.17) |
| First Year Progression for 13-year onset (D) | −0.16 (−0.24, −0.08) |
| Difference in first year progression for children who are a year apart in onset age (D) | 0.07 (0.05, 0.09) |
The estimates assume a baseline SPHEQ of −0.75 D and an equal proportion of males and females. The estimates also assume the following mix of race/ethnicity: Asian American 5.5%, Black 14.2%, Hispanic 26.8%, Native American 0.8%, White 52.7%. CI, confidence interval.
Table 5.
Differences Between Racial/Ethnic Groups in Progression Over the First 3 Years After First Myopic Visit in the CLEERE Study
| Comparison | Estimate for Onset at 7 Years (in D) With 95% CI | Estimate for onset at 10 Years (in D) With 95% CI |
|---|---|---|
| Three-year progression for an Asian American child | −1.93 (−2.18, −1.67) | −1.43 (−1.63, −1.24) |
| Three-year progression for a Black child | −1.05 (−1.34, −0.76) | −0.56 (−0.77, −0.34) |
| Three-year progression for a Hispanic child | −1.46 (−1.71, −1.22) | −0.97 (−1.13, −0.81) |
| Three-year progression for a Native American child | −1.45 (−1.80, −1.10) | −0.96 (−1.22, −0.69) |
| Three-year progression for a White child | −1.74 (−2.01, −1.48) | −1.25 (−1.43, −1.06) |
| Difference between 3-year progression for an Asian American child and a Black child | −0.88 (−1.13, −0.63) | −0.88 (−1.13, −0.63) |
| Difference between 3-year progression for an Asian American child and a Hispanic child | −0.46 (−0.67, −0.26) | −0.46 (−0.67, −0.26) |
| Difference between 3-year progression for an Asian American child and a Native American child | −0.48 (−0.78, −0.18) | −0.48 (−0.78, −0.18) |
| Difference between 3-year progression for an Asian American child and a White child | −0.19 (−0.42, 0.04) | −0.19 (−0.42, 0.04) |
The estimates presume an age at myopia-onset visit of either 7 or 10 years and a baseline SPHEQ of −0.75 D. The estimates also presume an equal proportion of male and female sex. CI, confidence interval.
As an evaluation of how the multivariate model performs, the model was used to predict the SPHEQ progression of children whose data were not used to fit the model (a test set) because they were myopic at study entry. The evaluation data are presented in Table 6 as the mean prediction error, with prediction error defined as the SPHEQ predicted by the model minus the observed SPHEQ. The mean prediction error for those subjects in whom we did not observe the myopia onset was between −0.10 and −0.23 D with each year seen. Standard deviations increased with time, reaching ±1.45 D in the children for whom we did not observe myopia onset. The distribution of prediction errors depicted in Figure 2 widen with time.
Table 6.
Mean Prediction Error for a Set of Myopic Subjects Without an Observed Myopia Onset Visit Who Were Not Included in the Development of the Main Model
| Time Relative to the First Visit (yr) | Mean ± SD (n) in Subjects for Whom Onset was Not Observed |
|---|---|
| 1 | −0.10 ± 0.47 (442) |
| 2 | −0.20 ± 0.72 (359) |
| 3 | −0.23 ± 0.89 (236) |
| 4 | −0.16 ± 1.15 (167) |
| 5 | −0.16 ± 1.30 (123) |
| 6 | −0.17 ± 1.45 (82) |
Prediction error is the difference of the predicted SPHEQ minus the observed SPHEQ.
Figure 2.
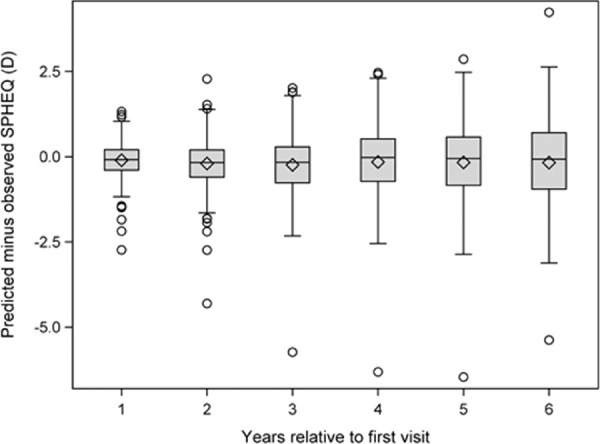
Prediction error estimates based on data not contributing to the development of the model.
Discussion
Although several of the factors that can specifically be used to predict those children whose myopia is most likely to progress have been considered previously, no study to date has had the diverse sample and longitudinal data to evaluate multiple groups at one time. This analysis confirmed and quantified the amount that myopia progression differed by sex, age at first myopic visit, and race/ethnicity. For Asian Americans, 3-year progression differed significantly from 3-year progression for Blacks, Native Americans, and Hispanics. Beyond confirmation of the influence of these risk factors, we also found that the number of myopic parents, time reading, or time in outdoor/sports activities did not influence the rate of progression after multivariate adjustment.
It is somewhat difficult to compare our results with other studies because we identified the incident myopes and then presented myopia progression information as a function of time from the myopia-onset visit. Most studies use prevalent myopes to present progression data as a function of age and have little or no reliable information about the age at the myopia-onset visit. Comparisons of progression rates at a particular age are not direct comparisons because the age at the myopia-onset visit may not be the same across the ages at study entry. In an attempt to evaluate this question in our sample, we compared progression rates for children at different ages as a function of their age at their myopia-onset visit. The model progression rates for a given age, sex, and race/ethnicity did not vary by age at the myopia-onset visit by a clinically meaningful amount (within ±0.065 D). The impact of age of myopia onset on the eventual amount of myopia comes from an earlier onset leading to a longer window for myopia progression. These results are similar to those reported previously by Chua et al.28 who presented data evaluating risk of high myopia (more myopia than −5.00 D). Although younger age of onset led to a more myopic SPHEQ at age 11 years, the slope of the progression curves from the various ages of onset were essentially parallel at each age.28 These results are consistent with those in Figure 1 in which progression is faster at younger ages. The eventual amount of myopia is greater if the myopia-onset visit occurred at a younger age, but the rate of progression at a particular age is largely independent of this age of myopia onset. This pattern supports the use of the final multivariate model in Table 3, even if myopia onset is not directly observed. The SPHEQ at the first visit would be substituted for SPHEQ at myopia onset, and the child's age would be substituted for age of onset. As shown in Table 6 and Figure 2, the model predicts the average rate of myopia progression within 0.25 D in children in which age of onset is unknown, although the variability in the predicted amount of myopia increases with years of progression.
Few studies provide information regarding the details of myopia progression by subgroups of their sample. A recent meta-analysis reported on myopia progression rates among Asian and European (includes 20% Black and Asian ancestry) urban children who wore single-vision spectacles.29 The summary estimate for 1-year myopia progression among European children was −0.55 D, whereas the summary estimate for those of Asian background was −0.82 D. The meta-analysis also found that the Asian children progressed faster than the European children over 3 years, with the difference in progression between the groups increasing with each year, that is, after 1 year the difference between the 2 groups was −0.31 D, after 2 years the difference was −0.49 D, and after 3 years the difference was −0.58 D.29 Our 3-year difference between Asian American and White children was −0.19 D. Although the reason for the different effects of European and Asian ancestry between studies is difficult to identify, one possibility is the different environments. All children in CLEERE resided in the United States and attended mostly suburban schools as opposed to the meta-analysis in which some of the children lived in Asia and all resided in cities. Near work and time outdoors did not affect the myopia progression rate in CLEERE, a finding that might not hold in other samples.
The COMET data provide a comparison for 3-year progression among American children because the two studies include the same ethnic groups (with the exception of the Native American children, who were not represented in COMET).16 Using the 3-year progression rates unadjusted for age of onset, COMET Asian Americans progressed −1.71 D, whereas CLEERE Asian Americans progressed −1.83 D. Progression in Hispanics was −1.27 D in COMET children versus −1.30 D in CLEERE children, whereas White children progressed −1.48 D and −1.46 D in COMET and CLEERE, respectively. Black children in COMET progressed −1.28 D compared with −0.85 D in CLEERE children. The progression rates are remarkably consistent between COMET and CLEERE, making the reason for the difference in Black children difficult to pinpoint.
Among studies in Asia with 3-year data presented from 7 years of age; the estimates of myopia progression rate were higher than those from Asian American subjects in our CLEERE Study (−1.93 D). In the Chua et al.28 study, 3-year progression from 7 to 10 years was −2.52 D, and Saw et al.18 reported a −2.02 D progression over the same time, an estimate that is included in the current study 95% confidence interval (Table 5). Some of the difference may be because of geographic location or outdoor exposure prior to myopia onset, consistent with similar discrepancies reported by Rose et al.30 between Chinese children of Australian and Singaporean descent.
Studies with a single-vision lens wearer group can provide a comparison group to identify risk factors for myopia progression. Younger age was significantly associated with faster myopia progression in a number of studies, with the younger children (generally younger than 10 years) progressing −0.20 D/yr to −0.70 D/yr faster than older children.9,11,13,16,18,31 In one Singaporean study, progression decreased with age, with an average myopia progression per year of −0.85 D for 7-year-olds, −0.66 D for 8-year-olds, −0.51 D for 9-year-olds, −0.32 D for 10-year-olds, and −0.20 D for 11-year-olds.7 Another Singaporean study that investigated myopia progression over 3 years in children aged 7 to 9 years at study entry found myopia progression of −0.80 D/yr for 7-year-olds and −0.57 D/yr for 9-year-olds.18
Yang et al.13 found −0.20 D faster progression among female subjects. Myopia progression was −0.16 D faster among females than males in the COMET Study regardless of treatment group,16 and the Singapore Cohort Study for Risk Factors of Myopia (SCORM) found a similar difference.18 Saw et al.7 found a similar myopia progression between Singaporean males and females (−0.59 D/yr vs. −0.60 D/yr). Our data indicate that females had a faster rate of annual progression compared with males, by an average of 0.093 D. This small female-male difference in progression is therefore a common finding.
Interestingly, the number of myopic parents or the amount of time spent in outdoor/sports activity did not significantly improve the ability of the multivariate model to predict myopia progression. The absence of a relationship between time spent in outdoor/sports activity and myopic progression was one we have previously reported from the CLEERE Study data.32 Other cohort analyses have found similar results using a similar outcome of progression of cycloplegic SPHEQ among those who are myopic,7,33,34 however, a few studies have found an association with time outdoors. Saxena et al.35 reported a protective odds ratio for more than 14 hours a week of outdoor activity, which represented a very small proportion of total participants. There was also no information about progression by activity, making this study's results difficult to apply.35 Pärssinen et al.36 presented the results of their 23-year follow-up on 240 participants that showed a statistically significant interaction between follow-up visits (based on overlapping age categories) and time outdoors. There are no progression values, but from their Figure 3 the effect appears quite small, reaching a difference of perhaps a third of a diopter between the high and low activity groups in the age range of 25 to 39 years. Wu et al.37 failed to find an effect of their outdoor recess treatment in the randomized controlled trial from 2013, whereas their small group of existing myopes (n = 71) showed a significant effect in their 2018 trial.38 Presently, the preponderance of data are consistent with the meta-analysis conducted by Xiong et al.39 indicating a lack of effect of time outdoors on myopia progression. CLEERE was one of the first longitudinal studies to report the protective effect of time outdoors on the risk of myopia onset.40 Others in various parts of the world have confirmed the same effect.37,41–43 One could postulate that perhaps there are different mechanisms that distinguish the onset of myopia from the progression of myopia, but this requires more study.32,44
A related issue to time outdoors is seasonal variation in progression. Donovan et al.45 reported on two groups of subjects from two Chinese trials who were assessed for either winter/summer progression or spring/autumn progression. They found one group of subjects experienced a progression rate higher in the winter/summer 6 months that the second group experienced in the spring/autumn. The study lacked accompanying activity information. COMET also discussed seasonal variation and showed lower progression in the summer months, based on 6-month cycles of noncycloplegic autorefraction.46 They attributed this to no school and more time outdoors during the summer. Similarly, Cui et al.47 found a correlation between myopic progression and day length averaged over the 6 months prior to the visit. They did not have corresponding activity data but indicated that they found it unlikely to be due to variations in schoolwork because in Denmark school vacations are interspersed throughout the year. If it is not time in school, the hours of daylight or the spectral composition of light might be an alternative explanation as to why progression lags in the summer. Not only have daily fluctuations in the composition of light been found, but seasonal variations exists whereby the absolute and relative contribution of blue wavelengths increase in the summer.48
Having myopic parents was also not related to the rate of myopia progression. This is a different result than reported by Kurtz et al.17 from COMET and Saw et al.,7 who both found an increasing progression rate with a greater number of myopic parents. One reason for the discrepancy might be enrollment bias because of different sources of subjects. Both the COMET and Saw et al.7 samples were myopic children recruited for a clinical trial. CLEERE sought children from schools for observation only. The majority of CLEERE subjects were nonmyopic at study entry; neither parents nor investigators had any prior knowledge of future myopia or, if myopic, the future progression rate. A child with two myopic parents and rapid myopia progression may have a higher probability of enrolling in a clinical trial than for an observational study begun at the time that the child was nonmyopic. CLEERE results suggest that having a family history of myopia may have more influence on whether one becomes myopic than it does on the rate of progression once myopia occurs.32,40
Education is an often discussed risk factor for myopia, including the ecologic analyses looking at world ranking of countries by various metrics of achievement.44 What is not clear is what “education” represents, being an amorphous construct of multiple components. Attempts to quantify the relation between near work activities and myopia progression have been equivocal. Several studies have not found an association between measures of time spent in near work and progression,7,32,33,37,38 whereas others have.35,38 Education encompasses many visual factors, including but not limited to focusing distance,49 text concentration,50 amount of time spent doing an activity or taking breaks from doing an activity,49 and the level and quality of lighting.51–54 All these have been cited as the source of the education effect, resulting in the “education effect” being a broadly defined classification, at best.
The CLEERE Study benefits from a large sample size as well as representation from multiple racial/ethnic groups. The study is limited by the restricted range of ages between 7 and 14 years. Follow-up was not long enough to observe either progression in the late teens or early adulthood or the cessation of progression. The CLEERE Study represents a sample of volunteer subjects in which sites preferentially recruited certain racial/ethnic groups, that is, it is not a population-based study. Differences between sites other than ethnicity, such as light exposure, socioeconomic status, suburban versus rural settings, and the many unknowns that may underlie differences in “education” are factors that could have an impact as well. Despite these limitations, other studies’ data support the generalizability of CLEERE Study results. The Sydney Myopia Study has published cross-sectional results similar to CLEERE with respect to the association between less time outdoors among myopic children and the lack of correlation between hours of reading and hours of outdoor/sports activity, and of reading in general.55–58 Another limitation was the change in autorefractors that took place in the CLEERE Study in 2001. This change should have little effect on the model or these results, however, as data from each instrument are represented across ages. The average difference across CLEERE Study visits between autorefractor values before and after adjustment for the change in instrument was negligible at 0.01 ± 0.10 D.59 An additional possible limitation with respect to the classification of race/ethnicity lies in the categories used by the National Institutes of Health in 1997, when data collection began. Categories used at that time grouped together race/ethnic groups that may not be appropriately combined for myopia research, for example, different Asian heritages. This may result in an underreporting of progression for certain groups. Additionally, the predictive ability of the model decreased as time from increased, as is apparent in the increase in error variability documented in Table 6.
Conclusions
We found that Asian American children exhibited faster myopic progression than Black, Hispanic, and Native American children, and that myopic progression was faster at younger ages and among girls across all racial/ethnic groups. These predictions may be useful in sample size planning for clinical trials of treatments designed to slow the progression of myopia given a particular sample composition. It may also help identify those myopic children who would most benefit from treatments that slow the progression of myopia.
Acknowledgments
The authors thank Eric R. Ritchey, PhD (University of Houston College of Optometry), and Noel Brennan, MScOptom, PhD (Vistakon, Johnson & Johnson Vision Care, Inc.), for their useful feedback during manuscript preparation.
Supported by the National Eye Institute/National Institutes of Health Grants U10-EY08893 and R24-EY014792, the Ohio Lions Eye Research Foundation, the E.F. Wildermuth Foundation, and Johnson & Johnson Vision Care Inc.
The CLEERE Study Group (as of March 2012):
Clinical Centers
Franklin Primary Health Center, Inc.: Sandral Hullett, MD, MPH (Principal Investigator, 1997–2006); Robert N. Kleinstein, OD, MPH, PhD (Co-Investigator, 1997–2006); Janene Sims, OD (Optometrist, 1997–2001 and 2004–2006); Raphael Weeks, OD (Optometrist, 1999–2006); Sandra Williams (Study Coordinator, 1999–2006); LeeAndra Calvin (Study Coordinator, 1997–1999); Melvin D. Shipp, OD, MPH, DrPH (Co-Investigator, 1997–2004).
University of California, Berkeley School of Optometry, Berkeley, CA: Nina E. Friedman, OD, MS (Principal Investigator, 1999–2001); Pamela Qualley, MA (Study Coordinator, 1997–2001); Donald O. Mutti, OD, PhD (Principal Investigator, 1996–1999); Karla Zadnik, OD, PhD (Optometrist, 1996–2001).
University of Houston College of Optometry: Ruth E. Manny, OD, PhD (Principal Investigator, 1997–2006); Suzanne M. Wickum, OD (Optometrist, 1999–2006); Ailene Kim, OD (Optometrist, 2003–2006); Bronwen Mathis, OD (Optometrist, 2002–2006); Mamie Batres (Study Coordinator, 2004–2006); Sally Henry (Study Coordinator, 1997–1998); Janice M. Wensveen, OD, PhD (Optometrist, 1997–2001); Connie J. Crossnoe, OD (Optometrist, 1997–2003); Stephanie L. Tom, OD (Optometrist, 1999–2002); Jennifer A. McLeod (Study Coordinator, 1998–2004); Julio C. Quiralte (Study Coordinator, 1998–2005); Gaby Solis (Study Coordinator, 2005–2006).
Southern California College of Optometry, Fullerton, CA: Susan A. Cotter, OD, MS (Principal Investigator, 2004–2006, Optometrist, 1997–2004); Julie A. Yu, OD (Principal Investigator, 1997–2004; Optometrist 2005–2006); Raymond J. Chu, OD, MS (Optometrist, 2001–2006); Carmen N. Barnhardt, OD, MS (Optometrist 2004–2006); Jessica Chang, OD (Optometrist, 2005–2006); Kristine Huang, OD, MPH (Optometrist, 2005–2006); Rebecca Bridgeford (Study Coordinator, 2005–2006); Connie Chu, OD (Optometrist, 2004–2005); Soonsi Kwon, OD (Optometrist, 1998–2004); Gen Lee (Study Coordinator, 1999–2003); John Lee, OD (Optometrist, 2000–2003); Robert J. Lee, OD (Optometrist, 1997–2001); Raymond Maeda, OD (Optometrist, 1999–2003); Rachael Emerson (Study Coordinator, 1997–1999); Tracy Leonhardt (Study Coordinator, 2003–2004).
University of Arizona, Department of Ophthalmology and Vision Science, Tucson, AZ: J. Daniel Twelker, OD, PhD (Principal Investigator, 2000–2010); Mabel Crescioni, DrPh (Study Coordinator, 2009–2010); Dawn Messer, OD, MPH (Optometrist, 2000–2010); Denise Flores (Study Coordinator, 2000–2007); Rita Bhakta, OD (Optometrist, 2000–2004); Katie Garvey, OD (Optometrist, 2005–2008); Amanda Mendez Roberts, OD (Optometrist, 2008–2010).
Resource Centers
Chairman's Office, The Ohio State University College of Optometry, Columbus, OH: Karla Zadnik, OD, PhD (Chairman, 1997–present); Jodi M. Malone Thatcher, RN (Study Coordinator, 1997–2012).
Videophakometry Reading Center, The Ohio State University College of Optometry, Columbus, OH: Donald O. Mutti, OD, PhD (Director, 1997–present); Huan Sheng, MD, PhD (Reader, 2000–2006); Holly Omlor (Reader, 2003–2006); Meliha Rahmani, MPH (Reader, 2004–2007); Jaclyn Brickman (Reader, 2002–2003); Amy Wang (Reader, 2002–2003); Philip Arner (Reader, 2002–2004); Samuel Taylor (Reader, 2002–2003); Myhanh T. Nguyen, OD, MS (Reader, 1998–2001); Terry W. Walker, OD, MS (Reader, 1997–2001); Vidhya Subramanian, MS (Reader, 2006–2007).
Optometry Coordinating Center, The Ohio State University College of Optometry, Columbus, OH: Lisa A. Jones-Jordan, PhD (Director, 1997–present); Linda Barrett (Data Entry Operator, 1997–2007); John Hayes, PhD (Biostatistician, 2001–2007); G. Lynn Mitchell, MAS (Biostatistician, 1998–present); Melvin L. Moeschberger, PhD (Consultant, 1997–2010); Loraine Sinnott, PhD (Biostatistician, 2005–present); Pamela Wessel (Program Coordinator, 2000–2017); Julie N. Swartzendruber, MA (Program Coordinator, 1998–2000); Austen Tanner (Data Entry Operator, 2008–2010).
Project Office, National Eye Institute, Rockville, MD: Donald F. Everett, MA.
Committees: Executive Committee: Karla Zadnik, OD, PhD (Chairman); Lisa A. Jones-Jordan, PhD; Robert N. Kleinstein, OD, MPH, PhD; Ruth E. Manny, OD, PhD; Donald O. Mutti, OD, PhD; J. Daniel Twelker, OD, PhD; Susan A. Cotter, OD, MS.
Disclosure: L.A. Jones-Jordan, None; L.T. Sinnott, None; R.H. Chu, None; S.A. Cotter, None; R.N. Kleinstein, None; R.E. Manny, None; D.O. Mutti, None; J.D. Twelker, None; K. Zadnik, Nevakar, Inc.
References
- 1.Vitale S, Ellwein L, Cotch MF, Ferris FL 3rd, Sperduto R.. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol. 2008; 126: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung SK, Lee JH, Kakizaki H, Jee D.. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in Seoul, South Korea. Invest Ophthalmol Vis Sci. 2012; 53: 5579–5583. [DOI] [PubMed] [Google Scholar]
- 3.He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB.. Refractive error and visual impairment in urban children in southern china. Invest Ophthalmol Vis Sci. 2004; 45: 793–799. [DOI] [PubMed] [Google Scholar]
- 4.Lam CS, Lam CH, Cheng SC, Chan LY.. Prevalence of myopia among Hong Kong Chinese school children: changes over two decades. Ophthalmic Physiol Opt. 2012; 32: 17–24. [DOI] [PubMed] [Google Scholar]
- 5.Edwards MH, Li RW, Lam CS, Lew JK, Yu BS.. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci. 2002; 43: 2852–2858. [PubMed] [Google Scholar]
- 6.Fan DS, Lam DS, Lam RF, et al.. Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest Ophthalmol Vis Sci. 2004; 45: 1071–1075. [DOI] [PubMed] [Google Scholar]
- 7.Saw SM, Nieto FJ, Katz J, Schein OD, Levy B, Chew SJ.. Factors related to the progression of myopia in Singaporean children. Optom Vis Sci. 2000; 77: 549–554. [DOI] [PubMed] [Google Scholar]
- 8.Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH.. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009; 116: 572–579. [DOI] [PubMed] [Google Scholar]
- 9.Hasebe S, Ohtsuki H, Nonaka T, et al.. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008; 49: 2781–2789. [DOI] [PubMed] [Google Scholar]
- 10.Shih YF, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LL.. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol Scand. 2001; 79: 233–236. [DOI] [PubMed] [Google Scholar]
- 11.Tan DT, Lam DS, Chua WH, Shu-Ping DF, Crockett RS.. One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005; 112: 84–91. [DOI] [PubMed] [Google Scholar]
- 12.Saw SM, Chua WH, Gazzard G, Koh D, Tan DT, Stone RA.. Eye growth changes in myopic children in Singapore. Br J Ophthalmol. 2005; 89: 1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Lan W, Ge J, et al.. The effectiveness of progressive addition lenses on the progression of myopia in Chinese children. Ophthalmic Physiol Opt. 2009; 29: 41–48. [DOI] [PubMed] [Google Scholar]
- 14.Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K.. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS. 2008; 12: 332–339. [DOI] [PubMed] [Google Scholar]
- 15.Gwiazda J, Hyman L, Hussein M, et al.. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003; 44: 1492–1500. [DOI] [PubMed] [Google Scholar]
- 16.Hyman L, Gwiazda J, Hussein M, et al.. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005; 123: 977–987. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz D, Hyman L, Gwiazda JE, et al.. Role of parental myopia in the progression of myopia and its interaction with treatment in COMET children. Invest Ophthalmol Vis Sci. 2007; 48: 562–570. [DOI] [PubMed] [Google Scholar]
- 18.Saw SM, Tong L, Chua WH, et al.. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005; 46: 51–57. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G.. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci. 2019; 96: 556–567. [DOI] [PubMed] [Google Scholar]
- 20.Chia A, Lu QS, Tan D.. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016; 123: 391–399. [DOI] [PubMed] [Google Scholar]
- 21.Walline JJ, Walker MK, Mutti DO, et al.. Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: the BLINK randomized clinical trial. JAMA. 2020; 324: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yam JC, Li FF, Zhang X, et al.. Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) Study: phase 2 report. Ophthalmology. 2020; 127: 910–919. [DOI] [PubMed] [Google Scholar]
- 23.Seddon JM, Sahagian CR, Glynn RJ, Sperduto RD, Gragoudas ES.. Evaluation of an iris color classification system. The Eye Disorders Case-Control Study Group. Invest Ophthalmol Vis Sci. 1990; 31: 1592–1598. [PubMed] [Google Scholar]
- 24.Kleinstein RN, Mutti DO, Manny RE, Shin JA, Zadnik K.. Cycloplegia in African-American children. Optom Vis Sci. 1999; 76: 102–107. [DOI] [PubMed] [Google Scholar]
- 25.Zadnik K, Mutti DO, Friedman NE, Adams AJ.. Initial cross-sectional results from the Orinda Longitudinal Study of Myopia. Optom Vis Sci. 1993; 70: 750–758. [DOI] [PubMed] [Google Scholar]
- 26.Walline JJ, Zadnik K, Mutti DO.. Validity of surveys reporting myopia, astigmatism, and presbyopia. Optom Vis Sci. 1996; 73: 376–381. [DOI] [PubMed] [Google Scholar]
- 27.Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K.. Parental myopia, near work, school achievement, and children's refractive error. Invest Ophthalmol Vis Sci. 2002; 43: 3633–3640. [PubMed] [Google Scholar]
- 28.Chua SY, Sabanayagam C, Cheung YB, et al.. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016; 36: 388–394. [DOI] [PubMed] [Google Scholar]
- 29.Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith EL 3rd, Holden BA.. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci. 2012; 89: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose KA, Morgan IG, Smith W, Burlutsky G, Mitchell P, Saw SM.. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008; 126: 527–530. [DOI] [PubMed] [Google Scholar]
- 31.Fulk GW, Cyert LA, Parker DE.. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000; 77: 395–401. [DOI] [PubMed] [Google Scholar]
- 32.Jones-Jordan LA, Sinnott LT, Cotter SA, et al.. Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci. 2012; 53: 7169–7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li SM, Li H, Li SY, et al.. Time outdoors and myopia progression over 2 years in Chinese children: the Anyang Childhood Eye Study. Invest Ophthalmol Vis Sci. 2015; 56: 4734–4740. [DOI] [PubMed] [Google Scholar]
- 34.Oner V, Bulut A, Oruc Y, Ozgur G.. Influence of indoor and outdoor activities on progression of myopia during puberty. Int Ophthalmol. 2016; 36: 121–125. [DOI] [PubMed] [Google Scholar]
- 35.Saxena R, Vashist P, Tandon R, et al.. Incidence and progression of myopia and associated factors in urban school children in Delhi: the North India Myopia Study (NIM Study). PLoS One. 2017; 12: e0189774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pärssinen O, Kauppinen M, Viljanen A.. The progression of myopia from its onset at age 8-12 to adulthood and the influence of heredity and external factors on myopic progression. A 23-year follow-up study. Acta Ophthalmol. 2014; 92: 730–739. [DOI] [PubMed] [Google Scholar]
- 37.Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK.. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013; 120: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 38.Wu PC, Chen CT, Lin KK, et al.. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018; 125: 1239–1250. [DOI] [PubMed] [Google Scholar]
- 39.Xiong S, Sankaridurg P, Naduvilath T, et al.. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017; 95: 551–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K.. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007; 48: 3524–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guggenheim JA, Northstone K, McMahon G, et al.. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci. 2012; 53: 2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.French AN, Morgan IG, Mitchell P, Rose KA.. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology. 2013; 120: 2100–2108. [DOI] [PubMed] [Google Scholar]
- 43.He M, Xiang F, Zeng Y, et al.. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015; 314: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 44.Morgan IG, French AN, Ashby RS, et al.. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018; 62: 134–149. [DOI] [PubMed] [Google Scholar]
- 45.Donovan L, Sankaridurg P, Ho A, et al.. Myopia progression in Chinese children is slower in summer than in winter. Optom Vis Sci. 2012; 89: 1196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gwiazda J, Deng L, Manny R, Norton TT, Group CS. Seasonal variations in the progression of myopia in children enrolled in the correction of myopia evaluation trial. Invest Ophthalmol Vis Sci. 2014; 55: 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui D, Trier K, Munk Ribel-Madsen S. Effect of day length on eye growth, myopia progression, and change of corneal power in myopic children. Ophthalmology. 2013; 120: 1074–1079. [DOI] [PubMed] [Google Scholar]
- 48.Thorne HC, Jones KH, Peters SP, Archer SN, Dijk DJ.. Daily and seasonal variation in the spectral composition of light exposure in humans. Chronobiol Int. 2009; 26: 854–866. [DOI] [PubMed] [Google Scholar]
- 49.Huang PC, Hsiao YC, Tsai CY, et al.. Protective behaviours of near work and time outdoors in myopia prevalence and progression in myopic children: a 2-year prospective population study. Br J Ophthalmol. 2020; 104: 956–961. [DOI] [PubMed] [Google Scholar]
- 50.Zylbermann R, Landau D, Berson D.. The influence of study habits on myopia in Jewish teenagers. J Pediatr Ophthalmol Strabismus. 1993; 30: 319–322. [DOI] [PubMed] [Google Scholar]
- 51.Hua WJ, Jin JX, Wu XY, et al.. Elevated light levels in schools have a protective effect on myopia. Ophthalmic Physiol Opt. 2015; 35: 252–262. [DOI] [PubMed] [Google Scholar]
- 52.Norton TT, Siegwart JT Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006; 47: 4687–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith EL 3rd, Hung LF, Huang J.. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012; 53: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landis EG, Yang V, Brown DM, Pardue MT, Read SA.. Dim light exposure and myopia in children. Invest Ophthalmol Vis Sci. 2018; 59: 4804–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ip JM, Huynh SC, Robaei D, et al.. Ethnic differences in the impact of parental myopia: findings from a population-based study of 12-year-old Australian children. Invest Ophthalmol Vis Sci. 2007; 48: 2520–2528. [DOI] [PubMed] [Google Scholar]
- 56.Ip JM, Saw SM, Rose KA, et al.. Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci. 2008; 49: 2903–2910. [DOI] [PubMed] [Google Scholar]
- 57.Ojaimi E, Rose KA, Morgan IG, et al.. Distribution of ocular biometric parameters and refraction in a population-based study of Australian children. Invest Ophthalmol Vis Sci. 2005; 46: 2748–2754. [DOI] [PubMed] [Google Scholar]
- 58.Rose KA, Morgan IG, Ip J, et al.. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008; 115: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 59.Mutti DO, Mitchell GL, Hayes JR, et al.. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006; 47: 837–846. [DOI] [PubMed] [Google Scholar]


