Abstract
Background:
Neuroticism is a heritable trait that contributes to the vulnerability to depression. We used polygenic risk scores (PRS) to examine genetic vulnerability to neuroticism and its associations with reward/punishment processing in a clinical sample with mood, anxiety, and substance use disorders. It was hypothesized that higher PRS for neuroticism is associated with attenuated neural responses to reward/punishment.
Method:
Four hundred sixty-nine participants were genotyped and their PRSs for neuroticism were computed. Associations between PRS for neuroticism and anticipatory processing of monetary incentives were examined using functional magnetic resonance imaging.
Results:
Individuals with higher PRS for neuroticism showed less anticipatory activation in the left amygdala and caudate region to incentives regardless of incentive valence. Further, these individuals exhibited altered sensitivity to gain/loss processing in the right anterior insula. Higher PRSs for neuroticism were also associated with reduced processing of gains in the precuneus.
Limitations:
The study population consisted of a transdiagnostic sample with dysfunctions in positive and negative valence processing. PRS for neuroticism may be correlated with current clinical symptoms due to the vulnerability to psychiatric disorders.
Conclusions:
Greater genetic loading for neuroticism was associated with attenuated anticipatory responsiveness in reward/punishment processing with altered sensitivity to valences. Thus, a higher genetic risk for neuroticism may limit the degree to which positive and/or negative outcomes influence the current mood state, which may contribute to the development of positive and negative affective dysfunctions in individuals with mood, anxiety, and addictive disorders.
Keywords: neuroticism, reward, sensitivity, fMRI, PRS, genetics
1. Introduction
Neuroticism is a heritable personality trait considered to be a risk factor for developing major depression, anxiety disorders, and other psychiatric disorders (Kotov et al., 2010; Malouff et al., 2005; Ormel et al., 2013). Individual difference in neuroticism is associated with differences in affective processing. It has been posited that neuroticism contributes to greater sensitivity to negative stimuli, resulting in high emotional arousal and emotional response to negative stimuli (Costa & McCrae, 1980, 2008; Suls & Martin, 2005; Wilson et al., 2006). As individuals with neuroticism are also more sensitive to potential loss, they tend to avoid risk (Lahey, 2009; Widiger & Oltmanns, 2017). At the neural level, neuroticism was associated with increased amygdala activation to emotional stimuli (Cremers et al., 2010; Servaas et al., 2013; Stein et al., 2007) and risk-taking decisions (Paulus et al., 2003; Preuschoff et al., 2008; Von Siebenthal et al, 2020). These results indicate that neuroticism is characterized by altered processing of negative affect at both behavioral and neural levels.
In addition, psychiatric disorders with high neuroticism are known for blunted responsivity to positive stimuli (Alloy et al., 2016; Nelson et al., 2013). A growing body of neuroimaging literature has shown decreased neural responses to reward as well as altered neural reward circuitry in depression (Luking et al., 2016; Pechtel et al., 2013; Robbins, 2016), anxiety (Elman et al., 2009; Guyer et al, 2012; Sailer et al., 2008), and substance use disorders (Cooper et al., 2017; Volkow et al., 2010). These findings suggest that neuroticism could be associated with positive affective dysfunction as well as negative affective dysfunction. However, the direct investigation on neuroticism and altered processing of positive affect is rather scarce, except for recent studies on altered electrophysiological sensitivity to reward and inappropriate use of reward information with increased neuroticism (Rupprechter et al., 2020; Speed et al., 2018).
While neuroticism has been predominantly associated with high sensitivity to negative affect, stress, threat, and the proneness to adverse life outcomes (Lahey, 2009; Ozer & Benet-Martinez, 2006), the study findings on altered processing of reward with neuroticism imply that neuroticism may underlie altered sensitivity to reward/punishment. Altered approach-avoidance behavior with compromised sensitivity to reward/punishment has been considered as one of the dysfunctional characteristics of various psychiatric disorders (Aupperle & Paulus, 2010, Ironside et al., 2020; Nelson et al., 2013). Neuroticism may mediate altered approach-avoidance behavior via differential sensitivity to reward/punishment with dysfunctional valence processing. Nonetheless, it is unknown whether neuroticism influences neural processing of sensitivity to reward/punishment through valence responsivity, despite the importance of understanding neuroticism in relation to the vulnerability for psychiatric disorders.
Since Eysenck and Prell’s (1951) seminal article, heredity is considered to contribute to individual differences in neuroticism (Nagel et al., 2018a; Smith et al., 2016). Previous studies have shown substantial genetic associations between neuroticism and psychiatric disorders (Hettema et al., 2006; Okbay et al., 2016). The association between neuroticism and differential depression treatment outcomes is also thought to be mediated by genetic factors (de Moor et al, 2012; Kendler et al., 1993). Personality traits are known to be polygenic, in which multiple genes collectively contribute to the effect of the trait, accounting for small variances across the genome (Amare et al., 2018; Zwir et al., 2019). In this regard, the polygenic risk score (PRS) can be a useful tool for investigating neuroticism. Using the PRS approach, we investigated whether genetic risk for neuroticism modulated the processing of gain (i.e., reward) and loss (i.e., threat of punishment) (hereafter reward processing inclusively) on a monetary incentive task with functional magnetic resonance imaging (fMRI). Due to the importance of the motivational component in reward processing, we focused on anticipatory neural responses to incentive cues. The present study included a transdiagnostic group along with healthy controls to examine how a range of genetic propensity for neuroticism was associated with reward processing in the broad context of psychopathology beyond depression and anxiety, along with healthy individuals.
We investigated (1) the association of PRS for neuroticism with reward processing across valence (gain & loss); (2) the association of PRS for neuroticism with sensitivity to reward/punishment by comparing motivational valence (gain vs. loss); and (3) the association of PRS for neuroticism with the processing of each valence (gain or loss). In light of previous studies showing attenuated reward processing in individuals with mood and anxiety disorders, we hypothesized that PRS for neuroticism would modulate overall neural responses to monetary incentives. We also predicted that relative sensitivity to motivational valence would be moderated by PRS for neuroticism. Further, we expected altered reward responsivity by incentive valence with PRS for neuroticism. Given the exploratory nature of the study, a whole-brain approach was adopted for studying the relationship between polygenic risk for neuroticism and neural reward processing.
2. METHODS AND MATERIALS
Participants
The participants in this study were from the first 500 participants in the Tulsa-1000 (T-1000) cohort, a naturalistic study that aimed to longitudinally follow 1000 individuals with mood, anxiety, substance use, and/or eating disorders, as well as healthy volunteers (Victor et al., 2018). Target population individuals were considered eligible for the T-1000 study if they fulfilled any of the following criteria: Patient Health Questionnaire (PHQ-9: Kroenke et al., 2001) ≥ 10 and/or Overall Anxiety Severity and Impairment Scale (OASIS: Campbell-Sills et al., 2009) ≥ 8, and/or Drug Abuse Screening Test (DAST-10: McCabe et al., 2006) score > 2, and/or Eating Disorder Screen (SCOFF: Morgan et al., 2000) score ≥ 2. Healthy controls were individuals who did not screen positive for the inclusion measures. Exclusion criteria of the T1000 study were (1) positive results on a drug screening test; (2) lifetime bipolar, schizophrenia spectrum, antisocial personality, or obsessive-compulsive disorders; (3) active suicidal ideation; (4) moderate to severe traumatic brain injury; (5) severe/unstable medical concerns; (6) change in psychiatric medication dose in the last 6 weeks; and (7) MRI contraindications. Participants provided written informed consent and received financial compensation for their involvement. All procedures were approved by the Western Institutional Review Board. The present study was based on the data from 469 participants after excluding those with excessive head-motion (n = 11) or incomplete genetic or MRI data (n = 20). Participants’ demographic and clinical information is displayed in Table 1.
Table 1.
Demographic and clinical characteristics of the study sample
| HC (N = 55) |
Target Population (N = 414) |
p-value | |||
|---|---|---|---|---|---|
| Age, M (SD) | 32.4 | (11.2) | 34.4 | (10.4) | |
| Male, N (%) | 27 | (49%) | 138 | (33%) | 0.02 |
| Ethnicity, N (%) | |||||
| Hispanic or Latino | 4 | (7%) | 15 | (4%) | |
| Not Hispanic or Latino | 50 | (91%) | 392 | (94%) | |
| Unspecified | 1 | (2%) | 7 | (2%) | |
| Race, N (%) | |||||
| White | 42 | (76%) | 287 | (69%) | |
| American Indian or Alaska Native | 7 | (12.5%) | 79 | (19%) | |
| Black or African American | 2 | (3.5%) | 28 | (7%) | |
| Asian or Pacific Islander | 2 | (3.5%) | 2 | (0.5%) | |
| “Other”, unspecified | 2 | (3.5%) | 18 | (4.5%) | |
| Education, N (%) | |||||
| Less than high school | 0 | (0%) | 46 | (0%) | |
| High school degree or equivalent (GED) | 7 | (13%) | 88 | (17%) | |
| Some college, no degree | 22 | (40%) | 139 | (28%) | |
| College or higher | 26 | (47%) | 138 | (55%) | |
| No response | 0 | (0%) | 3 | (0%) | |
| Income, M (SD) | $59,848 | ($57,373) | $51,785 | ($73,017) | |
| PRS-Neuroticism, M (SD) | −0.26 | (1.01) | 0.03 | (0.99) | 0.04 |
| BFI-Neuroticism, M (SD) | 16.4 | (5.53) | 28.0 | (6.66) | <0.001 |
| PHQ-9, M (SD) | 0.93 | (1.40) | 10.43 | (6.17) | <0.001 |
| OASIS, M (SD) | 1.31 | (1.93) | 8.38 | (4.40) | <0.001 |
| DAST, M (SD) | 0.12 | (0.39) | 3.14 | (3.72) | <0.001 |
| SCOFF, M (SD) | 0.09 | (0.29) | 1.02 | (1.27) | <0.001 |
Abbreviations: HC, Healthy Comparison; PRS-Neuroticism, Polygenic Risk Score for Neuroticism; BFI-Neuroticism, Big Five Inventory Neuroticism; PHQ-9, Patient Health Questionnaire; OASIS, Overall Anxiety Severity and Impairment Scale; DAST, Drug Abuse Screening Test; SCOFF, eating disorders screening questionnaire.
Polygenic Risk Score (PRS) for neuroticism
Participants’ blood samples were genotyped by RUCDR Infinite Biologics using Illumina Infinium Global Screening Array-24 (v.2.0) BeadChip arrays. The McCarthy Group Tool (HRC or 1000G Imputation preparation and checking, v.4.2.11) was used to compare genotypes to the Haplotype Reference Consortium (HRC) release 1.1 reference panel to check for mismatches in the strand, ID names, position, alleles, and ref/alt assignments. The resultant sample data were subjected to quality control using PLINK (v.1.9, https://www.cog-genomics.org/plink2; v.2.0, https://www.cog-genomics.org/plink/2.0) at the Laureate Institute for Brain Research. SNPs were excluded if (1) call rates were lower than 2%; (2) SNPs were duplicated; (3) SNPs did not meet Hardy-Weinberg equilibrium. Individuals were also excluded if closely related participants () were identified within the sample. Imputation was performed using the Michigan Imputation Server (Das et al., 2016). Genome information was imputed from 569,641 to 40,359,612 SNPs.
The base data describing the strength and significance of the association of each SNP with neuroticism was obtained from a genome-wide association study (GWAS) performed by the Center for Neurogenomics and Cognitive Research Complex Trait Genetics Lab on data from the UK Biobank (Nagel et al., 2018b). Neuroticism was quantified in the base data as a neuroticism sum score of 12 items. The GWAS was conducted using 380,060 participants, and the resulting GWAS summary included 10,846,943 SNPs. PRS for neuroticism was derived using the software PRSice-2 (Choi et al., 2019). Clumping was performed in PRSice to account for linkage disequilibrium (LD) between SNPs using standard parameters (250 kilobases, r2 = 0.1, p = 1), and 144,443 SNPs remained after clumping. The p-values provided in the GWAS summary were used to determine which SNPs to include in the calculation of the PRS. Several p-value thresholds were considered (5*10−8, 5*10−7, 5*10−6, 5*10−5, 5*10−4, 0.001, 0.005, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, & 1), and each resulting PRS was compared to participant neuroticism score from the Big Five Inventory (BFI). Because the thresholds between 0.05 and 1 shared comparable fit to the phenotype (Supplementary Figure 1), with no a-priori method to determine the best threshold, we opted to choose the most stringent threshold of 0.05 among the thresholds that explained greater than 0.025 of the variance. Principle Component Analysis (PCA) was used to adjust for population stratification (FlashPCA2, Abraham et al., 2017). PRSs for neuroticism were standardized across participants (M = 0, SD = 1).
MID task
The Monetary Incentive Delay task (MID) was used as an imaging experiment paradigm for investigating reward-related neural activity (Knutson et al., 2001). The study design included valence (2: gain, loss) x magnitude (3: high, low, no) of the incentive resulting in 6 incentive conditions: high-gain (+$5), low-gain (+$1), no-gain (+$0), high-loss (-$5), low-loss (-$1), and no-loss (-$0). On each trial, participants were presented a visual object cue (2 s) in which the shape of the cue indicated valence (circle: gain, square: loss) and the position of a horizontal line in the cue indicated magnitude (top: $5, middle: $1, bottom: $0). Following a varied delay period (2.25 –3 s), a target (triangle) prompted a button-press response. Participants were instructed to respond to the target as fast as they could, for either obtaining potential gain or avoiding potential loss. The target duration was calibrated to each participant such that participants would succeed on approximately 66% of trials. Feedback (2 s) followed the target and indicated the outcome (i.e., amount earned or lost). The task was comprised of two runs totaling 90 trials with 15 trials per condition (~19 m). Participants performed the task in the scanner.
Neuroimaging data acquisition and preprocessing
Two identical GE MR750 3T scanners in the same site equipped with 8 RF channel phased array coils were used to acquire both T1-weighted 3D high-resolution anatomical images (MP-RAGE pulse sequence, FOV 240 × 192mm, TR/TE = 5/2.012ms, 186 axial slices) and T2*-weighted echo-planar images (flip angle 78°, FOV 240 × 240mm, TR/TE = 2000/27ms, axial plane) per volume. Each volume comprised of 39 slices (2.9mm thick, 1.875 × 1.875mm voxels) acquired in an interleaved sequence. Functional imaging data were collected in two runs, each with 281 volumes (~ 9m 22s).
Preprocessing and statistical analyses of MRI data were performed using the Analysis of Functional NeuroImages software suite (AFNI, https://afni.nimh.nih.gov/). The first 3 EPI volumes were discarded for signal stabilization. For preprocessing, imaging data were despiked, slice-time corrected to the first slice, co-registered to a T1-weighted anatomical image, and motion-corrected. Time points with large head movements were censored (ENORM > 0.3). Imaging data were also normalized to the standard MNI space with resampling of 2-mm isotropic voxels and smoothed with an isotropic 4-mm FWHM Gaussian kernel.
Data analysis
A two-level general linear model was employed to analyze functional imaging data focused on neural responses to incentive cues during delay periods. First, six event regressors were constructed on a subject to model the response for an upcoming incentive: high-gain (+$5), low-gain (+$1), no-gain (+$0), high-loss (-$5), low-loss (-$1), and no-loss (-$0). The BOLD response to an incentive cue was convolved with a delta function for 4-s spanning from the presentation of the cue. Regressors of noninterest were included for the first four polynomial terms and the six motion parameters. For the group-level analyses, the contrasts of incentive valences were constructed by comparing high-incentive to no-incentive trials: gain (+$5 > +$0) and loss (-$5 > -$0). Then, the relationship between PRS for neuroticism and anticipatory reward processing was examined using a multivariate ANCOVA model (3dMVM) with age, sex, and race as covariates.1 That means, the gain and loss contrasts of valence served as two events-of-interests varied by PRS for neuroticism with other covariates in the analysis model (within-subject: valence (2), between-subject: PRS, age, sex, race). The main effect of PRS for neuroticism was estimated by collapsing valences. An interaction between PRS and valence was estimated by comparing the difference between gain versus loss by PRS. The effect of PRS for neuroticism was also estimated to each valence, gain and loss. A cluster-extent threshold of α < .05 (k > 43) was set based on the estimated ACF parameters of the group level error terms using 3dFWHMx and 3dClustSim with a voxel-wise threshold of p < .001. Cluster effects were further examined for each valence following a-priori hypotheses by extracting beta coefficients from suprathreshold clusters and applying the same model as was used for voxel-wise statistics. In addition, the association between cluster activation and psychiatric symptom severity (depression & anxiety) was also examined to see the relationship of genetic risk for neuroticism in depression and anxiety disorders. Besides, a supplementary model was constructed with the addition of an individual’s psychiatric diagnosis as a covariate to see if a psychiatric disorder contributed to the effect of PRS for neuroticism. Except for the inclusion of psychiatric diagnosis in the model, all statistical procedures were identical to those of the main model described above. Behavioral data, hit responses and reaction times (RT), were also analyzed analogously to the analysis of imaging data.
3. RESULTS
Participant characteristics.
Both treatment-seeking individuals and healthy volunteers participated in the study. PRSs for neuroticism were higher in treatment-seeking individuals compared to healthy volunteers (Table 1). Treatment-seeking individuals also showed higher neuroticism on BFI-Neuroticism. These individuals were more depressed and more anxious than healthy volunteers.2
Behavioral results.
Hit responses and RTs to cues by valence and magnitude are shown in Supplementary Table 2.3 Participants made more hits to $5 trials than $0 trials, F[1,464] = 5.36, p = 0.01, ŋp2 = 0.0045 [95% CI: 0, 0.01]. The interaction between valence and magnitude in hits was significant F[1,464] = 4.77, p = .03, ŋp2 = 0.0034 [95% CI: 0, 0.01], but participants made more hits to gain trials only marginally, p = .05. Participants responded faster to gains than losses, F[1,464] = 10.62, p = 0.001, ŋp2 = 0.0076 [95% CI: 0, 0.02]. An interaction showed that participants responded to gains faster in $5 trials, F[1,464] = 12.62, p < 0.001, ŋp2 = 0.009 [95% CI: 0, 0.02]. PRS for neuroticism was related to neither hits nor RTs (ps > .25). The amount earned was also not related to PRS for neuroticism (p > .57).
fMRI results.
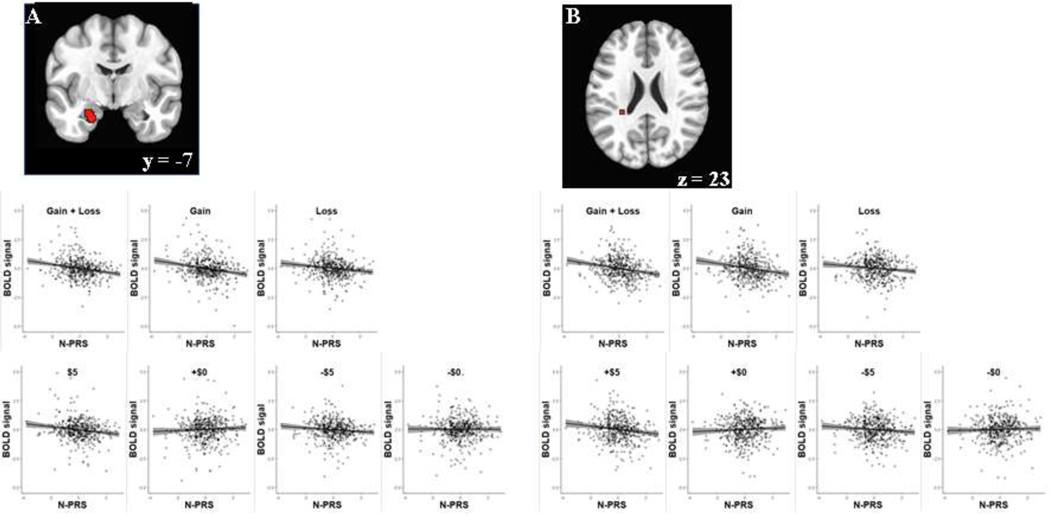
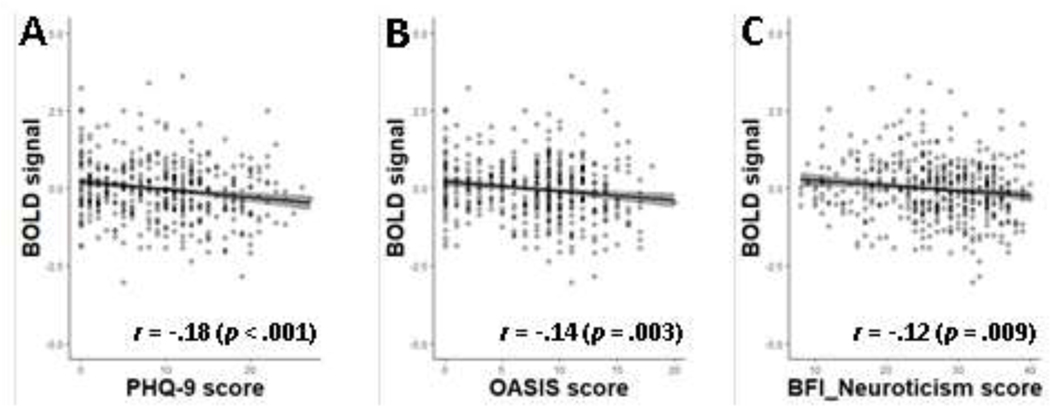
Neural correlates of PRS for neuroticism in reward processing across the valence of incentives (gain & loss) were found with diminished anticipatory activity for an upcoming incentive in the basolateral part of the left amygdala (−23,−7,−21), standardized β = −0.2048 [95% CI: −0.2938, −0.1158], t[467] = −4.52, and the region adjacent to the left caudate tail (−29,−37,23), standardized β = −0.2086 [95% CI: −0.2975, −0.1197], t[467] = −4.61 as shown in Figure 1. Follow-up tests showed that neural activations corresponding to anticipatory gain processing and loss processing were also reduced with the increase of PRSs for neuroticism in the amygdala (gain: standardized β = −0.1877 [95% CI: −0.2770, −0.0983], t[467] = −4.13; loss: standardized β = −0.1507 [95% CI: −0.2406, −0.0608], t[467] = −3.29) and the caudate tail area (gain: standardized β = −0.1820 [95% CI: −0.2714, −0.0926], t[467] = −4.00; loss: standardized β = −0.1427 [95% CI: −0.2327, −0.0527], t[467] = −3.12). In addition, those individuals with greater neural activity in the caudate tail region reported lower scores on depression (PHQ-9) and anxiety (OASIS) as well as BFI-Neuroticism (Figure 2).
Figure 1.
Brain regions showing the association of PRS for neuroticism (N-PRS) with reward processing across valence (A: Amygdala, B: Caudate). The gray shaded area in regression plot represents 95% confidence interval.
Figure 2.
Relationship between caudate activity and symptom severity (A: PHQ-9, B: OASIS, C: BFI-Neuroticism). Gray shading represents 95% confidence interval.
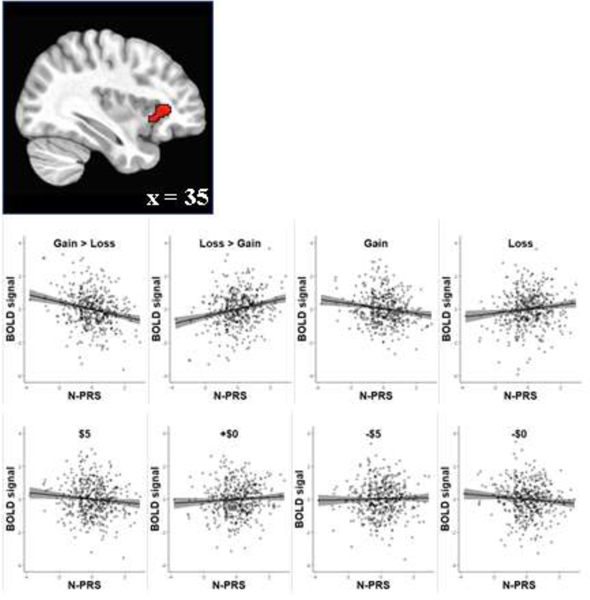
To examine the sensitivity to reward/punishment (gain vs. loss) we investigated the interaction between PRS and incentive valence. A large cluster in the dorsal anterior to the middle part of the right insula (35,31,5; 168 voxels) showed the association between PRS for neuroticism and sensitivity to gain versus loss during the delay period, standardized β = −0.2312 [95% CI: −0.3197, −0.1428], t[467] = −5.14 (Figure 3). Specifically, individuals with high PRS for neuroticism showed greater activation to loss relative to gain. Further, individuals with high PRS for neuroticism relative to low neuroticism PRS showed less activation to anticipating gains (standardized β = −0.1514 [95% CI: −0.2413, −0.0615], t[467] = −3.31) but showed slightly more activation to anticipating losses (standardized β = 0.1219 [95% CI: 0.0316, 0.2121], t[467] = 2.65). At the same time, high PRS for neuroticism individuals showed slightly larger activation to anticipating the positive neutral condition (+$0) and slightly less activation to anticipating the negative neutral condition (-$0). Taken together, these results show that the anterior insula in individuals with high PRS for neuroticism is more sensitive to anticipating losses than gains.
Figure 3.
Association of PRS for neuroticism (N-PRS) with gain/loss sensitivity in anterior insula. Gray shading represents 95% confidence interval
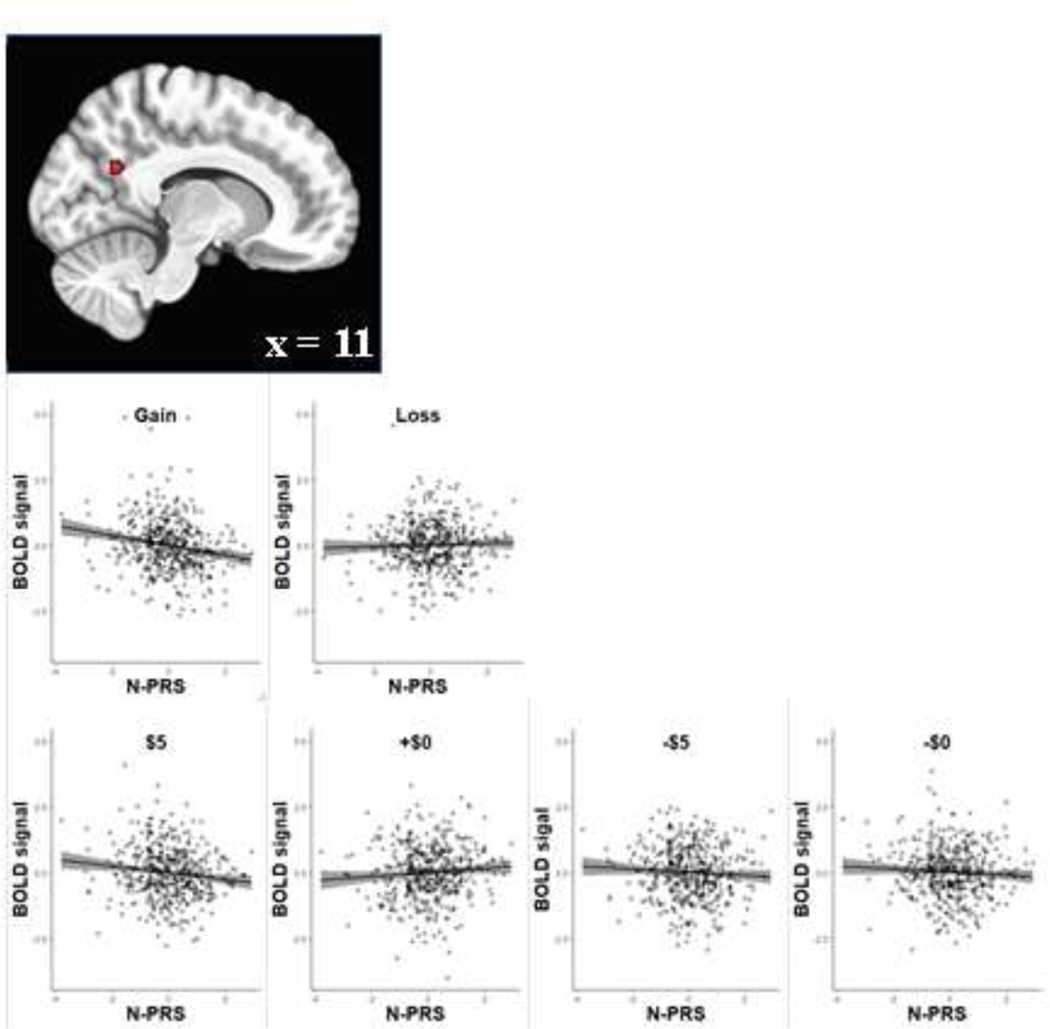
The relationship between PRS for neuroticism and valence processing was probed for gain and loss, individually. PRS for neuroticism was inversely associated with activation to the cue signaling potential gain in the posterior part of the right precuneus (11,−57,27), standardized β = −0.2028 [95% CI: −0.2919, −0.1138], t[467] = −4.48 (Figure 4). However, the examination of PRS for neuroticism and anticipatory loss processing did not yield any significant cluster. Moreover, the supplementary model with psychiatric diagnosis yielded similar patterns of findings described above (Supplementary Figure 2).
Figure 4.
Association of PRS for neuroticism (N-PRS) with gain processing. Gray shading represents 95% confidence interval.
4. DISCUSSION
This study investigated the association of polygenic risk for neuroticism with neural processing to reward or punishment using an fMRI paradigm of monetary incentives with a group of treatment-seeking individuals and healthy volunteers. The present study yielded three key findings. First, individuals with higher PRSs for neuroticism showed lower activations in the amygdala and caudate regions during anticipation of incentives regardless of gain or loss. Second, individuals with higher PRSs for neuroticism exhibited altered neural sensitivity between gain cues versus loss cues in the right anterior insula. Third, individuals with higher PRS for neuroticism revealed attenuated anticipatory responses to gains in the right precuneus. Taken together, these findings suggest that individuals with high genetic loading for neuroticism are less sensitive to anticipatory gains and losses in subcortical regions but are more sensitive to losses in the anterior insular cortex.
Based on the MID neuroimaging data, there was an inverse relationship between PRSs for neuroticism and neural activity for reward processing was found in the basolateral amygdala and the region near the caudate tail. The nature of monetary incentives presumably called upon arousal for motivating an appropriate approach to potential gain and avoidance from potential loss (Knutson & Greer, 2008). Decreasing activations in the amygdala by increased PRSs reflect lower arousal for anticipating an upcoming event regardless of valence, which implies dysregulation of arousal for motivating approach-avoidance behavior. This association is in line with previous findings of the basolateral amygdala connecting with the use of reward cue and reward value (Ambroggi et al., 2008; Wassum & Izquierdo, 2015).
The caudate nucleus as a part of the ventral striatum is known for its involvement in reward processing (Delgado et al., 2000; Rupprecheter et al., 2020; Tricomi et al., 2004). Reduced activity across gain and loss cues evidenced inefficient anticipatory reward processing with increased genetic risk for neuroticism. Further, negative correlations between neural activity and symptom measures of depression and anxiety indicate that attenuated activity may represent impaired reward processing linked to manifested neuroticism beyond genetic risk. As symptom severity of a disorder is more likely to be the outcome of interacting genetic and environmental contributions to the disorder, the negative relationship with symptom measures could reflect altered reward processing associated with manifested neuroticism in which genetic risk for neuroticism is counted. In fact, BFI-neuroticism scores also showed an inverse relationship with neural activity in this region.
The anterior insula has been linked to not only gain and loss but also greater gain over loss as well as greater loss over gain on incentive anticipation (Knutson & Greer, 2008; Wilson et al., 2018). The anterior insula has also been involved in processing uncertainty (Critchley et al., 2001; Paulus et al., 2003; Preuschoff et al. 2008). The association between PRS for neuroticism and motivational valence (gain vs. loss anticipation) was apparent in the right dorsal anterior insula. Differential sensitivity to potential yet uncertain gain relative to loss and vice versa by PRSs suggests that genetic risk for neuroticism may underlie altered sensitivity to reward/punishment. The opposite patterns of sensitivity to upcoming incentives with PRS for neuroticism in the anterior insula during the anticipatory period, negative relationship between genetic risk for neuroticism and neural activity to gain over loss and positive relationship between genetic risk for neuroticism and neural activity to loss over gain, may imply double calamity of neuroticism, reduced sensitivity to positive valence but heightened sensitivity to negative valence.
The role of the precuneus in visuospatial information processing is well established (Cavanna & Trimble, 2006; Schott et al., 2019). In the study task, maximizing monetary outcome was contingent upon visual processing of object cues. Decreased precuneus activation to gain cue was likely to reflect diminished visuospatial processing to the object cue signaling reward with increased PRSs for neuroticism. The result conveys the deleterious effect of neuroticism in perceiving and detecting potential rewards in connection with blunted responses to positive stimuli in depression. On the contrary, no significant cluster was found in the processing of potential loss associated with PRSs. The interpretation of this null finding warrants further investigations.
To the best of our knowledge, this is the first fMRI study examining the association of PRS for neuroticism with reward-related processing in the brain. We found that PRS for neuroticism modulates reward processing via altered responsivity to reward valence. Nonetheless, the present findings should be considered with the limitations. First, the clinical cohort in the study was comprised of various populations who may have disorders in both positive and negative affect. While the transdiagnostic populations provided the basis for studying PRS for neuroticism in the broad context of psychopathology, the mixed nature of the cohort may limit implications of the current findings to specific disorders. Second, genetic risk for neuroticism may be inherently intertwined with symptoms of mood and affective disorders, as neuroticism is known to increase the vulnerability to psychiatric disorders including depression. Therefore, the interpretation of the current findings should be considered in the context of the intercorrelation between PRS for neuroticism and psychiatric disorders. On a related note, symptom scores from the depression group did not show a significant relationship with neural activity in any clusters reported in this study whereas PRSs for neuroticism from the depression group did show significant correlations with neural activations in all clusters in the same directions reported above (Supplementary Figure 3).
5. CONCLUSION
The present study demonstrates that PRS for neuroticism modulates reward processing in the brain. Genetic loading of neuroticism attenuated anticipatory reward processing whether it involved reward or punishment. Further, genetic propensity for neuroticism mediated sensitivity to reward/punishment via altered reactivity to gain over loss and loss over gain. Finally, increased genetic risk for neuroticism was associated with reduced processing of reward cues. Collectively, these findings illustrate the relationship of genetic risk for neuroticism to both positive and negative valence dysfunctions. Considering the adaptive value of integrating contingencies of motivational valences for positive and negative outcomes, the current findings have significant implications for understanding genetic risk for neuroticism in daily life as well as psychopathology.
Supplementary Material
Supplementary Figure 1. Variances of BFI-Neuroticism score explained by PRS for neuroticism
Supplementary Figure 2.Brain regions showing associations between PRS for neuroticism and reward processing and corresponding BOLD signals from the model with psychiatric diagnosis [A: PRS for neuroticism across valence, B: Interaction between PRS for neuroticism and gain/loss sensitivity, C: PRS for neuroticism in gain processing]
Supplementary Figure 3. Associations of neural activation with PRS for neuroticism and depression symptom severity in depression group [A: Amygdala, B: Caudate, C: Insula, D: Precuneus]
Highlights.
We examined the Polygenic Risk Score (PRS) for neuroticism in neural reward processing.
Higher PRS for neuroticism is associated with attenuated processing of gain and loss.
Higher PRS for neuroticism is linked to altered sensitivity to both gain and loss.
Genetic propensity for neuroticism modulates reward processing.
Acknowledgments
The ClinicalTrials.gov identifier for the clinical protocol associated with data published in the current paper is NCT02450240, “Latent Structure of Multi-level Assessments and Predictors of Outcomes in Psychiatric Disorders”.
The Tulsa 1000 Investigators include the following contributors: Robin Aupperle, Ph.D., Jerzy Bodurka, Ph.D., Salvador Guinjoan, M.D., Sahib S. Khalsa, M.D., Ph.D., Jonathan Savitz, Ph.D., Jennifer Stewart, Ph.D.
Conflict of Interest
MP is an advisor to Spring Care, Inc., a behavioral health startup, and he has received royalties for an article about methamphetamine in UpToDate.
Disclosure
MP is an advisor to Spring Care, Inc., a behavioral health startup, and he has received royalties for an article about methamphetamine in UpToDate.
Role of the Funding Source
This work has been supported in part by The William K. Warren Foundation, by NIH/National Institute of Mental Health grants K23MH112949 (to SSK) and K23MH108707 (to RLA), and the National Institute of General Medical Sciences Center Grant Award Number 1P20GM121312. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The funder had no role in study design, in the collection, analysis, and interpretation of data, in the writing of the manuscript, or in the decision to submit the paper for publication.
Author Statement
The authors have read and approved the final version of the manuscript. We confirm that it is the authors’ original work, has not received prior publication, and is not under consideration for publication elsewhere. KF and RK computed polygenic risk scores. HP, RK, and MP performed data analysis. HP and MP drafted the manuscript. KF, RK, TV, HY, and WT provided critical revisions for intellectual content.
Footnotes
An alternative model in which 10 principal components for population stratification were used instead of race yielded similar patterns of results with smaller number of voxels.
Participant information by each group is shown in Supplementary Table 1.
Four participants were removed from analysis (n=465) due to incomplete behavioral data
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham G, Qiu Y, Inouye M. 2017. FlashPCA2: principal component analysis of Biobank-scale genotype datasets. Bioinformatics, 33, 2776–2778. 10.1093/bioinformatics/btx299 [DOI] [PubMed] [Google Scholar]
- Alloy LB, Olino T, Freed RD, Nusslock R. 2016. Role of reward sensitivity and processing in major depressive and bipolar spectrum disorders. Behav. Ther 47, 600–621. 10.1016/j.beth.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amare AT, Schubert O, Tekola-Ayele F, Hsu Y, Sangkuhl K, Jenkins G, … Baune BT 2018. Association of the polygenic scores for personality traits and response to selective serotonin reuptake inhibitors in patients with major depressive disorder. Front. Psychiatry, 9, 65. 10.3389/fpsyt.2018.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM 2008. Basolateral amygdala neurons facilitate reward-seeking behaviors by exciting nucleus accumbens neurons. Neuron. 59, 648–661. 10.1016/j.neuron.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Paulus MP 2010. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin. Neurosci 12, 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Norman SB, Craske MG, Sullivan G, Lang AJ, Chavira DA, Bystritsky A, Sherbourne AJ, Roy-Byrne P, Stein MB 2009. Validation of a brief measure of anxiety-related severity and impairment: the overall anxiety severity and impairment scale (OASIS). J. Affect. Disord 112, 92–101. 10.1016/j.jad.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, O’Reilly PF 2019. PRSice-2: Polygenic Risk Score Software for Biobank-Scale Data. GigaScience, 8, 7. 10.1093/gigascience/giz082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR 2006. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 129, 564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Cooper S, Robinson AJ, Mazei-Robison MS 2017. Reward circuitry in addiction. Neurotherapeutics. 14, 687–697. 10.1007/s13311-017-0525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR 2008. The revised NEO personality inventory (NEO-PI-R). in Boyles G, Matthews G, Saklofske D The SAGE handbook of personality theory and assessment: Volume 2 — Personality measurement and testing, Sage Publications Inc., London, pp 179–199. [Google Scholar]
- Costa PT, McCrae RR 1980. Influence of extraversion and neuroticism on subjective well-being: Happy and unhappy people. J. Pers. Soc. Psychol 38, 668–678. 10.1037/0022-3514.38.4.668 [DOI] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol MJ, van der Wee NJ, Veltman DJ, Roelofs K. 2010. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage, 49, 963–970. 10.1016/j.neuroimage.2009.08.023 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ 2001. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron 29, 537–545. 10.1016/S0896-6273(01)00225-2 [DOI] [PubMed] [Google Scholar]
- Das S, Forer L, Schonberr S, Sidore C, Locke AE, Kwong A. … Fuchsberger C. 2016. Next-generation genotype imputation service and methods. Nat. Genet 48, 1284–1287. 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA 2000. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol 84, 3072–3077. 10.1152/jn.2000.84.6.3072 [DOI] [PubMed] [Google Scholar]
- de Moor MH, Costa PT, Terracciano A, Krueger RF, de Geus EJ, Toshiko T, … Boomsma DI 2012. Meta-analysis of genome-wide association studies for personality. Mol. Psychiatry 17, 337–49. 10.1038/mp.2010.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK 2009. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry, 2009. 66, 1083–1090. 10.1016/j.biopsych.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ, Prell DB 1951. The inheritance of neuroticism: an experimental study. J. Ment. Sci 97, 441–465. 10.1192/bjp.97.408.441 [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, Perez-Edgar K, Fox NA, Pine DS, Ernst M. 2012. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am. J. Psychiatry, 169, 205–212. 10.1176/appi.ajp.2011.11010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS 2006. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am. J. Psychiatry, 163, 857–864. 10.1176/ajp.2006.163.5.857 [DOI] [PubMed] [Google Scholar]
- Ironside M, Amemori K. McGrath C, Pedersen ML, Kang MS, Amemori S, Frank MJ, Graybiel AM, Pissagalli DA 2020. Approach-avoidance conflict in major depressive disorder: congruent neural findings in humans and nonhuman primates. Biol. Psychiatry, 87, 399–408. 10.1016/j.biopsych.2019.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. 1993. A longitudinal twin study of personality and major depression in women. Arch. Gen. Psychiatry, 50, 853–862. 10.1001/archpsyc.1993.01820230009001 [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. 2001. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12, 3683–3687 10.1097/00001756-200112040-00016 [DOI] [PubMed] [Google Scholar]
- Knutson B, & Greer SM 2008. Anticipatory affect: neural correlates and consequences for choice. Philos. Trans. R. Soc. Lond. B. Biol. Sci 363, 3771–3786. 10.1098/rstb.2008.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. 2010. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychol. Bull 136, 768–821. 10.1037/a0020327 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB 2001. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med 16, 606–613 10.1046/j.15251497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB. 2009. Public health significance of neuroticism. Am. Psychol 64, 241–258. 10.1037/a0015309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, & Barch DM 2016. Reward processing and risk for depression across development. Trends. Cogn. Sci 20, 456–468. 10.1016/j.tics.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouff JM, Thorsteinsson EB, Schutte NS 2005. The relationship between the five-factor model of personality and symptoms of clinical disorders: A meta-analysis. J. Psychopathol. Behav. Assess 27, 101–114. 10.1007/s10862-005-5384-y [DOI] [Google Scholar]
- McCabe SE, Boyd CJ, Cranford JA, Slayden J, Lange JE, Reed MB, Ketchie JM, Scott MS 2007. Alcohol involvement and participation in residential learning communities among first-year college students. J. Stud. Alcohol Drugs 68, 722–726. 10.15288/jsad.2007.68.722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JF, Reid F, Lacey JH 2000. The SCOFF questionnaire: a new screening tool for eating disorders. West. J. Med 172, 164–165. 10.1136/ewjm.172.3.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Jansen PR, Stringer S, Watanabe K, de Leeuw CA, Bryois J. … Posthuma D. 2018a. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat. Genet, 50, 920–927. 10.1038/s41588-018-0151-7 [DOI] [PubMed] [Google Scholar]
- Nagel M, Watanabe K, Stringer S, Posthuma D, van der Sluis S. 2018b. Item-level analyses reveal genetic heterogeneity in neuroticism. Nat. Commun 9, 905. 10.1038/s41467-018-03242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, McGown SK, Sarapas C, Robinson-Andrew EJ, Altman SE, Campbell ML, Gorka SM, Katz AC, Shankman SA 2013. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. J. Abnorm. Psychol 122, 662–671. 10.1037/a0033982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Baselmans BML, De Neve J-E, Turley P, Nivard MG, Fontana MA, …, Cesarini D 2016. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat. Genet 48, 624–33. 10.1038/ng.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Jeronimus BF, Kotov M, Riese H, Bos EH, Hankin B. 2013. Neuroticism and common mental disorders: Meaning and utility of a complex relationship. Clin. Psychol. Rev 33, 686–697. 10.1016/j.cpr.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer DJ, Benet-Martinez V. 2006. Personality and the prediction of consequential outcomes. Ann Rev Psychol. 57, 401–421. 10.1146/annurev.psych.57.102904.190127 [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB 2003. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage, 19, 1439–48. 10.1016/s1053-8119(03)00251-9 [DOI] [PubMed] [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA 2013. Blunted reward responsiveness in remitted depression. J Psychiatr Res. 47, 1864–1869. 10.1016/j.jpsychires.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. 2008. Human insula activation reflects risk prediction errors as well as risk. J. Neurosci 28, 2745–2752. 10.1523/JNEUROSCI.4286-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW 2016. Illuminating anhedonia. Science 351, 24–25. 10.1126/science.aad9698 [DOI] [PubMed] [Google Scholar]
- Rupprechter S, Romaniuk L, Seies P, Hirose Y, Hawkins E, Sandu A, … Steele JD 2020. Blunted medial prefrontal cortico-limbic reward-related effective connectivity and depression. Brain, 143, 1946–1956. 10.1093/brain/awaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer U, Robinson S, Fischmeister FPS, Konig E, Oppenauer Cl., Lueger-Schuster B., Moser E, Kryspin-Exner I, Bauer H. 2008. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia, 46, 2836–2844. 10.1016/j.neuropsychologia.2008.05.022 [DOI] [PubMed] [Google Scholar]
- Schott BH, Wüsternberg T, Lücke E, Pohl I, Richter A, Seidenbecher CI … Richardson-Klavehn A 2019. Gradual acquisition of visuospatial associative memory representations via the dorsal precuneus. Hum. Brain Mapp 40, 1554–1570. 10.1002/hbm.24467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, Velde JVD, Costafreda SG, Horton P, Ormel J, Riese H, Aleman A. 2013. Neuroticism and the brain: A quantitative meta‐analysis of neuroimaging studies investigating emotion processing. Neurosci. Biobehav. Rev, 37, 1518–1529. 10.1016/j.neubiorev.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Smith DJ, Escott-Price V, Davies G, Bailey MES, Colodro-Conde L, Ward J, . . . O’Donovan MC 2016. Genome-wide analysis of over 106000 individuals identifies 9 neuroticism-associated loci. Molecular Psychiatry, 21, 749–757. 10.1038/mp.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed BC, Nelson BD, Levinson AR, Perlman G, Klein DN, Kotov R, Hajcak G. 2018. Extraversion, neuroticism, and the electrocortical response to monetary rewards in adolescent girls. Biol Psychol. 136, 111–118. 10.1016/j.biopsycho.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP 2007. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. A. J. Psychiatry 164, 318–327. 10.1176/ajp.2007.164.2.318 [DOI] [PubMed] [Google Scholar]
- Suls J, Martin R. 2005. The daily life of the garden‐variety neurotic: Reactivity, stressor exposure, mood spillover, and maladaptive coping. J. Pers 73(6), 1485–1510. 10.1111/j.1467-6494.2005.00356.x [DOI] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA 2004. Modulation of caudate activity by action contingency. Neuron, 41, 281–292. 10.1016/S0896-6273(03)00848-1 [DOI] [PubMed] [Google Scholar]
- Victor TA, Khalsa SS, Simmons WK, Feinstein JS, Savitz J, Aupperle RL, Yeh HW, Bodurka J, Paulus MP 2018. Tulsa 1000: a naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open 24, e016620. 10.1136/bmjopen-2017-016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomaso D, Telang F, Baler R. 2010. Addiction: Decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays, 32, 748–755. 10.1002/bies.201000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Siebenthal Z, Boucher O, Lazzouni L, Taylor V, Martinu K, Roy M, Rainville P, Lepore F, Nguyen DK 2020. Expected value and sensitivity to punishment modulate insular cortex activity during risky decision making. Sci. Rep 10, 11920. 10.1038/s41598-020-68644-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A. 2015. The basolateral amygdala in reward learning and addiction. Neurosci. Biobehav. Rev 57, 271–283. 10.1016/j.neubiorev.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiger TA, & Oltmanns JR 2017. Neuroticism is a fundamental domain of personality with enormous public health implications. World Psychiatry. 16, 144–145. 10.1002/wps.20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EJ, MacLeod C, Mathews A, Rutherford EM 2006. The causal role of interpretive bias in anxiety reactivity. J. Abnorm. Psychol 115, 103–111. 10.1037/0021843X.115.1.103 [DOI] [PubMed] [Google Scholar]
- Wilson RP, Colizzi M, Bossong MG, Allen P, Kempton M, Bhattacharyya S. 2018. The neural substrate of reward anticipation in health: A meta-analysis of fMRI findings in the monetary incentive delay task. Neuropsychol. Rev 28, 496–506. 10.1007/s11065-018-9385-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwir I, Mishra P, Del-Val C, Gu CC, de Erausquin GA, Lehtimaki T, Cloninger CR 2019. Uncovering the complex genetics of human personality: response from authors on the PGMRA Model. Mol. Psychiatry 10.1038/s41380-019-0399-z [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Variances of BFI-Neuroticism score explained by PRS for neuroticism
Supplementary Figure 2.Brain regions showing associations between PRS for neuroticism and reward processing and corresponding BOLD signals from the model with psychiatric diagnosis [A: PRS for neuroticism across valence, B: Interaction between PRS for neuroticism and gain/loss sensitivity, C: PRS for neuroticism in gain processing]
Supplementary Figure 3. Associations of neural activation with PRS for neuroticism and depression symptom severity in depression group [A: Amygdala, B: Caudate, C: Insula, D: Precuneus]