Abstract
Preferential oxidation of primary hydroxyls in unprotected sugars and sugar amino acids is reported using inexpensive and readily available reagents. This method offers a specific oxidation protocol for a variety of carbohydrates. The stereochemical integrity of the starting materials was preserved and a simple workup yielded the products in good yields with high purity. The procedure is compatible with base sensitive groups like Fmoc. Both mono and disaccharides undergo oxidation regioselectively.
Keywords: Selective Oxidation, Sugar amino acids (SAAs), Disaccharides, Trichloroisocyanuric acid (TCICA), TEMPO
1. Introduction
Peptide drug candidates have inherent drawbacks such as poor bioavailability, solubility, and oral stability, as well as shorter elimination times (Yusuf et al, 2018). These are typically addressed by the incorporation of unnatural amino acids such as amino acids derived from sugars (SAAs), various linkers, and other scaffolds. When introduced into the peptide chain, SAAs not only enhance the solubility in water but dramatically alter the H-bonding and folding of the peptide chain due to their rigidity and availability of additional hydroxyl groups (Yashoda et al, 2016). In addition, SAAs play a vital role in nature occurring as antibiotics, glycosidase inhibitors, and have been used as structural constraining entities (Martin et al, 2013; Chakraborty et al 2002; Gruner et al 2002; Lohof et al, 1999; Elsa et al, 2001; Jacquelyn &Thomas 2002). Many SAAs have been exploited as templates and synthons in the design of peptidomimetics of biologically active peptides (Frank et al, 2002; Sibylle et al, 2001). NMR studies have shown that cyclo-[Phe-Pro-Phe-D-Trp-Lys-Thr-], adopted a β-turn when pyranoid SAAs replaced the dipeptide, Phe-Pro (Erich et al, 1996). Another report revealed that the introduction of a furanose SAA in somatostatin analogs caused apoptosis in both drug-sensitive and multi-drug resistant tumor cells (Sybille et al, 2001). When D-Phe-Val was replaced with an SAA in the sequence, cyclo[-Arg-Gly-Asp-D-Phe-N(Me)Val, targeting integrin receptors, the peptidomimetic showed high affinity for one sub-type of integrins (Renate et al, 2003). Recently published work indicated that the introduction of an SAA in a linear peptide chain as a linker not only improved its solubility but also provided an approach to probe caspase inhibitor activity using molecular imaging techniques without compromising its biological activity (Helen et al, 2013). Sugar amino acid scaffolds have been also utilized in cyclic peptide chains (Johan and Nilsson, 2005) to create an artificial receptor, suggesting that SAAs have enormous potential in artificial receptor designs. These considerations warrant a simple procedure and easy access to sugar amino acids from readily available polyhydroxy amino alcohols/amino sugars.
2. Results and Discussion
During our work on the synthesis of peptide libraries, we also became interested in the use of SAAs as linkers or scaffolds to improve the pharmacological profiles and solubility of peptides. SAAs could provide a handle to conjugate radioactive tracers or dyes through the N-terminus or C-terminus to study in vivo/in vitro binding abilities of the SAAs containing substrates to their intended targets (Roland et al, 2004). Unfortunately, the SAAs of 2-deoxy-2-amino saccharides are not commercially available and require multistep protection and deprotection strategies to prepare the Fmoc-amino protected acids amenable for automated synthesis (Laiqiang Ying & Jacquelyn Gervay-Hague, 2004). Extensive efforts in this area are detailed (Guillaume et al, 2017), employing TEMPO as the catalyst and various oxidizing agents. However, these reported procedures use a wide range of temperatures, solvents, and laborious workups with inconsistent yields. They are also limited to the oxidation of polysaccharides bearing no base-sensitive groups. Other reported methods for these transformations involve, either the use of expensive oxidizing agents (Kochkar et al, 2000) and/or temperature control or procedures that were again not compatible with base- and acid-sensitive functionalities in the molecule (Arian et al, 1995). These limitations were nicely addressed in a recent report, offering an alternative route to these sugar acids (Mahipal et al, 2018). Surprisingly, this report does not include the preparation of SAAs and in some cases failed to yield the required carbohydrate acids. After scouring the literature for an efficient method to access SAAs, we came across a potential protocol that used trichloroisocyanuric acid (TCICA) in combination with TEMPO and NaBr as catalysts (Lidia et al 2003) to oxidize primary hydroxyls to the corresponding carboxylic acids. However, this procedure also oxidized secondary hydroxyls to the corresponding ketones making it less attractive for oxidation of carbohydrates. This method did not also explore the oxidation of Fmoc-amino alcohols to their respective acids, required for automated peptide synthesis. We believed that the oxidation of N-protected amino sugars with TCICA/TEMPO to produce SAAs could be achieved by careful manipulation of reaction conditions and reactants without the need to protect the hydroxyls. The oxidizer is economical and easier to handle compared to NaOCl/NaClO2, which require precise concentrations and are unstable at RT in aqueous solutions over long periods. Initial attempts to prepare the known Fmoc sugar amino acid derived from galactose (Roland et al, 2004) were not successful. The published oxidation method resulted in an inseparable mixture of products though the expected material was one of the major components offering some encouragement. Careful examination of the crude reaction mixture by LC/MS revealed the presence of the expected acid [M+H, 430] and other products with mass units of 432 [M+H], 428[M+H], 464 [M+H], and 508 [M+H] plus the unreacted starting material. Initial oxidation of hydroxymethyl to aldehyde followed by the formation of hemihydrate could result in M+H 432, and further oxidation of the aldehyde to acid followed by another oxidation of a hydroxyl to ketone could explain the mass of 428 [M+H]. We surmised that the mass units 464 and 508 could result from N-halogenation of the Fmoc-amino acid under acidic conditions similar to acetamide by dihaloisocyanuric acid (Scott, 2007). This assumption was confirmed, when the crude mixture was treated with sodium bisulfite for 30 min and reanalyzed. LC/MS showed the disappearance of peaks corresponding to 464 and 508, enhancing the signal intensity of the desired acid [M+H 430]. When the pH of the reaction was carefully monitored, it suddenly dropped to about 2.0 during the addition of TCICA. This observation was also noted during the oxidation of polysaccharides using NaOCl/TEMPO by Arian et al (1995). Lower pH coupled with the reduced solubility of NaHCO3 at 0°C in acetone/water might result in increased concentrations of HOCl/HOBr in the reaction, contributing to other side products observed. After exploring various ratios of the oxidizing agent, catalysts, and base, we were gratified to discover that substantially increasing the amount of bicarbonate and controlling the amounts of catalysts and the oxidizer achieved the desired results at RT. We also found that using water/acetonitrile instead of water/acetone as the reaction solvent shortened the reaction times with the diverse sugars evaluated. The use of water-miscible solvents like dioxane, DME, and/or THF seemed to promote N-halogenation. Completion of the reaction was observed in less than 2h in all cases examined. Monitoring the progress of the reaction by LC/MS indicated that the required acid was the major component (>90%) with less than 5% of starting material and other impurities. The other major product was the reduced oxidizing agent, cyanuric acid.
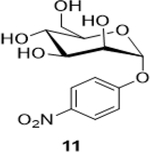
The following table summarizes our initial results with different carbohydrate substrates. 2-Acetamido-2-deoxy-D-glycopyranosides are the most observed structural units in oligosaccharides and glycoconjugates and are associated with a wide range of biological processes (Raymond 1996). Hence, we selected some common 2-acetamidopyranoses (Entries 1–3, Petrovic et al, 2006) for our oxidation procedure and were delighted to find that they underwent clean oxidation to provide the acids as their sodium salts with high purities. Next, we explored the oxidation of C1-nitrophenyl pyranoses (Entries 3–6). These derivatives have been extensively used to study enzyme activities of β-glycosidases (Elaine et al, 2016) that are involved in the natural degradation of sugars. In addition, the reduction of the nitro group followed by Fmoc protection of the amine would offer a range of unnatural SAAs, that could be exploited as linkers or scaffolds in peptide libraries. Oxidation was again very clean and yielded the corresponding acids as their sodium salts in good isolated yields and with no epimerization at the C1 (Rajendra et al, 1978). Entry 7 was reported as an intermediate for the synthesis of a radiolabeled ligand to elucidate the activities of the enzyme Caspase-3 (Helen et al, 2013). Our procedure produced the desired carboxylic acid in good isolated yields with a simple column chromatography compared to the reported procedure (Roland et al, 2004). Finally, disaccharides were subjected to this oxidation protocol and found to undergo oxidation to carboxylic acids without any notable side products (Entries 8 & 9; Madeleine et al, 2011 and Kobayashi et al, 1994), which may prove useful in the design of new heparin mimics (Danzhou et al, 1994). The products from the oxidation were supported by observed analytical data. Both the proton and 13C NMRs conclusively proved that the configuration was retained at the susceptible C1 carbon of the sugars.
4. Conclusion
This optimized method offers a convenient approach to sugar-derived carboxylic acids and amino acids from commercially available unprotected substrates. Base sensitive groups like Fmoc- and C1-4-nitrophenoxy were well tolerated in our procedure. This oxidation protocol does not require temperature control, uses an economical oxidizing agent and standard column chromatography is sufficient to isolate the products in good yields. Recent work (Thomas et al, 2019) demonstrates that peptide chains could be assembled using 1-chloro-3,5-dimethoxy triazine as the coupling agent, which tolerates the unprotected hydroxyls. Current work reported here could provide an efficient tool to prepare SAAs suitable for automated synthesis. Work is in progress to synthesize other sugar-based amino acids for incorporation into peptide libraries.
5. Experimental
General:
NMR data were recorded on a Varian 400 MHz NMR spectrometer. Mass spectra were obtained on an Agilent 1200 series instrument under ESI conditions. High-Resolution Mass Spectral data were recorded on a Varian Xevo G2-XSQTOF Mass Spectrometer. Purification of the products was carried out with a Teledyne Isco Combiflash® Rf+ instrument. The starting sugars were purchased from Carbosynth, LLC and used as received except for derivative 13 (Roland Haubner, et al 2004). All other chemicals and solvents were procured from Millipore-Sigma and were used as received. The purity of the fractions was checked with Agilent 1200 series LC connected to a Mass Spectrometer. Samples were analyzed by LC/MS under the following conditions; column: Agilent Zobrax RP C18; 3.5 microns; 4.6 × 50 mm; elution rate: 1.0 mL/min; gradient: 0% B for 3min and then ramped up to 100%B over 5 min; solvent A-water with 0.1% TFA (v/v) and solvent B- acetonitrile with 0.1% TFA (v/v); detection @ 210nm
General procedure for oxidation (representative example; detection @210 nm)
Methyl 2-acetamido-2-deoxy-α-D-mannopyranose (0.168g, 0.5 mmol) was dissolved in 5.0 mL of water and 1.0 mL of acetonitrile. Powdered sodium bicarbonate (0.63g, 7.5 mmol) was added and the mixture was stirred vigorously. Sodium bromide (0.025 mmol, 2.5 mg) and TEMPO (0.8mg, 0.005 mmol) were added and stirring was continued. Trichloroisocyanuric acid (0.244g, 1.05 mmol) was added in portions over 5 min. with vigorous stirring at RT. The reaction was followed by LC/MS and after completion, 0.5 mL of ethanol was added to the reaction mixture, and stirring was continued for 1h. The crude mixture was diluted with 50.0 mL of water and freeze-dried. The resulting solid was dissolved in a minimal amount of water, filtered through a 0.2m filter and the product was isolated via reverse phase (C18) flash chromatography. Yield: 108.0 mg (80%)
Purification of sugar acids
Method A (Products 2,4,6,8,12,16 & 18): The crude product in about 5.0 ml of water was injected onto a 275.0g flash C18 RP column (RediSep® Rf High-Performance Gold) and eluted with water until all the expected products were eluted. Elution rate: 60.0 ml/min; Detection @ 210 nm. Pure fractions were collected and freeze-dried to yield the acids as fluffy colorless solids.
Method B (Product 14): A linear gradient of 0–100% acetonitrile in water over 30 min was used with detection @ 280nm. Pure fractions (LC/MS) were collected and freeze-dried to yield the product as a colorless solid.
Analytical Data for the products
Sodium (2S,3S,4R,5R,6R)-5-acetamido-3,4-dihydroxy6-methoxytetrahydro-2H-pyran2-carboxylate (2): (Method A; 68%); m. p. 158–160°C (m/d); 1H NMR (Deuterium Oxide, 400 MHz): δ (ppm) 4.52 (d, J=8.5 Hz, 1H), 4.02 (d, J=9.6 Hz, 1H), 3.81 – 3.71 (m, 1H), 3.69 – 3.55 (m, 2H), 3.51 (s, 3H), 2.05 (s, 3H); 13C NMR (101 MHz): δ (ppm) 174.69, 172.16, 101.95, 74.38, 73.35, 71.46, 57.26, 55.04, 22.06. M. S. [M+H] 250.1; HRMS: Calcd for C9H14NO7 (M-H) 248.0770; Found: 248.0770
Sodium (2S,3R,4R,5R,6S)-5-acetamido-3,4-dihydroxy-6-methoxytetrahydro-2H-pyran-2-carboxylate (4): (Method A; 75%); m. p. 183–187°C (m/d); 1H NMR (Deuterium Oxide, 400 MHz): δ (ppm) 4.84 (d, J=3.7 Hz, 2H), 4.19 (dd, J=11.1, 3.7 Hz, 1H), 3.95 (dd, J=11.1, 3.3 Hz, 1H), 3.39 (s, 3H), 2.06 (s, 3H); 13C NMR (101 MHz): δ (ppm) 175.63, 174.61, 98.06, 71.27, 69.90, 67.93, 55.28, 49.55, 21.90.; M. S. [M+H] 250.1; HRMS : Calcd for C9H14NO7 (M-H) 248.0770; Found: 248.0772
Sodium (2S,3S,4S,5R,6R)-3,4,5-trihydroxy-6-(4-nitrophenoxy)tetrahydro-2H-pyran-2-carboxylate (6): (Method A; 80%); m. p. 225–228°C (m/d); 1H NMR (Deuterium Oxide, 400 MHz): δ (ppm) 4.25 (dd, J=4.5, 1.7 Hz, 1H), 3.88 (dd, J=9.7, 4.6 Hz, 1H), 3.81 (d, J=9.7 Hz, 1H), 3.60 (t, J=9.7 Hz, 1H), 3.31 (s, 3H), 1.96 (s, 3H); 13C NMR (D2O, 101 MHz): δ (ppm) 176.50, 174.65, 99.90, 73.32, 69.13, 68.86, 54.93, 54.92, 52.04, 21.89. M. S. [M+H] 250.1; HRMS: Calcd for C9H14NO7 (M-H) 248.0770; Found: 248.0777
Sodium (2S,3S,4S,5R,6R)-3,4,5-trihydroxy-6-(4-nitrophenoxy)tetrahydro-2H-pyran-2-carboxylate (8): (Method A: 71%); m. p. 235–237°C (m/d); 1H NMR (Deuterium Oxide, 400 MHz): δ (ppm) 8.33 – 8.24 (m, 2H), 7.37 – 7.29 (m, 2H), 5.85 (d, J=3.d7 Hz, 1H), 4.04 – 3.94 (m, 2H), 3.85 (ddd, J=9.8, 3.6, 0.7 Hz, 1H), 3.62 (ddd, J=9.8, 8.9, 0.7 Hz, 1H); 13C NMR (D2O, 101 MHz): δ (ppm) 175.93, 161.36, 142.37, 126.02, 116.70, 96.55, 73.07, 72.64, 71.72, 70.72.; M. S. [M-H] 314.1; HRMS: Calcd for C12H13NO9 (M-H) 314.0512; Found: 314.0512
Sodium (2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(4-nitrophenoxy)tetrahydro-2H-pyran-2-carboxylate (10): (Method A; 67%); m. p. 258–261° C (m/d); 1H NMR (Deuterium Oxide, 400 MHz): δ (ppm) 8.18 – 8.09 (m, 2H), 7.23 – 7.15 (m, 2H), 5.75 (d, J=3.7 Hz, 1H), 4.26 – 4.17 (m, 2H), 4.06 (dd, J=10.3, 3.4 Hz, 1H), 3.95 (dd, J=10.4, 3.7 Hz, 1H); 13C NMR (101 MHz): δ (ppm) 174.96, 161.67, 142.18, 126.00, 116.61, 96.79, 72.57, 70.53, 69.41, 67.51; M. S. [M-H] 314.1; HRMS: Calcd for C12H13NO9 (M-H) 314.0512; Found: 314.0516
Sodium (2S,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(4-nitrophenoxy)tetrahydro-2H-pyran-2-carboxylate (12): (Method A; 65%); m. p. 275–279°C; 1H NMR (Deuterium Oxide, 400 MHz): δ (ppm) 8.36 – 8.29 (m, 2H), 7.42 – 7.35 (m, 2H), 5.90 (d, J=2.4 Hz, 1H), 4.29 – 4.23 (m, 1H), 4.18 (dp, J=6.3, 2.9 Hz, 1H), 4.06 – 4.00 (m, 2H); 13C NMR (101 MHz): δ (ppm) 176.02, 160.99, 151.13, 142.25, 126.00, 116.57, 97.48, 74.07, 70.09, 69.26, 68.67; M. S. [M-H] 314.1; HRMS: Calcd for C12H13NO9 (M-H) 314.0512; Found: 314.0512
Sodium(2S,3R,4R,5R,6R)-6-(((((9H-fluoren-9-yl)methoxy)carbonyl)amino)methyl)-3,4,5-trihydroxytetrahydro-2H-pyr an-2-carboxylate (14): (Method B; 73%); m. p. 195–200°C (m/d); 1H NMR (400 MHz, DMSO-d6) δ 7.90 (d, J = 7.5 Hz, 3H), 7.71 (d, J = 7.3 Hz, 3H), 7.43 (t, J = 7.4 Hz, 4H), 7.35 (t, J = 7.4 Hz, 3H), 4.31 (d, J = 7.3 Hz, 2H), 4.25 (q, J = 7.2 Hz, 2H), 4.00 (s, 1H), 3.77 (d, J = 16.1 Hz, 2H), 3.58 (dd, J = 13.0, 6.4 Hz, 2H), 3.37 (d, J = 15.6 Hz, 3H), 3.22 – 3.17 (m, 1H), 3.14 – 3.06 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ 172.06, 156.63, 143.90, 140.69, 127.60, 127.12, 125.21, 120.09, 79.45, 78.91, 74.59, 70.68, 68.43, 65.61, 46.68, 42.04; M. S. [M+H] 430.1, [M+Na] 452.2; HRMS: Calcd for C22H22NO8(M-H) 428.1345; Found: 428.1346; Lit: 13C NMR (125 MHz, DMSO-d6) 170.2 (COOH), 156.2 (Fmoc-CO), 143.9 – 120.1 (aromatic carbons), 78.62, 77.1, 73.8, 70.2, 67.9, 65.4, 46.8 and 42.2
Sodium(2S,3S,4R,5R,6R)-6-(benzyloxy)-3-(((2R,3R,4S,5R,6S)-6-carboxylato-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-4,5-dihydroxytetrahydro-2H-pyran-2-carboxylate (16): (Method A, 68%); m. p. 240–245°C (m/d); 1H NMR (Deuterium Oxide, 400 MHz): δ (ppm) 7.56 – 7.41 (m, 5H), 4.99 (d, J=11.6 Hz, 1H), 4.76 (d, J=5.4 Hz, 1H), 4.61 (d, J=8.0 Hz, 1H), 4.47 (d, J=7.7 Hz, 1H), 4.24 (d, J=3.5 Hz, 1H), 4.12 (s, 1H), 3.89 (d, J=9.7 Hz, 1H), 3.81 – 3.72 (m, 2H), 3.67 (d, J=9.1 Hz, 1H), 3.59 (dd, J=9.9, 7.8 Hz, 1H), 3.45 (t, J=8.6 Hz, 1H); 13C NMR (101 MHz): δ (ppm) 175.16, 174.50, 136.45, 131.17, 128.74, 128.24, 102.73, 100.80, 81.19, 75.67, 75.46, 74.60, 72.55, 71.35, 70.59, 70.04; M. S. [M-H] 459.2; HRMS: Calcd for C19H23O13 (M-H) 459.1139; Found: 459.1143
Sodium(2S,3S,5R,6R)-3-(((2S,3R,4S,5R,6S)-6-carboxylato-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-4,5-dihydro xy-6-(4-nitrosophenoxy)tetrahydro-2H-pyran-2-carboxylate (18): (Method A; 73%); m. p. 208–212°C (m/d); 1H NMR (Deuterium Oxide, 400 MHz): δ (ppm) 8.32 (d, J=9.3 Hz, 2H), 7.36 (d, J=9.3 Hz, 2H), 5.88 (d, J=3.7 Hz, 1H), 5.67 (d, J=3.9 Hz, 1H), 4.26 (t, J=9.3 Hz, 1H), 4.01 – 3.91 (m, 2H), 3.77 (t, J=9.5 Hz, 1H), 3.65 – 3.57 (m, 1H), 3.47 (t, J=9.6 Hz, 1H); 13C NMR (101 MHz): δ (ppm) 176.86, 175.08, 161.29, 142.45, 126.08, 116.79, 97.45, 96.43, 75.51, 73.48, 72.98, 72.32, 72.06, 71.43, 70.86; M. S. [M-H] 490.2; HRMS: Calcd for C18H20NO15 (M-H) 490.0833; Found: 490.08839
Scheme.

Table I.
Substrates used for oxidation
| Entry | Substrate | Product | Yieldsa/Time in minb |
|---|---|---|---|
| 1 |

|

|
68 (30) |
| 2 |

|

|
75 (30) |
| 3 |

|

|
80 (30) |
| 4 |

|

|
71 (60) |
| 5 |

|

|
67 (60) |
| 6 |
|

|
65 (60) |
| 7 |

|

|
73 (120) |
| 8 |

|
|
68 (90) |
| 9 |

|

|
73 (90) |
isolated yield;
Reaction time
Acknowledgments
This work was supported by intramural research funds provided by NHLBI/NIH. The authors are grateful to Dr. Burchelle Blackman for acquiring the HRMS data reported in this article and for the editorial assistance provided by Dr. Carolyn Woodroofe.
References:
- de N. Arian, E. J., Arie CB, & van B. Herman (1995). Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans. Carbohydrate Research, 269, 89–98. 10.1016/0008-6215(94)00343-E [DOI] [Google Scholar]
- Chakraborty TK, Jayaprakash S, & Ghosh S (2002). Sugar Amino Acid Based Scaffolds - Novel Peptidomimetics and Their Potential in Combinatorial Synthesis. Comb. Chem. High Throughput Screen, 5(5), 373–387. 10.2174/1386207023330200 [DOI] [PubMed] [Google Scholar]
- Elaine C, Letícia M, de S. Zanphorlin, F. H. M., José AD, Alex CG, Carla BM, … Roberto R (2016). A novel cold-adapted and glucose-tolerant GH1 β-glucosidase from Exiguobacterium antarcticum B7. International Journal of Biological Macromolecules, 82, 375–380. 10.1016/j.ijbiomac.2015.09.018 [DOI] [PubMed] [Google Scholar]
- Elsa L, Matthias S, Sibylle G, & Horst K (2001). Cyclic Homooligomers from Sugar Amino Acids: Synthesis, Conformational Analysis, and Significance. J. Am. Chem. Soc, 123(34), 8189–8196. 10.1021/ja010181k [DOI] [PubMed] [Google Scholar]
- von R. Erich G., Elisabeth L, Gerhard H, Matthias H, & Horst K (1996). Synthesis and Conformational Analysis of Linear and Cyclic Peptides Containing Sugar Amino Acids. J. Am. Chem. Soc, 118(42), 10156–10167. 10.1021/ja961068a [DOI] [Google Scholar]
- Frank S (2002). Glycosamino Acids: Building Blocks for Combinatorial Synthesis—Implications for Drug Discovery. Angew. Chem. Int. Ed, 41, 230–253. [DOI] [PubMed] [Google Scholar]
- Gruner SAW, Locardi E, Lohof E, & Kessler H (2002). Carbohydrate-Based Mimetics in Drug Design: Sugar Amino Acids and Carbohydrate Scaffolds. Chem Rev, 102(2), 491–514. 10.1021/cr0004409 [DOI] [PubMed] [Google Scholar]
- Guillaume P, Carlo P, Cédric D, Lucio M, Pascal D, Andrea F, … Philippe M (2017). TEMPO-mediated oxidation of polysaccharides: An ongoing story. Carbohydrate Polymers, 165, 71–85. 10.1016/j.carbpol.2017.02.028 [DOI] [PubMed] [Google Scholar]
- Jacquelyn GH, Thomas M, & Weathers Jr. (2002). Pyranosyl sugar amino acid conjugates: their biological origins, synthetic preparations, and structural characterization. Journal of Carbohydrate Chemistry, 21(7–9), 867–910. 10.1081/CAR-120016491 [DOI] [Google Scholar]
- Johan FB, & Ulf JN (2005). Cyclic peptides containing a δ-sugar amino acid-synthesis and evaluation as artificial receptors. Tetrahedron, 61, 863–874. 10.1016/j.tet.2004.11.024 [DOI] [Google Scholar]
- Kobayashi K, Kakishita N, Okada M, Akaike T, & Usui T (1994). Chemo-Enzymatic Synthesis of a Glycopolymer Carrying Clustered-N-ACETYL-β-lactosamine Moieties. Journal of Carbohydrate Chemistry, 13(5), 753–766. 10.1080/07328309408011678 [DOI] [Google Scholar]
- Kochkar H, Lassalle L, Morawietz M, & Hölderich WF (2000). Regioselective Oxidation of Hydroxyl Groups of Sugar and Its Derivatives Using Silver Catalysts Mediated by TEMPO and Peroxodisulfate in Water. Journal of Catalysis, 194(2), 343–351. 10.1006/jcat.2000.2927 [DOI] [Google Scholar]
- Lidia De L., Giampaolo G, Simonetta M, & Andrea P (2003). Trichloroisocyanuric/TEMPO Oxidation of Alcohols under Mild Conditions: A Close Investigation. J. Org. Chem, 68(12), 4999–5001. 10.1021/jo034276b [DOI] [PubMed] [Google Scholar]
- Lohof E, Burkhart F, Born MA, Planker E, & Kessler H (1999). Sugar amino acids and carbohydrates as scaffolds and peptidomimetics. Advances in Amino Acid Mimetics and Peptidomimetics, 2, 263–292. 10.1016/S1874-5113(99)80011-2 [DOI] [Google Scholar]
- Madeleine P, Emilio J, Cocinero NM, Brina B, Benjamin G, Davis R, … John PS (2011). Isotopic Hydration of Cellobiose: Vibrational Spectroscopy and Dynamical Simulations. J. Phys. Chem. A, 115, 9498–9509. 10.1021/jp112109p [DOI] [PubMed] [Google Scholar]
- Mahipal Y, Charles LL, & Ramanarayanan K (2018). Effect of temperature modulations on TEMPO-mediated regioselective oxidation of unprotected carbohydrates and nucleosides. Bioorganic & Medicinal Chemistry Letters, 28, 2759–2765. 10.1016/j.bmcl.2018.01.066 [DOI] [PubMed] [Google Scholar]
- Marttijn R, Mark O, George WJF, & Michela IS (2013). A compendium of cyclic sugar amino acids and their carbocyclic and heterocyclic nitrogen analogues. Amino Acids, 45, 613–689. 10.1007/s00726-013-1521-1 [DOI] [PubMed] [Google Scholar]
- Petrovic V, Car Z, Prugovecki B, Tomic S, & Matkovic-Calogovic D (2006). Synthesis of Acylated Methyl 2-Acetamido-2-Deoxy-α-D-Mannopyranosides. Journal of Carbohydrate Chemistry, 25(8–9), 685–695. 10.1080/07328300601039351 [DOI] [Google Scholar]
- Rajendra M, Srivatsava NH, Fred RS, & Bernard W (1978). Preparation of (aryl α-l-idopyranosid)uronic acids. Carbohydrate Research, 60(1978), 315–326. 10.1016/S0008-6215(78)80038-X [DOI] [Google Scholar]
- Raymond AD (1996). Glycobiology: Toward Understanding the Function of Sugars. Chem. Rev, 96(2), 683–720. 10.1021/cr940283b [DOI] [PubMed] [Google Scholar]
- van W. Renate, M., Luciana M, Cornelis A, Kees E, Gregg S, van R. Mark, … Mark O (2003). Conformational Analysis of Furanoid ε-Sugar Amino Acid Containing Cyclic Peptides by NMR Spectroscopy, Molecular Dynamics Simulation, and X-ray Crystallography: Evidence for a Novel Turn Structure. J. Am. Chem. Soc, 125, 10822–10829. 10.1021/ja035461+ [DOI] [PubMed] [Google Scholar]
- Roland H, Bertrand K, Christian M, Wolfgang AW, Horst K, Hans-Jürgen W, & Markus S (2004). [18F]Galacto-RGD: Synthesis, Radiolabeling, Metabolic Stability, and Radiation Dose Estimates. Bioconjugate Chem, 15, 61–69. 10.1021/bc034170n [DOI] [PubMed] [Google Scholar]
- Sibylle A, Gruner W, Gyorgy K, Richard S, Aniko V, & Horst K (2001). Sugar Amino Acid Containing Somatostatin Analogues that Induce Apoptosis in Both Drug-Sensitive and Multidrug-Resistant Tumor Cells. Organic Letters, 3(23), 3723–3725. 10.1021/ol0166698 [DOI] [PubMed] [Google Scholar]
- Su H, Chen G, Gangadharmath U, Gomez LF, Liang QW, Mu FR, … Hartmuth CK, (2013). Evaluation of [18F]-CP18 as a PET Imaging Tracer for Apoptosis. Molecular Imaging, and Biology, 15, 739–747. 10.1007/s11307-013-0644-9 [DOI] [PubMed] [Google Scholar]
- Thomas M, Bocan RGS, Jennifer L, Brown J, Akuoku F, Falguni B, … Darci RS (2019). Characterization of Brain Inflammation, Apoptosis, Hypoxia, Blood-Brain Barrier Integrity and Metabolism in Venezuelan Equine Encephalitis Virus (VEEV TC-83) Exposed Mice by In Vivo Positron Emission Tomography Imaging. Viruses, 11, 1052. 10.3390/v11111052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgil SC, Corbu A, & Arseniyadis S (2008). N-Bromoacetamide. Encyclopedia of Reagents for Organic Synthesis. 10.1002/047084289X.rb268.pub2 [DOI] [Google Scholar]
- Yang DZ, Reedijk J, van B. Stella, S. E. G., & Wang AHJ (1994). 5’-CGA Motif Induces Other Sequences To Form Homo Base-Paired Parallel-Stranded DNA Duplex: The Structure of (G-A)n Derived from Four DNA Oligomers Containing (G-A)3 Sequence. J. Am. Chem. Soc, 116, 1565–1566. 10.1021/ja00083a050 [DOI] [Google Scholar]
- Yashoda KS, Faiyaz A, Pancham SK, Siriwardena A, Ravi SA, & Tushar KC (2016). Influence of Linker Length on Conformational Preferences of Glycosylated Sugar Amino Acid Foldamers. Chembiochem, 17(19), 1839–1844. 10.1002/cbic.201600386 [DOI] [PubMed] [Google Scholar]
- Ying LQ, & Gervay-Hague J (2004). Synthesis of N-(fluoren-9-ylmethoxycarbonyl)glycopyranosylamine uronic acids. Carbohydrate Research, (339), 367–375. 10.1016/j.carres.2003.10.018 [DOI] [PubMed] [Google Scholar]
- Yusuf A, Haggag AAD, Mohamed AO, & Sanaa A. El-G. (2018). Biomedical Journal of Scientific and Technical Research, 8(4). 10.26717/BJSTR.2018.08.0016 [DOI] [Google Scholar]


