Abstract
Pleural empyema represents a significant healthcare burden due to extended hospital admissions and potential requirement for surgical intervention. This study aimed to assess changes in incidence and management of pleural empyema in England over the past 10 years and the potential impact of influenza on rates.
Hospital Episode Statistics data were used to identify patients admitted to English hospitals with pleural empyema between 2008 and 2018. Linear regression was used to analyse the relationship between empyema rates and influenza incidence recorded by Public Health England. The relationship between influenza and empyema was further explored using serological data from a prospective cohort study of patients presenting with pleural empyema.
Between April 2008 and March 2018 there were 55 530 patients admitted with pleural empyema. There was male predominance (67% versus 33%), which increased with age. Cases have increased significantly from 4447 in 2008 to 7268 in 2017. Peaks of incidence correlated moderately with rates of laboratory-confirmed influenza in children and young adults (r=0.30). For nine of the 10 years studied, the highest annual point incidence of influenza coincided with the highest admission rate for empyema (with a 2-week lag). In a cohort study of patients presenting to a single UK hospital with pleural empyema/infection, 24% (17 out of 72) had serological evidence of recent influenza infection, compared to 7% in seasonally matched controls with simple parapneumonic or cardiogenic effusions (p<0.001).
Rates of empyema admissions in England have increased steadily with a seasonal variation that is temporally related to influenza incidence. Patient-level serological data from a prospective study support the hypothesis that influenza may play a pathogenic role in empyema development.
Short abstract
Rates of pleural empyema are increasing in English hospitals without any improvement in patient outcomes. This study also found that male predominance of empyema increased with age, and rates increased in winter and during peaks of influenza. https://bit.ly/3oiJ870
Background
Pleural empyema is a serious condition defined by the collection of infected fluid around the lung. It constitutes a considerable healthcare burden given the requirement of chest tube drainage, intrapleural fibrinolytics or thoracic surgery for resolution. Epidemiological studies have suggested that the incidence of pleural empyema is increasing [1–3]. No studies have assessed the potential impact of respiratory virus infections on empyema development.
The link between viral influenza and secondary bacterial pneumonia has been demonstrated in epidemiological, observational and animal studies [4]. The relationship between influenza and empyema is less well described, although it has a biological and epidemiological basis [5–7]. Such a link would explain any seasonal variation seen in empyema incidence and its perceived tendency to affect a younger population than pneumonia.
The aim of this study was to assess the change in incidence and management of pleural empyema in England over the past 10 years using Hospital Episode Statistics (HES) data. Using data from Public Health England (PHE), we compared weekly rates of influenza to empyema admissions. To examine this relationship further, we analysed a prospectively collected cohort of patients presenting with empyema for serological evidence of recent influenza A and B and respiratory syncytial virus (RSV) infection.
Methods
Hospital Episode Statistics
Data were extracted from the HES database, which contains information on all acute care hospital admissions in England. In the HES dataset, each hospital admission contains fields for the principal reason for the admission, alongside other secondary or subsidiary diagnoses, coded using the International Classification of Diseases (ICD-10) codes.
For this study, inpatient hospital admissions between April 1, 2008 and March 31, 2018 with the code J86 (pyothorax) in any diagnostic position were requested. This code, appearing in any diagnostic position, has been previously validated for positive predictive value and sensitivity for true clinical diagnosis of empyema (86% and 72%, respectively) [8]. We identified codes for pleural surgical procedures performed during the inpatient hospital admissions (codes validated by the Society for Cardiothoracic Surgery in Great Britain and Ireland) [9], namely “surgical drain” including open drainage and fenestration of pleura (T08.1, T08.3, T08.8, T08.9), “excision” including thoracoplasty and decortication (T01.1, T01.3, T07.1) and “other surgery” including unspecified open operation on the pleura (T09.8, T09.9, T10.8). To avoid double coding due to re-admissions and transfers, new episodes occurring within a 31-day period from the original episode were classified to the same admission. Admissions with missing age data or a recorded age of >100 years were removed from the dataset due to probable coding errors.
Public Health England
National weekly incidence of influenza (per 100 000) between April 1, 2008 and March 31, 2018 were provided by PHE from the influenza surveillance programme. A variety of data sources are collated to provide accurate information on circulating influenza strains and estimates of burden on primary and secondary care [10]. Using an external validated database such as this, which uses multiple sources to generate incidence figures, was seen preferable to relying on the HES database alone.
Statistical analysis: epidemiological data
Descriptive statistics are presented to provide the overall number and demographics of patients diagnosed with pleural empyema. We split patients into paediatric and adult populations, with adults further split into those aged 18–59 years and ≥60 years to account for age-related differences in empyema and influenza. To compare rates of viral influenza to rates of empyema, episodes from the HES dataset were aggregated to make them comparable to the PHE data. Annual statistics were calculated from April 1 to ensure that each whole UK winter period (defined as months December, January and February) was reported within the same year. We analysed the relationship between empyema and viral influenza using linear regression. Quadratic year terms were included to account for the concurrent annual increase in viral influenza and empyema throughout the study period. To ensure that our results were robust to potential lag effects, we ran sensitivity analyses with influenza lagged by 1 week behind that recorded by PHE.
Prospective cohort study: patients
To further evaluate the relationship between viral influenza and pleural empyema we analysed the serum and pleural fluid from patients recruited to a prospective cohort study for evidence of influenza A and B and RSV infection. In brief, consecutive patients referred to a single centre pleural service in the South West of England between 2009 and 2016 with an undiagnosed effusion were recruited. All patients had a diagnostic thoracentesis as part of normal clinical care and consented to having their demographic data recorded, as well as serum and pleural fluid stored (in a −70°C freezer). The final diagnosis was agreed by two independent consultant respiratory physicians based on all the available clinical, histological and radiological information at 12 months (refer to supplementary material for pre-specified diagnostic criteria).
For this research, the stored serum and pleural fluid of 72 patients with a final diagnosis of pleural infection was analysed en bloc with results compared to controls of simple parapneumonic effusions (SPPE) (n=29) and effusions secondary to cardiac dysfunction (n=49). Controls were seasonally matched to empyema cases (presented within the same fortnight) to avoid confounding from background changes in respiratory virus prevalence.
Prospective cohort study: serum and pleural fluid testing
Complement fixation testing (CFT) was performed on patient serum from a sample taken at the time of thoracentesis. We used a CFT titre of ≥1:128 as evidence of recent infection. Differences in the rates between the diagnostic categories were compared using a Chi-squared test.
Pleural fluid samples were tested for the presence of respiratory viruses, including influenza A and B, RSV A and B, parainfluenzavirus types 1–4, human metapneumoviruses, adenoviruses, rhinoviruses, enteroviruses, parechoviruses and bocaviruses by real-time PCR using a TaqMan low-density array, as described previously [11].
Results
Hospital Episode Statistics for empyema
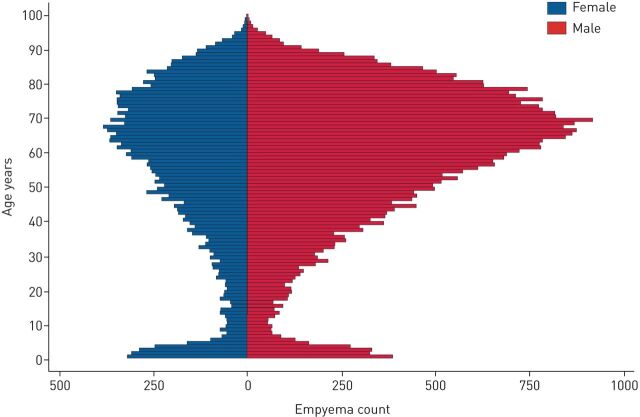
Between April 1, 2008 and March 31, 2018, there were 55 530 episodes in which patients were admitted to English hospitals with empyema. The median age was 62 years with a bimodal distribution. There was a male predominance in empyema across the cohort (67.3% compared to 32.7%), although this only appeared after the age of 18 years (table 1 and figure 1).
TABLE 1.
Patient demographics for empyema (J86) admissions from April 2008 to April 2018
| Age | All | ||
| 0–17 years | ≥18 years | ||
| Cases | 4473 | 51 057 | 55 530 |
| Age years | 4 (2–9) | 64 (51–75) | 62 (46–74) |
| Male | 2397 (53.6) | 34 945 (68.5) | 37 342 (67.3) |
| Outcomes | |||
| Hospital length of stay days | 12 (7–17) | 15 (6–28) | 14 (7–27) |
| Surgical management | 371 (8.3) | 7409 (14.5) | 7780 (14.0) |
| Inpatient mortality | 41 (0.9) | 7601 (14.9) | 7642 (13.8) |
Data are presented as n, median (interquartile range) or n (%).
FIGURE 1.
Age distribution of empyema episodes split by sex.
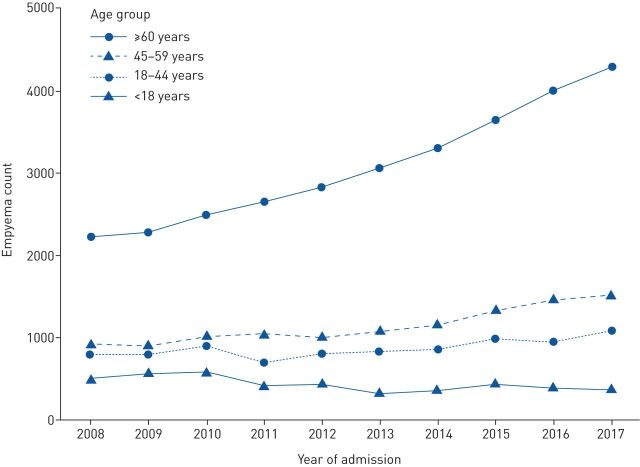
There was an increase in empyema rates across the 10-year period from 2008 to 2018 (table 2). This increase was mainly due to an increase in the over-60s, with empyema-related episodes in that group almost doubling across the same timeframe (from 2212 to 4287). In contrast, rates in the paediatric group (<18 years) fell by a quarter (from 510 to 376) (figure 2). The incidence of empyema, calculated per 100 000 hospital admissions, rose throughout the study period from 6.44 per 100 000 in 2008 to 8.38 per 100 000 in 2017. Mortality and surgery rates among patients with empyema remained consistent throughout the study period at ∼14%. Length of stay for patients with empyema also remained consistent at ∼14 days per episode.
TABLE 2.
Yearly changes in empyema incidence, management and mortality
| Year # | ||||||||||
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
| Total empyema count | ||||||||||
| All ages | 4447 | 4573 | 5002 | 4812 | 5087 | 5314 | 5697 | 6415 | 6811 | 7268 |
| <18 years | 510 | 573 | 591 | 423 | 438 | 336 | 372 | 448 | 393 | 376 |
| 18–44 years | 794 | 808 | 898 | 699 | 806 | 830 | 855 | 992 | 953 | 1082 |
| 45–59 years | 931 | 907 | 1019 | 1048 | 1021 | 1089 | 1170 | 1338 | 1472 | 1523 |
| ≥60 years | 2212 | 2285 | 2494 | 2642 | 2822 | 3059 | 3300 | 3637 | 3996 | 4287 |
| Empyema per 100 000 hospital admissions | 6.4 | 6.3 | 6.7 | 6.4 | 6.7 | 6.8 | 7.0 | 7.7 | 8.0 | 8.4 |
| Mortality % of total count of empyema | 13.7 | 14.0 | 14.0 | 13.5 | 13.7 | 14.8 | 15.2 | 13.2 | 14.2 | 12.7 |
| Surgery % of total count of empyema | 15.1 | 14.4 | 14.3 | 14.0 | 14.1 | 13.8 | 14.2 | 12.8 | 13.2 | 14.5 |
| Hospital LOS for empyema cases | 14.2 (6.6–27.58) | 14.5 (6.90–27.0) | 14.6 (6.8–27.07) | 14.1 (6.6–26.9) | 14.3 (6.4–27.0) | 14.5 (6.6–27.4) | 14.8 (6.7–27.4) | 14.23 (6.3–26.6) | 14.2 (6.4–26.6) | 13.6 (6.4–25.0) |
| Ratio of winter versus rest of year | 1.4 | 1.1 | 1.5 | 1.1 | 1.3 | 1.1 | 1.4 | 1.1 | 1.3 | 1.3 |
Data are presented as n or median (interquartile range). LOS: length of stay. #: years start April 1.
FIGURE 2.
Trend of incidence of empyema April 2008 to April 2018 (split by age categories).
There was seasonal variance in empyema with rates increasing by a quarter overall in winter months (December, January, February). This was particularly marked in the paediatric and young adult populations with rates more than doubling in winter months (supplementary material).
Influenza rates from PHE and empyema admission
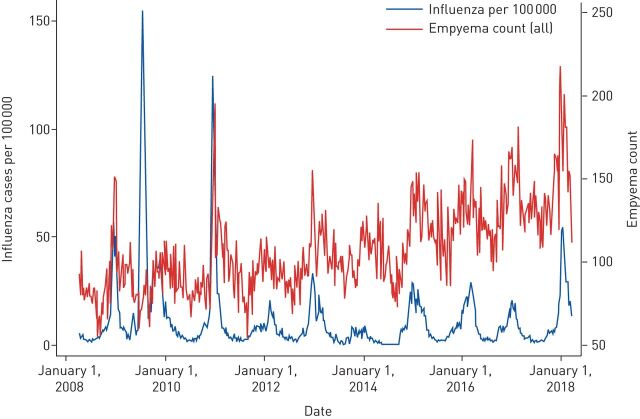
When compared on a week-by-week basis, empyema and influenza rates per 100 000 during the study period were moderately correlated (r=0.24 for empyema counts; r=0.30 for empyema incidence per 100 000). Results from the regression analyses of empyema counts shows that each one case increase in influenza per 100 000 people was associated with an additional β=0.25 (95% CI 0.16–0.34) empyema cases in the HES. Converted to incidence rates, which are more robust to changes in recording practices over the study period, each one case increase in influenza per 100 000 people was associated with an additional increase in empyema of β=0.076 (95% CI 0.051–0.102) per 100 000 people. As indicated by the R2 statistics, most of the variation in empyema was attributable to week and year differences (70.6% for counts; 68.8% for incidence per 100 000), although influenza rates provided some additional explanatory power (1.86% for counts; 2.22% for incidence per 100 000). For nine of the 10 years studied, the highest annual point incidence of influenza nationally coincided with the highest admission rate for empyema (within 2 weeks) (figure 3).
FIGURE 3.
Influenza incidence per 100 000 and empyema count, April 2008 to April 2018.
Decomposing these total population associations into age groups reveals differing patterns by age. Correlations between empyema counts and influenza rates per 100 000 were stronger for children (r=0.52) than for adults aged 18–59 years (r=0.33) and those aged ≥60 years (r=−0.019). The R2 statistics from the models reveal that rates of influenza per 100 000 explain a greater proportion of additional variance over and above week and year trends for children (8.49%) than adults (4.77%) and the elderly (0%).
The results of the sensitivity analysis investigating the lag effects (supplementary material) are consistent with our main results; each one case increase in influenza per 100 000 people is associated with an additional 0.27 (95% CI 0.18–0.35) counts of influenza cases, while each one case increase in influenza per 100 000 people is associated with an additional increase in empyema of 0.082 (95% CI 0.056–0.107) per 100 000 people.
Prospective cohort study
Baseline characteristics of patients included in the cohort study, including viral testing results, are presented in table 3. There was strong evidence of a difference in influenza infection rates between groups; out of the 72 patients who presented with empyema, 17 (24%) had serological evidence of recent influenza infection compared to two (7%) and two (4%) in the SPPE and cardiogenic effusion groups, respectively (p=0.0017). Patients presenting with cardiogenic effusions had a higher rate of RSV positivity, compared to the empyema and SPPE groups.
TABLE 3.
Baseline characteristics and serological results of the 72 patients with empyema and 78 controls
| Empyema | SPPE | Cardiac effusions | |
| Patients | 72 | 29 | 49 |
| Age years | 59 (43–72) | 68 (53–80) | 75 (67–82) |
| Male/female % | 49/23 | 16/13 | 34/15 |
| Right/left % | 40/32 | 17/12 | 38/11 |
| Symptom duration days | 10 (4–16) | 4 (2–6) | 35 (10–78) |
| Serum complement fixation testing | |||
| Influenza all | 17 (24) | 2 (7) | 2 (4) |
| Influenza A | 14 (20) | 1 (3) | 2 (4) |
| Influenza B | 3 (4) | 1 (3) | 0 (0) |
| RSV | 2 (3) | 2 (7) | 6 (12) |
| Age range of serology-positive patients years | 38–90 | 41–85 | 49–87 |
Data are presented as n, median (interquartile range) or n (%). SPPE: simple parapneumonic effusions; RSV: respiratory syncytial virus.
There was no evidence of respiratory viruses detectable by real-time PCR within any of the pleural fluid samples themselves (not shown).
Discussion
This study assessed the changes in rates of pleural empyema in England over the past decade and possible association with influenza. Data from HES have shown a significant increase of empyema rates over the study period, as well as average seasonal increases in winter months of ∼25%. The year-on-year increases were driven by rising rates among the elderly; rates were stable among the middle-aged and fell among children. Influenza rates were associated with empyema over and above seasonal changes among the paediatric and middle-aged populations. Accompanying patient-level data from a prospective study of adults presenting with pleural effusions found serological evidence of recent influenza A infection in a quarter of patients presenting with empyema.
Finley et al. [1] used the Canadian Discharge Abstract Database to identify hospital admissions with empyema (ICD-10 code J86) from 1995 to 2003. They demonstrated a significant increase in empyema rates especially in the paediatric population with a marked 463% increase in 1–4-year-olds during the study period. They hypothesised that due to the introduction of the eight-valent pneumococcal vaccine during the study period there had been a serotype shift to more invasive strains of pneumococcus alongside a reduction in overall pneumococcal infections. Other paediatric studies from Scotland and Spain have shown a similar increase following the introduction of the pneumococcal vaccine [12, 13]. Another North American based study used pleural empyema hospital discharge codes between 1996 and 2008 [3]. Similarly, they found that the rates increased during the study period by two-fold. Although the most marked increase was not in the paediatric population, but in 40–64-year-olds, despite examining the same study period in countries with a similar pneumococcal conjugate vaccine (PCV) vaccination introduction. Søgaard et al. [14] assessed rates of pleural empyema in Denmark between 1997 and 2011 (excluding the paediatric population) and found that rates rose steadily across the study period in the elderly, although absolute numbers were small (<500 cases per year).
This current study has shown that, in England, overall rates of pleural empyema have shown a year-on-year increase from 4447 in 2008 to 7268 in 2017. However, this increase is most marked in the population aged ≥60 years, which showed a 194% increase over the decade. By contrast, in children, rates began to fall after 2010 from 591 to 376 per year. This reassuring trend may be related to the 2011 introduction of the PCV13 vaccine for children in the UK, which has an extended coverage compared to PCV7 and most importantly now covers more invasive strains that are more likely to cause invasive pneumococcal disease (e.g. serotype 1). The increase in adults age ≥60 years is worrying and has several potential explanations. Firstly, the ageing population within England is likely to be increasing susceptibility to respiratory infections. There has been a slight increase in the average age of the cohort over the past decade, so although a likely contributing factor, it is unlikely to be the primary explanation. Secondly, there has been an increased prescribing of immunosuppressive agents and a rise in the prevalence of diabetes mellitus, both of which are well-recognised risk factors for pleural empyema [15]. Finally, there could be an overdetection bias with the more widespread use of computed tomography imaging in hospitals leading to the detection of parapneumonic effusions that would previously not have been identified as empyema and managed as pneumonia. Although overdetection is a possibility, the management and outcomes of pleural empyema have altered little in a decade. There was no significant change in the proportion of inpatient mortality or surgical rates (both between 12% and 14%). Hospital length of stay was consistent at 14 days, which is marginally longer than previous estimates which are based on randomised trials and are therefore likely to exclude particularly unwell patients with more extended length of stays.
Both epidemiological studies and randomised trial data on pleural empyema have found it to be a male-predominant disease. In the Multicenter Intrapleural Sepsis Trial (MIST) 1 and 2 studies combined, the ratio of males to females was 2.37 (450:190), and in the Canadian epidemiological study discussed earlier the ratio was 2.23 [16, 17]. Despite being well recognised, this phenomenon has never been explained. Hypotheses range from different health-seeking behaviours of males compared to females (with males delaying presentation with pneumonia), or poorer dental hygiene in males leading to bacterial translocation. This study has again confirmed the male predominance, but has shown that the ratio increases significantly with patient age. Boys and girls aged ≤15 years are affected roughly equally (1.13:1). After adolescence, the ratio rises gradually to a peak of 2.36:1 in the over-60s.
This is the first epidemiological study to demonstrate the increase in pleural empyema rates during winter months. The increased rates were particularly marked in the <18-years and 19–60-years age groups that had a ∼1.3 and ∼1.8 increase in cases, respectively. Given the seasonality of viral influenza as well as historic associations with empyema we correlated weekly rates of the two conditions. Using linear regression, there was significant positive association between incidence rates, which was stronger in the younger age groups. Most strikingly, the highest annual point incidence of influenza each year coincided with the highest annual admission rate for empyema (within 2 weeks) for nine of the 10 years studied, with the 2009 summer influenza pandemic being the only exception. The patient-level data from the cohort study of patients presenting with pleural effusions has shown patients presenting with empyema had significantly higher seroprevalence of recent influenza A infection. Although serological testing at a single time point should not be used as a diagnostic tool for the presence of active or recent viral infection the significant difference in titres between seasonally matched groups is of note and adds weight to the hypothesis that influenza is leading to a proportion of empyema cases [18].
This study has multiple data sources in an attempt to provide assurance of its conclusions. However, there are several limitations to consider. First, due to a lack of individual level data measuring both empyema and influenza, our analysis relied upon aggregated statistics. It is therefore possible that our results reflect common processes that underlie rates of empyema and influenza. There is also a risk of inaccuracy in the HES coding of pleural empyema. However, given the severity of the condition, requirement for hospital admission, pleural instrumentation and extended inpatient stays, we anticipated that it would be relatively robust. We have previously published a validation study which demonstrated that the positive predictive value and sensitivity of the J86 code for pleural empyema within HES was 86% and 72%, respectively (analysis published separately) [8]. Therefore, it is more likely that the figures reported in this study are an underestimate of the true burden of this condition. Given the lack of change over time in the demographics, mortality and management of empyema within HES (which is in keeping with data from prospective trials of the condition), there is no evidence to suggest that coding practice has changed over time. Data on the incidence of influenza was provided by PHE, which uses multiple sources to generate an estimate of disease burden [10]. This is widely seen as the most accurate assessment nationally, and given that two completely separate data sources correlated so closely this supported our hypothesis of a causative role of influenza. Finally, the prospective study used to further investigate the role between influenza and empyema is single-centre and the numbers are relatively small for epidemiological assessment. However, given that the stored serum and pleural fluid analysed is from the largest known prospective study of pleural effusions worldwide, this was seen as the best possible validation cohort available.
In conclusion, this is the first epidemiological assessment of pleural empyema in England which has demonstrated that incidence is rising in the elderly. The male predominance of the condition increases with age for reasons that remain unclear. In addition, this is the first study to show the marked increase in empyema incidence in winter months which is temporally associated with influenza. The relationship between influenza and empyema is an important finding and should be investigated further.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-03546-2020.SUPPLEMENT (288.4KB, pdf)
Shareable PDF
Acknowledgements
The authors would like to thank Henry Steer (Respiratory Dept, Gloucestershire Hospitals NHS Foundation Trust, UK) for his guidance when developing the original research question.
Footnotes
This article has supplementary material available from erj.ersjournals.com
Data availability: Data are available from NHS Digital and Public Health England.
Authors and contributors: D.T. Arnold, F.W. Hamilton, T.T. Morris, R.A. Payne, P. Muir and N.A. Maskell generated the research question and analysis plan. T.T. Morris and F.W. Hamilton performed the statistical analysis. T. Suri, I.B. Vipond and V. Frost performed the laboratory analysis of samples. A. Morley, A.R. Medford and N.A. Maskell were responsible for curating/collating the prospective study samples and patient diagnoses. All authors were involved in the final manuscript preparation.
Conflict of interest: D.T. Arnold has nothing to disclose.
Conflict of interest: F.W. Hamilton has nothing to disclose.
Conflict of interest: T.T. Morris has nothing to disclose.
Conflict of interest: T. Suri has nothing to disclose.
Conflict of interest: A. Morley has nothing to disclose.
Conflict of interest: V. Frost has nothing to disclose.
Conflict of interest: I.B. Vipond has nothing to disclose.
Conflict of interest: A.R. Medford has nothing to disclose.
Conflict of interest: R.A. Payne has nothing to disclose.
Conflict of interest: P. Muir has nothing to disclose.
Conflict of interest: N.A. Maskell has nothing to disclose.
Support statement: D.T. Arnold is funded by a National Institute for Health Research Doctoral Research Fellowship (DRF-2018-11-ST2-065) for this research project. This publication presents independent research funded by NIHR. Population Health Sciences, University of Bristol, has a Data Sharing Agreement (DSA; NIC-1785-X7K1V) with the HSCIC for HES Admitted Patient Care (inpatient/day case) data for the financial years 2007/8 to 2016/17, and for outpatient data for the financial years 2010/11 to 2016/17. The purchase of these data was funded by NIHR CLAHRC West. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Finley C, Clifton J, Fitzgerald JM, et al. . Empyema: an increasing concern in Canada. Can Respir J 2008; 15: 85–89. doi: 10.1155/2008/975312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farjah F, Symons RG, Krishnadasan B, et al. . Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg 2007; 133: 346–351. doi: 10.1016/j.jtcvs.2006.09.038 [DOI] [PubMed] [Google Scholar]
- 3.Grijalva CG, Zhu Y, Nuorti JP, et al. . Emergence of parapneumonic empyema in the USA. Thorax 2011; 66: 663–668. doi: 10.1136/thx.2010.156406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol 2017; 8: 1041. doi: 10.3389/fmicb.2017.01041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wörnle M, Sauter M, Kastenmüller K, et al. . Role of toll-like receptor 3, RIG-I, and MDA5 in the expression of mesothelial IL-8 induced by viral RNA. Appl Biochem Biotechnol 2010; 160: 1179–1187. doi: 10.1007/s12010-009-8643-7 [DOI] [PubMed] [Google Scholar]
- 6.Bender JM, Ampofo K, Sheng X, et al. . Parapneumonic empyema deaths during past century, Utah. Emerg Infect Dis 2009; 15: 44–48. doi: 10.3201/eid1501.080618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ampofo K, Herbener A, Blaschke AJ, et al. . Association of 2009 pandemic influenza A (H1N1) infection and increased hospitalization with parapneumonic empyema in children in Utah. Pediatr Infect Dis J 2010; 29: 905–909. doi: 10.1097/INF.0b013e3181df2c70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton F, Arnold D. Accuracy of clinical coding of pleural empyema: a validation study. J Eval Clin Pract 2020; 26: 79–80. doi: 10.1111/jep.13184 [DOI] [PubMed] [Google Scholar]
- 9.National Thoracic Surgery Activity and Outcomes Report. Henley-on-Thames, Dendrite Clinical Systems, 2008–2018, 2018. [Google Scholar]
- 10.Zhao H, Green H, Lackenby A, et al. . A new laboratory-based surveillance system (Respiratory DataMart System) for influenza and other respiratory viruses in England: results and experience from 2009 to 2012. Euro Surveill 2014; 19: 20680. doi: 10.2807/1560-7917.ES2014.19.3.20680 [DOI] [PubMed] [Google Scholar]
- 11.Ordóñez-Mena JM, Fanshawe TR, Butler CC, et al. . Relationship between microbiology of throat swab and clinical course among primary care patients with acute cough: a prospective cohort study. Fam Pract 2020; 37: 332–339. doi: 10.1093/fampra/cmz093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roxburgh CSD, Youngson GG, Turner SW. Trends in pneumonia and empyema in Scottish children in the past 25 years. Thorax 2006; 61: Suppl. 2, S041. [DOI] [PubMed] [Google Scholar]
- 13.Obando I, Camacho-Lovillo MS, Porras A, et al. . Sustained high prevalence of pneumococcal serotype 1 in paediatric parapneumonic empyema in southern Spain from 2005 to 2009. Clin Microbiol Infect 2012; 18: 763–768. doi: 10.1111/j.1469-0691.2011.03632.x [DOI] [PubMed] [Google Scholar]
- 14.Søgaard M, Nielsen RB, Nørgaard M, et al. . Incidence, length of stay, and prognosis of hospitalized patients with pleural empyema: a 15-year Danish nationwide cohort study. Chest 2014; 145: 189–192. doi: 10.1378/chest.13-1912 [DOI] [PubMed] [Google Scholar]
- 15.Corcoran JP, Psallidas I, Gerry S, et al. . Prospective validation of the RAPID clinical risk prediction score in adult patients with pleural infection: the PILOT study. Eur Respir J 2020; 56: 2000130. doi: 10.1183/13993003.00130-2020 [DOI] [PubMed] [Google Scholar]
- 16.Maskell NA, Davies CW, Nunn AJ, et al. . U.K. controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352: 865–874. 10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]
- 17.Rahman NM, Maskell NA, West A, et al. . Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365: 518–526. doi: 10.1056/NEJMoa1012740 [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention . Influenza Testing Methods. www.cdc.gov/flu/professionals/diagnosis/table-testing-methods.htmDate last accessed: September 9, 2020. Date last updated: August 10, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-03546-2020.SUPPLEMENT (288.4KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-03546-2020.Shareable (324.8KB, pdf)