Abstract
Background
Monoclonal antibodies (mAbs) are laboratory‐produced molecules derived from the B cells of an infected host. They are being investigated as a potential therapy for coronavirus disease 2019 (COVID‐19).
Objectives
To assess the effectiveness and safety of SARS‐CoV‐2‐neutralising mAbs for treating patients with COVID‐19, compared to an active comparator, placebo, or no intervention. To maintain the currency of the evidence, we will use a living systematic review approach.
A secondary objective is to track newly developed SARS‐CoV‐2‐targeting mAbs from first tests in humans onwards.
Search methods
We searched MEDLINE, Embase, the Cochrane COVID‐19 Study Register, and three other databases on 17 June 2021. We also checked references, searched citations, and contacted study authors to identify additional studies. Between submission and publication, we conducted a shortened randomised controlled trial (RCT)‐only search on 30 July 2021.
Selection criteria
We included studies that evaluated SARS‐CoV‐2‐neutralising mAbs, alone or combined, compared to an active comparator, placebo, or no intervention, to treat people with COVID‐19. We excluded studies on prophylactic use of SARS‐CoV‐2‐neutralising mAbs.
Data collection and analysis
Two authors independently assessed search results, extracted data, and assessed risk of bias using the Cochrane risk of bias tool (RoB2). Prioritised outcomes were all‐cause mortality by days 30 and 60, clinical progression, quality of life, admission to hospital, adverse events (AEs), and serious adverse events (SAEs). We rated the certainty of evidence using GRADE.
Main results
We identified six RCTs that provided results from 17,495 participants with planned completion dates between July 2021 and December 2031. Target sample sizes varied from 1020 to 10,000 participants. Average age was 42 to 53 years across four studies of non‐hospitalised participants, and 61 years in two studies of hospitalised participants.
Non‐hospitalised individuals with COVID‐19
Four studies evaluated single agents bamlanivimab (N = 465), sotrovimab (N = 868), regdanvimab (N = 307), and combinations of bamlanivimab/etesevimab (N = 1035), and casirivimab/imdevimab (N = 799). We did not identify data for mortality at 60 days or quality of life. Our certainty of the evidence is low for all outcomes due to too few events (very serious imprecision).
Bamlanivimab compared to placebo
No deaths occurred in the study by day 29. There were nine people admitted to hospital by day 29 out of 156 in the placebo group compared with one out of 101 in the group treated with 0.7 g bamlanivimab (risk ratio (RR) 0.17, 95% confidence interval (CI) 0.02 to 1.33), 2 from 107 in the group treated with 2.8 g (RR 0.32, 95% CI 0.07 to 1.47) and 2 from 101 in the group treated with 7.0 g (RR 0.34, 95% CI 0.08 to 1.56). Treatment with 0.7 g, 2.8 g and 7.0 g bamlanivimab may have similar rates of AEs as placebo (RR 0.99, 95% CI 0.66 to 1.50; RR 0.90, 95% CI 0.59 to 1.38; RR 0.81, 95% CI 0.52 to 1.27). The effect on SAEs is uncertain. Clinical progression/improvement of symptoms or development of severe symptoms were not reported.
Bamlanivimab/etesevimab compared to placebo
There were 10 deaths in the placebo group and none in bamlanivimab/etesevimab group by day 30 (RR 0.05, 95% CI 0.00 to 0.81). Bamlanivimab/etesevimab may decrease hospital admission by day 29 (RR 0.30, 95% CI 0.16 to 0.59), may result in a slight increase in any grade AEs (RR 1.15, 95% CI 0.83 to 1.59) and may increase SAEs (RR 1.40, 95% CI 0.45 to 4.37). Clinical progression/improvement of symptoms or development of severe symptoms were not reported.
Casirivimab/imdevimab compared to placebo
Casirivimab/imdevimab may reduce hospital admissions or death (2.4 g: RR 0.43, 95% CI 0.08 to 2.19; 8.0 g: RR 0.21, 95% CI 0.02 to 1.79). We are uncertain of the effect on grades 3‐4 AEs (2.4 g: RR 0.76, 95% CI 0.17 to 3.37; 8.0 g: RR 0.50, 95% CI 0.09 to 2.73) and SAEs (2.4 g: RR 0.68, 95% CI 0.19 to 2.37; 8.0 g: RR 0.34, 95% CI 0.07 to 1.65). Mortality by day 30 and clinical progression/improvement of symptoms or development of severe symptoms were not reported.
Sotrovimab compared to placebo
We are uncertain whether sotrovimab has an effect on mortality (RR 0.33, 95% CI 0.01 to 8.18) and invasive mechanical ventilation (IMV) requirement or death (RR 0.14, 95% CI 0.01 to 2.76). Treatment with sotrovimab may reduce the number of participants with oxygen requirement (RR 0.11, 95 % CI 0.02 to 0.45), hospital admission or death by day 30 (RR 0.14, 95% CI 0.04 to 0.48), grades 3‐4 AEs (RR 0.26, 95% CI 0.12 to 0.60), SAEs (RR 0.27, 95% CI 0.12 to 0.63) and may have little or no effect on any grade AEs (RR 0.87, 95% CI 0.66 to 1.16).
Regdanvimab compared to placebo
Treatment with either dose (40 or 80 mg/kg) compared with placebo may decrease hospital admissions or death (RR 0.45, 95% CI 0.14 to 1.42; RR 0.56, 95% CI 0.19 to 1.60, 206 participants), but may increase grades 3‐4 AEs (RR 2.62, 95% CI 0.52 to 13.12; RR 2.00, 95% CI 0.37 to 10.70). 80 mg/kg may reduce any grade AEs (RR 0.79, 95% CI 0.52 to 1.22) but 40 mg/kg may have little to no effect (RR 0.96, 95% CI 0.64 to 1.43). There were too few events to allow meaningful judgment for the outcomes mortality by 30 days, IMV requirement, and SAEs.
Hospitalised individuals with COVID‐19
Two studies evaluating bamlanivimab as a single agent (N = 314) and casirivimab/imdevimab as a combination therapy (N = 9785) were included.
Bamlanivimab compared to placebo
We are uncertain whether bamlanivimab has an effect on mortality by day 30 (RR 1.39, 95% CI 0.40 to 4.83) and SAEs by day 28 (RR 0.93, 95% CI 0.27 to 3.14). Bamlanivimab may have little to no effect on time to hospital discharge (HR 0.97, 95% CI 0.78 to 1.20) and mortality by day 90 (HR 1.09, 95% CI 0.49 to 2.43). The effect of bamlanivimab on the development of severe symptoms at day 5 (RR 1.17, 95% CI 0.75 to 1.85) is uncertain. Bamlanivimab may increase grades 3‐4 AEs at day 28 (RR 1.27, 95% CI 0.81 to 1.98). We assessed the evidence as low certainty for all outcomes due to serious imprecision, and very low certainty for severe symptoms because of additional concerns about indirectness.
Casirivimab/imdevimab with usual care compared to usual care alone
Treatment with casirivimab/imdevimab compared to usual care probably has little or no effect on mortality by day 30 (RR 0.94, 95% CI 0.87 to 1.02), IMV requirement or death (RR 0.96, 95% CI 0.90 to 1.04), nor alive at hospital discharge by day 30 (RR 1.01, 95% CI 0.98 to 1.04). We assessed the evidence as moderate certainty due to study limitations (lack of blinding). AEs and SAEs were not reported.
Authors' conclusions
The evidence for each comparison is based on single studies. None of these measured quality of life. Our certainty in the evidence for all non‐hospitalised individuals is low, and for hospitalised individuals is very low to moderate. We consider the current evidence insufficient to draw meaningful conclusions regarding treatment with SARS‐CoV‐2‐neutralising mAbs.
Further studies and long‐term data from the existing studies are needed to confirm or refute these initial findings, and to understand how the emergence of SARS‐CoV‐2 variants may impact the effectiveness of SARS‐CoV‐2‐neutralising mAbs. Publication of the 36 ongoing studies may resolve uncertainties about the effectiveness and safety of SARS‐CoV‐2‐neutralising mAbs for the treatment of COVID‐19 and possible subgroup differences.
Plain language summary
Are laboratory‐made, COVID‐19‐specific monoclonal antibodies an effective treatment for COVID‐19?
Key messages
‐ We do not know whether antibodies (the body’s natural defence against disease) made in a laboratory and all the same as one another (monoclonal) and designed to target COVID‐19, are an effective treatment for COVID‐19 because we assessed only six studies exploring different treatments in different types of patients.
‐ We identified 36 ongoing studies that will provide more evidence when completed.
‐ We will update this review regularly as more evidence becomes available.
What are ‘monoclonal’ antibodies?
Antibodies are made by the body as a defence against disease. However, they can also be produced in a laboratory from cells taken from people who have recovered from a disease.
Antibodies that are designed to target only one specific protein – in this case, a protein on the virus that causes COVID‐19 – are ‘monoclonal’. They attach to the COVID‐19 virus and stop it from entering and replicating in human cells, which helps to fight the infection. Monoclonal antibodies have been used successfully to treat other viruses. They are thought to cause fewer unwanted effects than convalescent plasma, which contains a variety of different antibodies.
What did we want to find out?
We wanted to know if COVID‐19 specific monoclonal antibodies are an effective treatment for COVID‐19. We looked at whether they:
‐ reduced the number of deaths from any cause;
‐ improved symptoms or made them worse;
‐ increased admissions to hospital; and
‐ caused any serious or other unwanted effects.
What did we do?
We searched for studies that investigated one or more monoclonal antibodies to treat people with confirmed COVID‐19 compared with placebo (sham treatment), another treatment or no treatment. Studies could take place anywhere globally and include participants of any age, gender or ethnicity, with mild, moderate or severe COVID‐19.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and size.
What did we find?
We found six active studies including a total of 17,495 people. Four studies investigated non‐hospitalised people with no symptoms or mild COVID‐19. Two studies investigated hospitalised people with moderate to severe COVID‐19. Studies took place across the world. Three studies were funded by pharmaceutical companies. The monoclonal antibodies they studied were bamlanivimab, etesevimab, casirivimab and imdevimab, sotrovimab, regdanvimab. We did not identify data for mortality at 60 days and quality of life.
Non‐hospitalised people, with no symptoms or mild COVID‐19 (four studies)
One study investigated different doses of bamlanivimab (465 people), compared to placebo.
We don’t know whether bamlanivimab:
‐ increases or reduces the number of deaths because no participants died within 30 days of treatment;
‐ causes more or fewer serious unwanted effects because there were few events.
Bamlanivimab may reduce the number of admissions to hospital within 30 days of treatment compared to placebo.
‐ May cause slightly fewer unwanted effects than placebo.
‐ We did not find data for improved symptoms or worsened symptoms.
One study investigated a combination of bamlanivimab and etesevimab (1035 people), compared to placebo.
‐ Bamlanivimab and etesevimab may reduce the number of deaths and admissions to hospital.
‐ May cause slightly more unwanted effects.
‐ May cause more serious unwanted effects.
For treatment with bamlanivimab alone or in combination with etesevimab we did not find data for improved symptoms or worsened symptoms.
One study (phase 1/2 with 799 people) investigated different doses of casirivimab combined with imdevimab, compared to placebo.
‐ Casirivimab combined with imdevimab may reduce the number of hospital admissions or death.
‐ We don't know whether casirivimab and imdevimab causes more unwanted (grades 3 and 4) and serious unwanted effects than placebo because there were too few deaths to allow us to make a judgment.
‐ We did not find data for the number of people who died at day 30 and development of severe symptoms.
‐ We did not include results from phase 3 (5607 people) of this study, because of high risk of bias, as it was not clear which participants were included in the analysis.
One study (583 people) investigated sotrovimab, compared to placebo.
We don't know whether sotrovimab:
‐ increases or reduces the number of deaths and people requiring invasive mechanical ventilation or dying, because there were too few deaths to allow us to make a judgment.
‐ Sotrovimab may reduce the number of people requiring oxygen, unwanted (grades 3 to 4) and serious unwanted effects;
‐ may have little or no effect on unwanted effects (all grades).
Another study (327 people) investigated different doses of regdanvimab (40 mg/kg and 80 mg/kg), compared to placebo.
‐ Regdanvimab at either dose may reduce the number of admissions to hospital or death.
‐ May increase unwanted events (grades 3 to 4).
‐ Regdanvimab at a dose of 80 mg/kg may reduce unwanted effects (all grades) and 40 mg/kg may have little to no effect.
‐ We don't know whether regdanvimab increases or decreases the number of deaths, requirement for invasive mechanical ventilation, and serious unwanted effects, because there were too few events to allow us to make a judgment.
Hospitalised people with moderate to severe COVID‐19 (2 studies)
One study (314 people) investigated bamlanivimab compared to placebo.
‐ We don’t know whether bamlanivimab increases or decreases the number of deaths due to any cause up to 30 or 90 days after treatment because there were too few deaths to allow us to make a judgment (6 deaths with bamlanivimab and 4 deaths with placebo in 314 people).
‐ Bamlanivimab may slightly increase the development of severe COVID‐19 symptoms five days after treatment and the number of people with unwanted effects.
‐ Bamlanivimab may have little to no effect on time until discharge from hospital.
‐ We don’t know whether bamlanivimab causes serious unwanted effects by day 30 because the study was small and reported few serious unwanted effects.
Another study (9785 people) investigated casirivimab combined with imdevimab, compared to standard of care.
‐ Casirivimab combined with imdevimab has probably little to no effect on the number of deaths, requirement for invasive mechanical ventilation or death, and hospital discharge alive.
‐ We did not find data for unwanted and serious unwanted effects.
What are the limitations of the evidence?
Our confidence in the evidence is low because we found only six studies, and they did not report everything we were interested in, such as the number of deaths within 60 days and quality of life. We found 36 ongoing studies. When they are published, we will add their results to our review. These results are likely to change our conclusions and will also help us understand how new variants affect how well monoclonal antibodies work.
How up to date is this evidence?
The evidence is up to date to 17 June 2021.
Summary of findings
Background
Description of the condition
The clinical syndrome novel coronavirus disease 2019 (COVID‐19) is a rapidly spreading infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2; WHO 2020a). Declared a pandemic on 11 March 2020, COVID‐19 is unprecedented in comparison to previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), with 813 and 858 deaths, respectively (WHO 2007; WHO 2019). Despite intensive international efforts to contain its spread, it has resulted in more than 198 million confirmed cases and more than 4.2 million deaths worldwide up to 3 August 2021 (WHO 2020b), impacting severely on healthcare facilities, healthcare workers, and medical equipment resources.
Since 23 March 2020, weekly hospitalisation rates in the USA fluctuated between 3 and 17.2 per 100,000 population, with a recent peak increase at the end of December 2020 (CDC 2020). Risk for severe disease, hospitalisation and mortality is higher for individuals aged 65 years or older, smokers and individuals with certain underlying medical conditions, such as cancer, chronic kidney disease, chronic obstructive pulmonary disease (COPD), heart conditions, immunocompromised state, obesity, sickle cell disease or type 2 diabetes mellitus (Huang 2020; Liang 2020; WHO 2020a; Williamson 2020). COVID‐19 case fatality ratios varied widely between countries and reporting periods, from 0.0% to more than 25% (Johns Hopkins 2021). However, these numbers may be misleading as they tend to overestimate the infection fatality ratio (the probability of dying for an infected individual) due to varying testing frequency, lag in reporting dates, and variations in case definitions, especially at the beginning of a pandemic where the main focus lies on severe cases (WHO 2020c). A recent estimate of the infection fatality ratio of SARS‐CoV‐2 based on seroprevalence data from the general population of a country ranged between 0.00% and 1.54% for 51 different locations (corrected median: 0.23%; Ioannidis 2020).
The median incubation time is estimated to be between five and six days, and 97.5% of symptomatic cases develop symptoms within 11.5 days of exposure (Lauer 2020). Signs and symptoms can include sore throat, cough, fever, headache, fatigue, and myalgia (muscle pain) or arthralgia (joint pain) (Struyf 2020). Other symptoms include shortness of breath, chills, nausea or vomiting, diarrhoea, nasal congestion, haemoptysis (coughing up blood), and conjunctival congestion (WHO 2020a). The proportion of infected people with mild disease is around 80% (Wu 2020). Of those, 20% may remain completely asymptomatic (Buitrago‐Garcia 2020), 14% are affected by severe disease, and 5% experience critical disease with intensive care unit (ICU) admittance due to respiratory failure, septic shock, or multiple organ dysfunction (Wu 2020). Due to the extent of the pandemic and a current lack of potent medication and vaccines, targeted immunotherapies such as monoclonal antibodies (mAbs) raise hopes for combating the disease.
Description of the intervention
Monoclonal antibodies (mAbs) are laboratory‐produced molecules derived from natural B cells of hosts who have experienced or been injected with the antigen of interest, to substitute antibodies that are able to mimic a person's own immune attack (Bayer 2019). While traditional serum therapy uses antibodies derived from more than one type of white cell (polyclonal), mAbs target only a single, predetermined epitope, thereby generally showing fewer adverse events, such as serum sickness and anaphylaxis (Marston 2018). More than 75 mAbs have already been licensed by the US Food and Drug Administration (FDA) for a spectrum of medical conditions, especially in oncology and immunology (Kaplon 2020; Lu 2020b).
The main principle of the production of monoclonal antibodies follows this sequence:
isolation and identification of antibodies against the desired antigen;
selection of the best candidate antibodies;
mass production of these antibodies;
and consequently, testing of these antibodies.
The first step, the method to create, isolate and identify potential antibodies, has evolved markedly over time. The first mAb, a mouse IgG2a antibody, was licensed in 1986 to prevent kidney transplant rejection by targeting CD3 on T‐lymphocytes. Early mAbs were generated using a hybridoma technique, which involves the extraction of B‐lymphocytes from the spleen of an immunised animal (e.g. a mouse), fusion with immortal myeloma cells and in vitro culturing of the cells (‐omab; Bayer 2019; Liu 2014). Antii‐mouse antibody reactions in patients, and advances in genetic engineering led to the development of chimeric mAbs. These use the same hybridoma technique but replace the constant region of the mouse antibodies with equivalent proteins from human antibodies (~ 65% human, identified by ending ‐ximab; Bayer 2019). In humanised mAbs, all regions except the complementary‐determining regions (CDRs) that create contact with the antigen, are replaced with its human equivalents (~ 95% human, identified by the ending ‐zumab; Bayer 2019). Both latter strategies sometimes result in low antibody specificity. The most recent generation which aims at resolving this issue, called fully humanised or human mAbs (identified by the ending ‐umab), can be generated by two routes. The first route follows the hybridoma technique, but uses genetically‐altered mice that carry human antibody genes (Bayer 2019). The second route, called phage display, involves the isolation of B‐lymphocytes from human blood, obtaining the genetic information of the CDRs by polymerase chain reaction (PCR), and inserting it into bacteriophages that are used to infect Escherichia coli. The E coli generate a cell library that can be more easily screened for ideal candidate antibodies (Liu 2014).
Ideally, mAbs intended for clinical practice should be easily mass‐produced, show high potency (high antigen binding activity), be stable (prolonged shelf‐life), have a long half‐life, and should not elicit a strong immune response (Marovich 2020). Immunogenicity varies by drug dosage, route of administration, possible contamination, structural features, and humanness of the mAbs, as well as by patient characteristics, such as age, genetic background, and related diseases (Lu 2020b). The strategy to target a single epitope can, depending on the specific target, be utilised both for halting disease progression and as temporary prophylaxis for groups with increased antigen exposure, such as healthcare workers, or those at increased risk of severe disease and mortality (Marston 2018). This may also interrupt the chain of infections. A mixture of mAbs, 'mAbs cocktails', may be more efficient for some antigens, so that they do not escape or mutate.
Although the majority of mAbs are licensed and used to treat chronic diseases, mAbs have also been used to treat infectious diseases, such as Zaire Ebola virus (Antibody Society 2020), respiratory syncytial virus, anthrax and Clostridioides difficile, which highlights the great potential of mAbs for the treatment of COVID‐19 (Marovich 2020).
How the intervention might work
SARS‐CoV‐2 stems from the coronavirus family, characterised by a positive‐sense, single‐stranded RNA (ribonucleic acid; Lu 2020a). The spike proteins on its envelope, which give the virus its name, play a critical role in enabling it to enter a host cell by two mechanisms (Hoffmann 2020; Ou 2020). The human angiotensin‐converting enzyme 2 (ACE2) receptor on the spike protein binds to ACE2 proteins that are found throughout the body, but with higher expression in respiratory epithelial cells, type I and II alveolar cells in the lungs, oral cavity, kidneys, testes and intestines (Tolouian 2020). This activates the S proteins' fusion machinery, which inserts into the cellular plasma membrane, brings the viral membrane into proximity and fuses them to create a portal to deposit the virus RNA genome into the host cell, where it starts replicating (Glaunsinger 2020; Tolouian 2020).
Although the exact cellular mechanisms are yet to be uncovered, it is hypothesized that the ACE2 protein plays additional roles in COVID‐19 disease severity, especially lung injury. Excessive Inflammation (the so‐called 'cytokine storm' seen in some cases), especially interleukin 1 and 6 (IL‐1, IL‐6), activates the kallikrein‐kinin system, which in several steps produces des‐Arg9‐BK (DABK). DABK is a ligand that binds to the bradykinin 1 receptor (B1R), responsible for controlling vascular permeability. Usually, ACE2 proteins play the role of guardians of B1R activity by counteracting DABK. Due to internalisation of ACE2 by SARS‐CoV‐2, they are unable to fulfill their role. In addition, inflammation increases the expression of B1Rs. Both overstimulation and overexpression of B1Rs may lead to increased plasma leakage, which in turn activates more plasma kallikrein–kinin, creating a loop to facilitate local angioedema in the lung, and is potentially responsible for acute respiratory distress syndrome (ARDS) (Van de Veerdonk 2020a; Van de Veerdonk 2020b; Buszko 2020).
Due to the importance of this pathway in viral replication and symptom severity, the focus of current research on SARS‐CoV‐2‐neutralising monoclonal antibodies is on blocking the binding of SARS‐CoV‐2 to the ACE2 receptor on human cells by targeting the receptor‐binding domain on the spike protein of the virus (Marovich 2020). The first COVID‐19‐specific mAb, bamlanivimab, has been tested in humans since June 2020 (Eli Lilly and Co 2020; NCT04427501). Interim results show a possible lower symptom burden and fewer hospitalisations, with a favourable safety profile in the mAbs group as compared with placebo (Chen 2020).
Studies have examined the effect of previously established immune‐modulatory mAbs, such as: IL‐6 and IL‐1 receptor antagonists (tocilizumab, sarilumab; canakinumab, RPH‐104; Catanzaro 2020), and kallikrein‐targeting mAbs (e.g. lanadelumab), in controlling the cytokine storm. In this review, we focus on SARS‐CoV‐2‐neutralising mAbs only. As of 10 November 2020, at least 120 SARS‐COV‐2‐neutralising mAbs targeting the spike protein are in discovery, development or testing phases, and 16 are in clinical trial phases (Chinese Antibody Society 2020; Yang 2020). Many questions, besides their potential clinical effectiveness and most favourable timing of administration, remain to be addressed. For example: their adverse events, the duration of protection, and possible mutations of the virus that lead to antibody resistance (for example, cluster 5 linked to mink farming, with previously unseen mutations; WHO 2020d). Changes to the virus may mean the viruses are no longer neutralised or cleared by the mAb, and may instead lead to increased severity of the infection, termed 'antibody‐dependent enhancement (ADE)' (Lee 2020).
Why it is important to do this review
The drastically increasing numbers of COVID‐19 cases worldwide and the enduring scarcity of treatment options threatens to burden health systems and increase mortality. Many ongoing studies are investigating pharmacological treatment options, but up to now only a few options have shown an effect. With the exception of corticosteroids in people on ventilation (Lamontagne 2020; WHO 2020e) and remdesivir (Beigel 2020; NIH 2020), supportive care remains the only current treatment option, in addition to oxygen therapy in mild cases and invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) in severe cases (NIH 2020). Multiple vaccines against SARS‐CoV‐2 have been approved in several countries. For example: BNT162b2 by Pfizer‐BioNTech, mRNA‐1273 by Moderna and Janssen, COVID‐19 Vaccine by Johnson&Johnson (FDA 2021a), AZD1222 by AstraZeneca (AstraZeneca 2020), CoronaVac by Sinovac (WHO 2021a). However, their distribution takes time and not everyone may be willing to be vaccinated. In addition, vaccines may not work equally well for everyone. Immunocompromised individuals, for example, may show a lower immune response to the vaccine (Monin‐Aldama 2021) and once infected, may be unable to clear the virus, which can lead to long bouts of illness and forms a source for the emergence of new variants (Choi 2020; Kemp 2021). In unvaccinated nosocomial infections, passive immunisation with SARS‐CoV‐neutralising mAbs may be a possibility to prevent a severe disease course.
Since November 2020, the emergence of several specific variants that have evolved independently from one another has caused concerns (WHO 2020f). Variant B.1.1.7 (also known as 20I/501Y.V1 or VOC 202012/01, now called alpha) was first identified in the UK and spread rapidly throughout the UK, becoming the dominant variant (Volz 2020). It has now spread globally with 114 countries (Volz 2020). B.1.1.7 is associated with increased transmissibility, and early evidence suggests an increase in disease severity (Muik 2021; Tang 2020). Another variant, B.1.351 (also known as 20H/501Y.V2 and now called beta), was initially identified in South Africa. It may also be more transmissible, and has shown resistance to antibody neutralisation (Zhou 2021) and some vaccine resistance (Madhi 2021). Variant P.1 (now called gamma), first identified in Brazil, might reduce antibody neutralisation (Wang 2021). Early in vitro findings suggest that bamlanivimab may efficiently neutralise the lineage B.1.1.7. However, no neutralising effect could be detected against variant B.1.351 (Widera 2021). Variant B.1.617, first found in India and so far categorised by the WHO as a variant of interest, consists of double mutations on the spike protein of the virus. These mutations may decrease binding to mAbs and their neutralisation capability (Cherian 2021; WHO 2021b). Monoclonal antibody cocktails or broadly acting SARS‐CoV‐2 mAbs such as VIR‐7831 (Starr 2021) may be developed to avoid virus escape.
The urgent need to evaluate therapies with a theoretical mechanism of action against SARS‐CoV‐2 persists due to the limited range of therapeutic options once infected, and the prevalence of individuals who may show an inhibited response to vaccines (e.g. when undergoing chemotherapy) . mAbs are one such option. We will conduct this systematic review as a living evidence synthesis to identify, track, evaluate and update information quickly on research efforts regarding SARS‐CoV‐2‐neutralising mAbs. We will include information on SARS‐CoV‐2‐neutralising mAbs in the early stages of clinical development, as well as providing information on the effectiveness and safety of these mAbs from randomised controlled trials (RCTs) to gain a complete picture of the field. Extensive work in the field of systematic reviews for interventions for COVID‐19 has already been undertaken. This work has included immunomodulatory mAbs. There has been one Cochrane Review concerning interleukin‐6 agonists (Ghosn 2021) and a non‐Cochrane review that aimed to summarise all studies on immunotherapies (e.g. Mansourabadi 2020)).
Various relevant projects may feed into the current undertaking. A research initiative supported by the World Health Organization (WHO) and Cochrane is mapping and evaluating all RCTs for COVID‐19, including studies on mAbs (WHO/Cochrane 2020). Another interesting resource is the COVID‐19 antibody therapeutics tracker (Yang 2020). This is a collaboration between the Chinese Antibody Society and the Antibody Society that includes mAb studies in different phases (Antibody Society 2020; Chinese Antibody Society 2020). In addition, the Australian clinical guideline is continually being updated for emerging treatments and already includes recommendations on bamlanivimab and casirivimab plus imdevimab (National COVID‐19 Clinical Evidence Taskforce 2020).
The current systematic review will fill gaps by identifying, describing, evaluating, and (if available RCTs are identified), meta‐analysing all evidence for SARS‐CoV‐2‐neutralising mAbs. It will provide a frequently updated summary of the evidence on mAb development.
Objectives
To assess the effectiveness and safety of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐neutralising monoclonal antibodies (mAbs) for treating patients with COVID‐19 compared to an active comparator, placebo, or no intervention. To maintain the currency of the evidence, we will use a living systematic review approach.
A secondary objective is to track newly developed SARS‐CoV‐2‐targeting mAbs from first tests in humans onwards.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) because this study design provides the best evidence for experimental therapies in highly‐controlled therapeutic settings. For RCT data, we used the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a). We had planned to include non‐standard RCT designs, such as cluster‐randomised studies (methods as recommended in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b)) and cross‐over studies, but have not identified any. In cross‐over studies, we would have only considered results from the first period before cross‐over because COVID‐19 is not a chronic condition, and its exact course and long‐term effects are yet to be defined.
To track the development of specific mAbs targeting SARS‐CoV‐2, we included all prospectively registered studies, including non‐randomised studies of interventions (NRSIs) on SARS‐CoV‐2‐neutralising mAbs in humans. We excluded animal studies, pharmacokinetic studies, and in vitro studies.
We included the following formats if sufficient information was available on study design, characteristics of participants, interventions, and outcomes.
Full‐text publications.
Preprint articles.
Abstract publications.
Results published in study registries.
We included preprints and conference abstracts to have a complete overview of the ongoing research activity, especially for tracking newly emerging SARS‐CoV‐2‐neutralising antibodies. We did not apply any limitation with respect to the length of follow‐up. We screened platform trials and we are continually following these because they may add new treatment arms.
Types of participants
For RCTs, we included participants with a confirmed diagnosis of COVID‐19 (virus antigens or RNA detected). We did not exclude any studies based on age, gender, ethnicity, disease severity, or setting (in‐ or outpatients). We excluded studies that evaluate mAbs for treatment of other coronavirus diseases such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), or other viral diseases, such as influenza. If studies enrolled populations with mixed viral diseases, we had planned to only include them if study authors provided subgroup data for COVID‐19.
For tracking of emerging mAbs by listing non‐randomised studies, we also accepted studies in healthy individuals, which are often used for safety assessments in order to gain a complete picture of the current developments.
Types of interventions
We included the following interventions.
SARS‐CoV‐2‐neutralising mAbs.
'Antibody cocktails' that include SARS‐CoV‐2‐neutralising mAbs.
We included the following comparisons for studies with a control arm.
Any mAb therapy compared with a control intervention, for example, drug treatments (including, but not limited to hydroxychloroquine, remdesivir), standard or hyperimmune immunoglobulin, convalescent plasma, or others. Co‐interventions were allowed but must have been comparable between intervention groups.
Any mAb therapy compared with no treatment or placebo. Co‐interventions were allowed but must have been comparable between intervention groups.
We also included studies that compare several mAbs with each other and another treatment, placebo or no treatment, and we included studies that compare several doses of one type of mAb with another treatment, placebo or no treatment.
We excluded SARS‐CoV‐2‐neutralising mAbs used for prevention of COVID‐19 and we excluded mAbs that are not specifically designed to treat COVID‐19 (such as nivolumab, tocilizumab, canakinumab, etc.).
Types of outcome measures
We evaluated core outcomes based on the Core Outcome Measures in Effectiveness Trials (COMET) Initiative for COVID‐19 patients and additional outcomes (COMET 2020; Marshall 2020).
We stratified analyses by disease severity, thereby separating non‐hospitalised (asymptomatic or mild disease) and hospitalised individuals (moderate to severe disease) with COVID‐19 according to the WHO clinical progression scale (Marshall 2020; see Figure 1). At the later stage, reduction of viral replication may no longer be the main driver of the disease, instead, inflammation and coagulopathy may be more important (Marovich 2020).
1.
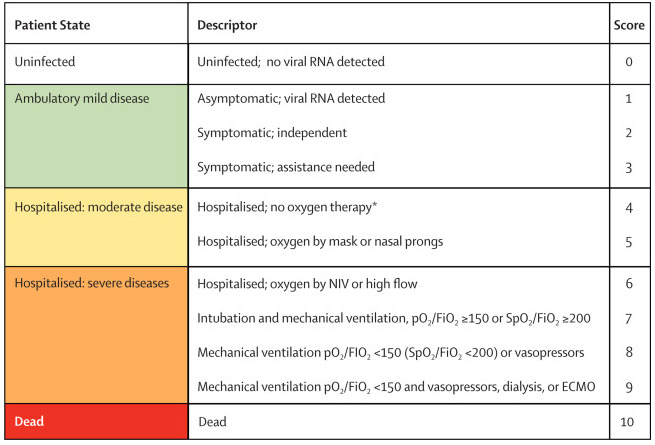
ECMO: extracorporeal membrane oxygenation; FiO2: fraction of inspired oxygen; NIV: non‐invasive ventilation; pO2: partial pressure of oxygen; RNA: ribonucleic acid; SpO2: oxygen saturation
aWHO Clinical Progression Scale from The Lancet Infectious diseases: Marshall 2020. Copyright © 2020 Elsevier Ltd. All rights reserved: reproduced with permission.
bIf hospitalised for isolation only, record status for ambulatory patient.
Timing of outcome measurement
In case of time‐to‐event analysis (e.g. for mortality, time to discharge from hospital, and time to improvement of symptoms), we included the outcome measure based on the longest follow‐up time. We also collected information on outcomes from all other time points reported in the publications.
We included adverse events occurring during active treatment, as well as long‐term adverse events. If sufficient data were available, we grouped the measurement time points of eligible outcomes. For example, we grouped adverse events and serious adverse events into those measured directly after treatment (up to seven days after treatment), medium‐term outcomes (up to 15 days after treatment), and longer‐term outcomes (more than 30 days after treatment).
Primary outcomes
Effectiveness of SARS‐CoV‐2‐neutralising mAbs
All‐cause mortality at up to 30 days
All‐cause mortality at up to 60 days
-
Clinical progression, improvement of symptoms, or development of severe symptoms according to the WHO scale
WHO Clinical Progression Scale (Marshall 2020), measured daily over the course of the study (Figure 1); or
-
assessed as individual items included in the Progression Scale (need for respiratory support, duration):
oxygen by mask or nasal prongs
oxygen by non‐invasive ventilation or high‐flow nasal cannula
intubation and mechanical ventilation
mechanical ventilation or vasopressors, high‐flow oxygen
mechanical ventilation and vasopressors, dialysis or extracorporeal membrane oxygenation (ECMO)
Quality of life, including fatigue, assessed with standardised scales, for example, WHOQOL‐100, at up to seven days; up to 30 days, and longest follow‐up available
Admission to hospital or death for non‐hospitalised and hospital discharge and alive for hospitalised participants
Safety outcomes
Number of participants with adverse events (all grades, grades 1 to 2, grades 3 to 4)
Number of participants with serious adverse events
Secondary outcomes
Length of hospital stay (for those admitted to hospital)
Admission to intensive care unit (ICU)
Length of ICU stay
Viral clearance, assessed with reverse transcription‐polymerase chain reaction (RT‐PCR) test for SARS‐CoV‐2 at baseline, up to three, seven, and 15 days
Time to sustained recovery (for hospitalised participants)
Neurologic dysfunction (for hospitalised participants)
Thromboembolic events
Renal failure
Search methods for identification of studies
We carried out weekly searches for completed and ongoing studies in all languages to limit language bias. We conducted weekly checks for newly emerging mAbs and changing terminology regarding mAbs included in the search strategy. We adapted the strategy where necessary.
Electronic searches
For the identification of studies on the effectiveness and safety of SARS‐CoV‐2‐neutralising mAbs, we designed search strategies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2021). Information Specialist Ina Monsef developed the search strategy based on input by clinicians. It was reviewed by Information Specialist Carolyn Dorée. Due to the international urgency for research on therapies for COVID‐19, we assumed that the abstracts of clinical studies would have been published in English. If the full‐text publication was published in a language that lay outside the abilities of our team (English, German, Dutch, French, Italian, Malay, and Spanish), we planned to involve Cochrane TaskExchange to identify people who are able to translate (taskexchange.cochrane.org). We restricted the database search to records added since 1 January 2020, as the first studies on COVID‐19 were registered on 23 January 2020 (Zhu 2020). We searched the following databases up to 17 June 2021.
MEDLINE (via Ovid; 1 January 2020 to 17 June 2021; Appendix 1)
Embase (via Ovid; 1 January 2020 to 17 June 2021; Appendix 2)
-
Cochrane COVID‐19 Study Register (covid-19.cochrane.org; inception to 17 June 2021; Appendix 3) including:
PubMed, daily updates
Embase.com, weekly updates
ClinicalTrials.gov (www.clinicaltrials.gov), daily updates
World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch), weekly updates
medRxiv (www.medrxiv.org), weekly updates
Cochrane Central Register of Controlled Trials (CENTRAL), monthly updates
PubMed (for epublications ahead of print only; 1 January 2020 to 17 June 2021; Appendix 4)
Epistemonikos COVID‐19 L*VE Platform (app.iloveevidence.com/loves; inception to 17 June 2021; Appendix 5)
World Health Organization COVID‐19 Global literature on coronavirus disease (bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov; inception to 17 June 2021; Appendix 6)
To track ongoing research efforts and address the potential influence of publication bias on our conclusions, we searched relevant study registries to find ongoing and completed, but not yet published studies. If results were uploaded into the study registry that had not yet been published elsewhere, we integrated these data for the current review and will add or replace data in future updates of this review in case of publication.
To identify prospectively registered platform trials that may add a mAb arm during the course of the study (such as the RECOVERY trial (RECOVERY 2020) or the REMAP‐CAP study(REMAP‐CAP 2020)), we conducted a separate search with monthly updates in the Cochrane COVID‐19 Study Register (Appendix 7). We listed the eligible studies in the section named Studies awaiting classification and regularly checked whether they had added additional treatment arms that include mAbs.
Between submission and publication, we conducted a shortened RCT‐only search up to 30 July 2021 (Appendix 8).
Searching other resources
-
We checked the reference lists of:
all identified studies;
relevant review articles; and
current treatment guidelines for further literature.
We conducted forwards citation searching on the included studies via Google Scholar.
We contacted experts in the field, drug manufacturers, and regulatory agencies in order to retrieve information on unpublished studies.
-
We compared our results with results from projects that aim to track COVID‐19 intervention research, such as:
covid-nma.com/dataviz;
chineseantibody.org/covid-19-track.
Data collection and analysis
Selection of studies
Two review authors (NK, CH, KLC, ZK, ET, MP, NS) independently screened search results for eligibility by reading the abstracts using the Covidence software (Covidence). Following the living review approach, we screened any new citations retrieved by the weekly searches immediately. Abstracts that both review authors found eligible, or abstracts that they disagreed upon or rated as uncertain, were obtained as full‐text publications for further discussion. Two review authors assessed the full‐text articles of selected studies. If the two review authors were unable to reach consensus, we consulted all review authors who were involved in study selection to reach a final decision.
The search for platform trials was conducted every two months (from November 2020 to July 2021). To identify potential platform trials, two review authors (NK, NS, CH) independently screened the results in Endnote X9.
We documented the study selection process in a flow chart, as recommended in the PRISMA statement (Moher 2009), and showed the total numbers of retrieved references and the numbers of included and excluded studies. We listed all studies that we excluded after full‐text assessment and the reasons for their exclusion in the Characteristics of excluded studies section.
Data extraction and management
We treated RCTs differently from non‐RCTs. For RCTs, we conducted data extraction according to the guidelines proposed by Cochrane (Li 2020). Two review authors (NK, MP, CH) independently extracted data using a customised data extraction form developed in Microsoft Excel (Microsoft 2018). We resolved disagreements by discussion. If no agreement was obtained, we involved a third review author to resolve the disagreement.
We extracted the following information if reported.
General information: author, title, source, publication date, country, language, duplicate publications.
Study characteristics: study design, setting and dates, source of participants, inclusion/exclusion criteria, treatment cross‐overs, compliance with assigned treatment, length of follow‐up.
Participant characteristics: age, gender, ethnicity, number of participants recruited/allocated/evaluated, additional diagnoses, the severity of disease, previous treatments, concurrent treatments, complementary medicine (e.g. quercetin, elderberry, zinc).
Interventions: type of mAbs, the target of mAbs, dose, frequency, timing, duration and route of administration, setting (e.g. inpatient, ambulant), duration of follow‐up.
For RCT studies: control intervention, dose, frequency, timing, duration and route of administration, setting, duration of follow‐up.
Outcomes: as specified under Types of outcome measures.
'Risk of bias' assessment: study design, confounding, the definition of risk estimates, selection bias, attrition bias, detection bias, reporting bias.
We included non‐RCTs for tracking of emerging mAbs from first‐in‐human studies onwards, without the aim to meta‐analyse, and extracted characteristics as outlined in Table 8.
1. Main study characteristics of included RCTs.
| Study | Intervention | Population | Country | N randomised up to data cut‐off | Status |
| ACTIV‐3 | Bamlanivimab (7.0 g) | Inpatients, moderate disease | USA, Denmark, Singapore, Spain, India, Poland, Switzerland, UK | 314 | Interim analysis, bamlanivimab arm stopped for futility, recruitment ongoing for other arms |
| BLAZE‐1 (phase 2) | Bamlanivimab (0.7 g, 2.8 g, 7.0 g) bamlanivimab/etesevimab (2.8 g + 2.8 g) |
Outpatients, mild disease | USA, Puerto Rico (added after publication of interim results) | 592 | Recruiting, estimated enrolment 4000 participants Estimated study completion: May 2021 |
| Weinreich (phase 1/2) | Casirivimab/imdevimab (REGN10933/ REGN10987; 2.4 g, 8 g) | Outpatients, mild disease | USA, Chile, Mexico, Romania | 275 | Interim analysis, recruiting, estimated enrolment 6420 participants Estimated study completion: August 2021 |
| Weinreich (phase 3) | Casirivimab/imdevimab (REGN10933/ REGN10987; 1200 mg, 2400 mg, ) | Outpatients, mild disease | USA, Mexico, Chile, Romania | 592 | Interim analysis, recruiting, estimated enrolment 6420 participants Estimated study completion: August 2021 |
| RECOVERY | Casivirimab/imdevimab (4g, 4g) | Hospitalised patients, moderate or severe disease | UK | 9185 | Preprint publication, recruiting, estimated enrolment 40000 participants, estimated study completion: December 2031 |
| COMET‐ICE | Sotrovimab (VIR‐7831; GSK4182136; 500 mg) | Outpatients, mild disease | USA, Brazil, Canada, Spain | 583 | Preprint publication, interim analysis, data collection ongoing; Estimated completion date: July 2021 |
| Eom 2021 | Regdanvimab (CT‐P59; 40 mg/kg, 80 mg/kg) | Outpatients, mild disease | South Korea, Romania, Spain and USA | 327 | recruiting, estimated enrolment: 1020 participants Estimated completion date: September 2021 |
Assessment of risk of bias in included studies
We assessed the risk of bias in RCTs by using the Risk of Bias 2.0 (RoB 2) tool (Sterne 2019), for the effect of the assignment to the intervention (the intention‐to‐treat (ITT) effect). The outcomes that we assessed were those specified for inclusion in the summary of findings tables.
Two review authors (NK, CH, KLC, MP, ET, ZK, MN) independently assessed the risk of bias for each outcome. In case of discrepancies among judgments and inability to reach consensus, we consulted a third review author to reach a final decision. We assessed the following types of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021c).
Bias arising from the randomisation process
Bias due to deviations from the intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
For cross‐over studies, we had planned to use the RoB 2 tool, as outlined in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b), because we would have only considered results from the first period before cross‐over.
For cluster‐RCTs, we had planned to add a domain to assess bias arising from the timing of identification and recruitment of participants in relation to the timing of randomisation as recommended in the RoB 2 guidance for cluster‐randomised studies (Eldridge 2021) and in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b).
To address these types of bias, we used the signalling questions recommended in RoB 2 to reach a judgment using the following options.
'Yes': if there is firm evidence that the question is fulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
Probably yes': a judgment has been made that the question is fulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'No': if there is firm evidence that the question is unfulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'Probably no': a judgment has been made that the question is unfulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'No information': if the study report does not provide sufficient information to allow any judgment.
We used the algorithms proposed by RoB 2 to assign each domain one of the following levels of bias.
Low risk of bias.
Some concerns.
High risk of bias.
Subsequently, we derived an overall risk of bias rating for each prespecified outcome in each study in accordance with the following suggestions.
'Low risk of bias': we judge the study to be at low risk of bias for all domains for this result.
'Some concerns': we judge the study to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain.
'High risk of bias': we judge the study to be at high risk of bias in at least one domain for the result or we judge the trial to have some concerns for multiple domains in a way that substantially lowers confidence in the results.
We used the RoB 2 Excel tool to implement RoB 2 (available from riskofbias.info) and stored and presented our detailed RoB 2 assessments as supplementary online material (available at: zenodo.org/record/4746642#.YJl0OLUzY2w).
Measures of treatment effect
For continuous outcomes, we planned to record the mean, standard deviation, and total number of participants in both treatment and control groups. Where continuous outcomes used the same scale, we performed analyses using the mean difference (MD) with 95% confidence intervals (CIs). For continuous outcomes measured with different scales, we had planned to perform analyses using the standardised mean difference (SMD). For interpreting SMDs, we would have re‐expressed SMDs in the original units of a particular scale with the most clinical relevance and impact (e.g. clinical symptoms with the WHO Clinical Progression Scale (Marshall 2020)).
For dichotomous outcomes, we recorded the number of events and participants in both treatment and control groups to obtain the pooled risk ratio (RR) with a 95% CI (Deeks 2021). We had planned to use Peto odds ratio (OR) if the number of observed events was small (less than 5% of sample per group, Deeks 2021). However, because there was very little difference in the effect estimate whether RR or Peto OR was used, for consistency and interpretability we decided to report RRs.
If available, we extracted and reported hazard ratios (HRs) for time‐to‐event outcomes (e.g. time to death). If HRs were not available, we would have made every effort to estimate the HR as accurately as possible from available data using the methods proposed by Parmar and Tierney (Parmar 1998; Tierney 2007). If sufficient studies provided HRs, we would have used HRs rather than RRs or MDs in a meta‐analysis, as they provide more information.
Unit of analysis issues
The aim of this review was to summarise studies that analyse data at the level of the individual. We would have accepted cluster‐randomised studies for inclusion. We collated multiple reports of one study so that the study, and not the report, was the unit of analysis.
Studies with multiple treatment groups
As recommended in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a), for studies with multiple treatment groups of the same intervention (i.e. dose, route of administration), we evaluated whether study arms were sufficiently homogeneous to be combined. When arms could not be pooled, we compared each arm with the common comparator separately. For pairwise meta‐analyses, we had planned to split the ‘shared’ group into two or more groups depending on the number of treatment arms and included two or more (reasonably independent) comparisons. For this purpose, for dichotomous outcomes, both the number of events and the total number of participants would have been divided, and for continuous outcomes, the total number of participants would have been divided with unchanged means and standard deviations (SDs).
Dealing with missing data
Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions suggests a number of potential sources for missing data, which we have taken into account: at study level, at outcome level, and at summary data level (Deeks 2021). At all levels, it is important to differentiate between data 'missing at random', which may often be unbiased, and 'not missing at random', which may bias the study and consequently, the review results.
We contacted three principal investigators from included RCTs (ACTIV‐3; BLAZE‐1 (phase 2); Weinreich (phase 1/2)) and requested data for our prioritised outcomes. We received two responses, from ACTIV‐3 and Weinreich (phase 1/2) that they would be unable to provide the requested information, but both stated that a more complete outcome set will be reported in follow‐up publications. The authors of BLAZE‐1 (phase 2) did not respond. As no additional data were provided, we explicitly decided that data were missing at random.
Assessment of heterogeneity
We planned to assess the heterogeneity of treatment effects between studies using a Chi² test with a significance level at P < 0.1. We would have used the I² statistic (Higgins 2003), to quantify possible heterogeneity (I² statistic between 30% and 60% may signify moderate heterogeneity, I² statistic between 50% and 90% may signify substantial heterogeneity, and I² > 75% may signify considerable heterogeneity; Deeks 2021). If we considered heterogeneity to be substantial (I² > 50%), we had planned to explore potential causes through sensitivity and subgroup analyses. If we could not find a reason for heterogeneity, we would not have performed a meta‐analysis but had planned to comment on results from all studies and presented these in tables. As we did not perform meta‐analyses, we could not assess heterogeneity.
Assessment of reporting biases
As mentioned above, we searched study registries to identify completed trials that have not been published elsewhere to determine publication bias.
We intended to explore potential publication bias by generating a funnel plot and statistically testing this by conducting a linear regression test for meta‐analyses involving at least 10 studies (Page 2021). We would have considered P < 0.1 as significant for this test. As we identified fewer than 10 studies per comparison and did not pool the data in meta‐analysis, and we did not generate a funnel plot.
Data synthesis
If the clinical and methodological characteristics of individual studies had been sufficiently homogeneous, we would have pooled the data in meta‐analysis. However, th studies originate from different convalescents who were infected in different countries (e.g. China, USA) and at different time points. Although most of the included mAbs target the spike protein of the virus, they all target different epitopes on the spike protein. One mAb, sotrovimab, was derived from a SARS patient. Therefore, the affinity and stability might be very different from monoclonal antibody to monoclonal antibody. Due to the possibility of antibody‐dependent enhancement (Arvin 2020), which may be dose‐dependent, we had decided at the beginning of the review process that for the current version, we would not pool different doses of mAbs. We may change this for future updates if doses seem to be sufficiently homogeneous. Based on these decisions, we reported results separately per substance and substance combination. We did not perform a meta‐analysis, because we had only one study per comparison. We commented on the results narratively, with the results from all studies presented in forest plots to prepare for future updates. We used Review Manager Web (RevMan Web 2020) software to create forest plots (RevMan Web 2020). One review author entered the data into the software, and a second review author checked the data for accuracy.
If meta‐analysis had been possible, we would have used the random‐effects model for all analyses as we anticipated that true effects are related but are not the same for included studies. We planned to treat placebo and no treatment, which both usually include standard of care at different institutions and time points, as the same intervention. We would have performed analyses according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). We planned to analyse studies including different disease stages separately, grouping them into mild, moderate, severe, and critical. However, for this review, we decided to follow the classification of the WHO Progression Scale (Marshall 2020; Figure 1) and group into mild versus moderate and severe disease (outpatients with mild disease; and inpatients who are classified with moderate or severe disease according to the WHO Progression Scale). We would have assessed the effects of potential biases in sensitivity analyses (see Sensitivity analysis). For binary outcomes, we would have based our estimation on the between‐study variance on the calculation performed using the Mantel‐Haenszel method. We used the inverse variance method for continuous outcomes, outcomes that included data from cluster‐RCTs, or outcomes where HRs were available. We planned to explore heterogeneity where the I² statistic was more than 50% with subgroup analyses. If we could not find a cause for the heterogeneity, we planned to comment on the results as a narrative with the results from all studies presented in tables, instead of performing a meta‐analysis.
We presented non‐randomised, prospectively registered studies narratively in table form (see Appendix 9).
Subgroup analysis and investigation of heterogeneity
To explore heterogeneity, we planned to perform subgroup analyses of the following characteristics. The limited number of RCTs that provided results and the variation of SARS‐CoV‐2‐neutralising mAbs used across the studies prevented us from performing any pre‐planned subgroup analyses.
Age of participants (divided into applicable age groups, e.g. children; 18 to 65 years, 65 years and older).
Pre‐existing conditions (diabetes, respiratory disease, hypertension, immunosuppression).
Timing of first dose administration since symptom onset (up to three days, four to seven days and more than seven days).
Antibodies detected at baseline.
We had planned to use the tests for interaction to test for differences between subgroup results.
We could not perform subgroup analysis, because we identified only one study per comparison.
Sensitivity analysis
We had planned to perform sensitivity analysis of the following characteristics for our primary outcomes.
'Risk of bias' assessment components (studies with a low risk of bias or some concerns versus studies with a high risk of bias).
Comparison of preprints of COVID‐19 interventions versus peer‐reviewed articles.
Comparison of premature termination of studies with completed studies.
We could not perform sensitivity analysis, because we identified only one study per comparison.
Summary of findings and assessment of the certainty of the evidence
Summary of findings tables
We created summary of findings tables and evaluated GRADE for interventions evaluated in RCTs using the GRADEpro GDT software, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021). We created a separate table per mAb type.
For time‐to‐event outcomes, we calculated absolute effects at specific time points, as recommended in the GRADE guidance 27 (Skoetz 2020).
According to Chapter 14 of the updated Cochrane Handbook for Systematic Reviews of Interventions, the “most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes” should be included in the summary of findings table(s) (Schünemann 2021). We included outcomes prioritised according to the Core Outcome Set for intervention studies (COMET 2020) and patient‐relevant outcomes.
Non‐hospitalised individuals with COVID‐19 and asymptomatic or mild disease
All‐cause mortality (up to 30 and 60 days)
Clinical progression, improvement of symptoms, or development of severe symptoms according to the WHO Clinical Progression Scale (Marshall 2020)
Quality of life, assessed with standardised scales (e.g. WHOQOL‐100), at up to seven days, up to 30 days, and longest follow‐up available
Admission to hospital
Adverse events (all grades, grades 1 to 2, grades 3 to 4)
Serious adverse events
Hospitalised individuals with COVID‐19 and moderate to severe disease
All‐cause mortality (up to 30 and 60 days)
Clinical progression, improvement of symptoms or development of severe symptoms according to the WHO Clinical Progression Scale (Marshall 2020)
Quality of life, assessed with standardised scales (e.g. WHOQOL‐100) at up to seven days, up to 30 days, and longest follow‐up available
Hospital discharge
Adverse events (all grades, grades 1 to 2, grades 3 to 4)
Serious adverse events
Assessment of the certainty in the evidence
We used the GRADE approach to assess the certainty in the evidence for the outcomes listed in the previous section.
The GRADE approach uses five domains (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty in the body of evidence for each prioritised outcome. We followed the current GRADE guidance for these assessments in their entirety as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 14 (Schünemann 2021).
We used the overall 'Risk of bias' judgment, derived from the RoB 2 tool, to inform our decision on downgrading for risk of bias. We phrased the findings and certainty in the evidence as suggested in the informative statement guidance (Santesso 2020).
Methods for future updates
Living systematic review considerations
Our information specialist (IM) will provide us with new records each week, which two review authors will screen, extract, evaluate, and integrate following the guidance for Cochrane living systematic reviews (Cochrane LSR). We will run searches for platform trials monthly, and manually check platform trials that were previously identified and listed as 'studies awaiting classification' for additional treatment arms.
We will wait until the accumulating evidence changes one or more of the following components of the review before republishing the review.
The findings of one or more prioritised outcomes.
The credibility (e.g. GRADE rating) of one or more prioritised outcomes.
New settings, populations, interventions, comparisons, or outcomes studied.
We will review the review scope and methods approximately monthly, or more frequently if appropriate, in light of potential changes in COVID‐19 research (for example, when additional comparisons, interventions, subgroups or outcomes, or new review methods become available).
Results
Description of studies
Results of the search
We searched all databases and screened the resulting records weekly up to 17 June 2021, and then undertook an RCT‐only top‐up search up to 30 July 2021. Based on newly developing SARS‐CoV‐2‐specific mAbs, we have added terms to our search strategy; see Differences between protocol and review for the ongoing changes that are still continually being implemented. Our searches retrieved 14,408 records for the mAbs‐specific searches. After removing duplicates, we screened 11,516 records based on their titles and abstracts. We excluded 11,327 records that did not meet the prespecified inclusion criteria. Of the remaining 189 records, we included 95 records (of which 28 are listed for tracking; see Appendix 9).
6 RCTs (in 28 records) are included in this review, of which three are published as preprint only and one as journal publication with additional data from two preprints.
31 RCTs (in 39 records) on 18 different mAbs or mAb combinations are currently ongoing.
Our platform trial search yielded 2241 records. After removing 161 duplicates, we screened 2080 during title and abstract screening and looked at 138 registry records in more detail. Of these, we excluded 20 studies (27 records). We categorised the remaining studies as follows:
2 platform trials (29 attached records) are included (already identified by mAbs‐specific search), one of these has added new treatment arms and is thus also listed as an ongoing study;
4 platform trials (13 records) with at least one mAb as an experimental treatment are ongoing (already identified by mAbs‐specific search);
30 platform trials (69 records) that may potentially add a mAb during the course of the study are ongoing.
The study flow diagram in Figure 2 illustrates the study selection process according to PRISMA guidelines (Moher 2009).
2.

PRISMA flow diagram
Update search between submission and peer review
To keep our review as updated as possible, we conducted an additional search for RCTs in infected individuals only (18 June to 30 July 2021, search strategy: Appendix 8). From the 286 records, we assessed 13 full‐text articles (Figure 3); of these, eight were press releases and one an ongoing RCT in healthy participants. We included the following four studies:
3.

PRISMA flow diagram RCT search between 18 June and 30 July 2021
one ongoing RCT (NCT04952805);
one preprint from a not yet included study (O’Brien 2021);
two publications with updated results (ACTIV‐3; BLAZE‐1 (phase 3)).
We integrated the data from the already included studies with updated results. Results from O’Brien 2021 will be integrated into the next version of this review, and we have listed the study under Characteristics of studies awaiting classification.
Included studies
Randomised controlled trials in individuals with COVID‐19
Design and sample size
We included six randomised controlled trials (RCTs) according to our inclusion criteria, involving 17,495 randomised participants (ACTIV‐3; BLAZE‐1 (phase 2); COMET‐ICE; Eom 2021; RECOVERY; Weinreich (phase 1/2); Weinreich (phase 3)). Three studies were published as preprint only (COMET‐ICE; Eom 2021; RECOVERY), one study was published in three full‐text publications, one conference abstract (BLAZE‐1 (phase 2); BLAZE‐1 (phase 3)), another study was published as a journal article with additional data from one preprint (ACTIV‐3), and one study was published as a journal article with additional data from two preprints (Weinreich (phase 1/2); Weinreich (phase 3)).
All six included RCTs are still active or ongoing due to different reasons, e.g. follow‐up of participants (COMET‐ICE), addition of study arms with mAbs (ACTIV‐3; BLAZE‐1 (phase 3); RECOVERY), or part 2 or 3 of the study recruiting (Eom 2021 (80 mg/kg), Weinreich (phase 3)). The estimated study completion dates ranged from July 2021 to December 2031. All but one study (RECOVERY), were blinded. BLAZE‐1 (phase 2) and BLAZE‐1 (phase 3) are part of a phase 2 to 3 study currently recruiting participants with completion planned in June 2022 with an estimated number of 3160 participants. Similarly, Eom 2021 is a two‐part phase 2 to 3 study, currently recruiting participants into part 2 of the study with completion planned in September 2021 with 1020 participants. Results of a preplanned interim analysis of part 1 of the study were published in a preprint (Eom 2021). COMET‐ICE was a phase 2 to 3 study with an estimated completion date in July 2021. Recruitment was stopped on 10 March 2021 due to profound efficacy after 1057 participants had been randomised. There were separate data cut‐offs for efficacy and safety for the presented interim analysis. Weinreich (phase 1/2) and Weinreich (phase 3) was a continually enrolling phase 1 to 3 study currently recruiting new participants for phase 3. Phase 1 to 2 was completed and results were reported separately (Weinreich (phase 1/2)). Based on results from phase 1 to 2, the trial was amended in November 2020 and only participants with at least one risk factor for severe COVID‐19 were included and no longer randomised to 8.0 g casirivimab and imdevimab. In February 2021, participants were no longer randomised to placebo. Weinreich (phase 3) comprised three cohorts (cohort 1: ≥ 18 years, cohort 2: < 18 years, cohort 3: pregnant at randomisation), but results were reported for cohort 1 only. Completion for phase 3 is planned for November 2021 with 6420 participants.
The studies ACTIV‐3 and RECOVERY were platform trials with an adaptive design that allows adding and dropping experimental drugs during the course of the study. In ACTIV‐3, the bamlanivimab arm was stopped after interim analysis for futility. This study has added additional SARS‐CoV‐2‐neutralising mAb arms, which are further described in the section on ongoing studies. The estimated completion date is July 2022 with 10,000 participants. RECOVERY is an open‐label study with a planned completion date in December 2031 and an estimated enrolment of 40,000 participants. So far it has only one SARS‐CoV‐2 specific mAb treatment arm. The factorial design allowed randomisation of a single participant into between zero and four treatment arms based on predefined groups of treatment (i.e., convalescent plasma and casirivimab/imdevimab were in the same group, therefore mutually exclusive). An overview of included studies can be found in Table 8. A more detailed description of the methods, eligibility criteria, interventions, and outcomes is provided in the Characteristics of included studies.
Setting and participants
All studies were multicentre trials. The RECOVERY study was conducted at different sites in the UK. The other studies were conducted at different global sites in the USA and Puerto Rico (this site was added after publication of the interim analysis; BLAZE‐1 (phase 3)), the USA, Chile, Mexico, and Romania (Weinreich (phase 1/2); Weinreich (phase 3)), the USA, South Korea, Romania, Spain (Eom 2021), the USA, Canada, Brazil, Spain (COMET‐ICE) and the USA, Denmark, and Singapore (ACTIV‐3; sites in India, Poland, Spain, Switzerland, and the UK joined after publication of interim results).
In five studies, a confirmed SARS‐CoV‐2 infection was necessary for inclusion (ACTIV‐3; BLAZE‐1 (phase 2); COMET‐ICE; Eom 2021; Weinreich (phase 1/2); Weinreich (phase 3)). RECOVERY included participants with confirmed or suspected SARS‐CoV‐2 infection. Positive polymerase chain reaction (PCR) rates at baseline ranged from 80% in Weinreich (phase 1/2) and ACTIV‐3 to more than 93% in Eom 2021; Weinreich (phase 3) and RECOVERY. No concerning mutations were identified in viral RNA sequences from 255 participants in ACTIV‐3. In six participants deletions in codon 69‐70 were detected. Genomes in all but one person with alpha variant contained the B strain.
Four studies included non‐hospitalised participants with clinical symptoms of mild disease according to the definition of the WHO Clinical Progression Scale (Figure 1; BLAZE‐1 (phase 2); COMET‐ICE; Eom 2021; Weinreich (phase 1/2); Weinreich (phase 3)). In BLAZE‐1 (phase 2), the median age ranged from 39 to 46 years between the treatment groups, and 56.4% of participants were female. In the phase 3 part (BLAZE‐1 (phase 3)), the mean age was 53.8 standard deviation ((SD) = 16.8), and 52% were female. In Eom 2021, the median age ranged from 51 to 52 years (interquartile range (IQR) 40 to 61 years), and 49.2% were female. In Weinreich (phase 1/2); Weinreich (phase 3) and COMET‐ICE, the median age of the participants was 42 years (IQR 30 to 53 years), 50 years (IQR 38 to 59 years), and 53 years (IQR 18 to 96 years), and 52.9%, 51.3% and 54% of participants were female. Risk factors for severe COVID‐19 progression were present in 60.5% in Weinreich (phase 1/2), 67% in BLAZE‐1 (phase 2) and 99.7% in COMET‐ICE. In Weinreich (phase 3) all participants had at least one risk factor for severe COVID‐19 and in Eom 2021 73.44% had comorbidities at baseline.
Two studies included moderately to severely ill, hospitalised participants (ACTIV‐3; RECOVERY). The median age of the participants in ACTIV‐3 was 61 years (IQR 49 to 71) and 44% of participants were female. Differences between the intervention and control groups in terms of co‐existing illnesses were observed in this study. More participants in the intervention group compared to the placebo group suffered from diabetes (33% versus 24%), renal impairment (15% versus 6%), and heart failure (7% versus 1%). At baseline, 27% did not require supplementary oxygen, 57% received oxygen (either < 4 litres/minute or ≥ 4 litres/minute), and 15% received oxygen by noninvasive ventilation (NIV) or high‐flow device. No participants required invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) at baseline. In RECOVERY the median age of the participants was 61.9 years (IQR 14.4 to 14.6) and 37% were female. Fifty‐three percent of the participants had previous diseases, including diabetes (26.3%), heart disease (21%), chronic lung disease (23%), tuberculosis (0.35%), HIV (0.47%), severe liver disease (1.42%) and severe kidney impairment (5.2%). At baseline, 6.6% of the participants did not receive oxygen, 61.3% received simple oxygen, 26.2% received oxygen by NIV and 6% received invasive mechanical ventilation (IMV).
Baseline serum antibody status was negative for about 70% of the participants in Weinreich (phase 3), 50% in Weinreich (phase 1/2), and 30% in RECOVERY. Eom 2021 reported that less than 6% of the participants were tested positive for immunoglobulin G (IgG) on day 1. In ACTIV‐3, 50% of the participants were tested positive for neutralising antibodies (nAb) and 59% had detectable anti‐nucleocapsid (N) antibodies.
Interventions
We included six RCTs (ACTIV‐3; BLAZE‐1 (phase 2); BLAZE‐1 (phase 3); COMET‐ICE; Eom 2021; RECOVERY; Weinreich (phase 1/2); Weinreich (phase 3)). These included 17,495 randomised participants: 486 participants were assigned to receive varying doses of bamlanivimab (0.7 g, 2.8 g, 7.0 g, ACTIV‐3; BLAZE‐1 (phase 2)), 632 participants were assigned to receive combination therapy of bamlanivimab and etesevimab (2.8 g each; BLAZE‐1 (phase 2); BLAZE‐1 (phase 3)), 3600 participants were assigned to receive a combination of casirivimab and imdevimab at different doses (1.2 g, 2.4 g, 8.0 g; Weinreich (phase 1/2); Weinreich (phase 3)), 430 participants were assigned to receive sotrovimab (COMET‐ICE), and 216 participants were assigned to receive regdanvimab (0.04 g/kg or 0.08 mg/kg, Eom 2021). A total of 3150 control group participants were assigned to receive placebo infusions. In RECOVERY 4839 participants were allocated to receive a combination of casirivimab and imdevimab (8.0 g). Ninety percent of participants with completed follow‐up at time of analysis received the combination and 4946 participants were allocated to receive standard of care. Less than 1% of participants with completed follow‐up at time of analysis received a combination of casirivimab and imdevimab (8.0 g). All included substances target the spike protein of SARS‐CoV‐2.
BLAZE‐1 (phase 2) randomised participants into five groups who received either bamlanivimab monotherapy at a dose of 0.7 g, 2.8 g, 7.0 g, combination therapy of bamlanivimab and etesevimab in equal doses of 2.8 g, or placebo IV. Additional arms with different doses of bamlanivimab/etesevimab have been added during the course of the study, but do not have published results yet. In BLAZE‐1 (phase 3), participants were treated with a combination of bamlanivimab and etesevimab in equal doses of 2.8 g, or placebo IV. Concomitant treatment was not reported in any phase.
In COMET‐ICE, participants were randomised to either 0.5 g sotrovimab (also known as VIR‐7831) or single dose of placebo IV. Concomitant treatment was not reported.
Eom 2021 randomised participants into three groups who were treated with regdanvimab (also known as CT‐P59) at a dose of 0.04 g/kg or 0.08 g/kg or a single dose of placebo IV. Concomitant medications were taken by more than 10% of the participants in the three study arms included analgesics, antibiotics, antithrombotic agents, agents acting on the renin‐angiotensin system, beta‐blocking agents, corticosteroids, cough and cold preparations, lipid‐modifying agents and vitamins.
Weinreich (phase 1/2) randomised participants into three groups who were treated with a combination of casirivimab and imdevimab (manufactured under the trade name REGN‐COV2) in equal doses, at a dose of 2.4 g or 8.0 g or a single dose of placebo IV. No concomitant treatment was reported. Similarly, in Weinreich (phase 3) participants were randomised to receive 1.2 g, 2.4 g or 8.0 g of casirivimab and imdevimab or placebo IV. Concomitant treatment was not reported.
In ACTIV‐3, participants were randomised to either 7.0 g bamlanivimab or a single dose of placebo IV. Pre‐ and concomitant treatments in both study arms included remdesivir and when indicated, antibiotic, antifungal, antiviral, angiotensin‐converting enzyme (ACE) inhibitor or angiotensin‐receptor blocker (ARB), antiplatelet/anticoagulant, immune‐modulating medication, or supplemental oxygen.
RECOVERY randomised participants into two groups to receive either a single dose casirivimab/imdevimab (8.0 g) or standard of care. Pre‐ and concomitant treatments in both study arms included corticosteroids, aspirin, colchicine, azithromycin, remdesivir, tocilizumab or sarilumab, hydroxychloroquine, lopinavir‐ritonavir.
Funding and conflicts of interest
Three studies were funded by pharmaceutical companies (BLAZE‐1 (phase 2); COMET‐ICE; Eom 2021). Weinreich (phase 1/2) and Weinreich (phase 3) were supported by pharmaceutical companies and partly funded by public sources. In all four studies, the pharmaceutical company was involved in analysing and interpreting the data. Two studies were funded by public sources (ACTIV‐3; RECOVERY), with donations of study medications by the manufacturing pharmaceutical companies. However, RECOVERY explicitly reported that the medication sponsor had no role in study conduct and publication of results. Except for the preprints of Weinreich (phase 1/2) and Weinreich (phase 3), all authors reported their conflicts of interest.
Outcomes
In BLAZE‐1 (phase 2), the primary outcome was the change in SARS‐CoV‐2 viral load from baseline to day 11. Planned secondary outcomes were viral clearance at days 7, 11, 15, and 22 and time to SARS‐CoV‐2 clearance, symptom resolution and time to symptom resolution, symptom improvement and time to symptom improvement, COVID‐19‐related deterioration (hospitalisation, emergency room or death by days 29, 60 and 85), and change in symptom questionnaire score. Additional biocatalytic and pharmacokinetic/pharmacodynamic methods were conducted. Safety outcomes included treatment‐emergent adverse events rated according to the Medical Dictionary for Regulatory Activities (MedDRA) and serious adverse events. The term "treatment‐emergent adverse events" is used, which is defined as any "event that first occurred or worsened in severity after baseline", therefore we treated these as regular adverse events. The phase 3 publication reported the combined endpoint hospital admission or death as the primary outcome. Additional reported outcomes for bamlanivimab/etesevimab included: mortality, the combined endpoint hospitalisation, emergency department visit or death, change in viral load at day 7, time to sustained symptom resolution, reduction in viral load to days three and five, time to viral clearance, and adverse events.
In COMET‐ICE, the primary endpoint was hospitalisation ≥ 24 hours or death due to any cause up to day 29. Secondary efficacy endpoints included: the composite endpoint emergency room visit, hospitalisation or death, all‐cause mortality (at days 29, 60, 90), patient‐reported outcomes (mean change in inFLUenza Patient‐ Reported Outcome score (FLU PRO Plus)), changes in viral load, progression to supplemental oxygen up to day 29, incidence and titres of serum anti‐drug antibodies (ADA). Safety endpoints were adverse events (including adverse events of special interest) and serious adverse events. Patient‐reported outcomes, change in viral load, and various time points for all‐cause mortality and adverse events were not reported. Secondary and exploratory endpoints were excluded from this interim analysis, because the study was still ongoing.
In Eom 2021, the primary efficacy endpoint of part 1 was time to negative conversion up to day 28, time to clinical recovery up to day 14. Secondary efficacy endpoints (assessed up to days 7, 14, 28) were the proportion of patients with negative seroconversion; proportion of patients with clinical symptoms requiring hospitalisation, oxygen, therapy, or mortality; proportion of patients requiring supplemental oxygen; intensive care unit (ICU) transfer; all‐cause mortality; time to clinical recovery; duration of fever; hospital admission; need for IMV; patients requiring rescue therapy, time to National Early Warning Score 2 (NEWS2) of 0, scores of other known symptoms (vomiting, diarrhoea, loss of taste or smell), incidence of antibody‐dependent enhancement, viral serology for SARS‐CoV‐2 antibody. Safety outcomes were reported as treatment‐emergent adverse events (TEAEs; including TEAEs of special interest), treatment‐emergent serious adverse events (TESAEs). The endpoints duration of fever, time to National Early Warning Score 2 (NEWS2) of 0, scores of other known symptoms (vomiting, diarrhoea, loss of taste or smell) were not reported in the preprint, and the outcome proportion of patients with negative seroconversion was not reported up to day 7.
In Weinreich (phase 1/2), for the reported phase 2 data, the primary virological endpoint was time‐weighted average change in viral load from baseline through day 22, according to the statistical analysis plan; in the publication, this outcome was reported through day 7. Secondary endpoints were time to negative reverse transcription PCR (RT‐qPCR) test on nasopharyngeal swabs, change in viral shedding at each visit through day 29, time‐weighted average change from baseline in viral shedding (days 5, 7, 15, and 29), the percentage of participants with one or more and two or more medically attended visits through day 29, number of COVID‐19‐related medically attended visits by day 29, proportion of admissions to hospital, ICU admission, outpatient visits, percentage of participants requiring mechanical ventilation, days of hospitalisation due to COVID‐19 by day 29, all‐cause mortality, time to symptom onset and duration of symptoms. Only the primary virological endpoint, percentage of participants with one or more and two or more medically attended visits through day 29, number of COVID‐19‐related medically attended visits by day 29 and the proportion of admissions to hospital were reported in this final analysis of phase 1 to 2 in the form of a preprint. Safety outcomes were reported as TEAEs, adverse events of special interest and TESAEs. Weinreich (phase 3) reported for phase 3 the endpoints all‐cause mortality by day 29 (for the doses 1.2g and 2.4 g), percentage of participants requiring mechanical ventilation (for the doses 1.2 g and 2.4 g), hospital admissions, days of hospitalisation due to COVID‐19 by day 29 (for the doses 1.2 g and 2.4 g), admission to ICU (for the doses 1.2 g and 2.4 g), TEAEs all grades, TEAEs grades 3 to 4 and TESAEs.
In the ACTIV‐3 study, the primary outcomes were time to sustained recovery up to 90 days, defined as hospital discharge and being alive and home for at least 14 days, and two outcomes (pulmonary and pulmonary plus outcome) measured on 7‐level ordinal scales, adapted from the WHO. The pulmonary outcome was based on oxygen requirements and the pulmonary plus outcome was based on oxygen requirements in combination with organ dysfunction associated with the progression of the disease. The secondary outcomes were death from any cause, composite of sustained recovery and mortality through day 90, time to hospital discharge, days alive outside of acute care hospital, ordinal outcomes on days 14 and 28, change in National Early Warning (NEW) score through day 5, clinical organ failure, composite of death and clinical organ failure, and a composite of cardiovascular and thromboembolic events. The primary safety outcome was a composite outcome of death, serious adverse events, or grades 3 or 4 adverse events assessed through day five. Secondary outcomes included all‐cause mortality through day 90 and a composite outcome considering time to sustained recovery and mortality through day 90. In addition, infusion‐related reactions, serious adverse events or death, adverse events of any grade through day 7 and at days 14 and 28 were planned. All preplanned outcomes for this stage have been reported except days alive outside of acute care hospital and change in NEW score (reported as odds ratio (OR)).
In RECOVERY, outcomes were reported at day 28 after randomisation. The primary outcome was all‐cause mortality. Secondary outcomes were time to hospital discharge, use of IMV or death. Subsidiary outcomes were use of ventilation, duration of IMV, use of renal dialysis or haemofiltration, thrombotic events. Safety outcomes included cause‐specific mortality, major cardiac arrhythmia, major bleeding, early safety of antibody‐based therapy and non‐coronavirus infection. All preplanned outcomes, except non‐coronavirus infection were reported in the preprint.
Ongoing randomised controlled trials testing SARS‐CoV‐2‐specific mAbs
We identified 36 ongoing studies in addition to the included platform trial ACTIV‐3 which have added new mAbs. These are listed in Appendix 10, more information on each study can be found in the Characteristics of ongoing studies. Of these RCTs, four are ongoing platform trials without results on mAbs yet (ACTIV‐2; AGILE; DISCOVERY; OPTIMISE‐C19) and 32 are standard RCTs (EUDRACT2020‐003401‐60; NCT04411628; NCT04426695; NCT04551898; NCT04584697; NCT04593641; NCT04627584; NCT04631666; NCT04631705; NCT04634409; NCT04644120; NCT04644185; NCT04649515; NCT04666441; NCT04674566; NCT04683328; NCT04709328; NCT04723394; NCT04734860; NCT04748588; NCT04770467; NCT04771351; NCT04779879; NCT04780321; NCT04787211; NCT04787211; NCT04796402; NCT04796402; NCT04805671; NCT04805671; NCT04822701; NCT04822701; NCT04840459; NCT04840459; NCT04900428; NCT04900428; NCT04913675; NCT04952805), which evaluated 23 different SARS‐CoV‐2‐specific mAb types or mAb combinations (e.g., AZD7442, MAD0004J08, BRII‐196/BRII‐198, casirivimab/imdevimab, bamlanivimab/etesevimab, C135‐LS/C144‐LS). Most SARS‐CoV‐neutralising mAbs in humans have proceeded to phase 2 studies onwards.
Two of the ACTIV‐3 arms, BRII‐196/BRII‐198, and sotrovimab, were closed with 343 and 344 randomised participants each for futility, and results have not yet been published (NIH 2021).
Ongoing platform trials
ACTIV‐2 is being conducted in outpatients and has five active mAbs treatment arms (bamlanivimab, BRII‐196/BRII‐198, AZD7442 (IV or IM), C135‐LS/C144‐LS). The estimated study completion date is May 2023.
The studies ACTIV‐3, AGILE, and DISCOVERY include hospitalised patients and randomise(d) into various mAb treatment arms (ACTIV‐3: AZD7442, bamlanivimab, BRII‐196/BRII‐198 and VIR‐7831; AGILE: VIR‐7832 and VIR‐7831; DISCOVERY: AZD7442). In ACTIV‐3, the bamlanivimab arm was stopped for futility after an interim analysis. We included the published results in this review. Randomisation to VIR‐7831 and BRI196/BRII/198 has stopped, but participants are still being recruited for the AZD7442 arm. Results had not been published by the time of review writing. The estimated study completion date is July 2022. AGILE is expected to complete by April 2022. DISCOVERY is planned to complete in March 2023.
OPTIMISE‐C19 includes any COVID‐19 positive patient who is eligible for the examined mAbs under US Food and Drug Administration (FDA) emergency use authorization (EUA), which may change over time. Currently, the study randomises patients into bamlanivimab, bamlanivimab/etesevimab, or casirivimab/imdevimab treatment arms and is estimated to have completed by December 2022.
Studies awaiting classification: ongoing platform trials without SARS‐CoV‐2‐specific mAbs
To facilitate rapid testing of emerging treatments, adaptive platform trials have become more frequent, because they allow more flexibility in adding new treatment arms and dropping futile treatment arms without registration of a new study. From our specific search for these studies, we have identified five ongoing platform trials with at least one SARS‐CoV‐2‐specific mAb as a treatment arm (ACTIV‐2; ACTIV‐3; AGILE; DISCOVERY; OPTIMISE‐C19), which are also listed above as included studies. We identified an additional 30 ongoing platform trials without any SARS‐CoV‐2‐specific mAb treatment arms (ACCORD & ACCORD 2; ACOVACT; ACTIV‐1 IM; ACTT‐4; ANTICOV; ARCO‐Home; BEAT COVID‐19; BET‐A (ACTIV‐5); BET‐B (ACTIV‐5); CATALYST; CCAP; COLHEART‐19; COPPS; CORIMUNO; COVID MED; DEFINE; EU SolidAct; I‐SPY; NCT04359095; NCT04590586; PaTS‐COVID; PRINCIPLE; PROTECT‐Surg; REMAP‐CAP; SOLIDARITY; SWISSPED‐RECOVERY; TACTIC‐E; TACTIC‐R; TOGETHER‐3; VIRCO). For regular tracking, we decided to list them under the Studies awaiting classification section.
Excluded studies
Tracking of SARS‐CoV‐2‐specific mAbs under development
We excluded 27 studies (29 records) from our search on SARS‐CoV‐2‐specific mAbs because they were conducted on healthy participants; however, we listed these studies for tracking (details in Appendix 9).
23 RCTs (25 records) were excluded because they are being done on healthy participants (Track: NCT04852978; Track: ChiCTR2100042150; Track: jRCT2071200117; Track: NCT04896541; Track: NCT04429529; Track: NCT04441918; Track: NCT04441931; Track: NCT04479631; Track: NCT04479644; Track: NCT04483375; Track: NCT04507256; Track: NCT04519437; Track: NCT04525079; Track: NCT04532294; Track: NCT04533048; Track: NCT04537910; Track: NCT04561076; Track: NCT04567810; Track: NCT04590430; Track: NCT04592549; Track: NCT04691180; Track: NCT04700163; Track: NCT04932850)
4 studies (4 records) are non‐randomised studies (Track: NCT04617535; Track: NCT04701658; Track: NCT04603651; Track: NCT04656691)
Randomised controlled trials in healthy participants
The 23 RCTs on healthy participants examined 19 different mAbs or mAb combinations (ADM03820, anti‐SARS‐CoV‐2 chicken egg IgY, AZD7442, bamlanivimab, BGB‐DXP593, BRII‐196, BRII‐196/BRII‐199, BRII‐198, casirivimab/imdevimab, CT‐P59, C144‐LS/C‐135‐LS, etesevimab, HFB30132A, HLX70, JMB2002, MW33, SCTA01, TY027, MAD0004J08). Studies planned to include between 16 and 974 participants. All except ADM03820, anti‐SARS‐CoV‐2 chicken egg IgY, HFB30132A, JMB2002, and HLX70 are also being investigated in infected individuals (Appendix 9).
| Year of completion | Studies |
| 2020 | 7 (Track: NCT04441918; Track: NCT04441931; Track: NCT04525079; Track: NCT04533048; Track: NCT04537910; Track: NCT04567810; Track: NCT04483375), of these, 5 show completed but without results |
| 2021 | 11 (Track: ChiCTR2100042150Track: NCT04429529; Track: NCT04479631; Track: NCT04479644; Track: NCT04507256; Track: NCT04519437; Track: NCT04532294; Track: NCT04561076; Track: NCT04590430; Track: NCT04592549; Track: NCT04691180) |
| 2022 | 4 (Track: NCT04852978; Track: NCT04896541; Track: NCT04700163; Track: NCT04932850) |
One study did not report a planned completion date.
Non‐randomised studies
All four non‐randomised studies are US‐based prospective cohorts or expanded access studies that investigate single dose infusions (IV) of mAbs or mAb combinations in outpatients; three of these in participants with risk factors (Track: NCT04701658; Track: NCT04603651; Track: NCT04617535).
Track: NCT04701658 provides bamlanivimab (IV) and will compare the treatment with matched controls for their primary outcome hospitalisation or death by day 29. Completion is planned for June 2021 with 3000 participants. Track: NCT04603651 provides expanded access to bamlanivimab (IV), however, expanded access is no longer available according to clinicaltrials.gov. Track: NCT04656691 provided at‐home infusions of bamlanivimab by registered nurses and examines the frequency of hospitalisations and safety in 4000 participants (study completed according to clinicaltrials.gov). Track: NCT04617535 provides expanded access to casirivimab/imdevimab (IV), no further information regarding sample size or outcomes is reported.
Excluded studies
From the main search, we excluded 94 records (26 press releases and 48 studies) that did not match our inclusion criteria (Figure 2):
7 studies (17 records) investigated mAbs as prevention of SARS‐CoV‐2 infection (BLAZE‐2; NCT04452318; NCT04625725; STORM CHASER; NCT04859517; NCT04894474) or mAb fragments (NCT04514302);
2 studies (3 records) were no longer available in the study registry (NCT04766671; NCT04454398);
20 studies (25 records) with irrelevant interventions (9 studies not SARS‐CoV‐2‐specific mAb: EUDRACT2020‐002713‐17; FORCE; NCT04275245; NCT04341116; NCT04369469; NCT04415073; NCT04494724; NCT04516564; NCT04586153; 2 studies polyclonal Abs: NCT04453384; NCT04469179; 9 studies other interventions: COREG; COVERAGE; C‐SMART; MAS‐COVID; NCT04453384; NCT04494984; NCT04569786; NCT04574869; PANAMO);
16 studies (16 records) with ineligible study design (ChiCTR2000030012; Dhand 2021a; Dong 2021; Alam 2021; Bariola 2021; Beam 2021; Cohen 2021; Dale 2021; Dhand 2021b; Ganesh 2021; Karr 2021; Kutzler 2021; Rainwater‐Lovett 2021; Shirk 2021; Webb 2021; Yang 2020);
3 studies (7 records) were duplicates;
26 press releases of included or ongoing studies.
From the platform trial search, we excluded 20 studies (27 records) for the following reasons (Figure 2):
5 platform trials (5 records) explored treatments for prevention of SARS‐CoV‐2 or other drug classes only (ACTIV‐4; COVER HCW; CROWN CORONA; NCT04498273; PROFACT‐01);
9 studies (13 records) did not plan to add treatment arms (AMMURAVID; ASCOT‐ADAPT; COVERAGE; COVID_Aging; C‐SMART; jRCT2031190264; NCT04629703; PO‐COV‐III‐20; RESP301‐002);
6 platform trials (in 9 records) were completed without having added a mAb (ACTT; ACTT‐2; ACTT‐3; EUCTR2020‐001243‐15‐BE; NCT04354428; NCT04370262).
Risk of bias in included studies
Risk of bias in randomised controlled trials
We assessed methodological quality and risk of bias for each comparison and outcome of the six RCTs (ACTIV‐3; BLAZE‐1 (phase 2); COMET‐ICE; Eom 2021; RECOVERY; Weinreich (phase 1/2); Weinreich (phase 3)) that provided outcomes relevant for this review using the Risk of Bias 2.0 (RoB 2) tool (Sterne 2019), recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021c). Since there were separate publications as preprints on phases 1 to 2 (Weinreich (phase 1/2)) and phase 3 (Weinreich (phase 3)) for the Weinreich study, we assessed risk of bias separately for the relevant outcomes provided.
Please refer to the risk of bias table section after the 'Characteristics of studies' section for more detailed information on the risk of bias assessments for each outcome. The completed RoB 2 tool with responses to all assessed signalling questions is available online at: https://zenodo.org/record/5159915#.YQq7pYgzY2w.
Risk of bias in randomised controlled trials in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
Bamlanivimab compared with placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
We judged the risk of bias for BLAZE‐1 (phase 2), the only study assessing bamlanivimab (LY3819253, LY‐CoV555) in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease), to be low across outcomes: all‐cause mortality up to 30 days (see Table 90), admission to hospital or death (see Table 91), viral clearance at days 7 and 15 (see Table 92; Table 93), all grades of adverse events (see Table 94), and serious adverse events (see Table 95). We could not assess the risk of bias for all‐cause mortality up to 60 days, clinical progression/improvement of symptoms or development of severe symptoms, length of hospital stay, admission to ICU and length of ICU stay, viral clearance at day 3, adverse events (grades 1 to 2, and grades 3 to 4), quality of life (up to7 days, 30 days and longest follow‐up), renal failure and thromboembolic events, as BLAZE‐1 (phase 2) did not report these outcomes.
Risk of bias for analysis 1.1 Mortality by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 2, 0.7g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 257 out of 265 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. |
| BLAZE‐1 (phase 2, 2.8g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 263 out of 270 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 7.0g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 257 out of 265 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 1.2 Admission to hospital or death.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 2, 0.7g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 257 out of 265 participants randomised. | Low risk of bias | The measurement of the outcome was likely appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 2.8g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 263 out of 270 participants randomised. | Low risk of bias | The measurement of the outcome was likely appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 7.0g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 257 out of 265 participants randomised. | Low risk of bias | The measurement of the outcome was likely appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 1.3 Viral clearance at day 7.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 2, 0.7g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 5 participants in the treatment arm and of 16 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 2.8g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 8 participants in the treatment arm and of 16 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 7.0g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 5 participants in the treatment arm and of 16 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 1.4 Viral clearance at day 15.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 2, 0.7g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 13 participants in the treatment arm and of 29 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 2.8g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 12 participants in the treatment arm and of 29 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 7.0g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 10 participants in the treatment arm and of 29 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 1.5 Adverse events: all grades.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 2, 0.7g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 257 out of 265 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 2.8g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 263 out of 270 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 7.0g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 257 out of 265 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 1.6 Serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 2, 0.7g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 257 out of 265 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 2.8g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 263 out of 270 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| BLAZE‐1 (phase 2, 7.0g) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 257 out of 265 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Bamlanivimab/etesevimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
We judged the risk of bias for BLAZE‐1 (phase 2), the only study assessing the combination of bamlanivimab and etesevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease) to be high at the study level, and thus across the outcomes (all‐cause mortality by day 30, hospital admission or death, viral clearance at days 7 and 15, all grades adverse events and serious adverse events) because the staggered start of treatment arms caused the bamlanivimab/etesevimab arm to be randomised later than the placebo arm due to adjustment of the allocation ratio. As the standard of care is evolving quickly, groups may not be comparable. We could not assess the risk of bias for all‐cause mortality up to 60 days, clinical progression/improvement of symptoms or development of severe symptoms, length of hospital stay, admission to ICU and length of ICU stay, viral clearance at day 3, adverse events (grades 1 to 2, and grades 3 to 4), quality of life (up to seven days, 30 days and longest follow‐up), renal failure and thromboembolic events, as BLAZE‐1 (phase 2) did not report these outcomes. Due to the high risk of bias, reported outcomes were not included in the analysis but reported narratively instead.
For BLAZE‐1 (phase 3), we judged the risk of bias to be low across the outcomes: all‐cause mortality up to 30 days (see Table 96), admission to hospital or death (see Table 97), length of hospital stay, viral clearance at days 3, 7, and 15 (see Table 98; Table 99; Table 100), all grades adverse events (see Table 101) and serious adverse events (see Table 102). We could not assess the risk of bias for all‐cause mortality up to 60 days, clinical progression or development of severe symptoms, admission to ICU and length of ICU stay, viral clearance (at days 3, 7, and 15), adverse events (grades 1 to 2 and grades 3 to 4), quality of life (up to seven days, 30 days and longest follow‐up), renal failure and thromboembolic events, as BLAZE‐1 (phase 3) did not report these outcomes.
Risk of bias for analysis 2.1 Mortality by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 3) | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and an appropriate analysis was performed. | Low risk of bias | Data for this outcome was available for 1035 out of 1049 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 2.2 Admission to hospital or death.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 3) | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and an appropriate analysis was performed. | Low risk of bias | Data for this outcome was available for 1035 out of 1049 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result were analysed in accordance to the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 2.3 Viral clearance at day 3.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 3) | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and an appropriate analysis was performed. | Low risk of bias | Data for this outcome was available for nearly all participants (968 out of 1035 participants randomised). | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | We used data from Kaplan‐Meier curve to calculate cumulative frequency of viral clearance. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 2.4 Viral clearance at day 7.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 3) | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and an appropriate analysis was performed. | Low risk of bias | Data for this outcome was available for nearly all participants (968 out of 1035 participants randomised). | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | We used data from Kaplan‐Meier curve to calculate cumulative frequency of viral clearance. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 2.5 Viral clearance at day 15.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 3) | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and an appropriate analysis was performed. | Low risk of bias | Data for this outcome was available for nearly all participants (968 out of 1035 participants randomised). | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | We used data from Kaplan‐Meier curve to calculate cumulative frequency of viral clearance. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 2.6 Adverse events: all grades.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 3) | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and an appropriate analysis was performed. | Low risk of bias | Data for this outcome was available for 1035 out of 1049 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 2.7 Serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| BLAZE‐1 (phase 3) | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and an appropriate analysis was performed. | Low risk of bias | Data for this outcome was available for 1035 out of 1049 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Casirivimab/imdevimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
We judged the risk of bias for Weinreich (phase 1/2) (preprint), the only study assessing the combination of casirivimab and imdevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease) to be of low risk of bias across the outcomes grades 3 to 4 adverse events (see Table 104), and serious adverse events (see Table 105). For the outcome hospital admission or death (see Table 103) we judged the risk of bias to be of some concern because the statistical analysis plan and protocol were not provided with the preprint. We could not assess the risk of bias for: all‐cause mortality at up to 30 and 60 days, clinical progression/improvement of symptoms, length of hospital stay, admission to ICU and length of ICU stay, clinical progression/improvement of symptoms or development of severe symptoms, viral clearance (at days 3, 7, 15) and adverse events (all grades and grades 1 to 2), quality of life (up to seven days, 30 days and longest follow‐up), renal failure and thromboembolic events, as Weinreich (phase 1/2) did not report these outcomes.
Risk of bias for analysis 3.2 Adverse events: grade 3 and 4.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Weinreich (phase 1/2, 2.4 g) | Low risk of bias | Participants were centrally randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 520 out of 532 participants randomised. | Some concerns | There is no information whether measurement of the outcome was appropriate, but it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the previous protocol. There is no indication that the outcome measurement and the results have been selected. | Some concerns | For this outcome, there is a low risk of bias due to the randomisation process, due to deviations from the intended interventions, missing outcomes and due to selection of reported results. However, there are some concerns for risk of bias due to measurement of the outcome. |
| Weinreich (phase 1/2, 8.0 g) | Low risk of bias | Participants were centrally randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 522 out of 533 participants randomised. | Some concerns | There is no information whether measurement of the outcome was appropriate, but it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the previous protocol. There is no indication that the outcome measurement and the results have been selected. | Some concerns | For this outcome, there is a low risk of bias due to the randomisation process, due to deviations from the intended interventions, missing outcomes and due to selection of reported results. However, there are some concerns for risk of bias due to measurement of the outcome. |
Risk of bias for analysis 3.3 Serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Weinreich (phase 1/2, 2.4 g) | Low risk of bias | Participants were centrally randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 520 out of 532 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the previous protocol. There is no indication that the outcome measurement and the results have been selected. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| Weinreich (phase 1/2, 8.0 g) | Low risk of bias | Participants were centrally randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 520 out of 532 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the previous protocol. There is no indication that the outcome measurement and the results have been selected. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 3.1 Admission to hospital or death.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Weinreich (phase 1/2, 2.4 g) | Low risk of bias | Participants were centrally randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were probably unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 446 out of 532 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | SAP and protocol not provided for this preprint. There is no indication that the outcome measurement and the results have been selected. | Some concerns | For this outcome, there is a low risk of bias due to the randomisation process, due to deviations from the intended interventions, missing outcomes, measurement of the outcome. There are some concerns for risk of bias due to selection of reported results. |
| Weinreich (phase 1/2, 8.0 g) | Low risk of bias | Participants were centrally randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were probably unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 450 out of 533 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | SAP and protocol not provided for this preprint. There is no indication that the outcome measurement and the results have been selected. | Some concerns | For this outcome, there is a low risk of bias due to the randomisation process, due to deviations from the intended interventions, missing outcomes, measurement of the outcome. There are some concerns for risk of bias due to selection of reported results. |
For Weinreich (phase 3) (preprint), we judged the risk of bias to be high across the outcomes: mortality by day 30, clinical progression/improvement of symptoms, admission to hospital or death, length of hospital stay, admission to ICU, adverse events (all grades and grades 3 to 4) and serious adverse events, because participants without risk factors were excluded from analysis and it was unclear which participants were included in the analysis set. More participants were missing than the ones not receiving or discontinuing treatment. Furthermore, data for participants who received casirivimab/imdevimab at a dose of 8.0 g were not reported on all relevant outcomes. We could not assess the risk of bias for all‐cause mortality at up 60 days, length of ICU stay, development of severe symptoms, viral clearance (at days 3,7 and 15) and adverse events grades 1 to 2, quality of life (up to day 7 days, 30 days and longest follow‐up), renal failure and thromboembolic events, as Weinreich (phase 3) did not report these outcomes. Due to the high risk of bias, reported outcomes were not included in the analysis but reported narratively instead.
Sotrovimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
We judged the risk of bias for COMET‐ICE, the only study assessing sotrovimab in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease) to be some concerns across the outcomes: all‐cause mortality up to 30 days (see Table 106), development of severe symptoms according to WHO scale (see Table 107; Table 108), admission to hospital or death (see Table 109), admission to ICU (see Table 110) and for safety outcomes (adverse events (all grades and grades 3 to 4), and serious adverse events; see Table 111; Table 112; Table 113), because the trial was stopped preliminary and protocol or statistical analysis plan were not available. We could not assess the risk of bias for all‐cause mortality up to 60 days, clinical progression/improvement of symptoms, length of hospital stay, length of ICU stay, viral clearance (at days 3, 7, 15), adverse events grades 1 to 2, quality of life (up to seven days, 30 days and longest follow‐up), renal failure and thromboembolic events, as COMET‐ICE did not report these outcomes.
Risk of bias for analysis 4.1 Mortality by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| COMET‐ICE | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were probably unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 583 out of 583 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | The trial protocol and SAP were not available. We have some concerns regarding selection of the reported results because the trial was stopped preliminary. | Some concerns | For this outcome, there is a low risk of bias from the randomization process, due to deviations from intended interventions, due to missing outcomes and due to measurement of the outcome. However, there are some concerns for bias due to selection of the reported results. |
Risk of bias for analysis 4.2 Development of severe symptoms according to WHO scale (≥ 5, incl. death).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| COMET‐ICE | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were probably unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 583 out of 583 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | The trial protocol and SAP were not available. We have some concerns regarding selection of the reported results because the trial was stopped preliminary. | Some concerns | For this outcome, there is a low risk of bias from the randomization process, due to deviations from intended interventions, due to missing outcomes and due to measurement of the outcome. However, there are some concerns for bias due to selection of the reported results. |
Risk of bias for analysis 4.3 Development of severe symptoms according to WHO scale (≥ score 7, IMV, incl. death).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| COMET‐ICE | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were probably unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 583 out of 583 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | The trial protocol and SAP were not available. We have some concerns regarding selection of the reported results because the trial was stopped preliminary. | Some concerns | For this outcome, there is a low risk of bias from the randomization process, due to deviations from intended interventions, due to missing outcomes and due to measurement of the outcome. However, there are some concerns for bias due to selection of the reported results. |
Risk of bias for analysis 4.4 Admission to hospital or death.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| COMET‐ICE | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were probably unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 583 out of 583 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | The trial protocol and SAP were not available. We have some concerns regarding selection of the reported results because the trial was stopped preliminary. | Some concerns | For this outcome, there is a low risk of bias from the randomization process, due to deviations from intended interventions, due to missing outcomes and due to measurement of the outcome. However, there are some concerns for bias due to selection of the reported results. |
Risk of bias for analysis 4.5 Admission to ICU by day 29.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| COMET‐ICE | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were probably unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 583 out of 583 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | The trial protocol and SAP were not available. We have some concerns regarding selection of the reported results because the trial was stopped preliminary. | Some concerns | For this outcome, there is a low risk of bias from the randomization process, due to deviations from intended interventions, due to missing outcomes and due to measurement of the outcome. However, there are some concerns for bias due to selection of the reported results. |
Risk of bias for analysis 4.6 Adverse events: all grades.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| COMET‐ICE | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were probably unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 868 out of 868 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | The trial protocol and SAP were not available. We have some concerns regarding selection of the reported results because the trial was stopped preliminary and the time point measured was not entirely clear. | Some concerns | For this outcome, there is a low risk of bias from the randomization process, due to deviations from intended interventions, due to missing outcomes and due to measurement of the outcome. However, there are some concerns for bias due to selection of the reported results. |
Risk of bias for analysis 4.7 Adverse events: grade 3 and 4.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| COMET‐ICE | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were probably unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 868 out of 868 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | The trial protocol and SAP were not available. We have some concerns regarding selection of the reported results because the trial was stopped preliminary and the time point measured was not entirely clear. | Some concerns | For this outcome, there is a low risk of bias from the randomization process, due to deviations from intended interventions, due to missing outcomes and due to measurement of the outcome. However, there are some concerns for bias due to selection of the reported results. |
Risk of bias for analysis 4.8 Serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| COMET‐ICE | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were probably unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 868 out of 868 participants randomised. | Low risk of bias | The measurement of the outcome was probably appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | The trial protocol and SAP were not available. We have some concerns regarding selection of the reported results because the trial was stopped preliminary and the time point measured was not entirely clear. | Some concerns | For this outcome, there is a low risk of bias from the randomization process, due to deviations from intended interventions, due to missing outcomes and due to measurement of the outcome. However, there are some concerns for bias due to selection of the reported results. |
Regdanvimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
We judged the risk of bias for Eom 2021, the only study assessing regdanvimab in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease) to be low across the outcomes: mortality up to 30 days (see Table 114), development of severe symptoms (see Table 115), admission to hospital or death (see Table 116), admission to ICU (see Table 117) and viral clearance at day 15 (see Table 118), and safety outcomes (adverse events all grades (see Table 119), adverse events grades 3 to 4 (see Table 120) and serious adverse events (see Table 121)). We could not assess the risk of bias for all‐cause mortality up to 60 days, clinical progression/improvement of symptoms, length of hospital stay, length of ICU stay, viral clearance at days three and seven, adverse events grades 1 to 2, quality of life (up to seven days, 30 days and longest follow‐up), renal failure and thromboembolic events, as Eom 2021 did not report these outcomes.
Risk of bias for analysis 5.1 Mortality by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Eom 2021 (40 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 307 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| Eom 2021 (80 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 307 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 5.2 Development of severe symptoms according to WHO scale (≥ score 7, IMV, incl. death).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Eom 2021 (40 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 307 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| Eom 2021 (80 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 307 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 5.3 Admission to hospital or death by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Eom 2021 (40 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 307 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| Eom 2021 (80 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 307 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 5.4 Admission to ICU by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Eom 2021 (40 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 307 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| Eom 2021 (80 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 307 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 5.5 Viral clearance at day 15.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Eom 2021 (40 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 307 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| Eom 2021 (80 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 307 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 5.6 Adverse events: all grades.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Eom 2021 (40 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 325 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| Eom 2021 (80 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 325 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 5.7 Adverse events: grade 3 and 4.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Eom 2021 (40 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 325 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| Eom 2021 (80 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 325 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 5.8 Serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Eom 2021 (40 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 325 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
| Eom 2021 (80 mg/kg) | Low risk of bias | Participants were randomised via an Interactive Web Response System (IWRS) and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the intervention received. Participants with a negative PCR were excluded from analysis in both groups (intervention and placebo group) and one participant in the placebo arm did not receive the allocated intervention. The analysis was probably appropriate. | Low risk of bias | Data for this outcome was available for 325 out of 327 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias in randomised controlled trials in hospitalised individuals with COVID‐19 (moderate to severe disease)
Bamlanivimab compared to placebo in hospitalised individuals with COVID‐19 (moderate and severe disease)
Overall, we judged the risk of bias for ACTIV‐3, the only study that assessed bamlanivimab in hospitalised individuals, to be low across outcomes: all‐cause mortality by days 30 and 90 (see Table 122; Table 123), clinical progression/improvement of symptoms or development of severe symptoms (see Table 124; Table 125), hospital discharge (time‐to‐event and at day 5; see Table 126; Table 127), adverse events grades 3 to 4 (see Table 128), and serious adverse events by days 30 and 90 (see Table 129; Table 130), time to sustained recovery by day 90 (see Table 131), neurological dysfunction at day 28 (transient ischemic events see Table 132, acute delirium CVA see Table 133 and cerebrovascular event see Table 134), thromboembolic events (see Table 135) and renal dysfunction (see Table 136). We noted differences in the baseline characteristics of the participants between treatment groups, but we assumed that these differences are unlikely to indicate problems with the randomisation process. We could not assess the risk of bias for the outcome quality of life (up to 7 days, 30 days and longest follow‐up), admission to ICU and length of ICU stay, viral clearance (at days three, seven, and 15), and adverse events (all grades and grades 1 to 2) as these were not reported in the study.
Risk of bias for analysis 6.1 Mortality by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 314 out of 326 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.2 Mortality by day 90.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 168 out of 168 participants with sustained recovery. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.3 Development of severe symptoms: need for NIV, IMV, ECMO, or renal replacement therapy at day 5 (group 5, 6 or 7).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 311 out of 326 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.4 Development of severe symptoms: clinical status at day 5, intubation (group 6 at pulmonary scale).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 311 out of 326 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.5 Hospital discharge up to 26 October 2020.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 314 out of 326 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.6 Hospital discharge: at day 5.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 314 out of 326 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.7 Adverse events: grade 3 and 4 by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 314 out of 326 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.8 Serious adverse events by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 314 out of 326 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.9 Serious adverse events (90 day follow‐up).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 168 out of 168 participants with sustained recovery. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.10 Sustained recovery (90 day follow‐up).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for 314 out of 326 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.11 Neurological dysfunction by day 30: transient ischemic events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 17 participants in the treatment arm and of 16 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.12 Neurological dysfunction by day 30: acute delirium CVA.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 17 participants in the treatment arm and of 16 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.13 Neurological dysfunction by day 30: cerebrovascular event.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 17 participants in the treatment arm and of 16 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.14 Thromboembolic events by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 17 participants in the treatment arm and of 16 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Risk of bias for analysis 6.15 Renal dysfunction (or need for dialysis) by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| ACTIV‐3 | Low risk of bias | Participants were randomised via mass‐weighted urn randomisation. There are baseline differences, but they are unlikely to suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data of 17 participants in the treatment arm and of 16 participants in the control arm were missing. The study was double‐blinded and it is not likely that missingness in the outcome depended on its true value. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified analysis plan and the outcome was reported as planned in the protocol. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Casirivimab/imdevimab compared to placebo in hospitalised individuals with COVID‐19 (moderate and severe disease)
We judged the risk of bias for RECOVERY, the only study assessing casirivimab/imdevimab in hospitalised individuals to be high across the outcomes: all‐cause mortality up to 30 days (see Table 137), development of severe symptoms according to WHO scale (see Table 138), hospital discharge alive by day 30 (see Table 139), thromboembolic events (see Table 140) and renal dysfunction (need for dialysis) (see Table 141), because of the open‐label design of the study control group participants may have received concomitant treatment more quickly. We could not assess the risk of bias for the outcomes: all‐cause mortality at up to 60 days, clinical progression/improvement of symptoms, quality of life (up to seven days, 30 days, and longest follow‐up), safety outcomes (adverse events (all grades, grades 1 to 2, grades 3 to 4) and serious adverse events), length of hospital stay, admission to ICU, length of ICU stay, viral clearance (at days 3, 7, 15), time to sustained recovery, and neurologic dysfunction, as RECOVERY did not report these outcomes.
Risk of bias for analysis 7.1 Mortality by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| RECOVERY | Low risk of bias | Participants were randomised via web‐based randomisation and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | High risk of bias | Both participants and those delivering the intervention were aware of intervention received and nearly all participants received assigned intervention. The analysis was appropriate. Because of no standard treatment for COVID‐19, control group may have received concomitant treatment more quickly. | Low risk of bias | Data for this outcome was available for all 9785 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. It was unclear whether outcome assessors were unaware of the intervention received, but it is unlikely that knowledge of intervention received could have affected outcome measurement. | Low risk of bias | The data that produced this result was analysed and reported as planned in the protocol and SAP. | High risk of bias | For this outcome, there is a low risk of bias due to the randomisation process, due to missing outcomes, due to measurement of the outcome and due to selection of reported results. However, there is high risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 7.2 Development of severe symptoms: requirement for IMV or death by day 28 (WHO ≥ 7).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| RECOVERY | Low risk of bias | Participants were randomised via web‐based randomisation and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | High risk of bias | Both participants and those delivering the intervention were aware of intervention received and nearly all participants received assigned intervention. The analysis was appropriate. Because of no standard treatment for COVID‐19, control group may have received concomitant treatment more quickly. | Low risk of bias | Data for this outcome was available for 9198 out of 9785 participants randomised. 587 participants were on mechanical ventilation at randomisation and therefore excluded. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. It was unclear whether outcome assessors were unaware of the intervention received, but it is unlikely that knowledge of intervention received could have affected outcome measurement. | Low risk of bias | The data that produced this result was analysed and reported as planned in the protocol and SAP. | High risk of bias | For this outcome, there is a low risk of bias due to the randomisation process, due to missing outcomes, due to measurement of the outcome and due to selection of reported results. However, there is high risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 7.3 Hospital discharge alive by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| RECOVERY | Low risk of bias | Participants were randomised via web‐based randomisation and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | High risk of bias | Both participants and those delivering the intervention were aware of intervention received and nearly all participants received assigned intervention. The analysis was appropriate. Because of no standard treatment for COVID‐19, control group may have received concomitant treatment more quickly. | Low risk of bias | Data for this outcome was available for all 9785 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. It was unclear whether outcome assessors were unaware of the intervention received, but it is unlikely that knowledge of intervention received could have affected outcome measurement. | Low risk of bias | The outcome was pre‐specified and there is no indication for selective reporting. | High risk of bias | For this outcome, there is a low risk of bias due to the randomisation process, due to missing outcomes, due to measurement of the outcome and due to selection of reported results. However, there is high risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 7.4 Thromboembolic events by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| RECOVERY | Low risk of bias | Participants were randomised via web‐based randomisation and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | High risk of bias | Both participants and those delivering the intervention were aware of intervention received and nearly all participants received assigned intervention. The analysis was appropriate. Because of no standard treatment for COVID‐19, control group may have received concomitant treatment more quickly. | Low risk of bias | Data for this outcome was available for all 9785 participants randomised. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. It was unclear whether outcome assessors were unaware of the intervention received, but it is unlikely that knowledge of intervention received could have affected outcome measurement. | Low risk of bias | The data that produced this result was analysed and reported as planned in the protocol and SAP. | High risk of bias | For this outcome, there is a low risk of bias due to the randomisation process, due to missing outcomes, due to measurement of the outcome and due to selection of reported results. However, there is high risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 7.5 Renal dysfunction (or need for dialysis) by day 30.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| RECOVERY | Low risk of bias | Participants were randomised via web‐based randomisation and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomisation. | High risk of bias | Both participants and those delivering the intervention were aware of intervention received and nearly all participants received assigned intervention. The analysis was appropriate. Because of no standard treatment for COVID‐19, control group may have received concomitant treatment more quickly. | Low risk of bias | Data for this outcome was available for 9670 out of 9785 participants randomised. Participants on renal replacement therapy at randomisation were therefore excluded. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. It was unclear whether outcome assessors were unaware of the intervention received, but it is unlikely that knowledge of intervention received could have affected outcome measurement. | Low risk of bias | The data that produced this result was analysed and reported as planned in the protocol and SAP. | High risk of bias | For this outcome, there is a low risk of bias due to the randomisation process, due to missing outcomes, due to measurement of the outcome and due to selection of reported results. However, there is high risk of bias due to deviations from the intended interventions. |
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings 1. Bamlanivimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease).
| Bamlanivimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease) | |||||||
| Patient or population: non‐hospitalised individuals with COVID‐19 Setting: outpatient Intervention: bamlanivimab Comparison: placebo | |||||||
| Outcomes | Dose |
Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) | N° of participants (studies) | Certainty in the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with SARS‐CoV‐2‐specific mAb | ||||||
| Mortality by day 30 | Bamlanivimab 0.7 g | 0 per 1000 | 0 per 1000 | Not estimable | 101 bamlanivimab/156 placebo (1 RCT) |
⊕⊕⊖⊖ Lowa |
No events observed. We are uncertain whether 0.7 g bamlanivimab has any impact on mortality at up to day 30. |
| Bamlanivimab 2.8 g | 0 per 1000 | 0 per 1000 | Not estimable | 107 bamlanivimab/156 placebo (1 RCT) |
No events observed. We are uncertain whether 2.8 g bamlanivimab has any impact on mortality at up to day 30. | ||
| Bamlanivimab 7.0 g | 0 per 1000 | 0 per 1000 | Not estimable | 101 bamlanivimab/156 placebo (1 RCT) |
No events observed. We are uncertain whether 7.0 g bamlanivimab has any impact on mortality at up to day 30. | ||
| Mortality by day 60 | Not reported | ‐ | ‐ | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. |
| Clinical progression/improvement of symptoms | Not reported | ‐ | ‐ | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. |
| Quality of life | Not measured | ‐ | ‐ | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. |
| Admission to hospital by day 30 | Bamlanivimab 0.7 g | 58 per 1000 |
10 per 1000 (1 to 77) |
RR 0.17 (0.02 to 1.33) |
257 (1 RCT) |
⊕⊕⊖⊖ Lowb |
Bamlanivimab 0.7 g may decrease hospital admission at day 30. |
| Bamlanivimab 2.8 g | 58 per 1000 |
18 per 1000 (4 to 85) |
RR 0.32 (0.07 to 1.47) |
263 (1 RCT) |
Bamlanivimab 0.7 g may decrease hospital admission at day 30. | ||
| Bamlanivimab 7.0 g | 58 per 1000 |
20 per 1000 (5 to 90) |
RR 0.34 (0.08 to 1.56) |
257 (1 RCT) |
Bamlanivimab 0.7 g may decrease hospital admission at day 30. | ||
| Adverse events: all grades | Bamlanivimab 0.7 g | 269 per 1000 |
267 per 1000 (178 to 404) |
RR 0.99 (0.66 to 1.50) |
257 (1 RCT) |
⊕⊕⊖⊖ Lowb |
Bamlanivimab 0.7 g may have little to no effect on all‐grade adverse events. |
| Bamlanivimab 2.8 g | 269 per 1000 |
242 per 1000 (159 to 372) |
RR 0.90 (0.59 to 1.38) |
263 (1RCT) |
Bamlanivimab 2.8 g may have little to no effect on all‐grade adverse events. | ||
| Bamlanivimab 7.0 g | 269 per 1000 |
218 per 1000 (140 to 342) |
RR 0.81 (0.52 to 1.27) |
257 (1 RCT) |
Bamlanivimab 7.0 g may have little to no effect on all‐grade adverse events. | ||
| Serious adverse events | Bamlanivimab 0.7 g | 6 per 1000 |
3 per 1000 (0 to 80) |
RR 0.51 (0.02 to 12.47) |
257 (1 RCT) |
⊕⊕⊖⊖ Lowb |
There were too few who experienced serious adverse events to determine whether bamlanivimab 0.7 g made a difference. |
| Bamlanivimab 2.8 g | 6 per 1000 |
3 per 1000 (0 to 76) |
RR 0.48 (0.02 to 11.78) |
263 (1 RCT) |
There were too few who experienced serious adverse events to determine whether bamlanivimab 2.8 g made a difference. | ||
| Bamlanivimab 7.0 g | 6 per 1000 |
3 per 1000 (0 to 80) |
RR 0.51 (0.02 to 12.47) |
257 (1 RCT) |
There were too few who experienced serious adverse events to determine whether bamlanivimab 7.0 g made a difference. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk on the comparison group and the relative effect of the intervention (and its 95% confidence interval). CI: confidence interval; mAb: monoclonal antibody; RCT: randomised controlled trial; RR: risk ratio | |||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is the possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded two levels for very serious imprecision, no events observed, effect not estimable bDowngraded two levels for very serious imprecision, because of small sample size, low number of events and/or wide confidence interval.
Summary of findings 2. Bamlanivimab/etesevimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease).
| Bamlanivimab/etesevimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease) | ||||||
| Patient or population: non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease) Setting: outpatients Intervention: bamlanivimab/etesevimab Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with bamlanivimab/ etesevimab | |||||
| Mortality by day 30 | 19 per 1000 | 1 per 1000 (0 to 15) | RR 0.05 (0.00 to 0.81) |
1035 (1 RCT) | ⊕⊕⊝⊝ Lowa | Bamlanivimab/etesevimab may reduce all‐cause mortality by day 30. |
| Mortality by day 60 ‐ not reported | not reported | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. | |
| Clinical progression | not reported | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. | |
| Quality of life by day 30 | not measured | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. | |
| Admission to hospital or death | 70 per 1000 | 21 per 1000 (11 to 41) | RR 0.30 (0.16 to 0.59) | 1035 (1 RCT) | ⊕⊕⊝⊝ Lowa | Bamlanivimab/ etesevimab may decrease the risk hospital admission by day 30. |
| Adverse events: all grades | 116 per 1000 | 133 per 1000 (96 to 184) | RR 1.15 (0.83 to 1.59) | 1035 (1 RCT) | ⊕⊕⊝⊝ Lowb | Bamlanivimab/ etesevimab may have little to no effect on the risk of all grade adverse events. |
| Serious adverse events | 10 per 1000 | 14 per 1000 (4 to 42) | RR 1.40 (0.45 to 4.37) | 1035 (1 RCT) | ⊕⊕⊝⊝ Lowa | Bamlanivimab/ etesevimab may increase the risk of serious adverse events. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised controlled trial | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is the possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_422966277463976837. | ||||||
a Downgraded two levels for very serious imprecision due to low number of events and low sample size b Downgraded two levels for very serious imprecision, because of small sample size
Summary of findings 3. Casirivimab/imdevimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease).
| Casirivimab/imdevimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease) | |||||||
| Patient or population: non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease) Setting: outpatients Intervention: casirivimab/imdevimab Comparison: placebo | |||||||
| Outcomes | Dose | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with casirivimab/imdevimab | ||||||
| Mortality by day 30 | not reported | ‐ | ‐ | ‐ | ‐ | Only one study which was excluded from analysis reported results on this outcome. | |
| Mortality by day 60 | not reported | ‐ | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. | |
| Clinical progression: requirement of IMV (WHO 7, 8 or 9), 1.2g | not reported | ‐ | ‐ | ‐ | ‐ | Only one study which was excluded from analysis reported results on this outcome. | |
| Quality of life | not measured | ‐ | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. | |
| Admission to hospital or death | 1.2g | ‐ | ‐ | ‐ | ‐ | ||
| 2.4g | 22 per 1000 | 9 per 1000 (2 to 47) | RR 0.43 (0.08 to 2.19) | 446 (1 RCT) | ⨁⨁⊖⊖ Lowa |
Casirivimab/imdevimab at 2.4 g or 8.0 g may reduce the occurrence of hospital admissions or death at day 30. | |
| 8.0g | 22 per 1000 | 5 per 1000 (0 to 39) | RR 0.21 (0.02 to 1.79) | 450 (1 RCT) | |||
| Adverse events: grade 3‐4 | 1.2g | ‐ | ‐ | ‐ | ‐ | Only one study which was excluded from analysis reported results on this outcome. | |
| 2.4g | 15 per 1000 | 12 per 1000 (3 to 51) |
RR 0.76 (0.17 to 3.37) |
520 (1 RCT) | ⨁⨁⊖⊖ Lowa | There were too few who experienced am event to determine whether any dose of casirivimab/imdevimab made a difference | |
| 8.0g | 15 per 1000 | 8 per 1000 (1 to 42) | RR 0.50 (0.09 to 2.73) | 522 (1 RCT) | |||
| Adverse events: all grades | not reported | ‐ | ‐ | ‐ | ‐ | Only one study which was excluded from analysis reported results on this outcome. | |
| Serious adverse events | 1.2g | ‐ | ‐ | ‐ | ‐ | Only one study which was excluded from analysis reported results on this outcome. | |
| 2.4g | 23 per 1000 | 16 per 1000 (4 to 54) | RR 0.68 (0.19 to 2.37) | 520 (1 RCT) | ⨁⨁⊖⊖ Lowa | There were too few who experienced am event to determine whether any dose of casirivimab/imdevimab made a difference. | |
| 8.0g | 23 per 1000 | 8 per 1000 (2 to 38) | RR 0.34 (0.07 to 1.65) | 522 (1 RCT) | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised controlled trial | |||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is the possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Downgraded two levels due to very serious imprecision; low sample size and low number of events
Summary of findings 4. Sotrovimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease).
| Sotrovimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease) | ||||||
| Patient or population: non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease) Setting: outpatients Intervention: sotrovimab Comparison: placebo | ||||||
| Outcome |
Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) | № of participants** (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with sotrovimab | |||||
| Mortality by day 30 | 3 per 1000 |
1 per 1000 (0 to 28) |
RR 0.33 (0.01 to 8.18) | 583 (1 RCT) |
⊕⊕⊝⊝ Lowa | There were too few who experienced mortality to determine whether sotrovimab made a difference. |
| Mortality by day 60 | not reported | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. | |
| Clinical progression: oxygen requirement (≥ 5 WHO scale) | 65 per 1000 |
7 per 1000 (1 to 29) |
RR 0.11 (0.02 to 0.45) | 583 (1 RCT) |
⊕⊕⊝⊝ Lowb | Sotrovimab may reduce the number of participants with any oxygen requirement. |
| Clinical progression: IMV or death (≥ 7 WHO scale) | 10 per 1000 |
1 per 1000 (0 to 28) |
RR 0.14 (0.01 to 2.76) | 583 (1 RCT) |
⊕⊕⊝⊝ Lowa | There were too few who experienced an event to determine whether sotrovimab made a difference. |
| Quality of life by day 30 | not reported | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. | |
| Admission to hospital or death by day 30 | 72 per 1000 |
10 per 1000 (3 to 35) |
RR 0.14 (0.04 to 0.48) | 583 (1 RCT) |
⊕⊕⊝⊝ Lowb | Sotrovimab may reduce the occurrence of hospital admissions or death. |
| Adverse events: all grades | 194 per 1000 |
169 per 1000 (128 to 225) |
RR 0.87 (0.66 to 1.16) | 868 (1 RCT) |
⊕⊕⊝⊝ Lowb |
Sotrovimab may have little to no effect on the occurrence of all grade adverse events. |
| Adverse events: grades 3 and 4 | 62 per 1000 |
16 per 1000 (7 to 37) |
RR 0.26 (0.12 to 0.60) | 868 (1 RCT) |
⊕⊕⊝⊝ Lowb | Sotrovimab may reduce the occurrence of grade 3‐4 adverse events. |
| Serious adverse events | 59 per 1000 |
16 per 1000 (7 to 37) |
RR 0.27 (0.12 to 0.63) | 868 (1 RCT) |
⊕⊕⊝⊝ Lowb | Sotrovimab may reduce the occurrence of serious adverse events. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**This study included a total of 868 participants. All were 868 participants randmoised were included in the safety set, but only 583 participants were analysed in the efficacy set. CI: confidence interval; RR: risk ratio; RCT: randomised controlled trial | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is the possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded two levels for very serious imprecision, because of low sample size, very low number of events and very wide confidence interval
b Downgraded two levels for very serious imprecision, because of low sample size and/or low number of events
Summary of findings 5. Regdanvimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease).
| Regdanvimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease) | |||||||
| Patient or population: non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease) Setting: outpatient Intervention: regdanvimab Comparison: placebo | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with placebo | Risk with regdanvimab | ||||||
| Mortality by day 30 | Regdanvimab 40mg/kg CT‐P59 80 mg/kg |
0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | (1 RCT) | ⨁⨁⊖⊖ Lowa | No events observed. We are uncertain whether CT‐P59 has any impact on mortality at up to day 30. |
| Mortality by day 60 | not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Clinical progression: development of severe symptoms (≥ 7 WHO scale, IMV) | 40 mg/kg | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | (1 RCT) | ⨁⨁⊖⊖ Lowa | No events observed. We are uncertain whether 40 mg/kg regdanvimab has any impact on IMV requirement or death. |
| 80 mg/kg | 0 per 1000 | 0 per 1000 (0 to 0) | RR 3.00 (0.12 to 72.80) | 103 (1 study) | ⨁⨁⊖⊖ Lowb | There were too few who experienced IMV or death to determine whether CT‐P59, 80 mg/kg made a difference. | |
| Quality of life by day 30 | not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Admission to hospital or death by day 30 | 40 mg/kg | 87 per 1000 | 39 per 1000 (12 to 124) | RR 0.45 (0.14 to 1.42) | 204 (1 RCT) | ⨁⨁⊖⊖ Lowb | Regdanvimab, 40 mg/kg or 80 mg/kg may decrease hospital admission or death by day 30. |
| 80 mg/kg | 87 per 1000 | 49 per 1000 (17 to 140) | RR 0.56 (0.19 to 1.60) | 206 (1 RCT) | |||
| Adverse events: all grades | 40 mg/kg | 309 per 1000 | 297 per 1000 (198 to 442) | RR 0.96 (0.64 to 1.43) | 215 (1 RCT) | ⨁⨁⊖⊖ Lowb | Regdanvimab 40 mg/kg may have little to no effect on all grade adverse events. |
| 80 mg/kg | 309 per 1000 | 244 per 1000 (161 to 377) | RR 0.79 (0.52 to 1.22) | 220 (1 RCT) | Regdanvimab 80 mg/kg may reduce the occurrence of all grade adverse events. | ||
| Adverse events: grades 3 and 4 | 40 mg/kg | 18 per 1000 | 48 per 1000 (9 to 239) | RR 2.62 (0.52 to 13.12) | 215 (1 RCT) | ⨁⨁⊖⊖ Lowb | Regdanvimab 40 mg/kg or 80 mg/kg may increase the occurrence of grade 3 adverse events. |
| 80 mg/kg | 18 per 1000 | 36 per 1000 (7 to 195) | RR 2.00 (0.37 to 10.70) | 220 (1 RCT) | |||
| Serious adverse events by day 30 | not reported | 0 per 1000 | 0 per 1000 (0 to 0) |
not estimable | (1 RCT) | ⨁⨁⊖⊖ Lowb | We are uncertain whether regdanvimab, 40 mg/kg or 80 mg/kg has an effect on serious adverse events. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised controlled trial | |||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is the possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Downgraded two levels for very serious imprecision, because no events were observed, the sample size small and the effect not estimable
b Downgraded two levels for very serious imprecision, because few event(s) were observed and/or the sample size was small.
Summary of findings 6. Bamlanivimab compared to placebo in hospitalised individuals with COVID‐19 (moderate to severe disease).
| Bamlanivimab compared to placebo in hospitalised individuals with COVID‐19 (moderate to severe disease) | ||||||
| Patient or population: hospitalised individuals with COVID‐19 (moderate to severe disease) Setting: inpatient Intervention: bamlanivimab Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty in the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with bamlanivimab | |||||
| Mortality by day 30 | 26 per 1000 | 37 per 1000 (11 to 128) | RR 1.39 (0.40 to 4.83) | 314 (1 RCT) | ⊕⊕⊝⊝ Lowa | We are uncertain whether bamlanivimab has an effect on mortality at 28 days. |
| Mortality by day 90 | 73 per 1000 |
79 per 1000 (36 to 168) |
HR 1.09 (0.49 to 2.43) |
314 (1 RCT) |
⊕⊕⊝⊝ Lowa | Bamlanivimab may have little to no effect on mortality by day 90. |
| Clinical progression: need for NIV, IMV, ECMO, or renal replacement therapy at day 5 (group 5, 6 or 7) assessed with: 7‐point scale, lower better | 180 per 1000 | 211 per 1000 (135 to 333) | RR 1.17 (0.75 to 1.85) | 311 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Bamlanivimab may slightly increase the development of severe symptoms (assessed by the need for NIV, IMV, ECMO, or renal replacement therapy) at day 5. |
| Quality of life | not measured | ‐ | ‐ | We did not identify any study reporting this outcome. | ||
| Time to hospital discharge up to 26.10.2020, absolute effects for day 10 | 762 per 1000 | 752 per 1000 (664 to 816) | HR 0.97 (0.76 to 1.18) | 314 (1 RCT) | ⊕⊕⊝⊝ Lowa | Bamlanivimab may have little to no impact on hospital discharge until data cut off (26 October 2020). |
| Adverse events: grades 3 and 4 by day 30 | 179 per 1000 | 227 per 1000 (145 to 354) | RR 1.27 (0.81 to 1.98) | 314 (1 RCT) | ⊕⊕⊝⊝ Lowa | Bamlanivimab may slightly increase the risk of grade 3 and 4 adverse events. |
| Serious adverse events by day 30 | 33 per 1000 | 31 per 1000 (9 to 104) | RR 0.93 (0.27 to 3.14) | 314 (1 RCT) | ⊕⊕⊝⊝ Lowa | We are uncertain whether bamlanivimab has an effect the occurrence of serious adverse events. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; HR: hazard Ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is the possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_422871223304980961. | ||||||
a Downgraded two levels for very serious imprecision, because of wide confidence interval, low sample size and/or low number of events, imbalances in baseline characteristics.
a Downgraded one level for serious indirectness, because the time frame of 5 days was short for assessing clinical status.
Summary of findings 7. Casirivimab/imdevimab compared to usual care alone in hospitalised individuals with COVID‐19 (moderate to severe disease).
| Casirivimab/imdevimab compared to usual care in hospitalised individuals with COVID‐19 (moderate to severe disease) | ||||||
| Patient or population: hospitalised individuals with COVID‐19 (moderate to severe disease) Setting: inpatient Intervention: casirivimab/imdevimab Comparison: usual care alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty in the evidence (GRADE) | Comments | |
| Risk with usual care alone | Risk with casirivimab/imdevimab | |||||
| Mortality by day 30 | 236 per 1000 | 221 per 1000 (205 to 241) | RR 0.94 (0.87 to 1.02) | 9785 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Casirivimab/imdevimab 8.0 g has probably little to no effect on mortality by day 30 in the overall cohort of hospitalised participants. |
| Mortality by day 60 | not reported | ‐ | ‐ | We did not identify any study reporting this outcome. | ||
| Clinical progression: need for IMV or death (WHO ≥ 7) | 248 per 1000 | 238 per 1000 (223 to 258) | RR 0.96 (0.90 to 1.04) | 9198 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Casirivimab/imdevimab 8.0 g has probably little to no effect on IMV requirement or death by day 30 in the overall cohort of hospitalised participants. |
| Quality of life | not measured | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. | |
| Hospital discharge alive by day 30 | 690 per 1000 | 697 per 1000 (676 to 718) | RR 1.01 (0.98 to 1.04) | 9785 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Casirivimab/imdevimab 8.0 g has probably little to no effect on discharge from hospital alive by day 30 in the overall cohort of hospitalised participants. |
| Adverse events: grades 3 and 4 by day 30 | not reported | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. | |
| Serious adverse events by day 30 | not reported | ‐ | ‐ | ‐ | We did not identify any study reporting this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RCT: randomised controlled trial | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is the possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level due to serious risk of bias, the study was not blinded. Currently, there is no clearly defined standard of care for COVID‐19, therefore, a lack of blinding can have resulted in differential treatments/timings of treatment between arms.
SARS‐CoV‐2‐specific mAbs in non‐hospitalised individuals with COVID‐19 and asymptomatic or mild disease
Bamlanivimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
We present our certainty in the evidence for prioritised outcomes for the comparison bamlanivimab and placebo in non‐hospitalised individuals in Table 1. We included only one study for this comparison, which did not report the outcomes mortality by day 60, clinical progression or development of severe symptoms, and quality of life (BLAZE‐1 (phase 2)). Since all outcomes were evaluated separately for each treatment arm (0.7 g, 2.8 g, 7.0 g of bamlanivimab), we did not pool the data.
Primary outcomes
All‐cause mortality (by days 30 and 60)
One RCT (BLAZE‐1 (phase 2)) reported all‐cause mortality at day 29 for 465 participants.
None of the participants died by day 29 (101 participants in dose 0.7 g arm, 107 participants in 2.8 g arm, 101 participants in 7.0 g arm and 156 participants in placebo arm; effect estimates not estimable, low‐certainty evidence for all arms; Analysis 1.1). For each comparison, our main reason for downgrading was very serious imprecision due to no observed events in any of the groups. BLAZE‐1 (phase 2) did not report all‐cause mortality at up to 60 days.
1.1. Analysis.

Comparison 1: Bamlanivimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 1: Mortality by day 30
Admission to hospital or death
Admission to hospital was reported for 465 participants at day 29 (BLAZE‐1 (phase 2)). Data indicate that treatment with 0.7 g, 2.8 g or 7.0 g bamlanivimab may result in a decrease in admissions to hospital compared to treatment with placebo, with 10, 18 and 20 admissions per 1000 for the bamlanivimab groups as compared to 58 per 1000 in the placebo group (risk ratio (RR) 0.17, 95% confidence interval (CI) 0.02 to 1.33; RR 0.32, 95% CI 0.07 to 1.47; and RR 0.34, 95% CI 0.08 to 1.56, respectively; 1 RCT). For each comparison, our main reason for downgrading was very serious imprecision due to low sample size and low number of events.
Adverse events (all grades, grades 1 to 2, grades 3 to 4)
BLAZE‐1 (phase 2) reported the number of participants with one or more adverse events, which first occurred or worsened in severity after baseline measurement, for 465 participants. Since the data were evaluated separately for each treatment arm (0.7 g, 2.8 g, and 7.0 g bamlanivimab), we did not pool the data.
Treatment with 0.7 g, 2.8 g or 7.0 g bamlanivimab may result in little to no difference in the occurrence of adverse events (267, 242 and 218 per 1000) compared to treatment with placebo (269 per 1000; RR 0.99, 95% CI 0.66 to 1.50; RR 0.90, 95% CI 0.59 to 1.38; and RR 0.81, 95% CI 0.52 to 1.27, respectively; 1 RCT, 257, 263 and 257 participants; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Bamlanivimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 5: Adverse events: all grades
For each comparison, our main reason for downgrading was very serious imprecision due to low sample size, low number of events, and wide confidence intervals. Adverse events were coded according to MedDRA, the Medical Dictionary for Regulatory Activities, and were classified as mild, moderate and severe. Therefore, we could not summarise grades 1 to 2 and grades 3 to 4 adverse events.
Serious adverse events
BLAZE‐1 (phase 2) assessed serious adverse events for 465 participants. Since the data were evaluated separately for each treatment arm that received different doses of bamlanivimab (0.7 g, 2.8 g, 7.0 g), we have not pooled the data.
There were too few participants who experienced serious adverse events to determine whether treatment with 0.7 g, 2.8 g or 7.0 g bamlanivimab has an effect on serious adverse events (3 per 1000 for all arms) compared to treatment with placebo (6 per 1000; RR 0.51, 95% CI 0.02 to 12.47; RR 0.48, 95% CI 0.02 to 11.78; and RR 0.51, 95% CI 0.02 to 12.47, respectively; 1 RCT; low‐certainty evidence; Analysis 1.6). For each comparison, our main reason for downgrading was very serious imprecision due to low sample size, very low number of events, and very wide confidence intervals.
1.6. Analysis.

Comparison 1: Bamlanivimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 6: Serious adverse events
Additional outcomes
BLAZE‐1 (phase 2) did not report length of hospital stay, admission to ICU, length of ICU stay, thromboembolic events, or renal failure.
Viral clearance (up to days 3, 7 and 15)
We defined viral clearance as a negative RT‐PCR test after treatment and planned to include clearance up to three, seven and 15 days. BLAZE‐1 (phase 2), defined viral clearance as two consecutive negative RT‐PCR test results for SARS‐CoV‐ 2. Since the data were evaluated separately for each treatment arm that received different doses of bamlanivimab (0.7 g, 2.8 g, 7.0 g), we did not pool the data.
Viral clearance up to day 3: BLAZE‐1 (phase 2) did not report viral clearance up to day 3.
Viral clearance up to day 7: BLAZE‐1 (phase 2) assessed viral clearance up to day 7 for 444 participants in total. Evidence suggests that treatment with 0.7 g, 2.8 g, or 7.0 g bamlanivimab may have little to no effect on viral clearance at days 7, with 10 out of 99 participants in the 0.7 g arm, 12 out of 101 participants in the 2.8 g arm, and 8 out of 99 participants in the 7.0 g arm achieving viral clearance compared to 16 out of 145 in the placebo arm (RR 0.92, 95% CI 0.43 to 1.93; RR 1.08, 95% CI 0.53 to 2.18; and RR 0.73, 95% CI 0.33 to 1.65, respectively; 1 RCT; Analysis 1.3). The evidence is uncertain due to the small sample size and wide confidence intervals.
Viral clearance up to day 15: BLAZE‐1 (phase 2) assessed viral clearance at day 15 for 414 participants. Evidence suggests that treatment with 0.7 g, 2.8 g, or 7.0 g bamlanivimab may have little to no effect on viral clearance at day 15, with 25 out of 91 participants in the 0.7 g arm, 30 out of 97 participants in the 2.8 g arm, and 25 out of 94 participants in the 7.0 g arm achieving viral clearance compared to 34 out of 132 in the placebo group (RR 1.07, 95% CI 0.69 to 1.66; RR 1.20, 95% CI 0.79 to 1.82; and RR 1.03, 95% CI 0.66 to 1.61, respectively; 1 RCT; Analysis 1.4). The evidence is uncertain due to the small sample size and wide confidence intervals.
1.3. Analysis.

Comparison 1: Bamlanivimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 3: Viral clearance at day 7
1.4. Analysis.

Comparison 1: Bamlanivimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 4: Viral clearance at day 15
Bamlanivimab/etesevimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
We present our certainty in the evidence for prioritised outcomes for bamlanivimab/etesevimab compared with placebo in non‐hospitalised individuals in Table 2. This comparison was examined in a two‐part study (BLAZE‐1 (phase 2); BLAZE‐1 (phase 3)), reported in two separate publications and one conference abstract. We decided to not include the phase 2 part of the study, because the start of treatment arms was staggered, and the allocation ratio was adjusted. This resulted in a late start of the bamlanivimab/etesevimab arm on 22 August 2021, while the placebo group was mainly enrolled up to 21 August 2021, with only 13 participants having been enrolled and analysed after this date. Therefore, we report on phase 2 narratively only. The outcomes mortality by day 60, clinical progression or development of severe symptoms and quality of life were not reported.
Primary outcomes
All‐cause mortality (by days 30 and 60)
The phase 3 part reported data on 1035 participants. Bamlanivimab/etesevimab may reduce overall mortality by day 30 as compared with placebo (1 death per 1000 participants as compared to 19 per 1000 in the placebo group; RR 0.05, 95% CI 0.00 to 0.81; 1 RCT, 1035 participants; low‐certainty evidence; Analysis 2.1). Our main reason for downgrading was very serious imprecision due to the low number of events. BLAZE‐1 (phase 3) did not report all‐cause mortality by day 60.
2.1. Analysis.

Comparison 2: Bamlanivimab/etesevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 1: Mortality by day 30
In BLAZE‐1 (phase 2), no deaths occurred by day 29 (112 in bamlanivimab/etesevimab arm, 151 in placebo arm), mortality by day 60 was not reported.
Admission to hospital or death
BLAZE‐1 (phase 3) reported the outcome admissions to hospital for 1035 participants by day 29. Treatment with bamlanivimab/etesevimab (21 per 1000) may result in a decrease in admissions to hospital by day 29 compared to treatment with placebo (70 per 1000; RR 0.30, 95% CI 0.16 to 0.59; 1 RCT, 1035 participants; low‐certainty evidence; Analysis 2.2). Our main reason for downgrading was very serious imprecision due to low number of events.
2.2. Analysis.

Comparison 2: Bamlanivimab/etesevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 2: Admission to hospital or death
In BLAZE‐1 (phase 2), 1 out of 112 in the bamlanivimab/etesevimab and 9 out of 156 in the non‐concurrent placebo arm were hospitalised.
Adverse events (all grades, grades 1 to 2, grades 3 to 4)
BLAZE‐1 (phase 3) reported the number of participants with one or more adverse events, whichever first occurred or worsened in severity after baseline measurement for 1035 participants in this comparison.
Treatment with bamlanivimab/etesevimab may have little to no effect on the occurrence of adverse events (133 per 1000) compared to treatment with placebo (116 per 1000; RR 1.15, 95% CI 0.83 to 1.59; 1 RCT, 1035 participants; low‐certainty evidence; Analysis 2.6). Our main reason for downgrading was very serious imprecision due to the low sample size. Adverse events were coded according to MedDRA, the Medical Dictionary for Regulatory Activities, and were classified as mild, moderate, and severe. Therefore, we could not summarise grades 1 to 2 and grades 3 to 4 adverse events.
2.6. Analysis.

Comparison 2: Bamlanivimab/etesevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 6: Adverse events: all grades
In BLAZE‐1 (phase 2), 12 out of 112 in the bamlanivimab/etesevimab arm and 42 out of 156 in the non‐concurrent placebo arm experienced at least one adverse event.
Serious adverse events
BLAZE‐1 (phase 3) assessed serious adverse events for 1035 participants. Treatment with bamlanivimab/etesevimab may increase the occurrence of serious adverse events (14 per 1000) as compared with placebo (10 per 1000; RR 1.40, 95% CI 0.45 to 4.37; 1 RCT, 1035 participants; low‐certainty evidence; Analysis 2.7). Our main reason for downgrading was very serious imprecision due to the low number of events and wide confidence interval.
2.7. Analysis.

Comparison 2: Bamlanivimab/etesevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 7: Serious adverse events
In BLAZE‐1 (phase 2), 1 out of 112 participants in the bamlanivimab/etesevimab arm and 1 out of 156 in the placebo arm experienced an event.
Additional outcomes
BLAZE‐1 (phase 3) did not report the additional outcomes admission to ICU, length of ICU stay, thromboembolic events, and renal failure.
Length of hospital stay
The mean duration of hospitalisation was 7.3 days (SD = 6.4 days; 11 participants) in the bamlanivimab/etesevimab arm and 11.2 days (SD = 10.1 days, 33 participants) in the placebo arm.
Viral clearance (up to days 3, 7, and 15)
The study defined viral clearance as two consecutive negative RT‐PCR test results for SARS‐CoV‐ 2.
BLAZE‐1 (phase 3) reported a Kaplan‐Meier graph including the number of events per day, which we used to calculate cumulative frequency of viral clearance. Evidence from BLAZE‐1 (phase 3) suggests that more people may achieve viral clearance at days 3, 7, and 15 when treated with bamlanivimab/etesevimab compared with placebo (day 3: RR 2.12, 95% CI 1.16 to 3.86; day 7: RR 1.81, 95% CI 1.27 to 2.60; day 15: RR 1.34, 95% CI 1.07 to 1.67; 1 RCT, 968 participants).
In BLAZE‐1 (phase 2), 14 out of 100 in the experimental group and 16 out of 145 in the placebo group achieved viral clearance at day 7, and 34 out of 98 in the experimental group compared to 34 out of 132 participants in the placebo arm achieved viral clearance.
Casirivimab/imdevimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
For the comparison casirivimab/imdevimab versus placebo in non‐hospitalised individuals with COVID‐19, we identified one phase 1/2/3 study with published results (Weinreich (phase 1/2); Weinreich (phase 3)) from phase 1/2 in one full‐text and one preprint publication, and phase 3 in an additional preprint. The first part of the study randomised participants to either 2.4 g or 8.0 g treatment or placebo. In the course of phase 3 of the study on 14 November 2020, the treatment doses were amended, the 8 g arm was stopped and a 1.2 g arm was added. In addition, the eligibility was amended from participants with and without participants to participants with at least one risk factor for severe disease. For the first part of the study, we have considered the preprint with updated data, including 799 randomised participants. Of these, 665 were included in the modified full analysis set which included only participants who tested positive on central RT‐qPCR.
Due to very serious risk of bias, we decided to not pool the data from phase 3 with the remaining data and report these narratively only. In particular, we could not follow the participant flow throughout the study; it was unclear whether the 1040 participants without risk factors were initially randomised and if so, why data were not reported. In addition, the origin of the number of participants in the placebo group of the safety set is not clearly documented, and results for the 8.0 g casirivimab/imdevimab arm are reported arbitrarily. Only data for cohort 1 were provided. The concurrent placebo groups vary but participants overlap with each other. Results based on phase 1 are presented in Table 3.
Primary outcomes
All‐cause mortality (by days 30 and 60)
The phase 1/2 part of the study by Weinreich (phase 1/2) did not report all‐cause mortality by days 30 or 60.
In the phase 3 part of the study, the number of events in all treatment arms (1.2 g, 2.4 g and 8.0 g) was very low and does not allow us to explore whether casirivimab/imdevimab may have an effect on all‐cause mortality by day 29 when compared with placebo. In both the 1.2 g and 2.4 g treatment arms, one participant died out of 736 and 1355 participants, in the 8.0 g, no one died out of 625. The reported concurrent placebo groups varied, in the combined placebo group, 3 out of 1341 participants died.
Clinical progression or development of severe symptoms according to the WHO scale
The phase 1/2 part of the study did not report any data on clinical progression or development of severe symptoms that could be translated into an outcome based on the WHO progression scale, although IMV requirement was planned at the protocol stage.
The phase 3 part reported the number of participants that require IMV (WHO scale 7, 8, 9), which does not explicitly includes deaths, for the 1.2 g and 2.4 g casirivimab/imdevimab arms. The number of events was too low to explore the direction of the effect of casirivimab/imdevimab.
| Clinical progression to IMV | Casirivimab/imdevimab | Placebo | ||
| Events | Participants analysed | Events | Participants analysed | |
| 1.2 g | 1 | 736 | 2 | 748 |
| 2.4 g | 1 | 1355 | 6 | 1341 |
| 8.0 g | NR | NR | NR | NR |
In addition to the very serious bias, it is not explicitly mentioned whether the outcome includes death events and may therefore be prone to effect distortion in case people died before receiving IMV.
Admission to hospital or death
In the phase 1/2 part of the study, casirivimab/imdevimab at 2.4 g or 8.0 g may reduce the frequency of hospital admission or death compared with placebo (9 and 5 per 1000 compared to 22 per 1000 in the placebo group; 2.4 g: RR 0.43, 95% CI 0.08 to 2.19, 446 participants; 8.0 g: RR 0.21, 95% CI 0.02 to 1.79, 450 participants; 1 RCT; low‐certainty evidence; Analysis 3.1). Our main reason for downgrading was the low sample size and low number of events.
3.1. Analysis.

Comparison 3: Casirivimab/imdevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 1: Admission to hospital or death
The phase 3 part of the study reported the number of participants admitted to hospital or dead by day 29:
| Hospital admission or death | Casirivimab/imdevimab | Placebo | ||
| Events | Participants analysed | Events | Participants analysed | |
| 1.2 g | 7 | 736 | 24 | 748 |
| 2.4 g | 18 | 1355 | 62 | 1341 |
| 8.0 g | 13 | 625 | 38 | 593 |
Due to the very serious risk of bias, we are uncertain whether casirivimab/imdevimab has an effect on hospital admissions or death.
Adverse events
The phase 1/2 part of the study reported grades 3 to 4 adverse events only. There were too few events to determine whether casiribimab/imdevimab at 2.4 g or 8.0 g has an effect on the occurrence of grades 3 to 4 adverse events (12 and 8 per 1000 compared to 15 per 1000 for the placebo arm; 2.4 g: RR 0.76, 95% CI 0.17 to 3.37, 520 participants; 8.0 g: RR 0.50, 95% CI 0.09 to 2.73, 522 participants; 1 RCT; low‐certainty evidence; Analysis 3.2). We graded down due to very serious imprecision, the sample size was small with a low number of events.
3.2. Analysis.

Comparison 3: Casirivimab/imdevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 2: Adverse events: grade 3 and 4
Part 3 of the study reported all grades adverse events and grades 3 to 4 adverse events separately:
| Adverse events | Casirivimab/imdevimab | Placebo | |||
| Events | Participants analysed | Events | Participants analysed | ||
| All grades | 1.2 g | 59 | 827 | 189 | 1843 |
| 2.4 g | 142 | 1849 | |||
| 8.0 g | 85 | 1012 | |||
| Grades 3 to 4 | 1.2 g | 11 | 827 | 62 | 1843 |
| 2.4 g | 18 | 1849 | |||
| 8.0 g | 15 | 1012 | |||
| Serious adverse events | 1.2 g | 9 | 827 | 74 | 1843 |
| 2.4 g | 24 | 1849 | |||
| 8.0 g | 17 | 1012 | |||
We are uncertain whether casiriviamb/imdevimab 1.2 g, 2.4 g or 8.0 g has an effect on adverse events or serious adverse events.
Serious adverse events
The number of events in phase 1/2 of the study was too small to determine whether any dose of casirivimab/imdevimab (2.4 g, 8.0 g) may have an effect on the occurrence of serious adverse events compared with placebo (16 and 8 per 1000 as compared to 23 per 1000; 2.4 g: RR 0.68, 95% CI 0.19 to 2.37, 520 participants; 8.0 g: RR 0.34, 95% CI 0.07 to 1.65; 1 RCT, 522 participants; low‐certainty evidence; Analysis 3.3). We downgraded two levels for very serious imprecision; the sample size was low with a small number of events.
3.3. Analysis.

Comparison 3: Casirivimab/imdevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 3: Serious adverse events
Serious adverse events reported in the phase 3 preprint publication are listed under adverse events.
Additional outcomes
For this comparison, the following outcomes were not reported in any of the preprints: length of ICU stay, viral clearance, frequency of thromboembolic events, and frequency of renal failure.
Length of hospital stay
The phase 3 portion of the trial reported on the length of hospital stay for dose 1.2 and 2.4 g as mean days and standard deviation, however, the number of participants who contributed to this outcome was very low (1.2 g: N = 7, concurrent placebo N = 24; 2.4 g: N =18, concurrent placebo N = 62). The data for 8.0 g are not reported. It is unclear whether patients who died were accounted for (i.e., earlier death means a shorter hospital stay).
Admission to ICU
The phase 3 part of the trial reported the number of participants admitted to the ICU. However, the number of events was too low to explore whether casirivimab/imdevimab has an effect on this outcome (1.2 g: 3 out of 736, 7 out of 749 in the concurrent placebo arm; 2.4 g: 6 out of 1355 as compared to 18 out of 1341 in the concurrent placebo arm). The results for the 8.0 g arm was not reported and it is unclear whether competing events (i.e. deaths) were accounted for.
Sotrovimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
We present our certainty in the evidence for prioritised outcomes for the comparison sotrovimab compared with placebo in non‐hospitalised individuals in Table 4. The one eligible trial with results (COMET‐ICE) for this comparison has been stopped for profound efficacy by the data monitoring committee on 10 March 2021 with 1057 participants enrolled. The preprint, uploaded on 28 May 2021, included participants up to 19 January 2021 for efficacy (583) and up to 17 February 2021 for safety (868) for preplanned interim analysis, the sets vary substantially in the number of participants analysed.
Primary outcomes
All‐cause mortality (by days 30 and 60)
Only one participant in the placebo group had died by day 29 (291 participants in sotrovimab arm, 292 in placebo arm; 1 RCT, 583 participants; low‐certainty evidence; Analysis 4.1), therefore, there were too few events to determine whether treatment with 0.5 g sotrovimab has an effect on mortality at 30 days. Our main reason for downgrading was very serious imprecision due to few events, which led to a very wide confidence interval. COMET‐ICE did not report all‐cause mortality at up to 60 days.
4.1. Analysis.

Comparison 4: Sotrovimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 1: Mortality by day 30
Clinical progression or development of severe symptoms according to the WHO scale
COMET‐ICE reported provided information on several stages of severe or critical progression. Based on relevance, we decided to include two related endpoints that translate to development of severe symptoms based on the WHO progression scale, requirement of oxygen (≥ 5) and requirement of invasive mechanical ventilation (≥ 7), both including deaths.
Included in both analyses were 583 participants. Evidence suggests that sotrovimab may reduce the number of participants with oxygen requirement (WHO ≥ 5; 7 per 1000 participants) compared with placebo (65 per 1000; RR 0.11, 95% CI 0.02 to 0.45; 1 RCT, 583 participants; low‐certainty evidence; Analysis 4.2). For the outcome IMV requirement or death, the number of events was too small to determine whether sotrovimab may have an effect (1 per 1000 as compared to 10 per 1000 in the placebo group; RR 14, 95% CI 0.01 to 2.76; 1 RCT, 583 participants; low‐certainty evidence; Analysis 4.3). Our main reason for downgrading was the low sample size and low number of events.
4.2. Analysis.

Comparison 4: Sotrovimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 2: Development of severe symptoms according to WHO scale (≥ 5, incl. death)
4.3. Analysis.

Comparison 4: Sotrovimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 3: Development of severe symptoms according to WHO scale (≥ score 7, IMV, incl. death)
Admission to hospital or death
Sotrovimab may reduce the number of hospital admissions or death (10 per 1000) compared to placebo (72 per 1000; RR 0.14, 95% CI 0.04 to 0.48; 1 RCT, 583 participants; low‐certainty evidence; Analysis 4.4). Our main reason for grading down was the small sample size and low number of events.
4.4. Analysis.

Comparison 4: Sotrovimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 4: Admission to hospital or death
Adverse events (all grades, grades 1 to 2, grades 3 to 4)
Evidence suggests that treatment with sotrovimab compared to placebo may have little to no effect on the occurrence of any grade adverse events (169 per 1000 as compared to 194 per 1000 with placebo; RR 0.87, 95% CI 0.66 to 1.16; 1 RCT, 868 participants; low‐certainty evidence; Analysis 4.6). Occurrence of grades 3‐4 adverse events may be reduced by treatment with sotrovimab compared to placebo (16 versus 62 per 1000 for sotrovimab and placebo, respectively; RR 0.26, 95% CI 0.12 to 0.60; 1 RCT, 868 participants; low‐certainty evidence; Analysis 4.7). Grades 1 to 2 adverse events were not reported separately. We downgraded two levels due to the low sample size.
4.6. Analysis.

Comparison 4: Sotrovimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 6: Adverse events: all grades
4.7. Analysis.

Comparison 4: Sotrovimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 7: Adverse events: grade 3 and 4
Serious adverse events
Treatment with sotrovimab may reduce the occurrence of serious adverse events compared to placebo (16 per 1000 compared to 59 per 1000 participants with placebo; RR 0.27, 95% CI 0.12 to 0.63; 1 RCT, 868 participants; low‐certainty evidence; Analysis 4.8). We downgraded two levels due to very serious imprecision; the sample size was low with a small number of events.
4.8. Analysis.

Comparison 4: Sotrovimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 8: Serious adverse events
Additional outcomes
For the comparison sotrovimab versus placebo in non‐hospitalised individuals, we did not identify data for the outcomes length of hospital stay, length of ICU stay, viral clearance, thromboembolic events and renal failure.
Admission to ICU
The number of events is too low to determine whether treatment with sotrovimab reduces the number of participants admitted to ICU by day 29 (0 out of 291 in the sotrovimab group compared to 5 out of 292 in the sotrovimab group; RR 0.09, 95% CI 0.01 to 1.64; Analysis 4.5).
4.5. Analysis.

Comparison 4: Sotrovimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 5: Admission to ICU by day 29
Regdanvimab compared to placebo in non‐hospitalised individuals with COVID‐19 (asymptomatic or mild disease)
We present our certainty in the evidence for prioritised outcomes for the comparison regdanvimab compared with placebo in non‐hospitalised individuals in Table 5. We included only one study (Eom 2021) which reported on part 1 of a two‐part phase 2/3 study in form of a preprint. Mortality by day 60 and quality of life were not measured or reported, serious adverse events were reported as treatment‐emergent SAE; however, we considered this outcome as unreliable because no events were reported although hospitalisation events took place.
Primary outcomes
All‐cause mortality (by day 30 and 60)
None of the participants had died by day 28 (101 in the CT‐P59 40 mg/kg arm, 103 in the regdanvimab 80 mg/kg arm and 103 participants in placebo arm; effect estimate was not estimable; low‐certainty evidence; Analysis 5.1). Our main reason for downgrading was very serious imprecision due to no observed events in any group.
5.1. Analysis.

Comparison 5: Regdanvimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 1: Mortality by day 30
Eom 2021 did not report all‐cause mortality at up to 60 days.
Clinical progression or development of severe symptoms according to the WHO scale
The study reported the number of participants who had progressed to invasive mechanical ventilation or death, which translates into a WHO score of ≥ 7.
In the regdanvimab 40 mg/kg arm and placebo arm, no one required mechanical ventilation (among 101 participants in regdanvimab 40 mg/kg arm and 103 participants in placebo arm, effect not estimable; low‐certainty evidence; Analysis 5.2). There were too few participants who experienced progress to invasive mechanical ventilation to determine whether treatment with CT‐P59 80 mg/kg has an effect (1 in 103 in regdanvimab 80 mg arm, 0 in 103 in placebo arm; RR 3.00, 95% CI 0.12 to 72.89; 1 RCT, 206 participants; low‐certainty evidence; Analysis 5.2).
5.2. Analysis.

Comparison 5: Regdanvimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 2: Development of severe symptoms according to WHO scale (≥ score 7, IMV, incl. death)
For both comparisons, the main reason for downgrading was very serious imprecision due to very low number of events and resulting in very wide confidence intervals.
Admission to hospital or death by day 30
Treatment with either 40 mg/kg or 80 mg per kg regdanvimab may decrease the frequency of hospital admission or death by day 30 compared to placebo (39 or 49 per 1000 in the regdanvimab arms versus 87 per 1000 in the placebo arm; 40 mg/kg: RR 0.45, 95% CI 0.14 to 1.42; 1 RCT, 204 participants; 80 mg/kg: RR 0.56, 95% CI 0.19 to 1.60; 1 RCT, 206 participants; low‐certainty evidence; Analysis 5.3). Our main reason for downgrading was very serious imprecision due to the small sample size and small number of events.
5.3. Analysis.

Comparison 5: Regdanvimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 3: Admission to hospital or death by day 30
Adverse events (all grades, grades 1 to 2, grades 3 to 4)
Treatment with 40 mg/kg regdanvimab may have little to no effect on the occurrence of any grade adverse events compared with placebo (297 versus 309 per 1000 for regdanvimab and placebo, respectively; RR 0.96, 95% CI 0.64 to 1.43; 1 RCT, 215 participants; low‐certainty evidence; Analysis 5.6). Treatment with 80 mg/kg regdanvimab may reduce the occurrence of any grade adverse events compared with placebo (244 versus 309 per 1000; RR 0.79, 95% CI 0.52 to 1.22; 1 RCT, 220 participants; low‐certainty evidence). Our main reason for downgrading was the low sample size.
5.6. Analysis.

Comparison 5: Regdanvimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 6: Adverse events: all grades
Treatment with either 40 mg/kg or 80 mg/kg regdanvimab may increase the occurrence of grade 3 adverse events compared to placebo, no grade 4 adverse events took place (48 and 36 per 1000 compared to 18 per 1000 in the placebo group; 40 mg/kg: RR 2.62, 95% CI 0.52 to 13.12; 1 RCT, 215 participants; 80 mg/kg: RR 2.00, 95% CI 0.37 to 10.70; 1 RCT, 220 participants; low‐certainty evidence; Analysis 5.7). Our main reason for downgrading was the small sample size and low number of events.
5.7. Analysis.

Comparison 5: Regdanvimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 7: Adverse events: grade 3 and 4
Additional outcomes
The outcomes length of hospital stay, length of ICU stay, frequency of thromboembolic events, and frequency of renal failure were not available for this comparison.
Admission to ICU or death
None of the participants had been admitted to ICU by day 28 (101 in the CT‐P59 40 mg/kg arm, 103 in the CT‐P59 80 mg/kg arm and 103 participants in placebo arm; effect not estimable; Analysis 5.4).
5.4. Analysis.

Comparison 5: Regdanvimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 4: Admission to ICU by day 30
Viral clearance (up to day 3, 7 and 15)
Viral clearance up to day 3 and 7: not reported.
Viral clearance up to day 15: Eom 2021 assessed viral clearance at day 15 for 309 participants. Evidence suggests that treatment with regdanvimab may have little to no effect on viral clearance at day 15, with 68 participants in each treatment arm, as compared to 62 out of 103 participants in the placebo arm achieving viral clearance (RR 1.12, 95% CI 0.91 to 1.38 for 40 mg/kg; RR 1.10, 95% CI 0.89 to 1.35 for 80 mg/kg, respectively; 1 RCT; Analysis 5.5).
5.5. Analysis.

Comparison 5: Regdanvimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 5: Viral clearance at day 15
SARS‐CoV‐2‐specific mAbs in hospitalised individuals with COVID‐19 (moderate to severe disease)
Bamlanivimab compared to placebo in individuals with COVID‐19 (moderate to severe disease)
We present the certainty in the evidence for our prioritised outcomes for the comparison bamlanivimab versus placebo in hospitalised individuals with COVID‐19 in Table 6. We included one study published as one full text and one preprint publication. The preprint with the final analysis reported the subgroups for seronegative and seropositive participants at baseline for mortality at 90 days, sustained recovery and serious adverse events. Quality of life was not reported.
Primary outcomes
All‐cause mortality (by days 30 and 60)
One RCT reported all‐cause mortality by days 28 and 90 for 314 participants (ACTIV‐3).
We are uncertain whether bamlanivimab has an effect on all‐cause mortality at 28 days (37 per 1000) when compared to treatment with placebo (26 per 1000; RR 1.39, 95% CI 0.40 to 4.83; 1 RCT, 314 participants; low‐certainty evidence; Analysis 6.1). Our main reason for downgrading was very serious imprecision due to wide confidence interval, low sample size, and the low number of events.
6.1. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 1: Mortality by day 30
The 90‐day follow‐up resulted in a hazard ratio (HR) of 1.09 (95% CI 0.49 to 2.43; 1 RCT, 314 participants; low‐certainty evidence; Analysis 6.2), indicating that bamlanivimab may have little to no effect on mortality compared with placebo (79 per 1000 compared to 73 per 1000 with placebo). Our certainty in the evidence was low due to very serious imprecision; the sample size was small, resulting in a low number of events and large confidence intervals.
6.2. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 2: Mortality by day 90
The subgroup analysis of the primary study authors suggests that the effect may differ between seronegative and seropositive participants at baseline. In seronegative participants, 5.4% of participants with bamlanivimab as compared to 11.4% with placebo experienced an event (HR 0.46, 95% CI 0.14 to 1.48). In contrast, in the seropositive arm, 9.6% in the bamlanivimab arm as compared to 2.9% in the placebo arm experienced an event (HR 3.52, 95% CI 0.75 to 16.58).
Clinical progression or development of severe symptoms according to the WHO scale
ACTIV‐3 reported the proportion of participants in each pulmonary clinical status category at day 5 for 311 participants per category. From this, we extracted severe symptoms based on the need for NIV, IMV, ECMO, or renal replacement therapy at day 5 (category 5 to 7 on the pulmonary‐plus scale).
Bamlanivimab may result in a slight increase in severe symptoms at day 5 (211 per 1000) compared to placebo (180 per 1000; RR 1.17, 95% CI 0.75 to 1.85; 1 RCT, 311 participants; very low‐certainty evidence; Analysis 6.3). Our main reason for downgrading was serious indirectness due to a short time frame of five days and serious imprecision due to wide confidence interval and low sample size, and for indirectness due to the short time frame of five days only.
6.3. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 3: Development of severe symptoms: need for NIV, IMV, ECMO, or renal replacement therapy at day 5 (group 5, 6 or 7)
We additionally extracted clinical status at day 5 by the need for intubation (which corresponds to category six at the pulmonary scale). From 161 participants treated with bamlanivimab, eight needed intubation, compared to five out of 150 participants in the control group. Treatment with bamlanivimab may result in a slight increase in the need for intubation at day 5 compared to placebo (RR 1.49, 95% CI 0.50 to 4.46; 1 RCT, 311 participants; Analysis 6.4).
6.4. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 4: Development of severe symptoms: clinical status at day 5, intubation (group 6 at pulmonary scale)
Hospital discharge and alive
ACTIV‐3 reported the HR for hospital discharge until data cut‐off (26 October 2020) for 314 participants, stratified by disease severity and study site pharmacy and using the Fine and Gray method to correct for competing risks. Treatment with bamlanivimab may have little to no effect on time to hospital discharge (751 per 1000, based on control group risk at day 10) compared to treatment with placebo (762 per 1000; HR 0.97, 95% CI 0.78 to 1.20; 1 RCT, 314 participants; low‐certainty evidence; Analysis 6.6). Our main reason for downgrading was serious imprecision due to the low sample size.
6.6. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 6: Hospital discharge: at day 5
In addition to the HR, the study reported hospital discharge at day 5 for 314 participants. Evidence suggests there may be little to no difference between participants receiving bamlanivimab and placebo (RR 0.98, 95% CI 0.81 to 1.19; 1 RCT, 314 participants; Analysis 6.5).
6.5. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 5: Hospital discharge up to 26 October 2020
Quality of life (at up to seven and 30 days, and longest follow‐up, including fatigue)
We did not identify any study reporting this outcome.
Adverse events (all grades, grades 1 to 2, grades 3 to 4)
ACTIV‐3 reported grades 3 to 4 adverse events at day 28 for 314 participants. Bamlanivimab may result in a slight increase of grades 3 to 4 adverse events (227 per 1000) compared to treatment with placebo (179 per 1000; RR 1.27, 95% CI 0.81 to 1.98; 1 RCT, 314 participants; low‐certainty evidence; Analysis 6.7). Our main reason for downgrading was very serious imprecision due to wide confidence interval and low sample size. ACTIV‐3 did not report adverse events of all grades and grades 1 to 2.
6.7. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 7: Adverse events: grade 3 and 4 by day 30
Serious adverse events
ACTIV‐3 reported serious adverse events by day 28 for 314 participants. It is uncertain whether treatment with bamlanivimab has an effect on the occurrence of serious adverse events (31 per 1000) compared to treatment with placebo (33 per 1000); RR 0.93, 95% CI 0.27 to 3.14; 1 RCT, 314 participants; low‐certainty evidence; Analysis 6.7). Our main reason for downgrading was very serious imprecision due to wide confidence interval, low sample size, and low number of events.
Treatment with bamlanivimab may reduce the occurrence of SAE by day 90 compared to placebo (9 out of 163 compared to 12 out of 151; RR 0.7, 95% CI 0.29 to 1.67). However, the number of events is low, resulting in a broad confidence interval. The effect may differ between seronegative and seropositive participants as reported in the primary publication; in the seronegative group, 5.4% of participants who received bamlanivimab experienced an SAE compared with 10.1% in the placebo arm (HR 0.51, 95% CI 0.15 to 1.70). In the seropositive group, 6% of those allocated to bamlanivimab experienced an SAE compared to 5.8% in the placebo arm (RR 1.1, 95% CI 0.3 to 4.11).
Additional outcomes
ACTIV‐3 did not report results on length of hospital stay, admission to ICU, length of ICU stay, or viral clearance.
Time to sustained recovery
ACTIV‐3 assessed time to sustained recovery up to day 90. Treatment with bamlanivimab may result in little to no difference in time to sustained recovery compared to treatment with placebo (HR 0.99, 95% CI 0.80 to 1.23; 1 RCT, 314 participants; Analysis 6.10). The evidence is uncertain due to the small sample size.
6.10. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 10: Sustained recovery (90 day follow‐up)
Neurological dysfunction
This outcome was reported by ACTIV‐3 for 293 participants at day 28. Transient ischaemic events, acute delirium, and cerebrovascular events were reported separately. No transient ischaemic event occurred at day 28 in either group (RR not estimable; Analysis 6.11). Too few participants experienced acute delirium (4 out of 152 participants; RR 3.71, 95% CI 0.42 to 32.80) and cerebrovascular events (0 out of 152 participants; RR 0.31, 95% CI 0.01 to 7.53) to determine whether bamlanivimab has an effect compared to placebo (1 out of 141 participants; 1 RCT, 293 participants; Analysis 6.12; Analysis 6.13). The evidence is very uncertain due to the low number of events, small sample size and very wide confidence intervals.
6.11. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 11: Neurological dysfunction by day 30: transient ischemic events
6.12. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 12: Neurological dysfunction by day 30: acute delirium CVA
6.13. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 13: Neurological dysfunction by day 30: cerebrovascular event
Thromboembolic events
ACTIV‐3 assessed thromboembolic events until data cut‐off for 293 participants. Too few participants experienced thromboembolic events to determine whether bamlanivimab has an effect (3 out of 152 participants) compared with placebo (1 out of 141 participants; RR 2.78, 95% CI 0.29 to 26.44; 1 RCT, 293 participants; Analysis 6.14). The evidence is very uncertain due to the low number of events, small sample size, and very wide confidence intervals.
6.14. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 14: Thromboembolic events by day 30
Renal failure
ACTIV‐3 reported the need for dialysis at data cut‐off for 293 participants. Too few participants experienced need for dialysis to determine whether bamlanivimab has an effect (2 out of 152 participants) compared with placebo (0 out of 141 participants; RR 4.64, 95% CI 0.22 to 95.83; 1 RCT, 293 participants; Analysis 6.15). The evidence is very uncertain due to the low number of events, small sample size, and very wide confidence intervals.
6.15. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 15: Renal dysfunction (or need for dialysis) by day 30
Casirivimab/imdevimab and usual care compared to usual care alone in hospitalised individuals with COVID‐19 (moderate and severe disease)
The certainty in the evidence for our prioritised outcomes for the comparison casirivimab/imdevimab versus usual care in hospitalised individuals with COVID‐19 can be found in Table 7. The one inlcuded study, published as a preprint with 9785 participants enrolled to either casirivimab/imdevimab or usual care, did not report mortality at 60 days, quality of life, adverse events and serious adverse events. This study reported a hierarchical analysis, testing participants who have not produced antibodies at baseline first, and upon significance testing all participants including seropositive and participants with unknown status, we will report on this subgroup for prioritised outcomes, taken over from the preprint, narratively as well (serostatus: negative, positive, unknown).
The outcomes mortality by day 60, quality of life, adverse events and serious adverse events were not planned to be reported.
Primary outcomes
All‐cause mortality (by day 30 and 60)
Casirivimab/imdevimab has probably little to no effect on all‐cause mortality by day 28 when compared with usual care (221 per 1000 participants died as compared to 236 per 1000 in the usual care group; RR 0.94, 95% CI 0.87 to 1.02; 1 RCT, 9785 participants; moderate‐certainty evidence; Analysis 7.1). We downgraded one level due to serious risk of bias, which we rated as high based on the lack of blinding and a yet undefined standard of care, which may have led to differences in concomitant treatment (i.e. the timing of concomitant treatment).
7.1. Analysis.

Comparison 7: Casirivimab/imdevimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 1: Mortality by day 30
The subgroup analysis of the primary study authors suggests that in seronegative participants, mortality was lower in the casirivimab/imdevimab arm with 24% mortality compared to 30% mortality in the usual care group (RR 0.80, 95% CI 0.70 to 0.91). Among participants who were seropositive or with unknown serostatus, there was no difference between the treatment groups (seropositive: 16% compared to 15%, RR 1.09, 95% CI 0.95 to 1.26; unknown: 24% compared to 24%, RR 0.98, 95% CI 0.78 to 1.22; for casirivimab/imdevimab versus placebo).
Clinical progression or development of severe symptoms according to the WHO scale
RECOVERY reported the number of participants who required invasive mechanical ventilation or death by day 28 for those who were not yet ventilated at baseline. Casirivimab/imdevimab has probably little to no effect on IMV requirement by day 28 compared to usual care in the complete enrolled sample (238 in the casirivimab/imdevimab arm compared to 248 per 1000 receiving usual care; RR 0.96, 95% CI 0.90 to 1.04; 1 RCT, 9198 participants; moderate‐certainty evidence; Analysis 7.2). We downgraded one level due to serious risk of bias, which we rated as high based on the lack of blinding and a yet undefined standard of care, which may have led to differences in concomitant treatment (i.e., the timing of concomitant treatment).
7.2. Analysis.

Comparison 7: Casirivimab/imdevimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 2: Development of severe symptoms: requirement for IMV or death by day 28 (WHO ≥ 7)
Similar to the endpoint mortality, the subgroup analysis of the primary study authors suggests that for the seronegative group, the requirement of invasive mechanical ventilation was lower in the casirivimab/imdevimab arm than in the usual care arm (30% compared to 37%; RR 0.83, 95% CI 0.75 to 0.92). In the seropositive and unknown status groups, there was no difference between the treatment arms (seropositive: 19% compared to 17%, RR 1.10, 95% CI 0.97 to 1.24; unknown: 29% compared to 27%; RR 1.05, 95% CI 0.87 to 1.26).
Hospital discharge alive
In the complete randomised group, casirivimab/imdevimab has probably little to no effect on hospital charge alive by day 28 compared to placebo (697 compared to 690 per 1000; RR 1.01, 95% CI 0.98 to 1.04; 1 RCT, 9785 participants; moderate‐certainty evidence; Analysis 7.3). We downgraded one level due to serious risk of bias, which we rated as high based on the lack of blinding and a yet undefined standard of care, which may have led to differences in concomitant treatment (i.e., the timing of concomitant treatment).
7.3. Analysis.

Comparison 7: Casirivimab/imdevimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 3: Hospital discharge alive by day 30
The subgroup analysis of the primary study authors suggests that in the seronegative group, more participants were discharged alive by day 28 compared to usual care (64% compared to 58%, RR 1.19, 95% CI 1.08 to 1.30), whereas there was no difference between the arms in the seronegative and unknown group (seropositive: 75% versus 77%, RR 0.94, 95% CI 0.88 to 1.00; unknown: 63% versus 64%, RR 0.96, 95% CI 0.83 to 1.10).
Additional outcomes
RECOVERY did not report results on our additional outcomes admission to ICU, length of ICU stay, viral clearance, and neurological dysfunction.
Length of hospital stay
Length of hospital stay was reported as median duration of hospitalisation. In the complete randomised sample, the median duration was 10 days in the casirivimab/imdevimab group compared to 10 days in the usual care group, it is unclear whether the reported interval was the range or the interquartile range.
Thromboembolic events
Treatment with casirivimab/imdevimab has probably little to no effect on thrombotic events compared with usual care in the complete randomised group; 249 out of 4839 experienced an event in the intervention arm; 248 out of 4946 experienced an event under usual care (RR 1.03; 95% CI 0.86 to 1.22; 1 RCT, 9785 participants; Analysis 7.4).
7.4. Analysis.

Comparison 7: Casirivimab/imdevimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 4: Thromboembolic events by day 30
Renal dysfunction
Treatment with casirivimab/imdevimab has probably little effect on the requirement of renal replacement therapy for those not on renal replacement therapy at baseline, with 203 out of 4783 in the casirivimab/imdevimab arm experiencing an event compared with 201 out of 4887 in the usual care arm (RR 1.03, 95% CI 0.85 to 1.25; 1 RCT, 9670 participants; Analysis 7.5).
7.5. Analysis.

Comparison 7: Casirivimab/imdevimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 5: Renal dysfunction (or need for dialysis) by day 30
Discussion
Summary of main results
The aim of this review was to assess the effectiveness and safety of SARS‐CoV‐2‐neutralising neutralising monoclonal antibodies (mAbs) for the treatment of COVID‐19. We identified six randomised controlled trials (RCTs) (ACTIV‐3; BLAZE‐1 (phase 2); BLAZE‐1 (phase 3); COMET‐ICE; Eom 2021; RECOVERY; Weinreich (phase 1/2); Weinreich (phase 3)). Three studies were published as preprint only (COMET‐ICE; Eom 2021; RECOVERY). Weinreich (phase 1/2) or as a journal publication with additional data from two preprints (Weinreich (phase 1/2); Weinreich (phase 3)).
Four studies included outpatients receiving either bamlanivimab or bamlanivimab in combination with etesevimab (BLAZE‐1 (phase 2); BLAZE‐1 (phase 3)), a combination of casirivimab and imdevimab (under the trade name REGN‐COV2) (Weinreich (phase 1/2); Weinreich (phase 3)), regdanvimab (Eom 2021), or sotrovimab (COMET‐ICE). Two studies included hospitalised participants treated with bamlanivimab (ACTIV‐3) or a combination of casirivimab and imdevimab (RECOVERY).
All six included RCTs are active. However, most of them have stopped enrolment and participants are being followed‐up. One study is continuing observation of the respective arm (COMET‐ICE), two have added additional arms with different mAbs or doses (ACTIV‐3, BLAZE‐1 (phase 2)), and one continues with other treatments not relevant to this review (RECOVERY). BLAZE‐1 (phase 2), part of a phase 2 to 3 study examining three doses of bamlanivimab and combination therapy with etesevimab, published interim results on 592 randomised participants. Additionally, results from phase 3 of this study were reported for 1035 participants who received bamlanivimab/etesevimab or placebo (BLAZE‐1 (phase 3)). Regdanvimab at two different doses (0.04 g or 0.08g) compared to placebo was examined in Eom 2021, a two‐part phase 2 to 3 study. Results of a preplanned interim anlysis of the first part of the study, including 327 participants, were reported in a preprint. COMET‐ICE, another phase 2 to 3 study, examined sotrovimab compared to placebo and reported results of a preprint interim analysis on 583 participants. Recruitment was stopped on 10 March 2021 for benefit after 1057 participants had been randomised. There were separate data cut‐offs for efficacy and safety. Weinreich (phase 1/2) published a final analysis of phase 1/2 on 799 participants who were assigned to casirivimab plus imdevimab (2.4g or 8.0g) or placebo and results from the ongoing phase 3 (Weinreich (phase 3)) where 5607 participants were randomised to receive the combination casirivimab plus imdevimab (at three different doses 1.2g, 2.4g, 8.0g) or placebo. Based on results from Weinreich (phase 1/2), the trial was amended in November 2020 and only participants with at least one risk factor for severe COVID‐19 were included and no longer randomised to 8.0g casirivimab and imdevimab. In February 2021, participants were no longer randomised to placebo. ACTIV‐3, a platform trial, stopped recruitment to the bamlanivimab arm for futility after preplanned interim analysis, including 314 patients. In RECOVERY, another platform trial, 9785 participants were randomised to receive either a combination of casirivimab and imdevimab or usual care.
Non‐hospitalised individuals
Evidence suggests that the various doses of bamlanivimab may reduce admissions to hospital or death in outpatients (mild cases according to the previous World Health Organization (WHO) scale) compared to placebo, although the confidence intervals include both benefit and harm. Little to no effect may be seen on the occurrence of adverse events and viral clearance. The occurrence of serious adverse events was too rare to allow a judgment. All evidence should be interpreted with caution due to very serious imprecision; data are from one small study, event numbers are low and confidence intervals are wide.
For the mAb combination bamlanivimab and etesevimab compared to placebo in mild cases, evidence suggests a similar pattern to bamlanivimab. The combination may reduce admissions to hospital, but may have little to no effect on the occurrence of adverse events. We could not assess mortality in this study as there were no deaths, and the occurrence of serious adverse events was too rare to allow a judgment. Other preplanned outcomes for this review were not reported. Similar to the comparison for bamlanivimab alone in mild COVID‐19, data are from one small study only, so evidence should be considered uncertain due to very serious imprecision.
Although a preprint with phase 3 data for the comparison between multiple doses of casirivimab and imdevimab to placebo was available, we base our summary on the part 1/2 preprint only due to several uncertainties regarding the included participants. Casirivimab/imdevimab may reduce hospital admissions, however, the confidence interval includes both benefits and harms. For the outcomes grades 3 to 4 adverse events and serious adverse events, there were too few events to allow a judgment. No other outcomes were reported in part 1/2 of the study. Evidence should be considered uncertain due to very serious imprecision.
Sotrovimab may reduce the number of participants with oxygen requirement, hospitalisations or death, the number of hospital admissions or death, and the occurrence of grades 3 to 4 and serious adverse events compared to placebo, although the confidence intervals include both benefit and harms. Events for the outcomes mortality and invasive mechanical ventilation (IMV) requirement or death were too rare to allow a judgment on the effect. Data for this comparison should be interpreted with caution as they originate from one small study with low number of events and wide confidence intervals.
Evidence suggests that regdanvimab at either 40 mg or 80 mg/kg may reduce hospital admissions, and the 80 mg/kg dose may reduce adverse events when compared with placebo, although the confidence interval includes both benefits and harms. In contrast, regdanvimab may increase grade 3 adverse events, however, the confidence intervals include both benefit and harm. It may have little to no effect on viral clearance at day 15. We could not assess mortality, IMV requirement or death, serious adverse events, and admission to: intensive care unit (ICU), as no or few events took place. Due to the small sample size and low number of events, any evidence has to be interpreted with caution.
Hospitalised individuals
We are uncertain whether bamlanivimab has an effect on mortality and serious adverse events in hospitalised, moderate‐to‐severe COVID‐19 cases, compared to placebo. It may increase grades 3 to 4 adverse events compared to placebo but seemed to have little to no effect on hospital discharge, non‐invasive ventilation (NIV) or IMV requirement or death, and sustained recovery. The number of events for the outcomes neurological dysfunction, thromboembolic events and renal failure was too low to allow for judgment. Our certainty in the evidence is low due to very serious imprecision. Our certainty in the evidence for NIV or IMV requirement was further downgraded for indirectness due to the short time frame. The study authors reported results for 90‐day mortality and serious adverse events (SAEs) that suggest a difference in effect between participants who were seronegative as compared to seropositive at baseline; for seronegative, the effect may be in favour of bamlanivimab, the opposite tendency was observed in seropositive participants.
Evidence for casirivimab/imdevimab suggests that the treatment at a dose of 8.0 g may have little to no effect on all‐cause mortality, clinical progression to IMV or death, hospital discharge alive, thrombotic events, and renal replacement therapy requirement compared with usual care alone in the complete randomised cohort. We have moderate certainty in the evidence due to high risk of bias. In line with the subgroups from ACTIV‐3, when looked at seronegative participants at baseline only, the study authors found an effect, while no effect was found for participants who already seroconverted or with unknown status.
Ongoing studies
Thirty‐six RCTs examining 23 different SARS‐CoV‐2‐specific mAbs or mAb combinations are currently ongoing or completed without published results. Of these, all except one are being explored in phase 2 studies onwards. We identified five additional mAbs that have not yet been tested in RCTs as part of our tracking of emerging mAbs.
Overall completeness and applicability of evidence
Completeness of the evidence
Completeness within our included studies varied. ACTIV‐3 reported on all preplanned outcomes, and BLAZE‐1 (phase 2) on all outcomes, but not in all preplanned formats. These outcomes are, unfortunately, not our complete set of preplanned outcomes. BLAZE‐1 (phase 3); COMET‐ICE; Eom 2021; RECOVERY and Weinreich (phase 3) reported up to eight preplanned outcomes, but Weinreich (phase 3) did not fully report these for all doses. Weinreich (phase 1/2) reported only three preplanned outcomes. We contacted study authors, but they could not provide us with additional data or did not reply. As some studies were published as interim analyses or preprints, we expect that more data will become available with the final analysis and in full publications.
Applicability of the evidence
Overall, the evidence included in this review represents two distinct populations: individuals with mild disease, and individuals with moderate‐to‐severe disease who are already hospitalised due to COVID‐19. During the course of this review, the WHO progression scale has changed multiple times. Since the decision to use the scale in Figure 1, it was changed on 25 January 2021 to represent different cut‐offs and categories: mild, moderate, severe and critical disease based on severity of pneumonia instead of hospitalisation (WHO 2021c). We will consider adopting the most recent scale in a future update of this review.
Four RCTs examined SARS‐CoV‐2‐specific mAbs in outpatients with mild disease. The various treatment arms in the samples included between 63% to 100% of participants who have at least one risk factor for severe disease, which may be slightly overrepresented when compared to modelling estimates for the general population (Clark 2020). However, this subgroup would be in more urgent need for the prevention of serious adverse outcomes. This was reflected in the initial emergency use authorisation (EUA) that was provided by the US Food and Drug Administration (FDA) for the combination of casirivimab and imdevimab, bamlanivimab, and bamlanivimab with etesevimab. The EUA for bamlanivimab was revoked on 17 April 2021 per Eli Lilly's request for bamlanivimab alone due to an increase in virus variants that are resistant to bamlanivimab monotherapy (FDA 2021b).
The median time from symptom onset to randomisation ranged from two to six days (median five, range three to six days in BLAZE‐1 (phase 2); four days, range 0 to 29 days in BLAZE‐1 (phase 3); three days, rangetwo2 to four days in Eom 2021 and three days, range two to five days in Weinreich (phase 1/2)). We are unable to extrapolate the evidence from this time window to a longer period. It remains to be seen whether the time frame in the context of an RCT with predefined eligibility criteria (i.e. symptom onset within seven days of enrolment) can be transferred to a situation of possibly overwhelmed hospitals where space may be scarce for treatments that need intravenous infusions.
The platform trial that examined bamlanivimab in hospitalised, moderate‐to‐severe cases in the USA, Denmark and Singapore (ACTIV‐3), had less restrictive eligibility criteria with a maximum of 12 days being allowed between symptom onset and treatment. However, the population that is considered to quote: "require admission to hospital for acute medical care" may vary depending on context. Despite this possible source of heterogeneity, in general, we would consider evidence from ACTIV‐3 applicable to inpatients (moderate to severe disease). RECOVERY, which examined the combination casirivimab/imdevimab, allowed clinically suspected cases without a positive polymerase chain reaction(PCR) result to enter the study, but the number of non‐confirmed cases was low (< 1% PCR‐negative, ~ 2.3% unknown). The study did not limit inclusion based on time from symptom onset. However, time from symptom onset varied between the serostatus groups (seronegative: 7 days, 4 to 10; seropositive: 10 days, 7 to 13; unknown: 9 days, 6 to 13), underlining the assumption that SARS‐CoV‐2 specific mAbs may be more effective early in the course of the disease.
Over time, the virus has developed changes compared to the original strain. Some of these changes have led to increased resistance to viral neutralisation by antibody therapy (FDA 2021b; Madhi 2021; Zhou 2021), which led to the retraction of the EUA for bamlanivimab monotherapy (FDA 2021b).
BLAZE‐1 (phase 2) mentioned assessing viral strains at baseline, with only two out of the 613 included participants having detectable variants at baseline. The enrolment into the ACTIV‐3 bamlanivimab arm stopped in October 2020, before the wider spread of variants, and reported. Therefore, results from these early studies probably apply mainly to the wild type variant. It is important to note that the beta, gamma, delta, and epsilon variants, which are currently more widespread, have shown resistance to bamlanivimab using pseudovirus and clinical viral isolate assays (Hoffmann 2021b; McCallum 2021; Planas 2021; Wang 2021b). Etesevimab has shown diminished activity against the alpha and epsilon variant but remained active against beta and delta (Planas 2021; McCallum 2021).
In the study on sotrovimab, virus variants were not assessed in participants. In vitro studies indicate that sotrovimab probably remains active against the alpha, beta, gamma (Cathcart 2021), and epsilon (McCallum 2021) variants. The study examining regdanvimab has not assessed the virus strains participants were infected with. In vitro, regdanvimab maintained potency against the gamma variant, but showed reduced binding to the beta and epsilon variants (Ryu 2021; McCallum 2021).
RECOVERY and Weinreich (phase 1/2)/Weinreich (phase 3) did not explicitly assess the variants that affected their participants. However, the dominant strain at the time of RECOVERY enrolment was the B1.1.7 (alpha) variant in the UK, which has not shown resistance to casirivimab/imdevimab (Wang 2021b). In general, imdevimab and the combination casirivimab/imdevimab has not shown diminished performance against alpha, beta, gamma, delta and epsilon variants (Hoffmann 2021a; Planas 2021; Copin 2021; McCallum 2021). Whereas, casirivimab alone was less active against the beta (Wang 2021b) and delta variants (Hoffmann 2021a).
Overall, combination therapies may be more applicable because of the broader spectrum of neutralisation and a smaller possibility of viral escape (Copin 2021).
Quality of the evidence
We judged the risk of bias to be low for two studies (ACTIV‐3; Eom 2021), to be of some concerns for one study (COMET‐ICE), and high for two studies (RECOVERY; Weinreich (phase 3)) for all reported outcomes. In BLAZE‐1 (phase 2), we judged risk of bias to be of some concerns for the outcome hospital admission or death and for the other reported outcomes we judged risk of bias to be low. In Weinreich (phase 1/2) we judged risk of bias to be high for the outcomes hospital admission or death and adverse events grades 3 to 4, and for the other reported outcomes we judged risk of bias to be low. We excluded Weinreich (phase 3) from analysis due to the unclear participant flow diagram and potentially selected analyses.
All data included in the summary of findings tables resulted from analyses according to the intention‐to‐treat principle or modified intention‐to‐treat principle, as prespecified in the respective study protocol (exclusions if randomised by error, withdrawal before any study treatment or not positive on diagnostic reverse transcription polymerase chain reaction (RT‐PCR)). However, we rated the certainty in the evidence as low or very low for each prioritised outcome in all studies except RECOVERY, due to serious imprecision based on low number of events, small sample size, and resulting wide 95% confidence intervals (for details, see summary of findings tables) with evidence per comparison resulting from one study only. We downgraded one outcome, development of severe symptoms (NIV, IMV or extracorporeal membrane oxygenation (ECMO)), because of serious indirectness, as we considered follow‐up at day five as too short. For the results from RECOVERY, we rated down one level due to lack of blinding and the potential influence from participants or staff on additional treatment options, as usual care is constantly changing seen the rapid development of the pandemic.
Potential biases in the review process
The publication of preliminary results in form of press releases and preprints has become widespread during the COVID‐19 pandemic. While we had decided to include and report on preprints, we have not tracked all possible sources of press releases because quality may be low and reports may lack detail regarding participants and outcomes, although some are indexed in Epistemonikos.
In addition to regular RCTs, we performed a search for ongoing platform trials, as they may add treatments during the course of the study. We are regularly checking these studies via the registry, any related trial websites we are aware of, and press releases that are indexed in Epistemonikos. Up to now, this has proved useful in identifying new treatment arms with SARS‐CoV‐2‐specific mAbs in ACTIV‐2, ACTIV‐3, AGILE, RECOVERY, DISCOVERY, and OPTIMISE‐C19. We will run these searches and check for additional treatment arms monthly. Interestingly, in addition to large‐scale platform trials, flexible study designs such as combined phase 1/2/3 trials with preplanned interim analyses, the potential to change the treatment dose during the process, and the batch‐wise recruitment of participants according to new cases have become common, posing a challenge on estimating the arrival of new studies and the decision on when to potentially update this review in the future.
Due to the novelty of SARS‐CoV‐2, numerous interventions are currently under development. To maintain the currency of our evidence and to follow the newly developed mAbs that enter clinical studies in humans, we adapted our search strategy each week, based on a monoclonal antibody tracker (Chinese Antibody Society 2020). Recently, this tracker has not identified any new SARS‐CoV‐2‐specific mAbs. There may be two possible underlying reasons for this: 1) no mAbs have entered clinical trial phase, which is unlikely given the rapidly changing COVID‐19‐related treatment landscape, 2) updating the list may have been delayed or stopped. In the latter case, we may have missed studies that are identified only with a drug name that is currently unknown to us. However, we would count on the fact that clinical studies in an advanced phase and their publications would provide more keywords for identification by our search strategy. We, therefore, assume that we have identified and will identify important phase 2, 3, and 4 RCTs in individuals infected with SARS‐CoV‐2.
For feasibility reasons, we did an abbreviated search between submission and response to peer review. We included publications on already included studies, but listed one new study under Studies awaiting classification for integration into the next version of this review.
Before data extraction, we had decided not to pool different monoclonal antibodies/antibody cocktails due to the different epitopes they are targeting, and not to pool different doses of the same mAb as they may produce varying levels of antibody‐dependent enhancement. We acknowledge that combining across doses and substances may have provided insight into the class effect, however, this would limit its utility in clinical decision‐making by combining substances that target different pathways and may be used in different settings due to varying prevalence of variants to which a mAb may have become resistant.
In contrast to the protocol, we changed some of our outcomes based on input from the German clinical guideline, and we faced difficulties obtaining a clear definition for outcomes covered by the WHO Progression Scale (Differences between protocol and review). Of note, most of our predefined outcomes are prone to competing events (i.e. death), therefore we have changed and replaced these where possible, and noted down where not reported. We do not think this has introduced bias, as we chose the outcomes based on clinical interest rather than the effect estimate.
Agreements and disagreements with other studies or reviews
We identified low‐certainty evidence about the effects of bamlanivimab at doses of 0.7 g, 2.8 g, and 7.0 g, the combination of bamlanivimab and etesevimab, the of combination casirivimab and imdevimab, sotrovimab at a dose of 0.5 g, and regdanvimab at 40 mg/kg and 80 mg/kg compared to placebo in non‐hospitalised individuals with asymptomatic or mild disease. Further, we identified low‐ to very low‐certainty evidence that treatment with bamlanivimab in hospitalised individuals with moderate‐to‐severe disease is not effective for the treatment of COVID‐19, when compared to placebo; and moderate‐certainty evidence that casirivimab/imdeviman is not effective to treat COVID‐19 when compared to usual care in the complete allocated cohort of a study.
The identified studies published as full texts and preprints are in line with the evidence used in the Australian clinical guideline (National COVID‐19 Clinical Evidence Taskforce 2020). This guideline pooled the two studies that examined bamlanivimab, and did not consider the conference abstract on bamlanivimab/etesevimab. Another difference is that we decided to refrain from meta‐analysing participants with mild versus moderate and severe disease, as working mechanisms are anticipated to differ between early administration and administration later during the course of the disease. The prioritised outcomes differed from this review, the guideline included clinical recovery for regdanvimab and withdrawals due to adverse events for casirivimab/imdevimab in non‐hospitalised participants.
A difference in the mechanism between the granting of an emergency use authorization (EUA) and summaries in systematic reviews and recommendations by several national clinical guidance (e.g. Australia, Germany) should be noted. An EUA is possible only in the context of a declared emergency when no viable alternatives are available. It does not constitute the approval of a medical product but facilitates its availability when it is not yet approved or is approved for a different indication. This decision may be based on favourable interim or final analyses of an RCT and may be revoked once the justification of its issuance is no longer in place (FDA 2021c). In contrast, systematic reviews aim to synthesise all available evidence, usually from peer‐reviewed journals only, thus targeting the inclusion of mature data (although for the current review, mainly interim analyses from preprint or full‐text publications were available).
The efficacy and safety of SARS‐CoV‐2‐neutralising mAbs are not yet well‐characterised, with only a few studies having been published (BLAZE‐1 (phase 2); COMET‐ICE; Eom 2021; Weinreich (phase 1/2); ACTIV‐3; RECOVERY). Additional data in the form of full‐text publications are expected to be published soon, and several studies have completed recruitment, therefore new evidence will not be long in coming.
Authors' conclusions
Implications for practice.
The evidence for each comparison results from one study only, and we rated certainty in the evidence as low due to very serious imprecision for all comparisons except casirivimab/imdevimab versus usual care in hospitalised individuals (moderate certainty evidence). Therefore, the identified evidence is mostly insufficient to draw meaningful conclusions regarding treatment with any specific monoclonal antibody (mAb), and the disease stage in which mAbs should be used.
For non‐hospitalised participants (asymptomatic or mild disease), we are uncertain about the effectiveness and safety of bamlanivimab, the combination of bamlanivimab/etesevimab, casirivimab/imdevimab, sotrovimab, and regdanvimab compared to placebo. Information on patient‐relevant outcomes such as mortality, quality of life, and serious adverse events is either inconclusive or entirely lacking, although bamlanivimab alone and in combination with etesevimab, as well as casirivimab/imdevimab, sotrovimab, and regdanvimab may reduce the occurrence of hospital admission or death. Data on adverse events (AEs) vary for the substances, doses and even type of AE (all grades, grades 3‐4, serious adverse events). For all comparisons, the 95% confidence interval includes both benefit and harm and our certainty in the evidence is low. We acknowledge that the true effect may substantially vary from the reported effects.
For hospitalised participants (moderate‐to‐severe disease), bamlanivimab may have little to no effect on efficacy outcomes when compared to placebo, but it may increase the occurrence of severe symptoms and grade 3 to 4 adverse events. Casirivimab/imdevimab has probably no effect on mortality, progression to invasive mechanical ventilation (IMV), and hospital discharge alive by day 30. Unfortunately, we are lacking important information on AE and SAE for this comparison. Subgroup analyses within the studies suggest that it could be worth examining the subgroup of participants who have not developed antibodies at baseline.
Implications for research.
We did not conduct meta‐analyses because studies were heterogeneous with regard to different mAbs used for treatment, setting, and inconsistently reported outcomes. Moreover, only one of the included studies reported all‐cause mortality exceeding 60 days, and none reported quality of life. WHO Progression Scale outcomes are reported on various versions of the scale, at different time points and as different effect measures. This highlights the urgent need for adequately‐powered randomised controlled trials (RCTs) with comparable study arms, as well as the assessment and reporting of clinically relevant outcomes such as quality of life and long‐term adverse events.
Future research will need to consider whether the emergence of SARS‐CoV‐2 variants impacts the effectiveness of SARS‐CoV‐2‐neutralising mAbs. BLAZE‐1 (phase 2) explored resistance to four variants for bamlanivimab and etesevimab, and further research will be required as variants emerge.
We identified 36 ongoing RCTs. Publication of these ongoing studies may resolve some uncertainties and allow a better judgment regarding the effectiveness and safety of SARS‐CoV‐2‐neutralising mAbs for the treatment of COVID‐19.
History
Protocol first published: Issue 1, 2021
Notes
Parts of the review's methods section is adopted from templates of Cochrane Haematology and a similar protocol published by Piechotta 2020, and the corresponding reviews (Piechotta 2021).
Risk of bias
Acknowledgements
This review was published in collaboration with the Cochrane Central Editorial Service. We particularly thank Robin Featherstone (Information Specialist, Cochrane Editorial and Methods Department) and Sarah Hodgkinson (Associate Editor, Cochrane Editorial and Methods Department). We thank Carolyn Dorée (Information Specialist, Systematic Review Initiative, NHS Blood and Transplant Oxford) for peer reviewing the search strategy. We thank Helen Wakeford (Executive Editor, Cochrane Editorial & Methods Department) and Toby Lasserson (Deputy Editor in Chief) for their management of the editorial process and excellent support. We thank Denise Mitchell (Senior Copy‐Editor, Cochrane Editorial & Methods Department) and Heather Maxwell (Copy‐Editor) for copy‐editing the review.
We would also like to thank our peer reviewers, Mary Marovich (National Institutes of Allergy and Infectious Diseases, Bethesda, Maryland), Jolanta Piszczek (Clinical Pharmacy Specialist, Infectious Diseases and Antimicrobial Stewardship, University of British Columbia) and Debra Knauft (consumer referee) for their helpful suggestions.
The research was part of a project supported by the German Federal Ministry of Education and Research (NaFoUniMedCovid19, funding number: 01KX2021; part of the project "COVIM" and "CEOsys"). The contents of this document reflect only the authors' views and the German Ministry is not responsible for any use that may be made of the information it contains.
The research was supported by NHS Blood and Transplant and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Appendices
Appendix 1. Search strategy: MEDLINE via Ovid
# Searches
1 "spike protein, SARS‐CoV‐2".mp.
2 Coronavirus Infections/ or Coronavirus/
3 SARS‐CoV‐2/ or COVID‐19/
4 ("2019 nCoV" or 2019nCoV or coronavir* or coronovir* or COVID or COVID19 or HCoV* or "nCov 2019" or "SARS CoV2" or "SARS CoV 2" or SARSCoV2 or "SARSCoV 2").tw,kf.
5 "severe acute respiratory syndrome coronavirus 2".tw,kf,nm.
6 ((corona* or corono*) adj1 (virus* or viral* or virinae*)).tw,kf.
7 or/2‐6
8 *Antibodies, Monoclonal/
9 Antibodies, Neutralizing/
10 Antibodies, Viral/
11 ((antibod* or mAb* or nAb*) adj2 (therap* or treatment* or neutrali?ing or prevent* or protect* or prophylax*)).tw,kf.
12 Spike Glycoprotein, Coronavirus/
13 Binding, Competitive/
14 (compet* adj1 bind*).tw,kf.
15 ("spike protein*" or "s protein*" or "Spike (S) protein").mp.
16 ((cocktail* or mixture* or combination*) adj3 (mAb* or antibod* or nAb*)).tw,kf.
17 (LY3832479* or LY‐CoV016* or LY‐3832479* or LYCoV016* or JS016* or JS‐016* or etesevimab*).mp.
18 (REGN‐COV2* or REGN‐COV‐2* or REGN10933* or REGN10987* or REGN‐10933* or REGN‐10987* or REGEN‐COV2* or REGEN‐COV‐2* or REGEN10933* or REGEN10987* or REGEN‐10933* or REGEN‐10987* or casirivimab* or imdevimab*).mp.
19 (LY3819253* or LY‐3819253* or LY‐CoV555* or LYCoV555* or bamlanivimab* or banlanivimab*).mp.
20 (VIR7831* or VIR‐7831* or GSK4182136* or GSK‐4182136* or sotrovimab*).mp.
21 (AZD7442* or AZD‐7442* or AZD8895* or AZD‐8895* or tixagevimab* or COV2‐2196 or COV22196* or AZD1061* or AZD‐1061* or cilgavimab* or COV2‐2130* or COV22130*).mp.
22 (DXP‐593* or DXP593* or BGB‐DXP593* or BGBDXP593* or BGB‐DXP‐593*).mp.
23 (TY027* or TY‐027*).mp.
24 (CTP59* or CTP‐59* or regdanvimab*).mp.
25 (STI1499* or STI‐1499* or covi‐shield* or covishield* or COVI‐GUARD* or COVIguard*).mp.
26 (BRII196* or BRII‐196*).mp.
27 (SCTA01* or SCTA‐01*).mp.
28 (MW33* or MW‐33*).mp.
29 (BRII198* or BRII‐198*).mp.
30 (HFB30132A* or HFB‐30132A*).mp.
31 (ADM03820* or ADM‐03820*).mp.
32 (HLX70* or HLX‐70*).mp.
33 (STI2020* or STI‐2020* or COVIAMG* or COVI‐AMG*).mp.
34 (DZIF10c* or DZIF‐10c* or BI767551* or BI‐767551*).mp.
35 (COV2‐2381* or COV22381*).mp.
36 (ABBV‐47D11* or 47D11* or ABBV47D11*).mp.
37 (COR‐101* or COR101* or STE90‐C11*).mp.
38 (DXP‐604* or DXP604* or BGB‐DXP604* or BGBDXP604* or BGB‐DXP‐604*).mp.
39 (Chicken egg antibod* or egg yolk antibod* or IgY*).mp.
40 (VIR‐7832 or VIR7832 or GSK4182137* or GSK‐4182137*).mp.
41 (IDB003 or MD65 or MTX‐COVAB).mp.
42 (C144‐LS or C144LS or C‐135‐LS or C135‐LS or C135LS or SAB‐185 or SAB185 or JMB2002 or JMB‐2002 or TATX‐03 or TATX03 or NOVOAB‐20 or NOVOAB20 or NOVO‐AB‐20 or ABP‐300 or ABP300 or MW05 or MW‐05 or MW07 or MW‐07 or ACmab1 or covimax or adimab or CPI‐006 or CPI006).tw,kf,nm.
43 Single‐Chain Antibodies/
44 (nmabs or diabod* or dia‐bod* or nanobod* or nano‐bod* or microbod* or micro‐bod* or monobod* or mono‐bod*).tw,kf,nm.
45 (scFv* or "single‐chain*" or "heavy chain*" or HCAbs or SCAbs or FAB).tw,kf,nm.
46 or/8‐45
47 7 and (1 or 46)
48 (exp Animals/ or exp Animal Experimentation/ or exp Models, Animal/) not Humans/
49 47 not 48
50 limit 49 to yr="2020‐Current"
Appendix 2. Search strategy: Embase via Ovid
# Searches
1 coronavirinae/ or coronaviridae/ or coronaviridae infection/
2 coronavirus disease 2019/
3 Coronavirus infection/
4 sars‐related coronavirus/
5 "Severe acute respiratory syndrome coronavirus 2"/
6 ((corona* or corono*) adj1 (virus* or viral* or virinae*)).tw,kw.
7 ("2019 nCoV" or 2019nCoV or coronavir* or coronovir* or COVID or COVID19 or HCoV* or "nCov 2019" or "SARS CoV2" or "SARS CoV 2" or SARSCoV2 or "SARSCoV 2").tw,kw.
8 "Severe acute respiratory syndrome coronavirus 2".mp.
9 or/1‐8
10 Antibodies, Monoclonal/
11 Antibodies, Neutralizing/
12 Antibodies, Viral/
13 ((antibod* or mAb* or nAb*) adj2 (therap* or treatment* or neutrali?ing or prevent* or protect* or prophylax*)).tw,kw.
14 Spike Glycoprotein, Coronavirus/
15 Binding, Competitive/
16 (compet* adj1 bind*).tw,kw.
17 ("spike protein*" or "s protein*" or "Spike (S) protein").mp.
18 ((cocktail* or mixture* or combination*) adj3 (mAb* or antibod* or nAb*)).tw,kw.
19 (LY3832479* or LY‐CoV016* or LY‐3832479* or LYCoV016* or JS016* or JS‐016* or etesevimab*).mp.
20 (REGN‐COV2* or REGN‐COV‐2* or REGN10933* or REGN10987* or REGN‐10933* or REGN‐10987* or REGEN‐COV2* or REGEN‐COV‐2* or REGEN10933* or REGEN10987* or REGEN‐10933* or REGEN‐10987* or casirivimab* or imdevimab*).mp.
21 (LY3819253* or LY‐3819253* or LY‐CoV555* or LYCoV555* or bamlanivimab* or banlanivimab*).mp.
22 (VIR7831* or VIR‐7831* or GSK4182136* or GSK‐4182136* or sotrovimab*).mp.
23 (AZD7442* or AZD‐7442* or AZD8895* or AZD‐8895* or tixagevimab* or COV2‐2196 or COV22196* or AZD1061* or AZD‐1061* or cilgavimab* or COV2‐2130* or COV22130*).mp.
24 (DXP‐593* or DXP593* or BGB‐DXP593* or BGBDXP593* or BGB‐DXP‐593*).mp.
25 (TY027* or TY‐027*).mp.
26 (CTP59* or CTP‐59* or regdanvimab*).mp.
27 (STI1499* or STI‐1499* or COVI‐shield* or COVIshield* or COVI‐GUARD* or COVIguard*).mp.
28 (BRII196* or BRII‐196*).mp.
29 (SCTA01* or SCTA‐01*).mp.
30 (MW33* or MW‐33*).mp.
31 (BRII198* or BRII‐198*).mp.
32 (HFB30132A* or HFB‐30132A*).mp.
33 (ADM03820* or ADM‐03820*).mp.
34 (HLX70* or HLX‐70*).mp.
35 (STI2020* or STI‐2020* or COVIAMG* or COVI‐AMG*).mp.
36 (DZIF10c* or DZIF‐10c* or BI767551* or BI‐767551*).mp.
37 (COV2‐2381* or COV22381*).mp.
38 (ABBV‐47D11* or 47D11* or ABBV47D11*).mp.
39 (COR‐101* or COR101* or STE90‐C11*).mp.
40 (DXP‐604* or DXP604* or BGB‐DXP604* or BGBDXP604* or BGB‐DXP‐604*).mp.
41 (Chicken egg antibod* or egg yolk antibod* or IgY*).mp.
42 (VIR‐7832* or VIR7832* or GSK4182137* or GSK‐4182137*).mp.
43 (IDB003 or MD65 or MTX‐COVAB).mp.
44 (C144‐LS or C144LS or C‐135‐LS or C135‐LS or C135LS or SAB‐185 or SAB185 or JMB2002 or JMB‐2002 or TATX‐03 or TATX03 or NOVOAB‐20 or NOVOAB20 or NOVO‐AB‐20 or ABP‐300 or ABP300 or MW05 or MW‐05 or MW07 or MW‐07 or ACmab1 or covimax or adimab or CPI‐006 or CPI006).tw,kw.
45 single chain fragment variable antibody/
46 (nmabs or diabod* or dia‐bod* or nanobod* or nano‐bod* or microbod* or micro‐bod* or monobod* or mono‐bod*).mp.
47 (scFv* or "single‐chain*" or "heavy chain*" or HCAbs or SCAbs or FAB).tw,kw.
48 or/10‐47
49 9 and 48
50 (exp animal/ or nonhuman/) not exp human/
51 Animal experiment/ not (human experiment/ or human/)
52 or/50‐51
53 49 not 52
54 limit 53 to yr="2020 ‐Current"
55 limit 54 to medline
56 54 not 55
Appendix 3. Search strategy: Cochrane Covid‐19 Study Register
"Ly‐3832479" OR Ly3832479 OR "LY‐3832479" OR LY3832479 OR "LY‐CoV016" OR "REGN‐COV2" OR "REGEN‐COV2" OR REGN10933 OR REGN10987 OR REGEN10933 OR REGEN10987 OR casirivimab OR imdevimab OR "LY‐3819253" OR LY3819253 OR "LY‐CoV555" OR Bamlanivimab OR Banlanivimab OR "VIR‐7831" OR VIR7831 OR GSK4182136 OR "GSK‐4182136" OR sotrovimab OR AZD7442 OR "AZD‐7442" OR AZD8895 OR "AZD‐8895" OR tixagevimab OR "COV2‐2196" OR COV22196 OR AZD1061 OR "AZD‐1061" OR cilgavimab OR "COV2‐2130" OR COV22130 OR DXP593 OR "DXP‐593" OR "BGB‐DXP‐593" OR BGBDXP593 OR JS016 OR "JS‐016" OR etesevimab OR TY027 OR "TY‐027" OR CTP59 OR "CTP‐59" OR "CT‐P59" OR regdanvimab OR STI1499 OR "STI‐1499" OR "COVI‐guard" OR COVIguard OR BRII196 OR "BRII‐196" OR SCTA01 OR "SCTA‐01" OR MW33 OR "MW‐33" OR BRII198 OR "BRII‐198" OR HFB30132A OR "HFB‐30132A" OR ADM03820 OR "ADM‐03820" OR HLX70 OR "HLX‐70" OR STI2020 OR "STI‐2020" OR COVIAMG OR "COVI‐AMG" OR DZIF10c OR "DZIF‐10c" OR BI767551 OR "BI‐767551" OR "COV2‐2381" OR COV22381 OR "ABBV‐47D11" OR 47D11 OR ABBV47D11 OR "COR‐101" OR COR101 OR "STE90‐C11" OR "DXP‐604" OR DXP604 OR "BGB‐DXP604" OR BGBDXP604 OR "BGB‐DXP‐604" OR "chicken egg antibody" OR "egg yolk antibody" OR IgY* OR "VIR‐7832" OR VIR7832 OR GSK4182137 OR "GSK‐4182137" OR IDB003 OR MD65 OR "MTX‐COVAB" OR "C144‐LS" OR C144LS OR "C‐135‐LS" OR "C135‐LS" OR C135LS OR "SAB‐185" OR SAB185 OR JMB2002 OR "JMB‐2002" OR "TATX‐03" OR TATX03 OR "NOVOAB‐20" OR NOVOAB20 OR "NOVO‐AB‐20" OR "ABP‐300" OR ABP300 OR MW05 OR "MW‐05" OR MW07 OR "MW‐07" OR ACmab1 OR covimax OR adimab OR "CPI‐006" OR CPI006 OR ADG20 OR "ADG 20" OR diabod* OR nanobod* OR microbod* OR monobod* OR scFv* OR "single‐chain" OR "heavy chain" OR HCAbs OR SCAbs OR nmAb* OR "f(ab)"
Appendix 4. Search strategy: PubMed (ahead of print only)
#1 2019 nCoV[tiab] OR 2019nCoV[tiab] OR corona virus[tiab] OR corona viruses[tiab] OR coronavirus[tiab] OR coronaviruses[tiab] OR COVID[tiab] OR COVID19[tiab] OR nCov 2019[tiab] OR SARS‐CoV2[tiab] OR SARS CoV‐2[tiab] OR SARSCoV2[tiab] OR SARSCoV‐2[tiab] OR "COVID‐19"[Mesh] OR "Coronavirus"[Mesh:NoExp] OR "SARS‐CoV‐2"[Mesh] OR "COVID‐19"[nm] OR "severe acute respiratory syndrome coronavirus 2"[nm]
#2 (antibod*[Title/Abstract] OR mAb[Title/Abstract] OR mAbs[Title/Abstract] OR nAb[Title/Abstract] OR nAbs[Title/Abstract]) AND (therap*[Title/Abstract] OR treat*[Title/Abstract] OR neutrali*[Title/Abstract] OR prevent*[Title/Abstract] OR protect*[Title/Abstract] OR prophylax*[Title/Abstract]) OR (compet*[Title/Abstract] AND bind*[Title/Abstract]) OR (cocktail*[Title/Abstract] OR mixture*[Title/Abstract] OR combination*[Title/Abstract]) AND (mAb[Title/Abstract] OR mAbs[Title/Abstract] OR antibod*[Title/Abstract] OR nAb[Title/Abstract] OR nAbs[Title/Abstract]) OR "spike protein*"[Title/Abstract] OR "s protein*"[Title/Abstract] OR "Spike (S) protein"[Title/Abstract]
#3 (LY‐3832479 OR LY3832479 OR LY‐CoV016 OR REGN‐COV2 OR REGN10933 OR REGN10987 OR REGN‐10933 OR REGN‐10987 OR REGEN10933 OR REGEN10987 OR REGEN‐10933 OR REGEN‐10987 OR REGN‐CoV2* OR REGN‐CoV‐2* OR REGEN‐CoV2* OR REGEN‐CoV‐2* OR casirivimab OR imdevimab OR LY‐3819253 OR LY3819253 OR LY‐CoV555 OR Bamlanivimab OR Banlanivimab OR VIR‐7831 OR VIR7831 OR GSK4182136 OR GSK‐4182136 OR sotrovimab OR AZD7442 OR AZD‐7442 OR AZD1061 OR AZD‐1061 OR AZD8895 OR AZD‐8895 OR tixagevimab OR cilgavimab OR DXP593 OR DXP‐593 OR BGB‐DXP‐593 OR BGBDXP593 OR JS016 OR JS‐016 OR LY‐CoV016 OR etesevimab OR TY027 OR TY‐027 OR CTP59 OR regdanvimab OR STI1499 OR STI‐1499 OR COVI‐guard OR COVIguard OR BRII196 OR BRII‐196 OR SCTA01 OR SCTA‐01 OR MW33 OR MW‐33 OR BRII198 OR BRII‐198 OR HFB30132A OR HFB‐30132A OR ADM03820 OR ADM‐03820 OR HLX70 OR HLX‐70 OR STI2020 OR STI‐2020 OR COVIAMG OR COVI‐AMG OR DZIF10c OR DZIF‐10c OR BI767551 OR BI‐767551 COV2‐2381 OR COV22381 OR ABBV‐47D11 OR 47D11 OR ABBV47D11 OR COR‐101 OR COR101 OR STE90‐C11 OR DXP‐604 OR DXP604 OR BGB‐DXP604 OR BGBDXP604 OR BGB‐DXP‐604 OR „chicken egg antibod*" OR "egg yolk antibod*" OR IgY OR IgYs OR VIR‐7832 OR VIR7832 OR GSK4182137 OR GSK‐4182137 OR IDB003 OR MTX‐COVAB OR MD65 OR TATX‐03 OR NOVOAB‐20 OR "C144‐LS" OR C144LS OR "C‐135‐LS" OR "C135‐LS" OR C135LS OR "SAB‐185" OR SAB185 OR JMB2002 OR "JMB‐2002" OR "TATX‐03" OR TATX03 OR "NOVOAB‐20" OR NOVOAB20 OR "NOVO‐AB‐20" OR "ABP‐300" OR ABP300 OR MW05 OR "MW‐05" OR MW07 OR "MW‐07" OR ACmab1 OR covimax OR adimab OR "CPI‐006" OR CPI006 OR diabod* OR nanobod* OR microbod* OR monobod* OR scFv* OR "single‐chain" OR "heavy chain" OR HCAbs OR SCAbs OR nmAbs OR FAB)
#4 "spike protein*"[Title/Abstract] OR "s protein*"[Title/Abstract] OR "Spike (S) protein"[Title/Abstract]
#5 #1 AND (#2 OR #3)
#6 #4 OR #5
#7 (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb])
#8 ("animals"[mh] NOT "humans"[mh])
#9 #6 NOT (#7 OR #8) Filters: from 2020/1/1 ‐ 3000/12/12
Appendix 5. Search strategy: Epistemonikos COVID‐19 L*VE Platform
https://app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d?utm=epdb_en
the Coronavirus disease (COVID‐19) L*OVE by PICO: Prevention or treatment > Pharmacological interventions > Targeted therapies > Anti‐SARS‐CoV‐2 Mab > primary studies.
Appendix 6. Search strategy: World Health Organization COVID‐19 global literature on coronavirus disease
Advanced search and in the search field: title, abstract, subject
("Ly‐3832479" OR ly3832479 OR "LY‐3832479" OR ly3832479 OR "LY‐CoV016" OR "REGN‐COV2" OR "REGEN‐COV2" OR regn10933 OR regn10987 OR regen10933 OR regen10987 OR casirivimab OR imdevimab OR "LY‐3819253" OR ly3819253 OR "LY‐CoV555" OR bamlanivimab OR banlanivimab OR "VIR‐7831" OR vir7831 OR gsk4182136 OR "GSK‐4182136" OR sotrovimab OR azd7442 OR "AZD‐7442" OR azd8895 OR "AZD‐8895" OR tixagevimab OR "COV2‐2196" OR cov22196 OR azd1061 OR "AZD‐1061" OR cilgavimab OR "COV2‐2130" OR cov22130 OR dxp593 OR "DXP‐593" OR "BGB‐DXP‐593" OR bgbdxp593 OR js016 OR "JS‐016" OR etesevimab OR ty027 OR "TY‐027" OR ctp59 OR "CTP‐59" OR "CT‐P59" OR regdanvimab OR sti1499 OR "STI‐1499" OR "COVI‐guard" OR coviguard OR brii196 OR "BRII‐196" OR scta01 OR "SCTA‐01" OR mw33 OR "MW‐33" OR brii198 OR "BRII‐198" OR hfb30132a OR "HFB‐30132A" OR adm03820 OR "ADM‐03820" OR hlx70 OR "HLX‐70" OR sti2020 OR "STI‐2020" OR coviamg OR "COVI‐AMG" OR dzif10c OR "DZIF‐10c" OR bi767551 OR "BI‐767551" OR "COV2‐2381" OR cov22381 OR "ABBV‐47D11" OR 47d11 OR abbv47d11 OR "COR‐101" OR cor101 OR "STE90‐C11" OR "DXP‐604" OR dxp604 OR "BGB‐DXP604" OR bgbdxp604 OR "BGB‐DXP‐604" OR "chicken egg antibody" OR "egg yolk antibody" OR igy* OR "VIR‐7832" OR vir7832 OR gsk4182137 OR "GSK‐4182137" OR idb003 OR md65 OR "MTX‐COVAB" OR "C144‐LS" OR c144ls OR "C‐135‐LS" OR "C135‐LS" OR c135ls OR "SAB‐185" OR sab185 OR jmb2002 OR "JMB‐2002" OR "TATX‐03" OR tatx03 OR "NOVOAB‐20" OR novoab20 OR "NOVO‐AB‐20" OR "ABP‐300" OR abp300 OR mw05 OR "MW‐05" OR mw07 OR "MW‐07" OR acmab1 OR covimax OR adimab OR "CPI‐006" OR cpi006 OR ADG20 OR "ADG20" OR diabod* OR nanobod* OR microbod* OR monobod* OR scfv* OR "single‐chain" OR "heavy chain" OR hcabs OR scabs OR "F(ab)2" OR nmAb*)
Appendix 7. Search strategy: Cochrane Covid‐19 Study Register, search strategy for platform trials
Cochrane COVID‐19 Study Register via the Cochrane Register of Studies http://crsweb.cochrane.org/
1 (adaptive clinical trial):PT AND INREGISTER
2 (adaptive NEAR7 (trial OR stud*)) AND INREGISTER
3 (adaptive NEAR5 (design)) AND INREGISTER
4 master protocol OR master study AND INREGISTER
5 "cohort multiple randomized controlled trial*" AND INREGISTER
6 "cohort multiple randomised controlled trial*" AND INREGISTER
7 "cmRCT" AND INREGISTER
8 (("multi‐factorial" OR multifactorial) NEAR7 (trial OR stud*)) AND INREGISTER
9 (network NEAR2 trial) AND INREGISTER
10 (network NEAR2 stud*):ti AND INREGISTER
11 (additional NEAR2 (arm* OR drug* OR agent* OR treatment* OR intervention*)): TI,AB AND INREGISTER
12 "candidate agents": TI,AB AND INREGISTER
13 (new NEAR2 (arm* OR drug*)): TI,AB AND INREGISTER
14 (different NEXT (agent* OR drug* OR treatment*)): TI,AB AND INREGISTER
15 (multiple NEXT (agent* OR treatment* OR intervention*)): TI,AB AND INREGISTER
16 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
Appendix 8. Search strategy: RCT search between 18 June and 30 July 2021
MEDLINE (via Ovid)
1. "spike protein, SARS‐CoV‐2".af.
2. Coronavirus Infections/ or Coronavirus/
3. SARS‐CoV‐2/ or COVID‐19/
4. ("2019 nCoV" or 2019nCoV or coronavir* or coronovir* or COVID or COVID19 or HCoV* or "nCov 2019" or "SARS CoV2" or "SARS CoV 2" or SARSCoV2 or "SARSCoV 2").tw,kf.
5. "severe acute respiratory syndrome coronavirus 2".tw,kf,nm.
6. ((corona* or corono*) adj1 (virus* or viral* or virinae*)).tw,kf.
7. or/2‐6
8. *Antibodies, Monoclonal/
9. Antibodies, Neutralizing/
10. Antibodies, Viral/
11. ((antibod* or mAb* or nAb*) adj2 (therap* or treatment* or neutrali?ing or prevent* or protect* or prophylax*)).tw,kf.
12. Spike Glycoprotein, Coronavirus/
13. Binding, Competitive/
14. (compet* adj1 bind*).tw,kf.
15. ("spike protein*" or "s protein*" or "Spike (S) protein").mp.
16. ((cocktail* or mixture* or combination*) adj3 (mAb* or antibod* or nAb*)).tw,kf.
17. ("two mab" or "mabs" or "two nab" or "two nabs").tw,kw.
18. (LY3832479* or LY‐CoV016* or LY‐3832479* or LYCoV016* or JS016* or JS‐016* or etesevimab*).mp.
19. (REGN‐COV2* or REGN‐COV‐2* or REGN10933* or REGN10987* or REGN‐10933* or REGN‐10987* or REGEN‐COV2* or REGEN‐COV‐2* or REGEN10933* or REGEN10987* or REGEN‐10933* or REGEN‐10987* or casirivimab* or imdevimab*).mp.
20. (LY3819253* or LY‐3819253* or LY‐CoV555* or LYCoV555* or bamlanivimab* or banlanivimab*).mp.
21. (VIR7831* or VIR‐7831* or GSK4182136* or GSK‐4182136* or sotrovimab*).mp.
22. (AZD7442* or AZD‐7442* or AZD8895* or AZD‐8895* or tixagevimab* or COV2‐2196 or COV22196* or AZD1061* or AZD‐1061* or cilgavimab* or COV2‐2130* or COV22130*).mp.
23. (DXP‐593* or DXP593* or BGB‐DXP593* or BGBDXP593* or BGB‐DXP‐593*).mp.
24. (TY027* or TY‐027*).mp.
25. (CTP59* or CTP‐59* or CT‐P59* or regdanvimab*).mp.
26. (STI1499* or STI‐1499* or COVI‐GUARD* or COVIguard*).mp.
27. (BRII196* or BRII‐196*).mp.
28. (SCTA01* or SCTA‐01*).mp.
29. (MW33* or MW‐33*).mp.
30. (BRII198* or BRII‐198*).mp.
31. (HFB30132A* or HFB‐30132A*).mp.
32. (ADM03820* or ADM‐03820*).mp.
33. (HLX70* or HLX‐70*).mp.
34. (STI2020* or STI‐2020* or COVIAMG* or COVI‐AMG*).mp.
35. (DZIF10c* or DZIF‐10c* or BI767551* or BI‐767551*).mp.
36. (COV2‐2381* or COV22381*).mp.
37. (ABBV‐47D11* or 47D11* or ABBV47D11*).mp.
38. (COR‐101* or COR101* or STE90‐C11*).mp.
39. (DXP‐604* or DXP604* or BGB‐DXP604* or BGBDXP604* or BGB‐DXP‐604*).mp.
40. (Chicken egg antibod* or egg yolk antibod* or IgY*).mp.
41. (VIR‐7832 or VIR7832 or GSK4182137* or GSK‐4182137*).mp.
42. (IDB003 or MD65 or MTX‐COVAB).mp.
43. (C144‐LS or C144LS or C‐135‐LS or C135‐LS or C135LS or SAB‐185 or SAB185 or JMB2002 or JMB‐2002 or TATX‐03 or TATX03 or NOVOAB‐20 or NOVOAB20 or NOVO‐AB‐20 or ABP‐300 or ABP300 or MW05 or MW‐05 or MW07 or MW‐07 or ACmab1 or covimax or adimab or CPI‐006 or CPI006 or ADG20 or "ADG20").mp.
44. Single‐Chain Antibodies/
45. (nmabs or diabod* or dia‐bod* or nanobod* or nano‐bod* or microbod* or micro‐bod* or monobod* or mono‐bod*).tw,kf,nm.
46. (scFv* or "single‐chain*" or "heavy chain*" or HCAbs or SCAbs or FAB).tw,kf,nm.
47. or/8‐46
48. 7 and (1 or 47)
49. randomized controlled trial.pt.
50. controlled clinical trial.pt.
51. randomi?ed.ab.
52. placebo.ab.
53. drug therapy.fs.
54. randomly.ab.
55. trial.ab.
56. groups.ab.
57. or/49‐56
58. exp animals/ not humans/
59. 57 not 58
60. clinical trial, phase iii/
61. ("Phase 3" or "phase3" or "phase III" or P3 or "PIII").ti,ab,kw.
62. (60 or 61) not 58
63. 59 or 62
64. 7 and (1 or 47) and 63
65. limit 64 to yr="2020‐Current"
PubMed
#1 2019 nCoV[tiab] OR 2019nCoV[tiab] OR corona virus[tiab] OR corona viruses[tiab] OR coronavirus[tiab] OR coronaviruses[tiab] OR COVID[tiab] OR COVID19[tiab] OR nCov 2019[tiab] OR SARS‐CoV2[tiab] OR SARS CoV‐2[tiab] OR SARSCoV2[tiab] OR SARSCoV‐2[tiab] OR "COVID‐19"[Mesh] OR "Coronavirus"[Mesh:NoExp] OR "SARS‐CoV‐2"[Mesh] OR "COVID‐19"[nm] OR "severe acute respiratory syndrome coronavirus 2"[nm]
#2 (antibod*[Title/Abstract] OR mAb[Title/Abstract] OR mAbs[Title/Abstract] OR nAb[Title/Abstract] OR nAbs[Title/Abstract]) AND (therap*[Title/Abstract] OR treat*[Title/Abstract] OR neutrali*[Title/Abstract] OR prevent*[Title/Abstract] OR protect*[Title/Abstract] OR prophylax*[Title/Abstract]) OR (compet*[Title/Abstract] AND bind*[Title/Abstract]) OR (cocktail*[Title/Abstract] OR mixture*[Title/Abstract] OR combination*[Title/Abstract]) AND (mAb[Title/Abstract] OR mAbs[Title/Abstract] OR antibod*[Title/Abstract] OR nAb[Title/Abstract] OR nAbs[Title/Abstract]) OR "spike protein*"[Title/Abstract] OR "s protein*"[Title/Abstract] OR "Spike (S) protein"[Title/Abstract]
#3 (LY‐3832479 OR LY3832479 OR LY‐CoV016 OR REGN‐COV2 OR REGN10933 OR REGN10987 OR REGN‐10933 OR REGN‐10987 OR REGEN10933 OR REGEN10987 OR REGEN‐10933 OR REGEN‐10987 OR REGN‐CoV2* OR REGN‐CoV‐2* OR REGEN‐CoV2* OR REGEN‐CoV‐2* OR casirivimab OR imdevimab OR LY‐3819253 OR LY3819253 OR LY‐CoV555 OR Bamlanivimab OR Banlanivimab OR VIR‐7831 OR VIR7831 OR GSK4182136 OR GSK‐4182136 OR sotrovimab OR AZD7442 OR AZD‐7442 OR AZD1061 OR AZD‐1061 OR AZD8895 OR AZD‐8895 OR tixagevimab OR cilgavimab OR DXP593 OR DXP‐593 OR BGB‐DXP‐593 OR BGBDXP593 OR JS016 OR JS‐016 OR LY‐CoV016 OR etesevimab OR TY027 OR TY‐027 OR CTP59 OR CT‐P59 OR regdanvimab OR STI1499 OR STI‐1499 OR COVI‐guard OR COVIguard OR BRII196 OR BRII‐196 OR SCTA01 OR SCTA‐01 OR MW33 OR MW‐33 OR BRII198 OR BRII‐198 OR HFB30132A OR HFB‐30132A OR ADM03820 OR ADM‐03820 OR HLX70 OR HLX‐70 OR STI2020 OR STI‐2020 OR COVIAMG OR COVI‐AMG OR DZIF10c OR DZIF‐10c OR BI767551 OR BI‐767551 COV2‐2381 OR COV22381 OR ABBV‐47D11 OR 47D11 OR ABBV47D11 OR COR‐101 OR COR101 OR STE90‐C11 OR DXP‐604 OR DXP604 OR BGB‐DXP604 OR BGBDXP604 OR BGB‐DXP‐604 OR „chicken egg antibod*" OR "egg yolk antibod*" OR IgY OR IgYs OR VIR‐7832 OR VIR7832 OR GSK4182137 OR GSK‐4182137 OR IDB003 OR MTX‐COVAB OR MD65 OR TATX‐03 OR NOVOAB‐20 OR "C144‐LS" OR C144LS OR "C‐135‐LS" OR "C135‐LS" OR C135LS OR "SAB‐185" OR SAB185 OR JMB2002 OR "JMB‐2002" OR "TATX‐03" OR TATX03 OR "NOVOAB‐20" OR NOVOAB20 OR "NOVO‐AB‐20" OR "ABP‐300" OR ABP300 OR MW05 OR "MW‐05" OR MW07 OR "MW‐07" OR ACmab1 OR covimax OR adimab OR "CPI‐006" OR CPI006 OR diabod* OR nanobod* OR microbod* OR monobod* OR scFv* OR "single‐chain" OR "heavy chain" OR HCAbs OR SCAbs OR nmAbs OR ADG20 OR ADG 20 OR MAD0004J08)
#4 "spike protein*"[Title/Abstract] OR "s protein*"[Title/Abstract] OR "Spike (S) protein"[Title/Abstract]
#5 #1 AND (#2 OR #3)
#6 #4 OR #5
#7 (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb])
#8 randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab] OR randomized controlled trial [pt] OR controlled clinical trial [pt]
#9 #6 AND #7 AND #8
#10 ("animals"[mh] NOT "humans"[mh])
#11 #9 NOT #10 Filters: from 2020/1/1 ‐ 3000/12/12
Embase (via Ovid)
# Searches
1 coronavirinae/ or coronaviridae/ or coronaviridae infection/
2 coronavirus disease 2019/
3 Coronavirus infection/
4 sars‐related coronavirus/
5 "Severe acute respiratory syndrome coronavirus 2"/
6 ((corona* or corono*) adj1 (virus* or viral* or virinae*)).tw,kw.
7 ("2019 nCoV" or 2019nCoV or coronavir* or coronovir* or COVID or COVID19 or HCoV* or "nCov 2019" or "SARS CoV2" or "SARS CoV 2" or SARSCoV2 or "SARSCoV 2").tw,kw.
8 "Severe acute respiratory syndrome coronavirus 2".mp.
9 or/1‐8
10 Antibodies, Monoclonal/
11 Antibodies, Neutralizing/
12 Antibodies, Viral/
13 ((antibod* or mAb* or nAb*) adj2 (therap* or treatment* or neutrali?ing or prevent* or protect* or prophylax*)).tw,kw.
14 Spike Glycoprotein, Coronavirus/
15 Binding, Competitive/
16 (compet* adj1 bind*).tw,kw.
17 ("spike protein*" or "s protein*" or "Spike (S) protein").mp.
18 ((cocktail* or mixture* or combination*) adj3 (mAb* or antibod* or nAb*)).tw,kw.
19 ("two mab" or "mabs" or "two nab" or "two nabs").tw,kw.
20 (LY3832479* or LY‐CoV016* or LY‐3832479* or LYCoV016* or JS016* or JS‐016* or etesevimab*).mp.
21 (REGN‐COV2* or REGN‐COV‐2* or REGN10933* or REGN10987* or REGN‐10933* or REGN‐10987* or REGEN‐COV2* or REGEN‐COV‐2* or REGEN10933* or REGEN10987* or REGEN‐10933* or REGEN‐10987* or casirivimab* or imdevimab*).mp.
22 (LY3819253* or LY‐3819253* or LY‐CoV555* or LYCoV555* or bamlanivimab* or banlanivimab*).mp.
23 (VIR7831* or VIR‐7831* or GSK4182136* or GSK‐4182136* or sotrovimab*).mp.
24 (AZD7442* or AZD‐7442* or AZD8895* or AZD‐8895* or tixagevimab* or COV2‐2196 or COV22196* or AZD1061* or AZD‐1061* or cilgavimab* or COV2‐2130* or COV22130*).mp.
25 (DXP‐593* or DXP593* or BGB‐DXP593* or BGBDXP593* or BGB‐DXP‐593*).mp.
26 (TY027* or TY‐027*).mp.
27 (CTP59* or CTP‐59* or CT‐P59* or regdanvimab*).mp.
28 (STI1499* or STI‐1499* or COVI‐GUARD* or COVIguard*).mp.
29 (BRII196* or BRII‐196*).mp.
30 (SCTA01* or SCTA‐01*).mp.
31 (MW33* or MW‐33*).mp.
32 (BRII198* or BRII‐198*).mp.
33 (HFB30132A* or HFB‐30132A*).mp.
34 (ADM03820* or ADM‐03820*).mp.
35 (HLX70* or HLX‐70*).mp.
36 (STI2020* or STI‐2020* or COVIAMG* or COVI‐AMG*).mp.
37 (DZIF10c* or DZIF‐10c* or BI767551* or BI‐767551*).mp.
38 (COV2‐2381* or COV22381*).mp.
39 (ABBV‐47D11* or 47D11* or ABBV47D11*).mp.
40 (COR‐101* or COR101* or STE90‐C11*).mp.
41 (DXP‐604* or DXP604* or BGB‐DXP604* or BGBDXP604* or BGB‐DXP‐604*).mp.
42 (Chicken egg antibod* or egg yolk antibod* or IgY*).mp.
43 (VIR‐7832* or VIR7832* or GSK4182137* or GSK‐4182137*).mp.
44 (IDB003 or MD65 or MTX‐COVAB).mp.
45 (C144‐LS or C144LS or C‐135‐LS or C135‐LS or C135LS or SAB‐185 or SAB185 or JMB2002 or JMB‐2002 or TATX‐03 or TATX03 or NOVOAB‐20 or NOVOAB20 or NOVO‐AB‐20 or ABP‐300 or ABP300 or MW05 or MW‐05 or MW07 or MW‐07 or ACmab1 or covimax or adimab or CPI‐006 or CPI006 or ADG20 or ADG 20 or MAD0004J08).mp.
46 single chain fragment variable antibody/
47 (nmabs or diabod* or dia‐bod* or nanobod* or nano‐bod* or microbod* or micro‐bod* or monobod* or mono‐bod*).mp.
48 (scFv* or "single‐chain*" or "heavy chain*" or HCAbs or SCAbs).mp.
49 or/10‐48
50 Randomized controlled trial/
51 Controlled clinical study/
52 random*.ti,ab.
53 randomization/
54 intermethod comparison/
55 placebo.ti,ab.
56 (compare or compared or comparison).ti.
57 (open adj label).ti,ab.
58 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
59 double blind procedure/
60 parallel group$1.ti,ab.
61 (crossover or cross over).ti,ab.
62 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
63 (controlled adj7 (study or design or trial)).ti,ab.
64 (volunteer or volunteers).ti,ab.
65 trial.ti.
66 or/50‐65
67 phase 3 clinical trial/
68 ("Phase 3" or "phase3" or "phase III" or P3 or "PIII").tw,kw.
69 or/67‐68
70 (animal experiment/ or Animal experiment/) not (human experiment/ or human/)
71 (66 or 69) not 70
72 9 and 49 and 71
73 limit 72 to yr="2020 ‐Current"
74 limit 73 to medline
75 73 not 74
Cochrane Covid‐19 study register (https://covid‐19.cochrane.org/)
"Ly‐3832479" OR Ly3832479 OR "LY‐3832479" OR LY3832479 OR "LY‐CoV016" OR "REGN‐COV2" OR "REGEN‐COV2" OR REGN10933 OR REGN10987 OR REGEN10933 OR REGEN10987 OR casirivimab OR imdevimab OR "LY‐3819253" OR LY3819253 OR "LY‐CoV555" OR Bamlanivimab OR Banlanivimab OR "VIR‐7831" OR VIR7831 OR GSK4182136 OR "GSK‐4182136" OR sotrovimab OR AZD7442 OR "AZD‐7442" OR AZD8895 OR "AZD‐8895" OR tixagevimab OR "COV2‐2196" OR COV22196 OR AZD1061 OR "AZD‐1061" OR cilgavimab OR "COV2‐2130" OR COV22130 OR DXP593 OR "DXP‐593" OR "BGB‐DXP‐593" OR BGBDXP593 OR JS016 OR "JS‐016" OR etesevimab OR TY027 OR "TY‐027" OR CTP59 OR "CTP‐59" OR "CT‐P59" OR regdanvimab OR STI1499 OR "STI‐1499" OR "COVI‐guard" OR COVIguard OR BRII196 OR "BRII‐196" OR SCTA01 OR "SCTA‐01" OR MW33 OR "MW‐33" OR BRII198 OR "BRII‐198" OR HFB30132A OR "HFB‐30132A" OR ADM03820 OR "ADM‐03820" OR HLX70 OR "HLX‐70" OR STI2020 OR "STI‐2020" OR COVIAMG OR "COVI‐AMG" OR DZIF10c OR "DZIF‐10c" OR BI767551 OR "BI‐767551" OR "COV2‐2381" OR COV22381 OR "ABBV‐47D11" OR 47D11 OR ABBV47D11 OR "COR‐101" OR COR101 OR "STE90‐C11" OR "DXP‐604" OR DXP604 OR "BGB‐DXP604" OR BGBDXP604 OR "BGB‐DXP‐604" OR "chicken egg antibody" OR "egg yolk antibody" OR IgY* OR "VIR‐7832" OR VIR7832 OR GSK4182137 OR "GSK‐4182137" OR IDB003 OR MD65 OR "MTX‐COVAB" OR "C144‐LS" OR C144LS OR "C‐135‐LS" OR "C135‐LS" OR C135LS OR "SAB‐185" OR SAB185 OR JMB2002 OR "JMB‐2002" OR "TATX‐03" OR TATX03 OR "NOVOAB‐20" OR NOVOAB20 OR "NOVO‐AB‐20" OR "ABP‐300" OR ABP300 OR MW05 OR "MW‐05" OR MW07 OR "MW‐07" OR ACmab1 OR covimax OR adimab OR "CPI‐006" OR CPI006 OR ADG20 OR "ADG 20" OR diabod* OR nanobod* OR microbod* OR monobod* OR scFv* OR "single‐chain" OR "heavy chain" OR HCAbs OR SCAbs OR nmAb* OR "two mab" OR "mabs" OR "two nab" OR "two nabs" OR "ADG20" OR "ADG 20" OR "MAD0004J08"
Study characteristics: "Intervention assignment": Randomised/quasi‐randomised/unclear
1) "Intervention assignment": “Randomised” OR „unclear“
2) "Study design": "Parallel/Crossover" AND "Unclear"
WHO database (without Embase, ICTRP, Medline and PubMed)
Advanced search and in the search field: title, abstract, subject
("Ly‐3832479" OR Ly3832479 OR "LY‐3832479" OR LY3832479 OR "LY‐CoV016" OR "REGN‐COV2" OR "REGEN‐COV2" OR REGN10933 OR REGN10987 OR REGEN10933 OR REGEN10987 OR casirivimab OR imdevimab OR "LY‐3819253" OR LY3819253 OR "LY‐CoV555" OR Bamlanivimab OR Banlanivimab OR "VIR‐7831" OR VIR7831 OR GSK4182136 OR "GSK‐4182136" OR sotrovimab OR AZD7442 OR "AZD‐7442" OR AZD8895 OR "AZD‐8895" OR tixagevimab OR "COV2‐2196" OR COV22196 OR AZD1061 OR "AZD‐1061" OR cilgavimab OR "COV2‐2130" OR COV22130 OR DXP593 OR "DXP‐593" OR "BGB‐DXP‐593" OR BGBDXP593 OR JS016 OR "JS‐016" OR etesevimab OR TY027 OR "TY‐027" OR CTP59 OR "CTP‐59" OR "CT‐P59" OR regdanvimab OR STI1499 OR "STI‐1499" OR "COVI‐guard" OR COVIguard OR BRII196 OR "BRII‐196" OR SCTA01 OR "SCTA‐01" OR MW33 OR "MW‐33" OR BRII198 OR "BRII‐198" OR HFB30132A OR "HFB‐30132A" OR ADM03820 OR "ADM‐03820" OR HLX70 OR "HLX‐70" OR STI2020 OR "STI‐2020" OR COVIAMG OR "COVI‐AMG" OR DZIF10c OR "DZIF‐10c" OR BI767551 OR "BI‐767551" OR "COV2‐2381" OR COV22381 OR "ABBV‐47D11" OR 47D11 OR ABBV47D11 OR "COR‐101" OR COR101 OR "STE90‐C11" OR "DXP‐604" OR DXP604 OR "BGB‐DXP604" OR BGBDXP604 OR "BGB‐DXP‐604" OR "chicken egg antibody" OR "egg yolk antibody" OR IgY* OR "VIR‐7832" OR VIR7832 OR GSK4182137 OR "GSK‐4182137" OR IDB003 OR MD65 OR "MTX‐COVAB" OR "C144‐LS" OR C144LS OR "C‐135‐LS" OR "C135‐LS" OR C135LS OR "SAB‐185" OR SAB185 OR JMB2002 OR "JMB‐2002" OR "TATX‐03" OR TATX03 OR "NOVOAB‐20" OR NOVOAB20 OR "NOVO‐AB‐20" OR "ABP‐300" OR ABP300 OR MW05 OR "MW‐05" OR MW07 OR "MW‐07" OR ACmab1 OR covimax OR adimab OR "CPI‐006" OR CPI006 OR ADG20 OR "ADG 20" OR diabod* OR nanobod* OR microbod* OR monobod* OR scFv* OR "single‐chain" OR "heavy chain" OR HCAbs OR SCAbs OR nmAb* OR "two mab" OR "mabs" OR "two nab" OR "two nabs" OR "ADG20" OR "ADG 20" OR "MAD0004J08")
AND
(random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII")
Epistemonikos
Covid‐19 àprevention or treatment à procedures à passive immunization à Anti‐SARS‐CoV‐2 MaB à primary studies
results filtered by RCT
Appendix 9. Characteristics of studies for tracking (listed in excluded studies)
|
Study, drug name |
Sponsor/developer | Target | Phase | Design | Population/disease severity | Patient status | Time of administration | Route of administration | Number of participants | Status, planned completion date, or DOI if published |
| Prospectively registered, non‐randomised studies examining SARS‐CoV‐2‐neutralising mAbs | ||||||||||
| Track: NCT04603651, Bamlanivimab |
Eli Lilly and Company | SARS‐CoV‐2 S protein | Expanded access | Non‐randomised, expanded access | Mild | Outpatients | Within 3 days of confirmation of disease | Infusion (IV) | Not reported | Cinicaltrials.gov: no longer available (was available previously) |
|
Track: NCT04701658 Bamlanivimab |
Eli Lilly and Company | SARS‐CoV‐2 S protein | Phase 2 | Non‐randomised prospective cohort Study | Mild | Outpatients | Not reported | Infusion (IV) | Estimated enrollment: 3000 participants | Recruiting Estimated completion date: June 2021 |
|
Track: NCT04617535, Casirivimab plus imdevimab |
Regeneron Pharmaceuticals | SARS‐CoV‐2 S protein | Expanded access | Non‐randomised, compassionate use | Mild | Outpatients | Not reported | Infusion (IV), single‐dose | Not reported | Available |
|
Track: NCT04656691 Bamlanivimab |
Daniel Griffin, United Health Group; Eli Lilly and Company; Optum, Inc. |
SARS‐CoV‐2 S protein | Phase 4 | Single‐dose study using matched controls | Mild to moderate | Outpatients | Not reported | Infusion (IV), single dose | Actual enrolment: 4000 participants | Completed in April 2021 |
| RCTs in healthy participants | ||||||||||
|
Track: NCT04592549, ADM03820 |
Ology Bioservices | A mixture of 2 human IgG1 non‐competitive binding anti‐SARS‐CoV‐2 antibodies | Phase 1 | Randomised, sequential assignment, quadruple‐blind (participant, care provider, investigator, outcome assessor), placebo‐controlled, dose‐escalation trial | Healthy | Not applicable | Not applicable | Infusion (IV), infusion (IM) (4 subcohorts with different injection/dose) |
Estimated enrolment: 40 participants | Recruiting Estimated completion date 30 September 2021 |
|
Track: NCT04567810, Anti‐SARS‐CoV‐2 chicken egg IgY |
Stanford University | Not specified | Phase 1 | Randomised, double‐blind, sequential‐assignment | Healthy | Not applicable | Not applicable | Intranasally, multiple doses | Actual enrolment: 48 participants | Completed in December 2020, no results posted yet |
|
Track: NCT04507256, AZD7442 (AZD8895 + AZD1061) |
AstraZeneca, Parexel | SARS‐CoV‐2 S protein | Phase 1 | Randomised, double‐blind, placebo‐controlled and dose‐escalation study | Healthy | Not applicable | Not applicable | Infusion (IV and IM), 3 cohorts sequentially, 1 cohort co‐administration of AZD8895 + AZD1061 | Actual enrolment: 60 participants | Active, not recruiting Estimated completion date 25 October 2021 Press release: https://doi.org/10.1021/cen-09843-buscon13 |
|
Track: NCT04896541 AZD7442 (AZD8895 + AZD1061) |
AstraZeneca | SARS‐CoV‐2 S protein | Phase 1 | Randomised, double‐blinded, placebo controlled, single dose | Healthy | Not applicable | On day 1 | IM injections or IV administration | Estimated enrolment: 40 participants | Active, not recruiting, Estimated completion date July 4, 2022 |
| Track: NCT04537910, Bamlanivimab | Eli Lilly and Company | SARS‐CoV‐2 S protein | Phase 1 | Randomised, placebo‐controlled, participant‐ and investigator‐blind | Healthy | Not applicable | Not applicable | Subcutaneously | Actual enrolment: 25 participants | Completed, December 28, 2020 |
|
Track: NCT04532294, BGB‐DXP593 |
BeiGene | SARS‐CoV‐2 S protein | Phase 1 | Randomised, double‐blind, placebo‐controlled, single‐dose escalation study | Healthy | Not applicable | Not applicable | Infusion (IV) | Estimated enrolment: 30 participants | Completed, estimated completion date February 13, 2021 |
|
Track: NCT04479631, BRII‐196 |
Brii Biosciences Limited, TSB Therapeutics (Beijing) CO.LTD |
SARS‐CoV‐2 S protein | Phase 1 | Randomised, single‐blind, placebo‐controlled, single ascending dose‐escalation study | Healthy | Not applicable | Not applicable | Infusion (IV), single dose | Estimated enrolment: 16 participants | Completed Completion dateJanuary 29, 2021 |
|
Track: NCT04691180 BRII‐196 and BRII‐198 (2 arms with different dose) |
Brii Biosciences Limited, TSB Therapeutics (Beijing) CO.LTD | SARS‐CoV‐2 S protein | Phase 1 | Randomised, single‐blind, placebo‐controlled, single ascending dose‐escalation study | Healthy | Not applicable | Not applicable | Infusion (IV), single dose | Estimated enrolment: 24 participants | Recruiting Estimated completion date: August 2021 |
|
Track: NCT04479644, BRII‐198 |
Brii Biosciences Limited, TSB Therapeutics (Beijing) CO.LTD | SARS‐CoV‐2 S protein | Phase 1 | Randomised, single‐blind, placebo‐controlled, single ascending dose‐escalation study | Healthy | Not applicable | Not applicable | Infusion (IV), single dose | Estimated enrolment: 17 participants | Completed Completion date February 3, 2021 |
|
Track: jRCT2071200117 Casarivimab/ imdevimab |
Chugai Pharmaceutical Co., Ltd. | SARS‐CoV‐2 S protein | Phase 1 | Randomised, double‐blind, placebo‐controlled study | Not applicable | Healthy | Not applicable | Infusion (IV), single dose | Planned enrolment: 22 participants | Active, not recruiting |
|
Track: NCT04519437, Casirivimab/ imdevimab |
Regeneron Pharmaceuticals | SARS‐CoV‐2 S protein | Phase 1 | Randomised, double‐blind, placebo‐controlled | Healthy | Not applicable | Not applicable | Subcutaneously, repeated dose | Actual enrolment: 974 participants | Active, not recruiting Estimated completion date October 2021 Press release: https://www.aljazeera.com/economy/2020/11/17/regeneron-roche-tested-manufacture-of-covid-drug-trump-used |
|
Track: NCT04852978 Casarivimab/ imdevimab + vaccine |
Regeneron Pharmaceuticals | SARS‐CoV‐2 S protein | Phase 2 | Randomised, open‐label, parallel group study | Healthy | Not applicable | Not applicable | Infusion (IV) or subcutaneous administration | Estimated enrolment: 180 participants | Recruiting Estimated completion date August 30, 2022 |
|
Track: NCT04525079, Regdanvimab |
Celltrion | SARS‐CoV‐2 spike RBD | Phase 1 | Randomised, double‐blind, placebo‐controlled, parallel‐group, single ascending dose | Healthy | Not applicable | Not applicable | Not reported | Estimated enrolment: 32 participants | Recruiting Estimated completion date 30 November 2020 |
|
Track: NCT04441931, Etesevimab |
Eli Lilly and Company | SARS‐CoV‐2 S protein | Phase 1 | Randomised, placebo‐controlled, double‐blinded | Healthy | Not applicable | Not applicable | Infusion (IV) | Actual enrolment: 26 participants | Completed on October 2, 2020 |
|
Track: NCT04441918, Etesevimab |
Shanghai Junshi Bioscience Co., Ltd. | SARS‐CoV‐2 S protein | Phase 1 | Randomised, double‐blind, placebo‐controlled | Healthy | Not applicable | Not applicable | Infusion (IV), single dose | Estimated enrolment: 40 participants | Recruiting Estimated completion date December 2020 |
|
Track: ChiCTR2100042150 JMB‐2002 |
Shu Lan (Hang Zhou) Hospital | SARS‐CoV‐2 S protein | Phase 1 | Randomised, double‐blind, placebo‐controlled study | Not applicable | Healthy | Not applicable | Infusion (IV), single dose | Planned enrolment: 40 participants | Active, not yet recruiting |
| Track: NCT04590430, HFB30132A | HiFiBiO Therapeutics | SARS‐CoV‐2 S protein | Phase 1 | Randomised, double‐blind, placebo‐controlled, single ascending dose | Healthy | Not applicable | Not applicable | Infusion (IV), single dose | Estimated enrolment: 24 participants | Active, not recruiting Estimated completion date July 2021 |
|
Track: NCT04561076 HLX70 |
Hengenix Biotech Inc., Sanyou Biopharmaceuticals (Shanghai) Co., Ltd Shanghai ZJ Bio‐Tech Co., Ltd |
SARS‐CoV‐2 S protein | Phase 1 | Randomised, double‐blind, placebo‐controlled, dose‐escalation study | Healthy | Not applicable | Not applicable | Infusion (IV), single dose | Estimated enrolment: 24 participants | Not yet recruiting Estimated completion date: September 2021 |
|
Track: NCT04533048, MW33 |
Mabwell (Shanghai) Bioscience Co., Ltd. | SARS‐CoV‐2 S protein | Phase 1 | Randomised, double‐blind, placebo‐controlled | Healthy | Not applicable | Not applicable | Injection | Actual enrolment: 42 participants | Completed, on December 2, 2020 no results posted yet |
| Track: NCT04483375, SCTA01 |
Sinocelltech Ltd. | SARS‐CoV‐2 S protein | Phase1 | Randomised, double‐blinded, placebo‐controlled, single ascending dose | Healthy | Not applicable | Not applicable | Not reported, single dose | Actual enrolment: 33 participants | Completed, on November 17, 2020 no results posted yet |
|
Track: NCT04429529, TY027 |
Tychan Pte Ltd. | SARS‐CoV‐2 S protein | Phase 1 | Randomised, placebo‐ controlled, double‐blind, single ascending dose, time‐lagged | Healthy | Not applicable | Not applicable | Infusion (IV), single dose | Estimated enrolment: 32 participants | Completed On NCT04429529 |
|
Track: NCT04700163, C144‐LS and C‐135‐LS |
Rockefeller University | SARS‐CoV‐2 S protein | Phase 1 | Non‐randomised, open ‐abel, single‐dose, dose‐escalation study | Healthy | Not applicable |
Not reported | Infusion (IV) or SC; 5 arms with different injection/dose | Actual enrolment: 23 participants | Active, not recruiting Estimated completion date: June 2022 |
|
Track: NCT04932850, MAD0004J08 |
Toscana Life Sciences Sviluppo s.r.l. | SARS‐CoV‐2 S protein | Phase 1 | Randomised, placebo‐controlled, quadruple‐ blind, dose‐escalation study | Healthy | Not applicable | Not reported | Injection (Cohort 1 (48 mg), Cohort 2 (100 mg) and Cohort 3 (400 mg)) | Actual enrolment: 30 participants | Active, not recruiting Estimated completion date: October 2021 |
Appendix 10. Ongoing RCTs investigating SARS‐CoV‐2 neutralising mAbs
| Ongoing studies for SARS‐CoV‐2 neutralising mAbs | ||||
| mAb or mAb combination | Number | RCTs | Phase | Status, planned completion |
| bamlanivimab | 6 RCTs | NCT04411628 | Phase 1 | Completed |
| NCT04840459 | Phase 2 | Recruiting, Jan 2022 | ||
| ACTIV‐2 | Phase 2/3 (platform) | Recruiting, May 2023 | ||
| OPTIMISE‐C19 | Phase 3 (platform) | Recruiting, Dec 2022 | ||
| NCT04748588 | Phase 4 | Recruiting, Mar 2023 | ||
| NCT04796402 | Phase 4 | Recruiting, Dec 2021 | ||
| sotrovimab (VIR‐7831) | 4 RCTs | AGILE | Phase 1/2 (platform) | Recruiting, April 2023 |
| NCT04779879 | Phase 2 | Recruiting, April 2023 | ||
| ACTIV‐3 | Phase 3 (platform) | Arm stopped for futility, study continues, July 2022 | ||
| NCT04913675 | Phase 3 | Not yet recruiting, Nov 2022 | ||
| AZD7442 | 4 RCTs | ACTIV‐2 | Phase 2/3 (platform) | Recruiting, May 2023 |
| NCT04723394 | Phase 3 | Recruiting, Sep 2022 | ||
| ACTIV‐3 | Phase 3 (platform) | Recruiting, July 2022 | ||
| DISCOVERY | Phase 3 (platform) | Active, not recruiting March 2023 | ||
| BRII‐196/BRII‐198 | 4 RCTs | NCT04770467 | Phase 2 | Withdrawn, Oct 2022 |
| NCT04787211 | Phase 2 | Recruiting, Dec 2021 | ||
| ACTIV‐2 | Phase 2/3 (platform) | Recruiting, May 2023 | ||
| ACTIV‐3 | Phase 3 (platform) | Arm stopped for futility, study continues, July 2022 | ||
| casirivimab/imdevimab | 4 RCTs | NCT04426695 | Phase 1/2 | Active not recruiting, June 2021 |
| NCT04666441 | Phase 2 | Active not recruiting, Aug 2021 | ||
| NCT04840459 | Phase 2 | Recruiting, Jan 2022 | ||
| OPTIMISE‐C19 | Phase 3 | Recruiting, Dec 2022 | ||
| DZIF‐10c | 3 RCTs | NCT04631666 | Phase 1/2 | Active not recruiting, Aug 2021 |
| NCT04631705 | Phase 1/2 | Recruiting, Sep 2021 | ||
| NCT04822701 | Phase 2/3 | Terminated, June 2021 | ||
| SCTA01 | 3 RCTs | NCT04644185 | Phase 2/3 | Recruiting, Dec 2021 |
| NCT04683328 | Phase 2/3 | Not yet recruiting, Nov 2021 | ||
| NCT04709328 | Phase 2/3 | Not yet recruiting, March 2022 | ||
| STI‐2020 (COVI‐AMG) | 3 RCTs | NCT04584697 | Phase 1/2 | Withdrawn, April 2021 |
| NCT04734860 | Phase 2 | Recruiting, Nov 2021 | ||
| NCT04771351 | Phase 2 | Recruiting, Oct 2021 | ||
| regdanvimab (CT‐P59) | 2 RCTs | NCT04593641 | Phase 1 | Active not recruiting, Dec 2021 |
| EUDRACT2020‐003401‐60 | Phase 2/3 | Not updated | ||
| bamlanivimab/etesevimab | 2 RCTs |
NCT04634409 |
Phase 2 | Recruiting, Nov 2021 |
| OPTIMISE‐C19 | Phase 3 (platform) | Recruiting, Dec 2022 | ||
| ABBV‐47D11 | 1 RCT | NCT04644120 | Phase 1 | Active not recruiting, Aug 2021 |
| ADG20 | 1 RCT | NCT04840459 | Phase 2/3 | Recruiting, Jan 2022 |
| bamlanivimab/etesevimab/LY‐CoV1404 | 1 RCT | NCT04634409 | Phase 2 | Recruiting, Nov 2021 |
| bamlanivimab/sotrovimab | 1 RCT | NCT04634409 | Phase 2 | Recruiting, Nov 2021 |
| BGB‐DXP593 | 1 RCT | NCT04551898 | Phase 2 | Completed |
| COR‐101 | 1 RCT | NCT04674566 | Phase 1b/2 | Recruiting, June 2021 |
| C135‐LS/C144‐LS | 1 RCT | ACTIV‐2 | Phase 2/3 (platform) | Recruiting, May 2023 |
| etesevimab alone | 1 RCT | NCT04780321 | Phase 1/2 | Recruiting, Oct 2021 |
| LY‐CoV1404 | 1 RCT | NCT04634409 | Phase 2 | Recruiting, Nov 2021 |
| MW33 | 1 RCT | NCT04627584 | Phase 2 | Recruiting, Sep 2021 |
| STI‐2099 | 1 RCT | NCT04900428 | Phase 2 | Not yet recruiting, March 2022 |
| VIR‐7832 | 1 RCT | AGILE | Phase 1/2 (platform) | Recruiting, April 2022 |
| MAD0004J08 | 1 RCT | NCT04952805 | Phase 2/3 | Recruiting, August 2022 |
| Abbreviations: mAb: monoclonal antibody; RCT: randomised controlled trial | ||||
Data and analyses
Comparison 1. Bamlanivimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality by day 30 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Admission to hospital or death | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3 Viral clearance at day 7 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4 Viral clearance at day 15 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 Adverse events: all grades | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Serious adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.2. Analysis.

Comparison 1: Bamlanivimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 2: Admission to hospital or death
Comparison 2. Bamlanivimab/etesevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mortality by day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Admission to hospital or death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Viral clearance at day 3 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4 Viral clearance at day 7 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.5 Viral clearance at day 15 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6 Adverse events: all grades | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.7 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
2.3. Analysis.

Comparison 2: Bamlanivimab/etesevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 3: Viral clearance at day 3
2.4. Analysis.

Comparison 2: Bamlanivimab/etesevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 4: Viral clearance at day 7
2.5. Analysis.

Comparison 2: Bamlanivimab/etesevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 5: Viral clearance at day 15
Comparison 3. Casirivimab/imdevimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Admission to hospital or death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.2 Adverse events: grade 3 and 4 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.3 Serious adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Sotrovimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Mortality by day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2 Development of severe symptoms according to WHO scale (≥ 5, incl. death) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.3 Development of severe symptoms according to WHO scale (≥ score 7, IMV, incl. death) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.4 Admission to hospital or death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.5 Admission to ICU by day 29 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.6 Adverse events: all grades | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.7 Adverse events: grade 3 and 4 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.8 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Regdanvimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mortality by day 30 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2 Development of severe symptoms according to WHO scale (≥ score 7, IMV, incl. death) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.3 Admission to hospital or death by day 30 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.4 Admission to ICU by day 30 | 2 | 410 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.5 Viral clearance at day 15 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.6 Adverse events: all grades | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.7 Adverse events: grade 3 and 4 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.8 Serious adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
5.8. Analysis.

Comparison 5: Regdanvimab in non‐hospitalised individuals with COVID‐19 (asymptomatic and mild disease), Outcome 8: Serious adverse events
Comparison 6. Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Mortality by day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2 Mortality by day 90 | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.3 Development of severe symptoms: need for NIV, IMV, ECMO, or renal replacement therapy at day 5 (group 5, 6 or 7) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.4 Development of severe symptoms: clinical status at day 5, intubation (group 6 at pulmonary scale) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.5 Hospital discharge up to 26 October 2020 | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.6 Hospital discharge: at day 5 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.7 Adverse events: grade 3 and 4 by day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.8 Serious adverse events by day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.9 Serious adverse events (90 day follow‐up) | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.10 Sustained recovery (90 day follow‐up) | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.11 Neurological dysfunction by day 30: transient ischemic events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.12 Neurological dysfunction by day 30: acute delirium CVA | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.13 Neurological dysfunction by day 30: cerebrovascular event | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.14 Thromboembolic events by day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.15 Renal dysfunction (or need for dialysis) by day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
6.8. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 8: Serious adverse events by day 30
6.9. Analysis.

Comparison 6: Bamlanivimab in hospitalised individuals with COVID‐19 (moderate and severe disease), Outcome 9: Serious adverse events (90 day follow‐up)
Comparison 7. Casirivimab/imdevimab in hospitalised individuals with COVID‐19 (moderate and severe disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Mortality by day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.2 Development of severe symptoms: requirement for IMV or death by day 28 (WHO ≥ 7) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.3 Hospital discharge alive by day 30 | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7.4 Thromboembolic events by day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.5 Renal dysfunction (or need for dialysis) by day 30 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACTIV‐3.
| Study characteristics | ||
| Methods | Drug name: bamlanivimab Trial design: randomised, adaptive, blinded, controlled, multicentre, phase 3 trial Type of publication: journal publication (English) NCT number: NCT04501978 (date of trial registration: 3 August 2020)
Number of participants in bamlanivimab comparison:
Estimated enrolment: 10,000 participants Estimated completion date: 22 July 2022* |
|
| Participants | Setting
Eligibility criteria
Participant characteristics:
|
|
| Interventions | Intervention
Comparator
|
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
|
| Notes | * Additional mAb arms: VIR‐7831 (administered by IV infusion, randomisation stopped); BRII‐196/BRII‐198 (administered by IV infusion, randomisation stopped); AZD7442 (600 mg AZD7442 (300 mg AZD8895 + 300 mg AZD1061, single‐dose IV infusion) Developer: AbCellera, NIAID Vaccine Research Center, Eli Lilly and Company Funding: NIAID Conflicts of interest: AB:
|
|
BLAZE‐1 (phase 2, 0.7g).
| Study characteristics | ||
| Methods | see BLAZE‐1 (phase 2) | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
BLAZE‐1 (phase 2, 2.8g).
| Study characteristics | ||
| Methods | see BLAZE‐1 (phase 2) | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
BLAZE‐1 (phase 2, 7.0g).
| Study characteristics | ||
| Methods | see BLAZE‐1 (phase 2) | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
BLAZE‐1 (phase 2).
| Study characteristics | ||
| Methods | Drug name:
Trial design: randomised, double‐blind, placebo‐controlled, multipart, phase 2/3, single‐infusion study Type of publication: journal publication (English) NCT number: NCT04427501 (date of trial registration: 11 June 2020)
Number of participants:
Estimated enrolment: 3160 participants Estimated completion date: 31 May 2021 |
|
| Participants | Setting
Eligibility criteria
Participant characteristics
|
|
| Interventions | Interventions
Comparator:
|
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
|
| Notes | Developer:
Funding: Eli Lilly and Company Conflicts of interest:
|
|
BLAZE‐1 (phase 3).
| Study characteristics | ||
| Methods | Drug name:
Trial design: randomised, double‐blind, placebo‐controlled, multipart, phase 2/3, single‐infusion study Type of publication: journal publication (English) NCT number: NCT04427501 (date of trial registration: 11 June 2020)
Number of participants:
Estimated enrolment: 577 participants Estimated completion date: 24 June 2022 |
|
| Participants | Setting
Eligibility criteria
Participant characteristics
|
|
| Interventions | Interventions
Comparator:
|
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
|
| Notes | Developer:
Funding: Eli Lilly and Company Conflicts of interest:
|
|
COMET‐ICE.
| Study characteristics | ||
| Methods | Drug name: sotrovimab (VIR‐7831) Trial design: randomised, multicentre, double‐blind, placebo‐controlled, phase 2/3 study Type of publication: preprint (English) NCT number: COMET‐ICE (date of trial registration: 10 September 2020) Ongoing: active, not recruiting, updated 7 May 2021 Number of participants:
Estimated enrolment: 1360 participants Estimated completion date: July 2021 |
|
| Participants | Setting
Eligibility criteria
Participant characteristics
|
|
| Interventions | Intervention Sotrovimab (VIR‐7831, GSK4182136)
Comparator
|
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
|
| Notes | Developer: Vir Biotechnology, Inc.; GlaxoSmithKline Funding: Vir Biotechnology, Inc.; GlaxoSmithKline Recruitment status: active, not recruiting Conflicts of Interest:
|
|
Eom 2021.
| Study characteristics | ||
| Methods | Drug name: regdanvimab (CT‐P59) Trial design: randomised, parallel‐group, placebo‐controlled, quadruple‐blinded, phase 2/3 study. Results are from part 1 of a two‐part phase 2/3 study Type of publication: preprint publication (English) NCT number: NCT04602000 (date of trial registration: 26 October 2020) Ongoing: recruiting, updated 26 October 2020 Number of participants:
Estimated enrolment: 1020 participants Estimated completion date: September 2021 |
|
| Participants | Setting
Eligibility criteria
Participant characteristics
|
|
| Interventions | Intervention
Comparator
|
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
|
| Notes | Developer: Celltrion Funding: Celltrion Recruitment status: recruiting Other: 3 arms Conflicts of interest:
|
|
Eom 2021 (40 mg/kg).
| Study characteristics | ||
| Methods | see Eom 2021 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Eom 2021 (80 mg/kg).
| Study characteristics | ||
| Methods | See Eom 2021 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
RECOVERY.
| Study characteristics | ||
| Methods | Drug name: casirivimab/imdevimab Other drugs: lopinavir‐ritonavir, corticosteroid, hydroxychloroquine, azithromycin, convalescent plasma, tocilizumab, immunoglobulin, aspirin, colchicine, baricitinib, anakinra, dimethyl fumarate Trial design: randomised, multicentre, factorial design, open‐label platform trial Type of publication: preprint publication (English) NCT number: NCT04381936 (date of trial registration: 11 May 2020) Ongoing: recruiting, updated 5 May 2021 Number of participants:
Estimated enrolment: 40,000 participants Estimated completion date: December 2031 |
|
| Participants | Setting
Eligibility criteria
Participant characteristics
|
|
| Interventions | Intervention: casirivimab/imdevimab
Comparator
|
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional outcomes
|
|
| Notes | Funding: University of Oxford Recruitment status: recruiting Conflicts of interest:
|
|
Weinreich (phase 1/2, 2.4 g).
| Study characteristics | ||
| Methods | See Weinreich (phase 1/2). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Weinreich (phase 1/2, 8.0 g).
| Study characteristics | ||
| Methods | See Weinreich (phase 1/2). | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Weinreich (phase 1/2).
| Study characteristics | ||
| Methods | Drug name: casirivimab/imdevimab Trial design: randomised, double‐blind, placebo‐controlled, phase 1–3 clinical trial (continual enrolment); part 1‐2 reported here Type of publication: journal publication for part 1, preprint for part 2 (English) NCT number: NCT04425629 (date of trial registration: 11 June 2020)
Number of participants:
Estimated enrolment: 6420 participants Estimated completion date: November 2021 |
|
| Participants | Setting
Eligibility criteria Inclusion criteria
Exclusion criteria
Participant characteristics (FAS)
Disease severity:
Co‐morbidities:
|
|
| Interventions | Intervention
Comparator:
|
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
|
| Notes | Developer: Regeneron Pharmaceuticals Funding: Regeneron Pharmaceuticals Conflicts of interest:
|
|
Weinreich (phase 3).
| Study characteristics | ||
| Methods | Drug name:
Trial design: randomised, double‐blind, placebo‐controlled part 3 of a phase 1/2/3 study, initially 3 arms, reduced to two Type of publication: preprint (English) NCT number: NCT04425629 (date of trial registration: 11 June 2020)
Number of participants:
Estimated enrolment: 6420 participants Estimated completion date: 26 November 2021 |
|
| Participants | Setting
Inclusion criteria (Cohort 1)
For further details and exclusion criteria, see Weinreich (phase 1/2) Participant characteristics
|
|
| Interventions | Interventions
Comparator:
|
|
| Outcomes |
Safety outcomes
Additional study outcomes:
|
|
| Notes | Developer: Regeneron Pharmaceuticals Funding: Regeneron Pharmaceuticals Conflicts of interest:
|
|
aB: antibody; ACE: angiotensin‐converting enzyme;ADA: Adenosine deaminase; AE: adverse event; ARB: angiotensin‐ receptor blocker; AUC: area under the curve; BMI: body mass index; COPD: chronic obstructive pulmonary disease; ECMO: extracorporeal membrane oxygenation; EUA: emergency use authorization; ICU: intensive care unit; IV: intravenous; hIVIG: human intravenous immunoglobulin;IQR: interquartile range;ITTI: intention‐to‐treat in PCR‐positive participants; LAR: legally authorised representative; mAb: monoclonal antibody; NaCl: sodium chloride; NCI‐CTAE: Common Terminology Criteria for Adverse Events; NIAID: National Institute of Allergy and Infectious Diseases; NAT: nucleic acid test; NIAID: National Institute of Allergy and Infectious Diseases;NIH: National Institutes of Health; NIV: noninvasive ventilation; nmAb: neutralising monoclonal antibody; NSAID: nonsteroidal anti‐inflammatory drug; NYHA: New York Heart Association; PaO2/FiO2: ratio of arterial partial pressure of oxygen to fraction of inspired oxygen; PGIC: Patient Global Impression of Change of symptoms associated with COVID‐19; PGIS: Patient Global Impression of Severity of symptoms associated with COVID‐19; RT‐PCR: reverse transcription polymerase chain reaction; RT‐qPCR: quantitative reverse transcription polymerase chain reaction; SAE: serious adverse event; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; SpO2: oxygen saturation; TEAE: treatment‐emergent adverse event; WHO: World Health Organization; WHOQOL‐100: World Health Organization quality‐of‐life scale; WOCBP: women of childbearing potential
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTIV‐4 | Platform trial on other drug class: anti‐thrombotics |
| ACTT | Completed without adding relevant mAb |
| ACTT‐2 | Completed without adding relevant mAb |
| ACTT‐3 | Completed without adding relevant mAb |
| Alam 2021 | Ineligible study design: retrospective |
| AMMURAVID | Does not add treatment arms |
| ASCOT‐ADAPT | Does not add treatment arms |
| Bariola 2021 | Ineligible study design: retrospective |
| Beam 2021 | Ineligible study design: retrospective |
| BLAZE‐2 | SARS‐CoV‐2‐specific mAb examined for prophylaxis |
| ChiCTR2000030012 | Ineligible design: preclinical |
| Cohen 2021 | Ineligible study design: retrospective |
| COREG | Ineligible intervention: other intervention |
| COVERAGE | Ineligible intervention: other intervention; does not add treatment arms |
| COVER HCW | Platform trial, but prophylaxis |
| COVID_Aging | Does not add treatment arms |
| CROWN CORONA | Platform trial, but on other treatment class (vaccines) |
| C‐SMART | Ineligible intervention: other intervention; does not add treatment arms |
| Dale 2021 | Ineligible study design: retrospective |
| Dhand 2021a | Ineligible study design: unclear design, probably retrospective |
| Dhand 2021b | Ineligible study design: retrospective |
| Dong 2021 | Ineligible study design: unclear design, probably retrospective |
| EUCTR2020‐001243‐15‐BE | Completed without adding relevant mAb |
| EUDRACT2020‐002713‐17 | Ineligible intervention: mAb, not SARS‐CoV‐2‐specific |
| FORCE | Ineligible intervention: mAb, not SARS‐CoV‐2‐specific |
| Ganesh 2021 | Ineligible study design: retrospective |
| jRCT2031190264 | Does not add treatment arms |
| Karr 2021 | Ineligible study design: retrospective |
| Kutzler 2021 | Ineligible study design: retrospective |
| MAS‐COVID | Ineligible intervention: other intervention |
| NCT04275245 | Ineligible intervention: mAb, not SARS‐CoV‐2‐specific |
| NCT04341116 | Ineligible intervention: mAb, not SARS‐CoV‐2‐specific |
| NCT04354428 | Completed without adding relevant mAb |
| NCT04369469 | Ineligible intervention: mAb, not SARS‐CoV‐2‐specific |
| NCT04370262 | Ineligible intervention: other intervention |
| NCT04415073 | Ineligible intervention: mAb, not SARS‐CoV‐2‐specific |
| NCT04452318 | SARS‐CoV‐2‐specific mAb examined for prophylaxis |
| NCT04453384 | Ineligible intervention: polyclonal Ab |
| NCT04454398 | Study withdrawn according to trial registry |
| NCT04469179 | Ineligible intervention: polyclonal Ab |
| NCT04494724 | Ineligible intervention: mAb, not SARS‐CoV‐2 specific |
| NCT04494984 | Ineligible intervention: other intervention |
| NCT04497987 | SARS‐CoV‐2 specific mAb for prophylaxis |
| NCT04498273 | Platform trial, but for different drug class/prevention: thrombosis |
| NCT04514302 | Ab fragment |
| NCT04516564 | Ineligible intervention: mAb, not SARS‐CoV‐2‐specific, healthy participants |
| NCT04569786 | Ineligible intervention: other intervention |
| NCT04574869 | Ineligible intervention: other intervention |
| NCT04586153 | Ineligible intervention: mAb, not SARS‐CoV‐2‐specific |
| NCT04625725 | SARS‐CoV‐2‐specific mAb examined for prophylaxis |
| NCT04629703 | Does not add treatment arms |
| NCT04766671 | Registry entry no longer available, study probably withdrawn |
| NCT04859517 | SARS‐CoV‐2‐specific mAb examined for prophylaxis |
| NCT04894474 | SARS‐CoV‐2‐specific mAb examined for prophylaxis |
| PANAMO | Ineligible intervention: other intervention |
| PO‐COV‐III‐20 | Does not add treatment arms |
| PROFACT‐01 | Platform trial, but non‐presciption treatments |
| Rainwater‐Lovett 2021 | Ineligible study design: retrospective |
| RESP301‐002 | Does not add treatment arms |
| Shirk 2021 | Ineligible study design: retrospective |
| STORM CHASER | SARS‐CoV‐2‐specific mAb examined for prophylaxis |
| Track: ChiCTR2100042150 | Included for tracking but excluded for analysis: healthy participants |
| Track: jRCT2071200117 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04429529 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04441918 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04441931 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04479631 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04479644 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04483375 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04507256 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04519437 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04525079 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04532294 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04533048 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04537910 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04561076 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04567810 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04590430 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04592549 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04603651 | Included for tracking but excluded for analysis: non‐randomised study design |
| Track: NCT04617535 | Included for tracking but excluded for analysis: non‐randomised study design |
| Track: NCT04656691 | Included for tracking but excluded for analysis: non‐randomised study design; previously NCT04639479 |
| Track: NCT04691180 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04700163 | included for tracking but excluded for analysis: healthy participants |
| Track: NCT04701658 | Included for tracking but excluded for analysis: non‐randomised study design |
| Track: NCT04852978 | Track: mAbs in healthy volunteers |
| Track: NCT04896541 | Included for tracking but excluded for analysis: healthy participants |
| Track: NCT04932850 | Included for tracking but excluded for analysis: healthy participants |
| Webb 2021 | Ineligible study design: retrospective |
| Yang 2020 | Ineligible study design: preclinical |
Ab: antibody; mAb: monoclonal antibody; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2
Characteristics of studies awaiting classification [ordered by study ID]
ACCORD & ACCORD 2.
| Methods | Drug name: MEDI3506, bemcentinib, acalabrutinib, zilucoplan Trial design: controlled, randomised, open, parallel group, SOC control arm Identifiers: EUCTR 2020‐001736‐95 Target sample size: 1800 participants Planned completion date: 23 April 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: University Hospital Southampton NHS Foundation Trust, UK There are various sub‐protocols. |
ACOVACT.
| Methods | Drug name: clazakizumab, lopinavir/ritonavir, pentaglobin, remdesivir, rivaroxaban, chloroquine or hydroxychloroquine (stopped), RAS blockade, asunercept Trial design: randomised, parallel, active‐controlled, open‐label platform trial, 3 main study arms and 3 substudies (the main study arms are exclusive, while patients from the main study arms may participate in one or more substudies) NCT number: NCT04351724 Target sample size: 500 participants Planned completion date: March 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Medical University of Vienna |
ACTIV‐1 IM.
| Methods | Drug name: infliximab, abatacept, remdesivir, cenicriviroc Trial design: randomised, parallel assignment, masking: triple (participant, investigator, outcome assessor) NCT number: NCT04593940 Target sample size: 2160 participants Planned completion date: September 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: National Center for Advancing Translational Science (NCATS) and Biomedical Advanced Research and Development Authority |
ACTT‐4.
| Methods | Drug name: baricitinib, dexamethasone, remdesivir Trial design: multicenter, adaptive, randomised blinded controlled trial Identifiers: NCT04640168 Target sample size: 1500 participants Planned completion date: June 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: NIAID |
ANTICOV.
| Methods | Drug name: lopinavir plus ritonavir, hydroxychloroquine Trial design: randomised, adaptive platform trial, open‐label study Identifiers: PACTR202006537901307 Target sample size: 3000 participants Planned completion date: December 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: DNDi, Switzerland |
ARCO‐Home.
| Methods | Drug name: lopinavir, plaquenil, rezolsta, avigan Trial design: adaptive randomised trial Identifiers: EUCTR 2020‐001528‐32 Target sample size: 435 participants Planned completion date: 23 April 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Instituto Nazionale per le malattie infettive "Lazzaro Spallanzani" |
BEAT COVID‐19.
| Methods | Drug name: not further specified Trial design: Bayesian, adaptive, blinded, randomised platform trial Identifier: ACTRN12620000566932 Target sample size: 400 participants Planned completion date: 30 June 2023 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: NHMRC Clinical Trials Centre, University of Sydney |
BET‐A (ACTIV‐5).
| Methods | Drug name: remdesivir, risankizumab Trial design: multicentre, randomised, double‐blinded trial NCT number: NCT04583956 Target sample size: 200 participants Planned completion date: December 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: NIAID |
BET‐B (ACTIV‐5).
| Methods | Drug name: remdesivir plus lenzilumab Trial design: randomised, parallel assignment, double‐blind, multicentre platform trial NCT number: NCT04583969 Target sample size: 400 participants Planned completion date: 31 December 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention Remdesivir plus lenzilumab Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: NIAID |
CATALYST.
| Methods | Drug name: mylotarg, namilumab, remsima, infliximab Trial design: multicentre, randomised, placebo‐controlled clinical trial Identifier: ISRCTN40580903 Target sample size: not known Planned completion date: May 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: University of Birmingham |
CCAP.
| Methods | Drug name: convalescent anti‐SARS‐CoV‐2 plasma Trial design: multicentre, randomised, double‐blinded, placebo‐controlled trial NCT number: NCT04345289 Target sample size: 1100 participants Planned completion date: 15 June 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Thomas Benfield, Hvidovre University Hospital |
COLHEART‐19.
| Methods | Drug name: colchicine Trial design: randomised, open‐label, controlled trial NCT number: NCT04355143 Target sample size: 150 participants Planned completion date: September 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: University of California, Los Angeles |
COPPS.
| Methods | Drug name: acebilustat, camostat Trial design: pragmatic, multi‐arm, adaptive, phase 2, blinded, randomised, placebo‐controlled platform trial Identifiers: NCT04662086 Target sample size: 240 participants Planned completion date: March 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Stanford University |
CORIMUNO.
| Methods | Drug name: tocilizumab, and many more Trial design: randomised, controlled open‐label trials; subprotocols are registered with separate NCT numbers NCT number: NCT04324047 NCT04331808 ‐ tocilizumab (active, not recruiting) NCT04346797 ‐ eculizumab (recruiting) NCT04324073 ‐ sarilumab (active, not recruiting) NCT04341584 ‐ anakinra (completed) NCT04343144 ‐ nivolumab (not yet recruiting) NCT04345991 ‐ plasma (recruiting) NCT04344782 ‐ bevacizumab (not yet recruiting) NCT04344756 ‐ tinzaparin or unfractionated heparin (not yet recruiting) Target sample size: 1000 participants Planned completion date: 31 December 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes: not planned |
| Notes | Funding: Assistance Publique ‐ Hôpitaux de Paris |
COVID MED.
| Methods | Drug name: losartan Trial design: randomised, double‐blind, placebo‐controlled trial Identifier: NCT04328012 Target sample size: 100 participants Planned completion date: 1 August 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Bassett Healthcare |
DEFINE.
| Methods | Drug name: nafamostat mesilate, TD139 Trial design: randomised, single‐blinded clinical trial NCT number: NCT04473053 Target sample size: 60 participants Planned completion date: 3 December 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: University of Edinburgh |
EU SolidAct.
| Methods | Drug name: multiple drugs including baricitinib Trial design: randomized, multifactorial, adaptive platform trial NCT number: NCT04891133 Target sample size: 1900 participants Planned completion date: December 2025 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Sponsors: Oslo University Hospital; Institut National de la Santé Et de la Recherche Médicale, France; Epidemiological and Clinical Research Information Network |
I‐SPY.
| Methods | Drug name: remdesivir, cenicriviroc, icatibant, razuprotafib, apremilast, pulmozyme, IC14, celecoxib Famotidine, narsoplimab, aviptadil acetate, cyclosporine Trial design: multicentre, randomised, open‐label trial NCT number: NCT04488081 Target sample size: 1500 participants Planned completion date: November 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: QuantumLeap Healthcare Collaborative |
NCT04359095.
| Methods | Drug name: emtricitabine/tenofovir, colchicine pill, rosuvastatin Trial design: randomised, parallel‐assignment, open‐label trial Identifier: NCT04359095 Target sample size: 1200 participants Planned completion date: 31 May 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Universidad Nacional de Colombia |
NCT04590586.
| Methods | Drug name: apremilast, lanadelumab, zilucoplan Trial design: phase 3, randomised, quadruple blind platform trial Identifiers: NCT04590586 Target sample size: 1400 participants Planned completion date: August 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Amgen |
O’Brien 2021.
| Methods | Drug name: casirivimab/imdevimab Trial design: randomised, double‐blind, placebo‐controlled, two‐part phase 3 trial for prevention of SARS‐CoV‐2 infection among uninfected household contacts; here reported: PCR‐positive cases (Part B) Type of publication: preprint (English) Identifiers: NCT04452318 Number of participants:
|
| Participants | Setting
Eligibility criteria
Patient characteristics:
|
| Interventions | Intervention
|
| Outcomes | Outcomes Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: jointly managed by Regeneron, the Covid‐19 Prevention Network (CoVPN), and the National Institute of Allergy and Infectious Diseases (NIAID) Conflicts of interest: The study protocol was not published with the preprint publication. |
PaTS‐COVID.
| Methods | Drug name: ivermectin, aspirin Trial design: single‐blind, randomised, clinical trial Identifiers: NCT04703608 Target sample size: 1200 participants Planned completion date: July 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
Cohort 2:
|
| Notes | Funding: London School of Hygiene and Tropical Medicine |
PRINCIPLE.
| Methods | Drug name: hydroxychloroquine Trial design: parallel, cross‐over, randomised trial (the trial will initially be two‐arm, comparing usual care to usual care with hydroxychloroquine treatment) Identifiers: ISRCTN86534580 Target sample size: 3000 participants Planned completion date: April 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: University of Oxford |
PROTECT‐Surg.
| Methods | Drug name: lopinavir‐ritonavir, hydroxychloroquine Trial design: randomised, parallel, open‐label, multicentre trial Identifiers: NCT04386070 Target sample size: 6400 participants Planned completion date: May 2026 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: University of Birmingham |
REMAP‐CAP.
| Methods | Drug name: hydrocortisone, ceftriaxone, moxifloxacin or levofloxacin, piperacillin‐tazobactam, ceftaroline, amoxicillin‐clavulanate, macrolide, lopinavir/ritonavir, hydroxychloroquine, interferon‐β1a, anakinra, tocilizumab, sarilumab, vitamin C, therapeutic anticoagulation, simvastatin, convalescent plasma, eritoran, apremilast, aspirin, clopidogrel, prasugrel, ticagrelor Trial design: randomised, embedded, open‐label, multifactorial adaptive platform trial Identifiers: NCT02735707 Target sample size: 7100 participants Planned completion date: December 2023 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Sponsor: MJM Bonten |
SOLIDARITY.
| Methods | Drug name: interferon beta‐1a, remdesivir Trial design: multicentre, adaptive, randomised, open‐label, controlled trial Identifiers: NCT04330690; NCT04575064; ISRCTN83971151; and more, one registration per country Target sample size: 2900 participants Planned completion date: 18 May 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Sunnybrook Health Sciences Centre |
SWISSPED‐RECOVERY.
| Methods | Drug name: methylprednisolone sodium succinate, Human normal immunoglobulin (IVIg) Trial design: randomised, open‐label, controlled trial Identifiers: NCT04826588 Target sample size: 75 participants Planned completion date: April 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: University Children's Hospital Basel |
TACTIC‐E.
| Methods | Drug name: EDP1815, dapagliflozin, ambrisentan Trial design: randomised, parallel‐arm, open‐label platform trial Identifiers: NCT04393246 Estimated enrolment: 1407 participants Planned completion date: 15 June 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Cambridge University Hospitals NHS Foundation Trust |
TACTIC‐R.
| Methods | Drug name: ravulizumab, baricitinib Trial design: randomised, parallel arm, open‐label platform trial Identifiers: NCT04390464 Target sample size: 1167 participants Planned completion date: May 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Cambridge University Hospitals NHS Foundation Trust |
TOGETHER‐3.
| Methods | Drug name: lopinavir ritonavir, ascorbic acid Trial design: parallel, randomised, blinded trial Identifier: PACTR202007700757139 Target sample size: 420 participants Planned completion date: July 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes:
Safety outcomes
Additional study outcomes
|
| Notes | Funding: University of Washington |
VIRCO.
| Methods | Drug name: favipiravir Trial design: adaptive, randomised, placebo‐controlled, phase 2 trial Identifiers: NCT04445467 Target sample size: 190 participants Planned completion date: November 2020 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Notes | Funding: Bayside Health |
Ab: antibody; ACE: angiotensis‐converting enzyme; AE: adverse event; ALT: alanine aminotransferase;ARB: angiotensin receptor blocker; ARDS: acute respiratory distress syndrome; AST: aspartate aminotransferase; AUC: area under the curve; BMI: body mass index;BP: blood pressure; bpm: beats per minute: CAP: community‐acquired pneumonia; Child Pugh score: clinical measures of liver disease; Cmax: maximum observed serum concentration; CNS: central nervous system; COPD: chronic obstructive pulmonary disease; CT: computed tomography; DSMB: Data Safety Monitoring Board;ECG: echocardiogram; ECMO: extracorporeal membrane oxygenation; ED: emergency department;EQ‐5D: EuroQol 5 Dimensions; FDA: US Food and Drug Administration; GFR: glomrilar filtration rate; IV: intravenous; Hb: haemoglobin; hIVIG: human intravenous immunoglobulin;HR: heart rate; ICU: intensive care unit;IV: intravenous; IVIg: intravenous immunoglobulin LAR: legally authorised representative;LFTs: liver function tests; mAb: monoclonal antibody; NAAT: nucleic acid amplification test; NEWS: National Early Warning Score; NIAID: National Institute of Allergy and Infectious Diseases; NIV: noninvasive ventilation; NP: nasopharyngeal; NSAID: nonsteroidal anti‐inflammatory drug; NYHA: New York Heart Association; PaO2/FiO2: ratio of arterial partial pressure of oxygen to fraction of inspired oxygen; PCR: polymerase chain reaction;RAS: renin‐angiotensin system; RT‐PCR: reverse transcription‐polymerase chain reaction; SAE: serious adverse event; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; SC: subcutaneous; SpO2: oxygen saturation;TNF: tumor necrosis factor;ULN: upper limit of normal; WBC: white blood cell; WHOQOL: World Health Organization quality of life scale; SD: standard deviation; WOCBP: women of childbearing potential
Characteristics of ongoing studies [ordered by study ID]
ACTIV‐2.
| Study name | ACTIV‐2: a study for outpatients with COVID‐19 |
| Methods | Drug name
Other tested drugs: SNG001, camostat Trial design: multicentre, randomised, unblinded, adaptive platform trial Identifiers: NCT04518410 Target sample size: 2000 participants Planned completion date: May 2023 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | August 2020 |
| Contact information | Study Chair: David Smith, MAS University of California, San Diego |
| Notes | Funding: NIAID Recruitment status: recruiting Other: platform trial, may add treatment arms |
AGILE.
| Study name | AGILE |
| Methods | Drug name: VIR‐7831 and VIR‐7832 Other drugs: nomacopan, MK4482 Trial design: randomised, controlled, double‐blind platform trial (open‐label in phase 1) Identifiers: NCT04746183, EUDRACT 2020‐001860‐27 Target sample size: 200 participants Planned completion date: December 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention: VIR‐7831 and VIR‐7832 Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | July 2020 |
| Contact information | Helen E Reynolds, +44 (0)1517945553, AGILE%20(Early%20Phase%20Platform%20Trial%20for%20COVID‐19)" type="EXTERNAL">livagile@liv.ac.uk |
| Notes | Funding: University of Liverpool, UK Recruitment status: recruiting |
DISCOVERY.
| Study name | DISCOVERY |
| Methods | Drug name: AZD7442 Trial design: multicenter, adaptive, randomised trial NCT number: NCT04315948 Target sample size: 3100 participants Planned completion date: March 2023 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | March 2020 |
| Contact information | Florence Ader, Hospices Civils de Lyon |
| Notes | Funding: Institut National de la Santé Et de la Recherche Médicale, France |
EUDRACT2020‐003401‐60.
| Study name | EUDRACT2020‐003401‐60 |
| Methods | Drug name: CT‐P59 Trial design: randomised, controlled, parallel‐group, double‐blind study Identifiers: EUDRACT2020‐003401‐60 Target sample size: not reported Planned completion date: not reported |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention: CT‐P59
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 23 November 2020 |
| Contact information | Sung Hyun Kim; +82328505000; SungHyun.Kim@celltrion.com |
| Notes | Developer: Celltrion Inc. Funding: Celltrion Inc. Recruitment status: not reported |
NCT04411628.
| Study name | NCT04411628 |
| Methods | Drug name: bamlanivimab (LY3819253, LY‐CoV555) Trial design: randomised, placebo‐controlled, double‐blind, sponsor‐unblinded, single‐ascending dose, phase 1 study NCT number: NCT04411628 (date of trial registration: 2 June 2020) Target sample size: 24 participants (actual enrolment) Completion date: 26 August 2020 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention Bamlavinimab (LY3819253, LY‐CoV555)
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 28 May 2020 |
| Contact information | Eli Lilly and Company, 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 |
| Notes | Developer: AbCellera, NIAID Vaccine Research Center, Eli Lilly and Company Funding: Eli Lilly and Company Recruitment status: completed |
NCT04426695.
| Study name | NCT04426695 |
| Methods | Drug name: casirivimab/imdevimab Trial design: randomised, parallel assignment, phase 1/phase 2/phase 3, quadruple masking NCT number: NCT04426695 (date of trial registration: 11 June 2020) Target sample size: 6900 participants Planned completion date: April 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention REGN‐CoV‐2 (REGN10933 (casirivimab) + REGN10987 (imdevimab))
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 11 June 2020 |
| Contact information | Clinical Trials Administrator, 844‐734‐6643, NCT04426695,%20R10933‐10987‐COV‐2066,%20Safety,%20Tolerability,%20and%20Efficacy%20of%20Anti‐Spike%20(S)%20SARS‐CoV‐2%20Monoclonal%20Antibodies%20for%20Hospitalized%20Adult%20Patients%20With%20COVID‐19" type="EXTERNAL">clinicaltrials@regeneron.com |
| Notes | Developer: Regeneron Pharmaceuticals Funding: Regeneron Pharmaceuticals Recruitment status: recruiting |
NCT04551898.
| Study name | NCT04551898 |
| Methods | Drug name: BGB‐DXP593 Trial design: randomised, double‐blind, placebo‐controlled, phase 2 study NCT number: NCT04551898 (date of trial registration: 16 September 2020) Actual enrolment: 180 participants Actual completion date: January 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention BGB‐DXP593
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 30 October 2020 |
| Contact information | BeiGene +1‐877‐828‐5568, clinicaltrials@beigene.com |
| Notes | Developer: BeiGene Funding: BeiGene Recruitment status: completed Other: 4 arms, follow‐up 85 days |
NCT04584697.
| Study name | NCT04584697 |
| Methods | Drug name: STI‐2020 (COVI‐AMG) Trial design: randomised, double‐blind, placebo‐controlled, phase 1/2 study NCT number: NCT04584697 (date of trial registration: 14 October 2020) Target sample size: 50 participants, actual enrolment: 0 participants Planned completion date: withdrawn, "Different study will be conducted" |
| Participants | Setting:
Eligibility criteria
|
| Interventions | Intervention STI‐2020 (COVI‐AMG) plus standard of care
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | December 2020 |
| Contact information | Mike Royal, MD, (858) 203‐4100 ext 4146, mroyal@sorrentotherapeutics.com |
| Notes | Developer: Sorrento Therapeutics, Inc. Funding: Sorrento Therapeutics, Inc. Recruitment status: not yet recruiting Other: withdrawn: "Different study will be conducted" |
NCT04593641.
| Study name | NCT04593641 |
| Methods | Drug name: regdanvimab (CT‐P59) Trial design: randomised, double‐blind, placebo‐controlled, parallel group, single ascending dose, phase 1 study NCT number: NCT4593641 (date of trial registration: 20 October 2020) Actual enrolment: 18 participants Planned completion date: December 2020 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention Regdanvimab (CT‐P59)
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 4 September 2020 |
| Contact information | JooHee Lee, +82‐32‐850‐5707, Celltrion |
| Notes | Developer: Celltrion Funding: Celltrion Recruitment status: active, not recruiting Other: 3 arms, alternative trial number: KCT0005896, EUDRACT‐2020‐003165‐19 |
NCT04627584.
| Study name | NCT04627584 |
| Methods | Drug name: MW33 Trial design: randomised, double‐blind, placebo‐controlled phase 2 study NCT number: NCT04627584 (date of trial registration: 13 November 2020) Target sample size: 150 participants Planned completion date: September 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention MW33
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | December 2020 |
| Contact information | Bei Zhao, +86‐18817773189, beizhao@mabwell.com Song Lu, 15216868129, song.lu@mabwell.com |
| Notes | Developer: Mabwell (Shanghai) Bioscience Co., Ltd. Funding: Mabwell (Shanghai) Bioscience Co., Ltd. Recruitment status: recruiting Other: 3 arms |
NCT04631666.
| Study name | NCT04631666 |
| Methods | Drug name: DZIF‐10c (BI 767551) Trial design: randomised, sequential assignment, phase 1/2a study NCT number: NCT04631666 (date of trial registration: 17 November 2020) Estimated enrolment: 57 participants Planned completion date: July 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention DZIF‐10c (BI 767551)
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | December 2020 |
| Contact information | Florian Klein, 0221 478 85801 ext 0049, florian.klein@uk‐koeln.de Henning Gruell, 0221 47896973 ext 0049, henning.gruell@uk‐koeln.de |
| Notes | Developer: University of Cologne, University Marburg and Boehringer Ingelheim Funding: University of Cologne, The Clinical Trials Centre Cologne, Boehringer Ingelheim Recruitment status: recruiting |
NCT04631705.
| Study name | NCT04631705 |
| Methods | Drug name: DZIF‐10c (BI 767551) Trial design: randomised, sequential assignment, phase 1/2a study NCT number: NCT04631705 (date of trial registration: 17 November 2020) Target sample size: 78 participants Planned completion date: July 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention:
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | December 2020 |
| Contact information | Florian Klein, +49‐221‐478‐96973, florian.klein@uk‐koeln.de Henning Gruell, +49‐221‐478‐85801, henning.gruell@uk‐koeln.de |
| Notes | Developer: University of Cologne, University Marburg and Boehringer Ingelheim Funding: University of Cologne, The Clinical Trials Centre Cologne, Boehringer Ingelheim Recruitment status: not yet recruiting Other: registry entry outcomes changed between November 2020 and March 2021 |
NCT04634409.
| Study name | BLAZE‐4 |
| Methods | Drug name:
Trial design: randomised, double‐blind, placebo‐controlled, phase 2 study NCT number: NCT04634409 (date of trial registration: 18 November 2020) Target sample size: 700 participants Planned completion date: June 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 29 October 2020 |
| Contact information | Eli Lilly and Company, 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559, ClinicalTrials.gov@lilly.com |
| Notes | Developer
Funding: Eli Lilly and Company Recruitment status: recruiting Other: 4 arms |
NCT04644120.
| Study name | NCT04644120 |
| Methods | Drug name: ABBV‐47D11 and ABBV‐2B04 Trial design: randomised, sequential assignment, double‐blind, placebo‐controlled, phase 1 study NCT number: NCT04644120 (date of trial registration: 25 November 2020) Estimated enrolment: 54 participants Planned completion date: 5 September 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 10 December 2020 |
| Contact information | ABBVIE Call Center, 844‐663‐3742, abbvieclinicaltrials@abbvie.com |
| Notes | Developer: AbbVie Funding: AbbVie Recruitment status: recruiting |
NCT04644185.
| Study name | NCT04644185 |
| Methods | Drug name: SCTA01 Trial design: randomised, adaptive, double‐blinded, placebo‐controlled phase 2/3 trial NCT number: NCT04644185 (date of trial registration: 25 November 2020) Target sample size: 795 participants Planned completion date: October 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention SCTA01 plus best supportive care
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | Estimated start date: 10 February 2021 |
| Contact information | Ji Qi, +86‐10‐5862 8288 ext 9360, ji_qi@sinocelltech.com |
| Notes | Developer: Sinocelltech Ltd. Funding: Sinocelltech Ltd. Recruitment status: not yet recruiting |
NCT04649515.
| Study name | NCT04649515 |
| Methods | Drug name: TY027 Trial design: randomised, placebo‐controlled, double‐blind, single‐dose, multi‐site, phase 3 study NCT number: NCT04649515 (date of trial registration: 2 December 2020) Target sample size: 1305 participants Planned completion date: December 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention TY027
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | December 2020 |
| Contact information | Justin Ng, +65 69046055, justin@tychanltd.com Anjali Baglody, +65 69046055, anjalib@tychanltd.com |
| Notes | Developer: Tychan Pte Ltd. Funding: Tychan Pte Ltd. Recruitment status: recruiting |
NCT04666441.
| Study name | NCT04666441 |
| Methods | Drug name: casirivimab/imdevimab Trial design: randomised, parallel assignment, quadruple‐blinded, phase 2 study NCT number: NCT04666441 (date of trial registration: 14 December 2020) Target sample size: 1,400 participants; actual enrolment: 1164 Planned completion date: August 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention REGN‐CoV‐2 (REGN10933 (casirivimab) + REGN10987 (imdevimab))
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 15 December 2020 |
| Contact information | Clinical Trials Administrator, 844‐734‐6643, clinicaltrials@regeneron.com |
| Notes | Developer: Regeneron Pharmaceuticals Funding: Regeneron Pharmaceuticals Recruitment status: active, not recruiting Other:
|
NCT04674566.
| Study name | NCT04674566 |
| Methods | Drug name: COR‐101 Trial design: randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐centre, first‐in‐human, phase Ib/II study NCT number: NCT04674566 (date of trial registration: 19 December 2020) Target sample size: 45 participants Planned completion date: June 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention COR‐101
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | April 2021 |
| Contact information | Corat Therapeutics Gmbh |
| Notes | Developer: Corat Therapeutics Gmbh Funding: Corat Therapeutics Gmbh Recruitment status: not yet recruiting |
NCT04683328.
| Study name | MASP3 |
| Methods | Drug name: SCTA01 Trial design: randomised, adaptive, double‐blinded, placebo‐controlled, multicentre phase 2/3 study NCT number: NCT04683328 (date of trial registration: 24 December 2020) Target sample size: 560 participants Planned completion date: 25 November 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention SCTA01 plus best supportive care
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 25 February 2021 |
| Contact information | Ji Qi, +86‐10‐5862 8288 ext. 9360, ji_qi@sinocelltech.com |
| Notes | Developer: Sinocelltech Ltd. Funding: Sinocelltech Ltd. Recruitment status: not yet recruiting |
NCT04709328.
| Study name | MAOP3 |
| Methods | Drug name: SCTA01 Trial design: randomised, adaptive, double‐blinded, placebo‐controlled, multicentre, phase 2/3 study NCT number: NCT04709328 Target sample size: 690 participants Planned completion date: March 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention SCTA01 plus standard of care
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | March 2021 |
| Contact information | Qiang Guo, 86‐10‐5862 8288, NCT04709328,%20SCTA01‐A301,%20To%20Evaluate%20SCTA01%20Treatment%20of%20High‐risk%20Outpatients%20With%20COVID‐19" type="EXTERNAL">qiang_guo@sinocelltech.com |
| Notes | Developer: Sinocelltech Ltd. Funding: Sinocelltech Ltd. Recruitment status: Not yet recruiting |
NCT04723394.
| Study name | NCT04723394 |
| Methods | Drug name: AZD7442 (AZD8895/tixagevimab + AZD1061/cilgavimab) Trial design: randomised, double‐blind, placebo‐controlled, multicenter, phase 3 study NCT number: NCT04723394 (date of trial registration: 5 January 2021) Target sample size: 1700 participants Planned completion date: May 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention AZD7442 (AZD8895/tixagevimab + AZD1061/cilgavimab)
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 22 January 2021 |
| Contact information | AstraZeneca Clinical Study Information Center, 1‐877‐240‐9479, NCT04723394, information.center@astrazeneca.com |
| Notes | Developer: AstraZeneca Funding: AstraZeneca Recruitment status: not yet recruiting |
NCT04734860.
| Study name | NCT04734860 |
| Methods | Drug name: STI‐2020 (COVI‐AMG) Trial design: randomised, double‐blind, placebo‐controlled, multicentre, phase 2 study NCT number: NCT04734860 (date of trial registration: 2 February 2021) Target sample size: 500 participants Planned completion date: November 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention STI‐2020 (COVI‐AMG)
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes:
Additional study outcomes:
|
| Starting date | March 2021 |
| Contact information | Mike Royal, (858) 203‐4100 ext 4146; NCT04734860,%20AMG‐COV‐201,%20Study%20to%20Evaluate%20a%20Single%20Dose%20of%20STI‐2020%20(COVI‐AMG%E2%84%A2)%20in%20Adults%20With%20Mild%20COVID‐19%20Symptoms" type="EXTERNAL">mroyal@sorrentotherapeutics.com |
| Notes | Developer: Sorrento Therapeutics, Inc. Funding: Sorrento Therapeutics, Inc. Recruitment status: Not yet recruiting |
NCT04748588.
| Study name | NCT04748588 |
| Methods | Drug name: bamlanivimab (LY3819253, LY‐CoV555) Trial design: randomised, open‐label, controlled, phase 4 study NCT number: NCT04748588 (date of trial registration: 10 February 2021) Target sample size: 648 participants Planned completion date: March 2023 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention Bamlanivimab (LY3819253, LY‐CoV555)
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 1 February 2021 |
| Contact information | Alain Tremblay, 4032103866, atrembla@ucalgary.ca Wendy Sligl, 780 492 8311, wsligl@ualberta.ca |
| Notes | Developer: Eli Lilly and Company Funding: University of Calgary; Sunnybrook Research Institute Recruitment status: not yet recruiting |
NCT04770467.
| Study name | NCT04770467 |
| Methods | Drug name: BRII‐196 and BRII‐198 Trial design: randomised, single‐blinded, placebo‐controlled, phase 2 study NCT number: NCT04770467 (date of trial registration: 25 February 2021) Target sample size: 56 participants Planned completion date: October 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention BRII‐196 and BRII‐198
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | March 2021 |
| Contact information | Lili Chen, +86 10 6299 8808, lili.chen@briibio.com |
| Notes | Developer: Brii Biosciences, Inc. Funding: Brii Biosciences, Inc. Recruitment status: not yet recruiting Other: 2 cohorts (mild‐to‐moderate and severe) |
NCT04771351.
| Study name | NCT04771351 |
| Methods | Drug name: STI‐2020 (COVI‐AMG) Trial design: randomised, double‐blinded, placebo‐controlled, phase 2 study NCT number: NCT04771351 (date of trial registration: 25 February 2021) Target sample size: 280 participants Planned completion date: September 2021 |
| Participants | Setting
Eligibility criteria:
|
| Interventions | Intervention STI‐2020 (COVI‐AMG)
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | March 2021 |
| Contact information | Mike Royal, (858) 203‐4100 ext 4146, mroyal@sorrentotherapeutics.com |
| Notes | Developer: Sorrento Therapeutics, Inc. Funding: Sorrento Therapeutics, Inc. Recruitment status: not yet recruiting |
NCT04779879.
| Study name | COMET‐PEAK |
| Methods | Drug name: Sotrovimab (VIR‐7831; Generation 1 and 2) Trial design: randomised, double‐blinded, parallel group, multicentre, phase 2 study (part A double‐blind, part B and C open‐label) NCT number: NCT04779879 (date of trial registration: 3 March 2021) Estimated enrollment: 340 participants Planned completion date: April 2023 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention VIR‐7831 (Generation 2)
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 18 February 2021 |
| Contact information | Study Inquiry, 415‐654‐5281, clinicaltrials@vir.bio |
| Notes | Developer: Vir Biotechnology, Inc. Funding: Vir Biotechnology, Inc.; GlaxoSmithKline Recruitment status: recruiting |
NCT04780321.
| Study name | NCT04780321 |
| Methods | Drug name: Etesevimab (JS016) Trial design: randomised, double‐blinded, placebo‐controlled, phase 1b/2 study NCT number: NCT04780321 (date of trial registration: 3 March 2021) Target sample size: 90 participants Planned completion date: 30 June 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention Etesevimab
Comparator
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 30 October 2020 |
| Contact information | Jingjing Sheng, 17317890616, NCT04780321,%20JS016‐002‐Ib/II,%20JS016%20(Anti‐SARS‐CoV‐2%20Monoclonal%20Antibody)With%20Mild%20and%20Moderate%20COVID‐19%20or%20SARS‐CoV‐2%20Asymptomatic%20Infection%20Subects" type="EXTERNAL">jingjing_sheng@junshipharma.com |
| Notes | Developer: Shanghai Junshi Bioscience Co., Ltd. Funding: Shanghai Junshi Bioscience Co., Ltd. Recruitment status: recruiting |
NCT04787211.
| Study name | NCT04787211 |
| Methods | Drug name: BRII‐196 and BRII‐198 Trial design: randomised, placebo‐controlled, single‐blinded, single‐dose, phase 2 study NCT number: NCT04787211 (date of trial registration: 8 March 2021) Target sample size: 24 participants (estimated enrollment) Estimated completion date: December 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention BRII‐196 and BRII‐198
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | March 2021 |
| Contact information | Lili Chen +86 10 6299 8808 lili.chen@briibio.com |
| Notes | Developer: Brii Biosciences Limited Funding: Brii Biosciences Limited Recruitment status: not yet recruiting Other: 3 arms |
NCT04796402.
| Study name | NCT04796402 |
| Methods | Drug name: Bamlanivimab (LY‐CoV555) Trial design: randomised, open‐label, phase 4 study NCT number: NCT04796402 (date of trial registration: 12 March 2021) Target sample size: 576 participants (estimated enrollment) Estimated completion date: 31 December 2021 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention Bamlanivimab
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 17 March 2021 |
| Contact information | Fraser Health Authority Fraser Health Region, British Columbia, Canada B‐Epic Study +1 236 332 9517 b‐epicstudy@fraserhealth.ca |
| Notes | Developer: Fraser Health Funding: Fraser Health Recruitment status: recruiting |
NCT04805671.
| Study name | NCT04805671 |
| Methods | Drug name: ADG20 Trial design: randomised, double‐blind, placebo‐controlled, single‐dose, phase 2/3 study NCT number: NCT04805671 (date of trial registration: 18 March 2021) Target sample size: 1084 participants (estimated enrollment) Estimated completion date: February 2023 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention ADG20
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | Estimated study start date: July 2021 |
| Contact information | +1 781‐819‐0080 ClinicalTrials@adagiotx.com |
| Notes | Developer: Adagio Therapeutics, Inc. Funding: Adagio Therapeutics, Inc. Recruitment status: not yet recruiting |
NCT04822701.
| Study name | NCT04822701 |
| Methods | Drug name: BI 767551 IV, BI 767551 inhalation Trial design: randomised, double‐blind, placebo‐controlled, phase 2/3 study (two phases)
NCT number: NCT04822701 (date of trial registration: 30 March 2021) Target sample size: 1500 participants (estimated enrollment) Estimated completion date: 30 June 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention BI 767551
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 21 April 2021 |
| Contact information | Boehringer Ingelheim 1‐800‐243‐0127 clintriage.rdg@boehringer‐ingelheim.com |
| Notes | Developer: Boehringer Ingelheim Funding: Boehringer Ingelheim Recruitment status: recruiting |
NCT04840459.
| Study name | NCT04840459 |
| Methods | Drug name: Bamlanivimab and Casirivimab + Imdevimab Trial design: non‐randomised, open‐label, phase 2 study NCT number: NCT04840459 (date of trial registration: 12 April 2021) Target sample size: 1000 participants (estimated enrolment) Estimated completion date: 31 January 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention Bamlanivimab
Casirivimab + Imdevimab
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 20 November 2020 |
| Contact information | Sohail Rao, MD +1 9563622387 s.rao@dhr-rgv.com Monica Betancourt‐Garcia, MD +1 956‐3623223. m.betancourt@dhr‐rgv.com |
| Notes | Developer: Sohail Rao, DHR Health Institute for Research and Development Funding: Sohail Rao Recruitment status: recruiting |
NCT04900428.
| Study name | NCT04900428 |
| Methods | Drug name: STI‐2099 (COVI‐DROPS) Trial design: randomised, double‐blind, placebo‐controlled, phase 2 study NCT number: NCT04900428 (date of trial registration: 25 May 2021) Target sample size: 350 participants (estimated enrolment) Estimated completion date: March 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention STI‐2099 (COVI‐DROPS)
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | July 2021 |
| Contact information | Mike Royal, MD (858) 203‐4100 ext 4146 mroyal@sorrentotherapeutics.com |
| Notes | Developer: Sorrento Therapeutics, Inc. Funding: Sorrento Therapeutics, Inc. Recruitment status: not yet recruiting |
NCT04913675.
| Study name | NCT04913675 |
| Methods | Drug name: Sotrovimab (VIR‐7831) Trial design: randomised, open‐label, phase 3 study NCT number: NCT04913675 (date of trial registration: 4 June 2021) Target sample size: 1020 participants (estimated enrollment) Estimated completion date: 29 November 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention: Sotrovimab (VIR‐7831)
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 9 June 2021 |
| Contact information | not provided |
| Notes | Developer: Vir Biotechnology, Inc., GlaxoSmithKline Funding: Vir Biotechnology, Inc. Recruitment status: not yet recruiting |
NCT04952805.
| Study name | NCT04952805 |
| Methods | Drug name: MAD0004J08 Trial design: randomised, placebo‐controlled, doubleb‐lind, multicentre, seamless adaptive, phase 2‐3 study NCT number: NCT04952805 (date of trial registration: 7 July 2021) Target sample size: 800 participants (estimated enrollment) Estimated completion date: 31 August 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention
MAD0004J08
Comparator:
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | 6 June 2021 |
| Contact information | Pierpaola Borgonovo 003903626331 info.studiclinici@opis.it |
| Notes | Developer: AchilleS Vaccines Srl Siena, Toscana Life Sciences Sviluppo s.r.l. Funding: Toscana Life Sciences Sviluppo s.r.l. Recruitment status: recruiting |
OPTIMISE‐C19.
| Study name | OPTIMISE‐C19 |
| Methods | Drug name: Bamlanivimab, Regeneron Casirivimab + Imdevimab, Bamlanivimab + Etesevimab Trial design: randomised, open‐label, adaptive platform trial NCT number: NCT04790786 (date of trial registration: March 2021) Target sample size: 5000 participants (estimated enrollment) Estimated completion date: December 2022 |
| Participants | Setting
Eligibility criteria
|
| Interventions | Intervention Bamlanivimab
Casirivimab + Imdevimab
Bamlanivimab + Etesevimab
|
| Outcomes | Efficacy outcomes
Safety outcomes
Additional study outcomes
|
| Starting date | March 10, 2021 |
| Contact information | Contact: David T Huang, MD, MPH(412) 647‐6818huangdt@upmc.eduContact: Kelsey Linstrum, MS203‐947‐6013linstrumk@upmc.edu |
| Notes | Funding: David T. Huang, MD, MPH Collaborators: University of Pittsburgh Medical CenterBerry Consultants Recruitment status: recruiting |
Ab: antibody; ACE: angiotensis‐converting enzyme; AE: adverse event; ALT: alanine aminotransferase;ARB: angiotensin receptor blocker; ARDS: acute respiratory distress syndrome; AST: aspartate aminotransferase; AUC: area under the curve; BMI: body mass index;BP: blood pressure; CAP: community‐acquired pneumonia; Cmax: maximum observed serum concentration; CNS: central nervous system; COVID‐19: coronavirus disease 2019; CT: computed tomography; ECG: electrocardiogram; ECMO: extracorporeal membrane oxygenation; ED: emergency department;eGFR: estimated glomerular filtration rate; FDA: US Food and Drug Administration; hIVIG: human intravenous immunoglobulin;HR: heart rate; ICU: intensive care unit;IM: intramuscular(ly); IV: intravenous; IVIg: intravenous immunoglobulin; kg: kilogram; LAR: legally authorised representative; mAb: monoclonal antibody; mg: milligram; mL: millilitre; NEWS: National Early Warning Score; NIAID: National Institute of Allergy and Infectious Diseases; NIV: noninvasive ventilation; NP: nasopharyngeal; NSAID: nonsteroidal anti‐inflammatory drug; NYHA: New York Heart Association; OP: oropharyngeal; PaO2/FiO2: ratio of arterial partial pressure of oxygen to fraction of inspired oxygen; PCR: polymerase chain reaction; PRO: patient‐reported outcomes; RBC: red blood cell; RNA: ribonucleic acid; RT‐PCR: reverse transcription‐polymerase chain reaction; RT‐qPCR: quantitative reverse transcription‐polymerase chain reaction; SAE: serious adverse event; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; SC: subcutaneous; SpO2: oxygen saturation;ULN: upper limit of normal; WBC: white blood cell; WHOQOL: World Health Organization quality of life scale; WOCBP: women of childbearing potential
Differences between protocol and review
Assessment of reporting bias
As we did not pool the data in meta‐analysis and included fewer than 10 trials, we did not generate a funnel plot to assess reporting bias.
Electronic searches
We stopped using the ‘Similar articles’ feature on PubMed, because we did not find new relevant references with this feature.
Data synthesis
We had planned to pool the data in meta‐analysis if clinical and methodological characteristics of individual studies were sufficiently homogeneous. The included studies were too heterogeneous regarding different monoclonal antibody types, doses, populations, or settings. We decided not to perform meta‐analyses and commented on the results instead.
We had planned at protocol stage to calculate Peto odds ratios for outcomes with few events (less than 5%). However, for interpretability and consistency throughout the review, we report risk ratios, and plan to do so for future updates of this review as well.
Assessment of risk of bias
We planned to use the Risk‐of‐bias VISualization tool (robvis) to generate risk of bias summary figures (McGuinness 2020), but instead we used Review Manager Web (RevMan Web 2020) to present risk of bias summary figures in the analysis and to produce interactive risk of bias tables for each outcome.
Types of participants
After discussion with clinical experts, we decided to perform separate analyses for populations with hospitalised individuals with COVID‐19 and moderate to severe disease, and for populations with non‐hospitalised individuals with COVID‐19 with asymptomatic and mild disease.
Types of outcome measures
In a group discussion with clinical experts, we revised our outcomes. We decided to add the outcomes thromboembolic events and renal failure for both populations. For adverse events, we have added all grade adverse events and grades 1 to 2.
For hospitalised individuals with moderate to severe COVID‐19, we changed the outcome hospital admission to hospital discharge. We added the outcomes time to sustained recovery and neurological dysfunction, as they were rated as important as part of a guideline consortium (CEOSys).
To account for possible bias due to competing events, we changed the outcome admission to hospital for non‐hospitalised individuals to admission to hospital or death; and hospital discharge for hospitalised participants to hospital discharge and alive.
Assessment of heterogeneity
We have changed the description of how we quantify heterogeneity. We now base it on the I² statistic to better reflect the Cochrane Handbook for Systematic Reviews of Interventions. In the light of future updates of this review, and based on peer review advice, we changed our criterion on when to perform subgroup analysis from I² > 80% to I² > 50%, reflecting substantial heterogeneity according to the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021).
Measures of treatment effect
We had planned to use Peto odds ratio if the number of observed events was small (less than 5% of sample per group). However, for consistency throughout the review, better interpretability, and lacking impact on the findings, we have decided to report risk ratios instead.
Subgroup analysis and investigation of heterogeneity
The subgroup analyses planned to explore:
age of participants (divided into applicable age groups, e.g. children; 18 to 65 years, 65 years and older)
pre‐existing conditions (diabetes, respiratory disease, hypertension, immunosuppression)
timing of first dose administration with illness onset
duration since symptom onset
antibodies detected at baseline
Due to the limited number of RCTs providing relevant data and the variation of SARS‐CoV‐2‐neutralising monoclonal antibodies used across the trials, we did not perform meta‐analyses and thus no subgroup analyses.
For future updates of the review, we will combine the subgroups 'timing of first dose administration with illness onset' and 'duration since symptom onset' and specify the splits of interest to be 'timing of first dose administration since symptom onset (up to 3 days, 4 to 7 days and more than 7 days)'.
Summary of findings tables
During the protocol stage, we had listed the following outcomes eligible for display in the summary of findings tables:
all‐cause mortality, up to 30 days
all‐cause mortality, until longest follow‐up (time‐to‐event)
clinical COVID‐19 symptoms, assessed with the WHO Clinical Progression Scale (Marshall 2020), within 30 days
quality of life, assessed with standardised scales (e.g. WHOQOL‐100) at up to 7 days, up to 30 days, and longest follow‐up available
adverse events (grade 3 to 4), including if possible, imputability of events
serious adverse events, including if possible, imputability of events
admission to hospital (for outpatients only)
Based on changes in the list of outcomes during protocol stage, we changed this to:
all‐cause mortality, up to 30 days
all‐cause mortality, up to 60 days
clinical COVID‐19 symptoms, assessed with the WHO Clinical Progression Scale (Marshall 2020), within 30 days, or development of severe symptoms if clinical progression scale not available; if available, we extracted IMV requirement or death
quality of life, assessed with standardised scales (e.g. WHOQOL‐100) at up to 7 days, up to 30 days, and longest follow‐up available)
admission to hospital (for outpatients only), hospital discharge (for inpatients only)
adverse events (all grades, grade 1‐2, grade 3‐4)
serious adverse events
Contributions of authors
NK: screening, data extraction, risk of bias assessment, meta‐analysis, writing the manuscript
CH: screening, data extraction, risk of bias assessment, meta‐analysis, writing the manuscript
KLC: screening, risk of bias assessment, clinical expertise
ET: screening, risk of bias assessment
ZK: screening, risk of bias assessment, characteristics of studies
MP: screening, data extraction, risk of bias assessment, clinical expertise and advice
MN: risk of bias assessment, characteristics of studies
VP: risk of bias assessment, methodological expertise
SS: conception of the protocol and review
SJV: clinical expertise
IM: development of the search strategy
CS: clinical expertise and advice
EMW: clinical expertise and advice
CS‐O: clinical expertise and advice
DJR: clinical expertise and advice
ZM: clinical expertise and advice
LJE: clinical and methodological expertise
NS: methodological expertise and advice, and conception and writing of the protocol and review
Sources of support
Internal sources
-
University Hospital of Cologne, Germany
Cochrane Cancer, Department I of Internal Medicine
-
NHS Blood and Transplant, UK
NHS Blood and Transplant
-
Monash University Australia, Australia
Transfusion Research Unit, Department of Epidemiology and Preventive Medicine
-
Haematology Society of Australia and New Zealand, Australia
Leukaemia Foundation and HSANZ Australia
External sources
-
Federal Ministry of Education and Research, Germany
NaFoUniMedCovid19 (funding number: 01KX2021); part of the project "COVIM"
-
Federal Ministry of Education and Research, Germany
This review is part of the CEOsys project funded by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021.
Declarations of interest
NK: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'COVIM', which was paid to the institution).
CH: none known
KLC: none known
ET: none known
ZK: none known
MP: none known
MN: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'COVIM', which was paid to the institution).
VP: none known
SS: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'COVIM' which was paid to the institution).
SJV: receives a PhD scholarship from the not‐for‐profit Sanquin blood bank.
IM: none known
CS: none known
EMW: none known
CS‐O: has declared to be employed by the not‐for‐profit Sanquin blood bank.
DJR: is a consultant haematologist for NHS Blood and Transplant.
ZM: is a haematologist at Monash University.
LJE: is a consultant haematologist for NHS Blood and Transplant.
NS: none known
* contributed equally
* contributed equally
New
References
References to studies included in this review
ACTIV‐3 {published data only}
- Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, Sandkovsky SM, et al. A neutralizing monoclonal antibody for hospitalised patients with COVID-19. New England Journal of Medicine 2021;384:905-14. [DOI: 10.1056/NEJMoa2033130] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, Sandkovsky U, et al . Clinical and virological response to a neutralizing monoclonal antibody for hospitalized patients with COVID-19. medrxiv [Preprint] 2021. [DOI: 10.1101/2021.07.19.21260559] [DOI] [PMC free article] [PubMed]
BLAZE‐1 (phase 2, 0.7g) {published data only}
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. New England Journal of Medicine 2020;384:229-37. [DOI: 10.1056/NEJMoa2029849] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325(7):632-44. [DOI: 10.1001/jama.2021.0202] [DOI] [PMC free article] [PubMed] [Google Scholar]
BLAZE‐1 (phase 2, 2.8g) {published data only}
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. New England Journal of Medicine 2020;384:229-37. [DOI: 10.1056/NEJMoa2029849] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325(7):632-44. [DOI: 10.1001/jama.2021.0202] [DOI] [PMC free article] [PubMed] [Google Scholar]
BLAZE‐1 (phase 2, 7.0g) {published data only}
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. New England Journal of Medicine 2020;384:229-37. [DOI: 10.1056/NEJMoa2029849] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325(7):632-44. [DOI: 10.1001/jama.2021.0202] [DOI] [PMC free article] [PubMed] [Google Scholar]
BLAZE‐1 (phase 2) {published data only}
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. New England Journal of Medicine 2020;384:229-37. [DOI: 10.1056/NEJMoa2029849] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325(7):632-44. [DOI: 10.1001/jama.2021.0202] [DOI] [PMC free article] [PubMed] [Google Scholar]
BLAZE‐1 (phase 3) {published data only}
- Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. New England Journal of Medicine 2021;-:-. [DOI: 10.1056/NEJMoa2102685] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M, Nirula A, Gottlieb RL, Azizad M, Mocherla B, Chen P, et al. Bamlanivimab + etesevimab for treatment of COVID-19 in high-risk ambulatory patients. Topics in Antiviral Medicine 2021;29(1):33, abstract no. 122. [Google Scholar]
COMET‐ICE {published data only}
Eom 2021 {published data only}
- Eom JS, Ison M, Streinu-Cercel A, Săndulescu O, Preotescu LL, Kim YS, et al. Efficacy and safety of CT-P59 plus standard of care: a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate SARS-CoV-2 infection. researchsquare.com/article/rs-296518/v1. [DOI: 10.21203/rs.3.rs-296518/v1] [DOI]
Eom 2021 (40 mg/kg) {published data only}
- Eom JS, Ison M, Streinu-Cercel A, Săndulescu O, Preotescu LL, Kim YS, et al. Efficacy and safety of CT-P59 plus standard of care: a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate SARS-CoV-2 infection. researchsquare.com/article/rs-296518/v1. [DOI: 10.21203/rs.3.rs-296518/v1] [DOI]
Eom 2021 (80 mg/kg) {published data only}
- Eom JS, Ison M, Streinu-Cercel A, Săndulescu O, Preotescu LL, Kim YS, et al. Efficacy and safety of CT-P59 plus standard of care: a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate SARS-CoV-2 infection. researchsquare.com/article/rs-296518/v1. [DOI: 10.21203/rs.3.rs-296518/v1] [DOI]
RECOVERY {published data only}
- Horby PW, Mafham M, Peto L, Campbell M, Pessoa-Amorim G, Spata E, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.06.15.21258542] [DOI]
Weinreich (phase 1/2, 2.4 g) {published data only}
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody cocktail in outpatients with COVID-19. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.06.09.21257915] [DOI]
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. New England Journal of Medicine 2021;384:238-51. [DOI: 10.1056/NEJMoa2035002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinreich (phase 1/2, 8.0 g) {published data only}
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody cocktail in outpatients with COVID-19. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.06.09.21257915] [DOI]
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. New England Journal of Medicine 2021;384:238-51. [DOI: 10.1056/NEJMoa2035002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinreich (phase 1/2) {published data only}
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody cocktail in outpatients with COVID-19. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.06.09.21257915] [DOI]
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. New England Journal of Medicine 2020;384:238-51. [DOI: 10.1056/NEJMoa2035002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinreich (phase 3) {published data only}
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody cocktail clinical outcomes study in COVID-19 outpatients. medRxiv [Preprint]. [DOI: 10.1101/2021.05.19.21257469] [DOI]
References to studies excluded from this review
ACTIV‐4 {published data only}
- NCT04505774. Anti-thrombotics for adults hospitalized with COVID-19 (ACTIV-4). clinicaltrials.gov/ct2/show/NCT04505774 (first received 10 August 2020).
ACTT {published data only}
- Euctr2020-001052-18-de. A multicenter, adaptive, randomised blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized adults - version for European Union/United Kingdom sites. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001052-18/DK (first received 20 March 2020).
- Galiuto L, Patrono C. Conflicting results on the efficacy of remdesivir in hospitalized COVID-19 patients: comment on the adaptive COVID-19 treatment trial. European Heart Journal 2020;41(46):4387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN13035264. Adaptive COVID-19 treatment trial in the EU & UK. www.isrctn.com/ISRCTN13035264 (first received 11 July 2020).
- NCT04280705. Adaptive COVID-19 treatment trial. clinicaltrials.gov/ct2/show/NCT04280705 (first received 25 September 2020).
ACTT‐2 {published data only}
- NCT04401579. Adaptive COVID-19 treatment trial 2 (ACTT-II). clinicaltrials.gov/ct2/show/NCT04401579 (first received 26 May 2020).
ACTT‐3 {published data only}
- NCT04492475. Adaptive COVID-19 treatment trial 3 (ACTT-3). clinicaltrials.gov/ct2/show/NCT04492475 (first received 30 July 2020).
Alam 2021 {published data only}
- Alam MM, Mahmud S, Aggarwal S, Fathma S, Al Mahi N, Shibli MS, et al. Clinical impact of the early use of monoclonal antibody LY-CoV555 (bamlanivimab) on mortality and hospitalization among elderly nursing home patients: a multicenter retrospective study. Cureus 2021;13(5):e14933. [DOI: 10.7759/cureus.14933] [DOI] [PMC free article] [PubMed] [Google Scholar]
AMMURAVID {published data only}EUCTR2020‐001854‐23
- EUCTR2020-001854-23. A study with immunotherapy for moderate COVID-19. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001854-23/IT (first received 26 June 2020).
ASCOT‐ADAPT {published data only}
- ACTRN12620000445976. Australasian COVID-19 trial: an adaptive platform trial (ASCOT-ADAPT). A multi-centre randomised adaptive platform clinical trial to assess clinical, virological and immunological outcomes in patients with SARS-CoV-2 infection (COVID-19). anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000445976 (first received 2 April 2020).
- NCT04483960. Australasian COVID-19 trial (ASCOT) adaptive platform trial. clinicaltrials.gov/ct2/show/NCT04483960 (first received 23 July 2020).
Bariola 2021 {published data only}
- Bariola JR, McCreary EK, Wadas RJ, Kip KE, Marroquin OC, Minnier T, et al. Impact of monoclonal antibody treatment on hospitalization and mortality among non-hospitalized adults with SARS-CoV-2 infection. Open Forum Infectious Diseases 2021;8(7):ofab254. [DOI: 10.1093/ofid/ofab254] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beam 2021 {published data only}
- Beam E, Destro Borgen MJ, Razonable RR. Infection prevention and control considerations for safe outpatient monoclonal antibody infusions in patients with coronavirus disease 2019 (COVID-19). Infection Control & Hospital Epidemiology 2021;-:1-2. [DOI: 10.1017/ice.2021.106] [DOI] [PMC free article] [PubMed] [Google Scholar]
BLAZE‐2 {published data only}
- NCT04497987. A study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in preventing SARS-CoV-2 infection and COVID-19 in nursing home residents and staff (BLAZE-2). clinicaltrials.gov/ct2/show/NCT04497987 (first received 4 August 2020).
ChiCTR2000030012 {published data only}
- ChiCTR2000030012. Development of anti-2019-nCoV therapeutic antibody from the recovered novel coronavirus pneumonia patients (COVID-19). www.chictr.org.cn/showproj.aspx?proj=49718 (first received 19 February 2020).
Cohen 2021 {published data only}
- Cohen MS, Wohl DA, Fischer WA, Smith DJ, Eron JJ. Outpatient treatment of SARS-CoV-2 infection to prevent COVID-19 progression. Clinical Infectious Diseases 2021;-:ciab494. [DOI: 10.1093/cid/ciab494] [DOI] [PMC free article] [PubMed] [Google Scholar]
COREG {published data only}
- NCT04602260. Functional recovery of hospitalised patients with COVID-19: the COREG extension study. clinicaltrials.gov/show/NCT04602260 (first received 26 October 2020).
COVERAGE {published data only}
- Duvignaud A, Lhomme E, Pistone T, Onaisi R, Sitta R, Journot V, et al. Home treatment of older people with symptomatic SARS-CoV-2 infection (COVID-19): a structured summary of a study protocol for a multi-arm multi-stage (MAMS) randomized trial to evaluate the efficacy and tolerability of several experimental treatments to reduce the risk of hospitalisation or death in outpatients aged 65 years or older (COVERAGE trial). Trials 2020;21(1):846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04356495. Treatments to decrease the risk of hospitalization or death in elderly outpatients with symptomatic SARS-CoV-2 infection (COVID-19). clinicaltrials.gov/ct2/show/NCT04356495 (first received 22 April 2020).
COVER HCW {published data only}
- NCT04561063. COVID-19 prophylaxis South Africa (COVER HCW). clinicaltrials.gov/ct2/show/NCT04561063 (first received 23 September 2020).
COVID_Aging {published data only}EUCTR2020‐001303‐16‐FR
- EUCTR2020-001303-16-FR. Efficacy of hydroxychloroquine, telmisartan and azithromycin on survival in elderly hospitalized patients with VIDOC-19: a randomized, multi-centre, adaptive, blinded study. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001303-16/FR (first received 29 March 2020).
CROWN CORONA {published data only}
- NCT04333732. Crown coronation: COVID-19 research outcomes worldwide network for CORONAvirus prevention (CROWN CORONA). clinicaltrials.gov/ct2/show/NCT04333732 (first received 3 April 2020).
C‐SMART {published data only}
- NCT04534725. COVID-19 prevention and treatment in cancer; a sequential multiple assignment randomised trial. clinicaltrials.gov/show/NCT04534725 (first received 1 September 2020).
Dale 2021 {published data only}
- Dale AP, Hudson M, Cullen T, Ellingson K, Davis K, Armenta D, et al. Administration of bamlanivimab to skilled nursing facility residents during a COVID-19 outbreak, January-February 2021, Arizona. Journal of the American Medical Directors Association 2021;S1525-8610(21):00422-9. [DOI: 10.1016/j.jamda.2021.04.023] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhand 2021a {published data only}
- Dhand A, Lobo SA, Wolfe K, Feola N, Nabors C. Bamlanivimab for treatment of COVID-19 in solid organ transplant recipients: early single-center experience. Clinical Transplantation 2021;35(4):00:e14245. [DOI: 10.1111/ctr.14245] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhand 2021b {published data only}
- Dhand A, Lobo SA, Wolfe K, Feola N, Lee L, Nog R, et al. Casirivimab-imdevimab for treatment of COVID-19 in solid organ transplant recipients: an early experience. Transplantation 2021;105(7):e68-e69. [DOI: 10.1097/TP.0000000000003737] [DOI] [PubMed] [Google Scholar]
Dong 2021 {published data only}
- Dong J, Zost SJ, Greaney AJ, Starr TN, Dingens AS, Chen EC, et al. Genetic and structural basis for recognition of SARS-CoV-2 spike protein by a two-antibody cocktail. bioRxiv [Preprint] 2021. [DOI: 10.1101/2021.01.27.428529] [DOI]
EUCTR2020‐001243‐15‐BE {published data only}EUCTR2020‐001243‐15‐BE
- EUCTR2020-001243-15-BE. COVID-19: a randomized, open-label, adaptive, proof-of-concept clinical trial of new antiviral drug candidates against SARS-CoV-2. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001243-15/BE (first received 27 March 2020).
EUDRACT2020‐002713‐17 {published data only}EUDRACT2020‐002713‐17
- EUDRACT2020-002713-17. A study to evaluate the safety and efficacy of MSTT1041A or UTTR1147A in patients with severe COVID-19 pneumonia. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-002713-17 (first received 13 July 2020).
FORCE {published data only}EUDRACT2020‐001686‐36
- NCT04371367. Avdoralimab an anti-C5aR antibody, in patients with COVID-19 severe pneumonia. clinicaltrials.gov/show/NCT04371367 (first received 1 May 2020).
Ganesh 2021 {published data only}
- Ganesh R, Pawlowski C, O'Horo JC, Arndt LL, Arndt R, Bell SJ, et al. Association of intravenous bamlanivimab use with reduced hospitalization, intensive care unit admission, and mortality in patients with mild to moderate COVID-19. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.05.23.21257670] [DOI] [PMC free article] [PubMed]
jRCT2031190264 {published data only}jRCT2031190264
- jRCT2031190264. A multicenter, adaptive, randomized blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized adults. jrct.niph.go.jp/latest-detail/jRCT2031190264 2020.
Karr 2021 {published data only}
- Karr E, Chung T, Burtson K, Markert R, Kelly D. Bamlanivimab use in a military treatment facility. Military Medicine 2021;-:usab188. [DOI: 10.1093/milmed/usab188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kutzler 2021 {published data only}
- Kutzler HL, Kuzaro HA, Serrano OK, Feingold A, Morgan G, Cheema F, et al. Initial experience of bamlanivimab monotherapy use in solid organ transplant recipients. Transplant Infectious Disease 2021;-:e13662. [DOI: 10.1111/tid.13662] [DOI] [PubMed] [Google Scholar]
MAS‐COVID {published data only}
- NCT04382651. Study of efficacy and safety of MAS825 in patients with COVID-19 (MAS-COVID). clinicaltrials.gov/ct2/show/NCT04382651 (first received 11 May 2020).
NCT04275245 {published data only}
- NCT04275245. Clinical study of anti-CD147 humanized meplazumab for injection to treat with 2019-nCoV pneumonia. ClinicalTrials.gov/show/NCT04275245 (first received 19 February 2020).
NCT04341116 {published data only}
- NCT04341116. Study of TJ003234 (anti-GM-CSF monoclonal antibody) in subjects with severe coronavirus disease 2019 (COVID-19). clinicaltrials.gov/show/NCT04341116 (first received 10 April 2020).
NCT04354428 {published data only}
- NCT04354428. Treatment for SARS-CoV-2 in high-risk adult outpatients. clinicaltrials.gov/ct2/show/NCT04354428 (first received 21 April 2020).
NCT04369469 {published data only}
- NCT04369469. Efficacy and safety study of IV ravulizumab in patients with COVID-19 severe pneumonia. clinicaltrials.gov/show/NCT04369469 (first received 30 April 2020).
NCT04370262 {published data only}
- NCT04370262. Multi-site adaptive trials for COVID-19. clinicaltrials.gov/ct2/show/NCT04370262 (first received 30 April 2020).
NCT04415073 {published data only}
- NCT04415073. A phase 2 study to evaluate axatilimab for hospitalized patients with respiratory involvement secondary to COVID-19. clinicaltrials.gov/show/NCT04415073 (first received 4 June 2020).
NCT04452318 {published data only}
- NCT04452318. Study assessing the efficacy and safety of anti-spike SARS CoV-2 monoclonal antibodies for prevention of SARS CoV-2 infection asymptomatic in healthy adults who are household contacts to an individual with a positive SARS-CoV-2 RT-PCR assay. clinicaltrials.gov/ct2/show/NCT04452318 (first received 30 June 2020).
NCT04453384 {published data only}
- NCT04453384. Study to evaluate the safety and efficacy of XAV-19 in patients with COVID-19 induced moderate pneumonia. clinicaltrials.gov/show/NCT04453384 (first received 1 July 2020).
NCT04454398 {published data only}
- NCT04454398. A randomized placebo-controlled study to evaluate STI-1499 (COVI-GUARD) in hospitalized patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04454398 (first received 1 July 2020).
NCT04469179 {published data only}
- NCT04469179. Safety, tolerability, and pharmacokinetics of SAB-185 in ambulatory participants with COVID-19. clinicaltrials.gov/ct2/show/NCT04469179 (first received 13 July 2020).
NCT04494724 {published data only}
- NCT04494724. Clazakizumab vs. placebo - COVID-19 infection. clinicaltrials.gov/show/NCT04494724 (first received 31 July 2020).
NCT04494984 {published data only}
- NCT04494984. A study to investigate the pharmacokinetics, efficacy and safety of INM005 in patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04494984 (first received 31 July 2020).
NCT04497987 {published data only}
- A study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in preventing SARS-CoV-2 infection and COVID-19 in nursing home residents and staff. clinicaltrials.gov/show/NCT04497987.
NCT04498273 {published data only}
- NCT04498273. COVID-19 positive outpatient thrombosis prevention in adults aged 40-80. clinicaltrials.gov/ct2/show/NCT04498273 (first received 4 August 2020).
NCT04514302 {published data only}
- NCT04514302. Safety and efficacy of anti-SARS-CoV-2 equine antibody fragments (INOSARS) for hospitalized patients with COVID-19. clinicaltrials.gov/show/NCT04514302 (first received 14 August 2020).
NCT04516564 {published data only}
- A study of AK119 (anti-CD73 antibody), a treatment for COVID-19, in healthy subjects. clinicaltrials.gov/show/NCT04516564 (first received 18 August 2020).
NCT04569786 {published data only}
- Dose ranging trial to assess safety and immunogenicity of V590 (COVID-19 vaccine) in healthy adults (V590-001). clinicaltrials.gov/show/NCT04569786 (first received 30 September 2020).
NCT04574869 {published data only}
- NCT04574869. A study of RLS-0071 in patients with acute lung injury due to COVID-19 pneumonia in early respiratory failure. clinicaltrials.gov/ct2/show/NCT04574869 (first received 5 October 2020).
NCT04586153 {published data only}
- NCT04586153. Study to assess the effect of meplazumab on COVID-19. clinicaltrials.gov/show/NCT04586153 (first received 14 October 2020).
NCT04625725 {published data only}
- NCT04625725. Phase III double-blind, placebo-controlled study of AZD7442 for pre-exposure prophylaxis of COVID-19 in adult. clinicaltrials.gov/ct2/show/NCT04625725 (first received 12 November 2020).
NCT04629703 {published data only}
- NCT04629703. Double-blind, randomized, placebo-controlled, adaptive design, multi-center phase 3 study to evaluate the efficacy and safety of fostamatinib in COVID-19 subjects. clinicaltrials.gov/ct2/show/NCT04629703 (first received 16 November 2020).
NCT04766671 {published data only}
- NCT04766671. An exploratory study to describe virological effect, safety, and pharmacokinetics of VIR-7831 monoclonal antibody in hospitalized participants with COVID-19. inclinicaltrials.com/covid-19/NCT04766671/.
NCT04859517 {published data only}
- NCT04859517. Evaluation of ADG20 for the prevention of COVID-19. clinicaltrials.gov/show/NCT04859517.
NCT04894474 {published data only}
- NCT04894474. A study to test whether BI 767551 can prevent COVID-19 in people who have been exposed to SARS-CoV-2. clinicaltrials.gov/show/NCT04894474.
PANAMO {published data only}
- NCT04333420. Randomized, controlled study of IFX-1 in patients with severe COVID-19 pneumonia (PANAMO). clinicaltrials.gov/ct2/show/NCT04333420 (first received 3 April 2020).
PO‐COV‐III‐20 {published data only}EUCTR2020‐001782‐37‐SK
- EUCTR2020-001782-37-SK. A multi-centre, adaptive, randomized, double-blind, placebo-controlled comparative clinical study of the safety and efficacy of 12 mg Polyoxidonium®, lyophilizate solution for injections (NPO Petrovax Pharm LLC, Russia) in patients with coronavirus disease (COVID-19). www.clinicaltrialsregister.eu/ctr-search/trial/2020-001782-37/SK (first received 15 June 2020).
PROFACT‐01 {published data only}
- NCT04621149. An outpatient study investigating non-prescription treatments for COVID-19 (PROFACT-01). clinicaltrials.gov/ct2/show/NCT04621149 (first received 9 November 2020).
Rainwater‐Lovett 2021 {published data only}
- Rainwater-Lovett K, Redd JT, Stewart MA, Calles NE, Cluff T, Fang M, et al. Real-world effect of monoclonal antibody treatment in COVID-19 patients in a diverse population in the United States. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.04.08.21254705] [DOI] [PMC free article] [PubMed]
RESP301‐002 {published data only}EUCTR2020‐002120‐37‐GB
- EUCTR2020-002120-37-GB. An open-label, adaptive randomized, controlled multicenter study to evaluate the efficacy and safety of RESP301+SOC vs SOC in hospitalized participants with COVID-19 requiring supplemental oxygen. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001072-15/GB (first received 19 March 2020).
Shirk 2021 {published data only}
- Shirk S, Kerr D, Saraceni C, Hand G, Terrenzi M, McDermott A, et al. The challenging but imperative path to monoclonal antibody use against COVID-19 for a small overseas military hospital. Military Medicine 2021;-:usab193. [DOI: 10.1093/milmed/usab193] [DOI] [PMC free article] [PubMed] [Google Scholar]
STORM CHASER {published data only}
- NCT04625972. Phase III double-blind, placebo-controlled study of AZD7442 for post- exposure prophylaxis of COVID-19 in adults (STORM CHASER). clinicaltrials.gov/show/NCT04625972 (first received 12 November 2020).
Track: ChiCTR2100042150 {published data only}
- ChiCTR2100042150. Phase I clinical study to evaluate the safety, tolerability, pharmacokinetic profile and immunogenicity of JMB-2002 injection in Chinese healthy subjects after intravenous infusion of single dose. www.chictr.org.cn/showprojen.aspx?proj=66096.
Track: jRCT2071200117 {published data only}
- jRCT2071200117. A phase 1 study of casavirimab and imdevimab in Japanese adult volunteers. rctportal.niph.go.jp/en/detail?trial_id=jRCT2071200117.
Track: NCT04429529 {published data only}
- NCT04429529. Safety of TY027, a treatment for COVID-19, in humans. clinicaltrials.gov/ct2/show/NCT04429529 (first received 12 June 2020).
Track: NCT04441918 {published data only}
- NCT04441918. Tolerability, safety, pharmacokinetic profile and immunogenicity of a recombinant humanized anti-SARS-CoV-2 monoclonal antibody (JS016) for injection in Chinese health subjects. clinicaltrials.gov/ct2/show/NCT04441918 (first received 22 June 2020).
Track: NCT04441931 {published data only}
- NCT04441931. A study of LY3832479 (LY-CoV016) in healthy participants. clinicaltrials.gov/ct2/show/NCT04441931 (first received 22 June 2020).
Track: NCT04479631 {published data only}
- NCT04479631. Safety, tolerability, and pharmacokinetics study of human monoclonal antibody BRII-196. clinicaltrials.gov/ct2/show/NCT04479631 (first received 21 July 2020).
Track: NCT04479644 {published data only}
- NCT04479644. Safety, tolerability, and pharmacokinetics study of human monoclonal antibody BRII-198. clinicaltrials.gov/ct2/show/NCT04479644 (first received 21 July 2020).
Track: NCT04483375 {published data only}
- NCT04483375. Safety, tolerability and pharmacokinetics of SCTA01, an anti-COVID-19 monoclonal antibody, in healthy Chinese subjects. clinicaltrials.gov/ct2/show/NCT04483375 (first received July 2020).
Track: NCT04507256 {published data only}
- NCT04507256. Study to evaluate the safety, tolerability and pharmacokinetics of AZD7442 in healthy adults. clinicaltrials.gov/ct2/show/NCT04507256 (first received 11 August 2020).
Track: NCT04519437 {published data only}
- NCT04519437. Study assessing the safety, tolerability, pharmacokinetics, and immunogenicity of repeated subcutaneous doses of anti-spike (S) SARS-CoV-2 monoclonal antibodies (REGN10933+REGN10987) in adult volunteers as related to COVID-19. clinicaltrials.gov/ct2/show/NCT04519437 (first received 19 August 2020).
Track: NCT04525079 {published data only}
- NCT04525079. To evaluate the safety, tolerability and pharmacokinetics of CT-P59 in healthy subjects. clinicaltrials.gov/ct2/show/NCT04525079 (first received 25 August 2020).
Track: NCT04532294 {published data only}
- NCT04532294. Safety, tolerability, pharmacokinetics, and immunogenicity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody in healthy subjects. clinicaltrials.gov/ct2/show/NCT04532294 (first received 31 August 2020).
Track: NCT04533048 {published data only}
- NCT04533048. A clinical study to evaluate MW33 injection. clinicaltrials.gov/ct2/show/NCT04533048 (first received 31 August 2020).
Track: NCT04537910 {published data only}
- NCT04537910. A study of LY3819253 (LY-CoV555) in healthy participants. clinicaltrials.gov/ct2/show/NCT04537910 (first received 3 September 2020).
Track: NCT04561076 {published data only}
- NCT04561076. Evaluate safety and pharmacokinetics of HLX70 in healthy adult volunteers. clinicaltrials.gov/ct2/show/NCT04561076 (first received 23 September 2020).
Track: NCT04567810 {published data only}
- NCT04567810. Safety, tolerability, and pharmacokinetics of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) chicken egg antibody (IgY) (COVID-19). clinicaltrials.gov/ct2/show/NCT04567810 (first received 29 September 2020).
Track: NCT04590430 {published data only}
- NCT04590430. Study to assess the safety, tolerability, and pharmacokinetics of HFB30132A against COVID-19 in healthy adults. clinicaltrials.gov/ct2/show/NCT04590430 (first received 19 October 2020).
Track: NCT04592549 {published data only}
- NCT04592549. Study of monoclonal antibody cocktail being tested for the prevention of COVID-19 [A phase 1, randomized, double-blind, placebo-controlled, dose escalation study to evaluate the safety, pharmacokinetics, and immunogenicity of ADM03820 in adults]. clinicaltrials.gov/ct2/show/NCT04592549 (first received 19 October 2020).
Track: NCT04603651 {published data only}
- NCT04603651. Expanded access program to provide bamlanivimab (LY3819253) for the treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04603651 (first received 27 October 2020).
Track: NCT04617535 {published data only}
- NCT04617535. Compassionate use of REGN-COV2 for the treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04617535 (first received 5 November 2020).
Track: NCT04656691 {published data only}
- NCT04656691. At-home infusion using bamlanivimab in participants with mild to moderate COVID-19 (UNITED). clinicaltrials.gov/ct2/show/NCT04656691.
Track: NCT04691180 {published data only}
- NCT04691180. A phase 1 study of human monoclonal antibodies, BRII-196 and BRII-198. clinicaltrials.gov/ct2/show/NCT04691180 (first received 31 December 2020). [CLINICALTRIALS.GOV: NCT04691180]
Track: NCT04700163 {published data only}
- NCT04700163. RU anti-SARS-CoV-2 mAbs in healthy volunteers. clinicaltrials.gov/ct2/show/NCT04700163 (first received 7 January 2021).
Track: NCT04701658 {published data only}
- NCT04701658. A real world study of bamlanivimab in participants with mild-to-moderate coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04701658 (first received 8 January 2021).
Track: NCT04852978 {published data only}
- NCT04852978. COVID-19 study to assess immunogenicity, safety, and tolerability of Moderna mRNA-1273 vaccine administered with casirivimab+imdevimab in healthy adult volunteers. clinicaltrials.gov/ct2/show/NCT04852978.
Track: NCT04896541 {published data only}
- NCT04896541. Phase I double-blind, placebo-controlled study of AZD7442. https://clinicaltrials.gov/ct2/show/NCT04896541.
Track: NCT04932850 {published data only}
- NCT04932850. Study to evaluate the safety and concentrations of monoclonal antibody against virus that causes COVID-19 disease. clinicaltrials.gov/ct2/show/NCT04932850.
Webb 2021 {published data only}
- Webb BJ, Buckel W, Vento T, Butler AM, Grisel N, Brown SM, et al. Real-world effectiveness and tolerability of monoclonal antibodies for ambulatory patients with early COVID-19. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.03.15.21253646] [DOI] [PMC free article] [PubMed]
Yang 2020 {published data only}
- Yang X, Pan Y, Song G, Wang J, Zhan S, Wu X, et al. Anti-SARS-CoV-2 monoclonal antibody 1E10. www.lens.org/lens/patent/147-382-786-055-641 2020. [EPISTEMONIKOS ID: 563468dd70e91a939051abfc89e7d250ead2636a]
References to studies awaiting assessment
ACCORD & ACCORD 2 {published data only}
- EUCTR2020-001736-95. ACCORD 2: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID 19 in hospitalised patients. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001736-95/GB (first received 23 April 2020). [DOI] [PMC free article] [PubMed]
- Wilkinson T, Dixon R, Page C, Carroll M, Griffiths G, Ho LP, et al. ACCORD: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID-19 in hospitalised patients: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):691. [DOI: 10.1186/s13063-020-04584-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACOVACT {published data only}
- Euctr2020-001302-30/AT. A multicenter, randomized, active controlled, open label, platform trial on the efficacy and safety of experimental therapeutics for patients with a lung disease caused by coronavirus infection. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001302-30/AT (first received 9 April 2020).
- NCT04351724. Austrian corona virus adaptive clinical trial (COVID-19). clinicaltrials.gov/ct2/show/NCT04351724 (first received 2 March 2021).
ACTIV‐1 IM {published data only}
- NCT04593940. Immune modulators for treating COVID-19. clinicaltrials.gov/ct2/show/NCT04593940 (first received 20 October 2020).
ACTT‐4 {published data only}
- NCT04640168. Adaptive COVID-19 treatment trial 4 (ACTT-4). clinicaltrials.gov/ct2/show/NCT04640168 (first received 23 November 2020).
ANTICOV {published data only}
- PACTR202006537901307. An open-label, multicentre, randomised, adaptive platform trial of the safety and efficacy of several therapies, including antiviral therapies, versus control in mild/moderate cases of COVID-19. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=12150 (first received 24 June 2020).
ARCO‐Home {published data only}
- EU/CTR/2020-001528-32. Adaptive randomised trial for therapy of corona virus disease 2019 at home with oral antivirals. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001528-32/IT (first received 24 June 2020).
BEAT COVID‐19 {published data only}ACTRN12620000566932
- ACTRN12620000566932. Randomised clinical trial of interventions for the treatment of COVID-19 in the community setting for high risk older people. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379801&isReview=true (first received 10 May 2020).
BET‐A (ACTIV‐5) {published data only}
- NCT04583956. Big effect trial (BET-A) for the treatment of COVID-19. www.clinicaltrials.gov/ct2/show/NCT04583956 (first received 12 October 2020).
BET‐B (ACTIV‐5) {published data only}
- NCT04583969. Big effect trial (BET-B) for the treatment of COVID-19. www.clinicaltrials.gov/ct2/show/NCT04583969 (first received 12 October 2020).
CATALYST {published data only}
- ISRCTN40580903. Which treatment could lessen the severity of a coronavirus infection when compared with usual care in an NHS setting? www.isrctn.com/ISRCTN40580903 (first received 14 May 2020).
CCAP {published data only}
- NCT04345289. Efficacy and safety of novel treatment options for adults with COVID-19 pneumonia. clinicaltrials.gov/ct2/show/NCT04345289 (first received 14 April 2020).
COLHEART‐19 {published data only}
- NCT04355143. Colchicine to reduce myocardial injury in COVID-19 (COLHEART-19). clinicaltrials.gov/ct2/show/NCT04355143 (first received 12 April 2020).
COPPS {published data only}
- NCT04662086. COVID-19 outpatient pragmatic platform study (COPPS) - master protocol. clinicaltrials.gov/ct2/show/NCT04662086 (first received 10 December 2020).
CORIMUNO {published data only}
- NCT04324047. Cohort multiple randomized controlled trials open-label of immune modulatory drugs and other treatments in COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04324047 (first received 27 March 2020).
COVID MED {published data only}
- NCT04328012. Comparison of therapeutics for hospitalized patients infected with SARS-CoV-2 in a pragmatic adaptive randomized clinical trial during the COVID-19 pandemic (COVID MED Trial). clinicaltrials.gov/show/NCT04328012 (first received 31 March 2020).
DEFINE {published data only}
- NCT04473053. Rapid experimental medicine for COVID-19. clinicaltrials.gov/ct2/show/NCT04473053 (first received 16 July 2020).
EU SolidAct {published data only}
- NCT04891133. EU SolidAct: an adaptive pandemic and emerging infection platform trial. clinicaltrials.gov/ct2/show/NCT04891133.
I‐SPY {published data only}
- NCT04488081. I-SPY COVID-19 trial: an adaptive platform trial for critically ill patients. clinicaltrials.gov/ct2/show/NCT04488081 (first received 27 July 2020).
NCT04359095 {published data only}
- NCT04359095. Effectiveness and safety of medical treatment for SARS-CoV-2 (COVID-19) in Colombia. clinicaltrials.gov/ct2/show/NCT04359095 (first received 24 April 2020).
NCT04590586 {published data only}
- NCT04590586. Study of multiple candidate agents for the treatment of COVID-19 in hospitalized patients. clinicaltrials.gov/ct2/show/NCT04590586 (first received 19 October 2020).
O’Brien 2021 {published data only}
- O’Brien M, Forleo-Neto E, Sarkar N, Isa F, Hou P, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination in early SARS-CoV-2 infection. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.06.14.21258569] [DOI]
PaTS‐COVID {published data only}
- NCT04703608. Prevention and treatment for COVID -19 (severe acute respiratory syndrome coronavirus 2 SARS-CoV-2) associated severe pneumonia in the Gambia (PaTS-COVID). clinicaltrials.gov/ct2/show/NCT04703608 (first received 11 January 2021).
PRINCIPLE {published data only}ISRCTN86534580
- ISRCTN86534580. A trial evaluating treatments for suspected coronavirus infection in people aged 50 years and above with pre-existing conditions and those aged 65 years and above. www.isrctn.com/ISRCTN86534580 (first received 20 March 2020).
PROTECT‐Surg {published data only}
- EUCTR2020-001448-24-GB. Preventing pulmonary complications in surgical patients at risk of COVID-19. clinicaltrialsregister.eu/ctr-search/trial/2020-001448-24/GB (first received 17 April 2020).
- NCT04386070. Preventing pulmonary complications in surgical patients at risk of COVID-19. clinicaltrials.gov/ct2/show/NCT04386070 (first received 13 May 2020).
REMAP‐CAP {published data only}
- Angus DC, Berry S, Lewis RJ, Al-Beidh F, Arabi Y, Van Bentum-Puijk W, et al. The randomized embedded multifactorial adaptive platform for community-acquired pneumonia (REMAP-CAP) study: rationale and design. Annals of the American Thoracic Society 2020;17(7):879-91. [DOI: 10.1513/AnnalsATS.202003-192SD] [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2015-002340-14-NL. Adaptive trial in severe pneumonia (REMAP-CAP). clinicaltrialsregister.eu/ctr-search/trial/2015-002340-14/NL (first received 13 July 2015).
- ISRCTN67000769. An international platform trial for severely ill patients with community-acquired pneumonia or COVID-19. www.isrctn.com/ISRCTN67000769 (first received 9 July 2020).
- NCT02735707. Randomized, embedded, multifactorial adaptive platform trial for community- acquired pneumonia (REMAP-CAP). clinicaltrials.gov/ct2/show/NCT02735707 (first received 13 April 2016).
SOLIDARITY {published data only}
- ISRCTN83971151. SOLIDARITY: an international randomized controlled trial to evaluate non-licensed COVID-19 treatments in addition to standard of care among hospitalised patients. www.isrctn.com/ISRCTN83971151 (first received 25 March 2020).
- NCT04330690. Treatments for COVID-19: Canadian arm of the SOLIDARITY trial. clinicaltrials.gov/ct2/show/NCT04330690 (first received 1 April 2020).
- NCT04575064. An International randomized trial of additional treatments for COVID-19 in hospitalized patients who are all receiving the local standard of care - WHO-SOLIDARITY-Germany. clinicaltrials.gov/ct2/show/NCT04575064 (first received 5 October 2020).
- Soto A, Quinones-Laveriano DM, Garcia PJ, Gotuzzo E, Henao-Restrepo AM. Rapid responses to the COVID-19 pandemic through science and global collaboration: the solidarity clinical trial. Revista Peruana de Medicina Experimental y Salud Publica 2020;37(2):356-60. [DOI: 10.17843/rpmesp.2020.372.5546] [DOI] [PubMed] [Google Scholar]
SWISSPED‐RECOVERY {published data only}
- NCT04826588. Randomised evaluation of COVID-19 therapy (RECOVERY) in children with PIMS-TS in Switzerland (SWISSPED-RECOVERY). clinicaltrials.gov/ct2/show/NCT04826588.
TACTIC‐E {published data only}
- NCT04393246. Multi-arm therapeutic study in pre-ICU patients admitted with COVID-19 - experimental drugs and mechanisms. clinicaltrials.gov/ct2/show/NCT04393246 (first received 19 May 2020).
TACTIC‐R {published data only}
- Kulkarni S, Fisk M, Kostapanos M, Banham-Hall E, Bond S, Hernan-Sancho E, et al. Repurposed immunomodulatory drugs for COVID-19 in pre-ICU patients - multi-arm therapeutic study in pre-ICU patients admitted with COVID-19 - repurposed drugs (TACTIC-R): a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):626. [DOI: 10.1186/s13063-020-04535-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04390464. Multi-arm therapeutic study in pre-ICU patients admitted with COVID-19 - repurposed drugs (TACTIC-R). clinicaltrials.gov/ct2/show/NCT04390464 (first received 15 May 2020).
TOGETHER‐3 {published data only}
- PACTR202007700757139. TOGETHER 3 trial. COVID-19. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=12194 (first received 14 July 2020).
VIRCO {published data only}
- NCT04445467. An adaptive clinical trial of antivirals for COVID-19 infection (VIRCO). clinicaltrials.gov/ct2/show/NCT04445467 (first received 24 June 2020).
References to ongoing studies
ACTIV‐2 {published data only}
- NCT04518410. ACTIV-2: a study for outpatients with COVID-19. clinicaltrials.gov/ct2/show/NCT04518410 (first received 19 August 2020).
AGILE {published data only}
- EUCTR2020-001860-27. AGILE: seamless phase I/IIa platform for the rapid evaluation of candidates for COVID-19 treatment. A randomised phase I/II study to determine the safety and effectiveness of multiple drugs for the treatment of COVID-19. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001860-27/GB (first received 17 April 2020).
- Griffiths G, Fitzgerald R, Jaki T, Corkhill A, Marwood E, Reynolds H, et al. AGILE-ACCORD: a randomized, multicentre, seamless, adaptive phase I/II platform study to determine the optimal dose, safety and efficacy of multiple candidate agents for the treatment of COVID-19: a structured summary of a study protocol for a randomised platform trial. Trials 2020;21(1):544. [DOI: 10.1186/s13063-020-04473-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
DISCOVERY {published data only}
- Ader F, Discovery French Trial Management Team. Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults. BMJ open 2020;10(9):e041437. [DOI: 10.1136/bmjopen-2020-041437] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04315948. Trial of treatments for COVID-19 in hospitalised adults (DisCoVeRy). clinicaltrials.gov/ct2/show/NCT04315948 (first received 20 March 2020).
EUDRACT2020‐003401‐60 {published data only}
- EUDRACT2020-003401-60. A phase 2/3, randomized, parallel-group, placebo-controlled, double-blind study to evaluate the efficacy and safety of CT-P59 in combination with standard of care in hospitalized patients with SARS-CoV-2 Iinfection (COVID19). www.clinicaltrialsregister.eu/ctr-search/trial/2020-003401-60/IT.
NCT04411628 {published data only}
- NCT04411628. A study of LY3819253 (LY-CoV555) in participants hospitalized for COVID-19. clinicaltrials.gov/ct2/show/NCT04411628 (first received 2 June 2020).
NCT04426695 {published data only}
- NCT04426695. Safety, tolerability, and efficacy of anti-Spike (S) SARS-CoV-2 monoclonal antibodies for hospitalized adult patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04426695 (first received 11 June 2020).
NCT04551898 {published data only}
- NCT04551898. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody BGB-DXP593 in participants with mild-to-moderate coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04551898 (first received 16 September 2020).
NCT04584697 {published data only}
- NCT04584697. Study to evaluate the safety, pharmacokinetics and efficacy of STI-2020 (COVI-AMG™) in outpatients with COVID-19. clinicaltrials.gov/ct2/show/NCT04584697 (first received 14 October 2020).
NCT04593641 {published data only}
- NCT04593641. A pilot phase 1, randomized, double-blind, placebo-controlled, parallel group, single ascending dose study to evaluate the safety, tolerability and virology of CT-P59 in patient with mild symptoms of SARS-CoV-2 infection. clinicaltrials.gov/ct2/show/NCT04593641 (first received 20 October 2020).
NCT04627584 {published data only}
- NCT04627584. A phase 2 clinical trial of MW33 injection to evaluate the efficacy and safety in patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04627584 (first received 13 November 2020).
NCT04631666 {published data only}
- NCT04631666. COVID-19 - administration of the SARS-CoV-2-neutralizing monoclonal antibody DZIF-10c (infusion). clinicaltrials.gov/ct2/show/NCT04631666 (first received 17 November 2020).
NCT04631705 {published data only}
- NCT04631705. COVID-19 - administration of the SARS-CoV-2-neutralizing monoclonal antibody DZIF-10c (inhalation). clinicaltrials.gov/ct2/show/NCT04631705 (first received 17 November 2020).
NCT04634409 {published data only}
- NCT04634409. A study of immune system proteins in participants with mild to moderate COVID-19 Illness. clinicaltrials.gov/ct2/show/NCT04634409 (first received 18 November 2020).
NCT04644120 {published data only}
- NCT04644120. Study to assess adverse events and how intravenous (IV) ABBV-47D11 moves through the body of adult participants hospitalized with coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04644120 (first received 25 November 2020).
NCT04644185 {published data only}
- NCT04644185. The efficacy and safety of SCTA01 in hospitalized patients with severe COVID-19. clinicaltrials.gov/ct2/show/NCT04644185 (first received 25 November 2020).
NCT04649515 {published data only}
- NCT04649515. Efficacy and safety of TY027, a treatment for COVID-19, in humans. clinicaltrials.gov/ct2/show/NCT04649515 (first received 2 December 2020).
NCT04666441 {published data only}
- NCT04666441. A study to assess the virologic efficacy of REGN10933+REGN10987 across different dose regimens in adult outpatients with SARS-CoV-2 infection. clinicaltrials.gov/ct2/show/NCT04666441 (first received 14 December 2020).
NCT04674566 {published data only}
- Evaluation of safety and tolerability of COR-101 in hospitalized patients with moderate to severe COVID-19. clinicaltrials.gov/ct2/show/NCT04674566 (first received 19 December 2020). [NCT04748588]
NCT04683328 {published data only}
- NCT04683328. The safety and efficacy of SCTA01 against COVID-19 in patients admitted to high dependence or intensive care. clinicaltrials.gov/ct2/show/NCT04683328 (first received 24 December 2020).
NCT04709328 {published data only}
- NCT04709328. An adaptive, randomized, double-blinded, placebo-controlled trial to evaluate SCTA01 in COVID-19. clinicaltrials.gov/ct2/show/NCT04709328 (first received 14 January 2021).
NCT04723394 {published data only}
- NCT04723394. Phase III study of AZD7442 for treatment of COVID-19 in outpatient adults. clinicaltrials.gov/ct2/show/NCT04723394 (first received 25 January 2021).
NCT04734860 {published data only}
- NCT04734860. Study to evaluate a single dose of STI-2020 (COVI-AMG™) in adults with mild COVID-19 symptoms. clinicaltrials.gov/ct2/show/NCT04734860 (first received 2 February 2021).
NCT04748588 {published data only}
- NCT04748588. Treatment of nosocomial COVID-19. clinicaltrials.gov/ct2/show/NCT04748588 (first received 10 February 2021).
NCT04770467 {published data only}
- NCT04770467. A safety and efficacy study of human monoclonal antibodies, BRII-196 and BRII-198 for the treatment of patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04770467 (first received 25 February 2021). [CLINICALTRIALS.GOV: NCT04770467]
NCT04771351 {published data only}
- Study to evaluate a single dose of STI-2020 (COVI-AMG) in hospitalized adults with COVID-19. www.clinicaltrials.gov/ct2/show/NCT04771351 (first received 25 February 2021). [CLINICALTRIALS.GOV: NCT04771351]
NCT04779879 {published data only}
- Safety, tolerability and pharmacokinetics of second generation VIR-7831 material in non-hospitalized participants with mild to moderate COVID-19 (COMET-PEAK). clinicaltrials.gov/ct2/show/NCT04779879 (first received 3 March 2021).
NCT04780321 {published data only}
- JS016 (anti-SARS-CoV-2 monoclonal antibody) with mild and moderate COVID-19 or SARS-CoV-2 asymptomatic infection subjects. clinicaltrials.gov/ct2/show/NCT04780321 (first received 3 March 2021).
NCT04787211 {published data only}
- NCT04787211. A phase 2 study of human monoclonal antibodies, BRII-196 and BRII-198. clinicaltrials.gov/ct2/show/NCT04787211.
NCT04796402 {published data only}
- NCT04796402. A study to assess if a medicine called bamlanivimab is safe and effective in reducing hospitalization due to COVID-19 (B-EPIC). clinicaltrials.gov/ct2/show/NCT04796402.
NCT04805671 {published data only}
- NCT04805671. Evaluation of ADG20 for the treatment of mild or moderate COVID-19 (STAMP). clinicaltrials.gov/ct2/show/NCT04805671.
NCT04822701 {published data only}
- NCT04822701. A study to test BI 767551 in people with mild to moderate symptoms of COVID-19. clinicaltrials.gov/ct2/show/NCT04822701.
NCT04840459 {published data only}
- NCT04840459. Use of monoclonal antibodies for the treatment of mild to moderate COVID-19 in non-hospitalized setting. clinicaltrials.gov/ct2/show/NCT04840459.
NCT04900428 {published data only}
- NCT04900428. Study to evaluate a single intranasal dose of STI-2099 (COVI-DROPS™) in outpatient adults with COVID-19 (UK). clinicaltrials.gov/ct2/show/NCT04900428.
NCT04913675 {published data only}
- NCT04913675. Intramuscular VIR-7831 (sotrovimab) for mild/moderate COVID-19. clinicaltrials.gov/ct2/show/NCT04913675.
NCT04952805 {published data only}
- NCT04952805. Clinical trial to select the dose and evaluate safety and efficacy of MAD0004J08 monoclonal antibody in adult patients with recently diagnosed asymptomatic to moderately severe COVID-19. ClinicalTrials.gov 2021.
OPTIMISE‐C19 {published data only}
- NCT04790786. UPMC OPTIMISE-C19 Trial, a COVID-19 Study (OPTIMISE-C19). clinicaltrials.gov/ct2/show/NCT04790786.
Additional references
Antibody Society 2020
- Reichert J. FDA approves inmazeb for ebola virus infection. www.antibodysociety.org/tag/approved-antibodies (accessed 5 November 2020).
Arvin 2020
- Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, Havenar-Daughton C, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020;584:353-3. [DOI: 10.1038/s41586-020-2538-8] [DOI] [PubMed] [Google Scholar]
AstraZeneca 2020
- AstraZeneca. AstraZeneca’s COVID-19 vaccine authorised for emergency supply in the UK. www.astrazeneca.com/media-centre/press-releases/2020/astrazenecas-covid-19-vaccine-authorised-in-uk.html (accessed 12 January 2021).
Bayer 2019
- Bayer V. An overview of monoclonal antibodies. Immunotherapy in Oncology 2019;35(5):150927. [DOI: 10.1016/j.soncn.2019.08.006] [DOI] [PubMed] [Google Scholar]
Beigel 2020
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19 — final report. New England Journal of Medicine 2020;383:1813-26. [DOI: 10.1056/NEJMoa2007764] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buitrago‐Garcia 2020
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci Aziz M, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLOS Medicine 2020;17(9):e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buszko 2020
- Buszko M, Park J-H, Verthelyi D, Sen R, Young HA, Rosenberg AS. The dynamic changes in cytokine responses in COVID-19: a snapshot of the current state of knowledge. Nature Immunology 2020;21(10):1146-51. [DOI: 10.1038/s41590-020-0779-1] [DOI] [PubMed] [Google Scholar]
Catanzaro 2020
- Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduction and Targeted Therapy 2020;5(1):84. [DOI: 10.1038/s41392-020-0191-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cathcart 2021
- Cathcart AL, Havenar-Daughton C, Lempp FA, Ma D, Schmid M, Agostini ML, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. [DOI: 10.1101/2021.03.09.434607] [DOI]
CDC 2020
- Centers for Disease Control and Prevention. COVIDView - a weekly surveillance summary of U.S. COVID-19 activity (Key updates for week 43). www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html (accessed 12 January 2020).
Chen 2020
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. New England Journal of Medicine 2020;384:229-37. [DOI: 10.1056/NEJMoa2029849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherian 2021
- Cherian S, Potdar V, Jadhav S, Yadav P, Gupta N, Das M, et al. Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. bioRxiv 2021. [DOI: 10.1101/2021.04.22.440932] [DOI] [PMC free article] [PubMed]
Chinese Antibody Society 2020
- Chinese Antibody Society. chineseantibody.org (accessed 9 November 2020).
Choi 2020
- Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. New England Journal of Medicine 2020;383:2291-3. [DOI: 10.1056/NEJMc2031364] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2020
- Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HH, Mercer SW, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Global Health 2020;8(8):E1003-17. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane LSR
- Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. community.cochrane.org/review-production/production-resources/living-systematic-reviews (accessed 2 November 2020).
COMET 2020
- Core outcome set developers’ response to COVID-19. www.comet-initiative.org/Studies/Details/1538 (accessed 2 November 2020).
Copin 2021
- Copin R, Baum A, Wloga E, Pascal KE, Giordano S, Fulton BO, et al. In vitro and in vivo preclinical studies predict REGEN-COV protection against emergence of viral escape in humans. bioRxiv. [DOI: 10.1101/2021.03.10.434834] [DOI]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, 2021. Available at covidence.org. [DOI: 10.1016/S1470-2045(20)30096-6] [DOI]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Eldridge 2021
- Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Giraudeau B, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0). Additional considerations for cluster-randomized trials (RoB 2 CRT). https://www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-cluster-randomized-trials (accessed June 11, 2021).
Eli Lilly and Co 2020
- Eli Lilly and Company. Lilly begins world's first study of a potential COVID-19 antibody treatment in humans [press release]. investor.lilly.com/news-releases/news-release-details/lilly-begins-worlds-first-study-potential-covid-19-antibody (accessed 5 November 2020).
Endnote X9 [Computer program]
- EndNote X9. EndNote. Clarivate, 2013.
FDA 2021a
- US Food and Drug Administration. COVID-19 vaccines. www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed 12 January 2021).
FDA 2021b
- US Food and Drug Administration. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab (accessed 27 April 2021).
FDA 2021c
- US Food and Drug Administration. Emergency use authorization. www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
Ghosn 2021
- Ghosn L, Chaimani A, Evrenoglou T, Davidson M, Graña C, Schmucker C, et al. Interleukin‐6 blocking agents for treating COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013881. [DOI: 10.1002/14651858.CD013881] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glaunsinger 2020
- Glaunsinger B. Lecture 2: "Coronavirus biology", from the course "COVID-19, SARS-CoV-2 and the pandemic" [video]. biology.mit.edu/undergraduate/current-students/subject-offerings/covid-19-sars-cov-2-and-the-pandemic/ (accessed 4 November 2020).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021a
- Higgins JP Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021b
- Higgins JP, Eldridge S, Li T. Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021c
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Hoffmann 2020
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181(2):271-280.e8. [DOI: 10.1016/j.cell.2020.02.052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffmann 2021a
- Hoffmann M, Hofmann-Winkler H, Krüger N, Kempf A, Nehlmeier I, Graichen L, et al. SARS-CoV-2 variant B.1.617 is resistant to Bamlanivimab and evades antibodies induced by infection and vaccination. bioRxiv 2021. [DOI: 10.1101/2021.05.04.442663] [DOI] [PMC free article] [PubMed]
Hoffmann 2021b
- Hoffmann M, Arora P, Groß R, Seidel A, Hörnich BF, Hahn AS, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell 2021;184(9):2384-93.e12. [DOI: 10.1016/j.cell.2021.03.036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2020
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioannidis 2020
- Ioannidis JP. Infection fatality rate of COVID-19 inferred from seroprevalence data. Bulletin of the World Health Organization 2021;99(1):19–33F. [DOI: 10.2471/BLT.20.265892] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johns Hopkins 2021
- Johns Hopkins University & Medicine. Mortality analysis. coronavirus.jhu.edu/data/mortality (accessed 11 March 2021).
Kaplon 2020
- Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020. mAbs 2019;12(1):e1703531. [DOI: 10.1080/19420862.2019.1703531] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemp 2021
- Kemp SA, Collier DA, Datir RP, Ferreira IA, Gayed S, Jahun A, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature 2021;592:277-82. [DOI: 10.1038/s41586-021-03291-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamontagne 2020
- Lamontagne F, Agoritsas T, Macdonald H, Leo Y-S, Diaz J, Agarwal A, et al. A living WHO guideline on drugs for COVID-19. BMJ 2020;370:m3379. [DOI: 10.1136/bmj.m3379] [DOI] [PubMed] [Google Scholar]
Lauer 2020
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Annals of Internal Medicine 2020;172(9):577–82. [DOI: 10.7326/M20-0504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2020
- Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nature Microbiology 2020;5:1185–91. [DOI: 10.1038/s41564-020-00789-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Li 2020
- Li T, Higgins JP, Deeks JJ. Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Liang 2020
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncology 2020;21(3):335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014
- Liu JK. The history of monoclonal antibody development - progress, remaining challenges and future innovations. Annals of Medicine and Surgery 2014;3(4):113-6. [DOI: 10.1016/j.amsu.2014.09.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2020a
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395(10224):565-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2020b
- Lu R, Hwang Y, Liu I, Lee C, Tsai H, Li H, et al. Development of therapeutic antibodies for the treatment of diseases. Journal of Biomedical Science 2020;27(1):1. [DOI: 10.1186/s12929-019-0592-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Madhi 2021
- Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 vaccine against the B.1.351 variant. New England Journal of Medicine 2021;384:1885-98. [DOI: 10.1056/NEJMoa2102214] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansourabadi 2020
- Mansourabadi AH, Sadeghalvad M, Mohammadi-Motlagh H-R, Rezaei N. The immune system as a target for therapy of SARS-CoV-2: a systematic review of the current immunotherapies for COVID-19. Life Sciences 2020;258:118185. [DOI: 10.1016/j.lfs.2020.118185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marovich 2020
- Marovich M, Mascola JR, Cohen MS. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA 2020;324(2):131-2. [DOI] [PubMed] [Google Scholar]
Marshall 2020
- Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI: 10.1016/S1473-3099(20)30483-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marston 2018
- Marston HD, Paules CI, Fauci AS. Monoclonal antibodies for emerging infectious diseases - borrowing from history. New England Journal of Medicine 2018;378(16):1469-72. [DOI: 10.1056/NEJMp1802256] [DOI] [PubMed] [Google Scholar]
McCallum 2021
- McCallum M, Bassi J, De Marco A, Chen A, Walls AC, Di Iulio J, et al. SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern. Science 2021;-:eabi7994. [DOI: 10.1126/science.abi7994 Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGuinness 2020
Microsoft 2018 [Computer program]
- Mircosoft Excel. Microsoft Corporation. Microsoft Corporation, 2018. office.microsoft.com/excel.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI] [PubMed] [Google Scholar]
Monin‐Aldama 2021
- Monin-Aldama L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Molino del Barrio I, Alaguthurai T, et al. Interim results of the safety and immune-efficacy of 1 versus 2 doses of COVID-19 vaccine BNT162b2 for cancer patients in the context of the UK vaccine priority guidelines. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.03.17.21253131] [DOI]
Muik 2021
- Muik A, Wallisch AK, Saenger B, Swanson KA, Muehl J, Chen W, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science 2021;371(6534):1152-3. [DOI: 10.1126/science.abg6105] [DOI] [PMC free article] [PubMed] [Google Scholar]
National COVID‐19 Clinical Evidence Taskforce 2020
- National COVID-19 Clinical Evidence Taskforce. Caring for people with COVID-19: supporting Australia’s healthcare professionals with continually updated, evidence-based clinical guidelines. covid19evidence.net.au/#living-guidelines (accessed 7 December 2020).
NCT04427501
- NCT04427501. A randomized, double-blind, placebo-controlled, phase 2/3 study to evaluate the efficacy and safety of LY3819253 and LY3832479 in participants with mild to moderate COVID-19 illness. clinicaltrials.gov/ct2/show/NCT04427501 (first received 11 June 2020).
NIH 2020
- National Institutes of Health. COVID-19 treatment guidelines - therapeutic management of patients with COVID-19 (version last updated on 9 October 2020). www.covid19treatmentguidelines.nih.gov/therapeutic-management (accessed 6 November 2020).
NIH 2021
- National Institutes of Health. NIH-sponsored ACTIV-3 clinical trial closes enrollment into two sub-studies. Available from: https://www.nih.gov/news-events/news-releases/nih-sponsored-activ-3-clinical-trial-closes-enrollment-into-two-sub-studies (accessed 29.07.2021).
Ou 2020
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Communications 2020;11(1):1620. [DOI: 10.1038/s41467-020-15562-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesiss. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI: ] [DOI] [PubMed] [Google Scholar]
Piechotta 2020
- Piechotta V, Valk SJ, Chai KL, Wood EM, Lamikanra A, Kimber C, et al. Safety and effectiveness of convalescent plasma or hyperimmune globulin for people with COVID-19: a rapid review. osf.io/dwf53 2020. [DOI: 10.17605/OSF.IO/DWF53] [DOI] [PMC free article] [PubMed]
Piechotta 2021
- Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Planas 2021
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of infectious SARS-CoV-2 variant B.1.617.2 to monoclonal antibodies and sera from convalescent and vaccinated individuals. bioRxiv 2021. [DOI: 10.1101/2021.05.26.445838] [DOI]
RECOVERY 2020
- Randomised Evaluation of COVID-19 Therapy. www.recoverytrial.net (accessed 16 November 2020). [CT.GOV: NCT04381936]
REMAP‐CAP 2020
- A randomised, embedded, multi-factorial, adaptive platform trial for community-acquired pneumonia. www.remapcap.org (accessed 16 November 2020). [CT.GOV: NCT02735707]
RevMan Web 2020 [Computer program]
- Cochrane Collaboration Review Manager Web (RevMan Web). Version 1.22.0. Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Ryu 2021
- Ryu D-K, Song R, Kim M, Kim Y-I, Kim C, Kim J-I, et al. Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant. Biochemical and Biophysical Research Communications 2021;566:135-40. [PMID: 10.1016/j.bbrc.2021.06.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl A, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014] [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ,et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Skoetz 2020
- Skoetz N, Goldkuhle M, Van Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and Evidence Profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI: 10.1016/j.jclinepi.2019.10.015] [DOI] [PubMed] [Google Scholar]
Starr 2021
- Starr TN, Czudnochowski N, Zatta F, Park YJ, Liu Z, Addetia A, et al. Antibodies to the SARS-CoV-2 receptor-binding domain that maximize breadth and resistance to viral escape. bioRxiv [Preprint]. [DOI: 10.1101/2021.04.06.438709] [DOI]
Sterne 2019
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
Struyf 2020
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2020
- Tang JW, Tambyah PA, Hui DS. Emergence of a new SARS-CoV-2 variant in the UK. Journal of Infection 2020;S0163-4453(20):30786-6. [DOI: 10.1016/j.jinf.2020.12.024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tolouian 2020
- Tolouian R, Vahed SZ, Ghiyasvand S, Tolouian A, Ardalan M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. Journal of Renal Injury Prevention 2020;9(2):e19. [DOI: 10.34172/jrip.2020.19] [DOI] [Google Scholar]
Van de Veerdonk 2020a
- Van de Veerdonk F, Netea MG, Van Deuren M, Van der Meer JW, De Mast Q, Bruggemann R, et al. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. Preprints.org 2020. [DOI: 10.20944/preprints202004.0023.v1] [DOI]
Van de Veerdonk 2020b
- Van de Veerdonk FL, Netea MG, Van Deuren M, Van der Meer JW, De Mast Q, Brüggemann RJ, et al. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 2020;9:e57555. [DOI: 10.7554/eLife.57555] [DOI] [PMC free article] [PubMed] [Google Scholar]
Volz 2020
- Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Transmission of SARS-CoV-2 lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. medRxiv [Preprint]. [DOI: 10.1101/2020.12.30.20249034] [DOI]
Wang 2021
- Wang P, Casner RG, Nair MS, Wang M, Yu J, Cerutti G, et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. biorxiv [Preprint]. [DOI: 10.1101/2021.03.01.433466v2] [DOI] [PMC free article] [PubMed]
Wang 2021b
- Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021;593:130-5. [DOI: 10.1038/s41586-021-03398-2] [DOI] [PubMed] [Google Scholar]
WHO/Cochrane 2020
- WHO/Cochrane. COVID-NMA Initiative - a living mapping and living systematic review of COVID-19 trials. covid-nma.com (accessed 6 November 2020).
WHO 2007
- World Health Organization. Cumulative number of reported probable cases of SARS. www.who.int/csr/sars/country/2003_07_11/en (accessed 13 April 2020).
WHO 2019
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). www.who.int/emergencies/mers-cov/en (accessed 13 April 2020).
WHO 2020a
- World Health Organization. Report of the WHO‐China Joint Mission on coronavirus disease 2019 (COVID‐19). www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report (accessed 6 November 2020).
WHO 2020b
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. covid19.who.int (accessed 11 June 2020).
WHO 2020c
- World Health Organization. Estimating mortality from COVID-19 - scientific brief. www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 (accessed 2 November 2020).
WHO 2020d
- World Health Organization. SARS-CoV-2 mink-associated variant strain – Denmark. www.who.int/csr/don/06-november-2020-mink-associated-sars-cov2-denmark/en (accessed 9 November 2020).
WHO 2020e
- World Health Organization. Corticosteroids for COVID-19 - living guidance 2 September 2020. www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed 5 November 2020).
WHO 2020f
- World Health Organization. SARS-CoV-2 variants. www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ 2020. [PubMed]
WHO 2021a
- World Health Organisation. WHO validates Sinovac COVID-19 vaccine for emergency use and issues interim policy recommendations. https://www.who.int/news/item/01-06-2021-who-validates-sinovac-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations.
WHO 2021b
- World Health Organization. Weekly epidemiological update on COVID-19 - 27 April 2021. www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---27-april-2021 (accessed 3 May 2021).
WHO 2021c
- World Health Organization. COVID-19 Clinical management - living guidance. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (last accessed 08.07.2021) 2021. [WHO/2019-nCoV/clinical/2021.1]
Widera 2021
- Widera M, Wilhelm A, Hoehl S, Pallas C, Kohmer N, Wolf T, et al. Bamlanivimab does not neutralize two SARS-CoV-2 variants carrying E484K in vitro. medRxiv [Preprint] (accessed 16 March 2021).
Williamson 2020
- Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430-6. [DOI: 10.1038/s41586-020-2521-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2020
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. [DOI: 10.1001/jama.2020.2648] [DOI] [PubMed] [Google Scholar]
Yang 2020
- Yang L, Liu W, Yu X, Wu M, Reichert JM, Ho M. COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19. Antibody Therapeutics 2020;3(3):205-12. [DOI: 10.1093/abt/tbaa020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2021
- Zhou D, Wanwisa Dejnirattisai W, Supasa P, Liu C, Mentzer AJ, Ginn HM, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 2021;184(9):2348-61. [DOI: 10.1016/j.cell.2021.02.037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2020
- Zhu R, Gao Y, Robert S, Gao J, Yang S, Zhu C. Systematic review of the registered clinical trials for coronavirus disease 2019 (COVID-19). Journal of Translational Medicine 2020;18:274. [DOI: 10.1186/s12967-020-02442-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kreuzberger 2021
- Kreuzberger N, Hirsch C, Chai KL, Piechotta V, Valk SJ, Estcourt LJ, et al . SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013825. [DOI: 10.1002/14651858.CD013825] [DOI] [PMC free article] [PubMed] [Google Scholar]


