Abstract
Objective:
Sudden unexpected death in epilepsy (SUDEP) is an unpredictable and devastating comorbidity of epilepsy that is believed to be due to cardiorespiratory failure immediately after generalized convulsive seizures.
Methods:
We performed cardiorespiratory monitoring of seizure-induced death in mice carrying either a p.Arg1872Trp or p.Asn1768Asp mutation in a single Scn8a allele—mutations identified from patients who died from SUDEP—and of seizure-induced death in pentylenetetrazole-treated wild-type mice.
Results:
The primary cause of seizure-induced death for all mice was apnea, as (1) apnea began during a seizure and continued for tens of minutes until terminal asystole, and (2) death was prevented by mechanical ventilation. Fatal seizures always included a tonic phase that was coincident with apnea. This tonic phase apnea was not sufficient to produce death, as it also occurred during many nonfatal seizures; however, all seizures that were fatal had tonic phase apnea. We also made the novel observation that continuous tonic diaphragm contraction occurred during tonic phase apnea, which likely contributes to apnea by preventing exhalation, and this was only fatal when breathing did not resume after the tonic phase ended. Finally, recorded seizures from a patient with developmental epileptic encephalopathy with a previously undocumented SCN8A likely pathogenic variant (p.Leu257Val) revealed similarities to those of the mice, namely, an extended tonic phase that was accompanied by apnea.
Interpretation:
We conclude that apnea coincident with the tonic phase of a seizure, and subsequent failure to resume breathing, are the determining events that cause seizure-induced death in Scn8a mutant mice.
Sudden unexpected death in epilepsy (SUDEP) is defined as the sudden, unexpected, nontraumatic, nondrowning death of a person with epilepsy, which is not due to status epilepticus, nor does a postmortem examination reveal another cause of death.1 SUDEP is the most common cause of death associated with epilepsy, accounting for between 8 and 17% of all epilepsy-related deaths.2 This number increases to 50% for patients whose seizures are refractory to treatment.3,4
Although numerous mechanisms have been proposed for SUDEP,3,5,6 a growing body of evidence supports postictal respiratory dysfunction as the primary cause of death for many cases. Apnea and oxygen desaturation have been reported in a large percentage of patients during and after convulsive and nonconvulsive seizures,7–12 and in 9 cases of SUDEP with adequate postictal cardiorespiratory monitoring, terminal apnea occurred prior to terminal asystole.13 It is believed that most SUDEP cases occur after generalized convulsive seizures13–17; thus, mouse models of SUDEP include those in which death occurs immediately after convulsive seizures. In such models, including CacnalaS218L, Lmx1bf/f/p, and Scn1aR1407X transgenic mice, apnea has also preceded terminal asystole.18–21 Of these, only the Scn1aR1407X mice represent a patient population known to be susceptible to SUDEP (ie, Dravet syndrome).18,22
The current study utilizes 3 transgenic mouse lines carrying gain-of-function Scn8a mutations, which were identified in several patients with developmental epileptic encephalopathy (DEE) who died of SUDEP23,24: either the germline p.Asn1768Asp (referred to as “D/+ mice”) or conditional p.Arg1872Trp (referred to as “W/+ mice”). In the presence of Emx1-Cre, the conditional p.Arg1872Trp mutation is expressed in forebrain excitatory neurons (referred to as “W/+Emx1-Cre mice”) and in the presence of EIIA-Cre it is expressed globally (referred to as “W/+EIIA-Cre mice”).25 Phenotypically, expression of either mutation recapitulates clinical features seen in patients, including spontaneous tonic–clonic seizures and seizure-induced death.25–27 Prior to the current study, peri-ictal cardiorespiratory monitoring of these pertinent mouse models had not been performed. We utilized the natural epileptogenesis of the W/+Emx1-Cre mice to examine the semiology of spontaneous nonfatal and fatal seizures, the inducible fatal seizures of the W/+EIIA-Cre mice to examine a causal relationship between apnea and seizure-induced death, and the inducible nonfatal seizures of the D/+ mice to observe tonic diaphragmatic contraction during the tonic phase.
We demonstrate that (1) sudden death in the Scn8a mutant mice and pentylenetetrazole (PTZ)-treated mice occurs after convulsive seizures in which apnea occurred coincident with the tonic phase; (2) death can be prevented by mechanical ventilation; (3) tonic phase apnea is necessary, but not sufficient, to produce seizure-induced death; (4) tonic diaphragm contraction may contribute to the fatal apnea; and (5) seizures recorded from a DEE patient with a novel SCN8A mutation also presented with apnea during the tonic phase.
Materials and Methods
Mutant Mice
All mice were housed and cared for in accordance with NIH guidelines in a 12-hour light/dark cycle with food and water ad libitum, and all procedures were carried out at the University of Virginia and approved by the Animal Care and Use Committee. Male mice homozygous for the conditional R1872W Scn8a mutant allele25 (Fig 1A) were crossed with female mice homozygous for either Emx1-Cre (Jax 005628)28 or EIIA-Cre (Jax 003724)29 to produce mice heterozygous for both alleles, resulting in expression of the R1872W mutation in forebrain excitatory neurons (W/+Emx1-Cre) or globally (W/+EIIA-Cre; see Fig 1B). Mice with germline N1768D Scn8a mutant allele were also used.27 Genotyping was performed to confirm the presence of the R1872W and N1768D mutant alleles as described previously,25,27 and for Emx1-Cre and EIIA-Cre alleles as directed by the Jackson Laboratories website. Male and female W/+Emx1-Cre and wild-type (WT) mice were used with no difference in seizure phenotype. Due to the preweaning age of the W/+EIIA-Cre mice, sex was not recorded.
FIGURE 1:
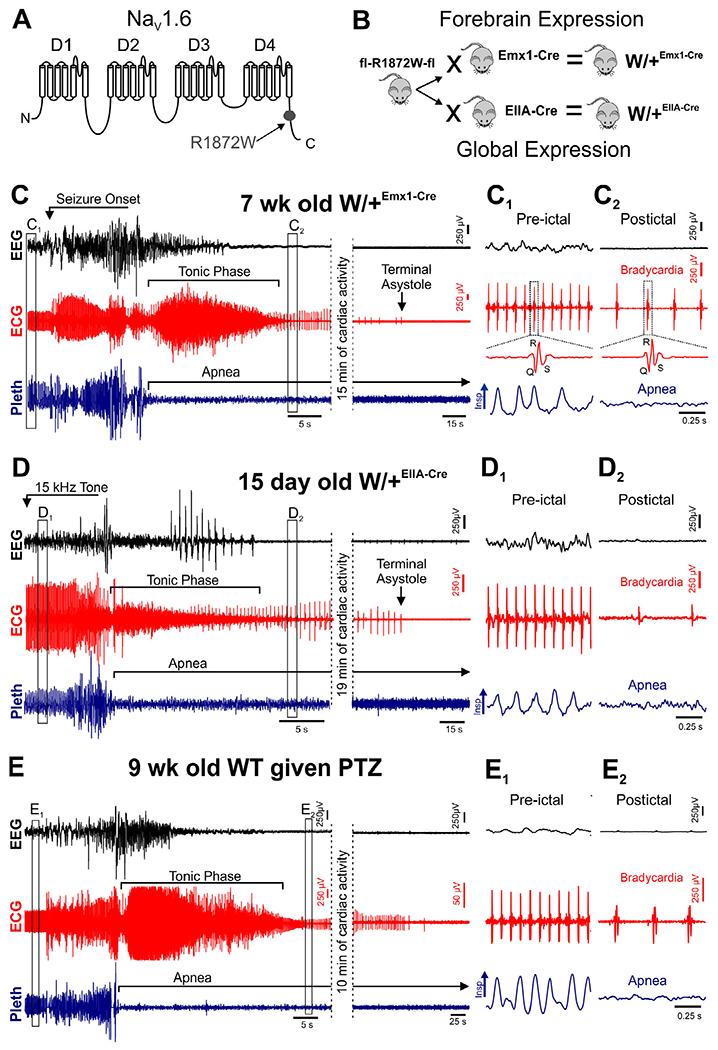
Seizure-induced death occurs when breathing does not resume after the tonic phase. (A) Diagram of SCN8A protein structure with location of R1872W mutation depicted. (B) Breeding scheme to generate W/+Emx1-Cre (top) and W/+EIIA-Cre (bottom) mice. Mice homozygous for the floxed R1872W mutant allele were bred with mice homozygous for Emx1-Cre or EIIA-Cre. The resulting offspring were heterozygous for both alleles. (C) Representative traces showing time from seizure onset to terminal asystole. (C–E) Recording of electroencephalogram (EEG), electrocardiogram (ECG), and plethysmogram (Pleth) during fatal spontaneous seizures of W/+Emx1-Cre mice (C), fatal audiogenic seizures of W/+EIIA-Cre mice (D), and fatal pentylenetetrazole (PTZ)-induced seizures of wild-type (WT) mice (E). (C1, C2) Expanded traces from corresponding boxes in C. (D1, D2) Expanded traces from corresponding boxes in D. (E1, E2) Expanded traces from corresponding boxes in E. Insp = inspiration.
Continuous Recording of Electroencephalogram, Electrocardiogram/Electromyogram, and Breathing
As previously reported, the W/+Emx1-Cre mice develop spontaneous tonic–clonic seizures at 4 weeks of age, and 75% succumb to seizure-induced death by 2 months.25 D/+ mice have a milder phenotype, with seizure onset at 8 to 16 weeks and only 50% lethality by 6 months.27 Custom headsets (Plastics One, Roanoke, VA) were implanted in 4- to 5-week-old W/+Emx1-Cre and 8- to 10-week-old D/+ mice using approved surgical techniques as previously described by our laboratories.18,25 Briefly, electroencephalographic (EEG) electrodes were placed over the parietal lobe unilaterally, and electrocardiographic (ECG) electrodes were placed in an approximate lead II configuration. In a subset of D/+ mice, ECG leads were replaced with leads that extended to contact the diaphragm, so as to record diaphragm electromyogram (EMG) as previously described.30 A reference electrode was placed over the cerebellum. Normal saline (8ml/kg, intraperitoneal [i.p.]) and ketoprofen (5mg/kg, i.p.) were given postoperatively. Mice were monitored, and additional ketoprofen was administered every 24 hours as needed. After recovery from surgery (minimum of 4 days), mice underwent continuous monitoring of EEG, ECG/EMG, and breathing in custom-designed plethysmography chambers that comply with guidelines for continuous housing described in the Guide for the Care and Use of Laboratory Animals,31 and as described previously.18 The same techniques were used for recording seizures from W/+EIIA-Cre mice at postnatal age 15 days. However, because W/+EIIA-Cre mice were used prior to weaning, due to their early lethality, they could only be allowed 24 hours to recover from surgery, because degradation of ECG signals was common due to parental grooming.
Audiogenic seizures were induced in W/+EIIA-Cre and D/+ mice with a 15kHz pure tone (~80–100dB), generated using Tone Generator software (NCH Software, Canberra, Australia), amplified with a Kinter K3118 stereo amplifier (Kinter USA, Amazon.com), and converted to sound using a small 3W speaker lowered into the plethysmography chamber. For mechanical ventilation experiments, a sonicator (Branson 200 ultrasonic cleaner) was placed next to the mouse cage and turned on to induce audiogenic seizures (see Video S5).
PTZ-induced seizures were generated by i.p. injection of 90mg/kg PTZ, which resulted in 100% lethality, as previously reported.32,33 PTZ (Sigma-Aldrich, St Louis, MO) was dissolved in normal sterile saline on the day of administration.
SCN8A Patient Video-EEG Monitoring and Breathing Analysis
Human studies were approved by the University of Virginia Institutional Review Board and performed under supervision of a faculty neurologist (H.P.G.). A patient with a pathogenic variant of SCN8A was admitted to the University of Virginia Medical Center (Charlottesville, VA) for continuous video-EEG scalp recordings (Natus, Pleasanton, CA) with electrodes placed according to the 10–20 system. Breathing movement was visualized using phase-based Eulerian video magnification motion processing with open-source software (http://people.csail.mit.edu/mrub/vidmag/) written for MATLAB (MathWorks, Natick, MA) as done previously.18
Statistical Analysis and Blinding
Because only mice expressing Scn8a mutations were used in this study, blinding to genotype was not necessary. For the quantification in Figure 5, measurements of tonic phase and apnea were done independent of one another. Quantification reported in Figure 7 was done with the researcher blinded to seizure outcome (ie, fatal or nonfatal). All statistics were performed using Prism 8 (GraphPad Software, San Diego, CA), presented as mean ± standard error of the mean, and defined as statistically detectable when p < 0.05. Comparison of survival proportions in Figures 2 and 4 was done using a 1-sided Fisher exact test. Comparisons between 2 groups were assessed by unpaired, 2-tailed Student t-test when residuals passed the D’Agnostino-Pearson Omnibus normality test and by the nonparametric Mann–Whitney test when residuals did not pass the D’Agnostino-Pearson Omnibus normality test. Comparisons between more than 2 groups were assessed by 1-way analysis of variance followed by Dunnett multiple comparison test when residuals passed the D’Agnostino-Pearson Omnibus normality test and by the nonparametric Kruskal–Wallis test for those that did not pass the D’Agnostino-Pearson Omnibus normality test. Details for statistical comparisons that are graphically depicted in figures are described in the figure legends. For comparisons only mentioned in the results section, statistics are reported in line.
FIGURE 5:
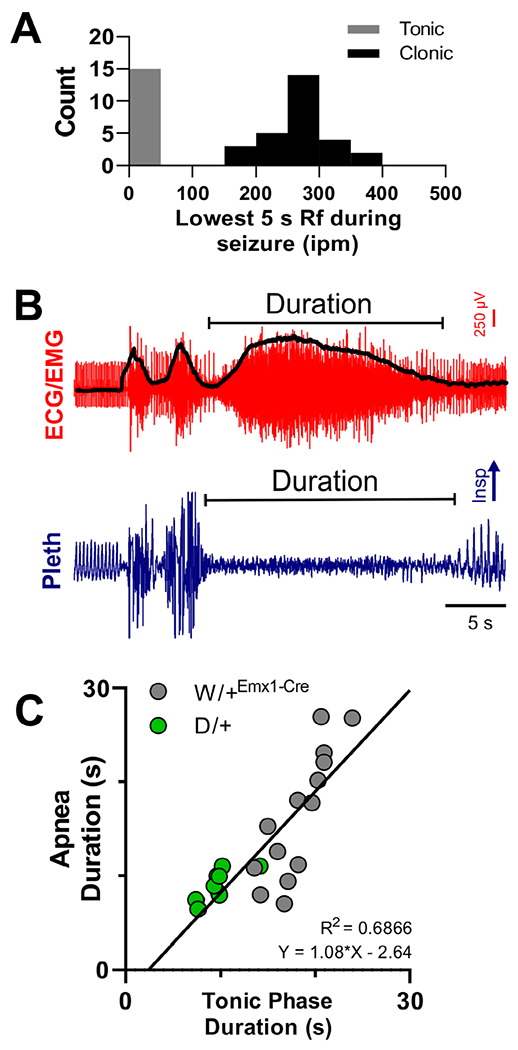
The tonic phase is coincident with apnea. (A) Histogram of the nadir of breathing rate for all nonfatal seizures. Breathing rate nadir fell into 2 distinct groups; tonic and clonic, with low (near zero) and high respiratory frequency (Rf), respectively. (B) Depiction of measurement of tonic phase and apnea delay and duration made from all nonfatal tonic seizures. Delay was measured from the seizure onset (ie, the first spike–wave) to apnea or tonic phase start (spike–wave/electroencephalographic [EEG] data not shown in image). The black line superimposed on the electrocardiogram (ECG)/electromyogram (EMG) trace is the same signal after rectification and smoothing. Lower points on the black line were used to determine the start and end of the tonic phase. (C) The duration of apnea is directly proportional to the duration of the tonic phase itself (R2 = 0.6886; n = 14 and 9 for W/+Emx1-Cre and D/+ mice, respectively). Insp = inspiration; ipm = inspirations per minute; Pleth = plethysmogram.
FIGURE 7:
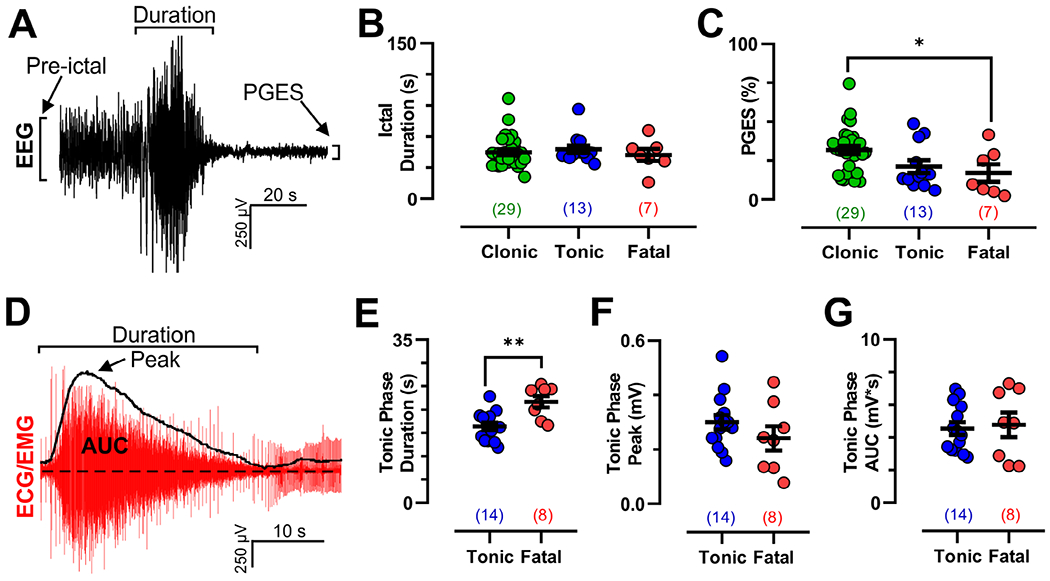
Electroencephalographic (EEG) and electromyographic (EMG) dynamics are similar for fatal and nonfatal tonic seizures in W/+Emx1-Cre mice. (A) Depiction of seizure duration, preictal amplitude, and postictal generalized EEG suppression (PGES) amplitude measurements from a peri-ictal EEG trace. (B) Seizure duration was no different across the 3 observed seizure types (Kruskal–Wallis test, K = 1.166, p = 0.5583). (C) PGES amplitude (as a fraction of preictal amplitude) was smaller for fatal compared to clonic seizures; however, there was no difference between tonic and fatal seizures (p = 0.0373 by Dunnett multiple comparison test after significant 1-way analysis of variance, F2,45 = 4.065, p = 0.0238). (D) Depiction of tonic phase duration, peak amplitude, and area under the curve (AUC) measurement from a peri-ictal electrocardiogram (ECG)/EMG trace from a tonic seizure. (E–G) Tonic phase duration was longer for fatal seizures (p = 0.0013, T = 3.729, df = 20, unpaired t test); however, there was no detectable difference in peak (p = 0.2407, T = 1.209, df = 20, unpaired t test) or AUC EMG activity (p = 0.7583, T = 0.3120, df = 20, unpaired t test). *p < 0.05, **p < 0.01. Values in parentheses indicate number of seizures analyzed in that dataset.
FIGURE 2:
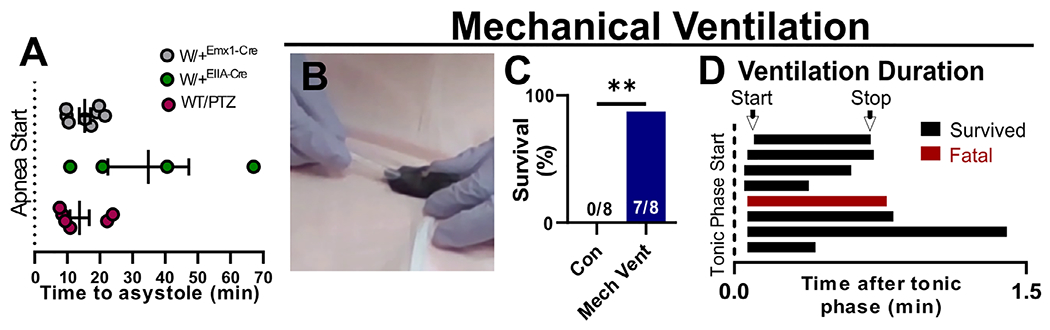
Apnea is the determining cause of seizure-induced death. (A) In the event of seizure-induced death, terminal apnea preceded terminal asystole in all mouse models. (B, C) Mechanical ventilation significantly increased survival of audiogenic seizures for W/+EIIA-Cre mice (**p = 0.0205, 2-sided chi-squared = 5.367). (D) For each mouse, ventilation was initiated within 6 seconds of the beginning of the tonic phase and was continued for 21 to 80 seconds. Con = control; PTZ = pentylenetetrazole; WT = wild type.
FIGURE 4:
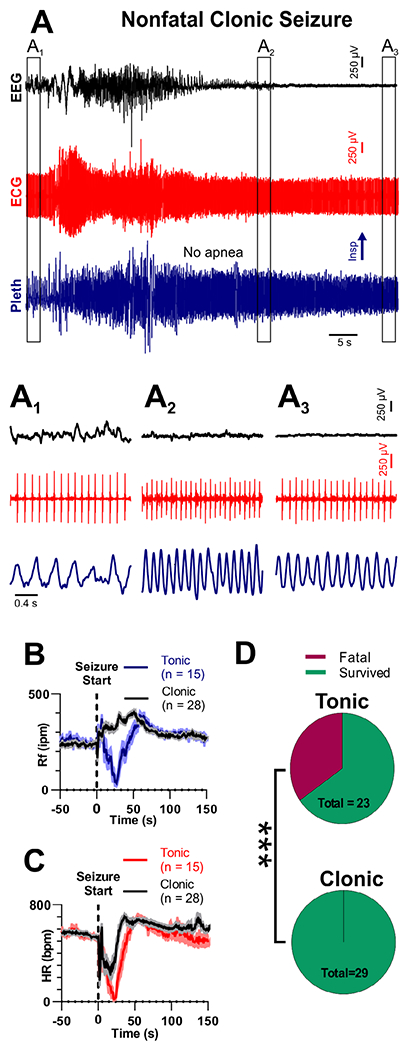
Clonic seizures do not produce death or apnea. (A) Recording of electroencephalogram (EEG), electrocardiogram (ECG), and plethysmogram (Pleth) during a clonic seizure in a W/+Emx1-Cre mouse. (A1–A3) Expanded views of boxes in A, depicting EEG, ECG, and breathing prior to, during, after the seizure. (B) Respiratory frequency (Rf) and (C) heart rate (HR) binned every second from 50 seconds before to 150 seconds after seizure initiation for tonic and clonic seizures. (D) Pie charts show tonic seizures are more likely to cause fatality than clonic seizures (***p = 0.007, 2-sided Fisher exact test). For B and C, solid lines represent mean, and shaded regions represent standard error of the mean. bpm = beats per minute; Insp = inspiration; ipm = inspirations per minute.
Results
Monitoring Seizures and Sudden Death in Scn8a Mutant Mice
Eight W/+Emx1-Cre mice were monitored for ECG, cortical EEG, and whole-body plethysmograph, which captured 44 nonfatal and 8 fatal seizures. Only seizures qualifying as R5 or above (ie, R5–R832,33) on a modified Racine scale were analyzed. These showed rearing and falling, tailraising, clonic and/or tonic convulsions, and increased ictal EEG amplitude followed by postictal generalized EEG suppression (PGES). Seizures were always separated by at least 1 hour, and never qualified as status epilepticus. Three subcategories of R5 or greater seizures were identified in the W/+Emx1-Cre mice: fatal tonic seizures (ie, seizure-induced death; Video S1), nonfatal tonic seizures (Video S2), and nonfatal clonic seizures (Video S3). Fatal tonic seizures were recorded from 8 W/+Emx1-Cre mice. A total of 44 spontaneous nonfatal seizures were recorded from 6 of the 8 W/+Emx1-Cre mice, of which 15 were tonic and 29 were clonic.
Four W/+EIIA-Cre mice were monitored for ECG, EEG, and plethysmography during audiogenic seizure-induced death. We found that high-frequency sound routinely caused seizures in W/+EIIA-Cre mice at 14-18 days of age. In all cases, these seizures resulted in sudden death with the same sequence of events as those experienced by W/+Emx1-Cre mice during spontaneous seizures. All W/+EIIA-Cre mice had convulsive seizures that included wild running followed by a tonic phase and death. The audio stimulus never induced observable seizure behavior in W/+Emx1-Cre or WT mice (data not shown).
To compare results obtained from the Scn8a mutant mice to a more widely used model of seizure-induced death, we monitored 7 WT (C57/B6J) mice for ECG, EEG, and plethysmography immediately before and after i.p. injection of 90mg/kg PTZ. All mice died from convulsive seizures with similar semiology to the fatal seizures of the Scn8a mutant mice, which included wild running followed by a tonic phase and death (Video S4).
Finally, 3 D/+ mice were monitored for ECG, EEG, and plethysmography during 14 spontaneously occurring nonfatal tonic seizures. Three additional D/+ mice had EEG, plethysmography, and diaphragm EMG measured (as previously described30) during audiogenic nonfatal tonic seizures. As per our previous report, both spontaneous and audiogenic seizures in D/+ mice were characterized by wild running followed by a tonic phase and recovery (manuscript under review).
Apnea Causes Sudden Death in Scn8a Mutant Mice
In all cases of sudden death, breathing was absent during the tonic phase of a convulsive seizure and never resumed (see Fig 1C–E). During and after the fatal seizure, cardiac activity continued, albeit at a lower rate (see Fig 1C2, D2, and E2). For W/+Emx1-Cre, W/+EIIA-Cre, and PTZ-treated WT mice, terminal asystole followed breathing cessation by 15.4 ± 1.7, 34.4 ± 12.4, and 13.8 ± 3.0 minutes, respectively (Fig 2A). Thus, the sequence of events did not differ when the Scn8a mutation was expressed globally (W/+EIIA-Cre mice) or selectively in forebrain neurons (W/+Emx1-Cre mice), or exogenously induced in otherwise genetically WT mice (ie, PTZ-treated WT mice).
Based on these observations, we hypothesized that apnea was the primary cause of death. To directly test this hypothesis, we induced audiogenic seizures in W/+EIIA-Cre mice that routinely resulted in death (0/8 survived with no intervention; Video S5, left side). When we induced audiogenic seizures in W/+EIIA-Cre mice and performed mechanical ventilation using a transfer pipet adapted to fit snugly around the nose and mouth (see Fig 2B; Video S5, right side), survival markedly improved (7/8 survived when ventilated; see Fig 2C). Ventilation was initiated within 6 seconds after the start of the tonic phase (see Fig 2D) and continued until the mouse began breathing on its own. The one mouse that did not survive was ventilated for 43 seconds before stopping due to lack of recovery. Because another mouse that survived was ventilated for 80 seconds, the single fatality may have resulted from premature termination of mechanical ventilation.
Tonic Phase Apnea Is Not Sufficient for Seizure-Induced Death
Nonfatal tonic seizures in W/+Emx1-Cre mice appeared nearly identical to fatal seizures, except that breathing resumed immediately postictally (compare Fig 3A to Fig 1C, D; Videos S1 and S2). Nonfatal seizures had a pronounced tonic phase, concurrent with apnea and bradycardia (see Fig 3). Following the tonic phase of nonfatal seizures, mice experienced tachycardia and tachypnea, which were different from the bradycardia and apnea observed after fatal tonic seizures. Our analysis likely underestimates heart rate during the tonic phase due to EMG activity obscuring the QRS complexes. However, at the end of the tonic phase, when QRS complexes were distinguishable from the EMG signal, heart rate was clearly reduced. Interestingly, there were no mice that had intermediate responses between complete and permanent respiratory failure, and rapid recovery. The mechanisms for conversion from reversible apnea to terminal apnea are unknown.
FIGURE 3:
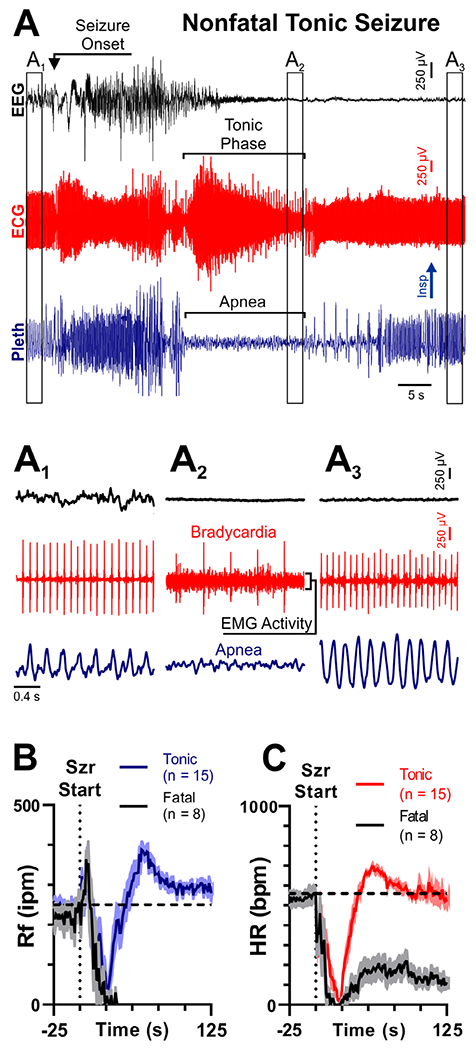
Transient apnea and bradycardia also occur during nonfatal tonic seizures. (A) Recording of electroencephalogram (EEG), electrocardiogram (ECG), and plethysmogram (Pleth) during a convulsive seizure in a W/+Emx1-Cre mouse. (A1–A3) Expanded views of traces from A, depicting EEG, ECG, and breathing at different time points. In A2, note the bradycardia, electromyographic (EMG) infiltration of the ECG, and apnea. (B, C) Respiratory frequency (Rf) and heart rate (HR) binned every second from 50 seconds before to 150 seconds after seizure (Szr) initiation for all tonic (red/blue) and fatal (black) seizures. For B and C, solid lines represent mean, and shaded regions represent standard error of the mean. Note that in nonfatal seizures, both breathing and HR increase beyond resting levels immediately after the seizure. bpm = beats per minute; Insp = inspiration; ipm = inspirations per minute.
Tonic Phase Apnea Is Necessary for Seizure-Induced Death
Clonic seizures were similar to tonic seizures with respect to EEG activity and most behavioral semiology (compare Fig 4A to Fig 3A; Videos S2 and S3). However, instead of a tonic phase denoted by hindlimb extension, clonic seizures had repetitive clonic movements in the upper extremities, visible in the ECG signal as small repetitive bursts of EMG activity (see Fig 4). Instead of apnea, tachypnea occurred during clonic seizures, with the minimum respiratory frequency (Rf) during clonic seizures being detectably higher than for tonic seizures (329 ± 12 and 75 ± 27 inspirations per minute [ipm], respectively; p < 0.0001, Mann–Whitney test). Both tonic and clonic seizures presented with bradycardia, which did not differ statistically (70 ± 21 vs 279 ± 60 beats per minute [bpm], respectively; p = 0.1244, Mann–Whitney test). Although clonic seizures represented a significant proportion of nonfatal seizures (29/44), no clonic seizure was fatal, and the probability of mortality was higher for tonic compared to clonic seizures.
Tonic Diaphragm Contraction Occurs during Tonic Phase Apnea
In W/+Emx1-Cre mice, ictal apnea is coincident with the tonic phase. Apnea did not occur during clonic seizures. Analysis of the minimum Rf for all nonfatal seizures from W/+Emx1-Cre mice (n = 43 seizures) identified 2 distinct groups; those with breathing rates of <100 or >100 ipm, corresponding to tonic and clonic seizures, respectively (Fig 5A). We hypothesized that tonic phase and apnea share an underlying mechanism, and thus a longer tonic phase should be correlated with longer apnea. We compared duration of the tonic phase and apnea, with data obtained from spontaneous tonic seizures recorded from D/+ mice included, as they present with the same behavioral and electrographic signatures, but with shorter apnea than W/+Emx1-Cre mice (9.0 ± 0.5 s and 16.4 ± 1.8 s for D/+ and W/+Emx1-Cre mice, respectively; p = 0.0045; unpaired t test, t = 3.182, df = 21). This allowed us to assess the relationship of tonic phase and apnea duration over a wider range. To estimate tonic phase duration, we rectified and smoothed the ECG/EMG signal and identified the lowest points preceding and following the tonic extension phase (see Fig 5B, black trace on top). Across all apneic seizures of the 2 mouse models, apnea duration was directly proportional to tonic phase duration (see Fig 5C; R2 = 0.6866). It is important to note that wild running occurs immediately prior to the tonic phase. The running produces noise in the plethysmography signal, making it impossible to know whether there is breathing during this short period. For this and other reasons, our determination of the time of onset of breathing cessation is imprecise, and it is possible that apnea starts prior to the tonic phase in some cases.
We hypothesized that tonic muscle contraction prolongs apnea. To test this, we recorded diaphragm muscle activity (diaEMG; as recently described30) during audiogenic seizures in D/+ mice. Our diaEMG was reliably synchronized with inspiratory activity recorded by plethysmography (Fig 6A). When we stimulated an audiogenic seizure, we observed a large amount of tonic diaEMG activity during the tonic phase (see Fig 6B). This observation was replicated in a total of 8 audiogenic seizures recorded from 3 D/+ mice (see Fig 6C).
FIGURE 6:
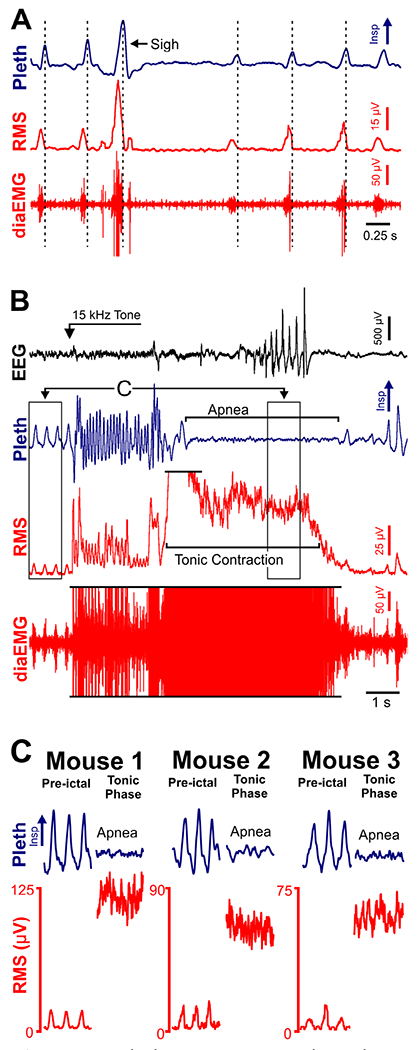
Tonic diaphragm activity is coincident with tonic phase apnea. (A) Plethysmogram (Pleth), raw diaphragm electromyogram (diaEMG), and root mean square amplitude of diaEMG (RMS) recorded from a D/+ mouse. (B) Electroencephalogram (EEG), Pleth, diaEMG, and RMS recorded during an audiogenic seizure from the same D/+ mouse as in A. Some of the diaEMG signal is clipped (indicated by black horizontal lines) to better depict lower amplitude, preictal signal. (C) Left, expanded 1-s traces from preictal and tonic phase time points indicated in B. Note inspiratory motor oscillation occurs during the preictal period and tonic contraction during the tonic phase. In middle and right panels, the same observation was made during seizures in 2 additional D/+ mice. Insp = inspiration.
Comparison of Fatal and Nonfatal Seizure Dynamics
The ictal duration and amplitude of PGES were compared between clonic, tonic, and fatal seizures from W/+Emx1-Cre mice (Fig 7A). There was no difference in ictal duration among the 3 seizure types (see Fig 7B). PGES, defined as the lowest EEG amplitude within 30 seconds of seizure termination as a percentage of preictal level, was detectably greater for fatal compared to clonic seizures, but was not detectably different between nonfatal tonic and fatal tonic seizures (see Fig 7C).
Duration, peak, and area under the curve (AUC) of EMG tonic activity were analyzed for tonic nonfatal and fatal seizures in W/+Emx1-Cre mice (see Fig 7D). Duration of tonic phase was longer for fatal seizures compared to nonfatal tonic seizures (see Fig 7E); however, no detectable differences in peak or AUC EMG activity were identified between the seizure types (see Fig 7F, G).
Tonic Phase Apnea in a Patient with SCN8A Epilepsy
An 18-month-old male patient was monitored for seizure characterization in the epilepsy-monitoring unit (EMU) at the University of Virginia Health System. Exome sequencing detected the heterozygous likely pathogenic variant p. Leu257Val (L257V) located in the pore-lining transmembrane segment S5 in domain 1 of Nav1.6 (Fig 8A). The corresponding leucine residue is conserved in the 3 paralogous human neuronal sodium channels as well as vertebrate and invertebrate homologs (see Fig 8B). Val257 is not present in the gnomAD database of 120,000 control exome sequences lacking early onset neurological disorders. Prediction software rates the L257V substitution as possibly detrimental (PolyPhen-2 score = 0.9) or damaging (Sorting Intolerant from Tolerant score = 0.037), with a high Combined Annotation Dependent Depletion score of 25.4, indicative of deleterious effects.
FIGURE 8:
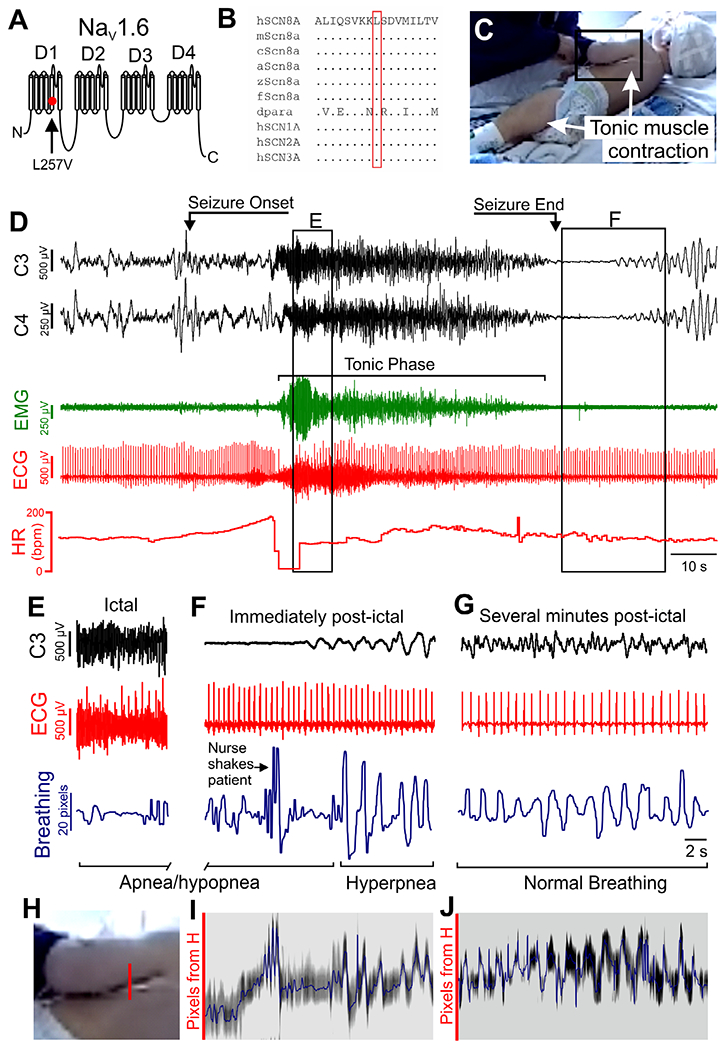
Apnea and bradycardia occur during tonic seizures in a patient with SCN8A epilepsy. (A) Diagram of SCN8A gene product (Nav1.6) structure, with location of L257V mutation depicted. (B) Evolutionary conservation of residue L257 in multiple species. a = anole; c = chicken; dpara = drosophila “paralytic”; f = fugu; h = human; m = mouse; z = zebrafish. Amino acids are indicated by the single letter code; dots represent identical to the human amino acid. (C) Image of 18-month-old patient during tonic phase of seizure. Note the tonic muscle contraction apparent in the quadricep and latissimus muscles (arrows). (D) Recording of C3 and C4 electroencephalographic (EEG), electromyographic (EMG), and electrocardiographic (ECG) leads during a convulsive seizure. See Video S6 for full video of the seizure. This seizure has a pronounced tonic phase, as indicated by elevated EMG activity (green signal). bpm = beats per minute; HR = heart rate. (E) At the beginning of tonic phase of the seizure, there was apparent apnea/hypopnea and bradycardia (box E in panel D). Signals shown are EEG, ECG, and breathing assessed with Eulerian video magnification analysis of breathing (blue trace, see below for description of analysis). (F) The same analysis at the end of the seizure showing a period of apnea/hypopnea followed immediately by hyperpnea when breathing recovers (box F in panel D). (G) Breathing analysis several minutes after the seizure, depicting normal, eupneic breathing in addition to normal ictal activity and ECG. (H) Expanded image of region in black box in C depicts column of pixels (red line segment) that were analyzed for breathing movements. (I, J) Grayscale time series of pixels of location depicted in H at the end of apnea (I) and several minutes postictal (J). The blue line corresponds to the midpoint of the band of lowest pixel intensity (binned over 5 pixels). Corresponding signals in F and G were bandpass filtered between 1 and 5Hz.
During EMU monitoring, a 12-minute cluster of 3 electroclinical seizures was recorded, and reviewed by a neurologist (H.P.G.). Each seizure in the cluster had a pronounced tonic phase, during which breathing motions were either absent or markedly diminished. The position of the patient during the first seizure provided clear visibility of the left side of the body, permitting video analysis of breathing movements (see Fig 8C, Video S6). This seizure lasted approximately 80 seconds, of which 60 seconds involved pronounced tonic muscle contraction (see Fig 8D), as indicated by the presence of a large amount of EMG activity in the ECG signal. The patient had tachycardia upon seizure onset followed by bradycardia at the tonic phase onset (see Fig 8D, bottom trace, heart rate).
To assess respiratory frequency and pattern, visualization of respiratory movements was aided by Eulerian video magnification34 as described previously.18 Dark pixels between the patient’s left arm and body were plotted over time to approximate breathing motions (see Fig 8). We were able to reliably analyze breathing with this method during 2 time periods during the seizure when view of the patient was not obstructed by caregivers. During the first period, occurring just after the start of the tonic phase, there were minimal breathing movements, clear tonic muscle contraction, and bradycardia. The second period occurred at the end of the seizure, with minimal breathing motions for >10 seconds prior to clear hyperpnea as breathing resumed (see Fig 8F; Video S6, apnea/hypopnea begins at 0:29 and hyperpnea begins at 1:26). These 2 periods of breathing analysis were different from the eupneic breathing observed several minutes after the seizure when PGES had subsided (see Fig 8G). Fourteen breaths were identified during this 26-second period, resulting in 32 breaths per minute, which is within the normal breathing frequency range for a child of 18 months (29–48 breaths per minute for the 10th and 90th percentiles, respectively).35
Discussion
In the present study, we characterize tonic phase apnea and demonstrate its role in spontaneous and evoked seizure-induced deaths using 3 mouse models of SCN8A epilepsy. Recordings of audiogenic seizure-induced deaths from W/+EIIA-Cre mice allowed us to identify breathing cessation as the primary cause of death. Chronic recording of W/+Emx1-Cre captured spontaneous nonfatal and fatal seizures, which demonstrate that tonic phase apnea is necessary but not sufficient for seizure-induced death. Death only occurred when the animal did not recover from postictal apnea. Recording of diaphragm EMG during audiogenic seizures of D/+ mice allowed us to determine that tonic phase apnea is coincident with tonic respiratory muscle contraction. Finally, data acquired from a patient with a novel SCN8A mutation demonstrates that tonic phase apnea can also occur in patients with SCN8A epilepsy.
Tonic Phase Apnea
In Scn8a mutant and PTZ-treated WT mice, a tonic phase was present in each case in which apnea and death occurred. We further show that tonic phase apnea occurs in a patient with a novel SCN8A mutated allele. This is consistent with the limited clinical literature assessing respiratory function during tonic muscle contractions that occur during seizures.36 Tonic phase apnea is likely common to many mouse models of epilepsy. Specifically, mouse models of Dravet syndrome18,37 and Kcna1−/−38 can demonstrate some apneas coinciding with elevated tonic EMG activity.
Our findings suggest that tonic phase apnea occurs, at least in part, due to tonic contraction of muscles involved in inspiration (ie, the diaphragm), which prevents the normal rhythmic contraction needed for alveolar ventilation. Although this concept is described in neurology textbooks,39 relevant breathing muscles have not been recorded during tonic seizures in humans or mice. Although tonic diaphragm contraction alone could produce an inspiratory breath hold, effectively an apnea, it should be noted that other respiratory muscles could be affected, including those that could cause airway obstruction, as has been reported for seizures induced with kainic acid in anesthetized rats.40
It is important to note that breathing did not resume immediately upon termination of diaphragm contraction for nonfatal seizures. In addition, wild running creates noise in the plethysmography signal immediately before the tonic phase begins, obscuring our ability to determine precise timing of apnea initiation. It is possible, even probable, that apnea is initiated before the tonic phase in some seizures. These periods of apnea outside the tonic phase indicate that mechanisms of central apnea independent of diaphragm contraction must also exist. One such mechanism could be inhibition of brain stem respiratory neural circuitry, as is observed by stimulation of the amygdala.41,42 Whatever the mechanism(s) of apnea, our evidence demonstrates a close temporal relationship between the tonic phase and apnea in both the Scn8a mutant and PTZ-treated mice; however, the directionality of this relationship is not clear. It is possible that apnea causes tonic contraction or that an unknown third process produces both effects. Because it is currently impossible to selectively suppress the tonic phase, it is not possible to determine the directionality of this relationship.
Apnea Is the Primary Cause of Death for Scn8a Mutant Mice
The MORTEMUS study is the most extensive compilation of cardiorespiratory recording during clinical SUDEP events to date. In 9 patients with adequate cardiorespiratory monitoring, terminal apnea occurred before terminal asystole.13 This sequence of events was replicated by both the spontaneous seizure-induced deaths of W/+Emx1-Cre mice and the audiogenic seizure-induced deaths of W/+EIIA-Cre mice; terminal apnea preceded terminal asystole by tens of minutes. Our interpretation is that apnea resulted in anoxia during and after the seizure, which eventually caused cardiac failure. This is supported by our report that WT mice exposed to anoxia succumb to cardiac failure on a similar time scale.18 In the present study, we were further able to demonstrate a causal relationship between apnea and sudden death by utilizing the audiogenic seizure-induced death of W/+EIIA-Cre mice. These audiogenic seizures were routinely fatal; however, mechanical ventilation greatly increased survival, underscoring the importance of apnea for seizure-induced death in this model.
Tonic Phase Apnea Always Occurs with Seizure-Induced Death in Scn8a Mutant Mice and Other Models of SUDEP
All observed instances of seizure-induced death of Scn8a mutant mice and PTZ-treated WT mice were preceded by seizures with a tonic phase, denoted by hindlimb extension, and coincident apnea. A number of SUDEP mouse models, including Scn1aR1407X, DBA1/2, Kcna1−/−, 129/SvTer, and Cacna1aS218L mice, experience fatal seizures that are classified as tonic, generally based on the observation of hindlimb extension.18–20,38, 43–45 However, in many studies of seizure-induced death, seizure-type is not specified, and we have observed a subset of fatal heat-induced seizures of Scn1aR1407X mice that were clonic.18 Thus, the relative predominance of tonic phase apnea during seizure-induced death across other epilepsy models remains to be determined. It is well known that ictal apnea does occur in many patients with nonconvulsive seizures,8–11 so the same may be true of mouse models.
Tonic Phase Apnea Is Not Sufficient to Produce Seizure-Induced Death in Scn8a Mutant Mice
Although all cases of seizure-induced death in the Scn8a mutant mice were preceded by a tonic seizure, there were many occurrences of tonic phase apnea in the W/+Emx1-Cre mice that were not fatal. Thus, tonic phase apnea appears necessary but not sufficient for death to occur in the Scn8a and PTZ models used in this study. For the W/+Emx1-Cre mice, recovery from tonic phase apnea was an all or none phenomenon; either breathing rapidly and fully recovered within seconds of tonic phase termination, or it failed completely to recover and death followed. There were no examples of partial or slow recovery. This suggests that a secondary central process responsible for breathing regulation must also fail in order to produce seizure-induced death. It is not unexpected that mechanisms of seizure-induced death and SUDEP will be multifactorial, and different SUDEP mechanisms may exist for different epilepsy etiologies. Other mechanisms that may inhibit recovery from tonic phase apnea include spreading depolarization to the brain stem, impaired homeostatic breathing (ie, central apnea), and cardiovascular dysfunction.2,46–48 Cardiac dysfunction appears to vary considerably in different mouse SUDEP models. For example, in W/+Emx1-Cre mice, tonic seizures induce transient asystole that occurs as rapidly as apnea (see Fig 3B, C), whereas in Scn1aR1407X mice, fatal seizures are associated with rapid, irreversible apnea, but with much slower development of bradycardia.18 Thus, future investigation should focus on mechanisms of tonic phase apnea, and uncovering the coexisting factors that impede breathing recovery after the tonic phase.
Supplementary Material
Acknowledgments
This work was supported by NIH National Institute of Neurological Disorders and Stroke grants R01 NS103090, R01 NS034509, U01 NS090414, and F31 NS110333, the Wishes for Elliott Foundation, and Citizens United for Research in Epilepsy.
Footnotes
Additional supporting information can be found in the online version of this article.
Potential Conflicts of Interest
Nothing to report.
References
- 1.Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012;53:227–233. [DOI] [PubMed] [Google Scholar]
- 2.Terra VC, Cysneiros R, Cavalheiro EA, Scorza FA. Sudden unexpected death in epilepsy: from the lab to the clinic setting. Epilepsy Behav 2013;26:415–420. [DOI] [PubMed] [Google Scholar]
- 3.Tolstykh GP, Cavazos JE. Potential mechanisms of sudden unexpected death in epilepsy. Epilepsy Behav 2013;26:410–414. [DOI] [PubMed] [Google Scholar]
- 4.Devinsky O Sudden, unexpected death in epilepsy. N Engl J Med 2011;365:1801–1811. [DOI] [PubMed] [Google Scholar]
- 5.Surges R, Henneberger C, Adjei P, et al. Do alterations in inter-ictal heart rate variability predict sudden unexpected death in epilepsy? Epilepsy Res 2009;87:277–280. [DOI] [PubMed] [Google Scholar]
- 6.Kang JY, Rabiei AH, Myint L, Nei M. Equivocal significance of postictal generalized EEG suppression as a marker of SUDEP risk. Seizure 2017;48:28–32. [DOI] [PubMed] [Google Scholar]
- 7.Vilella L, Lacuey N, Hampson JP, et al. Postconvulsive central apnea as a biomarker for sudden unexpected death in epilepsy (SUDEP). Neurology 2019;92:e171–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilella L, Lacuey N, Hampson JP, et al. Incidence, recurrence, and risk factors for peri-ictal central apnea and sudden unexpected death in epilepsy. Front Neurol 2019;10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacuey N, Zonjy B, Hampson JP, et al. The incidence and significance of periictal apnea in epileptic seizures. Epilepsia 2018;59: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bateman LM, Li C-S, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain 2008; 131:3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nashef L, Walker F, Allen P, et al. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry 1996;60:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacuey N, Hampson JP, Theeranaew W, et al. Cortical structures associated with human blood pressure control. JAMA Neurol 2018; 75:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson L, Farahmand BY, Persson P-G, et al. Risk factors for sudden unexpected death in epilepsy: a case control study. Lancet 1999; 353:888–893. [DOI] [PubMed] [Google Scholar]
- 15.Bird JM, Dembny KAT, Sandeman D, Butler S. Sudden unexplained death in epilepsy: an intracranially monitored case. Epilepsia 1997; 38:S52–S56.9092961 [Google Scholar]
- 16.Dasheiff RM, Dickinson LJ. Sudden unexpected death of epileptic patient due to cardiac arrhythmia after seizure. Arch Neurol 1986;43: 194–196. [DOI] [PubMed] [Google Scholar]
- 17.So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia 2000;41: 1494–1497. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y, Bravo E, Thirnbeck CK, et al. Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest 2018;128: 1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loonen ICM, Jansen NA, Cain SM, et al. Brainstem spreading depolarization and cortical dynamics during fatal seizures in Cacna1a S218L mice. Brain 2019;142:412–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen NA, Schenke M, Voskuyl RA, et al. Apnea associated with brainstem seizures in Cacna1aS218L mice is caused by medullary spreading depolarization. J Neurosci 2019;39:9633–9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol 2014;592:4395–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 2010;51:1650–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen J, Carvill GL, Gardella E, et al. The phenotypic spectrum of SCN8A encephalopathy. Neurology 2015;84:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagnon JL, Barker BS, Hounshell JA, et al. Pathogenic mechanism of recurrent mutations of SCN8A in epileptic encephalopathy. Ann Clin Transl Neurol 2016;3:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunton-Stasyshyn RKA, Wagnon JL, Wengert ER, et al. Prominent role of forebrain excitatory neurons in SCN8A encephalopathy. Brain 2019;142:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ottolini M, Barker BS, Gaykema RP, et al. Aberrant sodium channel currents and hyperexcitability of medial entorhinal cortex neurons in a mouse model of SCN8A encephalopathy. J Neurosci 2017;37: 7643–7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagnon JL, Jones JM, Meisler MH, et al. Convulsive seizures and SUDEP in a mouse model of SCN8A epileptic encephalopathy. Hum Mol Genet 2015;24:506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorski JA, Talley T, Qiu M, et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci 2002;22:6309–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakso M, Pichel JG, Gorman JR, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A 1996;93:5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hérent C, Diem S, Fortin G, Bouvier J. Absent phasing of respiratory and locomotor rhythms in running mice. eLife. 2020;9:e61919. 10.7554/elife.61919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academies Press, 2011. [Google Scholar]
- 32.DeVos SL, Goncharoff DK, Chen G, et al. Antisense reduction of tau in adult mice protects against seizures. J Neurosci 2013;33:12887–12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Erum J, Van Dam D, De Deyn PP. PTZ-induced seizures in mice require a revised Racine scale. Epilepsy Behav 2019;95:51–55. [DOI] [PubMed] [Google Scholar]
- 34.Wadhwa N, Freeman WT, Durand F, et al. Eulerian video magnification and analysis. Commun ACM 2016;60:87–95. [Google Scholar]
- 35.Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 2011;377: 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gastaut H, Roger J, Ocjahchi S, et al. An electro-clinical study of generalized epileptic seizures of tonic expression. Epilepsia 1963;4: 15–44. [DOI] [PubMed] [Google Scholar]
- 37.Kalume F, Westenbroek RE, Cheah CS, et al. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest 2013;123: 1798–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhaibar H, Gautier NM, Chernyshev OY, et al. Cardiorespiratory profiling reveals primary breathing dysfunction in Kcna1-null mice: implications for sudden unexpected death in epilepsy. Neurobiol Dis 2019;127:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyllie E Wyllie’s treatment of epilepsy. 6th ed. Alphen aan den Rijn, the Netherlands: Wolters Kluwer, 2015. [Google Scholar]
- 40.Nakase K, Kollmar R, Lazar J, et al. Laryngospasm, central and obstructive apnea during seizures: defining pathophysiology for sudden death in a rat model. Epilepsy Res 2016;128:126–139. [DOI] [PubMed] [Google Scholar]
- 41.Dlouhy BJ, Gehlbach BK, Kreple CJ, et al. Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J Neurosci 2015;35:10281–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nobis WP, Schuele S, Templer JW, et al. Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Ann Neurol 2018;83:460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin B, Dieuset G, Pawluski JL, et al. Audiogenic seizure as a model of sudden death in epilepsy: a comparative study between four inbred mouse strains from early life to adulthood. Epilepsia 2020;61:342–349. [DOI] [PubMed] [Google Scholar]
- 44.Faingold CL, Randall M, Tupal S. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy Behav 2010;17:436–440. [DOI] [PubMed] [Google Scholar]
- 45.Faingold CL, Randall M, Kommajosyula SP. Susceptibility to seizure-induced sudden death in DBA/2 mice is altered by adenosine. Epilepsy Res 2016;124:49–54. [DOI] [PubMed] [Google Scholar]
- 46.Mueller SG, Nei M, Bateman LM, et al. Brainstem network disruption: a pathway to sudden unexplained death in epilepsy? Hum Brain Mapp 2018;39:4820–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sainju RK, Dragon DN, Winnike HB, et al. Ventilatory response to CO2 in patients with epilepsy. Epilepsia 2019;60:508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol 2008;7: 1021–1031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


