Abstract
Conventional CD4+ and CD8+ T lymphocytes comprise a mixture of naive and memory cells. Generation and survival of these T-cell subsets is under strict homeostatic control and reflects contact with self–major histocompatibility complex (MHC) and certain cytokines. Naive T cells arise in the thymus via T-cell receptor (TCR)-dependent positive selection to self-peptide/MHC complexes and are then maintained in the periphery through self-MHC interaction plus stimulation via interleukin-7 (IL-7). By contrast, memory T cells are largely MHC-independent for their survival but depend strongly on stimulation via cytokines. Whereas typical memory T cells are generated in response to foreign antigens, some arise spontaneously through contact of naive precursors with self-MHC ligands; we refer to these cells as memory-phenotype (MP) T cells. In this review, we discuss the generation and homeostasis of naive T cells and these two types of memory T cells, focusing on their relative interaction with MHC ligands and cytokines.
T lymphocytes play a central role in adaptive immune responses. Conventional CD4+ and CD8+ T cells expressing αβ T-cell receptors (TCRs) are generated in the thymus via a process of positive and negative selection to a range of self-peptides bound to major histocompatibility complex class I (pMHC I) and class II (pMHC II) molecules (Klein et al. 2014). In this process, CD4+ CD8+ precursor thymocytes are tested for their relative TCR reactivity to pMHC ligands. A small proportion of cells with high avidity for these ligands is deleted, while the majority of thymocytes fail to interact with pMHC ligands and undergo “death by neglect.” The residual 1%–2% of double-positive (DP) thymocytes have moderate TCR affinity to self-antigens (Ags) and these cells are signaled to survive and migrate into the periphery as naive CD4+ and CD8+ T lymphocytes. In addition, another small subset of CD4+ CD8+ thymocytes with slightly higher affinity is signaled to differentiate into Foxp3+ CD4+ regulatory T cells (Tregs).
In the periphery, naive T lymphocytes have a resting CD44lo CD62Lhi CCR7hi phenotype and circulate in the lymphoid tissues as they screen antigen-presenting cells (APCs) for MHC ligands. When naive T cells recognize cognate foreign Ags, they proliferate extensively to give rise to CD44hi Ag-specific effector cells that serve to eliminate the pathogen concerned. After pathogen clearance, most effector cells die while a small fraction of these cells survive to form long-lived Ag-specific memory cells (Sprent and Surh 2002). Mature memory T cells express a high density of CD44 and comprise a mixture of central memory (Tcm), effector memory (Tem), and resident memory (Trm) cells (Seder and Ahmed 2003; Sheridan and Lefrançois 2011). Tcm cells are marked by expression of lymph node homing receptors, CD62L and CCR7, and reside largely in the lymphoid tissues, whereas Tem cells lack these markers and circulate mostly in nonlymphoid tissues. For Trm cells, they reside in nonlymphoid organs such as the intestines and lungs and provide a first line of defense against secondary infection. All of these subsets are long-lived cells with a slow turnover.
In addition to memory cells for foreign Ags, there is accumulating evidence that some T cells with a memory phenotype (MP) arise from naive precursors responding to self rather than foreign Ags (Sprent and Surh 2011). Thus, in normal conditions, T lymphocytes consist of foreign Ag-specific memory and self-driven MP cells as well as naive cells (Table 1). These cell populations are tightly regulated by homeostatic mechanisms that keep total peripheral T-cell numbers relatively constant throughout adult life (Surh and Sprent 2008). During acute infection, T cells specific for pathogens proliferate extensively to form effector cells, leading to a temporary increase in peripheral T-cell numbers; however, most effector T cells are rapidly eliminated, causing T-cell numbers to fall to their original level. Conversely, conditions of lymphopenia induce residual T cells to expand to restore normal T-cell numbers. In this review, we will discuss the various mechanisms that control such homeostasis of naive, foreign Ag-specific memory, and MP T-cell populations.
Table 1.
Classification of T lymphocytes in steady state
| Phenotype | Turnover | Stimulus | |
|---|---|---|---|
| Naive | CD44lo | Slow | Self-Ag |
| Foreign Ag-specific memory | CD44hi | Relatively slow | Foreign Ag |
| Memory-phenotype | CD44hi | Relatively slow + intermittently fast | Self-Ag |
CD4+ and CD8+ T cells in normal specific pathogen-free (SPF) mice consist of naive, foreign Ag-specific, and memory-phenotype cell subpopulations. Their typical phenotype, turnover, and stimuli for survival and turnover are shown.
NAIVE CELLS
TCR Signaling
As the result of positive selection in the thymus, mature naive T lymphocytes have relatively low TCR affinity for self-ligands and high affinity for cognate foreign Ags. Some of the self-pMHC ligands that induce positive selection of CD8+ and CD4+ T cells have been defined (Santori et al. 2002; Ebert et al. 2009; Lo et al. 2009), and it is well established that post-thymic contact with self-pMHC I and pMHC II ligands is important for controlling optimal homeostasis of peripheral CD8+ and CD4+ T cells, respectively (Fig. 1; Takeda et al. 1996; Tanchot et al. 1997; Dorfman et al. 2000). For CD4+ T cells, the requirement for post-thymic contact with self-MHC ligands for survival is most apparent in mice with total rather than partial absence of MHC II (Martin et al. 2003). Indeed, half-lives of peripheral naive CD8+ and CD4+ T lymphocytes are known to be reduced in the absence of MHC contact, especially for CD8+ T cells (Labrecque et al. 2001; Polic et al. 2001; Seddon and Zamoyska 2002).
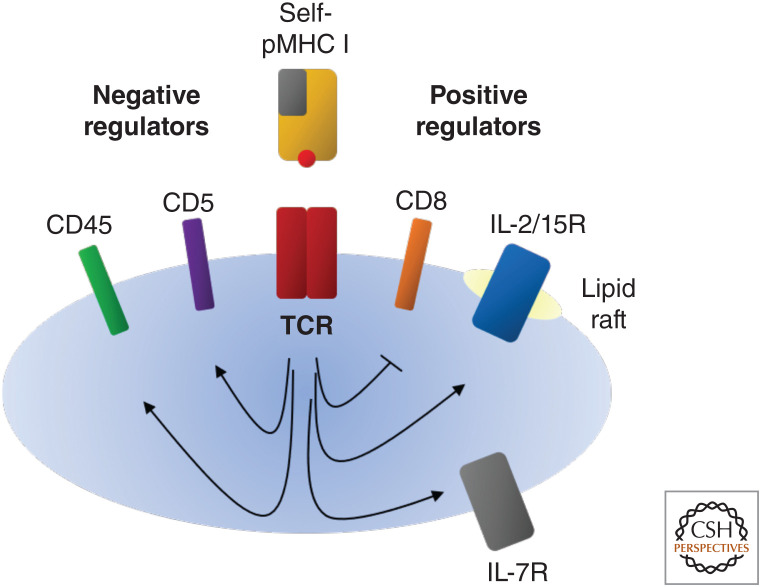
Figure 1.
Self-Ag recognition modulates T-cell receptor (TCR) sensitivity and cytokine reactivity in CD8+ T cells. Self-antigen (Ag) recognition promotes CD8+ T-cell survival, partly by enhancing interleukin-7 receptor (IL-7R) expression levels. Also, CD8+ T cells with strong reactivity to self-Ags have high levels of inhibitory CD5 and CD45 molecules and reduced expression of CD8. Altered expression of these molecules causes a reduction in TCR sensitivity as defined by short-term assays in vitro. However, despite reduced TCR sensitivity, CD5hi cells show enhanced expression of lipid rafts, thereby leading to augmented responsiveness to cytokines, notably IL-2 and IL-15. For this reason, CD5hi cells give stronger responses to Ags in vivo than CD5lo cells. This applies both to homeostatic proliferation to self-Ags in lymphopenic hosts and typical responses to foreign Ags.
To ensure post-thymic survival of naive T cells, it has generally been assumed that the ligands controlling positive selection in the thymus and T-cell survival in the periphery are the same (Ernst et al. 1999; Goldrath and Bevan 1999). Although this is clearly the case in some situations, it is now known that the MHC-bound peptides in the thymic cortex and the peripheral lymphoid organs are essentially different. Thus, positive selection in the thymus is directed in part to private peptides generated in cortical epithelial cells by the thymoproteasome for pMHC I and cathepsin L and thymus-specific serine protease (TSSP) for pMHC II (Nakagawa et al. 1998; Murata et al. 2007; Gommeaux et al. 2009; Klein et al. 2014). At least for CD8+ T cells, selection of cells by private cortical peptides is especially important for cells with relatively low self-affinity (Nitta et al. 2010; Xing et al. 2013), that is, cells with a low surface density of CD5, CD5 expression being a reliable marker for TCR affinity to self as discussed below. For CD5lo T cells, contact with self-ligands in the periphery presumably reflects cross-reactivity with the selecting peptides.
The notion that peripheral naive T cells require sustained self-pMHC recognition for their survival suggests that the population size of each T-cell clone, or the “space” available for each cell, is determined by the expression level of its corresponding self-ligand. Whereas self-Ags as a whole are abundantly expressed in the periphery, it has been suggested that individual APCs may express not all but only a partial set of endogenous peptides (Paul et al. 2013; Grossman et al. 2020). If this is the case, the niche available for each clone could be quite small. Supporting this idea, monoclonal CD4+ T cells adoptively transferred in large numbers tend to disappear rapidly, whereas cells injected in small numbers persist (Hataye et al. 2006); in part, this finding may reflect the relative levels of homeostatic cytokines in the recipients. In the steady state, the number of naive CD4+ T cells specific for a given foreign epitope is generally very low, varying from 10 to several hundred cells/mouse (Moon et al. 2007).
Under normal conditions, post-thymic contact of naive T cells with self-pMHC ligands does not induce overt T-cell activation, even for cells with relatively high self-reactivity. Such tolerance is due in part to relatively low levels of costimulatory molecules on APCs and inhibition by Tregs (Paul et al. 2013). Thus, acute depletion of Tregs induces up-regulation of B7 molecules on dendritic cells (DCs) and leads to autoimmunity (Kim et al. 2007; Yi et al. 2019). Hence, the quiescent state of naive T cells is partly due to extrinsic mechanisms. In addition, a T-cell-intrinsic mechanism of partial TCR desensitization occurs after positive selection (Grossman and Paul 2015). Such TCR “tuning” limits overt responsiveness to self-ligands and focuses TCR reactivity to foreign Ags. TCR tuning is known to correlate with surface levels of CD5, expression of CD5 being up-regulated during positive selection and maintained in the periphery (Azzam et al. 1998; Smith et al. 2001; Stamou et al. 2003). Under in vivo conditions, T cells with high CD5 expression give strong responses (see below) (Smith et al. 2001; Takada and Jameson 2009). Under in vitro conditions, however, CD5 acts as a negative regulator of TCR signaling, possibly via SHP-1 phosphatase (Tarakhovsky et al. 1995; Perez-Villar et al. 1999), although recent studies are against this idea (Dong et al. 2016). In addition to CD5, TCR signaling is also negatively regulated by surface expression of CD45, a phosphatase that induces tyrosine dephosphorylation of p56lck (Lck) (Cho et al. 2016).
Countering the negative activity of CD5 and CD45 on naive T cells, the presence of CD8 and CD4 molecules on these cells augments TCR signaling, largely by binding to nonpolymorphic epitopes on MHC I and MHC II molecules, respectively, thereby focusing Lck to the vicinity of the TCR (Sarmiento et al. 1980; Marrack et al. 1983; Gay et al. 1987; Rudd et al. 1988; Veillette et al. 1988; Turner et al. 1990; Park et al. 2007; Takada and Jameson 2009). In addition, certain microRNAs can augment TCR signaling. Thus, miR-181a increases TCR sensitivity for self-MHC during thymic selection and is then down-regulated in the periphery (Ebert et al. 2009). Together, these and the above positive and negative regulators cooperatively “fine-tune” the activation threshold for Ag-specific T-cell responses.
In addition to promoting survival, interaction of naive T cells with self-pMHC ligands can modulate TCR sensitivity (Fig. 1). For CD8+ T cells, depriving these cells from contact with pMHC I in a nonlymphopenic setting leads to a decline in CD5 levels but up-regulation of CD8 expression in vivo (Takada and Jameson 2009). These changes were found to improve responsiveness to foreign Ags, although only to weak Ags. Hence, although important for cell survival, T-cell contact with pMHC I can attenuate naive T-cell sensitivity by modulating CD8 levels. In addition, as mentioned above, TCR sensitivity in vitro can be reduced by high CD5 and CD45 expression. Thus, short-term extracellular signal-regulated kinase (ERK) phosphorylation and Ca2+ signaling induced by TCR ligation of CD8+ T cells is less marked for CD5hi CD45hi cells than for CD5lo CD45lo cells (Cho et al. 2016). However, reciprocal findings apply in vivo. Thus, CD5hi CD8+ T cells generally give stronger responses to foreign Ags in vivo than CD5lo cells (Cho et al. 2010). This discrepancy is explained by the finding that CD5hi CD45hi CD8+ T cells (i.e., cells with relatively strong self-reactivity) show enhanced expression of cell surface lipid rafts and are thereby more sensitive to the stimulatory effect of cytokines than low-affinity cells (Cho et al. 2010). Under in vivo conditions, the increased sensitivity of the high-affinity cells to cytokines counters the small reduction in their TCR sensitivity. In addition to regulation by CD5 and CD45, the TCR sensitivity of CD8+ T cells is also influenced by their initial contact with the thymoproteasome during development. Thus, although the latter is especially important for selection of low-affinity CD8+ T cells, even high-affinity clones such as OT-I display reduced TCR sensitivity when generated in a thymus lacking the thymoproteasome (Takada et al. 2015).
For CD4+ T cells, studies with TCR-transgenic T cells have shown that short-term blockade of TCR/MHC II interaction reduces TCR sensitivity and responsiveness to foreign Ags (Stefanová et al. 2002). Conversely, exposing naive TCR-transgenic CD4+ T lymphocytes to increased concentrations of the self-peptides that induced positive selection heightens responses to their cognate foreign Ags (Ebert et al. 2009; Lo et al. 2009). These findings indicate that steady-state contact of CD4+ T cells with pMHC II ligands augments responses to foreign Ags. As for CD8+ T cells, CD4+ T cells with high self-reactivity show enhanced reactivity to foreign Ags. Thus, CD5hi naive CD4+ T cells give stronger responses to pathogens in vivo than CD5lo cells (Mandl et al. 2013). The authors of this study favor a model where thymic selection by strong pMHC ligands has a direct effect in enhancing TCR interaction with agonist pMHC epitopes. An alternative idea, also applicable to CD8+ T cells, is that corecognition of self-peptides during responses to foreign pMHC ligands augments the avidity of T/APC interaction and thereby amplifies the response (Sprent and Surh 2011). Direct evidence on this important issue is needed.
IL-7 Signaling
In addition to TCR signaling, naive T cells require interleukin-7 (IL-7) for their survival. IL-7 is produced by bone marrow- and thymus-derived stromal cells and promotes survival of lymphoid progenitors (Namen et al. 1988; Sakata et al. 1990) as well as mature naive CD8+ and CD4+ T cells (Schluns et al. 2000; Tan et al. 2001). Thus, blocking IL-7 decreases the number of peripheral T cells in the absence of thymic output (Vivien et al. 2001; Kondrack et al. 2003), while IL-7-transgenic mice have increased naive cell counts without a major alteration in thymic cellularity (Mertsching et al. 1995; Kieper et al. 2002). The receptor for IL-7 (IL-7R) comprises heterodimers of IL-7Rα and the common γ chain (γc) (Kondo et al. 1994b) and is mainly expressed on T cells (Sudo et al. 1993; Kondo et al. 1994a). In T cells, IL-7 signaling augments expression of antiapoptotic factor Bcl-2, which counterbalances the effect of proapoptotic factors such as Bax and Bim (Akashi et al. 1997; Vivien et al. 2001; Khaled and Durum 2002; Khaled et al. 2002; Wojciechowski et al. 2007).
Because naive CD4+ and CD8+ T cells need to enter secondary lymphoid organs to receive survival signals from IL-7 (Link et al. 2007), this cytokine is thought to be largely cell membrane–bound and expressed in peripheral lymphoid organs. In support of this notion, IL-7 reporter mice show high IL-7 mRNA expression in fibroblastic reticular and lymphatic endothelial cells in peripheral lymph nodes (Alves et al. 2009; Mazzucchelli et al. 2009; Repass et al. 2009; Shalapour et al. 2010; Hara et al. 2012; Onder et al. 2012; Miller et al. 2013). IL-7 synthesis by these cells is limited and leads to strict competition between T cells and innate lymphoid cells for survival (Martin et al. 2017).
Ag-SPECIFIC MEMORY CELLS
Generation
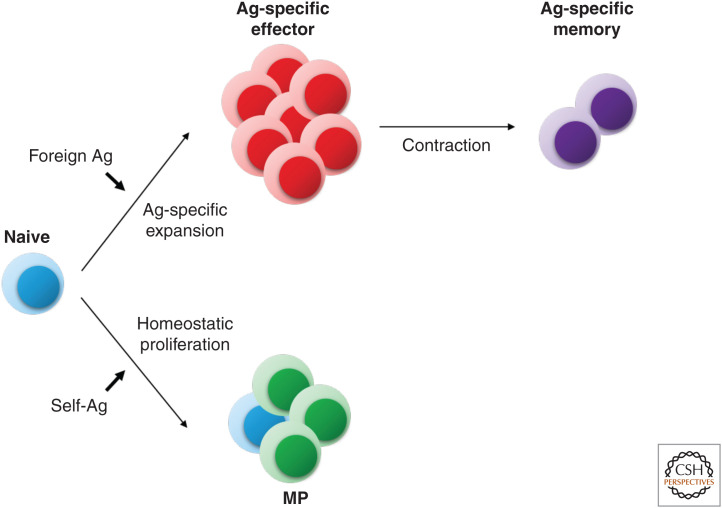
It is well documented that naive T-cell responses to foreign Ags involve TCR recognition of pMHC ligands (signal 1) plus joint interaction with costimulatory ligands on mature APCs (signal 2) together with stimulation by cytokines, especially IL-2 (signal 3) (Sprent and Surh 2002; Kaech and Cui 2012; Ratajczak et al. 2018). These three signals induce the responding T cells to undergo marked expansion and differentiation into effector cells. After pathogen clearance, >90% of the effector cells die rapidly, leading to a contraction phase where only a small fraction of cells survive as Ag-specific memory T cells (Fig. 2).
Figure 2.
Recognition of foreign (strong) versus self (weak) antigens (Ags) generates different outcomes. When naive T cells recognize foreign Ags in the presence of sufficient levels of costimulation and cytokines, they proliferate extensively to give rise to Ag-specific effector T cells. After pathogen clearance, most effector T cells die, while a small fraction of cells survives as Ag-specific memory T cells. On the other hand, when naive T cells recognize self-Ags, they undergo a relatively mild proliferative response; such homeostatic proliferation generates a population of cells with a memory phenotype (MP).
In the case of CD8+ memory T cells, the immune response generates a mixture of short-lived effector cells and memory precursor effector cells (SLECs and MPECs, respectively). Memory cell generation is complex and is driven in part by IL-7 via selective retention of IL-7R expression on MPECs (Joshi et al. 2007). However, many factors influence the fate of SLECs and MPECs, including TCR affinity, exposure time of contacting foreign Ags (Wherry et al. 1999; Prlic et al. 2006; Zehn et al. 2009), relative ligation of T-cell costimulatory molecules such as CD28, CD27, OX40, and 4-1BB (Hendriks et al. 2000, 2005; Mittrücker et al. 2001; Mousavi et al. 2008), and stimulation with cytokines such as IL-2, IL-21, interferon-γ (IFN-γ), and type I IFN (Marrack et al. 1999; Cheng et al. 2002; Blattman et al. 2003; Williams et al. 2006; Whitmire et al. 2007; Davis et al. 2008). Preferential generation of SLECs occurs with exposure to high concentrations of Ag (Wherry et al. 1999; Badovinac et al. 2004; Joshi et al. 2007; Zehn et al. 2009) and/or inflammatory cytokines such as IL-2, IFN-γ, and IL-12 (Badovinac et al. 2004; Cui et al. 2009; Kalia et al. 2010; Pipkin et al. 2010), whereas memory cells tend to be drawn from “latecomer” T cells that contact Ags during later stages of the immune response and thereby undergo relatively brief TCR ligation and exposure to inflammatory signals (Sprent 1994; D'Souza and Hedrick 2006; Sarkar et al. 2008).
The above findings apply largely to CD8+ T cells, and less is known about CD4+ memory T-cell generation (Marshall et al. 2011; Choi et al. 2013). As with CD8+ T cells, formation of memory CD4+ T cells is dependent on contact with various costimulatory molecules (Hendriks et al. 2000; Soroosh et al. 2007; Pagán et al. 2012). Likewise, very high concentrations of Ags favor terminal differentiation into Th1 effector cells rather than T follicular helper cells and memory cells. Thus, although CD4+ memory T-cell generation requires strong stimulation (Williams and Bevan 2004; Williams et al. 2008), most of these cells are derived from effector cells expressing low rather than high levels of the activation marker CD25 (a component of the IL-2 receptor) (Snook et al. 2018). This finding implies that, as for CD8+ T cells, CD4+ memory T cells arise from precursors subjected to relatively weak TCR signaling and low stimulation by cytokines.
Maintenance
Both for CD8+ and CD4+ T cells, Ag-specific memory cells are largely maintained in the absence of MHC recognition (Murali-Krishna et al. 1999; Swain et al. 1999), although tonic TCR/MHC contact may be required for optimal recall responses (Kassiotis et al. 2002). For CD8+ memory cells, the transition to MHC independence correlates with a mild reduction in TCR sensitivity but increased sensitivity to cytokines, relative to naive cells (Cho et al. 2016).
It is now well established that the survival of Ag-specific memory T cells depends crucially on contact with IL-7, both for CD8+ (Goldrath et al. 2002; Lenz et al. 2004) and CD4+ (Kondrack et al. 2003; Lenz et al. 2004; Purton et al. 2007) T cells. Thus, for CD8+ T cells, studies on the effects of IL-7 blockade and the use of genetically engineered mice with IL-7 signaling defects in mature T cells leads to progressive elimination of Ag-specific memory cells (Buentke et al. 2006; Carrio et al. 2007; Osborne et al. 2007). This finding reflects the capacity of IL-7 to up-regulate Bcl-2 (Kondrack et al. 2003; Carrio et al. 2007; Osborne et al. 2007) and thereby enables the cells to survive and undergo basal homeostatic proliferation (HP) (Goldrath et al. 2002; Lenz et al. 2004; Purton et al. 2007). Proliferation is slow and uniform, both for CD8+ and CD4+ memory T cells. For CD8+ memory cells, most of these cells have the CD62Lhi phenotype of Tcm, whereas typical memory CD4+ cells have a CD62Llo Tem phenotype.
Like IL-7, IL-15 plays a key role in the survival of memory T cells. IL-15 shares many biological properties with IL-2 (Grabstein et al. 1994) and, like IL-2, IL-15 binds to a receptor composed of an IL-2 receptor β chain and γc (Giri et al. 1994; Grabstein et al. 1994). This βγc receptor, the low-affinity receptor for both IL-2 and IL-15 (IL-2/15R), is expressed at a high level on memory T cells, particularly on CD8+ T cells (Zhang et al. 1998; Becker et al. 2002; Purton et al. 2007). Contact with IL-15 is required for optimal maintenance of memory CD8+ and CD4+ T lymphocytes, both by inducing Bcl-2 expression and stimulating basal cell turnover (Becker et al. 2002; Goldrath et al. 2002; Lenz et al. 2004; Purton et al. 2007). Reflecting higher expression of the βγc receptor on CD8+ than CD4+ memory cells, IL-15 is especially important for memory CD8+ T-cell survival (Zhang et al. 1998; Lenz et al. 2004).
The main cellular source of IL-15 is still unclear. Unlike IL-2, IL-15 is uniquely anchored to the surface of presenting cells via an IL-15 receptor α chain (IL-15Rα) to exert its biological function (Dubois et al. 2002; Sato et al. 2007). IL-15 and IL-15Rα are broadly expressed in hematopoietic and nonhematopoietic cells (Burkett et al. 2003; Schluns et al. 2004), and in the case of hematopoietic cells, these two molecules need to be expressed by the same cell to maintain memory T-cell homeostasis (Burkett et al. 2004; Sandau et al. 2004). IL-15 reporter mice have revealed CD8α+ DCs as the main source of peripheral IL-15 mRNA (Colpitts et al. 2012; Sosinowski et al. 2013; Cui et al. 2014), which is consistent with the observation that DCs express IL-15Rα (Dubois et al. 2005). In addition, one study using IL-15 knockin reporter mice suggested that fibroblastic reticular and blood endothelial cells are possible sources of IL-15 in peripheral lymph nodes (Cui et al. 2014). These stromal cell sources could be important because IL-15 production by radioresistant cells contributes to memory T-cell maintenance (Schluns et al. 2004).
MEMORY-PHENOTYPE CELLS
Definition
In normal unimmunized mice, mature T cells comprise a mixture of typical naive CD44lo cells plus a small proportion of cells with a CD44hi MP. In young mice, the CD44hi cell subpopulation accounts for approximately 10% of total CD4+ T cells and a slightly higher proportion of CD8+ cells. Because CD44hi cells closely resemble Ag-specific memory cells, it has tacitly been assumed that these cells arise through contact with a spectrum of environmental Ags derived from commensal microbes and/or food. Supporting this notion, CD44hi cells increase progressively with age and become the dominant population in old age (Dobber et al. 1992).
However, the simple idea that CD44hi T cells are generated in response to diverse foreign Ags has been called into question by the finding that CD44hi CD8+ and CD4+ T cells are present not only in specific pathogen-free (SPF) mice but also in germ-free (GF) and antigen-free (AF) animals, the latter lacking both commensal microflora and food Ags (Pereira et al. 1986; Dobber et al. 1992; Huang et al. 2005; Kim et al. 2016). Likewise, T cells with a similar phenotype exist in human cord blood and fetal spleen (Byrne et al. 1994; Szabolcs et al. 2003). These observations indicate that T cells with an MP can arise independently of foreign Ag stimulation, both in mice and humans. Notably, the MP CD8+ T cells, and to a lesser extent CD4+ cells, in unimmunized SPF mice have specificity for foreign Ags (as defined by specific tetramer staining) despite no previous contact with these Ags (Moon et al. 2007; Haluszczak et al. 2009); similar findings apply in humans (Su et al. 2013). These findings indicate that MP cells generated in the absence of foreign Ags have a broad TCR repertoire (Younes et al. 2011). In the case of CD8+ T cells, MP cells have also been termed “virtual” memory cells (Haluszczak et al. 2009).
With regard to turnover, typical MP T cells differ from Ag-specific memory T cells in comprising two broad subsets of cells proliferating at different rates (Tough and Sprent 1994; Younes et al. 2011). Thus, in normal unimmunized SPF mice, the majority of MP CD8+ T cells display a Tcm phenotype and have a slow turnover, but ∼30% have an activated phenotype and proliferate rapidly (Boyman et al. 2006a). Likewise, a considerable proportion (18%–35%) of CD4+ MP cells are in cell cycle, which is substantially higher than for Ag-specific memory cells (Younes et al. 2011). MP CD4+ T cells with a fast turnover also exist in human cord blood (Szabolcs et al. 2003). For both CD8+ and CD4+ MP cells, cells with a slow turnover closely resemble Ag-induced memory cells (see above). Also, MP cells are similar to Ag-induced memory cells in being in responsive to pathogens, and thereby may provide an important “preimmune” response to infection (see below). Nevertheless, these two memory subsets do display distinct differences, especially for CD8+ T cells. Thus, MP CD8+ T cells differ from Ag-induced memory cells in lacking expression of CD49d (integrin α4) and show only low synthesis of IFN-γ in response to TCR ligation (Haluszczak et al. 2009; Lee et al. 2013).
The above findings suggest that most of the MP T cells present in normal unimmunized SPF mice are generated in response to self-ligands rather than to foreign Ags (Fig. 2). Hence, MP T cells are a unique population distinct from conventional, foreign Ag-specific memory cells. In this respect, the frequency of MP (CD44hi) cells in SPF, GF, and AF mice is quite similar, and, at least for CD8+ T cells, >90% of CD44hi T cells in SPF mice lack CD49d expression (Kim et al. 2016; J Yi and T Kawabe, unpubl.). Nevertheless, it should be emphasized that, in marked contrast to mice raised in a clean environment, pet store mice and other mice housed in a “dirty” environment resemble adult humans in containing a high proportion of activated T cells with a Tem phenotype (Beura et al. 2016). The mechanisms controlling the homeostasis and function of the typical MP cells found in normal SPF mice are discussed below.
Generation
Information on the generation of MP cells has come largely from studies on the effects of transferring T cells to lymphopenic mice. Here, early studies documented that “homeostatic” proliferation of T cells following transfer to lymphopenic hosts applies not only to unseparated T cells but also to purified populations of naive T cells, both for CD8+ and CD4+ T cells (Ernst et al. 1999; Goldrath and Bevan 1999; Kieper and Jameson 1999; Oehen and Brduscha-Riem 1999; Viret et al. 1999; Murali-Krishna and Ahmed 2000). Such HP also applies to TCR-transgenic T cells and is demonstrable not only in constitutively lymphopenic hosts such as RAG-deficient, severe combined immunodeficient (SCID), and CD3-deficient hosts but also in normal mice following acute host T-cell depletion. Under these conditions, the injected naive T cells expand considerably and thereby return total T-cell numbers toward normal levels.
Most studies on HP have been conducted by transferring carboxy-fluorescein succinimidyl ester (CFSE)-labeled total, naive, or MP CD8+ or CD4+ T cells into congenic lymphopenic mice and then analyzing the donor cells several days to weeks later. Based on CFSE dilution, such proliferation has two distinct components: slow (homeostatic) and fast (spontaneous) (Table 2; Kieper et al. 2005; Min et al. 2005). Proliferation of naive T cells in acutely lymphopenic hosts—also termed lymphopenia-induced proliferation—is typically quite slow with cells dividing up to 3–4 times by 1 week after transfer and gradually increasing their CD44 levels but without up-regulation of activation markers such as CD25 and CD69 (Min et al. 2005); for CD4+ T cells, whether all of the proliferating cells eventually become CD44hi is still unclear (Kawabe et al. 2017; T Kawabe, unpubl.). This type of proliferation is generally stronger for CD8+ than CD4+ T cells. For both subsets, slow proliferation is mostly MHC-dependent, requiring contact with MHC I for CD8+ cells and MHC II for CD4+ cells (Ernst et al. 1999; Goldrath and Bevan 1999; Kieper and Jameson 1999; Viret et al. 1999; Tan et al. 2002). The rate of cell division by naive T cells is faster with CD5hi than CD5lo cells, both for polyclonal and TCR-transgenic cells (Kassiotis et al. 2003; Kieper et al. 2004). With regard to the stimulus for proliferation, early experiments established that slow HP can be induced by the ligands that induce initial positive selection in the thymus (Ernst et al. 1999; Goldrath and Bevan 1999). Also, it was shown that HP does not involve classic costimulation (Gudmundsdottir and Turka 2001; Prlic et al. 2001; Hagen et al. 2004; Kawabe et al. 2013), and that the rate of cell division is reduced by competition from other T cells (Ernst et al. 1999).
Table 2.
Homeostatic proliferation of naive T cells in lymphopenic specific pathogen-free (SPF) hosts
| Tempo of proliferation | Stimuli required | Generation of effector cells | Proliferation in lymphopenic hosts | |
|---|---|---|---|---|
| Acutely irradiated | RAG-deficient | |||
| Slow | Self–major histocompatibility complex (MHC) Interleukin-7 (IL-7) | – | ++++ | +++a |
| Fast | Self-MHC Costimulation IL-2/15 | + | + | ++++b |
When transferred into lymphopenic recipient mice, naive CD4+ and CD8+ donor T cells proliferate slowly or rapidly, depending on the host used. Characteristics of these two kinds of proliferation are shown.
aSlow proliferation is slightly less prominent in RAG-deficient than irradiated hosts, although only for polyclonal and not T-cell receptor (TCR)-transgenic T cells.
bFast proliferation is much less in germ-free (GF) and antigen-free (AF) than SPF mice (see text for details).
The early finding that slow HP of naive T cells was inhibited by host T cells led to speculation that, in addition to MHC ligands, proliferation also involved in contact with cytokines. This notion led to the subsequent discovery that HP is controlled largely by the relative concentration of IL-7, the level of this cytokine being much higher in lymphopenic than normal hosts (Schluns et al. 2000; Tan et al. 2001, 2002; Min et al. 2005; Martin et al. 2017). Collectively, these findings support a model where, during continuous interaction of naive T cells with self-MHC ligands, contact with above normal levels of IL-7 signals the cells to enter cell cycle and begin to proliferate. After expansion, the cells also become dependent on IL-15 (see below).
The above data apply to the typical slow rate of intermittent proliferation seen following naive T-cell transfer to lymphopenic hosts. As mentioned above, however, a proportion of T cells can undergo rapid proliferation and acquire a CD44hi phenotype in these hosts. Such fast proliferation is generally higher for CD4+ than CD8+ T cells and is more intense in mice that are constitutively lymphopenic such as RAG-deficient hosts than in mice made acutely lymphopenic (e.g., following irradiation) (Ernst et al. 1999). For CD4+ T cells, fast proliferation is abolished or greatly reduced in MHC II–deficient hosts (Ernst et al. 1999; Tan et al. 2002; Martin et al. 2003) and reflects contact with DCs (Do and Min 2009); likewise, fast proliferation of CD8+ T cells is MHC-dependent, although the data here are less clear-cut because of MHC I expression on the responding T cells (Cho et al. 2007) coupled with a small proportion of CD8+ T cells being MHC II–specific (Tyznik et al. 2004; Do and Min 2009). Unlike slow HP, fast proliferation requires costimulatory CD28 and OX40 signaling (Gudmundsdottir and Turka 2001; Hagen et al. 2004; Kawabe et al. 2013). Also, fast proliferation is considerably enhanced in hosts containing elevated levels of various γc cytokines, including IL-2, IL-4, IL-7, and/or IL-15 (Boyman et al. 2006b; Cho et al. 2007, 2010; Ramsey et al. 2008); conversely, proliferation is inhibited by transforming growth factor-β (TGF-β) (Zhang and Bevan 2012).
For SPF hosts, a key issue is whether fast proliferation is directed to foreign or self-Ags. This question was initially addressed by examining the rate of naive CD4+ T-cell proliferation in RAG knockout hosts maintained in a GF versus SPF environment. Here, the important finding was that fast proliferation was considerably lower (but not abolished) in GF than SPF hosts, whereas slow HP was similar in both hosts (Kieper et al. 2005). This finding strongly suggests that, for CD4+ T cells, the bulk of the cells proliferating in RAG-deficient hosts are proliferating in response to foreign Ags, namely, to microbial Ags. Because fast proliferation is limited in acutely irradiated hosts, the intense proliferation seen in constitutively lymphopenic RAG-deficient hosts is thought to reflect a “leaky” gut as the result of the chronic absence of protective mucosal T cells in these mice (Sheridan and Lefrançois 2011). Though lower than in RAG knockout mice, commensal-dependent fast proliferation does occur in irradiated hosts and generates α4β7+ cells that synthesize IL-17 (Kawabe et al. 2013).
Although the residual fast proliferation of naive CD4+ T cells in GF RAG-deficient hosts might be responding to food Ags, this possibility is unlikely because comparable proliferation is also seen in AF mice (Kieper et al. 2005; Yi et al. 2019). Moreover, the extent of fast proliferation can be augmented to high levels simply by increasing the number of T cells injected, thereby inducing strong activation of host APCs. These findings in GF and AF mice indicate that fast proliferation of CD4+ T cells can be prominent in these hosts and is directed selectively to self-pMHC ligands. Based on parallel studies in Foxp3-DTR mice given diphtheria toxin (DT) to deplete Tregs (Yi et al. 2019), fast proliferation of naive CD4+ T cells in hosts lacking Tregs, including RAG knockout hosts, is MHC II–restricted and directed to elevated levels of costimulation (CD80, CD86) on host APCs caused by the absence of Tregs. This finding follows previous evidence that fast proliferation in constitutively lymphopenic mice is inhibited by Tregs by IL-10-independent, CTLA4-dependent mechanisms (Bourgeois and Stockinger 2006; Winstead et al. 2008; Bolton et al. 2015; Yi et al. 2019, 2020). As for slow HP, fast proliferation is more prominent with CD5hi than CD5lo T cells, implying that the response is skewed to cells with the highest affinity for self-pMHC ligands (Kawabe et al. 2017; Yi et al. 2019). Based on studies with H2M knockout mice, proliferation includes reactivity to ubiquitous self-peptides.
In mice, fast proliferation is especially conspicuous in the neonatal period (Ichii et al. 2002; Min et al. 2003). Some of the proliferation during this period may be directed to foreign Ags, but rapid proliferation is also seen in AF neonates (K Frimpong-Boateng and CD Surh, pers. comm.). Because newborn mice are lymphopenic, much of the fast proliferation in neonates could be driven by the combination of increased availability of Ags and cytokines and the paucity of Tregs at birth (Jiang et al. 2006). Another interesting possibility is that the rapid tempo of proliferation in neonates is due in part to a lack of feedback inhibition by a mature T-cell repertoire. In this respect, studies in adult SPF mice have shown that fast proliferation is enhanced if the TCR repertoire of preexisting T cells is curtailed (Min et al. 2004). The model here—which is also applicable in adult mice—is that a polyclonal TCR repertoire, especially of memory T cells, is necessary to block contact of naive T cells with self-ligands on APCs (Do et al. 2012). Studies on the rate of donor T-cell proliferation in GF or AF hosts coinjected with polyclonal versus pauciclonal T cells will be needed to assess this notion.
In addition to the proliferation seen in lymphopenic hosts, T cells undergoing fast proliferation are also generated under lymphoreplete physiological conditions (e.g., in the spleen of adult AF mice [Pereira et al. 1986] and cord blood of humans [Szabolcs et al. 2003]). Notably, significant fast proliferation of naive CD4+ and CD8+ T cells is apparent following transfer to normal adult hosts (White et al. 2016; Kawabe et al. 2017); the responding cells in these hosts are largely CD5hi cells, and their progeny differentiate into typical MP cells that provide protection against infection (see below) (Kawabe et al. 2017). These findings provide direct support for the view that the component of MP cells found in normal mice is a reflection of an ongoing response to self-ligands.
Maintenance
Although the initial response of naive T cells to self-MHC ligands is TCR-dependent, subsequent proliferation of the cells appears to switch progressively to stimulation largely via cytokines, especially IL-7. Once fully formed, the resultant population of MP cells divides intermittently in response to background levels of both IL-7 and IL-15 (Tan et al. 2002; Purton et al. 2007; Younes et al. 2011). As for Ag-induced memory T cells, dependency on these two cytokines applies to both CD4+ and CD8+ MP cells, although IL-15 is more important for CD8+ cells than CD4+ cells, reflecting the higher density of IL-2Rβ on CD8+ cells. For CD8+ T cells, except for the minor MHC-dependent population of precursor cells, MP T-cell proliferation and survival are largely MHC-independent, which correlates with a mild reduction in their TCR sensitivity; whether this also applies to CD4+ T cells is less clear (Boyman et al. 2006a; Cho et al. 2016; Kawabe et al. 2017). For CD4+ MP cells, in addition to cytokines, their proliferation and survival require signaling via costimulatory molecules such as CD28 and OX40 (Yamaki et al. 2014; Kawabe et al. 2017).
Function
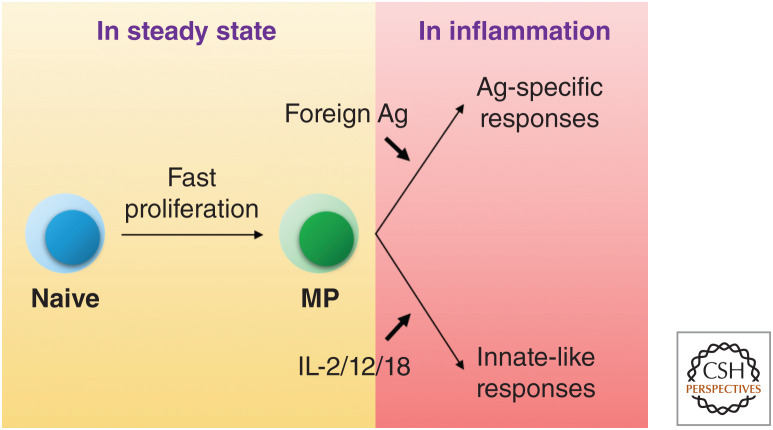
As mentioned earlier, MP cells resemble naive T cells in containing an extensive repertoire of cells reactive to foreign Ags and thus may be important in providing an accelerated Ag-specific response to pathogens relative to naive T cells (Fig. 3). This response might be conspicuous for cells in the early TCR-dependent phase of MP generation because fast-dividing T cells produce various types of effector cytokines/molecules such as IL-2, tumor necrosis factor-α (TNF-α), IFN-γ, IL-13, IL-17A, and Granzyme B in response to ex vivo TCR stimulation (Murali-Krishna and Ahmed 2000; Gudmundsdottir and Turka 2001; Min et al. 2003; Kieper et al. 2005; Min et al. 2005; Winstead et al. 2008; Kawabe et al. 2013; Yi et al. 2019). Indeed, TCR-transgenic MP CD8+ T lymphocytes generated in lymphopenic hosts showed better Ag-specific protection against pathogens than naive cells (Hamilton et al. 2006). Similarly, Ag-specific CD8+ MP cells defined by tetramer staining responded to their cognate Ags more rapidly than did their naive counterparts (Haluszczak et al. 2009). Whether CD4+ MP cells have this function in vivo is less clear (Kawabe et al. 2016).
Figure 3.
Steady-state generation of memory-phenotype (MP) cells and their immunological functions during infection. CD4+ and CD8+ MP cells are homeostatically generated from naive precursors. Once generated, they engage in typical antigen (Ag)-specific immune responses to pathogens. In addition, they exert innate-like effector activity by responding to cytokines such as interleukin (IL)-2, IL-12, and IL-18 and by producing effector cytokines in the absence of cognate Ag recognition.
In addition to adaptive memory function, MP cells also display innate immune function by several parameters (Fig. 3). Thus, for CD8+ T cells, MP cells produce only low amounts of IFN-γ after TCR ligation (Lee et al. 2013), but strong IFN-γ synthesis when stimulated with a cocktail of cytokines (IL-2, IL-12, IL-18) without TCR ligation, presumably reflecting enhanced cytokine receptor expression on MP cells (Haluszczak et al. 2009). Furthermore, as for bystander immunity mediated by conventional memory CD8+ T cells (Berg et al. 2003; Soudja et al. 2012; Chu et al. 2013; Schenkel et al. 2014), MP CD8+ T cells can induce strong non-Ag-specific host defense against pathogen infection in vivo (White et al. 2016). Likewise, whereas the evidence for bystander immunity by conventional memory CD4+ T cells is limited (Guo et al. 2015), CD4+ MP cells can generate effective innate IFN-γ responses, both in vitro (Hu and August 2008) and in vivo (Kawabe et al. 2017). Under in vivo conditions, IFN-γ responses by CD4+ MP cells are mediated by a T-bethi subset induced by background levels of IL-12; these cells provide non-Ag-specific protection against Toxoplasma gondii infection (Kawabe et al. 2020). Collectively, these findings provide increasing evidence that, like other innate immune subsets, MP CD8+ and CD4+ T cells provide an important first-line defense against infectious agents (Kawabe et al. 2018; Kedl and White 2018).
In addition to protection against pathogens, MP T cells may be important for maintaining self-tolerance. Thus, in parallel with typical CD4+ Tregs, a subset of MP CD8+ cells with an IL-2/15Rhi Ly49+ phenotype regulates the function of T follicular helper cells, thereby serving to prevent autoimmune disease (Kim et al. 2011).
Summary
To summarize the data on MP T cells, these cells arise continuously from naive precursors responding to self-pMHC ligands. Under steady-state conditions, small numbers of high-affinity CD4+ and CD8+ T cells are induced to proliferate transiently through contact with APCs and then differentiate into MP cells. This process is intensified when T-cell numbers are reduced, causing residual T cells to undergo compensatory, slow, MHC-restricted costimulation-independent expansion in response to increased host levels of stimulatory cytokines, mainly IL-7. In certain situations, altering the host environment can intensify T/APC interaction and drive fast proliferation. These conditions are extreme in T/B-cell-deficient RAG knockout and Treg-deficient, DT-treated Foxp3-DTR mice. Here, host APCs are activated to up-regulate costimulatory molecules by unregulated T cells, and together these cells stimulate production of very high levels of stimulatory cytokines such as IL-2 and IL-15. Under these conditions, the response to self-ligands can be as intense as the normal immune response to foreign Ags. Overall, the tempo of proliferation leading to MP cell formation is determined by the interplay of multiple factors, including relative TCR affinity of responding naive T cells, the influence of Tregs and possibly preexisting MP cells, the activation status of APCs, and the concentration of various cytokines and expression of receptors for these cytokines on T cells. Once formed, MP cells divide intermittently in response to cytokines and play an important role in providing rapid Ag-specific and -nonspecific responses to pathogens.
CONCLUDING REMARKS
As outlined in this review, CD8+ and CD4+ memory T cells comprise two broad subsets of cells, each derived from naive precursor cells but generated via essentially different pathways of differentiation. In normal adult animals, exposure to a multiplicity of environmental Ags generates an extensive repertoire of foreign Ag-specific memory cells that provide strong protective immunity against pathogens previously encountered. However, in early neonatal life and adults maintained in an SPF environment, Ag-induced memory T cells are rare and most T cells with an MP are the progeny of naive T cells responding to self-MHC ligands. These MP cells have a broad TCR repertoire and complement the naive adaptive immune system in giving rapid responses to pathogens; in particular, together with natural killer (NK) and other innate lymphoid cells, they provide an intermediary layer of immune activity between myeloid cell–mediated innate and lymphocyte-mediated adaptive immunity. Conceivably, manipulating MP cell generation and/or function might be useful to augment pathogen-targeted immunity or regulate autoimmune disease. Future studies will be needed to assess this possibility.
ACKNOWLEDGMENTS
We gratefully acknowledge the late C.D. Surh and W.E. Paul for their invaluable contribution to this field. We also thank R.N. Germain, B. Min, and A. Sher for their critical support. This work was supported by the National Health and Medical Research Council. The authors declare no competing financial interests.
Footnotes
Editors: David Masopust and Rafi Ahmed
Additional Perspectives on T-Cell Memory available at www.cshperspectives.org
REFERENCES
- Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89: 1033–1041. 10.1016/S0092-8674(00)80291-3 [DOI] [PubMed] [Google Scholar]
- Alves NL, Richard-Le Goff O, Huntington ND, Sousa AP, Ribeiro VS, Bordack A, Vives FL, Peduto L, Chidgey A, Cumano A, et al. 2009. Characterization of the thymic IL-7 niche in vivo. Proc Natl Acad Sci 106: 1512–1517. 10.1073/pnas.0809559106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. 1998. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med 188: 2301–2311. 10.1084/jem.188.12.2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. 2004. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol 5: 809–817. 10.1038/ni1098 [DOI] [PubMed] [Google Scholar]
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 195: 1541–1548. 10.1084/jem.20020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RE, Crossley E, Murray S, Forman J. 2003. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med 198: 1583–1593. 10.1084/jem.20031051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, et al. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532: 512–516. 10.1038/nature17655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. 2003. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 9: 540–547. 10.1038/nm866 [DOI] [PubMed] [Google Scholar]
- Bolton HA, Zhu E, Terry AM, Guy TV, Koh WP, Tan SY, Power CA, Bertolino P, Lahl K, Sparwasser T, et al. 2015. Selective Treg reconstitution during lymphopenia normalizes DC costimulation and prevents graft-versus-host disease. J Clin Invest 125: 3627–3641. 10.1172/JCI76031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois C, Stockinger B. 2006. CD25+CD4+ regulatory T cells and memory T cells prevent lymphopenia-induced proliferation of naive T cells in transient states of lymphopenia. J Immunol 177: 4558–4566. 10.4049/jimmunol.177.7.4558 [DOI] [PubMed] [Google Scholar]
- Boyman O, Cho JH, Tan JT, Surh CD, Sprent J. 2006a. A major histocompatibility complex class I-dependent subset of memory phenotype CD8+ cells. J Exp Med 203: 1817–1825. 10.1084/jem.20052495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. 2006b. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311: 1924–1927. 10.1126/science.1122927 [DOI] [PubMed] [Google Scholar]
- Buentke E, Mathiot A, Tolaini M, Di Santo J, Zamoyska R, Seddon B. 2006. Do CD8 effector cells need IL-7R expression to become resting memory cells? Blood 108: 1949–1956. 10.1182/blood-2006-04-016857 [DOI] [PubMed] [Google Scholar]
- Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. 2003. IL-15Rα expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci 100: 4724–4729. 10.1073/pnas.0737048100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. 2004. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med 200: 825–834. 10.1084/jem.20041389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JA, Stankovic AK, Cooper MD. 1994. A novel subpopulation of primed T cells in the human fetus. J Immunol 152: 3098–3106. [PubMed] [Google Scholar]
- Carrio R, Rolle CE, Malek TR. 2007. Non-redundant role for IL-7R signaling for the survival of CD8+ memory T cells. Eur J Immunol 37: 3078–3088. 10.1002/eji.200737585 [DOI] [PubMed] [Google Scholar]
- Cheng LE, Ohlen C, Nelson BH, Greenberg PD. 2002. Enhanced signaling through the IL-2 receptor in CD8+ T cells regulated by antigen recognition results in preferential proliferation and expansion of responding CD8+ T cells rather than promotion of cell death. Proc Natl Acad Sci 99: 3001–3006. 10.1073/pnas.052676899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Boyman O, Kim HO, Hahm B, Rubinstein MP, Ramsey C, Kim DM, Surh CD, Sprent J. 2007. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med 204: 1787–1801. 10.1084/jem.20070740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Kim HO, Surh CD, Sprent J. 2010. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity 32: 214–226. 10.1016/j.immuni.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Kim HO, Ju YJ, Kye YC, Lee GW, Lee SW, Yun CH, Bottini N, Webster K, Goodnow CC, et al. 2016. CD45-mediated control of TCR tuning in naive and memory CD8+ T cells. Nat Commun 7: 13373. 10.1038/ncomms13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, Crotty S. 2013. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol 190: 4014–4026. 10.4049/jimmunol.1202963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, Bevan MJ, Zehn D, Prlic M. 2013. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep 3: 701–708. 10.1016/j.celrep.2013.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpitts SL, Stoklasek TA, Plumlee CR, Obar JJ, Guo C, Lefrançois L. 2012. Cutting edge: the role of IFN-α receptor and MyD88 signaling in induction of IL-15 expression in vivo. J Immunol 188: 2483–2487. 10.4049/jimmunol.1103609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Joshi NS, Jiang A, Kaech SM. 2009. Effects of signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine 27: 2177–2187. 10.1016/j.vaccine.2009.01.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Hara T, Simmons S, Wagatsuma K, Abe A, Miyachi H, Kitano S, Ishii M, Tani-ichi S, Ikuta K. 2014. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc Natl Acad Sci 111: 1915–1920. 10.1073/pnas.1318281111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AM, Ramos HJ, Davis LS, Farrar JD. 2008. Cutting edge: a T-bet-independent role for IFN-α/β in regulating IL-2 secretion in human CD4+ central memory T cells. J Immunol 181: 8204–8208. 10.4049/jimmunol.181.12.8204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do JS, Min B. 2009. Differential requirements of MHC and of DCs for endogenous proliferation of different T-cell subsets in vivo. Proc Natl Acad Sci 106: 20394–20398. 10.1073/pnas.0909954106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do JS, Visperas A, Oh K, Stohlman SA, Min B. 2012. Memory CD4 T cells induce selective expression of IL-27 in CD8+ dendritic cells and regulate homeostatic naive T cell proliferation. J Immunol 188: 230–237. 10.4049/jimmunol.1101908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobber R, Hertogh-Huijbregts A, Rozing J, Bottomly K, Nagelkerken L. 1992. The involvement of the intestinal microflora in the expansion of CD4+ T cells with a naive phenotype in the periphery. Dev Immunol 2: 141–150. 10.1155/1992/57057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Somani AK, Love PE, Zheng X, Chen X, Zhang J. 2016. CD5-mediated inhibition of TCR signaling proceeds normally in the absence of SHP-1. Int J Mol Med 38: 45–56. 10.3892/ijmm.2016.2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman JR, Štefanová I, Yasutomo K, Germain RN. 2000. CD4+ T cell survival is not directly linked to self-MHC-induced TCR signaling. Nat Immunol 1: 329–335. 10.1038/79783 [DOI] [PubMed] [Google Scholar]
- D'Souza WN, Hedrick SM. 2006. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol 177: 777–781. 10.4049/jimmunol.177.2.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. 2002. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity 17: 537–547. 10.1016/S1074-7613(02)00429-6 [DOI] [PubMed] [Google Scholar]
- Dubois SP, Waldmann TA, Muller JR. 2005. Survival adjustment of mature dendritic cells by IL-15. Proc Natl Acad Sci 102: 8662–8667. 10.1073/pnas.0503360102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. 2009. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol 10: 1162–1169. 10.1038/ni.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11: 173–181. 10.1016/S1074-7613(00)80092-8 [DOI] [PubMed] [Google Scholar]
- Gay D, Maddon P, Sekaly R, Talle MA, Godfrey M, Long E, Goldstein G, Chess L, Axel R, Kappler J, et al. 1987. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nature 328: 626–629. 10.1038/328626a0 [DOI] [PubMed] [Google Scholar]
- Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. 1994. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J 13: 2822–2830. 10.1002/j.1460-2075.1994.tb06576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath AW, Bevan MJ. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 11: 183–190. 10.1016/S1074-7613(00)80093-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. 2002. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med 195: 1515–1522. 10.1084/jem.20020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommeaux J, Grégoire C, Nguessan P, Richelme M, Malissen M, Guerder S, Malissen B, Carrier A. 2009. Thymus-specific serine protease regulates positive selection of a subset of CD4+ thymocytes. Eur J Immunol 39: 956–964. 10.1002/eji.200839175 [DOI] [PubMed] [Google Scholar]
- Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. 1994. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science 264: 965–968. 10.1126/science.8178155 [DOI] [PubMed] [Google Scholar]
- Grossman Z, Paul WE. 2015. Dynamic tuning of lymphocytes: physiological basis, mechanisms, and function. Annu Rev Immunol 33: 677–713. 10.1146/annurev-immunol-032712-100027 [DOI] [PubMed] [Google Scholar]
- Grossman Z, Singh NJ, Simonetti FR, Lederman MM, Douek DC, Deeks SG, Kawabe T, Bocharov G, Meier-Schellersheim M, Alon H, et al. 2020. “Rinse and replace”: boosting T cell turnover to reduce HIV-1 reservoirs. Trends Immunol 41: 466–480. 10.1016/j.it.2020.04.003 [DOI] [PubMed] [Google Scholar]
- Gudmundsdottir H, Turka LA. 2001. A closer look at homeostatic proliferation of CD4+ T cells: costimulatory requirements and role in memory formation. J Immunol 167: 3699–3707. 10.4049/jimmunol.167.7.3699 [DOI] [PubMed] [Google Scholar]
- Guo L, Huang Y, Chen X, Hu-Li J, Urban JF Jr, Paul WE. 2015. Innate immunological function of TH2 cells in vivo. Nat Immunol 16: 1051–1059. 10.1038/ni.3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen KA, Moses CT, Drasler EF, Podetz-Pedersen KM, Jameson SC, Khoruts A. 2004. A role for CD28 in lymphopenia-induced proliferation of CD4 T cells. J Immunol 173: 3909–3915. 10.4049/jimmunol.173.6.3909 [DOI] [PubMed] [Google Scholar]
- Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. 2009. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med 206: 435–448. 10.1084/jem.20081829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. 2006. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol 7: 475–481. 10.1038/ni1326 [DOI] [PubMed] [Google Scholar]
- Hara T, Shitara S, Imai K, Miyachi H, Kitano S, Yao H, Tani-ichi S, Ikuta K. 2012. Identification of IL-7-producing cells in primary and secondary lymphoid organs using IL-7-GFP knock-in mice. J Immunol 189: 1577–1584. 10.4049/jimmunol.1200586 [DOI] [PubMed] [Google Scholar]
- Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. 2006. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science 312: 114–116. 10.1126/science.1124228 [DOI] [PubMed] [Google Scholar]
- Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. 2000. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol 1: 433–440. 10.1038/80877 [DOI] [PubMed] [Google Scholar]
- Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, Borst J. 2005. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol 175: 1665–1676. 10.4049/jimmunol.175.3.1665 [DOI] [PubMed] [Google Scholar]
- Hu J, August A. 2008. Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. J Immunol 180: 6544–6552. 10.4049/jimmunol.180.10.6544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Wei B, Velazquez P, Borneman J, Braun J. 2005. Commensal microbiota alter the abundance and TCR responsiveness of splenic naive CD4+ T lymphocytes. Clin Immunol 117: 221–230. 10.1016/j.clim.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, Arima M, Kuroda Y, Tokuhisa T. 2002. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol 3: 558–563. 10.1038/ni802 [DOI] [PubMed] [Google Scholar]
- Jiang Q, Su H, Knudsen G, Helms W, Su L. 2006. Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol 7: 6. 10.1186/1471-2172-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27: 281–295. 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Cui W. 2012. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12: 749–761. 10.1038/nri3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. 2010. Prolonged interleukin-2Rα expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32: 91–103. 10.1016/j.immuni.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Garcia S, Simpson E, Stockinger B. 2002. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol 3: 244–250. 10.1038/ni766 [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Zamoyska R, Stockinger B. 2003. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med 197: 1007–1016. 10.1084/jem.20021812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T, Sun SL, Fujita T, Yamaki S, Asao A, Takahashi T, So T, Ishii N. 2013. Homeostatic proliferation of naive CD4+ T cells in mesenteric lymph nodes generates gut-tropic Th17 cells. J Immunol 190: 5788–5798. 10.4049/jimmunol.1203111 [DOI] [PubMed] [Google Scholar]
- Kawabe T, Suzuki N, Yamaki S, Sun SL, Asao A, Okuyama Y, So T, Iwakura Y, Ishii N. 2016. Mesenteric lymph nodes contribute to proinflammatory Th17-cell generation during inflammation of the small intestine in mice. Eur J Immunol 46: 1119–1131. 10.1002/eji.201545907 [DOI] [PubMed] [Google Scholar]
- Kawabe T, Jankovic D, Kawabe S, Huang Y, Lee PH, Yamane H, Zhu J, Sher A, Germain RN, Paul WE. 2017. Memory-phenotype CD4+ T cells spontaneously generated under steady-state conditions exert innate TH1-like effector function. Sci Immunol 2: eaam9304. 10.1126/sciimmunol.aam9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T, Zhu J, Sher A. 2018. Foreign antigen-independent memory-phenotype CD4+ T cells: a new player in innate immunity? Nat Rev Immunol 18: 1. 10.1038/nri.2018.12 [DOI] [PubMed] [Google Scholar]
- Kawabe T, Yi J, Kawajiri A, Hilligan K, Fang D, Ishii N, Yamane H, Zhu J, Jankovic D, Kim KS, et al. 2020. Requirements for the differentiation of innate T-bethigh memory-phenotype CD4+ T lymphocytes under steady state Nat Commun 11: 3366. 10.1038/s41467-020-17136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedl RM, White JT. 2018. Foreign antigen-independent memory-phenotype CD4+ T cells: a new player in innate immunity? Nat Rev Immunol 18: 1. 10.1038/nri.2018.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled AR, Durum SK. 2002. Lymphocide: cytokines and the control of lymphoid homeostasis. Nat Rev Immunol 2: 817–830. 10.1038/nri931 [DOI] [PubMed] [Google Scholar]
- Khaled AR, Li WQ, Huang J, Fry TJ, Khaled AS, Mackall CL, Muegge K, Young HA, Durum SK. 2002. Bax deficiency partially corrects interleukin-7 receptor α deficiency. Immunity 17: 561–573. 10.1016/S1074-7613(02)00450-8 [DOI] [PubMed] [Google Scholar]
- Kieper WC, Jameson SC. 1999. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc Natl Acad Sci 96: 13306–13311. 10.1073/pnas.96.23.13306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, Surh CD. 2002. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med 195: 1533–1539. 10.1084/jem.20020067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieper WC, Burghardt JT, Surh CD. 2004. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol 172: 40–44. 10.4049/jimmunol.172.1.40 [DOI] [PubMed] [Google Scholar]
- Kieper WC, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang HQ, Dummer W, Shen H, Cebra JJ, Surh CD. 2005. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol 174: 3158–3163. 10.4049/jimmunol.174.6.3158 [DOI] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 8: 191–197. 10.1038/ni1428 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Wang X, Radfar S, Sproule TJ, Roopenian DC, Cantor H. 2011. CD8+ T regulatory cells express the Ly49 class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci 108: 2010–2015. 10.1073/pnas.1018974108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, Surh CD. 2016. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351: 858–863. 10.1126/science.aac5560 [DOI] [PubMed] [Google Scholar]
- Klein L, Kyewski B, Allen PM, Hogquist KA. 2014. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat Rev Immunol 14: 377–391. 10.1038/nri3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Ohashi Y, Tada K, Nakamura M, Sugamura K. 1994a. Expression of the mouse interleukin-2 receptor γ chain in various cell populations of the thymus and spleen. Eur J Immunol 24: 2026–2030. 10.1002/eji.1830240914 [DOI] [PubMed] [Google Scholar]
- Kondo M, Takeshita T, Higuchi M, Nakamura M, Sudo T, Nishikawa S, Sugamura K. 1994b. Functional participation of the IL-2 receptor γ chain in IL-7 receptor complexes. Science 263: 1453–1454. 10.1126/science.8128231 [DOI] [PubMed] [Google Scholar]
- Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. 2003. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med 198: 1797–1806. 10.1084/jem.20030735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque N, Whitfield LS, Obst R, Waltzinger C, Benoist C, Mathis D. 2001. How much TCR does a T cell need? Immunity 15: 71–82. 10.1016/S1074-7613(01)00170-4 [DOI] [PubMed] [Google Scholar]
- Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. 2013. Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci 110: 13498–13503. 10.1073/pnas.1307572110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. 2004. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc Natl Acad Sci 101: 9357–9362. 10.1073/pnas.0400640101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. 2007. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol 8: 1255–1265. 10.1038/ni1513 [DOI] [PubMed] [Google Scholar]
- Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. 2009. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol 10: 1155–1161. 10.1038/ni.1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. 2013. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity 38: 263–274. 10.1016/j.immuni.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Endres R, Shimonkevitz R, Zlotnik A, Dialynas D, Fitch F, Kappler J. 1983. The major histocompatibility complex-restricted antigen receptor on T cells. II: Role of the L3T4 product. J Exp Med 158: 1077–1091. 10.1084/jem.158.4.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Kappler J, Mitchell T. 1999. Type I interferons keep activated T cells alive. J Exp Med 189: 521–530. 10.1084/jem.189.3.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, Rutishauser R, Cui W, Kleinstein SH, Craft J, et al. 2011. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4+ cell properties during viral infection. Immunity 35: 633–646. 10.1016/j.immuni.2011.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Bourgeois C, Dautigny N, Lucas B. 2003. On the role of MHC class II molecules in the survival and lymphopenia-induced proliferation of peripheral CD4+ T cells. Proc Natl Acad Sci 100: 6021–6026. 10.1073/pnas.1037754100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, Spasova DS, Frimpong-Boateng K, Kim HO, Lee M, Kim KS, Surh CD. 2017. Interleukin-7 availability is maintained by a hematopoietic cytokine sink comprising innate lymphoid cells and T cells. Immunity 47: 171–182.e4. 10.1016/j.immuni.2017.07.005 [DOI] [PubMed] [Google Scholar]
- Mazzucchelli RI, Warming S, Lawrence SM, Ishii M, Abshari M, Washington AV, Feigenbaum L, Warner AC, Sims DJ, Li WQ, et al. 2009. Visualization and identification of IL-7 producing cells in reporter mice. PLoS ONE 4: e7637. 10.1371/journal.pone.0007637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertsching E, Burdet C, Ceredig R. 1995. IL-7 transgenic mice: analysis of the role of IL-7 in the differentiation of thymocytes in vivo and in vitro. Int Immunol 7: 401–414. 10.1093/intimm/7.3.401 [DOI] [PubMed] [Google Scholar]
- Miller CN, Hartigan-O'Connor DJ, Lee MS, Laidlaw G, Cornelissen IP, Matloubian M, Coughlin SR, McDonald DM, McCune JM. 2013. IL-7 production in murine lymphatic endothelial cells and induction in the setting of peripheral lymphopenia. Int Immunol 25: 471–483. 10.1093/intimm/dxt012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. 2003. Neonates support lymphopenia-induced proliferation. Immunity 18: 131–140. 10.1016/S1074-7613(02)00508-3 [DOI] [PubMed] [Google Scholar]
- Min B, Foucras G, Meier-Schellersheim M, Paul WE. 2004. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci 101: 3874–3879. 10.1073/pnas.0400606101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Yamane H, Hu-Li J, Paul WE. 2005. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol 174: 6039–6044. 10.4049/jimmunol.174.10.6039 [DOI] [PubMed] [Google Scholar]
- Mittrücker HW, Kursar M, Köhler A, Hurwitz R, Kaufmann SH. 2001. Role of CD28 for the generation and expansion of antigen-specific CD8+ T lymphocytes during infection with Listeria monocytogenes. J Immunol 167: 5620–5627. 10.4049/jimmunol.167.10.5620 [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. 2007. Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27: 203–213. 10.1016/j.immuni.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SF, Soroosh P, Takahashi T, Yoshikai Y, Shen H, Lefrançois L, Borst J, Sugamura K, Ishii N. 2008. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol 181: 5990–6001. 10.4049/jimmunol.181.9.5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K, Ahmed R. 2000. Cutting edge: naive T cells masquerading as memory cells. J Immunol 165: 1733–1737. 10.4049/jimmunol.165.4.1733 [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286: 1377–1381. 10.1126/science.286.5443.1377 [DOI] [PubMed] [Google Scholar]
- Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K. 2007. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 316: 1349–1353. 10.1126/science.1141915 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, Villadangos JA, Ploegh H, Peters C, Rudensky AY. 1998. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science 280: 450–453. 10.1126/science.280.5362.450 [DOI] [PubMed] [Google Scholar]
- Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S, et al. 1988. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature 333: 571–573. 10.1038/333571a0 [DOI] [PubMed] [Google Scholar]
- Nitta T, Murata S, Sasaki K, Fujii H, Ripen AM, Ishimaru N, Koyasu S, Tanaka K, Takahama Y. 2010. Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity 32: 29–40. 10.1016/j.immuni.2009.10.009 [DOI] [PubMed] [Google Scholar]
- Oehen S, Brduscha-Riem K. 1999. Naive cytotoxic T lymphocytes spontaneously acquire effector function in lymphocytopenic recipients: a pitfall for T cell memory studies? Eur J Immunol 29: 608–614. [DOI] [PubMed] [Google Scholar]
- Onder L, Narang P, Scandella E, Chai Q, Iolyeva M, Hoorweg K, Halin C, Richie E, Kaye P, Westermann J, et al. 2012. IL-7-producing stromal cells are critical for lymph node remodeling. Blood 120: 4675–4683. 10.1182/blood-2012-03-416859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LC, Dhanji S, Snow JW, Priatel JJ, Ma MC, Miners MJ, Teh HS, Goldsmith MA, Abraham N. 2007. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7Rα mutant mice. J Exp Med 204: 619–631. 10.1084/jem.20061871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán AJ, Pepper M, Chu HH, Green JM, Jenkins MK. 2012. CD28 promotes CD4+ T cell clonal expansion during infection independently of its YMNM and PYAP motifs. J Immunol 189: 2909–2917. 10.4049/jimmunol.1103231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, et al. 2007. “Coreceptor tuning”: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol 8: 1049–1059. 10.1038/ni1512 [DOI] [PubMed] [Google Scholar]
- Paul WE, Milner JD, Grossman Z. 2013. Pathogen-sensing, regulatory T cells, and responsiveness-tuning collectively regulate foreign- and self-antigen mediated T-cell responses. Cold Spring Harb Symp Quant Biol 78: 265–276. 10.1101/sqb.2013.78.020198 [DOI] [PubMed] [Google Scholar]
- Pereira P, Forni L, Larsson EL, Cooper M, Heusser C, Coutinho A. 1986. Autonomous activation of B and T cells in antigen-free mice. Eur J Immunol 16: 685–688. 10.1002/eji.1830160616 [DOI] [PubMed] [Google Scholar]
- Perez-Villar JJ, Whitney GS, Bowen MA, Hewgill DH, Aruffo AA, Kanner SB. 1999. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol 19: 2903–2912. 10.1128/MCB.19.4.2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. 2010. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32: 79–90. 10.1016/j.immuni.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polic B, Kunkel D, Scheffold A, Rajewsky K. 2001. How αβ T cells deal with induced TCRα ablation. Proc Natl Acad Sci 98: 8744–8749. 10.1073/pnas.141218898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M, Blazar BR, Khoruts A, Zell T, Jameson SC. 2001. Homeostatic expansion occurs independently of costimulatory signals. J Immunol 167: 5664–5668. 10.4049/jimmunol.167.10.5664 [DOI] [PubMed] [Google Scholar]
- Prlic M, Hernandez-Hoyos G, Bevan MJ. 2006. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med 203: 2135–2143. 10.1084/jem.20060928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. 2007. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med 204: 951–961. 10.1084/jem.20061805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey C, Rubinstein MP, Kim DM, Cho JH, Sprent J, Surh CD. 2008. The lymphopenic environment of CD132 (common γ-chain)-deficient hosts elicits rapid homeostatic proliferation of naive T cells via IL-15. J Immunol 180: 5320–5326. 10.4049/jimmunol.180.8.5320 [DOI] [PubMed] [Google Scholar]
- Ratajczak W, Niedźwiedzka-Rystwej P, Tokarz-Deptuła B, Deptuła W. 2018. Immunological memory cells. Cent Eur J Immunol 43: 194–203. 10.5114/ceji.2018.77390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repass JF, Laurent MN, Carter C, Reizis B, Bedford MT, Cardenas K, Narang P, Coles M, Richie ER. 2009. IL7-hCD25 and IL7-Cre BAC transgenic mouse lines: new tools for analysis of IL-7 expressing cells. Genesis 47: 281–287. 10.1002/dvg.20497 [DOI] [PubMed] [Google Scholar]
- Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF. 1988. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci 85: 5190–5194. 10.1073/pnas.85.14.5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata T, Iwagami S, Tsuruta Y, Teraoka H, Tatsumi Y, Kita Y, Nishikawa S, Takai Y, Fujiwara H. 1990. Constitutive expression of interleukin-7 mRNA and production of IL-7 by a cloned murine thymic stromal cell line. J Leukoc Biol 48: 205–212. 10.1002/jlb.48.3.205 [DOI] [PubMed] [Google Scholar]
- Sandau MM, Schluns KS, Lefrancois L, Jameson SC. 2004. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15Rα by the same cells. J Immunol 173: 6537–6541. 10.4049/jimmunol.173.11.6537 [DOI] [PubMed] [Google Scholar]
- Santori FR, Kieper WC, Brown SM, Lu Y, Neubert TA, Johnson KL, Naylor S, Vukmanović S, Hogquist KA, Jameson SC. 2002. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity 17: 131–142. 10.1016/S1074-7613(02)00361-8 [DOI] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. 2008. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med 205: 625–640. 10.1084/jem.20071641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M, Glasebrook AL, Fitch FW. 1980. Igg or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol 125: 2665–2672. [PubMed] [Google Scholar]
- Sato N, Patel HJ, Waldmann TA, Tagaya Y. 2007. The IL-15/IL-15Rα on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci 104: 588–593. 10.1073/pnas.0610115104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. 2014. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346: 98–101. 10.1126/science.1254536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrançois L. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 1: 426–432. 10.1038/80868 [DOI] [PubMed] [Google Scholar]
- Schluns KS, Klonowski KD, Lefrançois L. 2004. Transregulation of memory CD8 T-cell proliferation by IL-15Rα+ bone marrow-derived cells. Blood 103: 988–994. 10.1182/blood-2003-08-2814 [DOI] [PubMed] [Google Scholar]
- Seddon B, Zamoyska R. 2002. TCR signals mediated by Src family kinases are essential for the survival of naive T cells. J Immunol 169: 2997–3005. 10.4049/jimmunol.169.6.2997 [DOI] [PubMed] [Google Scholar]
- Seder RA, Ahmed R. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol 4: 835–842. 10.1038/ni969 [DOI] [PubMed] [Google Scholar]
- Shalapour S, Deiser K, Sercan O, Tuckermann J, Minnich K, Willimsky G, Blankenstein T, Hämmerling GJ, Arnold B, Schüler T. 2010. Commensal microflora and interferon-γ promote steady-state interleukin-7 production in vivo. Eur J Immunol 40: 2391–2400. 10.1002/eji.201040441 [DOI] [PubMed] [Google Scholar]
- Sheridan BS, Lefrançois L. 2011. Regional and mucosal memory T cells. Nat Immunol 12: 485–491. 10.1038/ni.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. 2001. Sensory adaptation in naive peripheral CD4 T cells. J Exp Med 194: 1253–1262. 10.1084/jem.194.9.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook JP, Kim C, Williams MA. 2018. TCR signal strength controls the differentiation of CD4+ effector and memory T cells. Sci Immunol 3: eaas9103. 10.1126/sciimmunol.aas9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroosh P, Ine S, Sugamura K, Ishii N. 2007. Differential requirements for OX40 signals on generation of effector and central memory CD4+ T cells. J Immunol 179: 5014–5023. 10.4049/jimmunol.179.8.5014 [DOI] [PubMed] [Google Scholar]
- Sosinowski T, White JT, Cross EW, Haluszczak C, Marrack P, Gapin L, Kedl RM. 2013. CD8α+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J Immunol 190: 1936–1947. 10.4049/jimmunol.1203149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudja SM, Ruiz AL, Marie JC, Lauvau G. 2012. Inflammatory monocytes activate memory CD8+ T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 37: 549–562. 10.1016/j.immuni.2012.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. 1994. T and B memory cells. Cell 76: 315–322. 10.1016/0092-8674(94)90338-7 [DOI] [PubMed] [Google Scholar]
- Sprent J, Surh CD. 2002. T cell memory. Annu Rev Immunol 20: 551–579. 10.1146/annurev.immunol.20.100101.151926 [DOI] [PubMed] [Google Scholar]
- Sprent J, Surh CD. 2011. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol 12: 478–484. 10.1038/ni.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamou P, de Jersey J, Carmignac D, Mamalaki C, Kioussis D, Stockinger B. 2003. Chronic exposure to low levels of antigen in the periphery causes reversible functional impairment correlating with changes in CD5 levels in monoclonal CD8 T cells. J Immunol 171: 1278–1284. 10.4049/jimmunol.171.3.1278 [DOI] [PubMed] [Google Scholar]
- Stefanová I, Dorfman JR, Germain RN. 2002. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature 420: 429–434. 10.1038/nature01146 [DOI] [PubMed] [Google Scholar]
- Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. 2013. Virus-specific CD4+ memory-phenotype T cells are abundant in unexposed adults. Immunity 38: 373–383. 10.1016/j.immuni.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H, Nishikawa S. 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci 90: 9125–9129. 10.1073/pnas.90.19.9125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. 2008. Homeostasis of naive and memory T cells. Immunity 29: 848–862. 10.1016/j.immuni.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Swain SL, Hu H, Huston G. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science 286: 1381–1383. 10.1126/science.286.5443.1381 [DOI] [PubMed] [Google Scholar]
- Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. 2003. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol 31: 708–714. 10.1016/S0301-472X(03)00160-7 [DOI] [PubMed] [Google Scholar]
- Takada K, Jameson SC. 2009. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J Exp Med 206: 2253–2269. 10.1084/jem.20082553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Van Laethem F, Xing Y, Akane K, Suzuki H, Murata S, Tanaka K, Jameson SC, Singer A, Takahama Y. 2015. TCR affinity for thymoproteasome-dependent positively selecting peptides conditions antigen responsiveness in CD8+ T cells. Nat Immunol 16: 1069–1076. 10.1038/ni.3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. 1996. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity 5: 217–228. 10.1016/S1074-7613(00)80317-9 [DOI] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci 98: 8732–8737. 10.1073/pnas.161126098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med 195: 1523–1532. 10.1084/jem.20020066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science 276: 2057–2062. 10.1126/science.276.5321.2057 [DOI] [PubMed] [Google Scholar]
- Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Muller W, Killeen N, Rajewsky K. 1995. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science 269: 535–537. 10.1126/science.7542801 [DOI] [PubMed] [Google Scholar]
- Tough DF, Sprent J. 1994. Turnover of naive- and memory-phenotype T cells. J Exp Med 179: 1127–1135. 10.1084/jem.179.4.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM, Brodsky MH, Irving BA, Levin SD, Perlmutter RM, Littman DR. 1990. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell 60: 755–765. 10.1016/0092-8674(90)90090-2 [DOI] [PubMed] [Google Scholar]
- Tyznik AJ, Sun JC, Bevan MJ. 2004. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J Exp Med 199: 559–565. 10.1084/jem.20031961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A, Bookman MA, Horak EM, Bolen JB. 1988. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 55: 301–308. 10.1016/0092-8674(88)90053-0 [DOI] [PubMed] [Google Scholar]
- Viret C, Wong FS, Janeway CA Jr. 1999. Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity 10: 559–568. 10.1016/S1074-7613(00)80055-2 [DOI] [PubMed] [Google Scholar]
- Vivien L, Benoist C, Mathis D. 2001. T lymphocytes need IL-7 but not IL-4 or IL-6 to survive in vivo. Int Immunol 13: 763–768. 10.1093/intimm/13.6.763 [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. 1999. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J Immunol 163: 3735–3745. [PubMed] [Google Scholar]
- White JT, Cross EW, Burchill MA, Danhorn T, McCarter MD, Rosen HR, O'Connor B, Kedl RM. 2016. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat Commun 7: 11291. 10.1038/ncomms11291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire JK, Eam B, Benning N, Whitton JL. 2007. Direct interferon-γ signaling dramatically enhances CD4+ and CD8+ T cell memory. J Immunol 179: 1190–1197. 10.4049/jimmunol.179.2.1190 [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. 2004. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol 173: 6694–6702. 10.4049/jimmunol.173.11.6694 [DOI] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441: 890–893. 10.1038/nature04790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Ravkov EV, Bevan MJ. 2008. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity 28: 533–545. 10.1016/j.immuni.2008.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstead CJ, Fraser JM, Khoruts A. 2008. Regulatory CD4+CD25+Foxp3+ T cells selectively inhibit the spontaneous form of lymphopenia-induced proliferation of naive T cells. J Immunol 180: 7305–7317. 10.4049/jimmunol.180.11.7305 [DOI] [PubMed] [Google Scholar]
- Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. 2007. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med 204: 1665–1675. 10.1084/jem.20070618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Jameson SC, Hogquist KA. 2013. Thymoproteasome subunit-β5 T generates peptide-MHC complexes specialized for positive selection. Proc Natl Acad Sci 110: 6979–6984. 10.1073/pnas.1222244110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaki S, Ine S, Kawabe T, Okuyama Y, Suzuki N, Soroosh P, Mousavi SF, Nagashima H, Sun SL, So T, et al. 2014. OX40 and IL-7 play synergistic roles in the homeostatic proliferation of effector memory CD4+ T cells. Eur J Immunol 44: 3015–3025. 10.1002/eji.201444701 [DOI] [PubMed] [Google Scholar]
- Yi J, Jung J, Hong SW, Lee JY, Han D, Kim KS, Sprent J, Surh CD. 2019. Unregulated antigen-presenting cell activation by T cells breaks self tolerance. Proc Natl Acad Sci 116: 1007–1016. 10.1073/pnas.1818624116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Kawabe T, Sprent J. 2020. New insights on T-cell self-tolerance. Curr Opin Immunol 63: 14–20. 10.1016/j.coi.2019.10.002 [DOI] [PubMed] [Google Scholar]
- Younes SA, Punkosdy G, Caucheteux S, Chen T, Grossman Z, Paul WE. 2011. Memory phenotype CD4 T cells undergoing rapid, nonburst-like, cytokine-driven proliferation can be distinguished from antigen-experienced memory cells. PLoS Biol 9: e1001171. 10.1371/journal.pbio.1001171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Lee SY, Bevan MJ. 2009. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458: 211–214. 10.1038/nature07657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. 2012. TGF-β signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat Immunol 13: 667–673. 10.1038/ni.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8: 591–599. 10.1016/S1074-7613(00)80564-6 [DOI] [PubMed] [Google Scholar]