Abstract
OBJECTIVE:
The objective of this study was to analyze the associations between the pro-inflammatory markers with the clinical outcomes of knee osteoarthritis (OA) in patients using resveratrol as an add-on treatment with meloxicam.
MATERIALS AND METHODS:
This was a double-blind controlled clinical investigation, with 110 eligible patients with OA assigned randomly to receive 15 mg a day meloxicam with either resveratrol 500 mg a day or placebo for 90 days. The standard tools for assessment of pain severity and physical functions were utilized. The tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 in the blood were evaluated. Spearman's correlation coefficient test was used to determine the significance of correlations.
RESULTS:
The regression analysis to determine the correlation between reductions of the inflammatory biomarkers with the amelioration of the clinical scores showed a nonsignificant weak correlation between these variables. Total clinical scores of each assessment tool that was used “Knee Injury and OA Outcome Score (KOOS) and WOMAC” displayed a weak and nonsignificant correlation with TNF-α, IL-1β blood level. The Spearman's correlation shows a relatively nonsignificant association between IL-6 levels and KOOS, WOMAC, and Visual Analog Scale scores after incorporating resveratrol as an adjuvant with meloxicam for 90 days.
CONCLUSION:
A weak and nonsignificant correlation between serum biomarkers and the clinical outcomes has been suggested in patients with painful knee OA treated with meloxicam and resveratrol.
Keywords: Association, biomarkers, correlation, score
Introduction
A painful articular complaint in osteoarthritis (OA) is accompanied by difficulty in movement and structural changes of the joints.[1] The pathological changes of OA are related to the excessive generation of signaling molecules such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α). The currently accepted concept of OA pathogenesis involves inflammation as an additional trigger of the OA progression.[2] This assumption will change the presentation of OA from noninflammatory disease to a complex chain of inflammation and cartilage degradation.[3] Inflammation is a potent contributor to osteoarthritic pain,[4] and the severity of pain has been linked with these inflammatory cytokines.[5] However, the exact relationship between those biomarkers and OA symptoms is relatively unexplored. Although previous studies have identified associations of many inflammatory cytokines with the destruction of cartilage, subchondral bone remodeling, synovitis,[3] and OA severity and progression.[6] Few attempts have reported the association of a particular cytokine to the levels of pain and its clinical scores; therefore, determining the relationships that link inflammatory biomarkers and knee complaint in interventional studies remains controversial. The positive correlations between synovium inflammation, joint effusion, and knee pain[7] characterize the significant impact of the inflammatory process in the initiation of knee pain.
Several authors have elucidated the contribution of cytokines in the generation of pain and clinical impairment through different mechanisms such as sensitization of joint nociceptors via mechanical stimulation and the mechanical hyperalgesia induction in the joints,[8] while others connected the loss of knee joint function to the elevation of pro-inflammatory cytokines.[9] The currently available conventional pharmacological drugs alleviated the pain and reduced the OA-associated inflammatory process. However, due to the relevant drug-related adverse effects, new approaches highlighted the role of herbal medicine as a choice in this regard. Resveratrol now became a core element in the treatment of musculoskeletal diseases. As predicted from clinical trials on RA management, resveratrol produces a significant decline in the main biomarkers of RA pathogenesis, including clinical and biochemical biomarkers.[10] To date, a small number of insufficient data are available concerning the potential effect of pro-inflammatory cytokines on the intensity of pain in painful knee OA utilizing interventional therapy. The present study aims to evaluate the correlation between the pro-inflammatory markers and the clinical outcomes in patients with painful knee OA during treatment with resveratrol as an add-on therapy with meloxicam.
Materials and Methods
Recruitment and ethical consideration
Patients with painful knee OA (n = 124) have been evaluated clinically, and all provided written consent for their participation. This study was designed a part of a Ph. D. program at the University of Sulaimani, Iraq. It was carried out in the Rehabilitation and Rheumatology Centre and one of the hospitals of Sulaimani city, north of Iraq. The research protocol was accepted by the ethical committee of the medical college of Sulaimani University (ID: 42-11-2016), following a 2000-revised version of the Helsinki Declaration.
Inclusion and exclusion criteria
Eligible patients with knee OA-associated pain were included in the present study. Their ages were ≥45 years, diagnosed with painful knee OA based on the American College of Rheumatology criteria (ACR)-1986 guideline.[11] They have mild-to-moderate pain in the Visual Analog Scale (VAS) >40, and their OA complaint lasted ≥6 months with Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) index above 50 out of 96 scales at baseline. Patients with rheumatic diseases, autoimmune disorders, immunologic diseases or other chronic pain conditions, malignancy, and earlier knee surgery were excluded.
Randomization and intervention
After meeting the inclusion criteria and signing the informed consent, all the participants were randomized using a simple randomization technique into two groups. The control group (Mlx + placebo), treated with meloxicam 15 mg (Boehringer Ingelheim, Germany), and a placebo capsule; specially prepared for the current study, once daily for 90 days. The second group (Mlx + Res) was treated with 500 mg/day resveratrol (Apollo Healthcare Resources, Singapore), in addition to 15 mg meloxicam for 90 days. The study was performed in a blinded manner to the investigators and participants. The participant's compliance was monitored by counting the number of unused capsules in the medication's bottles at each visit. The safety of medications was evaluated at all visits by the site investigator. The basic demographic data, the grade of knee OA (Kellgren–Lawrence grading system), and the duration of symptoms were reported for all the participants during the first visit.
Assessment of physical activity, function, and pain
Baseline values of the function and physical activity were evaluated at the first visit (day 0) and day 90 using the Knee Injury and OA Outcome Score (KOOS) scale[12] and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire,[13] while pain severity was evaluated using the Visual Pain Scale of 100 mm score graded from 0 (no pain) to 100 (severe pain).
Measurement of inflammatory markers
Serum values of the inflammatory cytokines IL-IB, IL-6, and TNF-α have been recorded at the first visit (day 0) and at the last visit (day 90) as previously described.[14] Briefly, two 5-ml blood were withdrawn from the patients at the baseline (day 0) and after intervention (day 90) to measure the blood value of TNF-α, IL-1β, and IL-6. After centrifugation at 5000 rpm for 10 min, then the serum was stored at −80°C until the time of biochemical assessment. The chemical assay was carried out using ELISA kits (Korain Biotech Co., Ltd., Shanghai, China) according to the instructions of the manufacturer manual.
Statistical analysis
The correlation of biomarkers with each outcome of interest (pain and inflammatory marker) was assessed by Spearman's correlation coefficient (nonparametric) test. The relationship between the clinical scores (measured by KOOS, WOMAC, and VAS) and the pro-inflammatory markers was assessed using the linear regression model. Multivariate regression test was utilized to evaluate the association between the changes in TNF-α, IL-1β, and IL-6 levels and the severity of knee pain, by including the changes in both baseline and 90-day values of TNF-α, IL-1β, and IL-6 as the main predictors of knee pain alteration.
Statistical analysis was done by GraphPad Software Inc., CA, USA. Age, sex, and body mass index plus other characteristics of the participants were recorded at the baseline. The Chi-square test was utilized for the analysis of categorical data. For continuous variables, two-way analysis of variance and paired t-test were utilized for the analysis. P < 0.05 was considered for statistical significance.
Results
After the clinical screening for eligibility, 110 OA patients were enrolled in the trial, and 14 OA patients were ruled out because they did not meet the inclusion criteria. Eighty-two participants completed the 90-day intervention, and 28 patients did not finish the study.
Participants' basic characteristics
In this study, the average age of participants was 58.2 ± 9.1 and 57.6 ± 8.2 years for Res + Mlx and placebo + Mlx, respectively (P = 0.77). The number of female patients was higher than males in both groups (37 in the Res + Mlx group vs. 18 in the group that taken placebo + Mlx). The BMI of both groups was fairly balanced (30.7 ± 5.6 kg/m2 for Res + Mlx vs. 32.1 ± 4.6 kg/m2 for placebo + Mlx; P = 0.1). The other baseline and clinical characteristics are shown in Table 1.
Table 1.
Baseline characteristics of the knee osteoarthritis patients treated with meloxicam supplemented with resveratrol (meloxicam + resveratrol) or meloxicam with placebo (meloxicam + placebo)
| Parameters | Res + Mlx (n=50) | Mlx + placebo (n=32) | P |
|---|---|---|---|
| Disease duration (year) | 3.5±3.2 | 3.9±2.9 | 0.45 |
| Disease grade, n (%) | |||
| Grade I | 7 (14) | 3 (9.4) | 0.12 |
| Grade II | 25 (50) | 15 (47) | |
| Grade III | 18 (36) | 14 (43.6) | |
| Baseline KOOS | 33.6±9.2 | 33.5±10.5 | 0.9 |
| Baseline WOMAC total score (0-96) | 59.6±13.7 | 59.3±7.4 | 0.2 |
| Baseline VAS-100 (mm) | 81.0±11.6 | 85.3±9.2 | 0.08 |
Mlx=Meloxicam, Res=Resveratrol, KOOS=Knee Injury and Osteoarthritis Outcome Score, WOMAC=Western Ontario and McMaster Universities Osteoarthritis Index, It is Osteoarthritis instead of Arthritis , VAS=Visual Analog Score
Clinical outcome measures
Clinical assessment and pain intensity were measured using the KOOS total score; 0 indicates worst while 100 indicates best; the WOMAC total score ranging from 0 (best) to 96 (worst); and VAS-100 mm scale. In Table 2, the administration of resveratrol improves significantly (P < 0.05) both KOOS and WOMAC total scores at the end of the treatment, compared with baseline values and those reported in the control group postintervention. However, the KOOS and WOMAC scores of the control group do not change significantly (P > 0.05) after 90 days. Furthermore, at day 90, the VAS score improved in the two groups; however, the improvement level in the treated participants with Res + Mlx was higher than that of the control group.
Table 2.
Effect of resveratrol as an adjuvant with meloxicam on the Knee Injury and Osteoarthritis Outcome Score, Osteoarthritis in WOMAC, and Visual Analog Score-100 in patients with mild-to-moderate knee osteoarthritis
| Score | Mlx + Res (n=50) |
Mlx + placebo (n=32) |
||
|---|---|---|---|---|
| Baseline | 90 days | Baseline | 90 days | |
| KOOS | 33.6±9.2a | 89.3±9.5*,c | 33.5±10.5a | 38.3±14.8a |
| WOMAC | 59.6±13.7a | 8.1±8.2*,c | 63.7±11.0a | 56.1±15.9a |
| VAS-100 | 81.0±11.6a | 18.6±10.8*,c | 85.3±9.2a | 65.7±16.8b |
*Significantly different compared with baseline values within the same group (paired t-test); values with different superscripts (a, b, c) are significantly different among different times and different groups (ANOVA; P<0.05), Values are presented as mean±SD. n=Number of patients, Mlx=Meloxicam, Res=Resveratrol, KOOS=Knee Injury and Osteoarthritis Outcome Score, Osteoarthritis in WOMAC, VAS=Visual Analog Score, SD=Standard deviation
The outcome of pro-inflammatory marker measurement
Supplementary addition of resveratrol to meloxicam therapy for 90 days remarkably reduces TNF-α, IL-1β, and IL-6 serum levels in OA patients (P < 0.05), comparing with day 0 level and with the placebo group.
Correlation between serum pro-inflammatory cytokines and clinical-related scores
Correlation of tumor necrosis factor-α with the clinical scores (Knee Injury and Osteoarthritis Outcome Score, WOMAC, and Visual Analog Scale)
Figure 1a shows the association between the levels of TNF-α and the KOOS, WOMAC, and VAS scores in the Mlx + placebo group at baseline. The TNF-α levels exhibited a negative and nonsignificant correlation pattern with the KOOS scores (r = −0.3, P = 0.11); meanwhile, a nonsignificant positive correlation was reported with the total WOMAC scores (r = 0.3, P = 0.13) and also nonsignificantly associated with the VAS scores (r = 0.3, P = 0.08) at the baseline.
Figure 1.
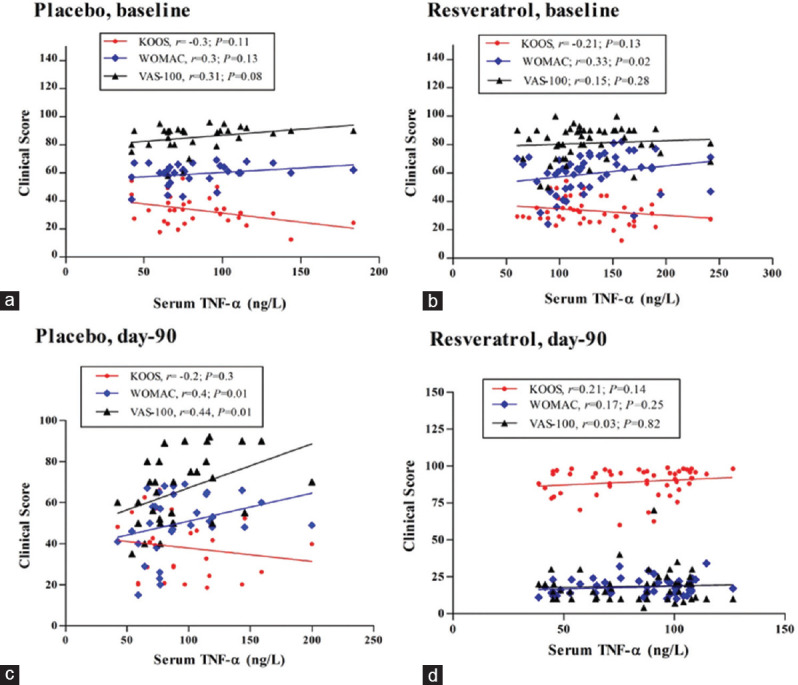
Spearman's correlation between serum levels of tumor necrosis factor-α and the clinical scores of Knee Injury and Osteoarthritis Outcome Score, WOMAC, and Visual Analog Scale in both treatment groups at baseline and after 90 days; r: Spearman's correlation coefficient; (a and c) Mlx+placebo group, (b and d) Mlx+Res group
A similar association pattern was obtained for the Mlx + Res group at the baseline [Figure 1b]. Using the previously mentioned markers, the level TNF-α shows a weak and nonsignificant association with the clinical symptoms at baseline. Total KOOS scores displayed a moderate-to-severe level of pain and physical impairments. Figure 1b shows a negative and weak correlation between serum level of TNF-α and KOOS scores, while a significant positive association was predicted between serum level of TNF-α and WOMAC scores (r = 0.33, P = 0.02). In addition, a very weak nonsignificant correlation was obtained between the pain intensity measured by VAS and serum TNF-α level (r = 0.15, P = 0.28).
The use of meloxicam in the Mlx + placebo group for 90 days makes few changes in the association between the serum levels of TNF-α and the clinical scores, as shown in Figure 1c (placebo, day 90); the KOOS score correlation coefficient shows a weak negative and nonsignificant value with the TNF-α (r = −0.2, P = 0.3). Meanwhile, correlation analysis of WOMAC and VAS scores with the serum levels of TNF-α indicates significant and positive associations (r = 0.4, P = 0.01; r = 0.44, P = 0.01), respectively.
Figure 1d demonstrates the association of TNF-α levels with the scores of KOOS, WOMAC, and VAS (r = 0.21, P = 0.14; r = 0.17, P = 0.25; r = 0.03, P = 0.82), respectively. On day 90, a remarkable reduction in the serum levels of TNF-α was recorded in the Mlx + Res group compared with the baseline values. However, the regression analysis to determine the correlation between such decreases of TNF-α with improving the clinical scores showed a nonsignificant weak correlation between these two variables.
Correlation between interleukin-1β and the clinical scores (Knee Injury and Osteoarthritis Outcome Score, WOMAC, and Visual Analog Scale)
In Figure 2a, IL-1β exhibited a nonsignificant, negative, and weak association with the total KOOS score (r = −0.16, P = 0.39) in the Mlx + placebo group at baseline. Meanwhile, a nonsignificant positive association with the WOMAC scores (r = 0.3, P = 0.09) and VAS scores (r = 0.35, P = 0.05) was reported in the same group at baseline.
Figure 2.
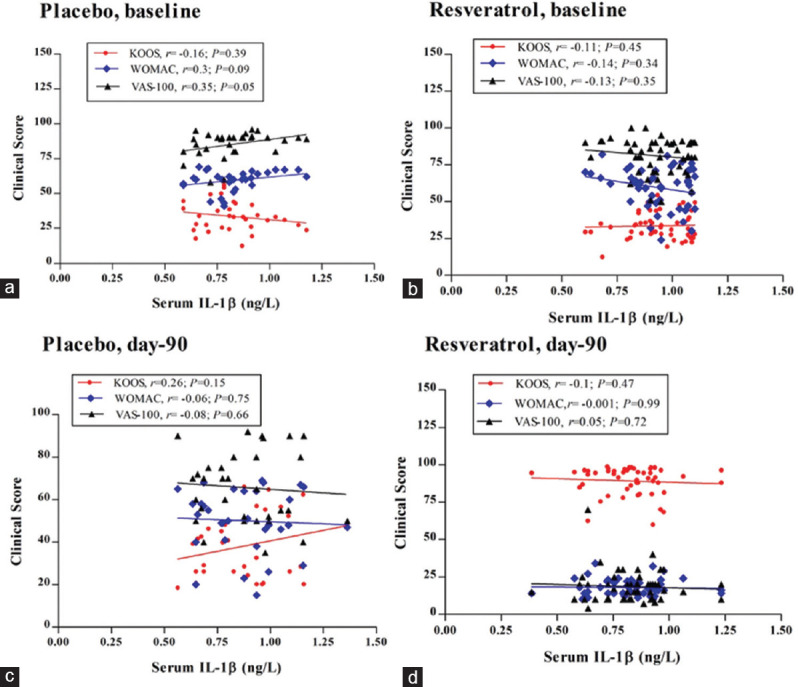
Spearman's correlation between serum level of interleukin-1β and clinical score of Knee Injury and Osteoarthritis Outcome Score, WOMAC, and Visual Analog Scale scores in both treatment groups after 90 days; r: Spearman's correlation coefficient; (a and c) Mlx+placebo group; (b and d) Mlx+Res group
The results characterized the association between the inflammatory biomarkers and the clinical scores of the Mlx + Res group at baseline [Figure 2b]. The serum IL-1β level shows a nonsignificant and a very weak negative correlation with the pain, stiffness, physical function, the function of daily activities, and quality of life, which are reflected by KOOS, WOMAC, and VAS scores at the baseline (r = −0.11, P = 0.45; r = −0.14, P = 0.34; r = −0.13, P = 0.35), respectively. After 90 days, the control group exhibits nonsignificant changes in Spearman's coefficient values [Figure 2c]. The regression coefficient value of KOOS score shows a weak positive nonsignificant correlation with the IL-1β levels (r = 0.26, P = 0.15), while regression analysis of WOMAC and VAS scores with the serum IL-1β levels displays a nonsignificant and weak negative correlation (r = −0.06, P = 0.75 and r = −0.08, P = 0.66, respectively). Figure 2d shows the regression analysis of the Mlx + Res group outcomes after 90 days. The total clinical scores of KOOS and WOMAC displayed a weak negative and nonsignificant correlation with the IL-1β levels (r = −0.1, P = 0.47; r = −0.001, P = 0.99, respectively); meanwhile, the VAS score shows a weak positive and nonsignificant association with the IL-1β levels (r = −0.05, P = 0.75).
Correlation of interleukin-6 with the clinical scores (Knee Injury and Osteoarthritis Outcome Score, WOMAC, and Visual Analog Scale)
Figure 3a-d shows the regression analysis of the data in Mlx + placebo and Mlx + Res groups before and after treatment. In Figure 3a, adjusting the analysis for IL-6 levels revealed a very weak negative association between the KOOS scores and IL-6 levels at the baseline (r = −0.02, P = 0.9); however, the association of this biomarker with the pain scores and the clinical assessment outcomes (WOMAC and VAS) was nonsignificant with weak positive values at the baseline (r = 0.1, P = 0.57; r = 0.15, P = 0.4, respectively). In comparison with the baseline values, regression analysis of the control group data after 90 days demonstrates relatively comparable outcomes [Figure 3c], where KOOS and WOMAC scores displayed very weak positive and nonsignificant correlations with the IL-6 levels (r = 0.1, P = 0.56; r = 0.11, P = 0.54, respectively). Meanwhile, the VAS outcome exhibits a relatively negative association with the serum concentrations of this cytokine (r = −0.25, P = 0.16). Figure 3b and d demonstrates a detectable correlation between the improvement of the clinical symptoms and the IL-6 levels at the baseline and after 3 months. In both figures, the Spearman's correlation shows a relatively weak and nonsignificant association between IL-6 levels and KOOS, WOMAC, and VAS scores at the baseline (r = −0.23, P = 0.11; r = 0.07, P = 0.63; r = 0.02, P = 0.9, respectively). Despite the remarkable reduction in the IL-6 values and the physical scores of the Mlx + Res group at day 90, a similar pattern of association has been observed after incorporating resveratrol as an adjuvant with meloxicam (r = −0.13, P = 0.11; r = 0.27, P = 0.06; r = 0.28, P = 0.05, respectively) [Table 3].
Figure 3.
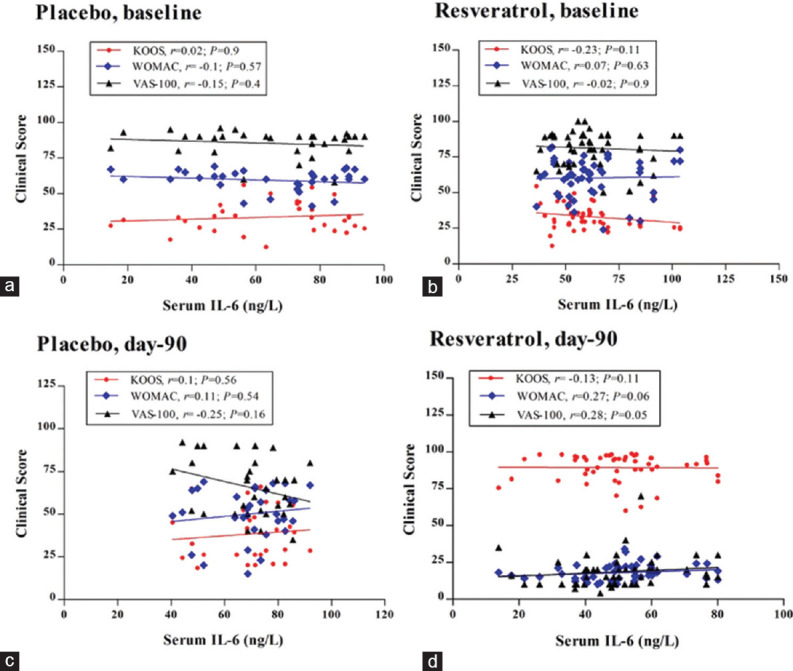
Spearman's correlation between serum level of interleukin-1 and clinical score of Knee Injury and Osteoarthritis Outcome Score, WOMAC, and Visual Analog Scale scores in both treatment groups after 90 days; r: Spearman's correlation coefficient; (a and c) Mlx+placebo group; (b and d) Mlx+Res group
Table 3.
Effect of resveratrol, as an adjuvant with meloxicam on serum level of tumor necrosis factor-α, interleukin-1β, and interleukin-6 in patients with mild-to-moderate knee osteoarthritis
| Biomarkers (ng/L) | Mlx + Res (n=50) |
Mlx + placebo (n=32) |
||
|---|---|---|---|---|
| Baseline | 90 days | Baseline | 90 days | |
| TNF-α | 141.1±122.9a | 96.6±97.3* | 100.1±76.4b | 95±34.9b |
| IL-1β | 1.16±0.96a | 1.13±1.04*,c | 0.91±0.46b | 0.97±0.41b |
| IL-6 | 79.6±78.4a | 75.1±94.5*,b | 70.1±40b | 72.1±38.1b |
*Significantly different compared with the baseline of the same group (P<0.05), values with different letters (a, b, c) are significantly different within the different groups (P<0.05), Values are presented as mean±SD. n=Number of patients, Res=Resveratrol, Mlx=Meloxicam, TNF-α=Tumor necrosis factor-α, IL-1β=Interleukin-1β, SD=Standard deviation
Discussion
This study investigated the expected improvement in the symptoms of OA by the supplementary addition of resveratrol to meloxicam and correlated the improvement in the blood values of the inflammatory biomarkers of patients who had a mild and moderate type of painful knee OA. The outcomes can support the pivotal role of inflammation as one of the etiologies of OA pathogenesis.
Currently, many studies provide pieces of evidence on the potential correlation between the circulating inflammatory cytokines and proteins that reflect changes in joint remodeling during OA-associated pain.[15] Many studies highlighted the relation of movement impairment “calculated by the WOMAC” with the elevation of pro-inflammatory cytokines.[9,16] In particular, many researchers reported the association of the increased IL-6 and TNF-α levels with clinical scores of painful knee OA.[9] In the present study, the major outcomes indicated that both baseline and posttreatment levels of TNF-α, IL-1β, and IL-6 are slightly linked to the gained pain relief assessed by total KOOS, WOMAC, and VAS scores. In general, studies that investigate the process of articular pain relief in interventional trials are lacking. However, many studies documented the significance of inflammation in pain development and the relevant clinical manifestation in OA. However, several studies have revealed inconsistent relations between knee joint complaint and IL-6, TNF-α, and C-reactive protein (CRP) levels.[17,18]
Furthermore, previous studies reported that IL-1β, TNF-α, and IL-6 are detectable during the early stages of OA[19,20] and associated with the radiographic outcome and destruction of articular cartilage in patients with or without knee complaint. These results proposed that the inflammatory mediators may have a role in the structural changes that occur in painful knee OA. Although the present study reported a remarkable reduction in the values of TNF-α, IL-1B, and IL-6 along with reducing KOOS, WOMAC, and VAS scores posttreatment compared with baseline values, the regression analysis using the Spearman test displayed a weak and nonsignificant correlation between those pro-inflammatory biomarkers and the reported relief of pain and improving the clinical manifestations. Therefore, it is likely that there is an involvement of some other proximate causes or mechanisms alongside inflammation associated with the pathogenesis of OA. Furthermore, resveratrol may engage another target molecule rather than pro-inflammatory cytokines to alleviate OA-associated pain reported by Tillu et al. who stated that resveratrol activates AMPK to attenuate ERK and mTOR signaling, the important biochemical pathways that sensitize many peripheral receptors.[21] In this regard, our finding seems inconsistent with that of a longitudinal study conducted by Stannus et al.[20] that revealed an association between the changes in serum levels of high-sensitivity CRP and TNF-α over 2.7 years with the elevated knee pain measured by the WOMAC scale. Moreover, it has been previously reported that IL-6 levels were not significantly associated with the improvement of WOMAC scores[22] while positively associated with the pain severity according to the outcomes of the VAS.[23] Many randomized clinical trials showed a parallel decline in IL-6 levels with the pain score and physical activity progression.[24] In addition, the baseline levels of IL-6 have been correlated with the alteration of pain severity during standing.[20] Meanwhile, Penninx et al. indicated that in patients with painful OA of the knee, TNF-α levels were not in line with changes in WOMAC-related knee pain, stiffness, and radiographic scores.[25] However, another clinical study reported a significant association between TNF-α and WOMAC scores while a weak significant association was predicted between IL-6 and the subscale of stiffness. These findings suggested that there are different ways for the involvement of inflammatory cytokines in synovitis of osteoarthritic knees; pain and TNF-α are well correlated, whereas joint function is correlated with IL-6.[26] Moreover, another meta-analysis data concluded that the changes in concentrations of many cytokines including TNF-α were correlated with the amelioration of pain severity after total knee arthroplasty in patients who had clinically significant pain relief after intra-articular administration of hyaluronic acid.[27] Moreover, intra-articular injection of hyaluronate OA patients demonstrated a negative correlation between synovial IL-1β levels and pain relief score over 6 months.[28] Based on our finding, the association of inflammatory factors with OA clinical scores may not positively translate the involvement of inflammation in pain etiologies.
The present study was the first clinical trial that describes correlation data between the levels of inflammatory cytokines and the clinical scores obtained by different types of validated instruments, including KOOS. The discrepancies between the present study and the previous reports might be because of the differences in sample size, design, and the limited duration of the study. Furthermore, the correlations were not adjusted for gender, age, body weight, and other potentially conflicting variables. In conclusion, this study demonstrated weak and nonsignificant correlations between serum levels of certain inflammatory biomarkers and the clinical outcome scores in patients with painful knee OA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The data of this study were abstracted from the PhD thesis submitted by Bushra Hassan Marouf to the Department of Pharmacology, College of Medicine, University of Sulaimani. The whole project was financially supported and ethically approved by the University of Sulaimani (Certificate ID: 42 on 21/11/2016). The authors would like to thank the kind technical supports from the Shar Teaching Hospital and the Rheumatology and Physical Rehabilitation Center in Sulaimani.
References
- 1.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–9. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 2.Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol. 2013;9:654–64. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saetan N, Honsawek S, Tanavalee A, Tantavisut S, Yuktanandana P, Parkpian V. Association of plasma and synovial fluid interferon-γ inducible protein-10 with radiographic severity in knee osteoarthritis. Clinic Biochem. 2011;44:1218–22. doi: 10.1016/j.clinbiochem.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890–6. doi: 10.1016/j.joca.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualized on MRI explain knee pain in knee osteoarthritis.A systematic review? Ann Rheum Dis. 2011;70:60–7. doi: 10.1136/ard.2010.131904. [DOI] [PubMed] [Google Scholar]
- 8.Uçeyler N, Schäfers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196:67–78. doi: 10.1007/s00221-009-1755-z. [DOI] [PubMed] [Google Scholar]
- 9.Miller GD, Nicklas BJ, Loeser RF. Inflammatory biomarkers and physical function in older, obese adults with knee pain and self-reported osteoarthritis after intensive weight-loss therapy. J Am Geriatr Soc. 2008;56:644–51. doi: 10.1111/j.1532-5415.2007.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khojah HM, Ahmed S, Abdel-Rahman MS, Elhakeim EH. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin Rheumatol. 2018;37:2035–42. doi: 10.1007/s10067-018-4080-8. [DOI] [PubMed] [Google Scholar]
- 11.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee: Diagnostic and therapeutic criteria committee of the American rheumatism association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 12.Örtqvist M, Roos EM, Broström EW, Janarv PM, Iversen MD. Development of the Knee Injury and Osteoarthritis Outcome Score for children (KOOS-Child): Comprehensibility and content validity. Acta Orthop. 2012;83:666–73. doi: 10.3109/17453674.2012.747921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellamy N, Buchnan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in subjects with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 14.Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: A longitudinal study. J Clin Endocrinol Metab. 2008;93:1952–8. doi: 10.1210/jc.2007-2325. [DOI] [PubMed] [Google Scholar]
- 15.Lourido L, Ayoglu B, Fernández-Tajes J, Oreiro N, Henjes F, Hellström C, et al. Discovery of circulating proteins associated to knee radiographic osteoarthritis. Sci Rep. 2017;7:137. doi: 10.1038/s41598-017-00195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, De Souza LP, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: A controlled analysis. Arthritis Rheum. 2008;59:1424–31. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- 17.Stürmer T, Brenner H, Koenig W, Günther KP. Severity and extent of osteoarthritis and low grade systemic inflammation as assessed by high sensitivity C reactive protein. Ann Rheum Dis. 2004;63:200–5. doi: 10.1136/ard.2003.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richette P, Poitou C, Garnero P, Vicaut E, Bouillot JL, Lacorte JM, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis. 2011;70:139–44. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- 19.Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-alpha are associated with radiographically approved osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18:1441–7. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: A prospective cohort study. Ann Rheum Dis. 2013;72:535–40. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- 21.Tillu DV, Melemedjian OK, Asiedu MN, Qu N, De Felice M, Dussor G, et al. Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol Pain. 2012;8:5. doi: 10.1186/1744-8069-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bas S, Finckh A, Puskas GJ, Suva D, Hoffmeyer P, Gabay C, et al. Adipokines correlate with pain in lower limb osteoarthritis: Different associations in hip and knee. Int Orthop. 2014;38:2577–83. doi: 10.1007/s00264-014-2416-9. [DOI] [PubMed] [Google Scholar]
- 23.Shimura Y, Kurosawa H, Sugawara Y, Tsuchiya M, Sawa M, Kaneko H, et al. The factors associated with pain severity in patients with knee osteoarthritis vary according to the radiographic disease severity: A cross-sectional study. Osteoarthritis Cartilage. 2013;21:1179–84. doi: 10.1016/j.joca.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. JAMA. 2013;310:1263–73. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penninx BW, Abbas H, Ambrosius W, Nicklas BJ, Davis C, Messier SP, et al. Inflammatory markers and physical function among older adults with knee osteoarthritis. J Rheumatol. 2004;31:2027–31. [PubMed] [Google Scholar]
- 26.Orita S, Koshi T, Mitsuka T, Miyagi M, Inoue G, Arai G, et al. Associations between pro-inflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet Disord. 2011;12:144. doi: 10.1186/1471-2474-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh JA, Noorbaloochi S, Knutson KL. Cytokine and neuropeptide levels are associated with pain relief in patients with chronically painful total knee arthroplasty: A pilot study. BMC Musculoskelet Disord. 2017;18:17. doi: 10.1186/s12891-016-1375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent HK, Percival SS, Conrad BP, Seay AN, Montero C, Vincent KR. Hyaluronic Acid (HA) viscosupplementation on synovial fluid inflammation in knee osteoarthritis: A Pilot Study. Open Orthop J. 2013;7:378–84. doi: 10.2174/1874325001307010378. [DOI] [PMC free article] [PubMed] [Google Scholar]


