Sir,
Tyrosine kinase inhibitors (TKI) are anticancer drugs effective in the targeted treatment of various malignancies. Second-generation TKI, nilotinib has been approved for the treatment of chronic myeloid leukemia (CML) expressing the Bcr-Abl mutation in patients with resistance to imatinib. Systemic side effects with nilotinib are rare, however, cutaneous side effects are common. A case of generalized keratosis pilaris (KP) induced by nilotinib is reported here.
A female, aged 40 years presented with multiple asymptomatic small raised lesions over the face, bilateral limbs, and upper back 10 days after taking capsule nilotinib 300 mg twice a day. There was a history of similar eruptions when nilotinib was first started when she developed localized KP lesions after 3 months of starting nilotinib after which she stopped nilotinib on her own and become a defaulter. During this period all her KP lesions resolved. As she stopped the drug her total leukocyte count increased to 96,500/μl so the patient was counseled and restarted nilotinib by the oncologist. When the patient restarted nilotinib, lesions appeared in 10 days with the generalized distribution. The patient is a known case of CML for 3 years and was on imatinib 400 mg twice a day for 2 months following which she developed a drug-induced rash with eosinophilia and systemic symptoms syndrome secondary to imatinib and hence it was stopped and shifted to 2nd-generation TKI nilotinib. Her family history for similar skin eruption was insignificant. There was neither history of any other medication taken simultaneously nor other illness. Examination revealed diffuse keratotic follicular papules over the extensor aspect of bilateral upper extremities face and upper back [Figure 1a-d]. Histopathological examination revealed follicular hyperkeratosis, dilated follicular infundibulum with keratotic plugging and mild perivascular lymphocytic infiltrate in upper dermis consistent with the diagnosis of KP [Figure 2a and b]. Naranjo scale of adverse drug reaction (ADR) probability was used to assess the causality and the score was 11 confirming that KP was definitely induced by the drug. This adverse event was reported to the Pharmacovigilance Program of India (PvPI). According to the WHO–UMC criteria, it was certainly an ADR secondary to nilotinib (PvPI Report No. IN-IPC-300454680). Based on the modified Hartwig and Siegel severity assessment scale, this ADR comes under the mild category (level 1). The patient was counseled and advised 10% urea containing emollient for local application. As this condition was tolerable and nilotinib has a substantial role in the treatment of CML, the drug was continued.
Figure 1.
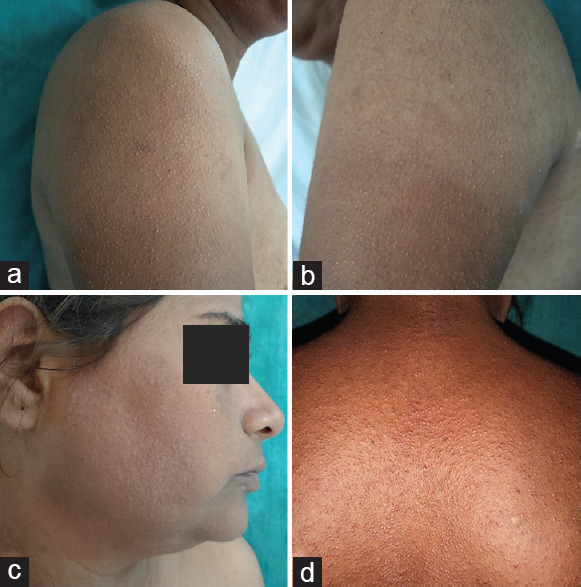
(a and b) Multiple keratotic follicular papules over bilateral upper extremity (c) face (d) and back
Figure 2.
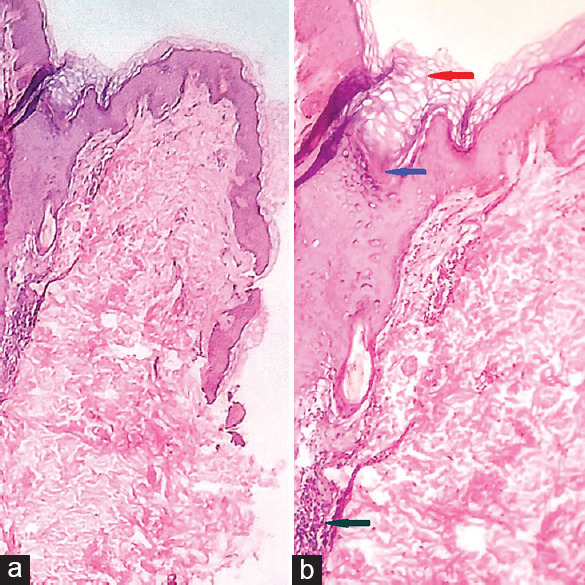
(a) scanner view showing hyperkeratotic epidermis and dermis (H and E x4) (b) Epidermis with follicular hyperkeratosis and infundibular dilatation (red arrow), hair follicle with keratin plug (blue arrow), mild perivascular lymphocytic infiltrate in the upper dermis (green arrow) (H and E x40)
Epidermal growth factor receptor (EGFR) inhibitors have antitumor properties as they cause attenuation in the EGFR signaling pathways leading to deregulation of cellular functions.[1] TKI belongs to the EGFR inhibitors group and causes blockage of adenosine triphosphate binding site of various tumor-producing tyrosine kinases and prohibits autophosphorylation of tyrosine molecules. This, in turn, deactivates the intracellular signal-transduction network causing cellular abnormalities.[1] Nilotinib is mainly reserved for patients who have failed to respond to first-line therapies or intolerant to imatinib. Among the nonhematological adverse events, cutaneous side effects are commonly observed after nilotinib which includes itching (2%–15%), cutaneous xerosis (0%–12%), hair loss (0%–6%), skin rash (22%–62%) mimicking maculopapular eruption, desquamation, KP-like lesions, milia, and rosacea-like eruption which may be pruritic or non-pruritic.[2] Chang et al., in 2017 had reviewed a total of ten cases of nilotinib-induced KP. In his study, the average duration of drug intake and the onset of KP lesion was 1–2 months and all the patients had generalized distribution with involvement of the face, extremity, and trunk which correlates with the present case.[3] KP is a common cutaneous disorder of abnormal follicular keratinization with autosomal dominant inheritance. Histopathology findings include dilated follicular infundibulum filled with orthokeratotic keratin plug at the orifice, sometimes a twisted hair shaft may be seen within the keratin material with mild perivascular mononuclear cell infiltrates in the adjacent dermis.[4] Nilotinib is thought to induce KP by modulating the tyrosine kinase receptors in the hair follicles. KP-like eruptions have also been reported with the use of RAF inhibitors vemurafenib and dabrafenib which are used in metastatic melanoma. They do so by paradoxical activation of the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathway in wild-type BRAF melanoma cells. Nilotinib has been known to have a low off-target anti-RAF protein action in-vitro. Thus, it may be deduced that vemurafenib and nilotinib induce KP by involving the common ERK/MAPK pathway.[5]
As KP is a mild dermatological condition of cosmetic significance only, prompt recognition, reassurance of patients with appropriate treatment, and collaboration with the oncologist are necessary for compliance to therapy and better outcome. We report this case as it is rare, recently documented cutaneous adverse event. Hence, in today's era of emerging drug reactions, every case should be reported to the pharmacovigilance department to increase awareness among treating physicians.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hu JC, Sadeghi P, Pinter-Brown LC, Yasher S, Chiu MW. Cutaneous side effects of epidermal growth factor receptor inhibitors: Clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317–26. doi: 10.1016/j.jaad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Drucker AM, Wu S, Busam KJ, Berman E, Amitay-Laish I, Lacouture ME. Rash with the multitargeted kinase inhibitors nilotinib and dasatinib: Meta-analysis and clinical characterization. Eur J Haematol. 2013;90:142–50. doi: 10.1111/ejh.12052. [DOI] [PubMed] [Google Scholar]
- 3.Chang CH, Shih LY, Lu PH. A case of nilotinib-induced keratosis pilaris-like perifollicular fibrosis with a brief review of the literature. Dermatol Sinica. 2017;35:163–5. [Google Scholar]
- 4.Michael D. Ioffreda. Inflammatory Diseases of Hair Follicles, Sweat Glands, and Cartilage. In: Elder DE, editor. Lever’s Histopathology of the Skin. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2008. p. 469. [Google Scholar]
- 5.Patel AB, Solomon AR, Mauro MJ, Ehst BD. Unique cutaneous reaction to second- and third-generation tyrosine kinase inhibitors for chronic myeloid leukemia. Dermatology. 2016;232:122–5. doi: 10.1159/000437383. [DOI] [PubMed] [Google Scholar]


