Abstract
BACKGROUND:
Anaphylaxis is a rapid-onset, multisystem, and potentially fatal hypersensitivity reaction with varied reports of prevalence, incidence, and mortality. There are limited cases reported of severe and/or fatal pediatric anaphylaxis.
OBJECTIVE:
This study describes the largest cohort of intensive care unit pediatric anaphylaxis admissions with a comprehensive analysis of identified triggers, clinical and demographic information, and probability of death.
METHODS:
We describe the epidemiology of pediatric anaphylaxis admissions to North American pediatric intensive care units (PICUs) that were prospectively enrolled in the Virtual Pediatric Systems database from 2010 to 2015. One hundred thirty-one PICUs in North America (United States and Canada) were queried for anaphylaxis International Classification of Diseases, Ninth Revision or International Classification of Diseases, Tenth Revision codes from the Virtual Pediatric Systems database from 2010 to 2015 in the United States and Canada. One thousand nine hundred eighty-nine patients younger than 18 years were identified out of 604,279 total number of patients admitted to a PICU in the database during this time frame.
RESULTS:
The primary outcome was mortality, which was compared with patient and admission data using Fisher exact test. Secondary outcomes (intubation, length of stay, mortality risk scores, systolic blood pressure, and pupillary reflex) were analyzed using the Kruskal-Wallis test or Wilcoxon rank-sum test, as appropriate. One thousand nine hundred eighty-nine patients with an anaphylaxis International Classification of Diseases code were identified in the database. One percent of patients died because of critical anaphylaxis. Identified triggers for fatal cases were peanuts, milk, and blood products. Peanuts were the most common trigger. Children were mostly male when younger than 13 years, and mostly female when 13 years and older. Average length of stay was 2 days. There was a higher proportion of Asian patients younger than 2 years or when the trigger was food.
CONCLUSIONS:
This is the largest study to describe pediatric critical anaphylaxis cases in North America and identifies food as the most common trigger. Death occurs in 1% of cases, with intubation occurring most commonly in the first hour. The risk for intensive care unit admission in children underscores the serious nature of anaphylaxis in this population.
Keywords: Pediatric, Adolescent, Anaphylaxis, ICU, Mortality, Peanut, Milk, Critically ill
INTRODUCTION
Anaphylaxis is a rapid-onset, multisystem, and potentially fatal hypersensitivity reaction with varied reports of prevalence (0.02%−15%), incidence (8.4–111.2 per 100,000 person-years), and mortality (0.33–1.06 per 1,000,000 person-years or 0.25%−0.33%).1,2 When restricted to pediatric patients, the estimated incidence is 10.5 per 100,000 person-years,3 with an estimated mortality of 0.096 per 1,000,000 person-years.4 The risk of fatal anaphylaxis has been likened to being fatally struck by lightning, with increased frequency in hospitalized patients (<1%) and food-allergic patients (1%).5 There is limited literature on critical or fatal pediatric anaphylaxis.
Among previous studies in the United States, only 2 describe pediatric anaphylaxis cases admitted to the intensive care unit (ICU). Dibs and Baker6 reported 55 anaphylaxis cases in Philadelphia showing many patients were younger than 6 years (42%), male (56%), and of black race (54%). Among them, 11 required ICU care (20%). The patients requiring ICU care had more anaphylaxis triggered by drugs or latex and via intravenous administration.6 The other report showed that, among Medicare and private insurance claims in the United States, anaphylaxis emergency department visits transferred to the ICU were most commonly caused by food (12%) and medication (14%). These patients were less likely to have a coincident asthma diagnosis and had younger age (<18 years) and venom as a trigger.7 Females made up 59% of those transferred to the ICU; however, only 5% of these patients were pediatric. ICU admissions also increased over time in this cohort, by 165% within the 9-year period.
There are several European and Asian studies that describe anaphylaxis cohorts including pediatric patients requiring ICU care. Among 133 anaphylaxis cases in a Madrid emergency department, many were pediatric patients, particularly infants (15%).8 A Danish study estimated mortality among anaphylaxis admissions to be less than 1%.9 The European Anaphylaxis Registry demonstrated that food was the main trigger of anaphylaxis in children with egg, milk, and nuts as the identified triggers in younger patients (younger than 10 years), and insects as the primary trigger in older patients (older than 10 years).10 In this cohort, only 6% required ICU care.10 A British study of 230 patients admitted to ICUs from 2005 to 2009 demonstrated a shift from equal sex distribution in pediatric patients to female predominance in adult cases.11 An Israeli cohort of 92 pediatric anaphylaxis patients had a mean age of 7.4 years, with food (milk and nuts) and medicines as the most common triggers (43% and 22%, respectively).12 Asthma was a diagnosis in 52% of patients in this cohort. Seven patients (7.6%) required intensive care in this cohort, but there were no fatalities reported.12 In addition to hospital and nation-specific epidemiological studies, regional and seasonal differences have also been reported. Within the United States and Australia, 3 reports documented fewer cases of anaphylaxis in the southern regions.13–15
Hypotension is considered an important marker for anaphylaxis severity and is therefore frequently reported. Vetander et al16 noted that hypotension occurred in 5% of patients, with only 1% requiring ICU care. Hypotension was also studied in a US mortality database, which estimated that rates varied between 3% and 15% among fatal anaphylaxis cases.17 Length of stay (LOS) has been reported for pediatric anaphylaxis hospital admissions overall; however, LOS for severe cases requiring ICU admissions or those with identified nonfood triggers have not yet been described. Rudder et al18 showed that LOS for food-induced pediatric anaphylaxis admissions ranged from 1.7 to 2.3 days between 2000 and 2009. LOS for an anaphylaxis admission is considered prolonged once the stay goes beyond 2 days. According to a national US database review, anaphylaxis admissions are prolonged in 8.2% of cases.19 Approximately 3% of cases required ICU admission and only 0.6% required intubation or positive pressure ventilation. ICU admission is risk stratified using several scoring algorithms including Pediatric Risk of Mortality 3 (PRISM III) and Pediatric Index of Mortality 2 (PIM2). These scores are based on physiologic data to determine probability of death (POD) for patients admitted to an ICU. To date, no published studies documenting anaphylaxis ICU admissions according to POD were found.
Characteristics of anaphylaxis among pediatric patients who require intensive care are underreported. The LOS and mortality risk have not been determined in children after an anaphylactic event. Our study expands on the existing knowledge of this important patient population, to include the largest cohort of ICU anaphylaxis admissions with a comprehensive analysis of identified triggers, clinical and demographic information, and POD.
METHODS
Study design and cohort
We describe the epidemiology of pediatric anaphylaxis admissions to 131 North American (US and Canada) pediatric intensive care units (PICUs) that were prospectively enrolled in the Virtual Pediatric Systems (VPS) database from 2010 to 2015. Among the available data for included PICUs, 75% (93 of 124) were teaching hospitals and 36% (45 of 124) are hospitals with Allergy and Immunology fellowship programs. The database was queried for an anaphylaxis International Classification of Diseases, Ninth Revision or International Classification of Diseases, Tenth Revision code (995.0, 995.6, 995.69, and 995.4). The study was approved by the Baylor College of Medicine Institutional Review Board.
Outcomes
The primary outcome for this study was mortality. Secondary outcomes included intubation, LOS, PIM2 (estimate of mortality risk based on scores assigned to data available at the time of ICU admission including systolic blood pressure, pupillary reaction, partial pressure of oxygen in arterial blood, base excess, mechanical ventilation in the first hour in ICU, elective admission to ICU, recovery from surgery, cardiac bypass admission, high-risk diagnosis, low-risk diagnosis),20 and PRISM III (a pediatric physiology-based score for mortality risk based on 21 physiologic variables, age, and operative status over 24 hours)21 scores, systolic blood pressure, and pupillary reflex. In our study, we compared the PIM2 and PRISM III POD, a number from 0 to 1 that allows for comparison on the same scale between scores. To identify risk factors, outcomes were compared with patient data (age, sex, race, origin, transport, operative status, pediatric cerebral performance category, pediatric overall performance category) and admission data (primary diagnosis, other diagnoses, referral and medical service data, discharge data, heart rate, noninvasive ventilation, oxygen therapy, and cardiopulmonary rescue).
Statistical analyses
Characteristics and outcomes were summarized using mean with SD, median with 25th and 75th percentiles, or frequency with percentage. Outcomes were compared with patient and admission characteristics using Kruskal-Wallis test, Wilcoxon rank-sum test, or Fisher exact test as appropriate. Statistical analyses were performed using Stata version 15 (StataCorp LLC, College Station, Texas).
RESULTS
In the VPS database, we identified 604,279 patients admitted to PICUs in North America, with 1989 patients with an anaphylaxis International Classification of Diseases, Ninth Revision or International Classification of Diseases, Tenth Revision diagnosis code (995.0, 995.6, 995.69, and 995.4) between 2010 and 2015.
Outcomes
Patient characteristics were compared with clinical markers to determine whether there were any groups that had a significantly different outcome (Table I).
TABLE I.
Association between age, hospital size, and asthma diagnosis on outcomes
| Variable | LOS (h) | PIM2 POD | PRISM III POD | Intubation | Pupillary reflex | Low SBP* | Death |
|---|---|---|---|---|---|---|---|
| All patients | 19.2 (13.7, 33.4)† | 0.94 (0.8, 1.6) | 0.3 (0.3–0.8) | 373 (19%) | 12 (0.6%) | 93 (5%) | 19 (1%) |
| Age (y) | P < 0.001‡ | P = 0.23 | P < 0.001‡ | P = 0.04‡ | P = 0.14 | P = 0.11 | P = 0.11 |
| <2 | 19.4 (13.9, 35.5) | 0.98 (0.8, 2.0) | 0.3 (0.3, 0.8) | 54 (21%) | 1 (0.4%) | 8 (3%) | 1 (0.4%) |
| 2–5 | 17.5 (13.2, 24.2) | 0.94 (0.82,1.2) | 0.3 (0.3–0.63) | 65 (14%) | 0 | 15 (3%) | 1 (0.2%) |
| 6–12 | 19.2 (13.7, 33.8) | 0.91 (0.79, 1.4) | 0.3 (0.3–0.63) | 119 (19%) | 5 (0.8%) | 40 (6%) | 7 (1.1%) |
| 13–18 | 20.4 (13.9, 36.5) | 0.94 (0.8, 3.0) | 0.49 (0.3, 1.0) | 135 (21%) | 6 (1%) | 30 (5%) | 10 (1.6%) |
| Hospital size (no. of pediatric beds) | P = 0.001‡ | P < 0.001‡ | P < 0.001‡ | P < 0.001‡ | P = 0.80 | P = 0.02‡ | P = 0.58 |
| <81 | 18 (12.5, 31.7) | 0.9 (0.78, 1.1) | 0.3 (0.3, 0.6) | 31 (12%) | 1 (0.4%) | 5 (2%) | 1 (0.4%) |
| 81–160 | 18.2 (12.7, 26.6) | 0.9 (0.79, 1.1) | 0.3 (0.3, 0.63) | 54 (14%) | 1 (0.3%) | 16 (4%) | 2 (0.5%) |
| 161–240 | 17.8 (12.0, 28.8) | 0.91 (0.79, 1.3) | 0.3 (0.3, 0.8) | 63 (20%) | 3 (1%) | 10 (3%) | 5 (1.6%) |
| 241–320 | 20.9 (14.4, 37.9) | 0.95 (0.83, 2.83) | 0.3 (0.3, 0.63) | 106 (21%) | 4 (0.8%) | 25 (5%) | 6 (1.2%) |
| >320 | 19.4 (13.9, 35) | 0.99 (0.83, 3.1) | 0.51 (0.3, 1.0) | 119 (23%) | 3 (0.6%) | 37 (7%) | 5 (1%) |
| Asthma§ | P = 0.79 | P < 0.001‡ | P = 0.26 | P = 0.003‡ | P = 1 | P = 0.001‡ | P = 0.8 |
| No diagnosis | 19.2 (13.7, 32.4) | 0.96 (0.82, 1.9) | 0.3 (0.3, 0.8) | 250 (17%) | 9 (0.7%) | 81 (6%) | 15 (1%) |
| Diagnosis | 19.2 (13, 34.8) | 0.89 (0.76, 1.18) | 0.3 (0.3, 0.8) | 123 (23%) | 3 (0.6%) | 12 (2%) | 4 (1%) |
| Trigger | P < 0.001 | P < 0.001 | P < 0.001 | P = 0.16 | P < 0.001 | P = 0.48 | P = 0.27 |
| Food | 17 (12.7, 25.2) | 0.91 (0.8, 1.1) | 0.3 (0.3, 0.51) | 126 (18%) | 18 (3%) | 5 (1%) | 7 (1%) |
| Drug | 28.1 (18.5, 54.7) | 0.96 (0.79, 3.5) | 0.51 (0.3, 1.1) | 11 (19%) | 5 (9%) | 0 | 0 |
| Serum | 23.5 (18.2, 46.3) | 1.6 (0.88, 4.3) | 1.2 (0.3, 4.5) | 16 (33%) | 8 (16%) | 1 (2%) | 2 (4%) |
| Venom | 19.7 (12.7, 37.0) | 0.9 (0.8, 1.0) | 0.3 (0.3, 0.63) | 4 (17%) | 2 (9%) | 0 | 0 |
| Unspecified | 20.2 (13.9, 36.2) | 0.95 (0.8, 2.8) | 0.45 (0.3, 1.0) | 216 (19%) | 50 (5%) | 6 (1%) | 10 (1%) |
Systolic blood pressure, calculated using the American College of Critical Care shock guideline.22
Median (25%, 75%).
P < .05.
According to International Classification of Diseases, Ninth Revision or International Classification of Diseases, Tenth Revision code.
Mortality.
Among 19 patients with fatal outcomes, the median age was 163 months (13.6 years) and LOS was 2.2 days (21.8 hours). Thirty-two percent had atopic diagnoses listed: 21% had a history of asthma, 5% had a history of eczema, and 5% had a history of drug or food allergy. A trigger for anaphylaxis was identified in 7 cases and was unidentified in the other 12 cases. Six were food (2 identified as peanut, 1 identified as milk, with 3 unspecified) and 1 was serum-triggered. Seventeen of 19 of these patients were intubated in the first hour of their admission to the ICU. Only 1 of the fatal anaphylaxis cases had an LOS of less than 1 day, so could potentially have arrested in the emergency department before transfer to the ICU.
PRISM III and PIM2.
In our cohort of pediatric anaphylaxis cases, PRISM III POD was affected by age. Adolescent children had a 19% higher PRISM III POD than did all other age groups. PIM2 was not significantly affected by age group categorization. There was a significant difference among race/ethnicity groups for both PIM2 (P = .006) and PRISM III (P = .003) POD scores. Hispanic patients were found to have a higher POD prediction by both PIM2 (18% higher) and PRISM III (19% higher) measures.
However, PIM2 POD did worsen according to hospital size; children in this cohort who were admitted to large hospitals (>320 pediatric beds) had a median PIM2 POD showing a 9% higher risk of dying compared with the whole cohort. The same relationship was seen for PRISM III POD, with 21% higher scores for children admitted to large hospitals.
Identified trigger was also found to vary with mortality probability scores (P < .001). Serum- and drug-triggered anaphylaxis had significantly worse PIM2 and PRISM III POD. The PIM2 POD for serum-triggered anaphylaxis was 60% higher than that for the total pediatric anaphylaxis cohort, whereas both drug- and serum-triggered anaphylaxis had an elevated PRISM III POD (20% and 90% higher, respectively, among groups).
Length of stay.
For most (75%) patients in this cohort, LOS in the ICU was not longer than 2 days (median, 21 hours). Specifically, for food-triggered anaphylaxis, LOS was 17 hours; adolescents tended to have a longer LOS (20 hours) as did Medicaid/Medicare-covered children (21 hours), children whose primary service was intensivists (18 hours), and large hospitals (20 hours). Children who had identifiable triggers had different LOS between groups (P < .001), with drug-triggered anaphylaxis resulting in longer ICU stay (28 hours) compared with food-triggered anaphylaxis (17 hours). Trigger, age, insurance type, primary provider, and hospital size all affected LOS, as presented in Table I. The average for the general cohort was 19 hours.
Intubation.
Children aged 2 to 5 years were less likely to be intubated (14% of 2–5-year-olds) than other age groups (19% overall; P = .037). Intubation was required more frequently for children in moderate- to large-sized hospitals (161 to >320 beds) in 23% of cases, versus 12% in the general cohort (P < .001). Having an asthma diagnosis led to increased likelihood of intubation (23% vs 17% children with no asthma diagnosis; P = .003). Intubation rate was not influenced by identified trigger.
Hypotension and other patient characteristics.
Hypotension (5% in the general cohort) was significantly affected by hospital size grouping and asthma status; children admitted to smaller sized hospitals (<81 beds, 2%; P = .015) or with asthma (2%; P = .001) were more likely to have hypotension. Children of all ages and identified triggers developed hypotension at similar rates. Grouping by sex, insurance type, medical service, season, or region did not have clinically significant effect on any of the other outcomes considered in this study. The rate of changes in pupillary reflex was similar when stratified by characteristic.
Patient characteristics
Table II presents the demographic and admission-related characteristics for these patients.
TABLE II.
Patient characteristics in study cohort
| Variable | n (%) |
|---|---|
| Number | 1989 |
| Age (mo), mean ± SD | 109 ± 65.6 |
| <1 mo | 4 (0.2) |
| 1–23 mo | 251 (13) |
| 2–5 y | 451 (23) |
| 6–12 y | 637 (32) |
| 13–18 y | 646 (32) |
| Male | 1140 (57) |
| Race or ethnicity | n = 1591 |
| Black | 331 (20) |
| American Indian/Indigenous | 10 (0.61) |
| Asian/Indian/Pacific Islander | 94 (6) |
| White/European/non-Hispanic | 835 (51) |
| Hispanic | 221 (13) |
| Other/mixed | 100 (6) |
| Unspecified | 57 (3) |
| Primary diagnosis of anaphylaxis | 1631 (82) |
| Other atopic conditions | |
| Asthma | 531 (27) |
| Eczema | 166 (8) |
| History of drug/food allergy | 185 (9) |
| Primary service — critical care (n = 873) | 780 (89) |
| Hospital size (no. of pediatric beds) | |
| 80 or fewer | 261 (13) |
| 81–160 | 375 (19) |
| 161–240 | 315 (16) |
| 241–320 | 510 (26) |
| More than 320 | 528 (27) |
| Payer (n = 776) | |
| Commercial/managed care | 575 (74) |
| Medicaid/Medicare | 159 (20) |
| Other | 42 (5) |
| Season of admission | |
| Winter | 425 (22) |
| Spring | 502 (26) |
| Summer | 484 (25) |
| Fall | 532 (27) |
Age, region, and race/ethnicity.
Mean age of this cohort was 9 years. Anaphylaxis occurred in 0.33% of patients younger than 18 years. This rate increased if patients were analyzed by age into 6- to 18-year olds (to 0.49%; P < .001), race/ethnicity (to 0.58%; P < .001) for Asian/Indian/Pacific Islanders, and region (to 0.44%; P < .001) for North-easterners (Figure 1). Fifty-seven percent of patients were males, and they were more commonly younger than 12 years (62%) than older than 13 years (P <.001; Figure 2). When comparing anaphylaxis episodes among VPS enrollees to all VPS enrollees in US census regions, anaphylaxis occurred more commonly in Northeast and Western regions (Figure 1; P < .001).
FIGURE 1.
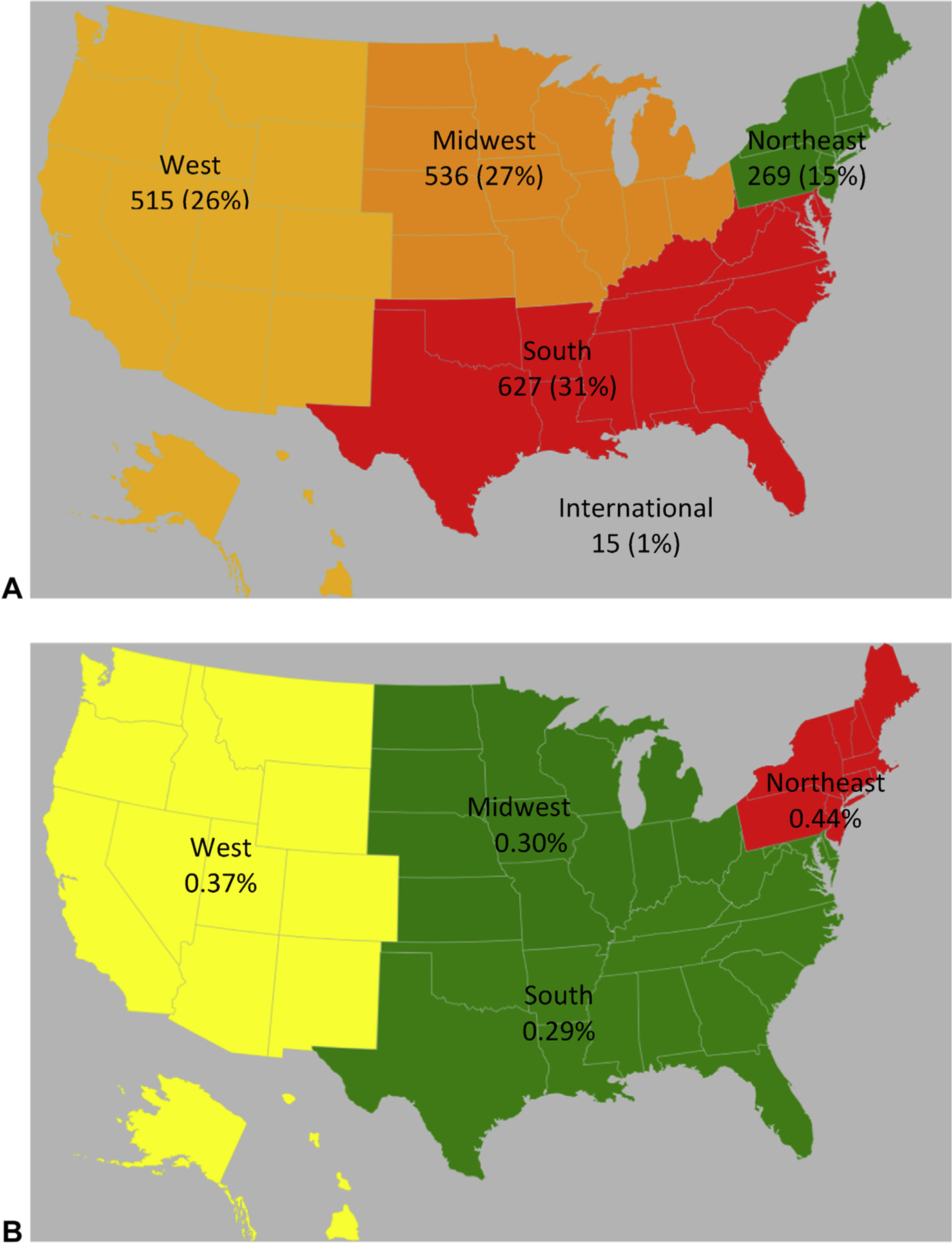
Prevalence according to US census region. Census regions are depicted on these maps. Regions are colored using a heat map with red>green. A, Absolute PICU anaphylaxis cases. B, Relative PICU anaphylaxis in comparison to total PICU admissions in the VPS database during this time frame, which were distributed as follows: West, 139,469 (23%); Midwest, 181,460 (30%); South, 214,542 (35%); Northeast, 66,563 (11%); International, 4,234 (0.7%).
FIGURE 2.
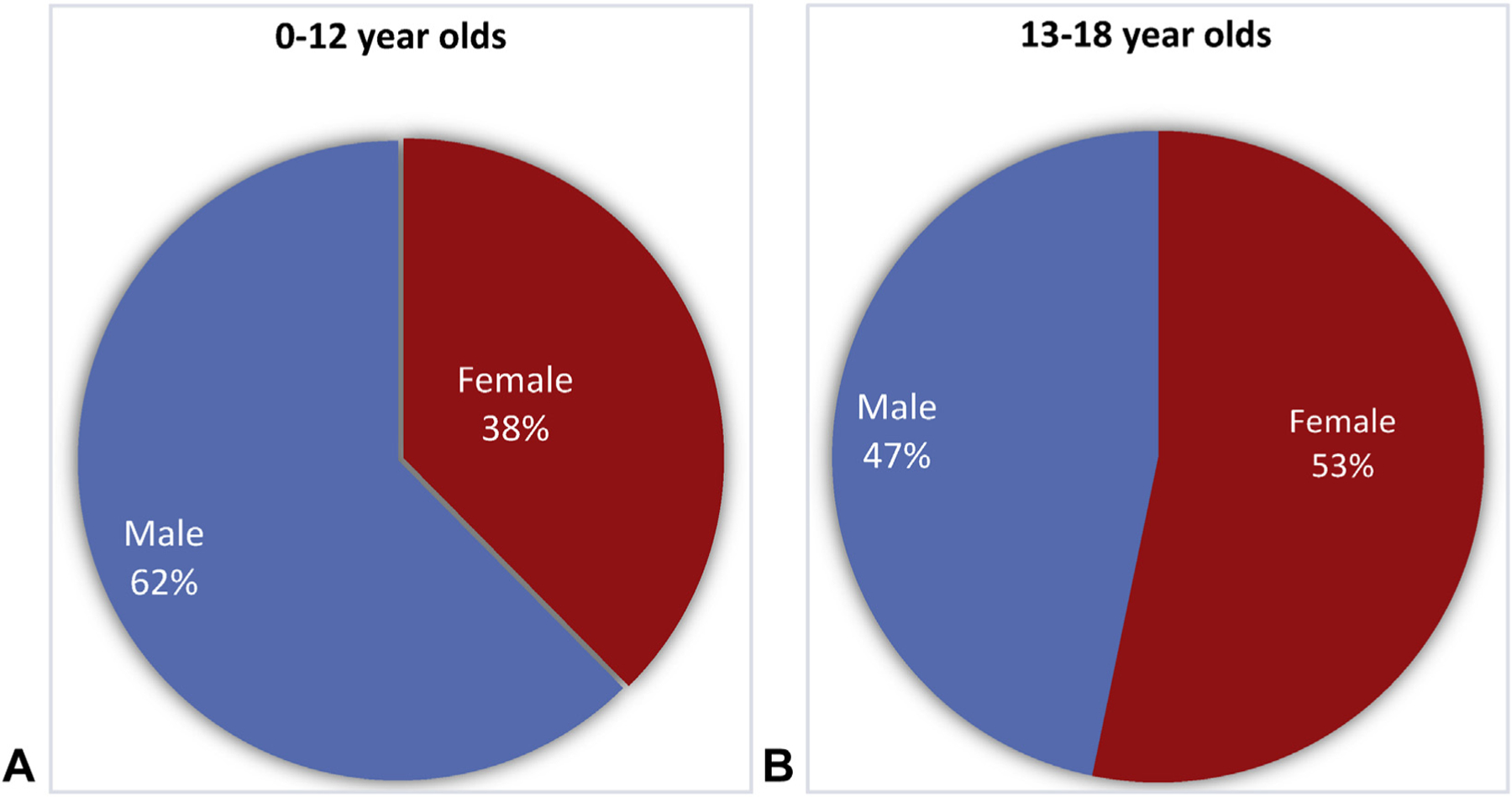
Sex distribution by age. A, (left) Children younger than 12 years were more likely to be male in this cohort. B, (right) Children older than 12 years were more likely to be female in this cohort (P < .001).
Among anaphylaxis patients, there were more Asian/Indian/Pacific Islanders younger than 2 years (21% vs 13% for all races) and fewer Asian/Indian/Pacific Islander adolescents (18% vs 32% for all races; P = .008). Children who identify as other or mixed race distinctly had more anaphylaxis than all races (32% vs 23%) between the ages of 2 and 5 years (P = .008). There were no significant differences noted when overall sex and race were compared.
Primary and secondary diagnoses.
Anaphylaxis was the primary diagnosis for 82% of patients in this cohort. When anaphylaxis was the secondary diagnosis, primary diagnoses most commonly included asthma with status asthmaticus (n = 47; 13.2%), pulmonary insufficiency (n = 32; 9%), angioneurotic edema (n = 18; 5%), acute lymphoid leukemia (n = 11; 3.1%), and sepsis with or without shock (n = 9; 2.5%). Other atopic conditions were listed as diagnoses in 27% (asthma), 9% (history of drug or food allergy), and 8% (eczema) of patients. Primary provider, hospital, size, and insurance type are described in Table II.
Identified triggers.
Most patients in our cohort did not have an identified trigger because it was either unknown or not documented, according to International Classification of Diseases, Ninth Revision or International Classification of Diseases, Tenth Revision code (995.0, 995.6, 995.69, and 995.4, 58%; Figure 3, A). Children who had an unknown trigger made up 15% of those with a history of food allergy (185). Because the VPS database includes only International Classification of Diseases codes, we cannot differentiate hereditary angioedema from allergic angioedema. Hereditary angioedema does not have its own International Classification of Diseases code. However, we can confirm that none of the cases on which we report include the code for “disorders of the complement system,” which might include patients with hereditary angioedema.
FIGURE 3.
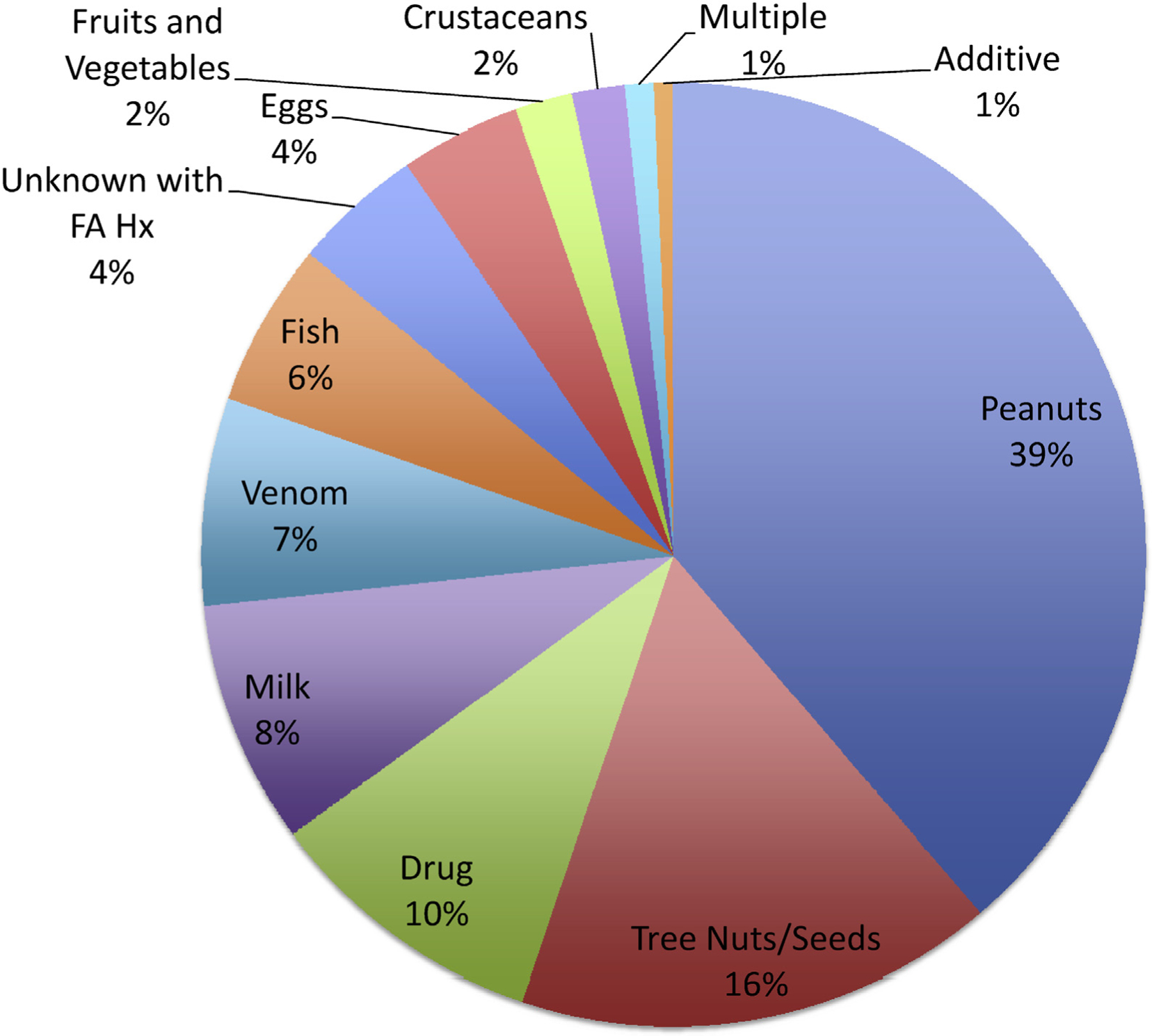
The most common identified trigger overall is peanut. The most common identified triggers for anaphylaxis among pediatric ICU patients from 2010 to 2015 in North America and Mexico were peanut, tree nut/seed, or drug.
When a trigger was identified for each ethnic group, there were significant differences between these groups (P = .003). Among patients with food-triggered anaphylaxis, they were more commonly Asian/Indian/Pacific Islander (44% vs 35% total) and less commonly Hispanic (25% vs 35% total). Mixed race patients had more anaphylaxis with an identified trigger (53% vs 42%; P = .003). When trigger was identified on the basis of sex, there were no significant differences found.
Of those patients with an identified trigger (42%), the most common was food (35%). All identified triggers, as well as those with an unidentified trigger and food allergy history, are depicted in a pie chart (Figure 3). The distribution of food-triggered anaphylaxis showed that these patients were younger compared with all other patients with or without identified trigger, with median age of 7.1 years versus 10.4 to 11.8 years for other trigger groups (Figure 4, A). Among the patients who were found to have anaphylaxis to food, peanuts were the most common trigger (45%; Figure 4, B). Eggs, milk, and tree nuts/seed-triggered anaphylaxis affected children at younger ages (medians, 2.2, 3.2, and 5.6 years, respectively), whereas peanuts, fruits/vegetables, and crustaceans affected older children (medians, 7.6, 11, and 13.4 years, respectively).
FIGURE 4.
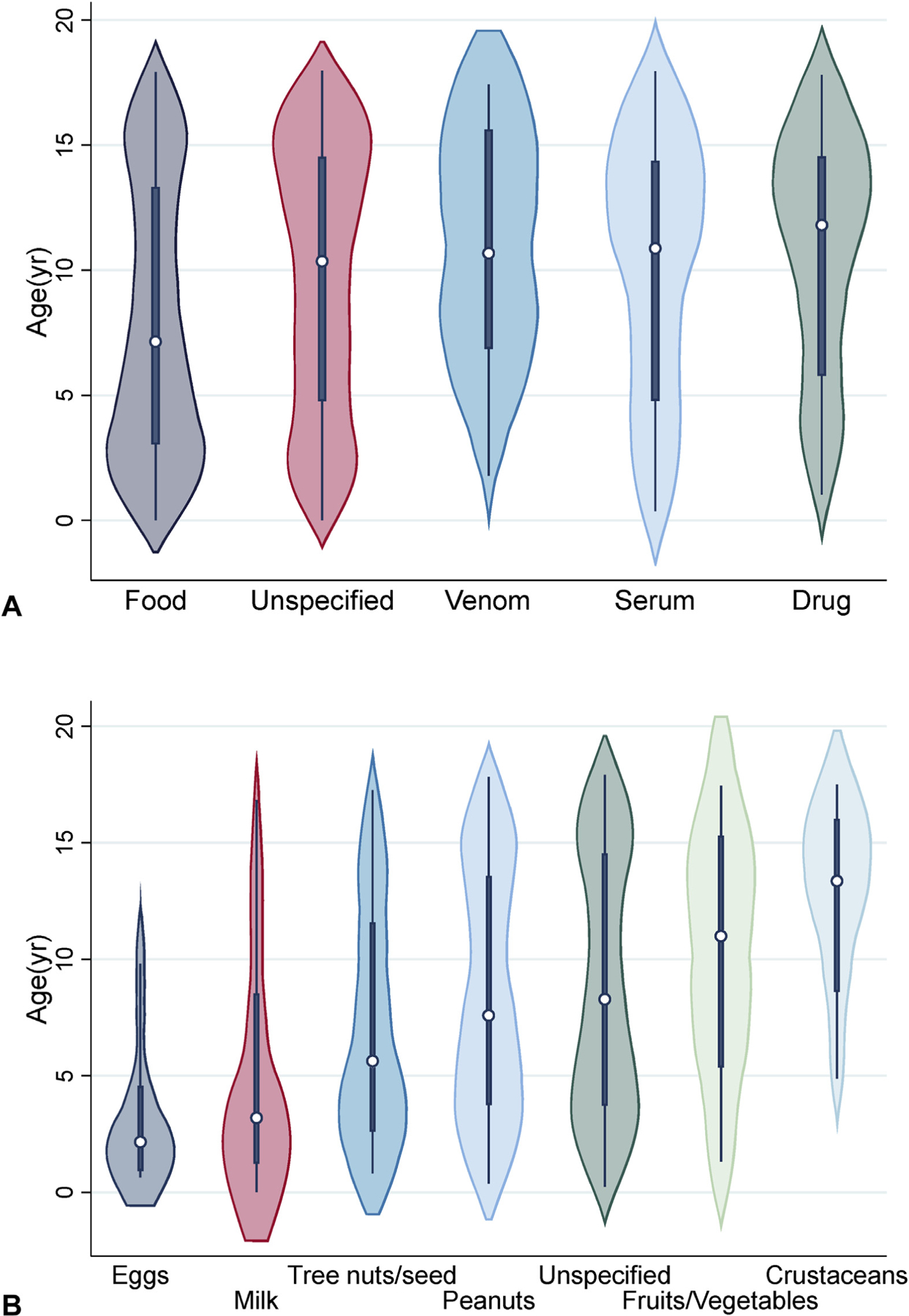
Distribution according to identified trigger (A) and food (B). n = 1989, P < .001. Box plots show median, 25% and 75%. Whiskers represent range for each variable. Figure 4, A, Frequency was as follows: Food (35%, n = 705), unspecified (58%, n = 1154), serum (2%, n = 48), drug (3%, n = 59), venom (1%, n = 23). B, Peanuts were the most common identified trigger (n = 235, 45%), followed by tree nuts/seeds (n = 100, 19%) and milk (n = 51, 9.8%). n = 478. Food trigger was based on International Classification of Diseases code.
There were significant differences among children with different race/ethnicities and triggers (P < .001). In blacks, fish (60%) and crustaceans (67%) were the most common triggers; in white children, tree nuts (59%) and milk (64%); in Asian children, eggs (10%) and tree nuts/seeds (9%); and in Hispanic patients, crustaceans (22%), eggs (20%), and fish (20%). There were also significant differences among children in different US regions with varying food triggers (P = .044). In Midwestern children, milk (46%) or crustaceans (45%) were the most common triggers; in Northeastern children, eggs (25%) and milk (22%); in Southern children, crustaceans (45%); and in Western children, tree nuts/seeds (30%) or peanuts (31%).
DISCUSSION
This study describes the largest cohort of ICU pediatric anaphylaxis admissions with a comprehensive analysis of mortality (primary outcome), identified triggers, clinical and demographic information, and POD.
We identified 1989 patients with critical anaphylaxis and 19 (1%) fatalities. Identified triggers for fatal cases were peanuts, milk, and blood products. Drug- and serum-triggered anaphylaxis led to worse outcomes. Food was the most common identified trigger, specifically peanut. These results suggest that anaphylaxis remains an important cause of critical illness and some children can have poor outcomes, including death.
This study describes factors more common among children who died, who had higher risk of death, required intubation, or were hypotensive. Older age (>12 years) was more common among children who died. A trigger was unidentified for most patients, due to either lack of documentation or identification. Mortality probability scores were worse for adolescent patients, Hispanic patients, those admitted to large hospitals, and those admitted for serum-triggered anaphylaxis. Mortality probability scores may have been worse at larger hospitals given higher acuity and higher rate of transfer from other community hospitals. These scores may be higher for serum-triggered anaphylaxis because these patients likely were already admitted to the hospital for a chronic illness. Intubation was less likely in children aged 2 to 5 years and more likely with a concomitant asthma diagnosis or those admitted to large-sized hospitals. Hypotension was more common in patients admitted to large-sized hospitals.
These results demonstrate that the overall burden of pediatric critical anaphylaxis in North America can be described according to demographic trends. It is relatively highest in the Northeastern and Western US census regions (Figure 2, B), there is a gender shift from male to female predominance when comparing young children to adolescents, and Asian children are more affected when younger than 2 years. Food trigger also varied according to region and race of patient. Milk and crustaceans were common in Midwestern children, crustaceans were more common in southern children, fish and crustaceans were common in black children, and tree nuts and milk were common in white children. This distribution may be affected by diet among certain regional and racial/ethnic groups. The overrepresentation of Asian patients reflects an increased rate of food allergy in Asian patients.23 The distribution of food triggers by patient age was similar to what has been described among pediatric food allergy diagnoses in the United States for all severities.10
This study is the largest reported cohort of pediatric anaphylaxis cases admitted to a PICU in North America. The largest study of pediatric critical anaphylaxis before this described 219 cases by Michelson et al.6,7,19 Our study allows for a closer look at the most critically ill hospitalized patients who suffer from anaphylaxis in North America (United States and Canada). Mortality probability scores were higher (median, −4.66) than those reported in the literature for pediatric critical asthma admissions (median, 0.5) over an 8-year period at 1 site in Hong Kong, which analyzed 116 patients retrospectively.24 This may suggest that patients with critical anaphylaxis are more seriously ill than patients with critical asthma, despite the overlap. LOS in this study (median, 21 hours) was much shorter for critical anaphylaxis than for critical asthma (median, 7 days).24 Hypotension in 5% of cases parallels the rate of hypotension in anaphylaxis cases that were described previously.15 These results highlight the serious nature of anaphylaxis. Future work will help us understand temporal changes and final diagnostic etiology of these cases.
This study is limited by the scope of the VPS database, which captures approximately one-fourth of all ICUs in the United States (>400) and 15 in Canada.25,26 It is also possible that those represented do not represent a random distribution of the more than 400 PICUs in the United States and Canada; thus, we did not extrapolate from our data to calculate incidence rates. Because of privacy regulations in a deidentified study, this study does not capture information about which years these events occurred. Another limitation of this study is that in most fatalities, the cause was not identified/reported.
This report is the largest report of critical pediatric anaphylaxis cases to date. We identified the important triggers—food, drugs, and blood products—that may increase the risk for a critical hospital admission in children, and we underscore the serious nature of anaphylaxis caused by any trigger. Further studies will be needed to understand the trends over time, incidence, and final diagnostic etiology of these cases. Overall, we report that anaphylaxis remains a significant cause of critical illness in pediatric patients and can lead to death for 1% of patients who are admitted to the ICU.
What is already known about this topic?
There are limited reports of prevalence, incidence, and mortality in pediatric anaphylaxis.
What does this article add to our knowledge?
This is the largest study to describe pediatric critical anaphylaxis cases in North America. Among pediatric anaphylaxis patients treated in intensive care units in the United States and Canada from 2010 to 2015, 1% died and food was the most common trigger.
How does this study impact current management guidelines?
The risk for intensive care unit admission in children underscores the serious nature of anaphylaxis in this population.
Acknowledgments
VPS data were provided by Virtual Pediatric Systems, LLC. No endorsement or editorial restriction of the interpretation of these data or opinions of the authors has been implied or stated.
Conflicts of interest:
C. M. Davis has received research grants from and signed contracts with Aimmune Therapeutics, Inc, DBV Technologies, Inc, Thermo Fisher Scientific, Inc, Nutricia North America, the National Institute of Allergy and Infectious Disease, Regeneron Pharmaceuticals, and DNAlytics,Inc; has served as a consultant or advisor with Aimmune Therapeutics, Inc, DBV Technologies, Inc, and Moonlight Therapeutics, Inc. K. Anagnostou has signed research contracts with Aimmune Therapeutics, Inc, and DBV Technologies Inc. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations used
- ICU
Intensive care unit
- LOS
Length of stay
- PICU
Pediatric intensive care unit
- PIM 2
Pediatric Index of Mortality 2
- POD
Probability of death
- PRISM III
Pediatric Risk of Mortality 3
- VPS
Virtual Pediatric Systems
REFERENCES
- 1.Tejedor Alonso MA, Moro Moro M, Múgica García MV. Epidemiology of anaphylaxis. Clin Exp Allergy 2015;45:1027–39. [DOI] [PubMed] [Google Scholar]
- 2.Yu JE, Lin RY. The epidemiology of anaphylaxis. Clin Rev Allergy Immunol. Available from: http://link.springer.com/10.1007/s12016-015-8503-x.AccessedMarch 1, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Bohlke K, Davis RL, DeStefano F, Marcy SM, Braun MM, Thompson RS, et al. Epidemiology of anaphylaxis among children and adolescents enrolled in a health maintenance organization. J Allergy Clin Immunol 2004;113:536–42. [DOI] [PubMed] [Google Scholar]
- 4.Simon MR, Mulla ZD. A population-based epidemiologic analysis of deaths from anaphylaxis in Florida. Allergy 2008;63:1077–83. [DOI] [PubMed] [Google Scholar]
- 5.Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract 2017;5: 1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dibs SD, Baker MD. Anaphylaxis in children: a 5-year experience. Pediatrics 1997;99:E7. [DOI] [PubMed] [Google Scholar]
- 7.Motosue MS, Bellolio MF, Van Houten HK, Shah ND, Campbell RL. Risk factors for severe anaphylaxis in the United States. Ann Allergy Asthma Immunol 2017;119:356–361.e2. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Perea A, Ameiro B, Morales C, Zambrano G, Rodríguez A, Guzmán M, et al. Anaphylaxis in the pediatric emergency department: analysis of 133 cases after an allergy workup. J Allergy Clin Immunol Pract 2017;5:1256–63. [DOI] [PubMed] [Google Scholar]
- 9.Jeppesen AN, Christiansen CF, Frøslev T, Sørensen HT. Hospitalization rates and prognosis of patients with anaphylactic shock in Denmark from 1995 through 2012. J Allergy Clin Immunol 2016;137:1143–7. [DOI] [PubMed] [Google Scholar]
- 10.Grabenhenrich LB, Dölle S, Moneret-Vautrin A, Köhli A, Lange L, Spindler T, et al. Anaphylaxis in children and adolescents: the European Anaphylaxis Registry. J Allergy Clin Immunol 2016;137:1128–1137.e1. [DOI] [PubMed] [Google Scholar]
- 11.Gibbison B, Sheikh A, McShane P, Haddow C, Soar J. Anaphylaxis admissions to UK critical care units between 2005 and 2009. Anaesthesia 2012;67:833–9. [DOI] [PubMed] [Google Scholar]
- 12.Hoffer V, Scheuerman O, Marcus N, Levy Y, Segal N, Lagovsky I, et al. Anaphylaxis in Israel: experience with 92 hospitalized children. Pediatr Allergy Immunol 2011;22:172–7. [DOI] [PubMed] [Google Scholar]
- 13.Camargo CA, Clark S, Kaplan MS, Lieberman P, Wood RA. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol 2007;120:131–6. [DOI] [PubMed] [Google Scholar]
- 14.Mullins RJ, Clark S, Camargo CA. Regional variation in epinephrine autoinjector prescriptions in Australia: more evidence for the vitamin Deanaphylaxis hypothesis. Ann Allergy Asthma Immunol 2009;103: 488–95. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan WJ, Graham D, Ma L, Baxi S, Phipatanakul W. Higher incidence of pediatric anaphylaxis in northern areas of the United States. J Allergy Clin Immunol 2009;124:850–852.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vetander M, Helander D, Flodström C, Östblom E, Alfvén T, Ly DH, et al. Anaphylaxis and reactions to foods in children—a population-based case study of emergency department visits. Clin Exp Allergy 2012;42:568–77. [DOI] [PubMed] [Google Scholar]
- 17.Jerschow E, Lin RY, Scaperotti MM, McGinn AP. Fatal anaphylaxis in the United States, 1999–2010: temporal patterns and demographic associations. J Allergy Clin Immunol 2014;134:1318–1328.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudders SA, Arias SA, Camargo CA. Trends in hospitalizations for food-induced anaphylaxis in US children, 2000–2009. J Allergy Clin Immunol 2014;134:960–962.e3. [DOI] [PubMed] [Google Scholar]
- 19.Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr 2015;167:719–724.e3. [DOI] [PubMed] [Google Scholar]
- 20.Slater A, Shann F, Pearson G, Paediatric Index of Mortality (PIM) Study Group. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 2003;29:278–85. [DOI] [PubMed] [Google Scholar]
- 21.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996;24:743–52. [DOI] [PubMed] [Google Scholar]
- 22.Davis AL, Carcillo JA, Aneja RK, Deymann AJ, Lin JC, Nguyen TC, et al. American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Crit Care Med 2017;45:1061–93. [DOI] [PubMed] [Google Scholar]
- 23.Hon K-L, Tang W-SW, Leung T-F, Cheung K-L, Ng P-C. Outcome of children with life-threatening asthma necessitating pediatric intensive care. Ital J Pediatr 2010;36:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki M, Peters RL, Koplin JJ, Field MJ, McWilliam V, Sawyer SM, et al. Risk factors for food allergy in early adolescence: the SchoolNuts study. J Allergy Clin Immunol Pract 2018;6:496–505. [DOI] [PubMed] [Google Scholar]
- 25.Odetola FO, Clark SJ, Freed GL, Bratton SL, Davis MM. A national survey of pediatric critical care resources in the United States. Pediatrics 2005;115:e382–6. [DOI] [PubMed] [Google Scholar]
- 26.Fowler RA, Abdelmalik P, Wood G, Foster D, Gibney N, Bandrauk N, et al. , Canadian Critical Care Trials Group; Canadian ICU Capacity Group. Critical care capacity in Canada: results of a national cross-sectional study. Crit Care 2015;19. Available from: http://ccforum.com/content/19/1/133.AccessedMarch 20, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


