Abstract
Increases in the D2 receptor high affinity state are associated with certain neurological disorders. We synthesized and characterized the high-affinity D2high ligand [3H]MCL-536 in competition binding against the D2/3 agonist R-(−)-N-n-propylnorapomorphine (NPA) and the D2/3 antagonist raclopride. The total binding of [3H]MCL-536 (minus that in the presence of 100 nM NPA) was measured by saturation binding in CHO cells expressing human D2long; the data yielded separable, nonsaturable nonspecific, and saturable specific components. The former represents an aporphine site common to NPA and [3H]MCL-536. The latter indicated specific binding to the total D2 receptors (both high and low-affinity states). [3H]MCL-536 had a Kd of 0.8 nM. In competition binding, NPA had a Ki of 0.16 nM, and a Ki of 0.9 nM for raclopride. Co-incubation with guanylylimidodiphosphate abolished binding to D2high. This unique profile makes radiolabeled MCL-536 a versatile tool for diagnostics and therapeutics, and may quantify D2high sites in schizophrenia and PD patients in vivo.
Keywords: Dopamine D2high receptor, Parkinson’s disease, schizophrenia, PET, aporphine, tritiated radioligand
Graphical Abstract
Dopamine receptors, especially those in the D2 family, play an important role in a variety of disorders involving the central nervous system. These include movement disorders such as Parkinson’s disease, restless leg syndrome (RLS), Huntington’s chorea, and multiple system atrophy (MSA),1 as well as psychoses including schizophrenia or Tourette’s syndrome, and other disorders including attention deficit disorder or addiction.2–6 The D2 receptor family includes the D2 receptor splice variants D2long and D2short7 as well as the D3 and D4 receptors; the D2 family of receptors are predominantly coupled to Gi/o proteins.8 G protein-coupled receptors have been reported to exist in high and low affinity states for dopamine. The existence of the dopamine D2 receptor in the high-affinity state (D2high) was first reported by De Lean and co-workers in 1982.9,10 The D2high receptor is thought to be the functional form of the D2 receptor to which endogenous neurotransmitter, dopamine, binds. The proportion of high-affinity and low-affinity receptors can be observed in vitro by using different assay conditions, and the receptors have different Ki binding affinities and show a biphasic competition curve. Despite some level of disagreement, the existence of the D2high receptor is widely accepted today. Evidence increasingly supports that the existence of the receptor in the high-affinity state, the functional or active state of the D2 receptor, is more important to pathophysiological processes than the expression of the receptor and its dysregulation.11,12 Thus, the development of high affinity and selective D2 receptor agonists, which are capable of distinguishing between the high and low affinity states of the D2 receptor, is important for research purposes as well as for early diagnosis and therapeutic intervention.
A number of ligands for the D2 receptor, both agonists as well as antagonists, are available to the research community. Among ligands which can selectively bind to the D2high receptor under appropriate assay conditions are antagonists such as raclopride, spiperone, N-methylspiperone (NMSP), domperidone and nemonapride. Likewise, a number of agonists also bind selectively to the D2high receptor: N-n-propylnorapomorphine (NPA), apomorphine (APO), PD-128907 ((+)-PHNO), 2-methoxy-N-n-propylnorapomorphine (MNPA), 7-OHDPAT, cabergoline, and bromocriptine. Most of these ligands are capable of identifying the D2high receptor. However, the proportion of the D2 receptor identified has been inconsistent and appears to depend on the ligand which is used. One problem which may complicate these findings is that these ligands are not selective for the D2 receptor, but may also bind to other receptors of the D2 family as well as with receptors for other neurotransmitters (Table 1).
Table 1.
Commercially Available 3H-Radioligands
| ligand | D2 Ki value (nM) | D3 Ki value (nM) | comments | |
|---|---|---|---|---|
| antagonists | spiperone | 0.06913a | 0.6113a | has D3/4, 5HT2A affinity15 |
| 0.0714b | has D3/4, 5HT2A affinity15 | |||
| N-methylspiperone | 0.19,16c 0.1717d | |||
| domperidone | 0.3013a | 9.513a | has D3 affinity | |
| nemonapride | 0.01718e | 0.05318e | α1 adrenoreceptor affinity19 | |
| raclopride | 0.618e | 1.0318e | has D3 affinity | |
| 1.314b | ||||
| agonists (D2high and D3high) | apomorphine | 2413a | 2013a | affinity to D122 |
| 120f | 2.620g | |||
| 0.520b | ||||
| 1.821f | ||||
| N-propylnorapomorphine (NPA) | 0.182f | 0.2520g | has D3 affinity17 | |
| 0.0920b | ||||
| 0.0617h | 0.0060717i | |||
| (+)-PHNO | 0.620f | 0.620g | D3 preferring | |
| 0.2420b | ||||
| MNPA | 0.0917h | 0.032617i | has D3 affinity17 | |
| 5.123f | ||||
| 7-OH-DPAT | 6124a | 0.7824a | has D3 affinity25 | |
| cabergoline | 1.6326j | 1.2726l | has D3 affinity | |
| 0.6126k | ||||
| 0.3621f | ||||
| bromocriptine | 4.8726j | 30.4526l | nonselective | |
| 2.926k | ||||
| 0.920b |
Rat D2, D3 expressed in CHO cells, [125I]iodosulpiride.
Human D2long expressed in CHO cells, [3H]domperidone.
Rat striatal homogenate, [3H]raclopride.
Human putamen homogenate, [3H]racropride.
Human D2, D3 expressed in CHO cells, [3H]nemonapride
Rat striatal homogenate, [3H]domperidone.
Human D3 expressed in CCL1 mouse fibroblasts, [3H]domperidone.
Human D2long expressed in HEK293T cells, [3H]N-methylspiperone.
Human D3 expressed in HEK293T cells, [3H]N-methylspiperone
Human putamen homogenate, [3H]spiperone.
Human putamen homogenate, [3H]raclopride.
Human putamen homogenate, [3H]PD128907.
For example, domperidone and raclopride also have a high affinity to D3 receptors.14,18 Spiperone and NMSP have significant affinity to D3 and D4 receptors, but also to 5HT2A receptors.15,16,18,27 The ligand nemonapride also interacts with alpha (α1) adrenoceptors in addition to D2 receptors.19 In vitro assay conditions also exert a huge effect on the results, as they can affect the accessibility of D2high receptors. Thus, the benzamide radioligands nemonapride and raclopride work only in the presence of sodium ions. At the same time, these ions interfere with the D2high affinity state.28,29 Interestingly, only domperidone, but neither spiperone nor raclopride has the capability to distinguish between D2high and D2low in whole, unfrozen rat anterior pituitary cells ex vivo.30
Since most dopamine antagonists have no inherent ability to distinguish between receptors in high- vs low-affinity states in vivo, they are not ideal for studies on neurotransmission and its pathophysiological changes which are associated with disease. Agonist ligands are preferable, because their preferential binding to the receptors in their high affinity state makes them an ideal tool for studying the role of the receptor state in the pathogenesis of disorders associated with the dopaminergic system, thus enabling more detailed studies and enhancing the knowledge of dopamine transmission and D2 receptor dynamics in these disorders.
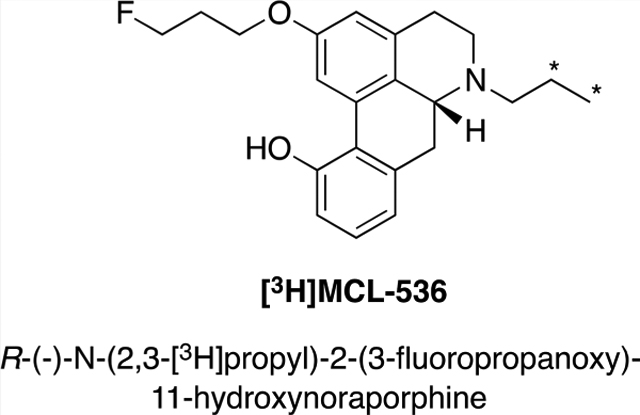
We have developed an aporphine agonist, R-(−)-2-(3-fluoropropanoxy-11-hydroxy-N-n-propyl(1,2-[3H])-noraporphine [3H]MCL-536, which binds with high affinity to dopamine D2 receptors but not to other receptors of the D2 family.31 Additionally, MCL-536 exhibited no affinity or low affinity for other receptors tested, including serotonin, α- and β-adrenergic, benzodiazepine, GABAA, muscarinic, sigma, kappa, and mu opioid receptors, as well as dopamine, serotonin, and norepinephrine transporters and translocator proteins (NIMH, Psychoactive Drug Screening Program, North Carolina). Herein, we describe the further characterization of this novel agonist ligand with regard to its binding affinities to human D2 receptors expressed in Chinese hamster ovary (CHO) cells.
RESULTS AND DISCUSSION
Saturation Curve of MCL-536 Binding to the Human D2 Long Receptor.
MCL-536 was evaluated in a saturation assay using cloned human D2long membranes in CHO cells; the presence of the dopamine D2 receptor agonist, NPA (100 nM) served to detect nonspecific binding. Human D2long is the isoform of the D2 receptor found at the postsynaptic terminal. The binding of [3H]MCL-536 to dopamine D2-receptor-containing membranes in CHO (Chinese Hamster Ovary cells) was found to have more than one component. The total binding of [3H]MCL-536 minus that bound in the presence of 100 nM NPA yielded two components that were graphically separable into a nonsaturable nonspecific component and a saturable specific component, as shown. The nonsaturable component, blocked by 100 nM NPA, represented an aporphine site common to NPA and [3H]MCL-536. The saturable component represented the specific binding to the total population of dopamine D2 receptors that were in the high-affinity state and the low-affinity state. At 50% of the specific binding of [3H]MCL-536 to the D2 receptor, a dissociation constant Kd of 0.8 nM could be determined (Figure 2).
Figure 2.
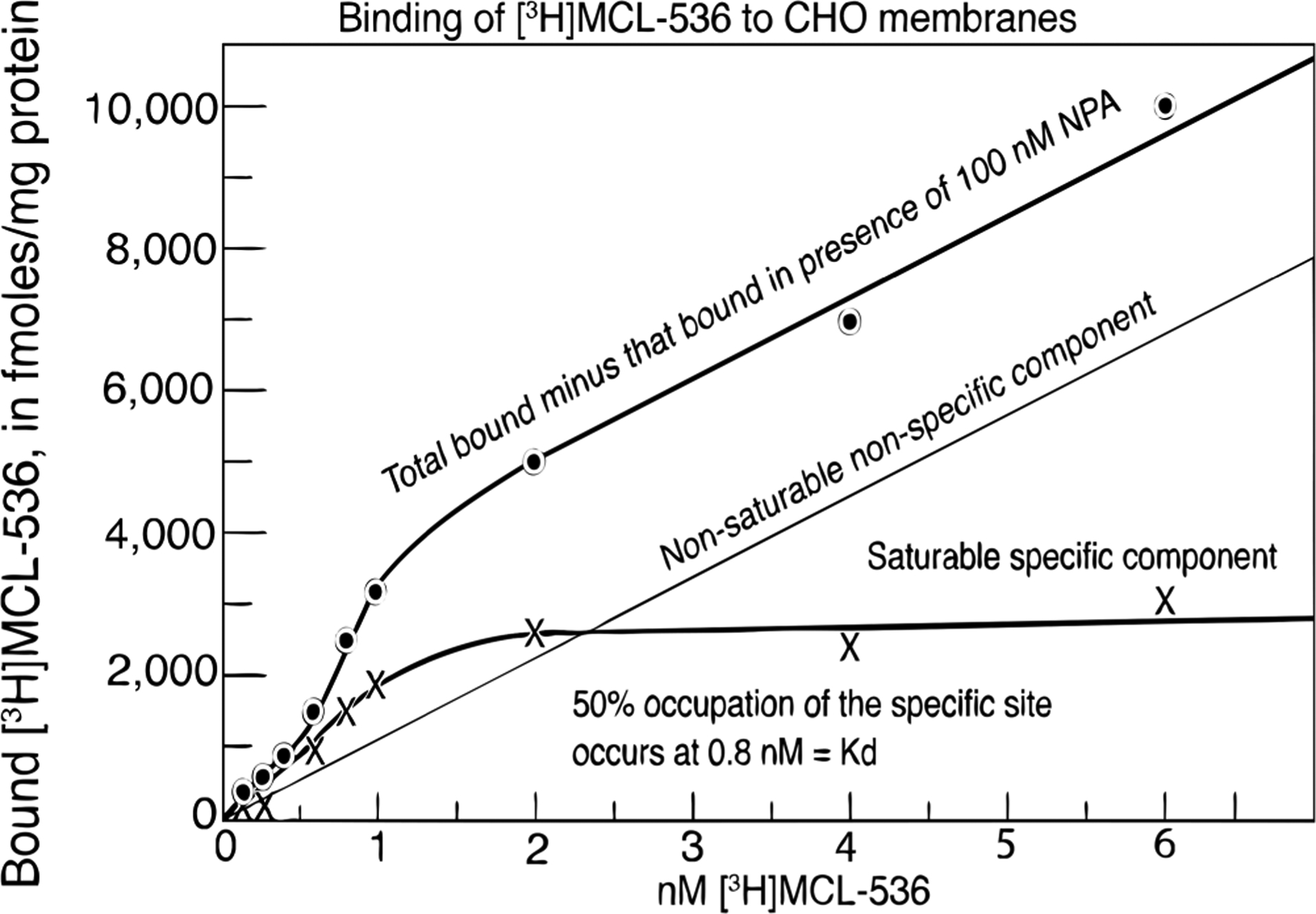
Binding of [3H]MCL-536 to dopamine D2 receptor expressed in CHO membranes (n = 5). Membranes of CHO cells were incubated with [3H]MCL-536 in increasing concentrations with or without 100 nM NPA. The total binding of [3H]MCL-536 minus [3H]MCL-536 in the presence of 100 nM NPA yielded a nonsaturable, nonspecific component (“apomorphine site”) and a saturable, specific component (total high- and low-affinity state D2 receptors). The dissociation constant, Kd, of [3H]MCL-536 for the D2 receptors occurred at the concentration where 50% of the receptors were occupied.
Competition of Agonist NPA and Antagonist Raclopride vs [3H]MCL-536.
[3H]-MCL-536 was tested in vitro for competition with NPA and raclopride. When R-(−)-N-n-propylnorapomorphine (NPA) was used as the competing ligand, it was found to have a Ki binding affinity of 0.16 nM against [3H]MCL-536 (Figure 3A). Raclopride had a Ki binding affinity of 0.90 nM against [3H]MCL-536 (Figure 3B). Nonradioactive MCL-536 had a Ki of 1.29 nM against [3H]MCL-536 (Figure 3C).
Figure 3.
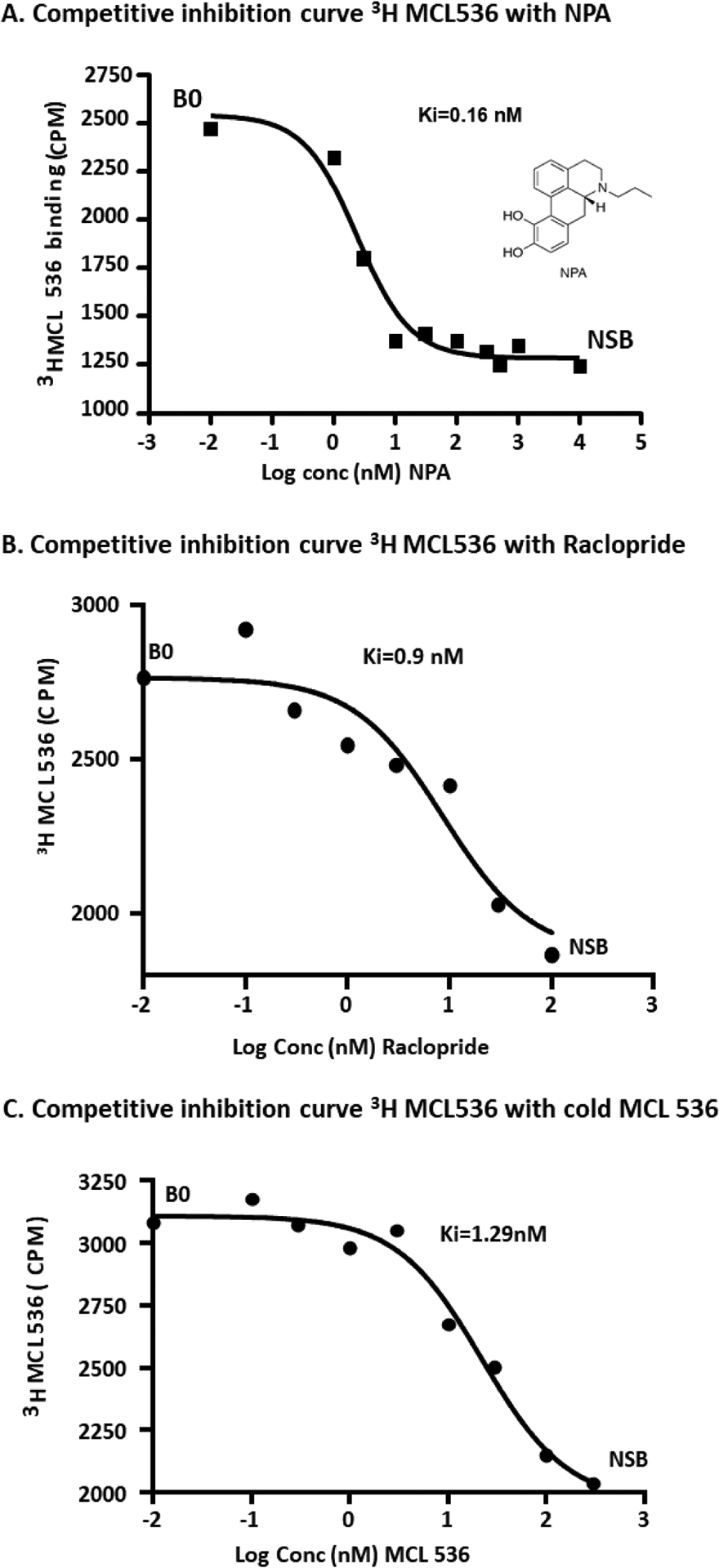
Curves illustrating competitive binding for [3H]MCL-536 vs NPA (A), for [3H]MCL-536 vs raclopride (B), and for nonradioactive MCL-536 (C) to the human cloned D2long receptor expressed in CHO cells (n = 5). Data were analyzed using GraphPad Prism software.
Abolition of D2High Receptors in the Presence of a Guanine Nucleotide (GppNHp).
The dopamine D2 receptor is a G protein-coupled receptor; its G protein α subunit binds GTP in the activated state. The receptor becomes inactivated when GTP is metabolized to GDP and Pi. The role of GppNHp is to maintain a more constant state of continued inactivation. The specific binding of [3H]MCL-536 was found to separate into two sites in the presence of 200 μM guanilylimidodiphosphate (GppNHp). The binding component removed by the guanine nucleotide represented the dopamine D2High receptors. The residual binding sites in the presence of the guanine nucleotide (straight line) appeared to represent nonspecific sites related to the aporphine structure of MCL-536. The D2 high state was abolished by incubation with GppNHp (Figure 4), confirming that [3H]MCL-536 is a D2high agonist. The proportion detected is in agreement with previously reported values.5 Thus, [3H]MCL-536 is able to identify the D2high receptor state.
Figure 4.
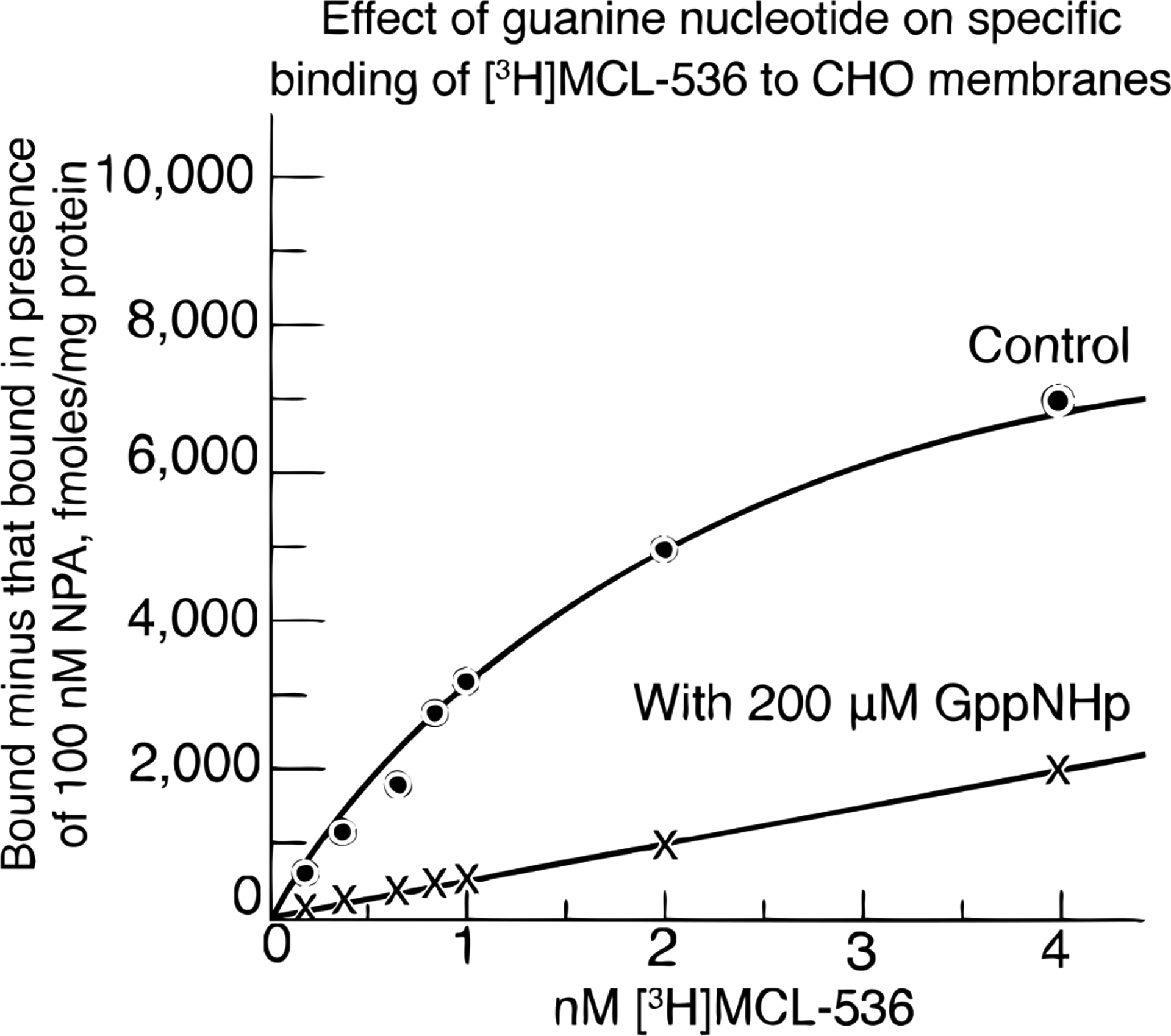
Specific binding of [3H]MCL-536 in the presence and absence of 200 μM guanilylimidodiphosphate (GppNHp) (n = 5).
There is increasing evidence that, in a number of neurological disorders involving the dopaminergic system, such as Parkinson’s disease or schizophrenia, more D2 receptors exist in the high-affinity state.32,33 However, the majority of commercially available D2 radioligands are antagonists, which cannot distinguish between active D2high and inactive D2low affinity states of the receptor in vivo. Additionally, none of the commercially available ligands are selective for the D2 receptor subtype, but also possess high affinities to other D2 family receptors (D3/4) or even other neurotransmitter receptors (5HT, alpha 1a, etc.). Thus, there is a demonstrated need for high-affinity, highly selective, agonist-based, [3H] radioligands for the D2 receptor.
Herein we describe here the synthesis and characterization of a selective and specific high affinity dopamine D2high receptor agonist, [3H]MCL-536. The advantages of this agonist are that (1) it has high selectivity for D2 over D3 and D4 (D2 Kd = 0.8 nM; Ki = 1.29 nM when [3H]MCL-536 is used; D3 Ki = 100 nM when [H]domperidone is used;23 D4 Ki = 53 nM when [3H]N-methylspiperone is used (NIMH, Psychoactive Drug Screening Program, North Carolina)); (2) it has no affinity or very low affinity for other receptors tested, including serotonin, α- and β-adrenergic, benzodiazepine, GABAA, muscarinic, sigma, kappa, and mu opioid receptors, as well as dopamine, serotonin, and norepinephrine transporters and translocator proteins (NIMH, Psychoactive Drug Screening Program, North Carolina); and (3) it detects the activated form, or high affinity state, of the D2 receptor, D2high (this study). This unique profile makes MCL-536 a versatile tool for research as well as for diagnostic and therapeutic purposes.
When [3H]MCL-536 was used in competition binding studies for D2high, NPA had a Ki value of 0.18 nM and raclopride had a Ki of 0.90 nM. Additionally, [3H]MCL-536 was shown to identify the D2high state. Thus, in its tritiated form, MCL-536 can be used experimentally to investigate D2 receptor activity and dynamics more effectively as a function of neurological conditions. In this regard, we are planning future studies to complete the preclinical evaluation of MCL-536 by confirming selectivity to D2high in different brain regions of the rat via autoradiography ex vivo, and to evaluate biodistribution in vivo.
Our ultimate goal is to develop MCL-536 as an 18F-radioligand for clinical PET neuroimaging. Most ligands which are currently available to the research community are 11C-labeled, including [11C]MNPA,17,34,35 [11C]NPA,36 and [11C]-(+)-PHNO.37,38 However, because the radioisotope 11C has a short half-life of only 20 min, its use is limited to on-site cyclotrons which are not readily available in all clinics. Furthermore, the ligands described above do not unambiguously identify the D2high receptor in its active state in vivo.39 This may be due to their long dissociation half-lives from the receptor, measured in vitro (from ~30 to ~600 s), compared to the short dissociation time of the G protein and the ensuing conversion of the receptor from its high- to low-affinity state (<1 s).33 Likewise, the development of an effective 18F-labeled D2 agonist radioligand has also encountered challenges in the N-alkyl radiofluorination of (+)-PHNO, e.g., significantly altered D2 binding compared to that of (+)-PHNO. Furthermore, N-alkyl radiofluorinated aporphine derivatives, including [18F]FNPA and [18F]FNEA, did not prove to be D2 agonists.40 Reports on the aminotetralin radioligands [18F]5-OH-FPPAT, [18F]5-OH-FHXPAT, and [18F]7-OH-FHXPAT describe their development as D2high and D3high ligands, respectively.41,42 Last year, a series of 18F-labeled chromanol derivatives were evaluated in rats, and one analogue ([18F]FEt-AMC13) possessed a striatum/cerebellum binding ratio of 2.08, although it exhibited a lower binding potential and it was less sensitive to competition with raclopride in comparison to previously reported agonist PET radioligands.43,44 Thus, development of an 18F-labeled D2 agonist which exhibits high selectivity and specificity for the D2high receptor for human use remains an important goal. Such a radioligand would be an invaluable tool for studying unusual activity of D2 receptor dynamics in the living brain, before extensive neurological changes occur, and before physical symptoms emerge. It could enable early diagnosis of dopamine-associated disorders, and at the same time also form a basis for more targeted and effective therapies for Parkinson’s disease, schizophrenia, addiction, and other neuropsychiatric disorders involving the D2high receptor. In summary, the new radioligand [3H]MCL-536 possesses subnanomolar binding affinity to human D2long and has proven to be a superior radioligand for in vitro evaluation in receptor binding assays. MCL-536 will be developed as 18F-labeled PET radiotracer, and being a selective, high affinity D2 agonist ligand, it also has the potential to replace the current standard therapies, as a lower-dose, potent oral medication for PD.
METHODS
Synthesis of R-(−)-N-Allyl-2-(3-fluoropropanoxy)-11-hydroxynoraporphine (MCL-565).
The N-allyl precursor to [3H]MCL-536, MCL-565, was synthesized in seven steps from commercially available morphine (Figure 1), and tritiated to produce [3H]MCL-536 at >99% purity and activity of 57.6 Ci/mmol.23,45–47
Figure 1.
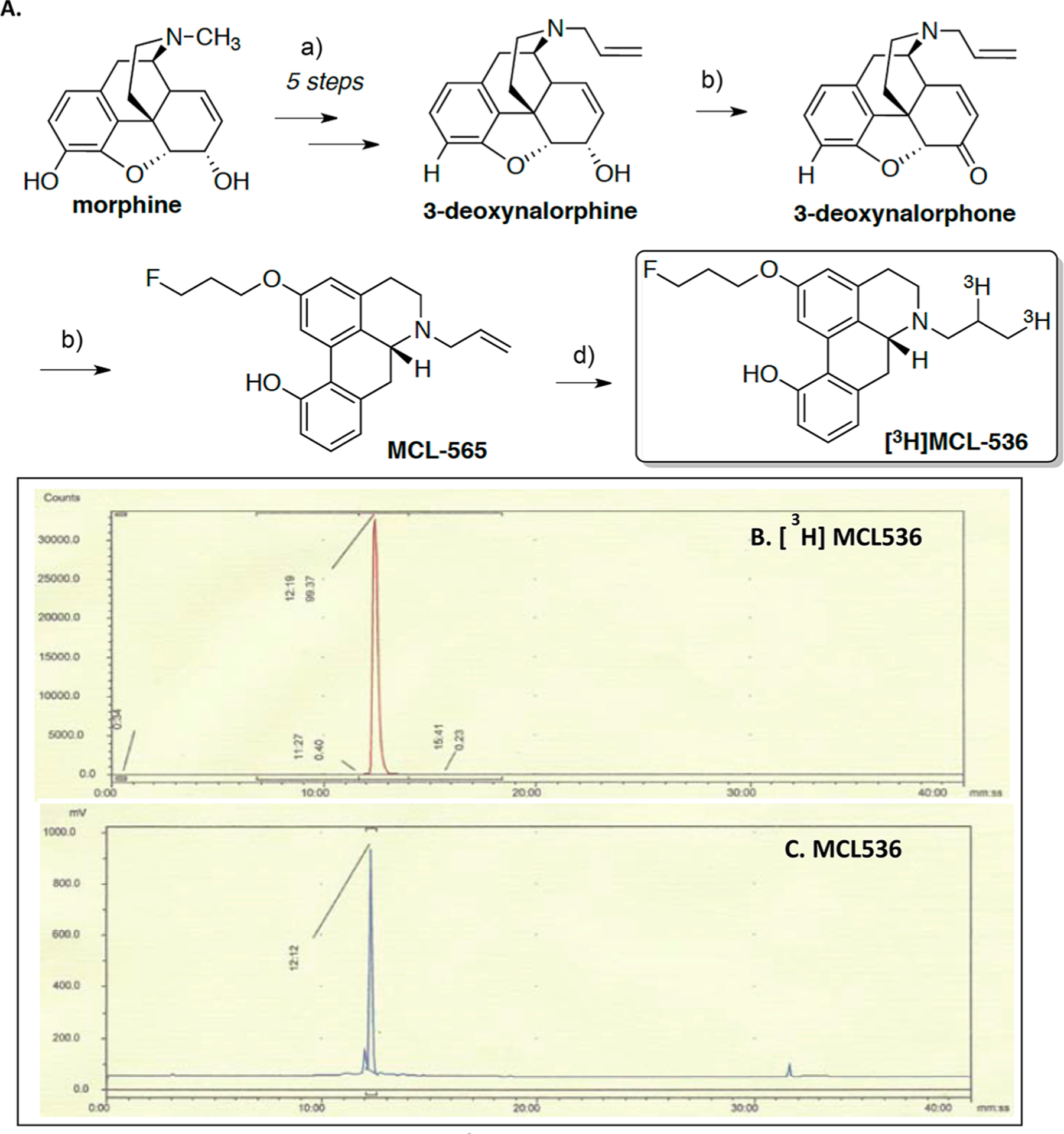
(A) Synthesis of allyl precursors for MCL-536. (B,C) HPLC chromatograms of (B) [3H]MCL-536 and (C) coinjected nonradioactive MCL-536. Reagents and conditions: (a) ref 46; (b) IBX, anhydrous DMF, rt, 76%; (c) 3-fluoropropanol, methanesulfonic acid, 95–105 °C, 44%; 1 N HCl/Et2O, 100%;23,45–47 (e) [3H]2, [Pd], 60 Ci/mmol.
3-Deoxynalorphone.
The oxidation of the allylic alcohol of 3-deoxynalorphine46 was carried out analogously as described by Sondergaard et al.48 To a dried vial was added 3-deoxynalorphine (307 mg; 1.016 mmol), followed by anhydrous DMF (5 mL) and 2-iodoxybenzoic acid (IBX) (313 mg; 1.12 mmol). The reaction mixture was stirred vigorously for 1 h at room temperature. Upon completion, the mixture was transferred to ethyl acetate (25 mL) and washed with water (80 mL), 10% aqueous sodium carbonate solution (2 × 80 mL), and brine (80 mL). The organic phase was separated and dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by silica gel flash column chromatography using 1:20 methanol/dichloromethane as eluent to afford the product as a pale yellow solid, 3-deoxynalorphone, 227 mg, yield 76%, mp 118–120 °C. 1H NMR (300 MHz, CDCl3) δ7.09−6.99 (m, 1H), 6.69−6.58 (m, 2H), 6.05 (dd, 1H, J1 = 3.00 Hz, J2 = 6.00 Hz), 5.28 (s, 1H), 5.22−5.15(brt, 2H, J = 6.00 Hz), 4.66 (s, 1H), 3.53 (dd, 1H, J1 = 3.00 Hz, J2 = 6.00 Hz), 3.29−3.13 (m, 3H), 3.07 (d,1H, J = 18.00 Hz), 2.78−2.67 (m, 2H), 2.34 (dd, 1H, J1 = 3.00 Hz, J2 = 6.00 Hz), 2.24 (td, 1H, J1 = 3.00 Hz, J2 = 12.00 Hz), 2.05 (ddd, 1H, J1 = 3.00 Hz, J2 = 12.00 Hz, J3 = 12.00 Hz), 1.79 (brd, 1H, J = 6.00 Hz). 13C NMR (75 MHz, CDCl3) δ 194.66, 157.31, 149.66, 135.64, 134.22, 132.56, 128.86, 127.68, 119.32, 117.90, 107.58, 87.80, 58.41, 56.75, 45.11, 43.10, 41.81, 34.20, 21.99.
R-(−)-N-Allyl-2-(3-fluoropropanoxy)-11-hydroxynoraporphine (MCL-565 Base).
To a 5 mL Wheaton microreactor were added 3-deoxynalorphone (94 mg; 0.32 mmol) and fluoropropanol (0.175 mL; 2.33 mmol). The mixture was stirred briefly at room temperature. Next, methanesulfonic acid (1.5 mL) was added dropwise and the resulting mixture was stirred at 80 °C for 30 min. Next, the mixture was poured into ice and water (30 mL) and the pH was adjusted to 8–9 with 28% aqueous NH4OH and the aqueous phase was extracted with dichloromethane (3 × 30 mL). The combined organic phase was washed with brine (20 mL) and dried over with anhydrous Na2SO4. After filtration, the solvent was removed in vacuo to afford a dark red foam (250 mg). The foam was purified by silica gel flash column chromatography using 1:30 methanol/dichloromethane as eluent to afford 53 mg of R-(−)-N-allyl-2-(3-fluoropropanoxy)-11-hydroxynoraporphine (MCL-565 base) as a buff foam, 44% yield, HPLC purity: >96%. 1H NMR (300 MHz, CDCl3) (base) δ 7.68 (s, 1H), 7.02 (brs, 1H), 6.81−6.72 (m, 2H), 6.60 (s, 1H), 6.01 (brs, 1H, likely to be –OH), 5.24 (t, J1 = 2.10 Hz, J2 = 1.20 Hz, 2H), 4.71 (brs, 1H), 4.55 (brs, 1H), 4.14−4.03 (m, 2H), 3.73 (brd, J = 1.20 Hz, 1H), 3.36 (d, J = 1.50 Hz, 1H), 3.23−2.90 (m, 5H), 2.73−2.55 (m, 2H), 2.45 (t, J1 = 1.20 Hz, 1H), 2.19 (brs, 1H), 2.10 (brs, 1H). 13C NMR (75 MHz, CDCl3) (base) δ 157.34, 153.26, 138.64, 134.87, 134.61, 133.05, 123.38, 127.95, 121.53, 120.71, 118.79, 116.89, 113.00, 112.19, 81.10 (d, J = 135 Hz), 63.55, 59.35, 57.75, 49.04, 35.30, 30.70 (d, J = 21.00 Hz), 29.50. 19F NMR (282 MHz, CDCl3) (base) δ 7.70 (tt, J1 = 27.00 Hz, J2 = 51.00 Hz).
MCL-565 free base was dissolved in a minimal amount of dichloromethane and treated with excess 1 N HCl in ether and crystallized to afford the HCl salt, 35 mg, off-white solid, HPLC purity (>97.6%), mp 169–171 °C (dec.).
Ligand Characterization Studies.
R-(−)-N-(2,3-[3H]Propyl)-2-(3-fluoropropanoxy)-11-hydroxynoraporphine, [3H]MCL-536, was synthesized (maximum specific activity 57.6 Ci/mmol; American Radiolabeled Chemicals, Inc. St. Louis, MO). The measurement of dopamine D2high receptors in cloned human D2long membranes in CHO cells in vitro was carried out as reported earlier.49 Briefly, crude D2long dopamine receptor membranes (5 ug/sample; EMD Millipore) were incubated with either MCL-536, R-(−)-N-n-propylnorapomorphine (NPA; final concentrations between 1 and 1000 nM) or raclopride (1 and 1000 nM) and [3H]MCL-536 (2 nM final concentration) in assay buffer (50 mM Tris-HC1 (pH 7.4 at 20 °C), 1 mM EDTA, 5 mM KCl, 1.5 mM CaC12, 4 mM MgC12, 10 Mm NaCl) for competition experiments and with or without a final concentration of 100 nM NPA for the saturation experiment to define nonspecific binding to the dopamine D2 receptors for 2 h at room temperature. The incubates were filtered, using a 48-well cell harvester (Brandel Inc.) and buffer-presoaked glass fiber filter mats (grade GF/B fired: size 4.5 × 12.25 in.: cat # FP-105, Brandel Inc.). Filter mats were rinsed with buffer for 15 s (7.5 mL buffer) and placed in scintillation minivials (7 mL, 16 × 54 mm) containing 4 mL of scintillant (PerkinElmer) each. Radioactivity was determined after 6 h in a Beckman LS 6500 scintillation counter. The specific binding of the [3H] ligand was defined as total binding minus binding in the presence of 100 nM NPA.
The Cheng–Prusoff equation50 was used to derive the dissociation constant (Ki value) of NPA from the concentration that inhibited 50% of the high-affinity component (IC50) or 50% of the low-affinity component for [3H]MCL-536. The form of the Cheng–Prusoff equation used was Ki = 5 − [IC50/(1 + C*/Kd)], where C* is the final concentration of the ligand and Kd is the dissociation constant of the ligand, as determined directly by independent experiments of saturation binding to the CHO cells (i.e., Scatchard plot).
To determine binding of the [3H] ligand to the D2 high receptor, [3H]MCL-536 was incubated in the presence of 200 μM GppNHp. Then 100 nM NPA was added to determine nonspecific binding. Measurement was carried out as described above.
ACKNOWLEDGMENTS
Off-target receptor binding profiles were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program (PDSP), Contract # HHSN-271-2013-00017-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA. MCL-565 was converted to [3H]MCL-536 by American Radiolabeled Chemicals, Inc. (St. Louis, MO), Dr. Surendra Gupta.
Funding
Financial support from the National Institutes of Health: R44-OD024615 (J.L.N.), R43-OD020186 (J.L.N.), R21-MH103718 (A.W.S.), and the Branfman Family Foundation (J.L.N.) are gratefully acknowledged.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Booij J, Tissingh G, Winogrodzka A, and van Royen EA (1999) Imaging of the dopaminergic neurotransmission system using single-photon emission tomography and positron emission tomography in patients with parkinsonism. Eur. J. Nucl. Med. Mol. Imaging 26 (2), 171–82. [DOI] [PubMed] [Google Scholar]
- (2).Kapur S, and Mamo D (2003) Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 27 (7), 1081–90. [DOI] [PubMed] [Google Scholar]
- (3).Volkow ND, Fowler JS, Wang GJ, and Swanson JM (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry 9 (6), 557–69. [DOI] [PubMed] [Google Scholar]
- (4).Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, Roder JC, Quirion R, Boksa P, Srivastava LK, Yanai K, Weinshenker D, and Sumiyoshi T (2006) Psychosis pathways converge via D2high dopamine receptors. Synapse 60 (4), 319–46. [DOI] [PubMed] [Google Scholar]
- (5).Seeman P, McCormick PN, and Kapur S (2007) Increased dopamine D2(High) receptors in amphetamine-sensitized rats, measured by the agonist [(3)H](+)PHNO. Synapse 61 (5), 263–7. [DOI] [PubMed] [Google Scholar]
- (6).Seeman P (2011) All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci. Ther 17 (2), 118–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, and Borrelli E (2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408 (6809), 199–203. [DOI] [PubMed] [Google Scholar]
- (8).Jackson DM, and Westlind-Danielsson A (1994) Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol. Ther 64 (2), 291–370. [DOI] [PubMed] [Google Scholar]
- (9).De Lean A, Kilpatrick BF, and Caron MG (1982) Dopamine receptor of the porcine anterior pituitary gland. Evidence for two affinity states discriminated by both agonists and antagonists. Mol. Pharmacol 22 (2), 290–7. [PubMed] [Google Scholar]
- (10).Sibley DR, De Lean A, and Creese I (1982) Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D-2 dopamine receptor. J. Biol. Chem 257 (11), 6351–61. [PubMed] [Google Scholar]
- (11).Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O’Dowd BF, George SR, Perreault ML, Mannisto PT, Robinson S, Palmiter RD, and Tallerico T (2005) Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc. Natl. Acad. Sci. U. S. A 102 (9), 3513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Frankle WG, Paris J, Himes M, Mason NS, Mathis CA, and Narendran R (2018) Amphetamine-Induced Striatal Dopamine Release Measured With an Agonist Radiotracer in Schizophrenia. Biol. Psychiatry 83 (8), 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Sokoloff P, Giros B, Martres MP, Bouthenet ML, and Schwartz JC (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347 (6289), 146–51. [DOI] [PubMed] [Google Scholar]
- (14).Seeman P, Tallerico T, and Ko F (2003) Dopamine displaces [3H]domperidone from high-affinity sites of the dopamine D2 receptor, but not [3H]raclopride or [3H]spiperone in isotonic medium: Implications for human positron emission tomography. Synapse 49 (4), 209–15. [DOI] [PubMed] [Google Scholar]
- (15).Metwally KA, Dukat M, Egan CT, Smith C, DuPre A, Gauthier CB, Herrick-Davis K, Teitler M, and Glennon RA (1998) Spiperone: influence of spiro ring substituents on 5-HT2A serotonin receptor binding. J. Med. Chem 41 (25), 5084–93. [DOI] [PubMed] [Google Scholar]
- (16).Hall H, Wedel I, Halldin C, Kopp J, and Farde L (1990) Comparison of the in vitro receptor binding properties of N-[3H]methylspiperone and [3H]raclopride to rat and human brain membranes. J. Neurochem 55 (6), 2048–57. [DOI] [PubMed] [Google Scholar]
- (17).Skinbjerg M, Namkung Y, Halldin C, Innis RB, and Sibley DR (2009) Pharmacological characterization of 2-methoxy-N-propylnorapomorphine’s interactions with D2 and D3 dopamine receptors. Synapse 63 (6), 462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hidaka K, Tada S, Matsumoto M, Ohmori J, Tasaki Y, Nomura T, Usuda S, and Yamaguchi T (1996) In vitro pharmacological profile of YM-43611, a novel D2-like receptor antagonist with high affinity and selectivity for dopamine D3 and D4 receptors. Br. J. Pharmacol 117 (8), 1625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Yang M, Verfurth F, Buscher R, and Michel MC (1997) Is alpha1D-adrenoceptor protein detectable in rat tissues? Naunyn-Schmiedeberg’s Arch. Pharmacol. 355 (4), 438–46. [DOI] [PubMed] [Google Scholar]
- (20).Seeman P, Ko F, Willeit M, McCormick P, and Ginovart N (2005) Antiparkinson concentrations of pramipexole and PHNO occupy dopamine D2(high) and D3(high) receptors. Synapse 58 (2), 122–8. [DOI] [PubMed] [Google Scholar]
- (21).Seeman P (2007) Antiparkinson therapeutic potencies correlate with their affinities at dopamine D2(High) receptors. Synapse 61 (12), 1013–8. [DOI] [PubMed] [Google Scholar]
- (22).Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, and Newman-Tancredi A (2002) Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J. Pharmacol. Exp. Ther 303 (2), 791–804. [DOI] [PubMed] [Google Scholar]
- (23).Sromek AW, Si YG, Zhang T, George SR, Seeman P, and Neumeyer JL (2011) Synthesis and Biological Evaluation of N-Fluoroalkyl and 2-Fluoroalkoxy Substituted Aporphines: Potential PET Ligands for Dopamine D(2) Receptors. ACS Med. Chem. Lett 2 (3), 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, and Sokoloff P (1992) Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-amino-tetralin. Proc. Natl. Acad. Sci. U. S. A 89 (17), 8155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Gonzalez AM, and Sibley DR (1995) [3H]7-OH-DPAT is capable of labeling dopamine D2 as well as D3 receptors. Eur. J. Pharmacol 272 (1), R1–3. [DOI] [PubMed] [Google Scholar]
- (26).Gerlach M, Double K, Arzberger T, Leblhuber F, Tatschner T, and Riederer P (2003) Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum. J. Neural Transm (Vienna) 110 (10), 1119–27. [DOI] [PubMed] [Google Scholar]
- (27).Lazareno S, and Nahorski SR (1982) Selective labelling of dopamine (D2) receptors in rat striatum by [3H]domperidone but not by [3H]spiperone. Eur. J. Pharmacol 81 (2), 273–85. [DOI] [PubMed] [Google Scholar]
- (28).Grigoriadis D, and Seeman P (1985) Complete conversion of brain D2 dopamine receptors from the high- to the low-affinity state for dopamine agonists, using sodium ions and guanine nucleotide. J. Neurochem 44 (6), 1925–35. [DOI] [PubMed] [Google Scholar]
- (29).Kohler C, Hall H, Ogren SO, and Gawell L (1985) Specific in vitro and in vivo binding of 3H-raclopride. A potent substituted benzamide drug with high affinity for dopamine D-2 receptors in the rat brain. Biochem. Pharmacol 34 (13), 2251–9. [DOI] [PubMed] [Google Scholar]
- (30).Seeman P (2008) Dopamine D2(High) receptors on intact cells. Synapse 62 (4), 314–8. [DOI] [PubMed] [Google Scholar]
- (31).Sromek AW, Zhang S, Akurathi V, Packard AB, Li W, Alagille D, Morley TJ, Baldwin R, Tamagnan G, and Neumeyer JL (2014) Convenient synthesis of 18F-radiolabeled R-(−)-N-n-propyl-2-(3-fluoropropanoxy-11-hydroxynoraporphine. J. Labelled Compd. Radiopharm 57 (14), 725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Richfield EK, Young AB, and Penney JB (1986) Properties of D2 dopamine receptor autoradiography: high percentage of high-affinity agonist sites and increased nucleotide sensitivity in tissue sections. Brain Res 383 (1–2), 121–8. [DOI] [PubMed] [Google Scholar]
- (33).Seeman P (2012) Dopamine agonist radioligand binds to both D2High and D2Low receptors, explaining why alterations in D2High are not detected in human brain scans. Synapse 66 (1), 88–93. [DOI] [PubMed] [Google Scholar]
- (34).Neumeyer JL, Gao YG, Kula NS, and Baldessarini RJ (1990) Synthesis and dopamine receptor affinity of (R)-(−)-2-fluoro-N-n-propylnorapomorphine: a highly potent and selective dopamine D2 agonist. J. Med. Chem 33 (12), 3122–4. [DOI] [PubMed] [Google Scholar]
- (35).Finnema SJ, Seneca N, Farde L, Shchukin E, Sovago J, Gulyas B, Wikstrom HV, Innis RB, Neumeyer JL, and Halldin C (2005) A preliminary PET evaluation of the new dopamine D2 receptor agonist [11C]MNPA in cynomolgus monkey. Nucl. Med. Biol 32 (4), 353–60. [DOI] [PubMed] [Google Scholar]
- (36).Hwang DR, Narendran R, Huang Y, Slifstein M, Talbot PS, Sudo Y, Van Berckel BN, Kegeles LS, Martinez D, and Laruelle M (2004) Quantitative analysis of (−)-N-(11)C-propylnorapomorphine in vivo binding in nonhuman primates. J. Nucl. Med 45 (2), 338–46. [PubMed] [Google Scholar]
- (37).Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, and Wilson AA (2006) High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol. Psychiatry 59 (5), 389–94. [DOI] [PubMed] [Google Scholar]
- (38).Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Houle S, Kapur S, and Wilson AA (2006) Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J. Neurochem 97 (4), 1089–103. [DOI] [PubMed] [Google Scholar]
- (39).Peng T, Zysk J, Dorff P, Elmore CS, Strom P, Malmquist J, Ding M, Tuke D, Werkheiser J, Widzowski D, Mrzljak L, and Maier D (2010) D2 receptor occupancy in conscious rat brain is not significantly distinguished with [3H]-MNPA, [3H]-(+)-PHNO, and [3H]-raclopride. Synapse 64 (8), 624–33. [DOI] [PubMed] [Google Scholar]
- (40).Finnema SJ, Bang-Andersen B, Wikstrom HV, and Halldin C (2010) Current state of agonist radioligands for imaging of brain dopamine D2/D3 receptors in vivo with positron emission tomography. Curr. Top. Med. Chem 10 (15), 1477–98. [DOI] [PubMed] [Google Scholar]
- (41).Shi B, Narayanan TK, Christian BT, Chattopadhyay S, and Mukherjee J (2004) Synthesis and biological evaluation of the binding of dopamine D2/D3 receptor agonist, (R,S)-5-hydroxy-2-(N-propyl-N-(5′-(18)F-fluoropentyl)aminotetralin ((18)F-5-OH-FPPAT) in rodents and nonhuman primates. Nucl. Med. Biol 31 (3), 303–11. [DOI] [PubMed] [Google Scholar]
- (42).Mukherjee J, Majji D, Kaur J, Constantinescu CC, Narayanan TK, Shi B, Nour MT, and Pan ML (2017) PET radiotracer development for imaging high-affinity state of dopamine D2 and D3 receptors: Binding studies of fluorine-18 labeled aminotetralins in rodents. Synapse 71 (3), e21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Shalgunov V, van Wieringen JP, Janssen HM, Fransen PM, Dierckx RA, Michel MC, Booij J, and Elsinga PH (2015) Synthesis and evaluation in rats of homologous series of [(18)F]-labeled dopamine D 2/3 receptor agonists based on the 2-aminomethylchroman scaffold as potential PET tracers. EJNMMI Res. 5 (1), 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).van Wieringen JP, Shalgunov V, Janssen HM, Fransen PM, Janssen AG, Michel MC, Booij J, and Elsinga PH (2014) Synthesis and characterization of a novel series of agonist compounds as potential radiopharmaceuticals for imaging dopamine D(2)/(3) receptors in their high-affinity state. J. Med. Chem 57 (2), 391–410. [DOI] [PubMed] [Google Scholar]
- (45).Hedberg MH, Johansson AM, Nordvall G, Yliniemela A, Li HB, Martin AR, Hjorth S, Unelius L, Sundell S, and Hacksell U (1995) R)-11-hydroxy- and (R)-11-hydroxy-10-methylaporphine: synthesis, pharmacology, and modeling of D2A and 5-HT1A receptor interactions. J. Med. Chem 38 (4), 647–58. [DOI] [PubMed] [Google Scholar]
- (46).Csutoras C, Zhang A, Bidlack JM, and Neumeyer JL (2004) An investigation of the N-demethylation of 3-deoxymorphine and the affinity of the alkylation products to mu, delta, and kappa receptors. Bioorg. Med. Chem 12 (10), 2687–90. [DOI] [PubMed] [Google Scholar]
- (47).Csutoras C, Zhang A, Zhang K, Kula NS, Baldessarini RJ, and Neumeyer JL (2004) Synthesis and neuropharmacological evaluation of R(−)-N-alkyl-11-hydroxynoraporphines and their esters. Bioorg. Med. Chem 12 (13), 3553–9. [DOI] [PubMed] [Google Scholar]
- (48).Sondergaard K, Kristensen JL, Palner M, Gillings N, Knudsen GM, Roth BL, and Begtrup M (2005) Synthesis and binding studies of 2-arylapomorphines. Org. Biomol. Chem 3 (22), 4077–81. [DOI] [PubMed] [Google Scholar]
- (49).Seeman P, and Guan HC (2009) Glutamate agonist LY404,039 for treating schizophrenia has affinity for the dopamine D2(High) receptor. Synapse 63 (10), 935–9. [DOI] [PubMed] [Google Scholar]
- (50).Cheng Y, and Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol 22 (23), 3099–108. [DOI] [PubMed] [Google Scholar]


