Abstract
There is now strong clinical and preclinical evidence that lymphocytes, e.g. CD8+ T cells, are key effectors of immunotherapy and that irradiation of large blood vessels, the heart, and lymphoid organs (including nodes, spleen, bones containing bone marrow, and thymus in children) causes transient or persistent lymphopenia. Furthermore, there is extensive clinical evidence, across multiple cancer sites and treatment modalities, that lymphopenia correlates strongly with decreased overall survival. At the moment, we lack quantitative evidence to establish the relationship between dose-volume and dose-rate to critical normal structures and lymphopenia. Therefore, we propose that data should be systematically recorded to characterize a possible quantitative relationship. This might enable us to improve the efficacy of radiotherapy and develop strategies to predict and prevent treatment-related lymphopenia. In anticipation of more quantitative data, we recommend the application of the principle of As Low As Reasonably Achievable (ALARA) to lymphocyte rich regions for radiotherapy treatment planning to reduce the radiation doses to these structures, thus moving toward “Lymphocyte-Sparing Radiotherapy”.
Keywords: Immunotherapy, lymphopenia, radiation oncology, organ at risk, lymphocytes, sparing Radiotherapy, LOAR
I. Introduction
The Pacific trial, a randomised phase 3 trial in non-metastatic, advanced NSCLC, represented a breakthrough in immuno-oncology treatment (IO) within radiation oncology, convincingly demonstrating that adjuvant IO, after normofractionated chemoradiotherapy, can improve progression-free survival (PFS)1. Remarkably, the radiotherapy (RT) schedules of the Pacific trial were neither standardised nor optimised, as these were based only on investigator or radiation oncologist choice for each individual patient (total dose 54 Gy - 74 Gy). Separately, it has been shown that RT is a double-edged sword regarding immune effects: it has both an immunostimulatory effect but also an immunosuppressive effect2. IO might reduce or overrule this RT-related immunosuppression1, 3. Furthermore, lower doses to the heart, circulating blood pool, and lymphoid organs are associated with reduced immunosuppressive effect3, 4. It can thus be hypothesised that an optimised RT protocol has the potential to decrease the immunosuppressive effects of RT, e.g. by reducing RT-related lymphopenia (LP).
Several studies have shown that low blood lymphocyte count at baseline, across a range of cancer types, is a negative predictor of outcome5–11. Furthermore, the presence of CD8+ tumour infiltrating lymphocytes (TILs) on pathology review is a well-established predictor of better overall survival12–14. Additionally, preclinical experiments with lymphocyte depletion, i.e. decreased CD4+ and CD8+ counts, have clearly established a causal relationship with reduced efficacy of radiotherapy and (radio)-IO15.
The effect of RT on LP is well-documented and has been extensively described for several decades16, 17. Typically, LP is a transient phenomenon with a recovery within three months after RT, but in certain cases it can continue to persist even years after treatment18 which has been correlated to RT dose, RT sites, (hyper)fractionation, adjuvant chemotherapy, and irradiated volume4, 11–13, 19–23. A causal relationship between RT-induced LP and adverse loco-regional control or survival has been speculated but not confirmed24.
II. The Radiobiology of Lymphocytes
Lymphocytes are located in the blood (circulating lymphocytes), in reservoir lymphoid organs such as the spleen, and the thymus (in children and teenagers), in lymph nodes, and in the bone marrow, which is continuously producing new lymphocytes. As noted, some tumours are infiltrated by lymphocytes. It is important to appreciate that lymphocytes are a highly heterogeneous cell population comprised of subgroups with different roles in the crosstalk of tumours and the host immune system. The most prominent cell type in anti-tumour immune responses are CD8+ effector T cells25, reflected in their prognostic significance26 and their use in adoptive T cell therapy27. TH1 polarised (CD4+)28, as well as CD4+ cytolytic T cells, have also been shown to induce strong anti-tumour responses29. On the other hand, regulatory T cells30 and TH2 polarised CD4+ T cells31 have mostly been linked to pro-tumour effects29. There is contradictory data on the role of TH17 T cells29 and cancer in cancer immune responses32, 33.
It has long been known that lymphocytes are the most radiosensitive cells of the hematopoietic system, as well as the entire body34. This radiosensitivity is surprising for a non-dividing cell type, but may be related to robust apoptotic response pathways. The lethal dose required to reduce the surviving fraction of circulating lymphocytes by 90% (LD90) is only 3 Gy35. 0.5 Gy already leads to significant cell death induction in lymphocytes. Such a dose could easily be reached in standard radiotherapy schedules. Yovino et al. found that with a standard treatment of 60 Gy in 30 fractions for glioblastoma treatment, during every fraction of radiotherapy, 5% of circulating cells receive >0.5 Gy36, summing up to >95% of circulating cells being exposed to >0.5 Gy over the six week treatment. The induced cell death is predominantly apoptosis37.
Importantly, different lymphocyte subtypes show distinct radiosensitivity38–40. Naïve CD8+ effector T cells are more sensitive than memory T cells37, 40, 41, while regulatory T cells are relatively resistant40, 42, 43. Furthermore, the state of T cells, the solid organs and the different location containing CD8+ T cells also influences radiosensitivity44, 45. T cells that are proliferating are more radio-resistant than T cells in other state44. With regard to the organs, the parenchymal CD8+ T cells in the solid lymphoid organs (lymph nodes and spleen) are found most radiosensitive, followed by those residing in liver and gut. The CD8+ T cells located intratumourally have a higher radio-resistance, an increased motility and IFN-ɣ secretion compared to circulating CD8+ T cells and T cells in unirradiated tumours45. This may be due to changes in the tumour microenvironment wherein TGF-β is a key regulator in making the intratumoral T cells more radio-resistant45. Similar differential effects have been observed concerning radiation dose rate46 with high dose rates leading to less lymphocyte death47, 48. These findings are well in line with clinical observations of decreased naïve T cells and enriched regulatory T cells in patients undergoing RT14, 49–51.
III. Analysis of the clinical literature
In many trials, the Common Terminology Criteria for Adverse Events (CTCAE) is used to differentiate between LP Grade 1 (<~1000 – 800/mm3), Grade 2 (<800 – 500/mm3), Grade 3 (<500 – 200 mm3), and Grade 4 (<200/mm3). Clinical factors that are associated with LP and key findings regarding LP for various cancers (glioblastoma (GBM), head and neck squamous cell carcinoma (HNSCC), nasopharyngeal cancer, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), breast cancer, oesophageal cancer, pancreatic cancer, hepatocellular cancer (HCC), cervical cancer)24 are summarized below.
a. Factors that influences LP
A disbalance in immuno-surveillance due to tumour suppressor systems can contribute to LP that is present before treatment14. Also immunosuppressive medication or cancer related treatment can lead to pre- and post-treatment LP, e.g. corticosteroids, tyrosine-kinase inhibitors (TKIs), and immune checkpoint inhibitor11, 14. In addition, patients with immune related conditions, such as multiple comorbidities, autoimmune diseases, genetic disorders in innate or adaptive defence, or patients with a poor WHO performance state are known to have worse PFS and OS, probably related to a sub-optimally functioning immune system.
Also, treatment factors such as radiotherapy and chemotherapy have been shown to influence incidence and severity of LP.
Firstly, RT in general results in a lymphocyte reduction. More specifically, hypo-fractionation results in less reduction than normo- or hyper-fractionation. Yuan et al. and Saito et al. have found in a breast and a palliative cohort respectively, that LP was correlated with the number of fractions, independent of overall dose52, 53. Secondly, irradiating larger Gross Tumour Volumes (GTV) in NSCLC patients has been associated with lower lymphocyte count but not with lower total leukocyte, neutrophil, or monocyte counts during RT24. Thirdly, if lymphopoietic sites or organs containing large blood volumes are within the PTV, it will contribute to (longer duration of) LP14. Several authors have also found that higher spleen irradiation doses (total dose of 50–60 Gy) were significantly correlated with more patients experiencing LP during RT for HCC or palliative RT53–56. Based on these results, Liu et al recommend sparing of the spleen during abdominal irradiation54. Furthermore, a lower heart and lung dose resulted in less LP57–60. Increasing the heart and long dose, severe loss of cardiopulmonary performance was seen in pre-clinical studies61–66. Lastly, another important factor is the use of concurrent chemotherapy. Concurrent chemotherapy has been shown to have an impact on the severity of LP22, whereas adjuvant chemotherapy may prolong the duration of LP22. Importantly, different chemotherapy agents differ in LP impact14.
b. Predictive/prognostic factors for OS after radiation induced LP
Many possible prognostic factors for OS and PFS have been investigated, including the role of LP. Ladbury et al. concluded that estimated dose of radiation to immune cells, Karnofsky performance status, not-otherwise-specified histology in NSCLC, lack of completion of chemotherapy9, 23, and smoking history23 are negative predictors for OS.
Disadvantageous prognostic factors for PFS and OS are baseline LP5–11, 14, early LP after chemotherapy treatment (5 or 15 days)14, LP after radiotherapy (RT)14 or LP after IO7. Post-treatment LP has been negatively associated with poor tumour specific outcome in multiple cancer types e.g. GBM, HNSCC, cervical, oesophageal, NSCLC, and pancreatic11.
c. Effect of combination treatment. (RT + chemo, RT + chemo and/or IO)
As described previously, RT alone can induce or worsen LP. However, combining RT with systemic treatment has an even bigger impact on LP and treatment outcome. Cho et al. found that RT + checkpoint inhibitor treated NSCLC patients with LP pre-IO treatment had a significant poorer PFS (2.2 vs 5.9 months) and OS (5.7 vs 12.1 months)10 compared to patients who had normal lymphocyte counts before IO treatment. Furthermore they found that RT significantly increased the LP before start of IO, however irradiating with SABR, proton beam therapy, hypo-fractionation or radiosurgery reduced the risk on (increasing) RT-induced LP10, 14, 60. The combination of RT with immunocytokines like IL2, IL7 or IL15 could eliminate LP due to their simulating effect to let the T cells develop, proliferate and survive14.
Joseph et al found that after concurrent chemo-radiotherapy the absolute lymphocyte count (ALC) dropped significantly compared to ALC pre-treatment4, but did not alter treatment outcome. In contrast, Grossman et al. observed worse tumour control and shorter OS in GBM patients with depleted CD4+ T cell counts pre- and post-chemo-radiotherapy treatment67. Furthermore, a prolonged duration of LP was also seen with RT. Similar results were found retrospectively by Wang et al, with almost 50% of SCLC patients experiencing severe LP and 70.4% prolonged lymphopenia of 3 months minimum after chemo-radiotherapy21. For reasons not currently well understood, LP following RT can last from several months up to several years, whereas LP seen after sepsis or even chemotherapy alone tends to resolve more quickly68, 69.
It is reasonable to hypothesise that transient LP has a different effect on the outcome than persistent LP. Thus, the negative influence of RT on LP might be abolished by combinatorial approaches with IO, which could result in differences in the timing, the length and probably the grade of LP. This effect also depends on type of IO agent applied. On the other hand, it might indicate that the effect of adding IO to RT schedules lies primarily in a better functioning immune system, which in turn will be crucial to slow down the pace of microscopic disease spread in at least some patients.
d. Modelling approaches to predict the incidence and severity of LP
Taking into account the negative effect of LP on clinical outcomes, it is important to identify high risk patients timely and possibly adapt the treatment. Models predicting grade 4 RT-induced LP during chemo(radio)therapy for oesophageal cancer, or acute and late LP for prostate cancer have already been published70, 71, although the prostate model is yet to be validated19, 72. Also for NSCLC, a predictive risk model has been developed where clinical and genetic factors, e.g. lung V5>48%, age >65 years, >40 pack-years, and XRCC1 rs25487 AA genotype, are associated with severe RT-induced LP73.
Several recent analyses have indicated that irradiation of cardio-vascular structures may lead not just to heart related morbidities but to unexplained reductions in OS following radiotherapy for NSCLC. A key question is whether this is mediated primarily through immune suppression. Contreras et al. showed that adjuvant chemotherapy and heart V50>25% are associated with lymphopenia at 4 months post RT3. Thor et al. observed that out-of-treatment-field regional recurrence was statistically linked to lymphopenia at 2 months post RT74. However, details of the relationship between patient/disease/treatment factors and lymphopenia, as well as the impact on disease progression remain elusive and need further study.
IV. Recommendations for clinical trials
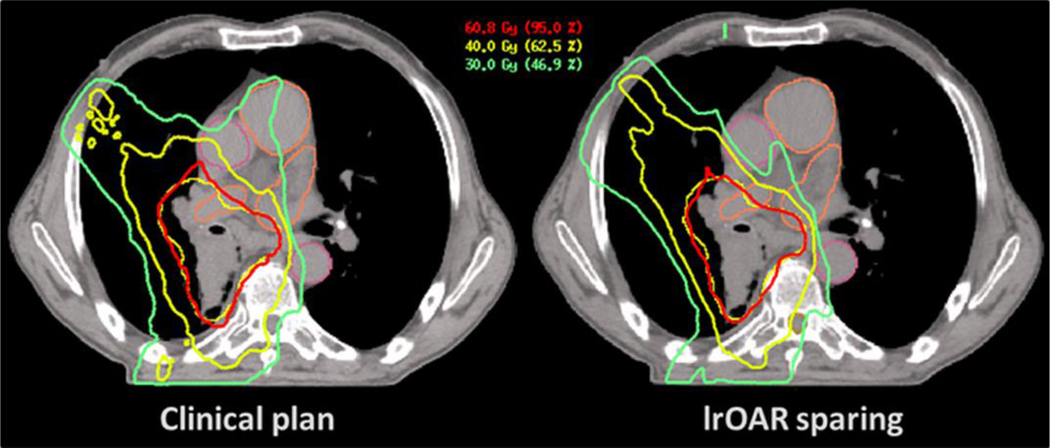
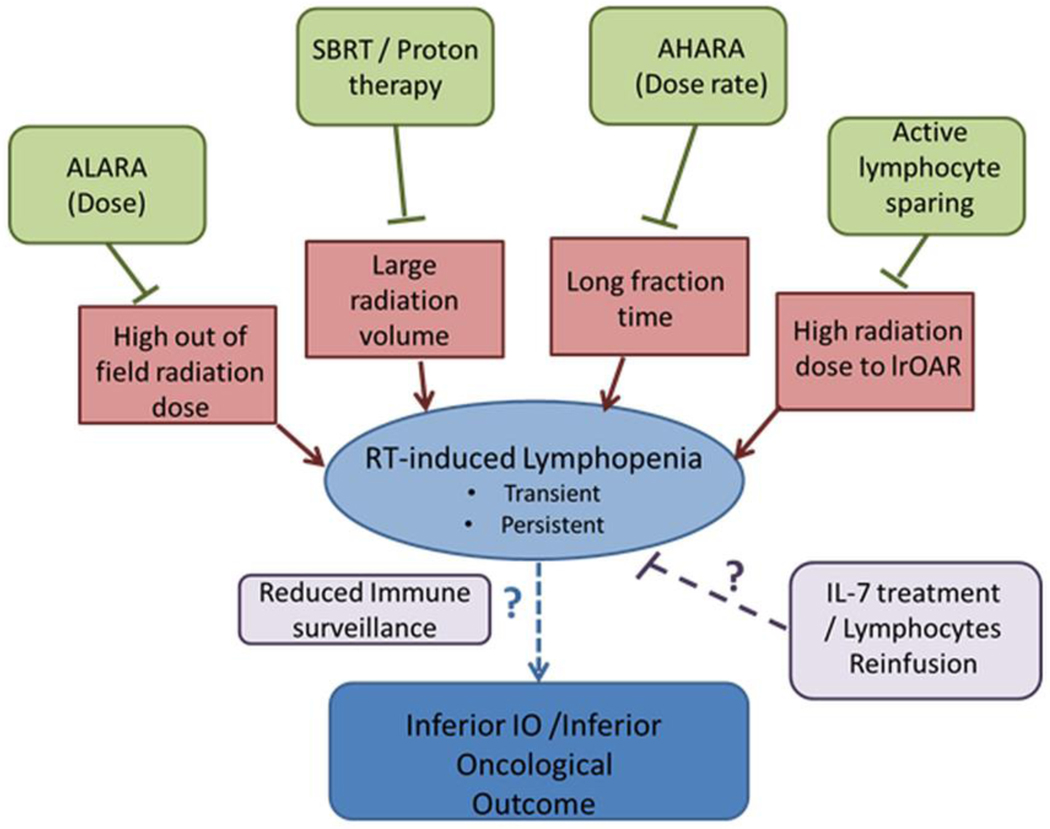
There is a large body of literature evidence showing that incidence and severity of LP are associated with patient and treatment characteristics, but also showing the importance for clinical outcomes. Moreover, we have identified 437 trials listed in clinicaltrial.gov combining IO with RT, September 2019, indicating that combining RT with IO is being increasingly adopted as treatment strategy. To improve clinical outcomes, but also to gain the most of RT-IO combination treatment, it is of utmost importance to establish recommendations for RT planning with regard to lymphocyte dose. However, as indicating absolute dose constraints is not (yet) possible, we propose to apply the As Low As Reasonably Achievable (ALARA) principle to Lymphocyte-related Organs At Risk (LOARs) without compromising irradiation of the PTV (see Figure 1 and 2) and keeping the constraints for “conventional” organs at risk such as lung, heart and spinal cord, as recommended in clinical protocols (Figure 3). Furthermore, systematic recording of dose-volume and dose-rate statistics for those LOARs, as well as longitudinal lymphocyte counts is recommended. These data, routinely available at most treatment centres, would allow the design of strategies to predict and to some extent prevent RT-induced LP. It would also help to answer the main remaining hypothesis whether maintaining and/or restoring optimal lymphocyte counts may improve treatment RT outcomes, or increase the efficacy of IO.
Figure 1:
Example of segmentation for lung cancer treatment: left: delineation of the Lymphocyte-related Organs At Risk (LOAR), right delineation of the LOAR and the planning target volume.
Figure 2:
A standard dose distribution of a clinically applied radiation treatment plan (left), and an example of an optimized radiation plan applying the (As Low As Reasonably Achievable) ALARA principle (right), demonstrating that sparing of LOAR is feasible without compromising dose coverage of the target volume or increasing dose to OARs important in clinical radiotherapy planning.
Figure 3:
Hypothetical model linking radiation to lymphopenia and to inferior oncological outcomes.
These data can only be obtained if relevant organs are systematically delineated. These include the large vessels, heart, and any irradiated lymphoid organs such as bone marrow (e.g. pelvic bones, vertebrae, large long bones), nodal regions not included in the CTV, spleen, and thymus in children. To facilitate the segmentation of large vessels, we propose to explore the use of contrast-enhanced computed tomography (CT), acquiring data during the early blood dominated phases. Automatic segmentation methods based on deep learning will certainly facilitate this process75. Dose, fractionation, dose rate, and mean doses to LOARs should be reported as a minimum. Blood can be seen as a “moving OAR”, therefore long irradiation times should be avoided. Instead, high dose rate irradiation, following the principle of “As High As Reasonably Achievable” (AHARA) should be favoured, e.g. using flattening filter-free irradiation76, 77.
V. Prospects
As it is clear that the role of the immune system is very important for clinical outcomes, much research currently focuses on unravelling the complex interplay between treatment characteristics and the immune system and how to influence this relationship. In an attempt to preserve the immune system from the effects of radiation and chemotherapy, lymphocytes were isolated before treatment, stored and administered again to the patient upon treatment completion (NCT01653834)67. Interestingly, the promising therapeutic effect of immunoadjuvant therapy with IL-7 (essential for lymphocyte proliferation and survival) has been explored in e.g. immunocompromised patient and in some cancer trials, however the data regarding IL-7 and LP during, pre, and post cancer treatment is scarce14, 78–82.
New imaging methods may also become important. New of magnetic resonance (MR) sequences may enable the investigator to quantify blood volume in vessels and organs using non-contrast MR imaging such as a venography technique or velocity-selective (VS) pulse trains83, 84. These new approaches will allow us not only to quantify blood volume without contrast in the vascular system but also in organs such as liver, brain and spleen. New positron emission tomography (PET) tracers that can precisely track CD8+ T cells are also under development85. Furthermore, the combination of new strategies and precise technological developments20, such as a magnetic resonance linear accelerator (MR-linac)86, will make it possible to not only more precisely identify and track LOARs, but also avoid or restrict radiation dose to these LOARs. To facilitate comparable analyses, new autosegmentation/AI methods could be distributed using portable container technology to extract dosimetric characteristics of the LOARs87.
VI. Conclusion
The breakthrough improvement in outcomes by IO alone, or in combination with RT, has renewed the interest of the scientific community in strategies to predict and avoid RT-associated LP that may be immunosuppressive. There is a convergence of preclinical and clinical evidence correlating unintentional irradiation of LOARs with LP and poor outcomes. Preclinical studies definitively show an established causal relationship between lymphocyte depletion and the effectiveness of IO. However, accurate, individualized NTCP models for LP are currently lacking. Therefore, we propose that the ALARA principle should be applied to LOARs, and dose-rates should be kept as high as practical to spare peripheral blood lymphocytes, in particular in the context of clinical trials combining RT with IO. Furthermore, we urge investigators of clinical RT trials with an immune component to systematically record the potentially-relevant dosimetric and hematopoietic parameters. Such unique data will hopefully lead to predictive models that will allow us to predict and prevent RT-induced LP in an individualised approach for each patient in order to answer the key unresolved question: whether maintaining and/or restoring optimal lymphocyte counts independently improves RT or IO outcomes.
Acknowledgments
Disclosure statement all authors:
Authors RL, DM, JD, CG, AW, CO, LD and PL have no conflict of interest within the submitted work FE was partly funded by the Else-Kroener-Fresenius Research Foundation under Grant 2015_Kolleg.14. FE has a research collaboration with Merck KgAa. FE has research and educational grants from Elekta, Philips, Siemens, Sennewald. JD is funded by the NIH and the breast cancer research foundation. Partially supported by NIH Core Grant P30 CA 008748. PL acknowledge financial support from ERC advanced grant (ERC-ADG-2015, n 694812 - Hypoximmuno) and the European Program H20202017 (ImmunoSABR – n 733008, PREDICT – ITN – n 766276).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
VII. References
- 1.Antonia SJ, Villegas A, Daniel D: Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 377:1919–1929, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Formenti SC, Demaria S: Systemic effects of local radiotherapy. Lancet Oncol 10:718–726, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contreras JA, Lin AJ, Weiner A: Cardiac dose is associated with immunosuppression and poor survival in locally advanced non-small cell lung cancer. Radiother Oncol 128:498–504, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Joseph N, McWilliam A, Kennedy J: Post-treatment lymphocytopaenia, integral body dose and overall survival in lung cancer patients treated with radical radiotherapy. Radiother Oncol 135:115–119, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Grassberger C, Hong TS, Hato T: Differential Association Between Circulating Lymphocyte Populations With Outcome After Radiation Therapy in Subtypes of Liver Cancer. Int J Radiat Oncol Biol Phys 101:1222–1225, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karantanos T, Karanika S, Seth B: The absolute lymphocyte count can predict the overall survival of patients with non-small cell lung cancer on nivolumab: a clinical study. Clin Transl Oncol 21:206–212, 2019. [DOI] [PubMed] [Google Scholar]
- 7.Diehl A, Yarchoan M, Hopkins A: Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget 8:114268–114280, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho WJ, Yarchoan M, Hopkins A: Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J Immunother Cancer 6:84, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki R, Wei X, Allen PK: Prognostic Significance of Total Lymphocyte Count, Neutrophil-to-lymphocyte Ratio, and Platelet-to-lymphocyte Ratio in Limited-stage Small-cell Lung Cancer. Clin Lung Cancer 20:117–123, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Cho Y, Park S, Byun HK: Impact of Treatment-Related Lymphopenia on Immunotherapy for Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys, 2019. [DOI] [PubMed] [Google Scholar]
- 11.Kleinberg L, Sloan L, Grossman S: Radiotherapy, Lymphopenia, and Host Immune Capacity in Glioblastoma: A Potentially Actionable Toxicity Associated With Reduced Efficacy of Radiotherapy. Neurosurgery 85:441–453, 2019. [DOI] [PubMed] [Google Scholar]
- 12.Yu PC, Long D, Liao CC: Association between density of tumor-infiltrating lymphocytes and prognoses of patients with gastric cancer. Medicine (Baltimore) 97:e11387, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim EM, Al-Foheidi ME, Al-Mansour MM: The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat 148:467–476, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Menetrier-Caux C, Ray-Coquard I, Blay JY: Lymphopenia in Cancer Patients and its Effects on Response to Immunotherapy: an opportunity for combination with Cytokines? J Immunother Cancer 7:85, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zegers CM, Rekers NH, Quaden DH: Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin Cancer Res 21:1151–1160, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Raben M, Walach N, Galili U: The effect of radiation therapy on lymphocyte subpopulations in cancer patients. Cancer 37:1417–1421, 1976. [DOI] [PubMed] [Google Scholar]
- 17.Newman GH, Rees GJ, Jones RS: Changes in helper and suppressor T lymphocytes following radiotherapy for breast cancer. Clin Radiol 38:191–193, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Petrini B, Wasserman J, Blomgren H: Blood lymphocyte subpopulations in breast cancer patients following radiotherapy. Clin Exp Immunol 29:36–42, 1977. [PMC free article] [PubMed] [Google Scholar]
- 19.Yang TJ, Oh JH, Apte A: Clinical and dosimetric predictors of acute hematologic toxicity in rectal cancer patients undergoing chemoradiotherapy. Radiother Oncol 113:29–34, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suryadevara CM, Desai R, Abel ML: Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology 7:e1434464, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Lu J, Teng F: Lymphopenia association with accelerated hyperfractionation and its effects on limited-stage small cell lung cancer patients’ clinical outcomes. Ann Transl Med 7:385, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin AJ, Campian JL, Hui C: Impact of concurrent versus adjuvant chemotherapy on the severity and duration of lymphopenia in glioma patients treated with radiation therapy. J Neurooncol 136:403–411, 2018. [DOI] [PubMed] [Google Scholar]
- 23.Ladbury CJ, Rusthoven CG, Camidge DR: Impact of Radiation Dose to the Host Immune System on Tumor Control and Survival for Stage III Non-Small Cell Lung Cancer Treated with Definitive Radiation Therapy. Int J Radiat Oncol Biol Phys 105:346–355, 2019. [DOI] [PubMed] [Google Scholar]
- 24.Venkatesulu BP, Mallick S, Lin SH: A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 123:42–51, 2018. [DOI] [PubMed] [Google Scholar]
- 25.Reiser J, Banerjee A: Effector, Memory, and Dysfunctional CD8(+) T Cell Fates in the Antitumor Immune Response. Journal of immunology research 2016:8941260, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galon J, Costes A, Sanchez-Cabo F: Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (New York, NY) 313:1960–1964, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Yee C, Thompson JA, Byrd D: Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proceedings of the National Academy of Sciences of the United States of America 99:16168–16173, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corthay A, Skovseth DK, Lundin KU: Primary antitumor immune response mediated by CD4+ T cells. Immunity 22:371–383, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Cantor H: CD4 T-cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer immunology research 2:91–98, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee A, Vasanthakumar A, Grigoriadis G: Modulating T regulatory cells in cancer: how close are we? Immunol Cell Biol 91:340–349, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Tatsumi T, Kierstead LS, Ranieri E: Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med 196:619–628, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Namm JP, Li Q, Lao X: B lymphocytes as effector cells in the immunotherapy of cancer. Journal of surgical oncology 105:431–435, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Morgan R, Podack ER: B cell regulation of anti-tumor immune response. Immunologic research 57:115–124, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Trowell OA: The sensitivity of lymphocytes to ionising radiation. J Pathol Bacteriol 64:687–704, 1952. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura N, Kusunoki Y, Akiyama M: Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res 123:224–227, 1990. [PubMed] [Google Scholar]
- 36.Yovino S, Kleinberg L, Grossman SA: The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 31:140–144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grayson JM, Harrington LE, Lanier JG: Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. Journal of immunology (Baltimore, Md : 1950) 169:3760–3770, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Williams JL, Patchen ML, Darden JH: Effects of radiation on survival and recovery of T lymphocyte subsets in C3H/HeN mice. Experimental hematology 22:510–516, 1994. [PubMed] [Google Scholar]
- 39.Zarybnicka L, Vavrova J, Havelek R: Lymphocyte subsets and their H2AX phosphorylation in response to in vivo irradiation in rats. International journal of radiation biology 89:110–117, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Pugh JL, Sukhina AS, Seed TM: Histone deacetylation critically determines T cell subset radiosensitivity. Journal of immunology (Baltimore, Md : 1950) 193:1451–1458, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabi Z, Spary LK, Coleman S: Resistance of CD45RA- T cells to apoptosis and functional impairment, and activation of tumor-antigen specific T cells during radiation therapy of prostate cancer. Journal of immunology (Baltimore, Md : 1950) 185:1330–1339, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Persa E, Balogh A, Safrany G: The effect of ionizing radiation on regulatory T cells in health and disease. Cancer letters 368:252–261, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Gururangan S, Reap E, Schmittling R: Regulatory T cell subsets in patients with medulloblastoma at diagnosis and during standard irradiation and chemotherapy (PBTC N-11). Cancer Immunol Immunother 66:1589–1595, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heylmann D, Badura J, Becker H: Sensitivity of CD3/CD28-stimulated versus non-stimulated lymphocytes to ionizing radiation and genotoxic anticancer drugs: key role of ATM in the differential radiation response. Cell Death Dis 9:1053, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arina A, Beckett M, Fernandez C: Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat Commun 10:3959, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gridley DS, Pecaut MJ, Dutta-Roy R: Dose and dose rate effects of whole-body proton irradiation on leukocyte populations and lymphoid organs: part I. Immunology letters 80:55–66, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Sterzing F, Munter MW, Schafer M: Radiobiological investigation of dose-rate effects in intensity-modulated radiation therapy. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al. ] 181:42–48, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Ware JH, Sanzari J, Avery S: Effects of proton radiation dose, dose rate and dose fractionation on hematopoietic cells in mice. Radiat Res 174:325–330, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckert F, Schaedle P, Zips D: Impact of curative radiotherapy on the immune status of patients with localized prostate cancer. Oncoimmunology, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sage EK, Schmid TE, Geinitz H: Effects of definitive and salvage radiotherapy on the distribution of lymphocyte subpopulations in prostate cancer patients. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al. ] 193:648–655, 2017. [DOI] [PubMed] [Google Scholar]
- 51.Schaue D, Comin-Anduix B, Ribas A: T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res 14:4883–4890, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan C, Wang Q: Comparative analysis of the effect of different radiotherapy regimes on lymphocyte and its subpopulations in breast cancer patients. Clin Transl Oncol 20:1219–1225, 2018. [DOI] [PubMed] [Google Scholar]
- 53.Saito T, Toya R, Matsuyama T: Dosimetric Predictors of Treatment-related Lymphopenia induced by Palliative Radiotherapy: Predictive Ability of Dose-volume Parameters based on Body Surface Contour. Radiol Oncol 51:228–234, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Zhao Q, Deng W: Radiation-related lymphopenia is associated with spleen irradiation dose during radiotherapy in patients with hepatocellular carcinoma. Radiat Oncol 12:90, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chadha AS, Liu G, Chen HC: Does Unintentional Splenic Radiation Predict Outcomes After Pancreatic Cancer Radiation Therapy? Int J Radiat Oncol Biol Phys 97:323–332, 2017. [DOI] [PubMed] [Google Scholar]
- 56.Chadha AS, Suh Y, Krishnan S: In Reply to Yazici et al. Int J Radiat Oncol Biol Phys 98:485–486, 2017. [DOI] [PubMed] [Google Scholar]
- 57.Shiraishi Y, Fang P, Xu C: Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol 128:154–160, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang P, Jiang W, Davuluri R: High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol, 2018. [DOI] [PubMed] [Google Scholar]
- 59.Fang P, Shiraishi Y, Verma V: Lymphocyte-Sparing Effect of Proton Therapy in Patients with Esophageal Cancer Treated with Definitive Chemoradiation. Int J Part Ther 4:23–32, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badiyan SN, Robinson CG, Bradley JD: Radiation Toxicity in Lung Cancer Patients: The Heart of the Problem? Int J Radiat Oncol Biol Phys 104:590–592, 2019. [DOI] [PubMed] [Google Scholar]
- 61.van Luijk P, Novakova-Jiresova A, Faber H: Radiation damage to the heart enhances early radiation-induced lung function loss. Cancer research 65:6509–6511, 2005. [DOI] [PubMed] [Google Scholar]
- 62.van Luijk P, Faber H, Meertens H: The impact of heart irradiation on dose-volume effects in the rat lung. Int J Radiat Oncol Biol Phys 69:552–559, 2007. [DOI] [PubMed] [Google Scholar]
- 63.van Rongen E, Tan CH, Durham SK: Late functional, biochemical and histological changes in the rat lung after fractionated irradiation to the whole thorax. Radiother Oncol 10:231–246, 1987. [DOI] [PubMed] [Google Scholar]
- 64.Ghobadi G, van der Veen S, Bartelds B: Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys 84:e639–646, 2012. [DOI] [PubMed] [Google Scholar]
- 65.Peterson LM, Evans ML, Thomas KL: Vascular response to fractionated irradiation in the rat lung. Radiat Res 131:224–226, 1992. [PubMed] [Google Scholar]
- 66.Ghobadi G, Bartelds B, van der Veen SJ: Lung irradiation induces pulmonary vascular remodelling resembling pulmonary arterial hypertension. Thorax 67:334–341, 2012. [DOI] [PubMed] [Google Scholar]
- 67.Feasibility of Lymphocyte Reinfusion in Newly Diagnosed High Grade Gliomas. clinicaltrial.gov. [https://clinicaltrials.gov/ct2/show/NCT01653834]
- 68.Dean RM, Fry T, Mackall C: Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol 26:5735–5741, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Llano A, Barretina J, Gutierrez A: Interleukin-7 in plasma correlates with CD4 T-cell depletion and may be associated with emergence of syncytium-inducing variants in human immunodeficiency virus type 1-positive individuals. J Virol 75:10319–10325, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sini C, Fiorino C, Perna L: Dose-volume effects for pelvic bone marrow in predicting hematological toxicity in prostate cancer radiotherapy with pelvic node irradiation. Radiother Oncol 118:79–84, 2016. [DOI] [PubMed] [Google Scholar]
- 71.van Rossum PSN, Deng W, Routman DM: Prediction of Severe Lymphopenia During Chemoradiation Therapy for Esophageal Cancer: Development and Validation of a Pretreatment Nomogram. Pract Radiat Oncol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Julie DA, Oh JH, Apte AP: Predictors of acute toxicities during definitive chemoradiation using intensity-modulated radiotherapy for anal squamous cell carcinoma. Acta Oncol 55:208–216, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie X, Lin SH, Welsh JW: Radiation-induced lymphopenia during chemoradiation therapy for non-small cell lung cancer is linked with age, smoking, lung V5, and XRCC1 rs25487 genotype in lymphocytes. Journal (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thor M, Montovano M, Hotca A: Are unsatisfactory outcomes after concurrent chemoradiotherapy for locally advanced non-small cell lung cancer due to treatment-related immunosuppression? Radiotherapy and Oncology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Q, Sun J, Ding H: Robust liver vessel extraction using 3D U-Net with variant dice loss function. Comput Biol Med 101:153–162, 2018. [DOI] [PubMed] [Google Scholar]
- 76.Yan Y, Yadav P, Bassetti M: Dosimetric differences in flattened and flattening filter-free beam treatment plans. J Med Phys 41:92–99, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dubois L, Biemans R, Reniers B: High dose rate and flattening filter free irradiation can be safely implemented in clinical practice. International journal of radiation biology 91:778–785, 2015. [DOI] [PubMed] [Google Scholar]
- 78.Francois B, Jeannet R, Daix T: Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight 3, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levy Y, Lacabaratz C, Weiss L: Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest 119:997–1007, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenberg SA, Sportes C, Ahmadzadeh M: IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. Journal of immunotherapy (Hagerstown, Md : 1997) 29:313–319, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sportes C, Babb RR, Krumlauf MC: Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res 16:727–735, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tredan O, Menetrier-Caux C, Ray-Coquard I: ELYPSE-7: a randomized placebo-controlled phase IIa trial with CYT107 exploring the restoration of CD4+ lymphocyte count in lymphopenic metastatic breast cancer patients. Ann Oncol 26:1353–1362, 2015. [DOI] [PubMed] [Google Scholar]
- 83.Aguirre-Reyes DF, Sotelo JA, Arab JP: Intrahepatic portal vein blood volume estimated by non-contrast magnetic resonance imaging for the assessment of portal hypertension. Magn Reson Imaging 33:970–977, 2015. [DOI] [PubMed] [Google Scholar]
- 84.Liu D, Xu F, Lin DD: Quantitative measurement of cerebral blood volume using velocity-selective pulse trains. Magn Reson Med 77:92–101, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colevas AD, Bedi N, Chang S: A study to evaluate immunological response to PD-1 inhibition in squamous cell carcinoma of the head and neck (SCCHN) using novel PET imaging with [18F]F-AraG. Journal of Clinical Oncology 36:6050–6050, 2018. [Google Scholar]
- 86.Tijssen RHN, Philippens MEP, Paulson ES: MRI commissioning of 1.5T MR-linac systems - a multi-institutional study. Radiother Oncol 132:114–120, 2019. [DOI] [PubMed] [Google Scholar]
- 87.Apte AP, Iyer A, Thor M: Library of model implementations for sharing deep-learning image segmentation and outcomes models. bioRxiv 773929, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]