Abstract
Background
We know that the brain damage resulting from traumatic and other insults is not due solely to the direct consequences of the primary injury. A significant and potentially preventable contribution to the overall morbidity arises from secondary hypoxic‐ischaemic damage. Brain swelling accompanied by raised intracranial pressure (ICP) prevents adequate cerebral perfusion with well‐oxygenated blood.
Detection of raised ICP could be useful in alerting clinicians to the need to improve cerebral perfusion, with consequent reductions in brain injury.
Objectives
To determine whether routine ICP monitoring in severe coma of any cause reduces the risk of all‐cause mortality or severe disability at final follow‐up.
Search methods
We searched the Cochrane Injuries Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (OvidSP), EMBASE (OvidSP), CINAHL Plus, ISI Web of Science (SCI‐EXPANDED & CPCI‐S), clinical trials registries and reference lists. We ran the most recent search on 22 May 2015.
Selection criteria
All randomised controlled studies of real‐time ICP monitoring by invasive or semi‐invasive means in acute coma (traumatic or non‐traumatic aetiology) versus clinical care without ICP monitoring (that is, guided only by clinical or radiological inference of the presence of raised ICP).
Data collection and analysis
Two authors (ET and RF) worked independently to identify the one study that met inclusion criteria. JR and RF independently extracted data and assessed risk of bias. We contacted study authors for additional information, including details of methods and outcome data.
Main results
One randomized controlled trial (RCT) meeting the selection criteria has been identified to date.
The included study had 324 participants. We judged risk of bias to be low for all categories except blinding of participants and personnel, which is not feasible for this intervention. There were few missing data, and we analysed all on an intention‐to‐treat basis.
Participants could be 13 years of age or older (mean age of sample 29; range 22 to 44), and all had severe traumatic brain injury, mostly due to traffic incidents. All were receiving care within intensive care units (ICUs) at one of six hospitals in either Bolivia or Ecuador. Investigators followed up 92% of participants for six months or until death. The trial excluded patients with a Glasgow Coma Score (GCS) equal to three and fixed dilated pupils on admission on the basis that they had sustained brain injury of an unsalvageable severity.
The study compared people managed using either an intracranial monitor or non‐invasive monitoring (imaging and clinical examination) to identify potentially harmful raised intracranial pressure. Both study groups used imaging and clinical examination measures.
Mortality at six months was 56/144 (39%) in the ICP‐monitored group and 67/153 (44%) in the non‐invasive group.
Unfavourable outcome (defined as death or moderate to severe disability at six months) as assessed by the extended Glasgow Outcome Scale (GOS‐E) was 80/144 (56%) in the ICP‐monitored group and 93/153 (61%) in the non‐invasive group.
Six percent of participants in the ICP monitoring group had complications related to the monitoring, none of which met criteria for being a serious adverse event. There were no complications relating to the non‐invasive group.
Other complications and adverse events were comparable between treatment groups, 70/157 (45%) in the ICP‐monitored group and 76/167 (46%) in the non‐invasive group.
Late mortality in both the monitored and non‐invasive groups was high, with 35% of deaths occurring > 14 days after injury. The authors comment that this high late mortality may reflect inadequacies in post‐ICU services for disabled survivors requiring specialist rehabilitation care.
Authors' conclusions
The data from the single RCT studying the role of routine ICP monitoring in acute traumatic coma fails to provide evidence to support the intervention.
Research in this area is complicated by the fact that RCTs necessarily assess the combined impact of measurement of ICP with the clinical management decisions made in light of this data. Future studies will need to assess the added value of ICP data alongside other information from the multimodal monitoring typically performed in intensive care unit settings. Additionally, even within traumatically acquired brain injury (TBI), there is great heterogeneity in mechanisms, distribution, location and magnitude of injury, and studies within more homogeneous subgroups are likely to be more informative.
Keywords: Humans; Cerebrovascular Circulation; Acute Disease; Brain Injuries; Brain Injuries/complications; Coma; Coma/physiopathology; Intracranial Hypertension; Intracranial Hypertension/physiopathology; Intracranial Pressure; Intracranial Pressure/physiology; Monitoring, Physiologic; Monitoring, Physiologic/methods
Plain language summary
For a person with a head injury who is in a coma, are there benefits to regularly monitoring the pressure inside the skull?
Background
The brain is situated in a rigid box (the skull) that cannot expand, so normal swelling from injury cannot occur. When brain swelling does occur, pressure inside the skull rises. This makes it harder for the heart to pump the oxygen‐rich blood into the brain needed for recovery. If treating physicians cannot control swelling, the lack of blood supply to the swollen brain can cause further brain damage. Efforts to avoid this damage can include regular monitoring of the pressure inside the skull (intracranial). There are different ways to monitor pressure. One commonly used method is to insert a small probe into the skull. But whenever something is put into the skull, there is a chance it may cause bleeding or an infection.
Search date
The evidence in this review was up to date as of May 2015.
Study characteristics
The one randomised controlled trial (RCT) identified included 324 participants, all of whom had sustained severe traumatic brain injury and were receiving care in intensive care units in South America. People in one group had a pressure monitoring device inserted into their skull. People in the control group did not receive the device. All participants had regular monitoring of pressure in the skull through observation by the treating doctors and nurses, and X‐rays.
Key results
We did not identify any (statistically significant) differences between the two groups at six months in relation to death or survival with severe disability. There were no important complications of ICP monitoring.
Future research
More research is needed into how routine monitoring of intracranial pressure can inform clinical care.
Background
Description of the condition
Studies in traumatic brain injury (TBI) first led to the insight that the damage and disability seen was not due solely to the direct consequences of the primary injury. Postmortem pathological data suggested that some additional brain damage occurred as a result of further hypoxic‐ischaemic damage. This raised the important possibility that inadequate cerebral blood supply in the early postinjury phase added to overall morbidity (Becker 1989). Given that this process occurs when the patient is in hospital, the damage was potentially amenable to remediation.
The brain's blood supply (cerebral perfusion) is dependent on the difference between the arterial blood pressure driving blood into the brain and the pressure of brain tissue within the skull (intracranial pressure, or ICP). The higher the ICP, the harder it is for the heart to drive blood into the brain, as it is enclosed in a rigid box (the skull). Thus detection and treatment of raised ICP can in certain circumstances lead to improved brain perfusion and a reduced risk of additional ischaemic brain damage.
Description of the intervention
Physicians typically monitor ICP continuously by using a pressure transducer, placed into either the subdural or subarachnoid space, the brain parenchyma itself or a cerebral ventricle via catheter. Although placement of the latter is more invasive, it allows the additional possibility of draining cerebrospinal fluid as a therapeutic response to raised ICP. However, subdural/subarachnoid placement of pressure transducers is technically simpler and more favoured among clinicians.
Data from an ICP monitor are typically displayed in real time in an ICU setting, and values over agreed thresholds may trigger specific medical or neurosurgical interventions. Some authors have additionally advocated the recording of ICP continuously to allow the estimation of a cumulative 'burden' of raised ICP and impaired cerebral perfusion over time.
Researchers and clinicians have proposed several non‐invasive techniques for estimating intracranial pressure over the years, including 'fontanometry' in infants with a patent anterior fontanelle, and measurements of intraocular and middle ear pressure in older children and adults. These techniques remain experimental, and this review confines itself to studies measuring ICP directly.
A number of early non‐randomised studies suggested that prompt resuscitation and painstaking management of raised intracranial pressure (ICP) in an intensive care unit (ICU) could reduce mortality and morbidity after traumatic brain injury (Becker 1977; Marshall 1979; Miller 1981). Whilst investigators initially emphasised elevations of ICP per se, attention has more recently switched to maintenance of the cerebral perfusion pressure (CPP), calculated as the difference between the mean systemic arterial pressure (MAP) and ICP (Robertson 1999; Rosner 1990). From this perspective, maintenance of adequate MAP may be useful in addition to interventions aimed at lowering ICP.
How the intervention might work
Potential benefits of having accurate real‐time ICP data in the management of coma might include:
improved detection of rising ICP, provided that monitoring can detect raised ICP more effectively than bedside assessment based on clinical or radiological data (this might be particularly pertinent in units where patients are electively paralysed as part of neurointensive care, as this masks some clinical indicators of rising ICP);
knowledge on the degree to which ICP is elevated, which can inform the selection of the best treatment options (in terms of aggressiveness) according to the severity of the situation.
Despite the development of relatively less invasive techniques for the measurement of ICP, potential hazards (including infection and haemorrhage) still remain associated with the placement of monitoring probes (Chambers 1990; Mayall 1984; Narayan 1982; Paramore 1994).
Benefit will only ensue from ICP monitoring if effective clinical responses exist, and these will depend on the mechanism of the raised ICP. Increases in ICP due to focal (localised) space‐occupying collections are more probable in the context of traumatic, rather than non‐traumatic coma, and these are potentially amenable to surgical evacuation. On the other hand, non‐traumatic causes of coma include brain infections (such as bacterial or viral meningoencephalitis or cerebral malaria), metabolic disturbances (such as diabetic ketoacidosis, hepatic encephalopathy and poisoning) and other medical conditions (such as preeclampsia), and in these situations physicians may have fewer effective options for addressing raised ICP. Therefore, it is necessary to separately analyse the benefits of monitoring ICP according to traumatic and non‐traumatic causes of coma.
The risk−benefit ratio of ICP monitoring may also depend on coma severity, as typically measured by the Glasgow Coma Score (GCS), with benefit increasing with severity (Jennett 1975). As with several other aspects of 'routine' neurointensive care of patients after acute brain injury, there is a wide variation in the use of ICP monitoring in routine clinical practice (Morris 2006).
Why it is important to do this review
Data from non‐randomised controlled trials supporting routine ICP monitoring have led to recommendations that it be included in published guidelines, such as BTF 2007, for the routine management of severe traumatic brain injury (Becker 1977; Marshall 1979; Miller 1981). In this regard, it is important to remember that ICP measurement is not an end in itself, but a tool to guide treatment of raised ICP and maintain cerebral perfusion. Any randomised controlled trial in this area will of necessity comprise both an approach to detecting raised ICP (i.e. invasive real‐time monitoring versus clinical assessment) and one or more approaches to treating it (i.e. a protocol for clinical intervention based on the measured or clinically inferred presence and degree of raised ICP), and it may be difficult to separate the effects of ICP measurement from the effects of its treatment.
This is an update of a Cochrane review first published in 2001 and last updated in 2010. The present version includes data from the first study identified that meets inclusion criteria.
Objectives
To determine whether routine ICP monitoring in severe coma of any cause reduces the risk of all‐cause mortality or severe disability at final follow‐up.
Methods
Criteria for considering studies for this review
Types of studies
-
Randomised controlled trials of ICP monitoring in acute coma versus no ICP monitoring (that is, with only clinical assessment, radiological assessment, or both, of ICP); studies must have:
a standardised approach to outcome assessment (i.e. assessment at a prespecified fixed time, or with sufficient data to enable time‐to‐event analysis); and
an adequate approach to allocation concealment.
Types of participants
Patients with acute severe coma (typically defined by an admission GCS of less than or equal to 8) of traumatic or non‐traumatic aetiology.
Types of interventions
Real‐time ICP monitoring using any invasive or semi‐invasive technique, including intraventricular catheters, subdural/subarachnoid space pressure transducers, or serial lumbar or ventricular taps with ICP measurement.
We excluded studies that used indirect estimations of ICP by imaging techniques (cranial CT, cranial ultrasound ± Doppler) and non‐invasive methods of ICP estimation (fontanometry, typanometry).
Types of outcome measures
Primary outcomes
All‐cause mortality at the end of the follow‐up period
Severe disability at the end of the follow‐up period. If the trial presented Glasgow Outcome Score (GOS), we defined severe disability as a GOS outcome of 'severe disability' or 'persistent vegetative state'. If trials presented other outcome scoring systems, we mapped these to the GOS (Jennett 1975).
Secondary outcomes
Adverse events including catheter‐related complications such as focal haemorrhage and local infection
Search methods for identification of studies
The searches were not restricted by date, language or publication status.
Electronic searches
The Cochrane Injuries Group Trials Search Co‐ordinator searched the following databases and registries.
Cochrane Injuries Group Specialised Register (up to 22 May 2015).
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) (2015, Issue 4).
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (1946 to May 2015).
EMBASE Classic + EMBASE (OvidSP) (1947 to May 2015).
CINAHL Plus (EBSCO) (1937 to May 2015).
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to May 2015).
ISI Web of Science: Conference Proceedings Citation Index‐ Science (CPCI‐S) (1990 to May 2015).
ClinicalTrials.gov (www.clinicaltrials.gov) (up to 22 May 2015).
WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/) (up to May 2015).
We report the full search strategies in Appendix 1.
We adapted the MEDLINE search strategy illustrated in Appendix 1 as necessary for each of the other databases. We used the Cochrane Highly Sensitive Search Strategies to identify randomised trials in MEDLINE and EMBASE (Lefebvre 2011).
Searching other resources
We handsearched the reference lists of all relevant trials and review articles, obtaining English‐language translations where necessary.
Data collection and analysis
The Injuries Group Trials Search Co‐ordinator (TSC) collated the search results and removed duplicates before passing them on to the authors for screening.
Selection of studies
For versions of the review up to 2008, one author (RF) along with previous editions' coauthors independently examined titles and abstracts for potentially relevant trials.
For the present update (2015), we ran two searches (2013 and 2015). In the first, one author (ET) screened titles and another (RF) examined the titles and abstracts selected for retrieval. For the second search in 2015, one author (RF) and a Cochrane Injuries Group staff member (Jane Dennis) independently screened results. We retrieved the full text of all reports that seemed to meet the inclusion criteria based on the title and abstract, and ET, RF and the Injuries Group staff member examined them to determine eligibility.
We independently applied selection criteria for this review, and in no case was there disagreement as to the eligibility of studies.
Data extraction and management
Two authors (JR, RF) independently extracted data on characteristics of included studies (including patient characteristics, intervention characteristics, setting); aspects of trial design (specifically those relevant to risk of bias judgments); methods of outcome measurement; and outcome data.
Assessment of risk of bias in included studies
Two authors (JR, RF) independently assessed the risk of bias in the one included study by using Cochrane's 'Risk of bias' tool (Higgins 2011a).
We assessed the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting, and we examined the trial description in order to characterise the risk of bias for each domain as 'low', 'high' or 'unclear'.
Measures of treatment effect
For dichotomous outcome data, we reported trial data as did the original investigators for the one trial that currently meets inclusion criteria.
Should meta‐analysis be possible in future, we plan to calculate risk ratios and 95% confidence intervals (CI) for dichotomous data and mean difference (MD) and 95% CI for continuous data for each trial (if outcomes are measured using the same instrument) and employ the standardised mean difference (SMD) if trial investigators use different measures.
Unit of analysis issues
The one study meeting inclusion criteria for this review allocated individuals rather than clusters and involved a two‐group comparison.
Should we identify trials involving multiple intervention groups in the future, we will follow the approach described in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Where appropriate, we will pool data from eligible intervention groups or eligible control groups to create a single pair‐wise comparison. Where such combination is not appropriate, we will treat the trial as two or more smaller trials, with data from each active group analysed in comparison with data from the shared comparison group. We will divide the data for the shared group proportionately to avoid double‐counting participants.
Neither cross‐over nor cluster‐randomised trials are likely to be conducted in this topic area, so we do not include plans for their analysis.
Dealing with missing data
In the event of missing data, it is our plan to contact investigators for information concerning any unexplained dropouts or exclusions from analysis. We will assess missing data and dropouts for each included study and report the number of participants who were included in the final analysis as a proportion of all randomised participants in each study. We will provide reasons, where known, for missing data in the narrative summary, as well as details of investigators' use of intention‐to‐treat (ITT) analysis.
Assessment of heterogeneity
We planned at protocol stage to assess the heterogeneity of treatment effect between trials with the Chi2 test, using the P value of 0.1 as the cut‐off point to establish statistical significance. If appropriate, we planned to calculate a weighted estimate of typical 'treatment' effects separately in traumatic and non‐traumatic groups, using the Mantel‐Haenzel odds ratio. For this review, there were insufficient data for such calculations, but we retain this aspect of the protocol for the sake of future updates.
Assessment of reporting biases
Should we identify at least ten studies that use the same outcome in future updates, we plan to investigate the potential of reporting (publication) bias using funnel plots. We recognise that asymmetry of the plots may indicate publication bias, although it may also represent a true relationship between trial size and effect size. If such a relationship is identified, we will examine the clinical diversity of the studies as a possible explanation (Egger 1997).
Data synthesis
In the future, if trials meeting inclusion criteria are too clinically heterogeneous to pool, we will describe the results narratively. Alternatively, if we consider a pooled analysis to be appropriate, we will combine effect estimates using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
Should data become available in the future, we plan to investigate subgroup analyses to examine the differential impact of ICP monitoring by coma aetiology (e.g. traumatic versus non‐traumatic causes of severe brain injury). Within the traumatic brain injury group, if data permit, we will perform subgroup analysis by injury type (e.g. on radiological criteria such as the Marshall classification; Marshall 1991).
Sensitivity analysis
In the future, if data permit, we will conduct sensitivity analyses to examine the impact of aspects of trial design including adequate allocation concealment and blinding of outcome assessors.
Results
Description of studies
Results of the search
We retrieved 507 references to screen for this update (Figure 1). We describe potentially relevant studies that were ultimately excluded, along with their reasons for exclusion, in the Characteristics of excluded studies table.
1.
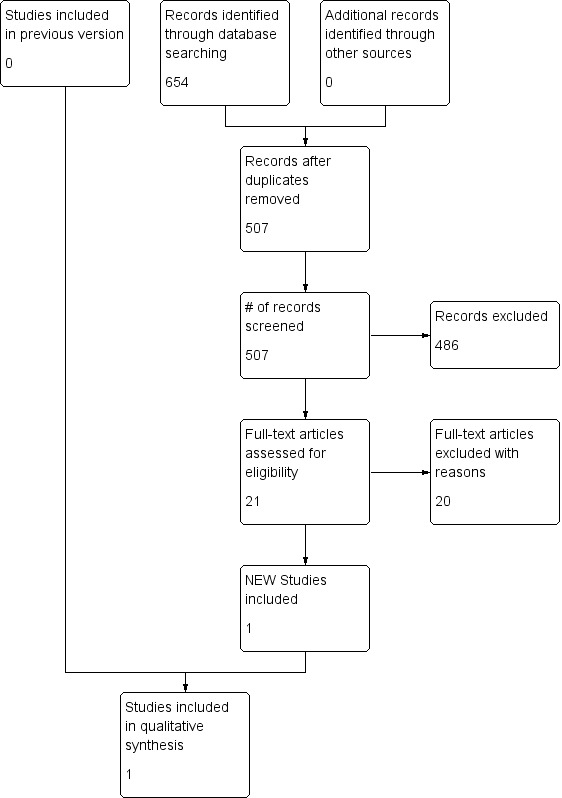
Study flow diagram for most recent search (May 2015)
Included studies
We identified one trial that met the protocol inclusion criteria (Chesnut 2012).
Design
Chesnut 2012 is a multicentre randomised controlled trial. Investigators employed stratified sequence generation, allocating participants according to centre, severity of injury and age.
Sample size
The sample size of the one included study was 324. Investigators planned the sample size "by means of simulation to provide 80% power to detect an increase of 20 percentage points in the percentage of patients with a good outcome or with moderate disability according to the GOS‐E [extended GOS]" (Chesnut 2012).
Setting
Chesnut 2012 took place in intensive care units (ICUs) at six South American hospitals (four in Bolivia and two in Ecuador).
Participants
Trialists randomised all participants either within 24 hours of injury (for patients with GCS < 8 on admission) or within 24 hours of deterioration (for patients deteriorating to GCS < 8 within 48 hours of injury). Median GCS motor score at randomisation was 4.0. Forty‐nine per cent of participants had localised brain injuries, and none were capable of following commands. Road traffic accidents involving cars or motorcycles were responsible for 51% of the injuries (Chesnut 2012, supplemental file table S6). Fewer than half of all participants were transported to the first admitting hospital by ambulance. Most were transferred to study hospitals from another admitting hospital. Median time from injury to arrival at a study hospital for all participants was 3.1 hours.
Interventions
Intervention: "Patients…assigned to the pressure monitoring group had an intraparenchymal monitor placed as soon as possible and were treated to maintain an intracranial pressure of less than 20 mmHg . . . Drainage of cerebrospinal fluid required ventriculostomy placement" (Chesnut 2012). Physicians were instructed to maintain an ICP < 20 mmHg and a cerebral perfusion pressure (mean arterial pressure minus ICP) of 50 to 70 mmHg.
Control: standard care, defined as care "in accordance with a protocol based on the pretrial standard for care at the three original participating hospitals". The supplemental data file in the online version of Chesnut 2012 contains a detailed description of this protocol. In addition to general care measures, interventions for raised ICP/inadequate cerebral perfusion pressure include sedation, ventilation to mild hypocapnia for the shortest possible time, and hyperosmolar therapies.
Outcomes
The primary, prespecified outcome for the included study was a composite measure of survival time, impaired consciousness and functional status at three and six months as well as neuropsychological status at six months (Carney 2012). A blinded outcomes assessor determined neuropsychological status. This composite measure was based on performance across 21 measures of functional and cognitive status and calculated as a percentile (with 0 indicating the worst performance, and 100 the best performance). This composite score incorporates GOS‐E outcomes, which are reported separately in the supplemental data.
The trial also reported data on mortality, adverse events and the use of brain‐specific treatments (e.g, hyperosmolar fluids, use of hyperventilation).
Excluded studies
Since publication of the last edition of this review (2010), we have identified five further publications relevant to the field, although none met review inclusion criteria. We describe the reasons for exclusion in the Characteristics of excluded studies table.
One study requires further comment: Kostic 2011 is a randomised trial comparing mortality in ICP monitored patients to non‐ICP monitored controls. It is a single centre RCT allocating patients to treatment arms by day of the week and thus has a high risk of bias. The primary outcome is survival, but the paper did not make it clear at which point trialists assessed survival. The study authors confirmed that the endpoint was survival to discharge from hospital and that the minimum length of stay was seven days. We excluded the study on grounds of inadequate allocation concealment.
We know of no relevant ongoing studies at present.
Risk of bias in included studies
Overall, we were satisfied by the precautions taken to minimise risk of bias in Chesnut 2012. Below, we report our assessments on individual aspects of trial design and conduct in accordance with Cochrane's 'Risk of bias' tool (Higgins 2011a).
Allocation
A data‐centre biostatistician created computer‐generated randomisation sequences, stratifying allocations by centre, severity of injury and age, so that numbers of participants at each centre were roughly equal after every two to four randomisations (Chesnut 2012). Investigators report that the two groups were similar at baseline with regard to all characteristics assessed at that point. We assessed there to be a low risk of bias for sequence generation.
Blinding
Blinding of participants and personnel to study arm is inherently impossible given the invasive nature of ICP monitor insertion, and consequently this trial is at a high risk of bias for this criterion. However, blinded examiners assessed outcomes, and moreover, the outcome of mortality was not at risk of bias, so we judged there to be a low risk of bias for the criterion of blinding of outcome assessors.
Incomplete outcome data
Trialists analysed outcome data on an intention‐to‐treat basis (model not described). The primary outcome measure was a composite percentile score amalgamating neurological and psychological scores across a range of domains, thus it was vulnerable to missing data. The authors considered this and attempted to account for it in order to keep participants in the study despite incomplete outcome data. Double entry and electronic data checks ensured accuracy. We assessed risk of attrition bias to be low.
Selective reporting
Reporting was consistent with the prospectively published protocol (Chesnut 2012); we therefore deemed this domain to be at low risk of bias.
Other potential sources of bias
We deemed the study to be at low risk for other bias. Investigators performed inter‐rater reliability analyses for the assessment of injury severity (Abbreviated Injury Scale, GCS) and CT scan interpretation. A commercial sponsor (Integra Life) provided ICP monitoring devices; however, investigators confirm the firm had "no role in the design or conduct of the study, the data analysis, or the writing of the manuscript" (Chesnut 2012).
Effects of interventions
Primary outcomes
Instead of mapping results to the GOS, we chose to report them as the original investigators did, using the GOS‐E. Although the scales are similar, they are not the same, and specifically the GOS outcomes of 'severe disability' and 'persistent vegetative state' do not exist in the GOS‐E.
All‐cause mortality at the end of the follow‐up period
Mortality at six months was 56/144 (39%) in the ICP‐monitored group and 67/153 (44%) in the non‐invasive group.
Unfavourable outcome (defined as death or moderate to severe disability as assessed by the GOS‐E (i.e. outcomes 1 to 6) was 80/144 (56%) in the ICP‐monitored group and 93/153 (61%) in the non‐invasive group.
For these outcomes, the differences between groups are not statistically significant.
Survival with severe disability at the end of the follow‐up period
Overall survival at six months was 88/144 (61%) in the ICP‐monitored group and 86/153 (56%) in the non‐invasive group, as described above.
Survival with severe disability (GOS‐E outcomes 2 to 4) at six months was 28/144 (20%) in the ICP‐monitored group and 26/153 (17%) in the non‐invasive group.
There was no difference in the study's primary outcome composite score between treatment groups (56th centile in the ICP‐monitored group and 53rd centile in the non‐invasive group).
For these outcomes, the differences between groups are not statistically significant.
Secondary outcomes
Six percent of participants in the ICP monitoring group had complications related to the monitoring, none of which met criteria for being a serious adverse event. There were no complications relating to the non‐invasive group.
Other complications and adverse events were comparable between treatment groups, 70/157 (45%) in the ICP‐monitored group and 76/167 (46%) in the non‐invasive group.
More people in the non‐invasive (imaging–clinical examination) group received specific treatments for raised ICP, such as administration of hyperosmolar fluids and the use of hyperventilation (4.8 versus 3.4 in the ICP‐monitored group, P = 0.002).
Discussion
Since the last update of this review, one trial meeting inclusion criteria has been published (Chesnut 2012). It is a randomised controlled trial comparing an ICP treatment strategy guided by continuously monitored ICP data versus care based on clinico‐radiological assessment of the presence of raised ICP. There was no evidence of difference in outcome between the two strategies.
Summary of main results
The one included study did not find any differences between the two groups in the primary outcome measures, mortality or severe disability. There is evidence that in the non‐invasive group (reliant on imaging and clinical examination), physicians tended to take more aggressive measures to treat suspected raised ICP. Since there are known and potential adverse consequences for some of these treatments (for example, excessive hyperventilation to reduce ICP by vasoconstriction can cause further cerebral ischaemia), the data suggest that accurate ICP data may allow more appropriate tailoring of treatments for raised ICP to the severity of an individual case.
Overall completeness and applicability of evidence
Trials related to ICP monitoring inevitably reflect the combined effects of both detecting and quantifying elevated ICP, on the one hand, and treating it through one or more approaches, on the other. There are also several proposed ways to analyse and interpret real‐time ICP data (Eide 2011; Robertson 1999). In any case, the value of ICP data may be greater in conjunction with other real‐time technologies for monitoring brain metabolic, energetic and functional status (Chesnut 2014; Le Roux 2014).
Chesnut 2012 took place in South America, and its authors raised the possibility that limited pre‐hospital services may have affected the data in ways that limit the generalisability of the findings to other countries (i.e. with more developed rapid response and pre‐hospital services). They further point out that suboptimal pre‐hospital care may have reduced the potential for optimal ICU care to confer benefit, although they maintain that overall mortality postadmission to ICU was comparable to that of other published studies.
A more important consideration may be the high rate of late mortality (> 14 days postinjury), which likely reflects deficiencies of post‐ICU, specialist care for severely disabled survivors in the early stages of rehabilitation after injury. However, a post hoc analysis of mortality at 14 days showed no significant difference between groups.
Quality of the evidence
Chesnut 2012 appears to be a well conducted study, largely meeting CONSORT standards for implementation and reporting and fulfilling intentions for recruited sample size.
Potential biases in the review process
We describe the rationale for excluding Kostic 2011 in Excluded studies.
Agreements and disagreements with other studies or reviews
In a recent meta‐analysis of the role of ICP monitoring in traumatic brain injury (Su 2014), the authors aggregate the results of six non‐randomised cohort studies excluded from this review, together with Chesnut 2012 and Kostic 2011. These authors report a summary odds ratio for prevention of mortality of 1.16 (95% CI 0.87 to 1.54), although they also acknowledge that the time at which mortality is reported varies between studies and is not reported in some. They interpret the data from Kostic 2011 as being mortality at six months, which is not our understanding after correspondence with the authors. Notwithstanding these issues, we support the conclusion of Su 2014 (consistent with Chesnut 2012, Chesnut 2013, Chesnut 2014) that there is no current evidence of reduced mortality from the implementation of routine ICP monitoring. Recent expert consensus statements, such as Stocchetti 2014, confirm that ICP monitoring should instead be targeted to groups with specific indications.
Authors' conclusions
Implications for practice.
The role of routine intracranial pressure (ICP) monitoring in the management of acute encephalopathy remains controversial, and there is wide variability in current clinical practice. Data from a single, large, well‐conducted RCT failed to provide evidence to inform clinical practice at this stage. The findings of observational studies demonstrating associations between ICP monitoring and improved survival should be interpreted with caution due to their non‐randomised nature (Shafi 2008, Bennett 2012; Farahvar 2012).
Implications for research.
For many years, commentators expressed pessimism that a formal randomised study would assess the use of routine ICP monitoring in the foreseeable future. The main grounds for this pessimism were the cost and logistics of undertaking a study of the requisite size as well as the opinion that the role of ICP monitoring was widely established and that evaluation of other newer interventions was now a priority. Disappointing subsequent experience with trials of novel neuroprotective strategies suggested that the effects of between‐centre variation in 'conventional' management may have effects on patients' outcomes which are of a comparable magnitude to these novel approaches (Clifton 2001; Reinert 1999). Suggestions of large effect sizes in non‐randomised studies, such as in Lane 2000, and indications that indiscriminate use of ICP monitoring might prolong ICU stay without improving outcome, as Cremer 2005 reported, restored some equipoise, enabling the important contribution to the literature that Chesnut 2012 provides.
Trials related to ICP monitoring inevitably reflect the combined effects of both detecting and quantifying elevated ICP, on the one hand, and treating it through one or more approaches, on the other. There are also several proposed ways to analyse and use real‐time ICP data in clinical care (Eide 2011; Lavinio 2011; Robertson 1999). In any case, the value of ICP data may be greater in conjunction with other real‐time technologies for monitoring brain metabolic, energetic and functional status (Chesnut 2014; Le Roux 2014).
Future work needs to acknowledge the very marked heterogeneity of pathologies even among people with traumatic brain injury (Saatman 2008). Indications for ICP monitoring vary between subgroups (Stocchetti 2014), and researchers should consider studies within clinico‐radiologically defined subgroups.
What's new
| Date | Event | Description |
|---|---|---|
| 15 February 2016 | Amended | One typographical error was corrected in the abstract, main results, section. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 8 September 2015 | New search has been performed | The search has been updated to 22 May 2015. One new study was included in the review. |
| 8 September 2015 | New citation required and conclusions have changed | One study has been added to the review. The authors of the review have changed. |
| 7 August 2015 | Amended | A 'Published Note' has been added, with a change to the inclusion criteria. In the future, trials eligible for inclusion must have adequate allocation concealment. |
| 15 October 2010 | Amended | Reference corrected. |
| 13 October 2009 | New citation required but conclusions have not changed | The search was updated to April 1 2009. No new studies have been included. The conclusions remain the same. The authors of the review have changed. |
| 27 March 2008 | Amended | Converted to new review format. |
| 12 April 2006 | New search has been performed | The searches were updated in April 2006; no new studies for inclusion were identified. |
Notes
Review authors' note, October 2015: In future versions of this review, we will calculate the risk ratio (rather than the odds ratio which was pre‐specified in the original protocol (Forsyth 2000)).
Acknowledgements
We thank Peter Baxter and Tracy Elliott for their input as coauthors of the first published version of this review.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategy
Cochrane Injuries Group Specialised Register (“intracranial pressure” or “cerebrospinal pressure” or “cerebrospinal fluid”) and (monitor*) and (coma*) CENTRAL (the Cochrane Central Register of Controlled Trials) #1MeSH descriptor Craniocerebral Trauma explode all trees #2MeSH descriptor Cerebrovascular Trauma explode all trees #3MeSH descriptor Brain Edema explode all trees #4(brain or cerebral or intracranial) next (oedema or edema or swell*) #5MeSH descriptor Glasgow Coma Scale explode all trees #6MeSH descriptor Glasgow Outcome Scale explode all trees #7MeSH descriptor Unconsciousness explode all trees #8glasgow next (coma or outcome) next (score or scale) #9(Unconscious* or coma* or concuss* or 'persistent vegetative state') near5 (injury* or trauma* or damage* or wound* or fracture*) #10"Rancho Los Amigos Scale" #11(head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) near3 (injur* or trauma* or damag* or wound* or fracture* or contusion*) #12Diffuse next axonal next injur* #13(head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) near3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure) #14MeSH descriptor Coma explode all trees #15(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) #16MeSH descriptor Intracranial Pressure explode all trees #17MeSH descriptor Cerebrospinal Fluid Pressure explode all trees #18intracranial near3 pressure #19cerebrospinal near5 pressure #20(#16 OR #17 OR #18 OR #19) #21MeSH descriptor Monitoring, Physiologic explode all trees #22(patient near3 monitor*) or (physiologic* near3 monitor*) #23monitor*:ti,ab #24(#21 OR #22 OR #23) #25(#15 AND #20 AND #24) MEDLINE (OvidSP) 1.exp Craniocerebral Trauma/ 2.exp Brain Edema/ 3.exp Glasgow Coma Scale/ 4.exp Glasgow Outcome Scale/ 5.exp Unconsciousness/ 6.exp Cerebrovascular Trauma/ 7.((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) adj5 (injur* or trauma* or damag* or wound* or fracture* or contusion*)).ab,ti. 8.((head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) adj5 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ti,ab. 9.(Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti. 10."rancho los amigos scale".ti,ab. 11.("diffuse axonal injury" or "diffuse axonal injuries").ti,ab. 12.((brain or cerebral or intracranial) adj3 (oedema or edema or swell*)).ab,ti. 13.((unconscious* or coma* or concuss* or 'persistent vegetative state') adj3 (injur* or trauma* or damag* or wound* or fracture*)).ti,ab. 14.exp coma/ 15.or/1‐14 16.exp Intracranial Pressure/ 17.exp Cerebrospinal Fluid Pressure/ 18.(intracranial adj3 pressure).ab,ti. 19.(cerebrospinal adj5 pressure).ab,ti. 20.16 or 17 or 18 or 19 21.exp Monitoring, Physiologic/ 22.((patient adj3 monitor*) or (physiologic* adj3 monitor*)).ab,ti. 23.22 or 21 24.23 and 20 and 15 25.randomi?ed.ab,ti. 26.randomized controlled trial.pt. 27.controlled clinical trial.pt. 28.placebo.ab. 29.clinical trials as topic.sh. 30.randomly.ab. 31.trial.ti. 32.25 or 26 or 27 or 28 or 29 or 30 or 31 33.(animals not (humans and animals)).sh. 34.32 not 33 35.34 and 24
EMBASE (OvidSP) 1.exp Brain Injury/ 2.exp Brain Edema/ 3.exp Glasgow Coma Scale/ 4.exp Glasgow Outcome Scale/ 5.exp Rancho Los Amigos Scale/ 6.exp Unconsciousness/ 7.((brain or cerebral or intracranial) adj5 (oedema or edema or swell*)).ab,ti. 8.((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) adj5 (injur* or trauma* or damag* or wound* or fracture* or contusion*)).ab,ti. 9.(Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti. 10.Rancho Los Amigos Scale.ab,ti. 11.((unconscious* or coma* or concuss* or 'persistent vegetative state') adj3 (injur* or trauma* or damag* or wound* or fracture*)).ti,ab. 12.Diffuse axonal injur*.ab,ti. 13.((head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ab,ti. 14.exp Coma/ 15.or/1‐14 16.exp Intracranial Pressure/ 17.exp Cerebrospinal Fluid Pressure/ 18.(intracranial adj3 pressure).ab,ti. 19.(cerebrospinal adj5 pressure).ab,ti. 20.16 or 17 or 18 or 19 21.exp monitoring/ 22.exp Patient Monitoring/ 23.((physiologic* adj3 monitor*) or patient* monitor*).ab,ti. 24.21 or 22 or 23 25.24 and 20 26.exp Intracranial Pressure Monitoring/ 27.25 or 26 28.27 and 15 29.exp Randomized Controlled Trial/ 30.exp controlled clinical trial/ 31.randomi?ed.ab,ti. 32.placebo.ab. 33.*Clinical Trial/ 34.randomly.ab. 35.trial.ti. 36.29 or 30 or 31 or 32 or 33 or 34 or 35 37.exp animal/ not (exp human/ and exp animal/) 38.36 not 37 39.28 and 38 CINAHL Plus (EBSCO) S1(MH "Monitoring, Intracranial Pressure") or (MH "Intracranial Pressure Monitoring (Iowa NIC)") S2(MH "Monitoring, Physiologic+") S3(physiologic* N3 monitor*) or (patient* N5 monitor*) S4S2 or S3 S5(MH "Intracranial Pressure") S6intracranial N3 pressure S7cerebrospinal N5 pressure S8S3 or S4 or S5 S9S4 and S8 S10S1 or S9 S11 MH clinical trials S12 PT clinical trial* S13 TX clinical N3 trial* S14 TI ( (singl* N3 blind*) or (doubl* N3 blind*) or (trebl* N3 blind*) or (tripl* N3 blind*) ) or TI ( (singl* N3 mask*) or (doubl* N3 mask*) or (trebl* N3 mask*) or (tripl* N3 mask*) ) or AB ( (singl* N3 blind*) or (doubl* N3 blind*) or (trebl* N3 blind*) ) or AB ( (singl* N3 mask*) or (doubl* N3 mask*) or (trebl* N3 mask*) or (tripl* N3 mask*) ) S15 TX randomi?ed N3 control* N3 trial* S16 MH placebos S17 TX placebo* S18 MH random assignment S19 TX random* N3 allocat* S20 MH quantitative studies S21 S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 S22 S10 and S21 S23TI ( head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran* ) and AB ( haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure ) S24AB ( head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran* ) and TI ( injur* or trauma* or damag* or wound* or fracture* or contusion* ) S25AB ( Unconscious* or coma* or concuss* or "persistent vegetative state" ) and TI ( injur* or trauma* or damag* or wound* or fracture* ) S26 (MH "Unconsciousness+") or (MH "Brain Concussion+") S27(MH "Brain Injuries+") S28(MH "Head Injuries+") S29S23 or S24 or S25 or S26 or S27 or S28 S30S22 and S29 ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED), Conference Proceedings Citation Index‐ Science (CPCI‐S) 1.TS=(intracranial pressure or cerebrospinal pressure or cerebrospinal fluid) AND TS=(monitor*) AND TS=(coma*) 2.TS=(clinical OR control* OR placebo OR random OR randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random) SAME TS=(trial* or group* or study or studies or placebo or controlled) 3.1 and 2
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chesnut 2012.
| Methods | Randomised controlled trial (multicentre: Ecuador and Bolivia) | |
| Participants | 324 patients aged ≥ 13 years with severe traumatic brain injury (mean age 29, range 22 to 44). The median GCS motor score at randomisation was 4.0. Traffic incidents accounted for the majority of injuries. Most patients were admitted to the study hospitals via another centre. Median time from injury to arrival at hospital for all participants was 3.1 hours Inclusion criteria: GCS < 8 on admission or within first 48 hours after injury; admission to study hospital within 24 hours of injury Exclusion criteria: GCS 3 and fixed dilated pupils on admission; age ≤ 12 years |
|
| Interventions | Guidelines based management protocol Active group: invasive ICP monitoring with protocol‐based clinical care to maintain measured ICP < 20 mmHg Control group: protocol based general clinical care with intervention for raised ICP as indicated by clinical examination or radiological data |
|
| Outcomes |
Primary outcomes:
Secondary outcomes: severe disability at the end of the follow‐up period. As part of the multi‐domain outcome, data investigators provided GOS‐E data at six months. Frequency of complications such as focal haemorrhage and local infection: adverse effect data were collected Additional outcomes: investigators also reported on the number of days spent in the ICU and the duration and intensity of medical treatments for raised ICP (e.g. administration of hyperosmolar fluids, use of hyperventilation) |
|
| Notes | The authors of the paper concluded that for patients with severe traumatic brain injury, care focused on maintaining monitored intracranial pressure at less than 20 mmHg was not shown to be superior to care based on imaging and clinical examination | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequences performed remotely by a data‐centre biostatistician at the University of Washington (USA) and stratified according to site, severity of injury and age, such that numbers of participants on each site were balanced after every two to four allocations. Investigators report that the two groups were similar at baseline with regard to all characteristics assessed at that point. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment according to protocol; the local coordinator logged onto a secure database and entered each patient's details after consent was obtained from a legally authorised representative. Once confirmation of eligibility was made and stratum determined (by the programme), the programme itself entered "the subject information on the randomisation log, retrieve[d] the next assignment for that center and stratum, enter[ed] that on the randomisation log, and sen[t] the assignment to the study co‐ordinator". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treating personnel cannot be blinded to the placement of an ICP monitor. Arguably, (unconscious) participants could be blinded although monitor placement leaves a surgical scar. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to assigned treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data was analysed on an intention‐to‐treat basis (model not described) |
| Selective reporting (reporting bias) | Low risk | Reporting was consistent with the pre‐published protocol |
| Other bias | Low risk | Integra Life Sciences donated catheters used in monitoring and provided other support to the trial, but investigators reported that the firm had "no role in the design or conduct of the study, the data analysis, or the writing of the manuscript" (Chesnut 2012). |
GCS: Glasgow Coma Score; GOS‐E: extended Glasgow Outcome Score; ICP: intracranial pressure; ICU: intensive care unit.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agbeko 2012 | 'Prospective, randomised, interventional cohort study' assessing impact of head elevation on CPP and ICP |
| Badri 2012 | Retrospective study of patients with traumatic brain injury; no non‐invasively monitored group |
| Batjer 1990 | RCT comparing three treatments for hypertensive putaminal haemorrhage, with ICP monitoring a component of management in one arm. Recruitment for the study was concluded early with 21 patients out of a target of 60 because of unacceptably poor outcomes in all treatment groups. |
| Bennett 2012 | Retrospective cohort study of children with traumatic brain injury |
| Brawanski 1983 | Uncontrolled intervention study examining effect of endoscopic third ventriculostomy in patients with communicating hydrocephalus |
| Cremer 2005 | Non‐randomised between‐centre comparative study without standardisation of other aspects of neurointensive care protocol |
| Eddy 1994 | Retrospective uncontrolled study of use of fibreoptic ICP monitoring in patients with closed head injury |
| Eide 2011 | RCT comparing the value of 2 indices derived from ICP monitoring (mean pressure versus wave amplitude) in guiding management in subarachnoid haemorrhage; no non‐invasive monitoring group |
| Farahvar 2012 | Non‐randomised study; reduced mortality at 14 days in ICP monitored group |
| Idriz 2007 | Randomised controlled trial of ICP monitoring (alone) against multimodal monitoring (ICP monitoring plus regional cerebral blood flow, focal and global brain tissue oxygenation, middle cerebral artery flow velocity and EEG); no non‐invasive group |
| Jacobs 1998 | Randomised study of role of prophylactic antibiotics during ICP monitoring; however, the decision to perform ICP monitoring per se was not randomised, and ICP monitoring was mandatory in more severely injured patients (study suggests increased rates of infective complications when prophylactic antibiotics used). |
| Kostic 2011 | RCT of intracranial pressure monitoring in 61 severe brain trauma patients. Excluded as study does not incorporate a standardised approach to outcome assessment. The primary outcome (mortality) is not reported at a uniform time postinjury, nor is patient‐level data available to enable time‐to‐event analysis. |
| Lane 2000 | Non‐randomised, retrospective review of admissions to neurosurgical centres in Ontario, Canada, showing that although in univariate analysis the use of ICP monitoring, together with other injury severity indices, were each associated with greater mortality, a multivariate analysis controlling for the other injury severity indices showed an association between the use of ICP monitoring and a lower mortality rate. |
| Robertson 1999 | RCT of neurointensive care targeted toward reduction of ICP versus a strategy targeted toward maintenance of cerebral perfusion pressure (mean arterial pressure minus intracranial pressure); no non‐invasive group |
| Sarnaik 1989 | Not an RCT; case series of 14 children with penetrating craniocerebral gunshot wounds |
| Saul 1982 | This study uses a previous study, conducted in 1977‐1978 as an historical control and compares this with a study conducted in 1979‐1980. This second group were randomised into a trial looking at barbiturate versus non‐barbiturate intervention when ICP reached 25 mmHg. The results of this are not reported, and there is no indication of whether this had any influence on the lower overall mortality rate of group 2. |
| Shafi 2008 | Retrospective, non‐randomised study showing independent association of use of ICP monitoring with reduced survival after controlling for some injury severity variables |
| Signorini 1999 | Case series of 110 patients with TBI designed to assess prognostic value of summary measures of secondary physiological insult in addition to baseline clinical variables. |
| Smith 1986 | RCT of 2 mannitol‐based treatment regimens (one targeted to elevations of ICP; the other independent of measured ICP) for raised ICP; ICP monitored in all patients. |
| Zanier 2007 | Validation of computerised continuous recording of ICP against manual ICP recording |
CCP: cerebral perfusion pressure; EEG: electroencephalogram; ICP: intracranial pressure; RCT: randomised controlled trial; TBI: traumatic brain injury.
Differences between protocol and review
This version (October 2015) of the review incorporates changes to bring it up to the standards of The Cochrane Handbook for Systematic Reviews of Interventions, including revised Methods that utilise the Cochrane 'Risk of bias' tool. In January 2015, Joseph Raper joined the author team. In the methods section, instead of mapping results to the GOS, we chose to report them as the original investigators did, using the GOS‐E. Although the scales are similar, they are not the same, and specifically the GOS outcomes of 'severe disability' and 'persistent vegetative state' do not exist in the GOS‐E.
Review authors' note, August 2015: In future versions of this review, in order for a study to be eligible for inclusion in the review, it must have adequate allocation concealment and have pre‐specified measurement time points, or sufficient data to enable time‐to‐event analysis.
Contributions of authors
ET and RF reviewed the new literature. JR and RF performed independent data extraction. RF revised the text of the review.
Sources of support
Internal sources
University of Sheffield, UK.
Newcastle University, UK.
External sources
No sources of support supplied
Declarations of interest
RF: none known.
JR: none known.
ET: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Chesnut 2012 {published data only}
- Carney N, Lujan S, Dikmen S, Temkin N, Petroni G, Pridgeon J, et al. Intracranial pressure monitoring in severe traumatic brain injury in Latin America: process and methods for a multi‐center randomized controlled trial. Journal of Neurotrauma 2012;29(11):2022‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial‐pressure monitoring in traumatic brain injury. New England Journal of Medicine 2012;367(26):2471‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Agbeko 2012 {published data only}
- Agbeko RS, Pearson S, Peters MJ, McNames J, Goldstein B. Intracranial pressure and cerebral perfusion pressure responses to head elevation changes in pediatric traumatic brain injury. Pediatric Critical Care Medicine 2012;13(1):e39‐47. [DOI] [PubMed] [Google Scholar]
Badri 2012 {published data only}
- Badri S, Chen J, Barber J, Temkin NR, Dikmen SS, Chesnut RM, et al. Mortality and long‐term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Medicine 2012;38(11):1800‐9. [DOI] [PubMed] [Google Scholar]
Batjer 1990 {published data only}
- Batjer HH, Reisch JS, Allen BC, Plaizier LJ, Su CJ. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomised trial. Archives of Neurology 1990;47(10):1103‐6. [DOI] [PubMed] [Google Scholar]
Bennett 2012 {published data only}
- Bennett TD, Riva‐Cambrin J, Keenan HT, Korgenski E K, Bratton SL. Variation in intracranial pressure monitoring and outcomes in pediatric traumatic brain injury. Archives of Pediatrics & Adolescent Medicine 2012;166(7):641‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brawanski 1983 {published data only}
- Brawanski A, Gaab M, Fuhrmeister U, Haubitz I. Spontaneous intracranial hemorrhage, intracranial pressure and indications of operation: a clinical follow‐up study. In: Jensen HP, Brock M, Klinger M editor(s). Acute Non‐Traumatic Intracranial Bleedings. Posterior Fossa Tumors in Infancy. Vol 11, Advances in Neurosurgery. Springer Berlin Heidelberg, 1983:90‐8. Available from: http://www.springer.com/gp/book/9783540125389. [Google Scholar]
Cremer 2005 {published data only}
- Cremer OL, Dijk GW, Wensen E, Brekelmans GJ, Moons KG, Leenen LP, et al. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Critical Care Medicine 2005;33(10):2207. [DOI] [PubMed] [Google Scholar]
Eddy 1994 {published data only}
- Eddy V, Vitsky J, Rutherford E, Morris J. Aggressive use of ICP monitoring is safe and alters patient care. American Journal of Surgery 1995;61(1):6. [PubMed] [Google Scholar]
Eide 2011 {published data only}
- Eide PK, Bentsen G, Sorteberg AG, Marthinsen PB, Stubhaug A, Sorteberg W. A randomized and blinded single‐center trial comparing the effect of intracranial pressure and intracranial pressure wave amplitude‐guided intensive care management on early clinical state and 12‐month outcome in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2011;69(5):1105‐15. [DOI] [PubMed] [Google Scholar]
Farahvar 2012 {published data only}
- Farahvar A, Gerber LM, Chiu YL, Carney N, Härtl R, Ghajar J. Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. Journal of Neurosurgery 2012;117(4):729‐34. [DOI] [PubMed] [Google Scholar]
Idriz 2007 {published data only}
- Idris Z, Ghani RI, Musa KI, Ibrahim MI, Abdullah M, Nyi NN, et al. Prognostic study of using different monitoring modalities in treating severe traumatic brain injury. Asian Journal of Surgery 2007;30(3):200‐8. [DOI] [PubMed] [Google Scholar]
Jacobs 1998 {published data only}
- Jacobs DG, Westerband A. Antibiotic prophylaxis for intracranial pressure monitors. Journal of the National Medical Association 1998;90(7):417‐23. [PMC free article] [PubMed] [Google Scholar]
Kostic 2011 {published data only}
- Kostic A. Re: Your paper Prognostic significance of intracranial pressure monitoring and intracranial hypertension in severe brain trauma patients [personal communication]. Email to: R Forsyth 13 March 2013. [PubMed]
- Kostic A, Stefanovic I, Novak V, Veselinovic D, Ivanov G, Veselinovic A. Prognostic significance of intracranial pressure monitoring and intracranial hypertension in severe brain trauma patients. Medicinski Pregled 2011;64(9‐10):461‐5. [PubMed] [Google Scholar]
Lane 2000 {published data only}
- Lane PL, Skoretz TG, Doig G, Girotti MJ. Intracranial pressure monitoring and outcomes after traumatic brain injury. Canadian Journal of Surgery 2000;43(6):442‐8. [PMC free article] [PubMed] [Google Scholar]
Robertson 1999 {published data only}
- Robertson CS, Valadka AB, Hannay HJ, Contant CF, Gopinath SP, Cormio M, et al. Prevention of secondary ischemic insults after severe head injury. Critical Care Medicine 1999;27(10):2086‐95. [DOI] [PubMed] [Google Scholar]
Sarnaik 1989 {published data only}
- Sarnaik A, Kopec J, Moylan P, Alvarez S, Candy A. Role of aggressive intracranial pressure control in management of pediatric craniocerebral gunshot wounds. Journal of Trauma 1989;29(10):1434‐7. [DOI] [PubMed] [Google Scholar]
Saul 1982 {published data only}
- Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. Journal of Neurosurgery 1982;56(4):498‐503. [DOI] [PubMed] [Google Scholar]
Shafi 2008 {published data only}
- Shafi S, Diaz‐Arrastia R, Madden C, Gentilello L. Intracranial pressure monitoring in brain‐injured patients is associated with worsening of survival. Journal of Trauma 2008;64(2):335‐40. [DOI] [PubMed] [Google Scholar]
Signorini 1999 {published data only}
- Signorini DF, Andrews PJ, Jones PA, Wardlaw JM, Miller JD. Adding insult to injury: the prognostic value of early secondary insults for survival after traumatic brain injury. Journal of Neurology, Neurosurgery, and Psychiatry 1999;66(1):26‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1986 {published data only}
- Smith HP, Kelly DL Jr, McWhorter JM, Armstrong D, Johnson R, Transou C, et al. Comparison of mannitol regimens in patients with severe head injury undergoing intracranial monitoring. Journal of Neurosurgery 1986;65(6):820‐4. [DOI] [PubMed] [Google Scholar]
Zanier 2007 {published data only}
- Zanier ER, Ortolano F, Ghisoni L, Colombo A, Losappio S, Stocchetti N. Intracranial pressure monitoring in intensive care: clinical advantages of computerized system over manual recording. Critical Care 2007;11(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Becker 1977
- Becker DP, Miller JD, Ward JD, Greenberg RP, Young HF, Sakalas R. The outcome from severe head injury with early diagnosis and intensive management. Journal of Neurosurgery 1977;47(4):491‐502. [DOI] [PubMed] [Google Scholar]
Becker 1989
- Becker DP. Common themes in head injury. In: Becker DP, Gudeman SK editor(s). Textbook of Head Injury. Philadelphia: WB Saunders, 1989:1‐22. [Google Scholar]
BTF 2007
- Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma, Critical Care. AANS/CNS, Bratton SL, et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. Journal of Neurotrauma 2007;24(Suppl. 1):S37‐44. [DOI] [PubMed] [Google Scholar]
Chambers 1990
- Chambers IR, Mendelow AD, Sinar EJ, Modha P. A clinical evaluation of the Camino subdural screw and ventricular monitoring kits. Neurosurgery 1990;26(3):421‐3. [DOI] [PubMed] [Google Scholar]
Chesnut 2013
- Chestnut RM. Intracranial pressure monitoring: headstone or a new head start. Intensive Care Medicine 2013;39(4):771‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chesnut 2014
- Chesnut R, Videtta W, Vespa P, Le Roux P for the Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. Intracranial pressure monitoring: fundamental considerations and rationale for monitoring. Neurocritical Care 2014;21(Suppl 2):S64‐84. [DOI: 10.1007/s12028-014-0048-y] [DOI] [PubMed] [Google Scholar]
Clifton 2001
- Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, et al. Lack of effect of induction of hypothermia after acute brain injury. New England Journal of Medicine 2001;344(8):556‐63. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; Vol. 315, issue 7109:629‐34. [DOI] [PMC free article] [PubMed]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jennett 1975
- Jennett B, Bond M. Assessment of outcome after severe brain damage. The Lancet 1975;1(7905):480–4. [DOI] [PubMed] [Google Scholar]
Lavinio 2011
- Lavinio A, Menon DK. Intracranial pressure: why we monitor it, how to monitor it, what to do with the number and whatʼs the future?. Current Opinion in Anaesthesiology 2011;24(2):117–23. [DOI] [PubMed] [Google Scholar]
Le Roux 2014
- Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy G, et al. The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: evidentiary tables: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocritical Care 2014;21(Suppl 2):S297‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Marshall 1979
- Marshall LF, Smith RW, Shapiro HM. The outcome with aggressive treatment in severe head injuries. Part I: The significance of intracranial pressure monitoring. Journal of Neurosurgery 1979;50(1):20‐5. [DOI] [PubMed] [Google Scholar]
Marshall 1991
- Marshall LF, Bowers‐Marshall S, Klauber MR, Berkum Clark M, Eisenberg HM, Jane JA, et al. A new classification of head injury based on computerized tomography. Journal of Neurosurgery 1991;75(Suppl):S14‐S20. [Google Scholar]
Mayall 1984
- Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy‐related infections. A prospective epidemiologic study. New England Journal of Medicine 1984;310(9):553‐9. [DOI] [PubMed] [Google Scholar]
Miller 1981
- Miller JD, Butterworth JF, Gudeman SK, Faulkner JE, Choi SC, Selhorst JB, et al. Further experience in the management of severe head injury. Journal of Neurosurgery 1981;54(3):289‐9. [DOI] [PubMed] [Google Scholar]
Morris 2006
- Morris KP, Forsyth RJ, Parslow RC, Tasker RC, Hawley CA. Intracranial pressure complicating severe traumatic brain injury in children: monitoring and management. Intensive Care Medicine 2006;32(10):1606‐12. [DOI] [PubMed] [Google Scholar]
Narayan 1982
- Narayan RK, Kishore PRS, Becker DP, Ward JD, Enas GG, Greenberg RP. Intracranial pressure: to monitor or not to monitor?. Journal of Neurosurgery 1982;56:650‐9. [DOI] [PubMed] [Google Scholar]
Paramore 1994
- Paramore CG, Turner DA. Relative risks of ventriculostomy infection and morbidity. Acta Neurochirurgica 1994;127(1‐2):79‐84. [DOI] [PubMed] [Google Scholar]
Reinert 1999
- Reinert MM, Bullock R. Clinical trials in head injury. Neurological Research 1999;21(4):330‐8. [DOI] [PubMed] [Google Scholar]
Rosner 1990
- Rosner MJ, Daughton S. Cerebral perfusion pressure management in head injury. Journal of Trauma 1990;30(8):933‐41. [DOI] [PubMed] [Google Scholar]
Saatman 2008
- Saatman KE, Duhaime AC, Bullock R, Maas AIR, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. Journal of Neurotrauma 2008;25(7):719‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stocchetti 2014
- Stocchetti N, Picetti E, Berardino M, Buki A, Chesnut RM, Fountas KN, et al. Clinical applications of intracranial pressure monitoring in traumatic brain injury: report of the Milan consensus conference. Acta Neurochirurgica 2014;156(8):1615‐22. [DOI] [PubMed] [Google Scholar]
Su 2014
- Su SH, Wang F, Hai J, Liu NT, Yu F, Zhu YH. The effects of intracranial pressure monitoring in patients with traumatic brain injury. PLOS One 2014;9(2):e87432. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Forsyth 2000
- Forsyth R, Baxter P. Routine intracranial pressure monitoring in acute coma. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002043] [DOI] [PubMed] [Google Scholar]
Forsyth 2001
- Forsyth R, Baxter P, Elliott T. Routine intracranial pressure monitoring in acute coma. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD002043] [DOI] [PubMed] [Google Scholar]
Forsyth 2003
- Forsyth RJ, Baxter P, Elliott T. Routine intracranial pressure monitoring in acute coma. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD002043] [DOI] [PubMed] [Google Scholar]
Forsyth 2006
- Forsyth RJ, Rodriguez B. Routine intracranial pressure monitoring in acute coma. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD002043] [DOI] [Google Scholar]
Forsyth 2010
- Forsyth RJ, Wolny S, Rodrigues B. Routine intracranial pressure monitoring in acute coma. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD002043.pub2] [DOI] [PubMed] [Google Scholar]


