Abstract
The actual role of SARS‐CoV‐2 in brain damage remains controversial due to lack of matched controls. We aim to highlight to what extent is neuropathology determined by SARS‐CoV‐2 or by pre‐existing conditions. Findings of 9 Coronavirus disease 2019 (COVID‐19) cases and 6 matched non‐COVID controls (mean age 79 y/o) were compared. Brains were analyzed through immunohistochemistry to detect SARS‐CoV‐2, lymphocytes, astrocytes, endothelium, and microglia. A semi‐quantitative scoring was applied to grade microglial activation. Thal‐Braak stages and the presence of small vessel disease were determined in all cases. COVID‐19 cases had a relatively short clinical course (0–32 days; mean: 10 days), and did not undergo mechanical ventilation. Five patients with neurocognitive disorder had delirium. All COVID‐19 cases showed non‐SARS‐CoV‐2‐specific changes including hypoxic‐agonal alterations, and a variable degree of neurodegeneration and/or pre‐existent SVD. The neuroinflammatory picture was dominated by ameboid CD68 positive microglia, while only scant lymphocytic presence and very few traces of SARS‐CoV‐2 were detected. Microglial activation in the brainstem was significantly greater in COVID‐19 cases (p = 0.046). Instead, microglial hyperactivation in the frontal cortex and hippocampus was clearly associated to AD pathology (p = 0.001), regardless of the SARS‐CoV‐2 infection. In COVID‐19 cases complicated by delirium (all with neurocognitive disorders), there was a significant enhancement of microglia in the hippocampus (p = 0.048). Although higher in cases with both Alzheimer's pathology and COVID‐19, cortical neuroinflammation is not related to COVID‐19 per se but mostly to pre‐existing neurodegeneration. COVID‐19 brains seem to manifest a boosting of innate immunity with microglial reinforcement, and adaptive immunity suppression with low number of brain lymphocytes probably related to systemic lymphopenia. Thus, no neuropathological evidence of SARS‐CoV‐2‐specific encephalitis is detectable. The microglial hyperactivation in the brainstem, and in the hippocampus of COVID‐19 patients with delirium, appears as a specific topographical phenomenon, and probably represents the neuropathological basis of the “COVID‐19 encephalopathic syndrome” in the elderly.
Keywords: COVID‐19, dementia, elderly, microglia, neurocognitive disorders, neuropathology
The neuropathological alterations strictly attributable to SARS‐CoV‐2 infection are overall modest, predominantly caused by innate immunity activation, without evidence of SARS‐CoV‐2‐specific encephalitis. All COVID‐19 cases presented remarkable activation of ameboid CD68‐positive microglia, forming perivascular infiltrates and parenchymal nodules, mostly in the brainstem. Although higher in cases with both Alzheimer's pathology and COVID‐19, cortical neuroinflammation is not related to COVID‐19 per se but mostly to pre‐existing neurodegeneration. Cases with dementia appear more prone to a strong microglial response causing delirium.
1. INTRODUCTION
SARS‐CoV‐2 infection continues to be a global threat, especially in people over the age of 65. In elderly subjects affected by dementia, the Coronavirus disease 2019 (COVID‐19) mortality is increased approximately three‐fold (1). In addition to pneumonia, the virus may induce an uncontrolled cytokine storm (mostly involving IL6, IL1β, TNF), causing a wide range of symptoms, including encephalopathy (2, 3). Mild symptoms, such as dizziness, lethargy, and psychomotor retardation may be part of the “sickness behaviour”, a nonspecific cytokine‐induced syndrome present in several infectious‐inflammatory states due to the activation of innate immunity (4, 5). On the other hand, severe manifestations including confusion, agitation, delirium and seizures may be caused by specific underlying encephalitis. When focusing on patients with dementia, delirium was found to frequently develop during the clinical course of COVID‐19, but in some cases, it represented the initial manifestation of the disease and heralded a worse prognosis (6, 7). Neurological manifestations could be immune‐mediated or caused by direct viral invasion of the CNS. SARS‐CoV‐2 may enter the CNS through: (1) the trans‐synaptic route, via the cranial nerves en route to the brainstem or via the olfactory bulbs to the basal frontal lobes; or (2) the endothelial‐astrocytic route, by crossing the blood brain barrier (2, 8). Previous studies on SARS‐CoV‐1 pathology reported viral localization in some brain neurons, but the topographical distribution was not described (9). Nonetheless, there is still no clear evidence of SARS‐CoV‐2 neurotropism, and the expression of angiotensin converting enzyme‐2 (ACE‐2) and transmembrane protease serine 2 (TMPRSS2), the main factors allowing the virus to enter cells (10), is generally low in the human brain (11). Although viral RNA may be detected in about 50% of cases, immunohistochemical staining has revealed the presence of scant viral proteins limited to isolated neurons and endothelial cells in the medulla oblongata of the CNS (12, 13). Additionally, no correlation has been found between the severity of neuropathological changes and the presence of viral protein or RNA in the brain (12). However, it should be considered that the presence of the virus could be influenced by time intervals between initial infection, death, autopsy and subsequent brain processing (12, 13).
Postmortem analyses of brains from clinically heterogeneous groups of COVID‐19 patients showed a wide range of neuropathological changes of different severity. These were more pronounced in those who manifested serious neurological complications, mostly elderly patients (14, 15). The neuropathological alterations are highly influenced by the COVID‐19 clinical course (e.g., presence of critical illness, hypoxia, sepsis). Apart from brain congestion, oedema and neuronal loss attributable to hypoxic phenomena, the neuropathological picture appears inconstant, showing a variety of alterations, mainly comprising 2 types of pathologies: (1) Vascular injuries, of either ischemic or haemorrhagic type, including macroscopic and microscopic lesions caused by clotting alterations, endotheliitis, or sudden blood pressure changes in critically ill patients (12, 13, 16, 17, 18, 19, 20, 21); and (2) Inflammatory processes, including ADEM‐like features and different patterns of immune‐induced meningoencephalitis with meningeal, perivascular, or parenchymal lymphomonocytic infiltrates (12, 14, 16, 18, 20, 21, 22, 23, 24, 25). Many authors have reported both types of pathologies, and nearly all papers describing inflammatory changes have demonstrated some degree of microglial activation, often associated with microglial nodules. Immune hyperactivation involving the innate immune system inside the brain (i.e., microglia) appears to be a key factor in the pathogenesis of neurological damage, particularly in the elderly or in patients with dementia. As recently pointed out, the exact mechanisms underpinning these neuropathological changes still remain unclear, and discovering the precise role played by SARS‐CoV‐2 will most likely have an important clinical relevance (26), particularly in older people whose brain pathology can be affected by several other concomitant conditions, including vascular comorbidities and pre‐existing neurodegenerative processes causing inflammation.
The objective of this study is to comprehensively describe the neuropathological alterations present in a series of clinically well‐documented COVID‐19 cases, comprising mostly older adults. We also aim to compare the brains of COVID‐19 and matched non‐COVID cases, both with and without Alzheimer's disease (AD), to verify where and how SARS‐CoV‐2 affects the inflammatory response in the brains of older people, and determine whether the inflammatory changes are mainly due to COVID‐19 or are also related to the presence of AD neuropathology.
2. MATERIALS AND METHODS
2.1. Study design, setting and participants
Twelve consecutive autopsies performed between 17th April to 4th June 2020 were considered for this study. The inclusion criteria were: (1) confirmed SARS‐CoV‐2 infection through pharyngeal swab; (2) continuous refrigeration of the cadaver at 4℃ until the time of the autopsy with adequate preservation of the sample material for histological analysis. The exclusion criteria consisted of a postmortem delay (PMD) longer than 13 days or inadequate preservation of the cadaver. In addition, 6 non‐COVID cases, provided by ABB (Abbiategrasso Brain Bank), were selected for comparison. They share similar clinico‐pathological features with COVID‐19 cases.
Autopsy human brain samples were provided by the Department of Legal Medicine and Forensic Sciences (Institute of Legal Medicine, University of Pavia) and the ABB (Golgi‐Cenci Foundation, Milan). During the COVID‐19 pandemic, autopsies have been generally discouraged by government regulations due to the risk of further spreading the disease through the handling and dissection of corpses (27). Despite these restrictions, the present COVID‐19 cases were subjected to forensic autopsies, ordered by the State Prosecutor in the hypothesis of medical mistakes or failure to comply with hygiene regulations. The samples were taken in accordance with the Italian law regarding processing of personal data. The reference law is the authorization n9/2016 of the guarantor of privacy, then replaced by Regulation (eu) 2016/679 of the European Parliament and of the Council. The ABB autopsy and sampling protocol (28) was approved by the Ethics Committee of the University of Pavia on October 6th, 2009 (Committee report 3/2009). The autopsies were performed in a room suitable for the examination of cadavers of people who died from infectious diseases and with the use of adequate personal protective equipment.
A retrospective review of medical charts was performed by two forensic medical doctors, a geriatrician and a neurologist in order to ascertain the clinical history. The mean COVID‐19 duration was 10 days (ranging from 0 to 32 days). Two neurologists with expertise in neuropathology, blinded to the clinical history, performed the neuropathological evaluation. The patients were clinically defined for the presence or absence of comorbidities, dementia, delirium, and sepsis. The DSM‐5 criteria were used to define the mental state and identify any pre‐existing cognitive dysfunction, namely major‐neurocognitive disorder (NCD) to indicate dementia, and mild‐NCD to designate Mild Cognitive Impairment (MCI) (29). confusion and inattention, accompanied by either disorganized thinking or altered level of consciousness, as indicated by the Confusion Assessment Method (CAM) (30). Early delirium was defined as delirium occurring before the onset of COVID‐19 typical symptoms (13), whereas late delirium appeared during disease course. Sepsis was considered a severe bacterial suprainfection with at least one positive blood culture.
After the harvesting of COVID‐19 brains, a fresh frontal sample was frozen for the subsequent quantitative Reverse Transcription‐PCR (qRT‐PCR) and droplet‐digital PCR (ddPCR) analysis for the detection of the viral RNA, while the rest of the brain was fixed in formalin for 15–30 days. Successively, the cerebrum and cerebellum were cut to produce coronal and sagittal sections respectively, and the brainstem was divided axially. The resulting 10 mm slices were then processed for paraffin inclusion. The processing of ABB cases followed a protocol previously described (28).
2.2. Quantitative PCR and Droplet Digital PCR analysis of SARS‐CoV‐2
Frozen slices were used for isolation of total RNA by Trizol reagent (Life Science Technologies, Monza, Italy) according to the manufacturer's instructions. TaqMan probes for SARS‐CoV‐2 target sequences N1 were used for the detection of viral RNA while RNase P (RP) was used as internal reference control. Primers use have been indicated by US Centers for Disease Control and Prevention [CDC 2019‐Novel Coronavirus (2019‐nCoV) Real‐Time RT‐PCR Diagnostic Panel]. The reaction mix used was composed by 5 µl of extracted RNA, 5 µl of Reliance One‐Step RT‐qPCR Supermix (BioRad, Richmond, CA), 1 µl of RT enzyme and 4 µl of water have been used for qPCR in CFX96 (BioRad, Richmond, CA). qPCR analysis has been considered valid in all samples in which RP gene has been detected. Positive samples were determinate by Cycle threshold (Ct) of N1 and N2 gene minor of 40.
Also Droplet Digital PCR (ddPCR) was used for the detection of SARS‐CoV‐2 using One‐Step RT‐ddPCR Advanced Kit for Probes (BioRad, Richmond, CA). 5 μl of Supermix, 2 μl of Reverse transcriptase, 1 μl of 300 mM DTT, 1 μl of Probe (1:40), 1 μl of both Forward and Reverse primers (1:10) and 4 μl of H2O were provided and 5 μl of starting RNA was added for each 20 μl reaction. This reaction has been used for droplet generation using QX200 droplet generator (BioRad, Richmond, CA). After PCR of targets presented in the droplets, the QX200 droplet reader (BioRad, Richmond, CA) was used to analyze each droplet individually, counting positive and negative droplets to establish absolute quantification of samples (concentration) and which was later analyzed using QuantaSoft 1.6 software (Bio‐rad, Richmond, CA). The multi‐well threshold tool was used in all the wells according to results of specificity assay in negative samples to discriminate between positive and negative droplets. The software automatically reported the copy number of each sample.
The oligonucleotides and probes used in RT‐ddPCR were the same used in RT‐qPCR.
2.3. Histology and immunohistochemistry (IHC)
All autopsies were conducted in the same manner. A total of 11 samples were collected from each sujects: 2 from the frontal lobe (1 from the anterior frontal area, including the rhinencephalon; and 1 from the posterior area, containing the gyrus cynguli and basal ganglia), 1 each from the remaining lobes (parietal, temporal and occipital), and 1 sample each from the olfactory bulbs, hippocampus and entorhinal cortex, cerebellum, midbrain, pons and medulla oblongata. Eight‐μm thick paraffin‐embedded sections from each brain areas were collected and stained with Hematoxylin and Eosin (H&E) and Luxol Fast Blue (LFB) to evaluate vascular, architectural, structural tissue abnormalities and myelin loss. To obtain a definite AD diagnosis, selected areas were stained for with AD neurodegenerative markers. To detect and grade the extent of β‐amyloid deposits, 4G8 antibody was used as indicated by Thal (31). To define the stage of phospho‐TAU aggregates, AT8 antibody was used as indicated by Braak (32). Successively, the severity of AD neuropathology was defined according to Montine's scheme (low‐intermediate‐high AD pathology) (33). To detect immunological activation and SARS‐CoV‐2 presence inside the brain, specific antibodies were utilized: CD68, CD3, CD20, and SARS‐CoV‐2 nucleocapsid (clone B46F) respectively for microglia, T&B lymphocytes, and the virus, respectively. For a comparison with previous works, the evaluation of lymphocytes was carried out through a semiquantitative system similar to that used by Matschke (none/4.7mm2; rare: 0–9; moderate: 10–49; abundant >49) (12). The highest grading observed was then reported. None or rare lymphocytes were considered as a condition of normal immunological surveillance.
Vascular and astrocytic markers (CD34 and GFAP) were also tested (see Table S1).
2.4. Semi‐quantitative analysis of microglial activation
A validated grading system for microglial activation in COVID‐19 is yet to be produced. Thus, based on previous works on microglia in relation to other diseases (HIV infection, AD, multiple sclerosis) (34, 35, 36), we coined a system that examined wide areas of tissue and was simple and easily reproducible. Microglial activation was evaluated through anti‐CD68 antibody using an optical analysis by two‐dimensional counting technique. Five different brain regions were considered: midbrain, pons, medulla oblongata, hippocampus, and frontal lobe (divided into cortex and white matter). Regarding the hippocampus, frontal cortical gray matter (FCGM), and frontal white matter (FWM), three representative areas of 4.7 mm2 were evaluated across each slide: upper left corner, center, and lower right corner. For the midbrain, pons and medulla, additional two fields were considered: upper right and lower left, thus capturing all four corners of each slide plus the center area. A low magnification (4x) was used to explore the area and higher magnifications (10‐20x) to judge the cell morphology and microglial activation status. In order to evaluate the extent of microglial activation, we did not consider only the number of microglial cells positive for CD68 immunoreactivity but also their amoeboid morphology, the presence of perivascular infiltrates and parenchymal clusters, namely, microglial nodules with 3 or more cells (37). This prompted us to use a manual scoring technique rather than automated quantification of the total antigen load, which would also include non‐amoeboid microglial cells. The degree of microglial activation was graded semi‐quantitatively using a 4‐point scale (0–3): 0 = absence of both perivascular infiltrate and microglial nodules and <20 amoeboid cells/reactive microglial cells; 1 = presence of at least one perivascular infiltrate or 1 micronodule or >20 amoeboid cells/reactive microglial cells; 2 = presence of 2–4 microglial nodules; and 3 = presence of >4 microglial nodules. Two different neuropathologists, blind to the clinical picture and each other's evalutions, separately and manually evaluated the inflammatory lesions (amoeboid cells, perivascular infiltrates and nodules) in order to determine the global semi‐quantitative scoring. Whenever discrepancies between the counts totaled by the two operators existed, the area in question was reassessed together until an agreement was reached. The scores obtained in each area were averaged, thus providing a single final value for each section, which was used for statistical analysis of the data.
2.5. Statistical analysis
COVID‐19 cases (with and without dementia) were compared with the control non‐COVID group (also with and without dementia). Moreover, COVID‐19 cases were grouped according to the presence or absence of delirium, and sepsis. Regarding delirium, COVID‐19 cases were also categorized based on its onset (early, late, and no delirium). Differences in microglial activation (i.e. microglial grading) between groups were compared using Mann‐Whitney U test or Kruskall‐Wallis, where appropriate. The Mood's Median Test was used to compare microglial activation between COVID‐19 cases with and without delirium, and with and without sepsis; this choice was made because the variables, in addition to having a skewed distribution, belong to a very low number of cases. The correlation between microglial activation and AD pathology (Braak and Thal stages) was assessed using Spearman's Rho correlation. p‐values <0.05 were considered significant. All statistical analyses were conducted using SPSS version 26.
3. RESULTS
3.1. General and clinical data
Nine (4 females, 5 males) of the 12 COVID‐19 subjects who underwent autopsy were included, 3 were excluded for excessive post‐mortem delay (PMD) or inadequate conservation of the cadaver. Death occurred 0 to 32 days after the onset of symptoms (mean: 10 days). The mean PMD in COVID‐19 cases was 7 days (range: 3–13). The mean PMD in ABB cases was 10.5 hours (range: 3–16).
The mean age of all 15 cases (9 COVID‐19 cases and 6 non‐COVID ABB matched controls) was 79 (range: 29–94); their clinical features are summarized in Table 1. Except for one case (Cov2), all COVID‐19 and ABB subjects had several comorbidities of varying severity, including pulmonary diseases, hypertension, diabetes, obesity and cancer. None of them had severe heart disease. Of the 9 COVID‐19 cases, 4 had a history of major‐NCD (dementia), 2 of mild‐NCD, and 3 without any NCD; 5 had a clinical course complicated by hyperactive or hypoactive delirium (3 had delirium as first COVID‐19 symptom, 2 during disease course; all had NCD, 3 major and 2 mild). All COVID‐19 cases developed severe lymphopenia and typical symptoms (fever‐cough‐dyspnea), except for Cov2, who was asymptomatic and died from haemorrhagic shock due to accident. Three had sepsis before death and Cov4 was treated in an intensive care unit; however, none underwent orotracheal intubation. Of the 6 ABB non‐COVID cases, 3 had a history of dementia (2 of them with previous episodes of hyperactive delirium), 1 had mild‐NCD, and 2 did not have any NCD. These subjects died of either of the following: heart failure, cachexia due to terminal dementia, or cancer.
TABLE 1.
Overall clinical and neuropathological characteristics
| Code | PMD (days/hours) | Demographics and clinical features | Neuropathological changes (immunohistochemistry) | SARS‐COV‐2 PCR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (y/o) | Anamnesis; cause of death | NCD | DEL | SEP | NP diagnosis | B | Ts | Microglia (CD68) | Lymphocytes (CD3‐CD20) | SARS‐COV‐2 | Vessels (CD34) | Gliosis (GFAP) | RT‐PCR | dd‐PCR | ||
| COV2 | 7 d | M | 29 | NR; hemorrhagic shock due to accident | No | No | No | No AD, no SVD | 0 | 0 | F‐HC:++ BS:+++ | Rare perivascular | Negative | Moderate tufts in BS | Mild, RA | n/a | n/a |
| COV4 | 5 d | M | 67 | Obesity, HTN, CVD; CIP, respiratory failure and sepsis | No | No | Yes | No AD, SVD | 0 | 0 | F‐HC:+ BS:+++ | Rare perivascular | Negative | Normal | Mild | − | + |
| COV6 | 11 d | F | 90 | HTN, COPD; respiratory failure | No | No | No | Low AD | 2 | 2 | F‐HC:+ BS:++ | Rare perivascular | Negative | Moderate tufts in BS | Mild | − | + |
| COV3 | 7 d | M | 87 | T2D, CVD; respiratory failure | Mild (VCI) | Hyper/Hypo, (early onset) | No | Low AD, SVD | 2 | 1 | F‐HC:++ BS:++ | Rare perivascular | Very rare cells in BS | Normal | Mild | − | + |
| COV10 | 7 d | M | 81 | AF, paraparesis (previous GBS); respiratory failure and sepsis | Mild (VCI) | Hypo, (late onset) | Yes | No AD, SVD | 1 | 0 | F‐HC:+ BS:++ | Rare perivascular | Negative | Rare tufts in BS | Perivascular | − | + |
| COV1 | 7 d | F | 74 | NR; respiratory failure | Major (AD) | Hyper, (early onset) | No | High AD | 6 | 4 | F‐HC:+++ BS:++ | None | Negative | Abundant tufts in BS | Perivascular | − | − |
| COV5 | 3 d | F | 94 | T2D, HTN, CVD, AF; sepsis | Major (AD + VaD) | Hypo, (early onset) | Yes | Intermediate AD, SVD | 4 | 3 | F‐HC:++ BS:++ | Rare perivascular | Negative | Moderate tufts in BS | Mild, RA | − | + |
| COV8 | 13 d | F | 83 | HTN; respiratory failure | Major (AD) | No | No | Intermediate AD | 4 | 3 | F‐ HC:+ BS:++ | None | Negative | Normal | Perivascular, RA | − | + |
| COV9 | 6 d | M | 92 | HTN, Cerebrovascular disease; respiratory failure | Major (AD + VaD) | Hyper/Hypo, (late onset) | No | Intermediate AD, SVD | 5 | 3 | F:++ HC:+++ BS:+++ | Rare perivascular | Negative | Abundant tufts in BS | Perivascular, RA | − | + |
| BB71 | 6 h | F | 79 | CVD; cardiogenic shock | No | No | No | Low AD | 2 | 1 | F‐HC:+ BS:++ | Rare perivascular | n/a | Rare tufts in BS | None | n/a | n/a |
| BB118 | 3 h | M | 79 | T2D; liver cancer | No | No | No | No AD | 1 | 1 | F‐HC:+ BS:+ | None | Negative control | Moderate tufts in BS | None | − | − |
| BB102 | 8 h | M | 79 | HTN, CVD, cerebrovascular disease; cachexia | Mild (VCI), hemiparesis | No | No | No AD, stroke and SVD | 0 | 1 | F‐HC:+ BS:+ | Rare parenchymal | n/a | Normal | None | n/a | n/a |
| BB137 | 16 h | F | 83 | HTN, CVD, cerebrovascular disease; CHF | Major (AD + VaD) | No | No | Intermediate AD, stroke | 6 | 3 | HC:+ F‐BS:++ | Rare perivascular | n/a | Rare tufts in BS | Severe | n/a | n/a |
| BB138 | 15 h | F | 85 | CVD, cerebrovascular disease; CHF | Major (AD + VaD) | Hyper, (prev. ep) | No | High AD, SVD | 5 | 5 | F‐HC:++ BS:++ | Moderate perivascular | n/a | Rare tufts in BS | Severe | − | − |
| BB210 | 15 h | F | 89 | HTN, COPD, cerebrovascular disease; cachexia | Major (AD) | Hyper, (prev. ep) | No | High AD | 5 | 5 | F:+++ HC‐BS:++ | Rare parenchymal | n/a | Moderate tufts in BS | Severe | n/a | n/a |
COVID‐19 cases are labeled as ‘COV’ and control cases are identified as ‘BB’ (for Brain Bank); PMD is measured in days (d) or hours (h).
Abbreviations: AD, Alzheimer's disease; AF, atrial fibrillation; B, Braak stage; BS, brainstem; COV, COVID‐19 (+); CHF, chronic heart failure; CIP, critical illness polyneuropathy; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; dd‐PCR, droplet digital polymerase chain reaction; DEL, delirium; F, frontal lobe; GBS, Guillain Barre Syndrome; GFAP, Glial fibrillary acidic protein; HC, hippocampus; HTN, hypertension; n/a, not available; NCD, neurocognitive disorder; NP, neuropathological; NR, nothing relevant in medical history; PMD, post mortem delay; prev. ep., previous episode; RA, reactive astrocytes; RT‐PCR, real‐time transcription‐polymerase chain reaction; SEP, sepsis; SVD, small vessel disease (moderate to severe); Ts, Thal stage; T2D, type 2 diabetes; T, temporal lobe; VaD, vascular dementia; VCI, vascular cognitive impairment.
3.2. Macroscopic and microscopic findings
Neuropathological features are summarized in Table 1, and divided in COVID‐19 and non‐COVID cases stratified according to the cognitive status.
3.2.1. COVID‐19 cases (n = 9)
All COVID‐19 brains did not show gross abnormalities, thrombosis of the large vessels or infarcts. Brain atrophy and enlarged perivascular spaces were observed in the 4 cases with dementia. Some of the most representative microscopic details of the COVID‐19 cases are displayed in Figure 1. In all cases, microscopic examination showed diffuse moderate to severe brain edema (Figure 1A) commonly seen as unspecific hypoxic and agonal change. H&E staining indicated loss of neurons in the cerebral cortex and hippocampus, and reveals inflammatory infiltrates (sporadically in the meninges and consistently in the brain tissue) forming perivascular cuffing (Figure 1B) and parenchymal nodules which are also identified by the CD68 microglial marker, particularly in the brainstem, cortex and hippocampus but also in basal ganglia (Figure 1C) and subependymal zone, (Figure 1D,E). In five out of nine cases, small vessel disease (SVD) was observed, including enlarged perivascular spaces, arteriolosclerosis, hemosiderin leakage and microbleeds in the cortex and basal ganglia. In addition, myelin loss in subcortical and deep white matter was detected by LFB (Figure 1F). Different degrees of AD pathology were noted in 6 cases, and a definite AD diagnosis was made in 4. The distribution of neuritic plaques in the cortex and hippocampus was comparable to that of microglial nodules. In 7 cases, rare perivascular T&B lymphocytes and sporadic B‐lymphocytes in some inflammatory nodules were found (Figure 1G–I), with no particular differences between AD and non‐AD cases. None of the COVID‐19 cases showed clear IHC positivity for SARS‐CoV2, except for Cov3, which exhibited very few SARS‐CoV‐2‐positive cells with neuronal morphology in the lower brainstem (Figure 1J,K). The microvasculature was characterized by a continuous and intense perivascular profile with no relevant alterations in the vascular endothelium. Unexpectedly, frequent foci of tuft‐like capillary proliferation were present in the brainstem of 6 COVID‐19 cases (Figure 1L,M; anti‐CD34): in two cases tufts were abundant (COV1,9: tufts in at least 3 of the 5 areas examined) while in others cases they were moderate (COV2,5,6: tufts in 2 areas) or rare (COV10: scant tufts in 1 area) (Table 1). These capillary tufts were observed in areas containing neuronal aggregates rather than fibers. GFAP immunoreactivity revealed astrogliosis associated with reactive astrocytes, especially in subjects with dementia (Figure 1N,N′). Enhanced labelling around blood vessels (enhanced astrocytic endfeet) was a peculiar feature in some COVID‐19 cases (Figure 1O).
FIGURE 1.
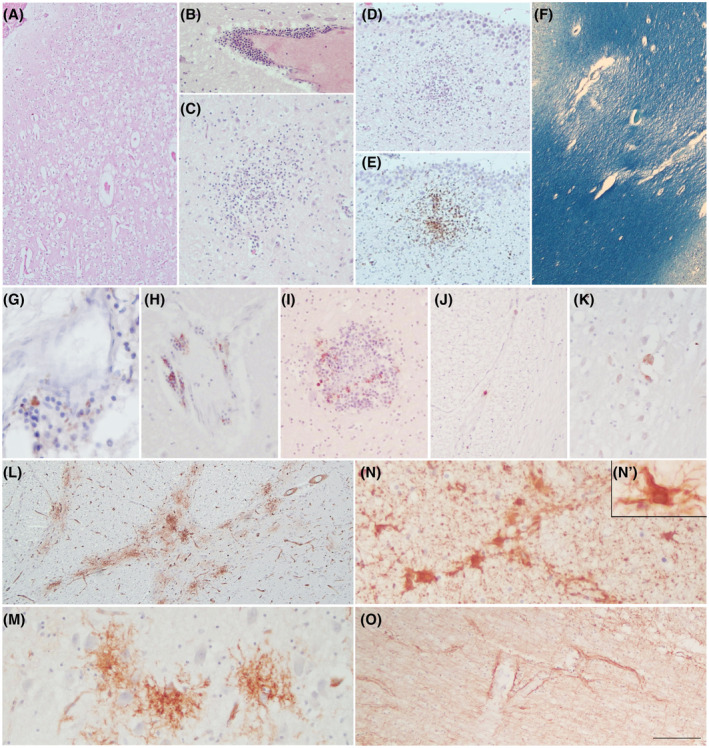
COVID‐19 neuropathology. H&E reveals diffuse cortical oedema (A), inflammatory perivascular infiltrates (B), and micro nodules in the basal ganglia (C) and subependymal zone (D), which are also identified with CD68 marker (E). LFB shows myelin loss in the subcortical WM due to SVD (F). Rare foci of perivascular T‐lymphocytes (G) and B‐lymphocytes (H) are identified using anti‐CD3 and anti‐CD20 antibodies, respectively. B‐lymphocytes are also observed within some inflammatory nodules (I). Rare SARS‐Cov‐2 positive cells are detected only in the lower brainstem of the one case (Cov3) (J, K).CD34 staining displays foci of abnormal tuft‐like capillary features, particularly frequent in the pons (L, M). GFAP reveals mild to moderate gliosis with frequent reactive astrocytes (N, N’). GFAP enhancement in astrocytic end feet around blood vessels is a peculiar picture (O). Scale bars: 512 μm (F); 288 μm (A, L); 180 μm (D, E); 106 μm (B, J, O); 76 μm (C, I); 47 μm (H, K, M, N); 31 μm (G); 17 μm (N’)
3.2.2. Control non‐COVID ABB cases (n = 6)
Non‐COVID cases with dementia showed diffuse brain atrophy at gross examination. Moreover, both BB102 and BB137 cases showed brain infarcts in the territories of the right middle cerebral artery and left posterior cerebral artery, respectively. SVD was present in 2 cases. Different degrees of AD pathology were noted in 4 cases, with a definite AD diagnosis in 3. Diffuse activation of microglia and microglial nodules were also observed in the non‐COVID ABB cases affected by AD; the distribution of parenchymal microglial nodules was similar to that of neuritic plaques. In 5 cases, rare perivascular T&B lymphocytes and sporadic parenchymal T lymphocytes were found. The 3 AD cases showed a higher number of lymphocytes. This phenomenon was not particularly prominent apart from one case (BB138), wherein a moderate presence of T lymphocytes was observed, especially in the hippocampus and mainly in perivascular zones. Normal vascular endothelium was identified using CD34 antibody. Although to a lesser extent in comparison with COVID‐19 cases, tuft‐like capillariy structures were also present in the pons of 5 out of the 6 non‐COVID cases (rare to moderate tuft‐like CD34 features; Table 1). Widespread astrogliosis was seen in all 3 AD cases.
3.2.3. Specific microglial features
Pictures of microglial activation are presented in Figure 2. The first row demonstrates the patterns of cortical and hippocampal microglial activation in subjects with and without COVID‐19, and with and without AD. Although a mild microglial reinforcement is detectable in AD cases who suffered from COVID‐19, AD cases with and without COVID‐19 are quite similar. In both, microglial nodules showed a topographical distribution comparable to that of amyloid and neuritic plaques (Figure 2A–C,E–G). Instead, the picture of non‐AD/non‐COVID cases is quite different with a normal microglial representation (Figure 2D,H). The second row displays the features of CD68‐positive cells (activated ameboid microglia) in COVID‐19 cases, forming perivascular infiltrates and parenchymal inflammatory nodules. They were particularly prominent in the olfactory bulbs, frontal cortex and hippocampus (Figure 2I–M), and even more prominent in the brainstem, where some macrophages engulfed neurons (neuronophagia) (Figure 2N–S).
FIGURE 2.
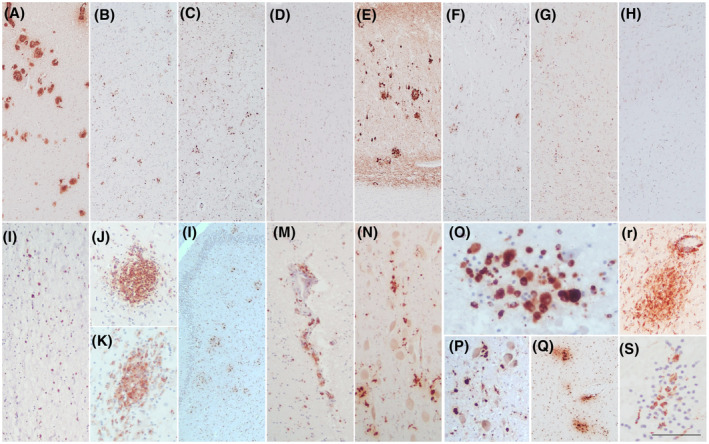
Microglial activation. The first row compares the neuropathological findings present in the frontal cortex (A–D) and the subiculum (E–H) of 2 AD cases (one AD/COVID‐19 and one AD/non‐COVID) and a non‐AD/non‐COVID case. The AD/COVID‐19 case shows amyloid plaques (A, E), as well as moderate to severe microglial activation and nodules (CD68+) in a topographical distribution similar to that of the plaques (B, F). This microglial activation does not appear different from that seen in the AD/non‐COVID case (C, G), while the same areas in the non‐AD/non‐COVID case are clearly diverse, with a normal microglial representation (D, H). The second row shows different pictures of activated microglia (CD68+) in COVID‐19 cases, including amoeboid cells, perivascular infiltrates and nodules in the olfactory bulb (I), frontal cortex (J, K), hippocampus (L, M), midbrain (N: red nucleus; O: detail of a nodule with neuronophagia in the upper right sector), pons (P: locus coeruleus Q & R: raphe nuclei), and medulla oblongata (S: DMV area). Scale bars: 315 μm (A‐H, L); 137 μm (I, M, N, P, Q); 60 μm (J, K, R); 48 μm (O, S)
3.3. Semi‐quantitative analysis of microglial activation
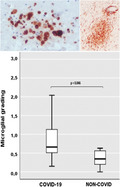
An overview of CD68 semi‐quantitative analysis revealed that, in COVID‐19 cases, the microglial activation was particularly enhanced in the brainstem, mainly in the pons. The comparison between the whole brainstem (midbrain + pons + medulla) of all 9 COVID‐19 and all 6 non‐COVID cases showed significantly greater microglial activation in COVID‐19 cases (p = 0.046). This did not happen at the level of the other hemispheric brain areas considered (frontal lobe and hippocampus) where the differences were modest and not significant (Table 2; Figure 3A,B).
TABLE 2.
Microglial grading
| CODE | Clinical features | AD pathology | Microglial activation grading | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal lobe | Hippocampus | Midbrain | Pons | MEDULLA OBLONGATA | |||||||||||||||||||||||||||
| COV | NCD | DEL | SEP | B | Ts | AD PATH | GM1 | GM2 | GM3 | WM1 | WM2 | WM3 | HC1 | HC2 | HC3 | MB1 | MB2 | MB3 | MB4 | MB5 | P1 | P2 | P3 | P4 | P5 | MO1 | MO2 | MO3 | MO4 | MO5 | |
| COV2 | + | − | − | − | 0 | 0 | No | ||||||||||||||||||||||||
| COV4 | + | − | − | + | 0 | 0 | No | ||||||||||||||||||||||||
| COV6 | + | − | − | − | 2 | 2 | Low | ||||||||||||||||||||||||
| COV3 | + | + | +* | − | 2 | 1 | Low | ||||||||||||||||||||||||
| COV10 | + | + | + | + | 1 | 0 | No | ||||||||||||||||||||||||
| COV1 | + | + | +* | − | 6 | 4 | High | ||||||||||||||||||||||||
| COV5 | + | + | + | + | 4 | 3 | Int | ||||||||||||||||||||||||
| COV8 | + | + | − | − | 4 | 3 | Int | ||||||||||||||||||||||||
| COV9 | + | + | +* | − | 5 | 3 | Int | ||||||||||||||||||||||||
| BB71 | − | − | − | n/a | 2 | 1 | Low | ||||||||||||||||||||||||
| BB118 | − | − | − | n/a | 1 | 1 | No | ||||||||||||||||||||||||
| BB102 | − | + | − | n/a | 0 | 1 | No | ||||||||||||||||||||||||
| BB137 | − | + | − | n/a | 6 | 3 | Int | ||||||||||||||||||||||||
| BB138 | − | + | (+) | n/a | 5 | 5 | High | ||||||||||||||||||||||||
| BB210 | − | + | (+) | n/a | 5 | 5 | High | ||||||||||||||||||||||||
COVID‐19 cases are labeled as ‘COV’ and control cases are identified as ‘BB’ (for Brain Bank).
Legend: + = present; +* = hyperactive delirium; (+) = previous episodes of delirium; − = absent.
Micronodule grading:  = 0/none;
= 0/none;  = 1/mild;
= 1/mild;  = 2/moderate;
= 2/moderate;  = 3/severe.
= 3/severe.
Abbreviations: AD PATH, AD pathology; AD, Alzheimer's Disease; B, Braak stage; COV, COVID‐19; DEL, delirium; GM, Gray matter; HC, hippocampus; Int, intermediate; MB, midbrain; MO, Medulla oblongata; n/a, not available; NCD, neurocognitive disorder; P, pons; SEP, sepsis; Ts, Thal stage; WM, whitematter.
FIGURE 3.
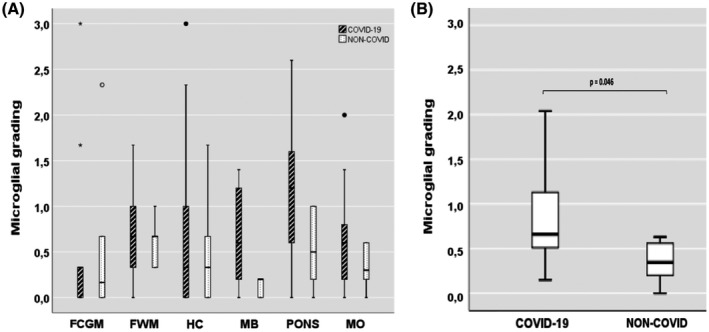
The degree of microglial activation in brain areas (A) and whole brainstem (B). (A) Microglial grading in COVID‐19 and non‐COVID cases per areas. (B) Comparison of microglial grading from the whole brainstem (midbrain+pons+medulla) between COVID‐19 and non‐COVID cases. Small circles represent out values, stars show far out values. FCGM, frontal cortical gray matter; FWM, frontal white matter; HC, hippocampus; MB, midbrain; MO, medulla oblongata
Considering the frontal cortex of the 9 COVID‐19 cases, the presence of activated microglia was significantly greater in those with AD (n = 4) compared to those without AD (n = 5) (p = 0.018; Figure 4). Likewise, considering the frontal cortex of the 6 non‐COVID cases, microglial scores were significantly higher in those with AD (n = 3) than in those without AD (n = 3) (p = 0.022; Figure 4). Moreover, subjects belonging to the non‐COVID/AD group (n = 3) had a significantly higher microglial activation than those of the COVID‐19/no AD group (n = 5) (p = 0.028; Figure 4). On the other hand, the comparison between the frontal cortical areas of AD cases with and without COVID‐19 did not reveal any significant differences (central columns in Figure 4; p = 0.979).
FIGURE 4.
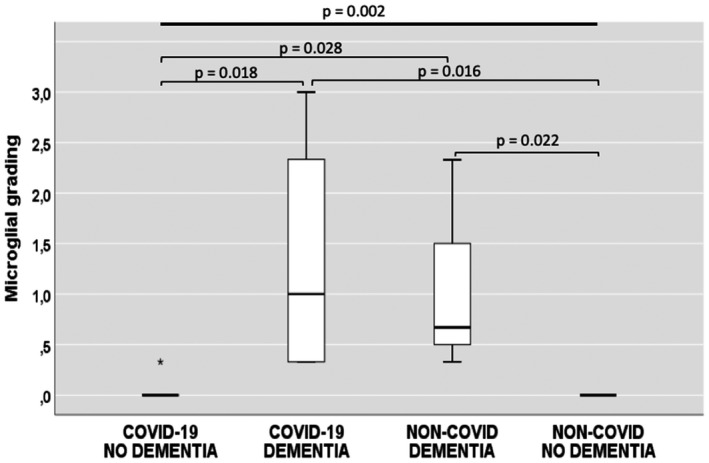
The degree of microglial activation in the frontal cortex considering the COVID‐19 and dementia groups. Boxplot showing average microglial grading in the frontal cortical gray matter of COVID‐19 and non‐COVID cases, with or without Alzheimer's Dementia (only significant p‐values are shown). Number of cases: COVID‐19/no dementia, n = 5; COVID‐19/dementia, n = 4; non‐COVID/dementia, n = 3; non‐COVID/no dementia, n = 3
Comparing the 5 COVID‐19 cases complicated by delirium (all of them had either mild‐ or major‐NCD) and the 4 COVID‐19 cases without delirium, a significantly stronger microglial activation was found in the hippocampus of cases with delirium (p = 0.048; Figure 5A). Interestingly, cases presenting with early delirium tended to exhibit more severe TAU pathology, but this trend did not reach statistical significance (p = 0.350; Figure 5B).
FIGURE 5.
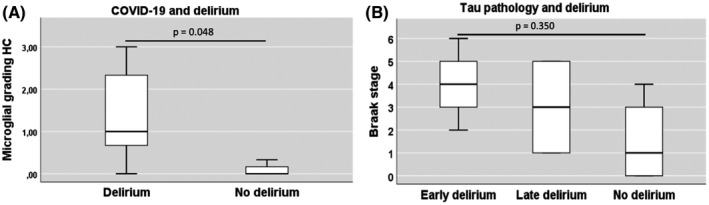
Microglial activation and Braak stage in COVID‐19 cases with delirium. (A) Boxplot showing microglial activation in the hippocampus of COVID‐19 cases comparing cases with and without delirium (delirium n = 5; no delirium n = 4). (B) Boxplot showing association between the degree of tau pathology and delirium onset in COVID‐19 cases. Early delirium n = 3, Late delirium n = 2, no delirium n = 4
No significant differences in microglial activation were found between COVID‐19 cases with and without sepsis. However, there was a trend to less microglial activation in the frontal white matter in those who had sepsis compared to those who did not (p = 0.16; data not shown).
Considering all cases, subjects with dementia showed significantly greater microglial activation in the cortex than subjects without dementia (p = 0.001), and a strong, positive monotonic correlation was found between microglial activation and both Braak (rs=0.767; p = 0.001) and Thal stages (rs=0.760; p = 0.001) (Figure 6).
FIGURE 6.
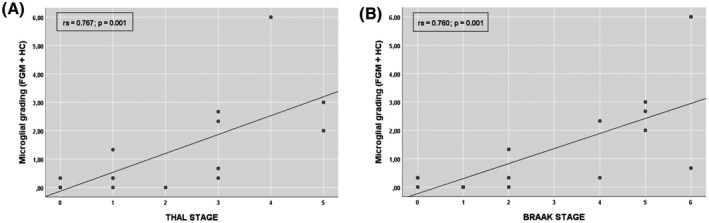
Microglial activation and Alzheimer's disease pathology. Scatter plot showing correlation between microglial activation and amyloid‐β burden (A) and tau pathology (B). FGM, frontal cortical gray matter; HC, hippocampus
3.4. SARS‐CoV‐2 PCR
None of the COVID‐19 cases showed qRT‐PCR‐positivity for SARS‐CoV‐2 RNA. Nonetheless, traces of viral RNA were detected in almost all cases (7 out of 8 cases tested), through a very sensitive technique (ddPCR) (Table 1).
3.5. General autoptic findings
COVID‐19 cases showed typical findings, including hypoxic‐ischemic damage in multiple organs and diffuse pneumonia with lung congestion or edema, frequent clots inside the vessels, and alveolar damage often associated with bacterial superinfection.
4. DISCUSSION
The main results of this study can be summarized in the following: (1) All COVID‐19 cases show remarkable non‐SARS‐CoV‐2‐specific changes including hypoxic‐agonal alterations (without fresh infarcts), and a variable degree of neurodegeneration and/or pre‐existent SVD (Figure 1); (2) The neuroinflammatory picture is dominated by the stimulated innate immune system (ameboid CD68 positive microglia), particularly prominent in the brainstem, while only scant lymphocytic presence and very few traces of SARS‐CoV‐2 have been detected (Figures 1 and 2); (3) Microglial activation in the brainstem is significantly greater in COVID‐19 cases than in non‐COVID cases (p = 0.046; Figure 3B), and all COVID‐19 cases without dementia show intense microglial activation particularly in the brainstem with a scarce cortical involvement (Table 2; Figure 4); (4) Microglial activation in the frontal cortex (Figure 4) and hippocampus is associated with NCD due to AD pathology rather than COVID‐19, although a slight microglial boosting occurs in the hippocampus (not statistically significant; Figure 2, Table 2); (5) In COVID‐19 cases complicated by delirium (all with some degree of NCD), there is a significantly higher microglial activation in the hippocampus (p = 0.048; Figure 5).
This research study has some limitations. It was conducted on a limited number of subjects, essentially elderly people, who happen to represent the most affected population and were thus deliberately chosen to be the focus of the study. Furthermore, PMD varies greatly among non‐COVID ABB controls and COVID‐19 subjects, but there was no evident impact on immunohistochemical reactions used for this study. Another limitation of this study is the lack of data regarding viral RNA data from the brainstem. Unfortunately, for safety reasons, as soon as the autopsy was performed, the entire brain was fixed, with the exception of a sample of the fronto‐basal cortex (chosen for its proximity to the olfactory epithelium). Thus, the available material from the brainstem was formalin‐fixed and paraffin‐embedded from the beginning, rendering RNA extraction really problematic. We were able to isolate some viral RNA from the paraffin‐embedded pons and run qPCR and ddPCR, but the results were not reliable due to the low quality of RNAs. The strength of the study is the availability of a detailed clinical history for each case, and the comparison between COVID‐19 and matched non‐COVID controls, to estimate to what extent does the virus or any other pre‐existing or concomitant disease contribute to the observed alterations, a question raised by some authors (12, 19, 26).
Fresh vascular lesions are described by other authors, mostly in patients with a history of atrial fibrillation, heart failure or mechanical ventilation (12, 18, 20). In contrast, our COVID‐19 cases do not show acute vascular lesions, probably, due to their clinical characteristics. Indeed, they had no pre‐existing severe heart disease, nor were they subjected to mechanical ventilation during their relatively short clinical course. This fact may suggest that acute vascular changes are not directly associated with COVID‐19 but mainly a consequence of heart complications, prolonged lung failure, and mechanical ventilation. In our series, as in Solomon's series (24), diffuse brain congestion, cortical edema, neuronal loss, and microvascular changes including microbleeds appear clearly attributable to non‐specific hypoxic phenomena or to pre‐existing SVD.
In all COVID‐19 subjects we studied, the monocyte‐microglia component clearly prevails over the lymphocyte component and there is no evidence of the activation of specific lymphocyte clones within the CNS. This picture is quite different from that of autoimmune or viral encephalitis in which frequent viral inclusions are detectable in the brain tissue and/or many lymphocytes are present (37, 38). To compare lymphocytic pattern, we have searched for lymphocytes in the 6 control non‐COVID cases and found an overall low number of them. Nonetheless, the 3 non‐COVID/AD cases present a slight increase in the number of T lymphocytes, a phenomenon that becomes particularly relevant in the BB138 case, which shows a moderate perivascular lymphocytic infiltrate in the hippocampus. These data are consistent with the findings of Zotova and Togo who reported a possible increase in T lymphocytes in AD, especially in the hippocampus (39, 40). In their COVID‐19 series, Matschke et al. reported a variable presence of T lymphocytes (from absent to mild or moderate) predominantly in the perivascular brainstem zones. Overall, the authors detected T lymphocytes more frequently than us, but it should be considered that only 5 of the 43 cases reported by Matschke had NCD (less than 12% of cases) (12), while our COVID‐19 series includes mainly elderly with NCD. Our observation of a lower number of lymphocytes in AD patients with COVID‐19 seems to confirm Rakic's work describing a diminution of T lymphocytes (brain immunosuppression) in cases suffering from both advanced AD and severe systemic infection (41). Furthermore, our COVID‐19 cases had marked lymphopenia, which in turn may have contributed to the reduced number of lymphocytes we have observed in the brain. Zotova and Togo did not find any correlation between the activation of innate immunity (microglia) and the presence of lymphocytes, and these two phenomena appear partially independent (39, 40). Precisely, COVID‐19 might induce dissociation of innate (microglia) from adaptive (lymphocytes) immunity. Indeed, apart from single peculiar cases showing specific and severe para‐infectious autoimmune pictures, such as hemorrhagic or disseminated encephalomyelitis (23, 42), non‐specific innate immunity activation (namely microglial activation) seems to be prevalent in the great majority of SARS‐CoV‐2‐induced brain alterations (12, 14, 16, 18, 21, 22). This phenomenon may be particularly pronounced in elderly patients who tend to have a worse prognosis due to immunological senescence (reduced adaptive immunity with decreased specific immune responses) and “inflammaging” (excessive inflammatory activation in aging) (43, 44, 45). At the same time, the elderly are prone to greater neurological injury, due to both cerebrovascular comorbidities and the presence of degenerative alterations recruiting inflammation and “priming” microglia. The role of inflammatory processes in the pathogenesis of dementias is well known. Specifically in AD, microglia and activated astrocytes are relevant for the disease pathogenesis and its histopathology (46, 47, 48, 49). Nonetheless, microglial cells are extremely dynamic and their topographical distribution and activation states are complex and dependent on multiple factors. This explains the different microglial patterns observed in different immunological situations. Frequently, an increase in microglial activity occurs concurrently with neurodegeneration, while in other situations a decrease is observed (40, 41).
Hence, the main question this study poses is: where and how does SARS‐CoV‐2 affect inflammatory response in the brain of older people with and without AD? A first consideration is that COVID‐19 induces significant microglial activation in the brainstem, regardless of cognitive status and age; in fact, it is also present in the Cov2 case (the only young subject in this series). Therefore, the microglial activation in brainstem seems to be a specific COVID‐19 effect (p = 0.046; Figure 3B). The particular intensity of microglial activation in such anatomical region is also confirmed by neuronophagia features, as observed by us (Figure 2O) and by other authors (16, 21). Our study combined with others authors’ study describing an important involvement of the brainstem (12, 14, 16, 21), provide evidence that the trans‐synaptic route through the cranial nerves may be the preferred point of entry of virions or viral antigens to the CNS. These viral particles then probably activate a response with a prevalent topographical distribution in this anatomic area. This interpretation is confirmed by the clear antigenic positivity for SARS‐CoV‐2 found by Matschke and colleagues in the lower cranial nerves. The clinical impact of these phenomena cannot be ascertained; however, it is possible that this inflammatory damage contributed to the lethargy, the neurovegetative alterations and the central component of respiratory failure manifested by some patients, as signs of brainstem dysfunction. On the other hand, the comparison between AD cases with and without COVID‐19 demonstrates relevant cortical microglial activation and microglial nodules in AD, regardless of the SARS‐CoV‐2 infection. The occurrence of COVID‐19 in AD cases appears to add only a slight boosting of the already present cortical microglial activation (Figures 2 and 4).
Nevertheless, microglial enhancement in the hippocampus appears to be clinically relevant in COVID‐19 patients affected by NCD. Particularly, the hippocampi of cases Cov1,3,9 who all suffered from hyperactive delirium show higher inflammatory changes, and COVID‐19 cases with delirium have a significantly higher microglial activation in the hippocampus than COVID‐19 cases without delirium (p = 0.048; Figure 5A). Moreover, the severity of TAU pathology seems to show a correlation trend with the precocity of delirium onset (Figure 5B). Such a correlation may be of some interest, even if it does not reach statistical significance due to the low number of cases. These data are consistent with Zotova's and Torvell's findings showing a positive correlation between microglial activation and TAU pathology in AD (40, 50). Also, our data confirm and reinforce what was reported in a previous interesting study describing the inflammatory brain changes of 9 elderly patients who died in close temporal association with an episode of delirium (51), just like our COVID‐19 cases. Probably for these reasons, COVID‐19 patients with NCD are more prone to the “COVID‐19 encephalopathic syndrome” with unfavorable clinical and prognostic consequences (6, 20, 52). Through a tragic “natural experiment”, SARS‐CoV‐2 is helping shed some light on the neuropathology underlying behavioral symptoms and delirium, as signs of limbic system dysfunction, which seem to be related to both degenerative load and microglial boosting in the hippocampus.
Regarding the role of sepsis, in our COVID‐19 cases there are no significant differences in microglial activation in relation to the absence or presence of severe bacterial superinfection. Nevertheless, it is interesting to note that our septic COVID‐19 cases show a clear trend towards less microglial activation in the white matter. Although not statistically significant, again due to the low number of cases (p = 0.16; Table 2), this trend is line with Rakic observation of microglial rarefaction in the white matter of advanced AD cases affected by severe bacterial infection (41). Even if the neuropathology of “COVID‐19 encephalopathic syndrome” resembles that of “septic encephalopathy”, which is also related to similar neurological manifestations including behavioral changes and delirium (22, 53, 54), we observed some important qualitative differences between the neuropathology described in septic patients (22, 53) and the one observed in our COVID‐19 cases. Indeed, microglial activation in sepsis is much more disseminated and intense in the white matter with scant nodules (53). Instead, our cases showed several microglial nodules in the cortex and in the lower brainstem, and less in the white matter. It is intriguing to hypothesize that this topography may have a specific reason. It might be caused by the degenerative burden in the cortex and by the presence of viral antigens in the lower brainstem, where traces of viral immunoreactivity was found by us and others (12, 13). From this standpoint, we chose to include in the study a young SARS‐CoV‐2 positive individual, with no known symptoms, who died in a tragic fatality upon returning from work (Cov2 case). His neuropathology shows microglial activation with a peculiar topography (moderate in the frontal WM and severe in the lower brainstem; Figure 2Q; Table 2). It is probable that, due to the absence of coexisting neurodegenerative lesions, neuroinflammation is not present in the brain cortex of this case, demonstrating that microglial reaction is case‐specific and related to the personal clinical history.
As noted by Kantonen and other authors, the microvascular changes are caused by virus‐triggered endotheliitis, hypoxia or pre‐existing SVD (18, 19, 20, 21). We do not observe a clear picture of endotheliitis. However, a number of our COVID‐19 cases (Cov1, 8, 9, 10) show perivascular gliosis with a very peculiar astrocytic endfeet enhancement, mainly at the capillary level, suggesting an inflammatory reinforcement of the blood‐brain barrier (Figure 1O; GFAP). Moreover, in several of our cases (Cov1, 2, 5, 6, 9, 10 as well as BB118, 71, 137, 138, 210), we caught another peculiar feature of the capillary endothelium attributable to focal chaotic proliferation leading to the formation of ramified tuft‐like structures. This alteration appears much more evident in the pons of COVID‐19 cases (Figure 1L,M; CD34 reaction) but, to a lesser extent, it is also common in non‐COVID cases. To the best of our knowledge, there are no similar descriptions for SARS‐CoV‐2 infection, mostly because CD34 staining for endothelium was not considered in previous studies. Instead, a very similar endothelial picture was observed in a case of encephalitis caused by the human BK polyomavirus (55), occasionally in AD brains (56), as observed by us, and in brain specimens from the temporal lobe of patients who previously underwent surgery for epilepsy (57). This CD34 picture has been described as “vascular buds” or “CD34‐positive clusters”, and, currently, we report it as “CD34‐tufts”. At the moment, the nature of these CD34‐tufts is uncertain, but we can say they do not seem to possess any neoplastic proliferative characteristics nor are they associated with ischemic type lesions. In fact, examining the same sections with HE, no particular alteration of the tissue is noted. It is not clear whether CD34 tufts can be considered a form of endotheliitis, but their topographical association with activated microglia is evident. Therefore, CD34 tufts constitute a histological phenomenon associated with inflammation, and it is not surprising to find them abundantly in association with COVID‐19, where they constitute a typical, although not specific, manifestation.
In line with the findings of other authors, our study confirms that the neuropathological alterations strictly attributable to the SARS‐CoV‐2 infection are overall modest, inflammatory in nature and prevalent in the brainstem (12, 14, 16, 21), where very rare viral inclusions were detected (Figure 1J,K). Although traces of viral antigens and viral RNA (possibly blood‐borne) have certainly been identified within the CNS by several authors (12, 13, 24) and also by us, such a few viral traces and scant lymphocytes argue against the presence of encephalitis and active viral replication within the CNS, and they do not suggest a neurotropism of SARS‐CoV‐2. The hallmarks of COVID‐19 brains, compared to matched non‐COVID brains, as they emerge from this series of elderly subjects (many of them with AD pathology), can be summarized as follows, in 3 points: (1) Generally, in the brain tissue, microglial reinforcement is observed with a low number of lymphocytes – suppression of adaptative immunity; (2) The brainstem is the area specifically most affected by microglial hyperactivation in all cases – specific topographical phenomenon; (3) COVID‐19 patients with NCD and delirium are characterized by microglial boosting in the hippocampus – microglial activation associated with TAU‐pathology. The last two points probably represent the neuropathological basis of the “COVID‐19 encephalopathic syndrome” in the elderly. This study essentially refers to patients of senile age, therefore, the generalizability of the results can only be referred to elderly subjects and should be interpreted with caution because of the small number of cases. The data would require confirmation from more extensive series. Nevertheless, the analysis carried out on these cases presents considerable points of interest for the interpretation of the interactions between neurodegenerative diseases, systemic inflammation and behavioral disorders, even beyond the SARS‐CoV‐2 infection. A better understanding of the pathogenesis of behavioral alterations in neuro‐cognitive disorders may have practical repercussions on therapeutic approaches for delirium. If delirium occurs in a context of systemic inflammation with microglial activation, it could greatly benefit from drugs capable of inhibiting microglia, such as simple corticosteroids. In this perspective, COVID‐19 can represent a useful “natural experiment” of severe systemic inflammation, from which much can be learned.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
AUTHOR CONTRIBUTIONS
Tino Emanuele Poloni and Valentina Medici contributed equally to the original research idea conception, study design, literature search, data collection, data analysis and interpretation, figure and table formatting, and writing of the manuscript. Matteo Moretti, Silvia Damiana Visonà, Alice Cirrincione, Arenn Faye Carlos, Annalisa Davin, Stella Gagliardi, and Orietta Pansarasa contributed to data collection and analysis and assisted in the writing of the text. Cristina Cereda, Livio Tronconi, and Antonio Guaita contributed to data analysis and critical revision. Mauro Ceroni supervised the study and assisted in the writing of the text. All authors provided intellectual content and critical review of the manuscript.
ETHICS APPROVAL
On February 27, 2020, the Italian Presidency of the Council of Ministers authorized the collection and scientific dissemination of data concerning the COVID‐19 epidemics by the Italian National Institute of Health and other public health institutions. Therefore, ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The present COVID‐19 cases were subjected to forensic autopsies, ordered by the State Prosecutor. The samples were taken in accordance with the Italian law regarding processing of personal data, and all the subjects were made anonymous. The reference law is the authorization n.9/2016 of the guarantor of privacy, then replaced by Regulation (eu) 2016/679 of the European Parliament and of the Council. In line with our institution's Human Research Ethics Committee and the BNE Code of Conduct, the ABB performs its activities following ethical standards. The ABB autopsy and sampling protocol was approved by the Ethics Committee of the University of Pavia on October 6th, 2009 (Committee report 3/2009). The study procedures were in accordance with the principles outlined in the Declaration of Helsinki of 1964 and the following amendments.
Supporting information
Supplementary Material
FIGURE S1 Pons of COV9 case is shown by H&E (A and C) and CD34 immunostaining (B and D). CD34 tufts are clearly evident both in 4x (B) and 10x magnification (D) particularly around neurons. In the same area H&E staining at 4x (A) shows a normal cytoarchitecture. The higher magnification image (10x C) confirms normal tissue without any cytological alteration
Supplementary Material
TABLE S1 List of the primary and secondary antibodies, their characteristics and dilutions
ACKNOWLEDGEMENTS
We would like to thank all brain donors who generously donated the noblest organ of their body. We deeply thank Intesa Sanpaolo for their financial contibution, and Mrs. Federica Zagari, Dr. Elena Rolandi, Mrs Valeria Marzagalli, Dr. Mauro Colombo and Dr. Arcangelo Ceretti for their valuable support and technical help. We are very grateful to Prof. Johannes Attems for his wise recommendations. We also thank the student Anna Milione who gave useful assistance and wrote her thesis on this topic. Ultimately, we wish to dedicate this work to Dr. Michela Mangieri and Prof. Antonio Osculati, who died prematurely, for their dedication and commitment to the Abbiategrasso Brain Bank’s activities.
Poloni TE, Medici V, Moretti M, Visonà SD, Cirrincione A, Carlos AF, et al. COVID‐19‐related neuropathology and microglial activation in elderly with and without dementia. Brain Pathology. 2021;31:e12997. 10.1111/bpa.12997
Tino Emanuele Poloni and Valentina Medici have contributed equally.
Funding information
This study was supported by Fondo di Beneficenza Intesa Sanpaolo (Italy). Project code: B/2020/0045
DATA AVAILABILITY STATEMENT
The dataset of this research is deposited in the official computer archive of the Golgi‐Cenci Foundation, and it is available upon request.
REFERENCES
- 1.Hariyanto TI, Putri C, Arisa J, Situmeang RFV, Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID‐19) pneumonia: a systematic review and meta‐analysis. Arch Gerontol Geriatr. 2021;93:104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dantzer R. Cytokine‐induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. [DOI] [PubMed] [Google Scholar]
- 5.Holmes C, Cunningham C, Zotova E, Culliford D, Perry VH. Proinflammatory cytokines, sickness behavior, and Alzheimer disease. Neurology. 2011;77:212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy M, Helfand BKI, Gou RY, Gartaganis SL, Webb M, Moccia JM, et al. Delirium in older patients with COVID‐19 presenting to the emergency department. JAMA Netw Open. 2020;3:e2029540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poloni TE, Carlos AF, Cairati M, Cutaia C, Medici V, Marelli E, et al. Prevalence and prognostic value of Delirium as the initial presentation of COVID‐19 in the elderly with dementia: an Italian retrospective study. EClinicalMedicine. 2020;26:100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baig AM, Sanders EC. Potential neuroinvasive pathways of SARS‐CoV‐2: deciphering the spectrum of neurological deficit seen in coronavirus disease‐2019 (COVID‐19). J Med Virol. 2020;92:1845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis virus transmission pathways. J Pathol. 2004;203:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iadecola C, Anrather J, Kamel H. Effects of COVID‐19 on the nervous system. Cell. 2020;183:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID‐19 in Germany: a post‐mortem case series. Lancet Neurol. 2020;19:919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS‐CoV‐2 invasion as a port of central nervous system entry in individuals with COVID‐19. Nat Neurosci. 2021;24:168–75. [DOI] [PubMed] [Google Scholar]
- 14.Al‐Sarraj S, Troakes C, Hanley B, Osborn M, Richardson MP, Hotopf M, et al. Invited Review: the spectrum of neuropathology in COVID‐19. Neuropathol Appl Neurobiol. 2021;47:3–16. [DOI] [PubMed] [Google Scholar]
- 15.Younger DS. Postmortem neuropathology in COVID‐19. Brain Pathol. 2020;e12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al‐Dalahmah O, Thakur KT, Nordvig AS, Prust ML, Roth W, Lignelli A, et al. Neuronophagia and microglial nodules in a SARS‐CoV‐2 patient with cerebellar hemorrhage. Acta Neuropathol Commun. 2020;8:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryce C, Grimes Z, Pujadas E, Ahuja S, Beth Beasley M, Albrecht R, et al. Pathophysiology of SARS‐CoV‐2: The Mount Sinai COVID‐19 autopsy experience. Mod Pathol. 2021;1:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaunmuktane Z, Mahadeva U, Green A, Sekhawat V, Barrett NA, Childs L, et al. Microvascular injury and hypoxic damage: emerging neuropathological signatures in COVID‐19. Acta Neuropathol. 2020;140:397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantonen J, Mahzabin S, Mäyränpää MI, Tynninen O, Paetau A, Andersson N, et al. Neuropathologic features of four autopsied COVID‐19 patients. Brain Pathol. 2020;30:1012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschenbaum D, Imbach LL, Rushing EJ, Frauenknecht KBM, Gascho D, Ineichen BV, et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID‐19. Neuropathol Appl Neurobiol. 2021;47(3):454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M‐H, Perl DP, Nair G, Li W, Maric D, Murray H, et al. Microvascular Injury in the Brains of Patients with Covid‐19. N Engl J Med. 2020;384:481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deigendesch N, Sironi L, Kutza M, Wischnewski S, Fuchs V, Hench J, et al. Correlates of critical illness‐related encephalopathy predominate postmortem COVID‐19 neuropathology. Acta Neuropathol. 2020;140:583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID‐19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)‐like pathology. Acta Neuropathol. 2020;140:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological features of Covid‐19. N Engl J Med. 2020;383:989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Weyhern CH , Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID‐19 outcomes. Lancet. 2020;395:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank S. Catch me if you can: SARS‐CoV‐2 detection in brains of deceased patients with COVID‐19. Lancet Neurol. 2020;19:883–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moretti M, Malhotra A, Visonà SD, Finley SJ, Osculati AMM, Javan GT. The roles of medical examiners in the COVID‐19 era: a comparison between the United States and Italy. Forensic Sci Med Pathol. 2021;17(2):262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poloni TE, Medici V, Carlos AF, Davin A, Ceretti A, Mangieri M, et al. Abbiategrasso brain bank protocol for collecting, processing and characterizing aging brains. J Vis Exp. 2020;2020:1–25. [DOI] [PubMed] [Google Scholar]
- 29.Raffaello C. Diagnostic and statistical manual of mental disorders (DSM‐5®). American Psychiatric Association. https://books.google.it/books?id=‐JivBAAAQBAJ. Accessed 15 Feb 2021. [Google Scholar]
- 30.Inouye SK, Van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990;113:941–8. [DOI] [PubMed] [Google Scholar]
- 31.Thal DR, Rüb U, Orantes M, Braak H. Phases of Aβ‐deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease‐associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National institute on aging‐Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghorpade A, Persidsky Y, Swindells S, Borgmann K, Persidsky R, Holter S, et al. Neuroinflammatory responses from microglia recovered from HIV‐1‐infected and seronegative subjects. J Neuroimmunol. 2005;163:145–56. [DOI] [PubMed] [Google Scholar]
- 35.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–62. [DOI] [PubMed] [Google Scholar]
- 36.Hendrickx DAE, van Eden CG , Schuurman KG, Hamann J, Huitinga I. Staining of HLA‐DR, Iba1 and CD68 in human microglia reveals partially overlapping expression depending on cellular morphology and pathology. J Neuroimmunol. 2017;309:12–22. [DOI] [PubMed] [Google Scholar]
- 37.Tröscher AR, Wimmer I, Quemada‐Garrido L, Köck U, Gessl D, Verberk SGS, et al. Microglial nodules provide the environment for pathogenic T cells in human encephalitis. Acta Neuropathol. 2019;137:619–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludlow M, Kortekaas J, Herden C, Hoffmann B, Tappe D, Trebst C, et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016;131:159–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol. 2002;124:83–92. [DOI] [PubMed] [Google Scholar]
- 40.Zotova E, Bharambe V, Cheaveau M, Morgan W, Holmes C, Harris S, et al. Inflammatory components in human Alzheimer’s disease and after active amyloid‐β42 immunization. Brain. 2013;136:2677–96. [DOI] [PubMed] [Google Scholar]
- 41.Rakic S, Hung YMA, Smith M, So D, Tayler HM, Varney W, et al. Systemic infection modifies the neuroinflammatory response in late stage Alzheimer’s disease. Acta Neuropathol Commun. 2018;6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akbar AN, Gilroy DW. Aging immunity may exacerbate COVID‐19. Science. 2020;369:256–7. [DOI] [PubMed] [Google Scholar]
- 44.Mueller AL, Mcnamara MS, Sinclair DA. Why does COVID‐19 disproportionately affect older people? Aging. 2020;12:9959–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salimi S, Hamlyn JM, Le Couteur D. COVID‐19 and crosstalk with the hallmarks of aging. J Gerontol A Biol Sci Med Sci. 2020;75:e34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felsky D, Roostaei T, Nho K, Risacher SL, Bradshaw EM, Petyuk V, et al. Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat Commun. 2019;10:409. 10.1038/s41467-018-08279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018;217:459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos LE, Beckman D, Ferreira ST. Microglial dysfunction connects depression and Alzheimer’s disease. Brain Behav Immun. 2016;55:151–65. [DOI] [PubMed] [Google Scholar]
- 49.Serrano‐Pozo A, Muzikansky A, Gómez‐Isla T, Growdon JH, Betensky RA, Frosch MP, et al. Differential relationships of reactive astrocytes and microglia to fibrillar amyloid deposits in alzheimer disease. J Neuropathol Exp Neurol. 2013;72:462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torvell M, Hampton DW, Connick P, MacLullich AMJ, Cunningham C, Chandran S. A single systemic inflammatory insult causes acute motor deficits and accelerates disease progression in a mouse model of human tauopathy. Alzheimers Dement. 2019;5:579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, De Rooij SEJA. Neuroinflammation in delirium: a postmortem case‐control study. Rejuvenation Res. 2011;14:615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis DHJ, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, et al. Delirium is a strong risk factor for dementia in the oldest‐old: a population‐based cohort study. Brain. 2012;135:2809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemstra AW, Groen in’t Woud JCM, Hoozemans JJM, van Haastert ES , Rozemuller AJM, Eikelenboom P, van Gool WA Microglia activation in sepsis: a case‐control study. J Neuroinflammation. 2007;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michels M, Danielski L, Dal‐Pizzol F, Petronilho F. Neuroinflammation: microglial activation during sepsis. Curr Neurovasc Res. 2014;11:262–70. [DOI] [PubMed] [Google Scholar]
- 55.Darbinyan A, Major EO, Morgello S, Holland S, Ryschkewitsch C, Monaco MC, et al. BK virus encephalopathy and sclerosing vasculopathy in a patient with hypohidrotic ectodermal dysplasia and immunodeficiency. Acta Neuropathol Commun. 2016;4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalaria RN, Kroon SN. Expression of leukocyte antigen CD34 by brain capillaries in Alzheimer’s disease and neurologically normal subjects. Acta Neuropathol. 1992;84:606–12. [DOI] [PubMed] [Google Scholar]
- 57.Blümcke I, Giencke K, Wardelmann E, Beyenburg S, Kral T, Sarioglu N, et al. The CD34 epitope is expressed in neoplastic and malformative lesions associated with chronic, focal epilepsies. Acta Neuropathol. 1999;97:481–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
FIGURE S1 Pons of COV9 case is shown by H&E (A and C) and CD34 immunostaining (B and D). CD34 tufts are clearly evident both in 4x (B) and 10x magnification (D) particularly around neurons. In the same area H&E staining at 4x (A) shows a normal cytoarchitecture. The higher magnification image (10x C) confirms normal tissue without any cytological alteration
Supplementary Material
TABLE S1 List of the primary and secondary antibodies, their characteristics and dilutions
Data Availability Statement
The dataset of this research is deposited in the official computer archive of the Golgi‐Cenci Foundation, and it is available upon request.


