Abstract
Photodynamic therapy (PDT) is a two-step procedure that involves the administration of special drugs, commonly called photosensitizers, followed by the application of certain wavelengths of light. The light activates these photosensitizers to produce reactive molecular species that induce cell death in tissues. There are numerous factors to consider when selecting the appropriate photosensitizer administration route, such as which part of the body is being targeted, the pharmacokinetics of photosensitizers, and the formulation of photosensitizers. While intravenous, topical, and oral administration of photosensitizers are widely used in preclinical and clinical applications of PDT, other administration routes, such as intraperitoneal, intra-arterial, and intratumoral injections, are gaining traction for their potential in treating advanced diseases and reducing off-target toxicities. With recent advances in targeted nanotechnology, biomaterials, and light delivery systems, the exciting possibilities of targeted photosensitizer delivery can be fully realized for preclinical and clinical applications. Further, in light of the growing burden of cancer mortality in low and middle-income countries and development of low-cost light sources and photosensitizers, PDT could be used to treat cancer patients in low-income settings. This short article introduces aspects of interfaces of intratumoral photosensitizer injections and nano-biomaterials for PDT applications in both high-income and low-income settings but does not present a comprehensive review due to space limitations.
Administration routes for oncologic photosensitizers.
Photodynamic therapy (PDT) is a photochemistry-based approach in which a photosensitizer is energized by red or near-infrared light to generate reactive oxygen species that kill or modulate target cells or tissues [1, 2]. A photosensitizer is defined as a chemical entity that absorbs incident light and imparts a physical and chemical change on another chemical entity. There are two main types of photosensitized reactions to produce reactive oxygen species. In the Type I reaction, a photosensitizer at an excited state reacts with the substrate to produce radical ions or radicals via electron transfer or hydrogen atom abstraction. In the presence of oxygen, the photosensitizer radical ion can transfer the electron to oxygen to produce superoxide radical anion (O2.-) or the radicals can further react to generate oxygenated species. In a Type II reaction, the excited photosensitizer directly transfers energy to the ground-state molecular oxygen without a radical ion intermediate to form singlet molecular oxygen (1O2). Common photosensitizers used in the practice of photodynamic therapy (PDT) include porphyrins, chlorins and dyes. These photosensitizers may be synthesized or induced endogenously in the heme biosynthetic pathway, such as 5-aminolevulinic acid (5-ALA)-induced protoporphyrin IX (PpIX). There are several reviews summarizing the history, development, and types of photosensitizers for PDT [3, 4].
Since Dougherty first reported successful PDT in a large cohort of cancer patients in 1978 [5], over 250 PDT clinical trials have been conducted to treat different types of cancer [6, 7]. Photosensitizers are typically given to cancer patients or animals via intravenous injection, topical application, and oral administration. Out of the 17 completed cancer PDT clinical trials that were supported by NIH or other U.S. Federal agencies, 10 involved intravenous injections of photosensitizers, 5 delivered photosensitizers topically, and 2 administered photosensitizers orally (ClinicalTrials.gov). While intravenous injection remains the mainstream route of photosensitizer administration for cancer (i.e., accounts for 12 out of 24 of the ongoing trials), alternative photosensitizer injection routes, such as intratumoral (NCT04552990) and intravesical (NCT03945162) injections, are also starting to gain traction in clinical practices (Figure 1). The most common intravenous photosensitizers include porfimer sodium (Photofrin®), WST-11 (TOOKAD® Soluble), verteporfin (Visudyne®, HS-201), temoporfin (tetra[m-hydroxyphenyl]chlorin, m-THPC, or Foscan®), and 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a (HPPH or Photochlor®) (Figure 2). While topical and oral administration of 5-aminolevulinic acid (5-ALA or Gliolan®) are broadly accepted due to their simplicity, safety, convenience, and cost effectiveness, other groups have also demonstrated the feasibility and benefits (e.g., reduced skin phototoxicity) of intravenous [8] and intravesical [9] 5-ALA injections in vivo.
Figure 1 |. Summary of photosensitizer administration routes for photodynamic therapy of cancer in clinical trials that are published by the U.S. National Library of Medicine.
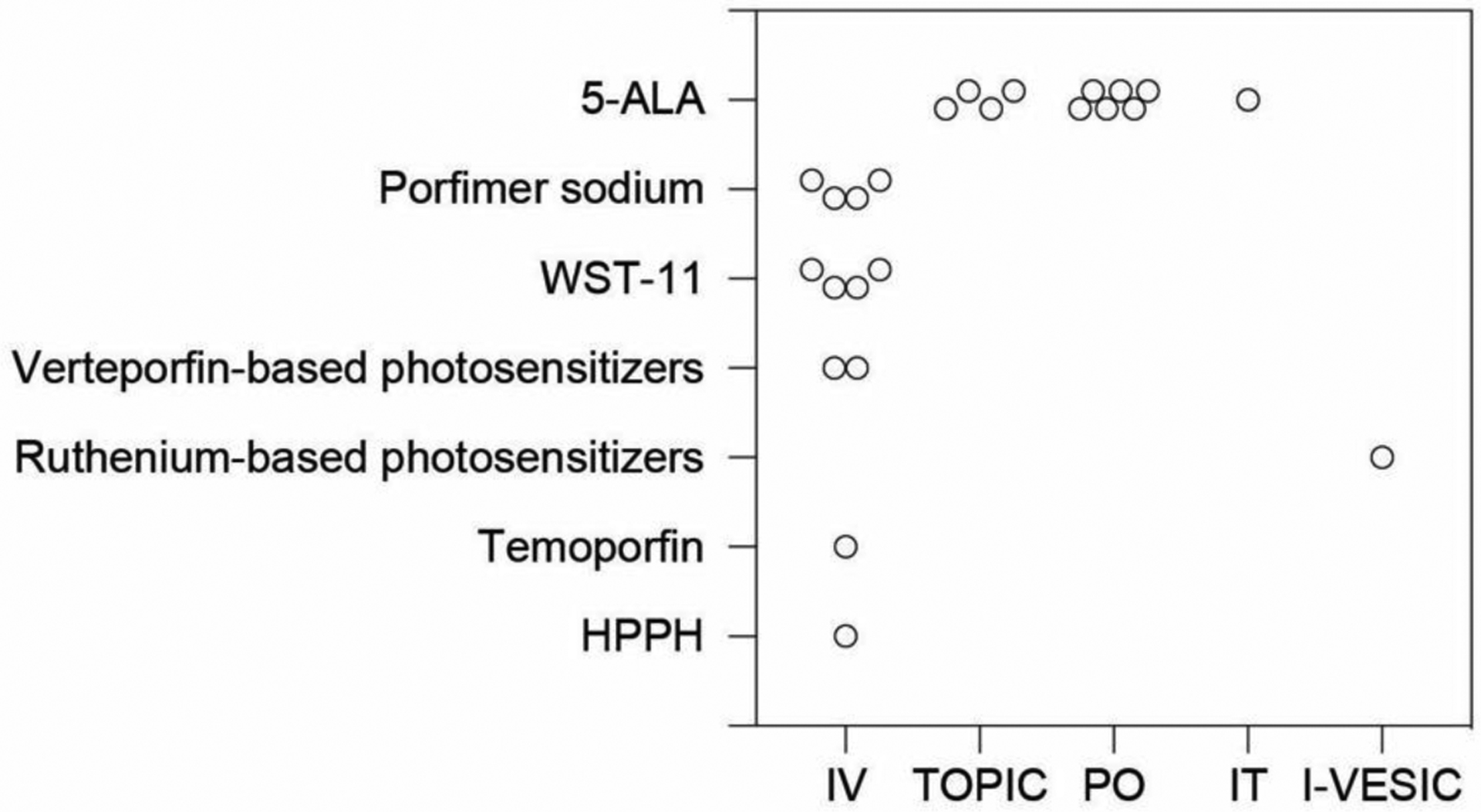
Data collected from the clinicaltrials.gov website show different photosensitizers (y-axis) and their respective administration routes (x-axis). Each dot in the plot represents a clinical trial that is Active (not recruiting), Enrolling by invitation, Recruiting, or Not yet recruiting. Our summary indicates a majority of photosensitizers are currently administered intravenously in the clinic, with the exception of 5-ALA. In the plot, clinical trials that are Suspended, Terminated, Completed, Withdrawn, or have Unknown status were not included. Photosensitizers include 5-aminolevulinic acid (5-ALA or Gliolan®), porfimer sodium (Photofrin®), WST-11 (TOOKAD® Soluble), verteporfin-based PS (e.g., Visudyne®, HS-201), ruthenium-based PS (TLD1433), temoporfin (tetra[m-hydroxyphenyl]chlorin, m-THPC, or Foscan®), and 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a (HPPH or Photochlor®). Routes of administration include intravenous (IV, administration within or into a vein or veins), topical (TOPIC, administration to a particular spot on the outer surface of the body), oral (PO, administration to or by way of the mouth), intratumor (IT, administration within a tumor), and intravesical (I-VESIC, administration within the bladder).
Figure 2 |. Chemical structures of clinically used photosensitizers described in Figure 1.
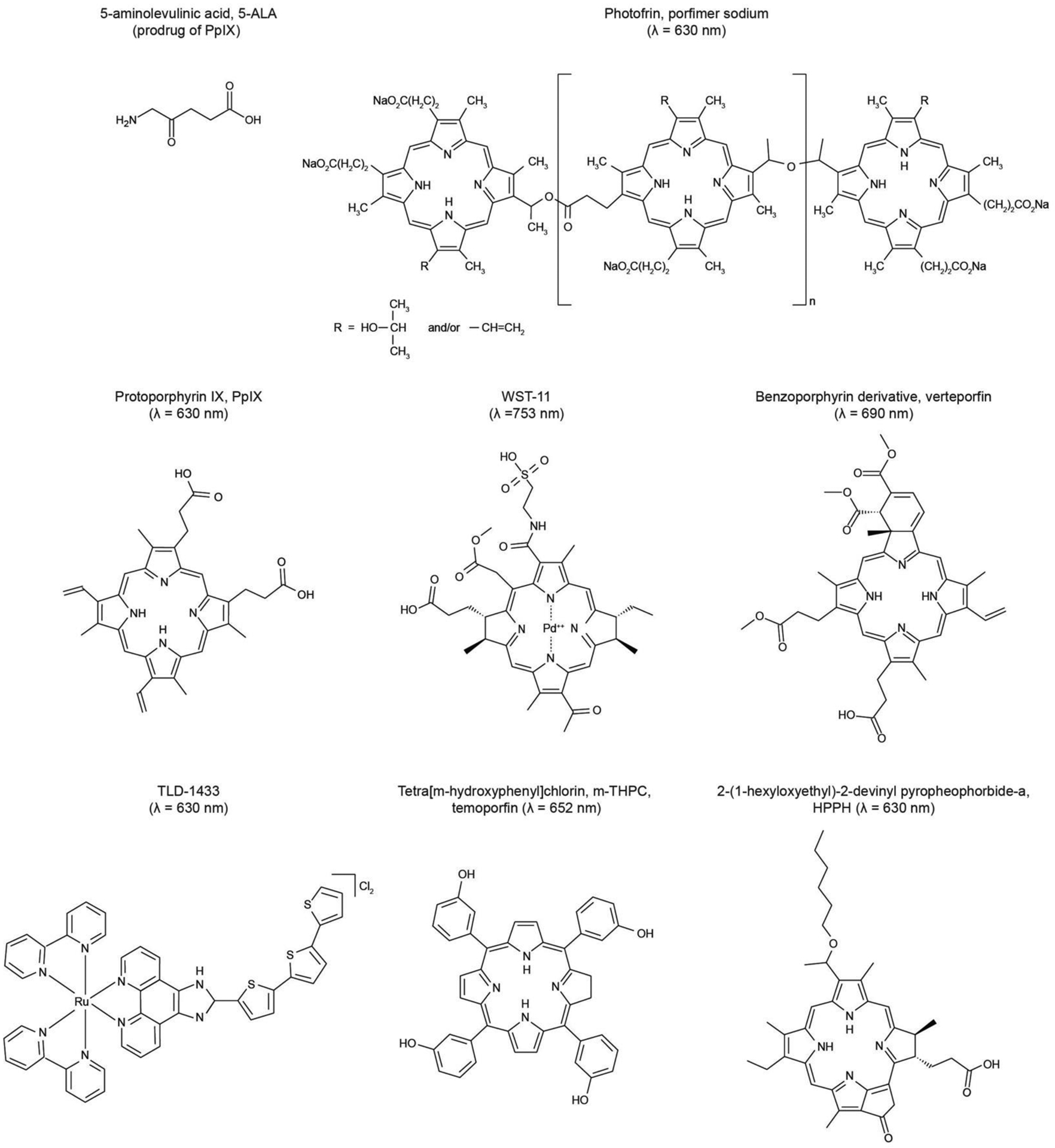
All the listed photosensitizers, besides the ruthenium-based photosensitizer TLD-1422, contain the tetrapyrrole macrocycle structure. The porphyrin-based photosensitizers (e.g., Photofrin, protoporphyrin IX) typically have a ring structure with 22 π-electrons, while the chlorin-type photosensitizer (i.e., tetra[m-hydroxyphenyl]chlorin) has one reduced double bond. These photosensitizers absorb light at red and near-infrared wavelengths, allowing for maximum penetration of light through tissues.
Advances in photosensitizer formulation, surgical device design, and light applicators [10] are creating tremendous opportunities to broaden photosensitizer injection routes to include intraperitoneal, intra-arterial, and intratumoral administrations. Intraperitoneal (or intracavitary) injection method has been reported to increase intratumoral photosensitizer concentrations compared to intravenous injection [11–14]. With significant progress being made towards antibody-photosensitizer conjugates (also known as photo-immunoconjugates) [15–18], we [19, 20] and others [21, 22] have shown that photo-immunoconjugates improve the selectivity and safety of photosensitizers, achieving a tumor-to-normal tissue ratio (T/N) of around 9–13 in ovarian cancer mouse models [19, 21] and reducing bowel phototoxicity [21]. Additionally, there have been key innovation in the development of photosensitizing biomolecules [18, 23], light scattering agents, and intracavitary balloon catheter light applicators [24–28] that, together with advances in photo-immunoconjugates, make intraperitoneal photosensitizer delivery a more promising route of administration in clinical practice [29].
Intra-arterial administration is another potential strategy to selectively deliver photosensitizers to diseased sites and reduce the drug-light interval (i.e., waiting time of light illumination after photosensitizer injection). The feasibility of intra-arterial photosensitizer injection has been demonstrated in veterinary medicine using canine [30, 31] and swine models [32]. For example, Moore et al. showed that intra-arterial infusion selectively delivers lipid-formulated QLT0074 photosensitizers to the prostate with photosensitizer concentrations up to 18 times higher than that in the surrounding bladder, rectum and urethral tissues [33]. In a canine case study with adenocarcinoma of the left paranasal sinus, the combination of PDT with oral administration of 5-aminolevulinic acid and intra-arterial injection of chemotherapy (carboplatin and doxorubicin) achieved complete remission and long-term survival (~2 year) after initial disease presentation [34]. In view of these results, further clinical studies are warranted to evaluate the intra-arterial injection of photosensitizers and chemotherapy for PDT-based combination treatments.
Benefits and challenges of intratumoral photosensitizer injection.
The intratumoral injection is particularly appealing for PDT of locally advanced, unresectable solid tumors [35]. First, directly injecting photosensitizers into a tumor can shorten the drug-light interval from a day to an hour [36] and reduce phototoxicity to normal tissues (e.g., skin), which are current drawbacks of intravenous photosensitizer injection [1]. Second, with growing clinical interest in interstitial PDT [37] and endoscopic ultrasound-guided PDT [38, 39], where one or more laser fibers are inserted into the tumor and/or margins, photosensitizers could be delivered intratumorally immediately prior to optical fibers insertion through the same puncture or endoscopic approach. Finally, the use of nano-biomaterials [40, 41] could further improve the distribution and retention of intratumorally injected photosensitizer, enhancing the safety, reliability, and efficacy of PDT. While direct intratumoral injections have significant advantages in photosensitizer delivery, several challenges remain to be addressed: 1) backflow (or retrograde flow) of photosensitizers along the catheter, 2) poor distribution, low retention, and quenching of photosensitizers within the tumor, 3) reabsorption of photosensitizers into the bloodstream, 4) lack of methods to quantitatively image photosensitizer distribution and concentrations, and 5) limited treatment efficacy with monotherapy. The following sections will provide examples of how novel formulations of photosensitizers, innovative light delivery systems, and PDT-based combination therapies could mitigate some of these challenges.
Intratumoral administration of photosensitizer-loaded nanoparticles to improve distribution and retention has been studied in vivo for many years. Liposomal photosensitizer is one of the most studied nanoformulations for PDT [42–45]. D’Hallewin et al. showed that intratumoral injection of liposomal formulation of meta-tetra(hydroxyphenyl)chlorin (Foslip®, 25 μL of 0.15 mg/mL) resulted in minimum reabsorption of meta-tetra(hydroxyphenyl)chlorin into the bloodstream (maxed at 1.5 ng/mg) [44]. Using intratumorally injected Foslip® and a drug-light interval of 24 hours, around 70% of the tumor was necrotic after PDT. While light activation of Foslip® did not result in a total cure likely due to the heterogeneous photosensitizer distribution, the authors suggested that repeated PDT sessions might be beneficial. Other organic and inorganic nanoparticles, such as polymeric nanoparticles [46, 47], upconversion nanoconstructs [48, 49], silica nanoparticles [50, 51], and gold nanoparticles [52, 53] are also promising for intratumoral administration of photosensitizers and PDT. For example, Hu et al. showed that zeolite nanocarriers co-packaged with catalase and methylene blue photosensitizer improve intratumoral photosensitizer delivery and singlet oxygen yield for enhanced PDT outcomes [54]. Specifically, adding 0.05 mg/mL of catalase into methylene blue-loaded zeolite nanocarriers improved the singlet oxygen yield by approximately 3.7-fold upon light activation (635 nm, 50mW/cm2, 5 min). Using a xenograft mouse model of human SW1990 pancreatic cancer, the authors also showed that zeolite nanocarriers decrease the diffusion rate and increase the retention time of methylene blue in tumors by nearly 3-fold. Light activation of zeolite nanocarriers loaded with catalase and methylene blue completely inhibited tumor growth for 18 days and achieved 100% morbidity-free survival. Pluronic (F127) nanocomposite hydrogels have also been used to prolong the retention of micellar pyropheophorbide a (PPa) and imidazole derivative in 4T1 murine breast tumors. The pluronic composite gel increased the retention of PPa for up to 14 days and improved PDT-induced tumor growth inhibition by 54% compared to intratumorally injected PPa, which was cleared after 4 days with PDT-induced tumor growth inhibition of only ~28% [55].
Implantable light sources and combination approaches for intratumoral photosensitizer injection
In addition to novel photosensitizer formulations that improve distribution and retention in target tissue, there have been recent developments in implantable light delivery systems that can perform repeated PDT. Clinically, light has been successfully delivered through chronically implantable balloon catheter systems to activate the photosensitizers for PDT [24–28]. Preclinically, Bansal et al. showed that a wireless light-emitting diode (LED) could be implanted near the MB49 bladder tumors to activate intratumorally injected chlorin e6 photosensitizers in mice [56]. Using wireless light delivery, two cycles of PDT suppressed MB49 tumor growth in vivo for 15 days. Multi-cycle PDT could be used to manage tumor burden post-surgery, particularly for residual or recurrent tumors.
It is increasingly evident that the most effective cancer treatments will likely involve a combination approach targeting multiple non-overlapping tumor growth and survival pathways. Photodynamic therapy and priming have been repeatedly shown to potentiate the efficacy of chemotherapy [57–60], immunotherapy [61–64], and biological agents [11, 65, 66]. We believe that intratumoral injection is an ideal method to deliver PDT-based combination regimen because the appropriate dosage can be optimized and easily controlled within a tumor for prolonged exposure. For example, Gupta et al. showed that nanoassemblies of anti-carcinoembryonic antigen (anti-CAE) monoclonal antibody and technetium-labeled hematoporphyrin derivative (PS-3) could be directly injected into murine Ehrlich ascites tumors for enhanced dye retention [67]. Tumor-selective accumulation of anti-CEA-PS-3 conjugates can be seen up to 2 hours after intratumoral administration. Non-specific diffusion of anti-CEA-PS-3 to the liver was observed at 6 hours after intratumoral injection and cleared from the system by 26 hours. No anti-CEA-PS-3 accumulation in muscle tissues was observed. Although PDT was not carried out in this study, these results suggest that cancer-targeted PDT can potentially be achieved soon after intratumoral administration of antibody-photosensitizer conjugates.
Future perspective: Is intratumoral photosensitizer injection an effective approach for global health PDT applications?
In low and middle-income countries (LMICs), where access to surgery, radiotherapy, and immunotherapy remains extremely limited, low-cost targeted therapies are desperately needed to reduce the global burden of cancer [68]. Currently, low-cost targeted therapies include cryoablation [69–72], thermocoagulation [71, 73], and ethanol ablation [74–77], which have been used to treat a variety of cancers, including cervical dysplasia [69, 71, 73], breast tumors [72], hepatocellular carcinoma [74–76], thyroid tumors [77], and renal tumors [70], among others. However, these therapies are currently only used to treat superficial dysplasia or small tumors (<3–5 cm in diameter) due to limited thermal diffusion [69, 71] or poor retention of ethanol within tumors [78, 79]. Recently, Morhard et al. demonstrated increased intratumoral retention of ethanol through: 1) slowing the infusion rate, which reduced backflow, and 2) adding the polymer ethyl cellulose to the injectate, which formed a gel in tissue, further improving retention of ethanol in a hamster cheek pouch model of oral squamous cell carcinoma [80, 81]. These approaches could also enhance intratumoral delivery of low-cost photosensitizers for implementation of PDT in LMICs. Additionally, Mallidi et al. developed an inexpensive, portable, battery-powered LED light source for use in LMICs and found there was no significant differences in necrotic volume in a xenograft murine model of human squamous cell carcinoma when compared with a standard, high-cost laser source [82].
In summary, intratumoral administration of photosensitizers could be achieved through minimally invasive techniques to significantly improve the T/N ratio, shorten the drug-light interval, and reduce any associated adverse events, compared to intravenous injection. Emerging intratumoral delivery platforms, such as nanoparticles and hydrogels, hold promise to further improve the retention and sustained delivery of photosensitizers, thereby enhancing the overall efficacy of PDT. With the development of low-cost light sources and intratumoral delivery of photosensitizers, PDT may provide an increasingly effective, yet affordable, method to treat cancer patients in both high-income countries and in LMICs.
Acknowledgments:
This work is supported by the National Institutes of Health (NIH) R21EB028508 and R01CA260340 grants (H.H.) and the University of Maryland startup funds (H.H., J.M.)
References
- 1.Celli JP, et al. , Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev, 2010. 110(5): p. 2795–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baptista MS, et al. , Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem Photobiol, 2017. 93(4): p. 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahamse H and Hamblin MR, New photosensitizers for photodynamic therapy. Biochem J, 2016. 473(4): p. 347–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lan M, et al. , Photosensitizers for Photodynamic Therapy. Adv Healthc Mater, 2019. 8(13): p. e1900132. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty TJ, et al. , Photoradiation therapy for the treatment of malignant tumors. Cancer Res, 1978. 38(8): p. 2628–35. [PubMed] [Google Scholar]
- 6.Kessel D, Photodynamic Therapy: A Brief History. J Clin Med, 2019. 8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alsaab HO, et al. , Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers (Basel), 2020. 12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loh CS, et al. , Oral versus intravenous administration of 5-aminolaevulinic acid for photodynamic therapy. Br J Cancer, 1993. 68(1): p. 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SC, et al. , 5-Aminolevulinic acid (ALA)-induced protoporphyrin IX fluorescence and photodynamic effects in the rat bladder: an in vivo study comparing oral and intravesical ALA administration. Lasers Surg Med, 1997. 20(3): p. 254–64. [DOI] [PubMed] [Google Scholar]
- 10.Yun SH and Kwok SJJ, Light in diagnosis, therapy and surgery. Nat Biomed Eng, 2017. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer G, et al. , Preclinical Evaluation of Cetuximab and Benzoporphyrin Derivative-Mediated Intraperitoneal Photodynamic Therapy in a Canine Model. Photochem Photobiol, 2020. 96(3): p. 684–691. [DOI] [PubMed] [Google Scholar]
- 12.Perry RR, et al. , Intravenous vs intraperitoneal sensitizer: implications for intraperitoneal photodynamic therapy. Photochem Photobiol, 1991. 53(3): p. 335–40. [DOI] [PubMed] [Google Scholar]
- 13.Veenhuizen RB, et al. , Foscan-mediated photodynamic therapy for a peritoneal-cancer model: drug distribution and efficacy studies. Int J Cancer, 1997. 73(2): p. 230–5. [DOI] [PubMed] [Google Scholar]
- 14.Cengel KA, Glatstein E, and Hahn SM, Intraperitoneal photodynamic therapy. Cancer Treat Res, 2007. 134: p. 493–514. [DOI] [PubMed] [Google Scholar]
- 15.Mew D, et al. , Photoimmunotherapy: treatment of animal tumors with tumor-specific monoclonal antibody-hematoporphyrin conjugates. J Immunol, 1983. 130(3): p. 1473–7. [PubMed] [Google Scholar]
- 16.Mitsunaga M, et al. , Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med, 2011. 17(12): p. 1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato K, et al. , Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci Transl Med, 2016. 8(352): p. 352ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inglut CT, et al. , Systematic Evaluation of Light-Activatable Biohybrids for Anti-Glioma Photodynamic Therapy. Journal of Clinical Medicine, 2019. 8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang HC, et al. , Immobilization of Photo-Immunoconjugates on Nanoparticles Leads to Enhanced Light-Activated Biological Effects. Small, 2018: p. e1800236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang BJ, et al. , Breaking the Selectivity-Uptake Trade-Off of Photoimmunoconjugates with Nanoliposomal Irinotecan for Synergistic Multi-Tier Cancer Targeting. J Nanobiotechnology, 2020. 18(1): p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spring BQ, et al. , Selective treatment and monitoring of disseminated cancer micrometastases in vivo using dual-function, activatable immunoconjugates. Proceedings of the National Academy of Sciences, 2014. 111(10): p. E933–E942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savellano MD and Hasan T, Targeting cells that overexpress the epidermal growth factor receptor with polyethylene glycolated BPD verteporfin photosensitizer immunoconjugates. Photochem Photobiol, 2003. 77(4): p. 431–9. [DOI] [PubMed] [Google Scholar]
- 23.Almeida-Marrero V, et al. , Porphyrinoid biohybrid materials as an emerging toolbox for biomedical light management. Chemical Society Reviews, 2018. 47(19): p. 7369–7400. [DOI] [PubMed] [Google Scholar]
- 24.Tatter SB, et al. , An inflatable balloon catheter and liquid 125I radiation source (GliaSite Radiation Therapy System) for treatment of recurrent malignant glioma: multicenter safety and feasibility trial. J Neurosurg, 2003. 99(2): p. 297–303. [DOI] [PubMed] [Google Scholar]
- 25.Friedberg JS, Radical pleurectomy and photodynamic therapy for malignant pleural mesothelioma. Ann Cardiothorac Surg, 2012. 1(4): p. 472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simone CB and Cengel KA, Photodynamic Therapy for Lung Cancer and Malignant Pleural Mesothelioma. Semin Oncol, 2014. 41(6): p. 820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huggett MT, et al. , Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer, 2014. 110(7): p. 1698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sindelar WF, et al. , Technique of photodynamic therapy for disseminated intraperitoneal malignant neoplasms. Phase I study. Arch Surg, 1991. 126(3): p. 318–24. [DOI] [PubMed] [Google Scholar]
- 29.Xu S, et al. , Photodynamic Diagnosis and Therapy for Peritoneal Carcinomatosis: Emerging Perspectives. Cancers (Basel), 2020. 12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Z, et al. , Interstitial photodynamic therapy of the canine prostate using intra-arterial administration of photosensitizer and computerized pulsed light delivery. J Urol, 2007. 178(1): p. 308–13. [DOI] [PubMed] [Google Scholar]
- 31.Abele JT, et al. , Prostate perfusion mapped by technetium-99m macroaggregated albumin after selective arterial injection. J Vasc Interv Radiol, 2015. 26(3): p. 418–25. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins MP, et al. , Intra-arterial photodynamic therapy using 5-ALA in a swine model. European Journal of Vascular and Endovascular Surgery, 1998. 16(4): p. 284–291. [DOI] [PubMed] [Google Scholar]
- 33.Moore RB, et al. , Photodynamic therapy of the canine prostate: intra-arterial drug delivery. Cardiovasc Intervent Radiol, 2008. 31(1): p. 164–76. [DOI] [PubMed] [Google Scholar]
- 34.Osaki T, et al. , Efficacy of 5-Aminolevulinic Acid in Photodynamic Detection and Photodynamic Therapy in Veterinary Medicine. Cancers (Basel), 2019. 11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Z, et al. , Photodynamic therapy for treatment of solid tumors--potential and technical challenges. Technol Cancer Res Treat, 2008. 7(4): p. 309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster TH, et al. , Intratumor administration of the photosensitizer pc 4 affords photodynamic therapy efficacy and selectivity at short drug-light intervals. Transl Oncol, 2010. 3(2): p. 135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafirstein G, et al. , Interstitial Photodynamic Therapy-A Focused Review. Cancers (Basel), 2017. 9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeWitt JM, et al. , Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest Endosc, 2019. 89(2): p. 390–398. [DOI] [PubMed] [Google Scholar]
- 39.Hanada Y, et al. , EUS-guided verteporfin photodynamic therapy for pancreatic cancer. Gastrointest Endosc, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obaid G, et al. , Photonanomedicine: a convergence of photodynamic therapy and nanotechnology. Nanoscale, 2016. 8(25): p. 12471–12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang HC and Hasan T, The “Nano” World in Photodynamic Therapy. Austin Journal of Nanomedicine & Nanotechnology, 2014. 2(3): p. 1020. [PMC free article] [PubMed] [Google Scholar]
- 42.Hamblin MR and Newman EL, Photosensitizer targeting in photodynamic therapy. II. Conjugates of haematoporphyrin with serum lipoproteins. J Photochem Photobiol B, 1994. 26(2): p. 147–57. [DOI] [PubMed] [Google Scholar]
- 43.Reddi E, et al. , Liposome- or LDL-administered Zn (II)-phthalocyanine as a photodynamic agent for tumours. I. Pharmacokinetic properties and phototherapeutic efficiency. Br J Cancer, 1990. 61(3): p. 407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Hallewin MA, et al. , Photodynamic therapy with intratumoral administration of Lipid-Based mTHPC in a model of breast cancer recurrence. Lasers Surg Med, 2008. 40(8): p. 543–9. [DOI] [PubMed] [Google Scholar]
- 45.Derycke AS and de Witte PA, Liposomes for photodynamic therapy. Adv Drug Deliv Rev, 2004. 56(1): p. 17–30. [DOI] [PubMed] [Google Scholar]
- 46.Zhang N, et al. , Multitriggered Tumor-Responsive Drug Delivery Vehicles Based on Protein and Polypeptide Coassembly for Enhanced Photodynamic Tumor Ablation. Small, 2016. 12(43): p. 5936–5943. [DOI] [PubMed] [Google Scholar]
- 47.Chepurna OM, et al. , Core-shell polymeric nanoparticles co-loaded with photosensitizer and organic dye for photodynamic therapy guided by fluorescence imaging in near and short-wave infrared spectral regions. J Nanobiotechnology, 2020. 18(1): p. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gnanasammandhan MK, et al. , Near-IR photoactivation using mesoporous silica-coated NaYF4:Yb,Er/Tm upconversion nanoparticles. Nat Protoc, 2016. 11(4): p. 688–713. [DOI] [PubMed] [Google Scholar]
- 49.Gao W, et al. , Photodynamic Therapy Induced Enhancement of Tumor Vasculature Permeability Using an Upconversion Nanoconstruct for Improved Intratumoral Nanoparticle Delivery in Deep Tissues. Theranostics, 2016. 6(8): p. 1131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng J, et al. , Hollow silica nanoparticles loaded with hydrophobic phthalocyanine for near-infrared photodynamic and photothermal combination therapy. Biomaterials, 2013. 34(32): p. 7905–12. [DOI] [PubMed] [Google Scholar]
- 51.Ashkbar A, et al. , Treatment of breast cancer in vivo by dual photodynamic and photothermal approaches with the aid of curcumin photosensitizer and magnetic nanoparticles. Sci Rep, 2020. 10(1): p. 21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terentyuk G, et al. , Gold nanorods with a hematoporphyrin-loaded silica shell for dual-modality photodynamic and photothermal treatment of tumors in vivo. Nano Research, 2014. 8: p. 325–337. [Google Scholar]
- 53.Huang H-C, et al. , Gold Nanoparticles in Cancer Imaging and Therapeutics. Nano LIFE, 2010. 01(03n04): p. 289–307 [Google Scholar]
- 54.Hu D, et al. , A catalase-loaded hierarchical zeolite as an implantable nanocapsule for ultrasound-guided oxygen self-sufficient photodynamic therapy against pancreatic cancer. Nanoscale, 2018. 10(36): p. 17283–17292. [DOI] [PubMed] [Google Scholar]
- 55.Luo L, et al. , Thermosensitive nanocomposite gel for intra-tumoral two-photon photodynamic therapy. J Control Release, 2019. 298: p. 99–109. [DOI] [PubMed] [Google Scholar]
- 56.Bansal A, et al. , In vivo wireless photonic photodynamic therapy. Proc Natl Acad Sci U S A, 2018. 115(7): p. 1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang HC, et al. , Photodynamic Therapy Synergizes with Irinotecan to Overcome Compensatory Mechanisms and Improve Treatment Outcomes in Pancreatic Cancer. Cancer Res, 2016. 76(5): p. 1066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang HC, et al. , Photodynamic Priming Mitigates Chemotherapeutic Selection Pressures and Improves Drug Delivery. Cancer Res, 2018. 78(2): p. 558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo D, et al. , Doxorubicin encapsulated in stealth liposomes conferred with light-triggered drug release. Biomaterials, 2016. 75: p. 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inglut CT, et al. , Photodynamic Priming Modulates Endothelial Cell-Cell Junction Phenotype for Light-activated Remote Control of Drug Delivery. IEEE J Sel Top Quantum Electron, 2021. 27(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saji H, et al. , Systemic antitumor effect of intratumoral injection of dendritic cells in combination with local photodynamic therapy. Clin Cancer Res, 2006. 12(8): p. 2568–74. [DOI] [PubMed] [Google Scholar]
- 62.Kleinovink JW, et al. , Combination of Photodynamic Therapy and Specific Immunotherapy Efficiently Eradicates Established Tumors. Clin Cancer Res, 2016. 22(6): p. 1459–68. [DOI] [PubMed] [Google Scholar]
- 63.He C, et al. , Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun, 2016. 7: p. 12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gollnick SO and Brackett CM, Enhancement of anti-tumor immunity by photodynamic therapy. Immunol Res, 2010. 46(1–3): p. 216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gallagher-Colombo SM, et al. , Erlotinib Pretreatment Improves Photodynamic Therapy of Non-Small Cell Lung Carcinoma Xenografts via Multiple Mechanisms. Cancer Res, 2015. 75(15): p. 3118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spring BQ, et al. , The role of photodynamic therapy in overcoming cancer drug resistance. Photochem Photobiol Sci, 2015. 14(8): p. 1476–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta S, et al. , Improved targeting of photosensitizers by intratumoral administration of immunoconjugates. Technol Cancer Res Treat, 2004. 3(3): p. 295–301. [DOI] [PubMed] [Google Scholar]
- 68.Ferlay J, et al. , Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer, 2015. 136(5): p. E359–86. [DOI] [PubMed] [Google Scholar]
- 69.Mariategui J, et al. , Comparison of depth of necrosis achieved by CO2- and N2O-cryotherapy. Int J Gynaecol Obstet, 2008. 100(1): p. 24–6. [DOI] [PubMed] [Google Scholar]
- 70.Cadeddu JA, Re: Salvage Percutaneous Cryoablation for Locally Recurrent Renal-Cell Carcinoma after Primary Cryoablation. J Urol, 2017. 197(3 Pt 1): p. 604. [DOI] [PubMed] [Google Scholar]
- 71.Maza M, et al. , Cervical Precancer Treatment in Low- and Middle-Income Countries: A Technology Overview. J Glob Oncol, 2017. 3(4): p. 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pusceddu C, et al. , Cryoablation In The Management Of Breast Cancer: Evidence To Date. Breast Cancer (Dove Med Press), 2019. 11: p. 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dolman L, et al. , Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia: a systematic review. BJOG, 2014. 121(8): p. 929–42. [DOI] [PubMed] [Google Scholar]
- 74.Kuang M, et al. , Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology, 2009. 253(2): p. 552–61. [DOI] [PubMed] [Google Scholar]
- 75.Ebara M, et al. , Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol, 2005. 43(3): p. 458–64. [DOI] [PubMed] [Google Scholar]
- 76.Huang GT, et al. , Percutaneous ethanol injection versus surgical resection for the treatment of small hepatocellular carcinoma: a prospective study. Ann Surg, 2005. 242(1): p. 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heilo A, et al. , Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab, 2011. 96(9): p. 2750–5. [DOI] [PubMed] [Google Scholar]
- 78.Koda M, et al. , Hepatic vascular and bile duct injury after ethanol injection therapy for hepatocellular carcinoma. Gastrointest Radiol, 1992. 17(2): p. 167–9. [DOI] [PubMed] [Google Scholar]
- 79.Facciorusso A, Serviddio G, and Muscatiello N, Local ablative treatments for hepatocellular carcinoma: An updated review. World J Gastrointest Pharmacol Ther, 2016. 7(4): p. 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morhard R, et al. , Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Sci Rep, 2017. 7(1): p. 8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morhard R, et al. , Understanding Factors Governing Distribution Volume of Ethyl Cellulose-Ethanol to Optimize Ablative Therapy in the Liver. IEEE Trans Biomed Eng, 2020. 67(8): p. 2337–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mallidi S, et al. , In vivo evaluation of battery-operated light-emitting diode-based photodynamic therapy efficacy using tumor volume and biomarker expression as endpoints. J Biomed Opt, 2015. 20(4): p. 048003. [DOI] [PMC free article] [PubMed] [Google Scholar]


