Abstract
Age-related macular degeneration (AMD) is a common, degenerative disease of the central retina affecting millions of elderly in the US alone and many more worldwide. A better understanding of the pathophysiology of AMD will be essential for developing new treatments. In this review we discuss the potential impact of complement complex deposition at the choriocapillaris of aging eyes and the relationship between choriocapillaris loss and drusen formation. We further propose a model that integrates genetic and anatomical findings in AMD, and suggest the implications of these findings for future therapies.
Keywords: age-related macular degeneration, choriocapillaris, choroid, complement, RPE
Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is a complex disease characterized by degenerative changes that are most severe in the macular region of the retina. It is characterized at its earliest stages by the formation of drusen beneath the retinal pigment epithelium (RPE), and may progress to severe atrophic degeneration, choroidal neovascularization, hemorrhage and scarring. Due to its detrimental effect on central vision, AMD is a major societal problem in the developed world, with a substantial impact on quality of life in the elderly[1, 2].
Over the last several years, genetic insights into the risk factors that contribute to AMD have emerged. These include genes that encode members of the complement system (most notably complement factor H (CFH), a principal inhibitor of complement activation, but also including CFHR genes, as well as CFB, C2, C3, and in some reports CFI and SERPING1)(reviewed in [3]). Despite recent advances in genetics, the precise mechanism of AMD is not clear, and only very recently has the impact of high- and low-risk genotypes on ocular tissues been studied.
The Complement system
The complement system is an evolutionarily ancient defense mechanism against pathogens. It is comprised of a large array of proteins that are present in the circulation and can be localized to ocular tissues[4]. The complement system can be activated by multiple pathways [5]; however, regardless of the initial pathway, complement activation results in the formation of a membrane spanning pore (the membrane attack complex, or MAC), which can lyse target pathogens. Significantly, when unchecked, the same machinery that is designed to lyse microbes can similarly damage eukaryotic cells [6].
In view of the genetic finding of polymorphisms in complement genes in AMD, understanding where components of the complement system are localized (and where they assert their physiological effects) in the aging macula is an important step. While other byproducts of complement activation may have important biological activities (e.g., proinflammatory anaphylatoxins [7–9]), the MAC is intuitively the endpoint in complement activation that could have the most profound effect on ocular cells.
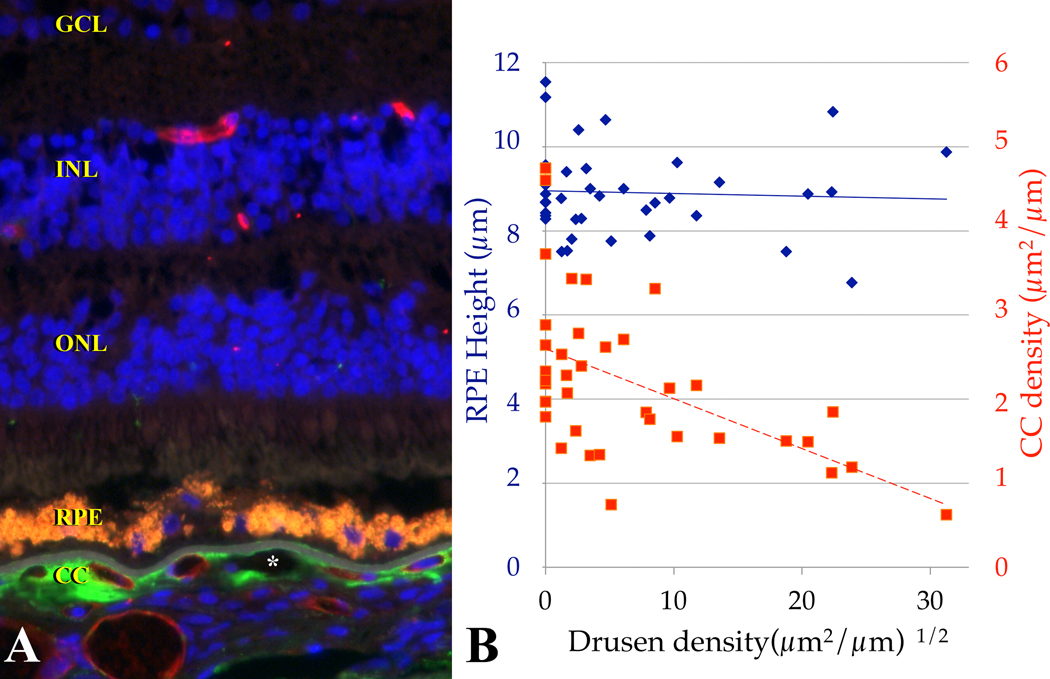
Solitary nodular drusen outside the macula show robust labeling with antibodies directed against the neoepitope of MAC. Basal deposits in the macula, however, show variable immunoreactivity with anti-MAC antibodies. In contrast, we and others have shown that domains surrounding the endothelial cells (EC) of the choriocapillaris appear to be the main sites of MAC deposition in the macula [8, 10–12](Figure 36.1A). While this observation does not preclude RPE injury by MAC as an important event in AMD pathogenesis, it does suggest that the cells under the greatest complement-mediated stress are choriocapillaris EC.
Figure 36.1.
(A) The choriocapillaris is the main site of MAC deposition in the macula (green fluorescence); red fluorescence is UEA-I lectin. RPE lipofuscin appears orange. The asterisk indicates a choriocapillaris ghost vessel. (B) Relationship between choriocapillaris endothelial cell density (open squares, maroon dashed trendline), RPE height (closed diamonds, blue solid trendline) and drusen density. Note that eyes with the lowest vascular density had the largest and most numerous drusen. Modified from [20]; copyright held by ARVO.
High risk genotypes associated with increased complement activation
The recent identification of genetic risk factors associated with AMD has created new opportunities to study disease pathophysiology. Notably, these findings may allow the discovery of molecular changes in the tissues of interest even before the onset of detectable disease. Studies on plasma and serum from AMD patients and controls have shown increased complement activation in individuals with high-risk genotypes[13]. ELISA analyses of MAC in human genotyped RPE-choroid samples show ~ 70% increase in eyes homozygous for the high risk CFH allele[14], with higher levels of MAC more closely associated with genotype than AMD status. These findings in aggregate may suggest that increased MAC deposition precedes an overt AMD phenotype, as discussed below.
Choriocapillaris EC loss occurs with increasing density of drusen
In addition to being the principal site of MAC localization, anatomical and functional changes occur at the level of the choriocapillaris in AMD. Prolonged filling of the choroid in early AMD was noted by Pauleikhoff and coworkers using both indocyanine green and fluorescein[15]. In laser Doppler flow studies, choroidal blood volume and choroidal blood flow are decreased in association with increasing drusen abundance[16]. Proteomic analyses of age-matched control tissues compared to AMD tissues found loss of choriocapillaris proteins CA4 and HLA-A, with persistence of RPE proteins CRALBP and RPE65[17]. In histological studies, McLeod and colleagues found loss of choriocapillaris and preservation of RPE outside of the neovascular lesion in eyes with wet AMD, whereas in atrophic AMD preservation of choroid with loss of the RPE was observed[18]. We employed human donor eyes labeled with the EC-binding lectin from Ulex europaeus (UEA-I) to detect live EC and discriminate them from unoccupied, “ghost” vessels in the choriocapillaris, and related these features to the abundance of drusen. We also assessed the height of the RPE as a measure of RPE viability. Measurement of AMD and control eyes in a masked fashion revealed that as drusen density increases, there is loss of choriocapillaris area and increase in the number of ghost vessels[19]. Superimposing the height of the RPE onto these measurements shows no correlation between RPE height and advancing drusen abundance (Figure 36.1B). However, it should be noted that the presence of an intact RPE monolayer of normal height does not mean that the RPE is completely normal. Morphological and biochemical changes in the RPE may be occurring in eyes with drusen that our measurements do not detect.
The relationship between vascular loss and accumulation of drusen suggests to us that vascular changes occur early in disease, especially since drusen tend not to form over vascular lumens. We recognize the alternative (and non-exclusive) possibility that the RPE, while still present, may fail to deliver necessary trophic factors to choriocapillaris when drusen develop, such that choriocapillaris loss occurs after drusen biogenesis. It is well-known that injury to the RPE can lead to loss of the choriocapillaris as a secondary phenomenon. Nevertheless, our experiments do indicate that there is a correlation between death of choriocapillaris EC and increasing drusen number and size. Further studies of selective choriocapillaris ablation in animals (see below) will refine the relationship between the RPE, Bruch’s membrane deposits, and death of the choriocapillaris.
Choriocapillaris loss and implications for therapy
The photoreceptor cells, RPE, and choriocapillaris function as an interdependent unit (recently reviewed in [20]). In light of the many roles of the RPE in maintaining retinal health, replacement of the RPE may be beneficial in AMD, and a number of trials have begun with this goal. RPE cells derived from allogeneic, embryonic or fetal sources, or more desirably from autogenic induced pluripotent stem cells, can now be placed into the subretinal space. However in the absence of patient-specific evidence of an intact choriocapillaris (becoming possible by improvements in OCT[21, 22]), we suggest caution with this approach. Replacement of photoreceptor cells and/or RPE cells on top of a depleted choriocapillaris may be fruitless.
A model of early AMD based on vascular loss
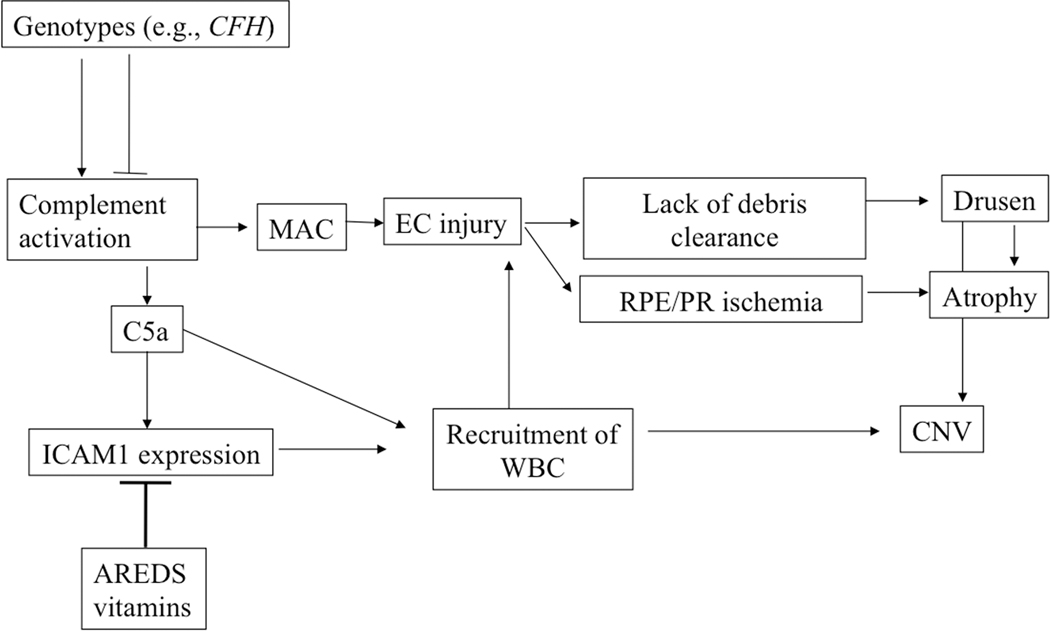
A model that attempts to synthesize the role of genetics, complement, vascular loss, drusen, and AMD pathogenesis is depicted in Figure 36.2. In this model, systemic and local complement activation occurs at the level of the choriocapillaris and this activation is more pronounced in eyes with high-risk CFH genotypes, as we have observed[14]. Increased complement activation in the choriocapillaris yields C5a anaphylatoxin, which activates VEGF expression in the RPE and ICAM1 expression in the choriocapillaris, promoting angiogenesis and leukocyte recruitment, respectively[7, 8]. The generation of MAC over the course of decades leads to loss of choriocapillaris EC (which may have a more limited repertoire of defensive proteins than the RPE). Focal loss of the choriocapillaris results in failure to remove debris from the RPE/Bruch’s membrane and development of drusen, as well as retinal and RPE hypoxia.
Figure 36.2.
Model for AMD pathogenesis. Please see text for details.
It is interesting in this context that a solution of AREDS vitamins has been shown to attenuate at least one step in this pathway (the upregulated ICAM1 expression by choriocapillary EC[23]).
Future directions:
One of the critical questions is the cause-effect relationship between drusen formation and EC loss in the choriocapillaris. A clear determination of the natural history of Bruch’s membrane deposit formation and EC loss needs to be established. Genetically tractable models of vascular dropout will offer insight into this relationship. A better understanding of the mechanisms by which healthy EC are lost in early AMD and the molecular basis of why drusen form in areas depleted of capillary lumens is crucial. Moreover, as noted above, the loss of choroidal EC in AMD suggests that ideal treatments of early AMD may require replacement of lost choroidal EC, through, for example, induced pluripotent stem cells. Advances in the generation, phenotyping, and replacement of these EC will be important in providing cell based therapies for this blinding disease.
Acknowledgments:
Supported in part by NIH grant EY-017451, EY-016822, the Sramek Foundation, and the Hansjoerg E.J.W. Kolder Professorship for Best Disease Research. We regret any omissions in the bibliography, made necessary by page restrictions.
REFERENCES
- 1.Cahill MT, Banks AD, Stinnett SS, Toth CA. Vision-related quality of life in patients with bilateral severe age-related macular degeneration. Ophthalmology 2005; 112(1):152–8. [DOI] [PubMed] [Google Scholar]
- 2.Coleman AL, Yu F, Ensrud KE, Stone KL, Cauley JA, Pedula KL, Hochberg MC, Mangione CM. Impact of age-related macular degeneration on vision-specific quality of life: Follow-up from the 10-year and 15-year visits of the Study of Osteoporotic Fractures. American journal of ophthalmology 2010; 150(5):683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorin MB. Genetic insights into age-related macular degeneration: controversies addressing risk, causality, and therapeutics. Molecular aspects of medicine 2012; 33(4):467–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhutto IA, Baba T, Merges C, Juriasinghani V, McLeod DS, Lutty GA. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. The British journal of ophthalmology 2011; 95(9):1323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunological reviews 2008; 223:300–16. [DOI] [PubMed] [Google Scholar]
- 6.Neher MD, Weckbach S, Flierl MA, Huber-Lang MS, Stahel PF. Molecular mechanisms of inflammation and tissue injury after major trauma--is complement the “bad guy”? Journal of biomedical science 2011; 18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proceedings of the National Academy of Sciences of the United States of America 2006; 103(7):2328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skeie JM, Fingert JH, Russell SR, Stone EM, Mullins RF. Complement component C5a activates ICAM-1 expression on human choroidal endothelial cells. Investigative ophthalmology & visual science 2010; 51(10):5336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B, Wei L, Meyerle C, Tuo J, Sen HN, Li Z, Chakrabarty S, Agron E, Chan CC, Klein ML, Chew E, Ferris F, Nussenblatt RB. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. Journal of translational medicine 2011; 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerl VB, Bohl J, Pitz S, Stoffelns B, Pfeiffer N, Bhakdi S. Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Investigative ophthalmology & visual science 2002; 43(4):1104–8. [PubMed] [Google Scholar]
- 11.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America 2005; 102(20):7227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seth A, Cui J, To E, Kwee M, Matsubara J. Complement-associated deposits in the human retina. Investigative ophthalmology & visual science 2008; 49(2):743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecker LA, Edwards AO. Genetic control of complement activation in humans and age related macular degeneration. Advances in experimental medicine and biology 2010; 703:49–62. [DOI] [PubMed] [Google Scholar]
- 14.Mullins RF, Dewald AD, Streb LM, Wang K, Kuehn MH, Stone EM. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Experimental eye research 2011; 93(4):565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pauleikhoff D, Spital G, Radermacher M, Brumm GA, Lommatzsch A, Bird AC. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Archives of ophthalmology 1999; 117(10):1353–8. [DOI] [PubMed] [Google Scholar]
- 16.Berenberg TL, Metelitsina TI, Madow B, Dai Y, Ying GS, Dupont JC, Grunwald L, Brucker AJ, Grunwald JE. The association between drusen extent and foveolar choroidal blood flow in age-related macular degeneration. Retina 2012; 32(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan X, Gu X, Crabb JS, Yue X, Shadrach K, Hollyfield JG, Crabb JW. Quantitative proteomics: comparison of the macular Bruch membrane/choroid complex from age-related macular degeneration and normal eyes. Molecular & cellular proteomics : MCP 2010; 9(6):1031–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investigative ophthalmology & visual science 2009; 50(10):4982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Investigative ophthalmology & visual science 2011; 52(3):1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Molecular aspects of medicine 2012; 33(4):295–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Lee K, Niemeijer M, Mullins RF, Sonka M, Abramoff MD. Automated segmentation of the choroid from clinical SD-OCT. Investigative ophthalmology & visual science 2012; 53(12):7510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smailhodzic D, Klaver CC, Klevering BJ, Boon CJ, Groenewoud JM, Kirchhof B, Daha MR, den Hollander AI, Hoyng CB. Risk alleles in CFH and ARMS2 are independently associated with systemic complement activation in age-related macular degeneration. Ophthalmology 2012; 119(2):339–46. [DOI] [PubMed] [Google Scholar]
- 23.Zeng S, Hernandez J, Mullins RF. Effects of antioxidant components of AREDS vitamins and zinc ions on endothelial cell activation: implications for macular degeneration. Investigative ophthalmology & visual science 2012; 53(2):1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]