Abstract
Purpose of Review:
This review provides an overview of the neurobiological mechanisms underlying opioid use disorder (OUD) drawing from genetic, functional and structural magnetic resonance imaging (MRI) research.
Recent findings:
Preliminary evidence suggests an association between OUD and specific variants of the DRD2, δ-opioid receptor 1 (OPRD1) and μ-opioid receptor 1 (OPRM1) genes. Additionally, MRI research indicates functional and structural alterations in striatal and corticolimbic brain regions and pathways underlying reward, emotion/stress and cognitive control processes among individuals with OUD.
Summary:
Individual differences in genetic and functional and structural brain-based features are correlated with differences in OUD severity and treatment outcomes, and therefore may potentially one day be used to inform OUD treatment selection. However, given the heterogeneous findings reported, further longitudinal research across different stages of opioid addiction is needed to yield a convergent characterization of OUD and improve treatment and prevention.
Keywords: addiction, neuroimaging, resting-state, fMRI, voxel-based morphometry, diffusion weighted imaging
Introduction
Non-medical opioid use is a serious global public health problem and is associated with adverse physical and psychosocial consequences for the individual. Regular and prolonged opioid use may result in opioid use disorder (OUD), a chronic relapsing disorder characterized by diminished control over drug use, and impaired behavioral and psychosocial functioning (1). Recent estimates indicate that nearly three million individuals in the United States were diagnosed with OUD in 2015, thereby placing enormous economic burden on the healthcare system. Furthermore, one-third of individuals with OUD undergoing treatment report using opioids before the age of 18 (2), and mortality rates attributed to opioid overdose have quadrupled since 1999 (3). Thus, improved prevention and treatment strategies are urgently needed.
Both pharmacological and behavioral interventions show promise in treating OUD. Medication-assisted treatments (MATs) with opioidergic agents such as methadone (agonist), buprenorphine (partial agonist) and naltrexone (antagonist) are widely used as first-line OUD treatment (4, 5). In addition, empirical evidence for cognitive behavioral therapy as an effective adjunct to MAT for OUD has grown (6–8). However, these evidence-based interventions still yield variable outcomes across individuals and the clinical course of OUD is often marred by multiple unsuccessful treatment attempts (9–13). Hence, further research is required to identify individual difference factors that confer risk and resilience for OUD acquisition and relapse in order to improve existing interventions and develop novel ones.
Factors underlying the onset, maintenance and relapse of OUD are multifold. Addiction research is starting to gain a deeper understanding of how psychological processes including emotions, cognitions and behaviors are neurally mapped and, in turn, how genetic and socioenvironmental factors influence neural development and functioning. Genetics research holds promise in identifying genetic variants that confer OUD risk and resilience and inter-individual differences in treatment response. Additionally, neuroimaging studies have demonstrated functional and structural neural alterations among OUD individuals, which may play a role in treatment outcomes.
Extant data suggest that the capacity to resist drug craving and remain abstinent depends on dynamic interactions between cognitive and inhibitory control systems including the prefrontal cortex (PFC) and subcortical limbic systems underlying reward, emotion/stress regulation and motivational processes relevant to drug-seeking and taking (14). Such insight has advanced neurobiological theories of OUD and substance use disorders (SUDs) more generally and may facilitate the search for reliable neural biomarkers that underlie OUD pathophysiology, track disorder severity and predict treatment response (15).
The focus of this present review is to highlight recent and notable findings in addictions neuroscience that afford greater understanding of OUD pathophysiology, and to discuss the clinical implications of these findings for improving prevention and treatment efforts. We specifically focus on genetic contributions to OUD and studies using functional and structural MRI to examine task-based neural activations, resting-state functional connectivity (RSFC), brain morphometry or white matter microstructural features.
Endogenous opioid system
The endogenous opioid system is composed of a group of receptors and peptides that are broadly distributed throughout the peripheral and central nervous systems (16). There are three main classes of G protein-coupled opioid receptors (mu(μ), MOR; delta(δ), DOR; and kappa(κ), KOR) (17, 18) which are stimulated by three families of endogenous opioid peptides (β-endorphins, enkephalins and dynorphins) (19). Although one gene encodes for each receptor (OPRM1, OPRD1 and OPRK1), there is greater than 60% overlap in their amino acid composition (20, 21). These receptors are highly expressed in brain areas responsible for reward, stress and analgesic processes (e.g. ventral tegmental area, VTA; nucleus accumbens, NAcc; prefrontal cortex, PFC; thalamus; hypothalamus and extended amygdala) and are implicated in the modulation of these circuits (22, 23). Opioid receptors can also be activated by exogenous alkaloid opiates such as morphine, heroin and other synthetic opioids (e.g., fentanyl).
MORs have been the most extensively studied because of their role in mediating the actions of clinically relevant analgesic agents and drugs of abuse (24). Activation of MORs stimulates VTA dopaminergic release in the ventral striatum and MPFC by inhibiting local γ-aminobutyric acid (GABA) interneurons (25). Additionally, MORs are found in pain-modulating descending pathways (e.g. periaqueductal gray area and medulla locus coeruleus) and, when activated, MORs in these sites directly inhibit neurons which block spinal cord pain transmission (20). Hence, MORs in two distinct pathways underpin euphoric and analgesic effects of opioids, which highlights both their therapeutic value in alleviating chronic pain and also their liability for abuse.
MORs are critically involved in encoding for rewarding and reinforcing drug effects, as demonstrated by the absence of physiological and rewarding effects of morphine in MOR knockout mice (16, 26). In contrast, self-administration of morphine is intact in DOR knockout mice, indicating that DORs are not essential for morphine reward (16). In addition, whereas DOR agonism in humans produces anxiolytic effects, KOR agonism produces dysphoria (16, 27) Thus, current neurobiological models of addictions emphasize the role of MORs during the consummatory phase of addiction (e.g., binge/intoxication), but the role of KORs and DORs during withdrawal and negative affective states (16). For a recent review, see (16).
Pharmacotherapy for OUD
Methadone, buprenorphine and naltrexone are FDA-approved opioidergic medications for long-term OUD treatment, but there are pharmacological differences among these medications (5). Methadone is a long-acting, synthetic full MOR agonist with a long half-life, which allows it to act as an opioid substitute while reducing the euphoria and withdrawal associated with opioids of abuse (28, 29). Buprenorphine, on the other hand, is a partial MOR agonist and a KOR antagonist, which diminishes euphoria in the presence of other MOR agonists (5). Buprenorphine may also be combined with naloxone, an opioid antagonist, in order to prevent abuse (i.e., Suboxone). Finally, naltrexone is a full competitive opioid receptor antagonist that blocks the rewarding effects of opioids and precipitates marked withdrawal if administered to active opioid users (28). Common forms of naltrexone for OUD include oral (daily) naltrexone and injectable (monthly) extended-release naltrexone (XR-NTX).
Among the three MATs, methadone is the most extensively studied. A Cochrane meta-analysis demonstrated that methadone yielded greater treatment retention and reduction in heroin use than no MAT for heroin-dependent individuals (30). Another Cochrane meta-analysis found that buprenorphine had to be administered at higher doses (>16mg) to yield significantly better treatment outcomes than placebo and achieve comparable efficacy to methadone (31). Whereas methadone treatment requires daily visits to a methadone clinic, recent findings suggest that buprenorphine can be effectively prescribed on an outpatient basis, and may represent a promising approach for encouraging treatment engagement and reducing opioid use among individuals with OUD presenting to the emergency department (32). Research support for naltrexone’s efficacy is mixed (33). Although a Cochrane meta-analysis did not demonstrate its efficacy in treatment retention or reduction in opioid use relative to placebo (34), extended-release naltrexone (XRNTX) has shown some promise in sustaining abstinence (35), with a recent randomized clinical trial finding XRNTX to be as effective as buprenorphine for treatment retention and short term abstinence from opioids (36). However, there are substantial barriers to successful XRNTX induction and many patients discontinue treatment prematurely, suggesting that further research is needed to improve the clinical utility of XRNTX in real-world settings (33). To date, no study has directly compared all three MATs. While the current evidence base suggests successful OUD management with methadone and buprenorphine, a meta-analysis of MAT treatment outcomes suggests that a substantial percentage of opioid-maintained individuals fail to sustain opioid abstinence (31). Additional research is needed to identify and address barriers to successful MAT treatment (37, 38). Moreover, there are currently no biomarkers to predict which pharmacotherapy or dose may be most effective at the individual level (39). For a comprehensive review of pharmacotherapies for OUD, see (28).
Pharmacotherapy research incorporating genetic and neuroimaging assessments may provide key insights into the neurobiology of OUD via identification of individual difference factors contributing to variability in treatment responses. In the following sections, we review recent findings from translational studies using these approaches.
Genetic research
Genetic variability may influence the complex phenotype of OUD vulnerability and treatment response. For instance, a single nucleotide polymorphism (SNP) at rs1076560 in the DRD2 gene, which is involved in the dopaminergic signaling pathway, has been associated with OUD in a sample of more than 1,300 European and African Americans (40). Additionally, specific variants in genes within the endogenous opioid system, particularly in the OPRM1 gene (e.g. rs1799971, A118G), have been associated with OUD (41, 42). In contrast, meta-analyses have not found significant associations between the OPRM1 rs1799971 genetic marker and initial risk for OUD (43, 44). Recently, Woodcock and colleagues proposed that, while genetic factors may contribute to OUD vulnerability, they are also likely to be implicated in different stages of opioid dependence (45). Consistent with this, they demonstrated that 118G allele carriers reported significantly greater heroin use consequences and multiple quit-attempts compared to 118A/A homozygotes in a sample of Caucasian male chronic heroin users.
Several studies have also identified genetic variants associated with better MAT outcomes, which may represent candidate markers of treatment response (46–48). For example, an intronic OPRD1 SNP, rs678849, differentially predicted methadone and buprenorphine treatment outcomes in African-American, but not European-American, participants such that methadone-maintained individuals with CC (vs. CT or TT) genotype had increased likelihoods of opioid-positive urine screens whereas buprenorphine-maintained individuals showed the opposite pattern (46). In a different study, sex-specific analyses revealed that two intronic OPRD1 SNPs predicted treatment outcome in buprenorphine-maintained individuals, such that females with CC (vs. A/A) genotype at rs529520 and G/G (vs. A/A or A/G) genotype at rs581111 had significantly lower opioid-positive urine tests (47). Other recent research demonstrated that methadone-maintained European-Americans with the A/A (vs. A/G and G/G) genotype at rs10485058 of the OPRM1 gene were less likely to have opioid-positive urine tests and that the A allele was also associated with lower self-reported relapse rates in an independent sample (48). Additionally, a genome-wide association study yielded a significant association between methadone dose and one SNP, located slightly upstream of the OPRM1 (49).
Collectively, the above data indicate that individual differences in genes encoding for aspects of dopaminergic and opioidergic functioning contribute to vulnerabilities for OUD. They further raise the possibility that inter-individual and sex-specific genotypic differences may one day be used to inform MAT selection and dosing requirements, thereby improving individualized OUD treatment efforts. However, prospective replications of these findings along with further testing in independent samples are warranted.
Current neurobiological theories of addiction and relevance to OUD
Prevailing neurobiological theories conceptualize addiction as a chronic, relapsing brain disorder in which initial, voluntary drug use progresses into compulsive, uncontrolled drug-seeking and taking (50). This process has been conceptualized as a three-stage cycle of binging/intoxication, negative emotionality and craving during withdrawal (14), and is associated with lasting neuroadaptations with prolonged drug use. During intoxication, drug-taking activates the endogenous opioid system and elicits large, rapid dopaminergic increases in mesocorticolimbic regions including midbrain (e.g., VTA), striatum (e.g., NAcc, dorsal striatum) and PFC (e.g., anterior cingulate and orbitofrontal cortices; ACC and OFC), which positively reinforces further drug-taking and strengthens conditioned associations between drug-related stimuli and the expectation of reward (51). With prolonged drug use, lasting neuroadaptations such as an overvaluation of drug-related stimuli and a decreased sensitivity to natural and non-drug-related rewards are thought to occur (14, 52).
Within the above framework, during an opioid-deprived state, the emergence of negative emotionality or dysphoria may perpetuate drug-seeking through negative reinforcement mechanisms (14). Similarly, during withdrawal, brain stress systems (e.g. corticotropin releasing factor, norepinephrine) and neural circuits (e.g. the extended amygdala) are implicated, producing aversive or stress-like states. Moreover, enhanced sensitivity to drug-related cues and negative emotional states characterizes increased drug cravings that may motivate further drug-seeking behavior and increase the likelihood of relapse (14). Continued drug-taking is likely mediated by dysfunctions in prefrontal cortical regions, which underpin executive functioning processes including cognitive and inhibitory control. Prolonged drug use likely contributes to impairments in decision-making and behavioral inhibition that diminish the ability to resist drug craving and maintain abstinence.
Current SUD diagnostic criteria and prevailing neurobiological theories of addiction emphasize shared neurobiological markers and psychological processes across different SUDs (1, 14). However, distinct neurobiological mechanisms have also been demonstrated across SUDs (53). Preclinical studies indicate that specific lesions to NAcc dopamine terminals inhibits cocaine, but not heroin self-administration (54). This suggests that independent neural systems may mediate the reinforcing effects of cocaine and heroin. Moreover, relapse propensity appears to be substance-specific and depends on the environmental setting. Preclinical and clinical studies indicate that heroin is preferentially used at home whereas the opposite pattern is true for cocaine (55, 56). Such contextualization of substance-specific affect was linked to a double dissociation of neural activity in the left PFC, left dorsal caudate and bilateral cerebellum (56). Greater increases in fronto-striatal-cerebellar activations were found when individuals imagined using heroin outside the home and cocaine at home (less preferred) compared to heroin at home and cocaine outside home (preferred).
Studies have also identified motivational differences underlying different substance-use behaviors. Individuals with OUD identify pain and physical discomfort as primary motives for drug use, whereas cocaine users describe their motivations for use as stemming from tempting urges (57). Consistent with this, ecological momentary assessment data indicate that decisions to use cocaine are preceded by drug exposure and by positive affective states but that opioid craving is preceded by negative affective states (58). A recent machine-learning study identified substance-specific behavioral markers for OUD and cocaine use disorder, in which the personality trait of impulsivity was a predictor of cocaine use disorder, but not OUD (59, 60). It is important to take into account such neural, behavioral, environmental and motivational factors that may distinguish OUD from other SUDs, as they could shed additional light on OUD pathophysiology and guide development of tailored, substance-specific treatments.
Neuroimaging research: Task-based fMRI
Reward processing and learning.
Task-based fMRI studies have demonstrated heightened corticolimbic neural responses to opioid-related stimuli among individuals with OUD, which may decrease following MATs and with extended abstinence (Figure 1) (61). Compared to controls, methadone-maintained individuals show greater neural responses in reward areas (e.g., NAcc) when viewing heroin-related (vs. neutral) cues, which were sustained in subsequent relapsers relative to non-relapsers at three month follow-up (62). Moreover, two cross-sectional studies demonstrated that, compared to long-term OUD abstainers, short-term abstainers exhibited increased neural activations in the ACC, MPFC and caudate (63) and in the hippocampus, insula, thalamus and dorsal striatum (64) when presented with heroin (vs. neutral) stimuli.
Figure 1 -.
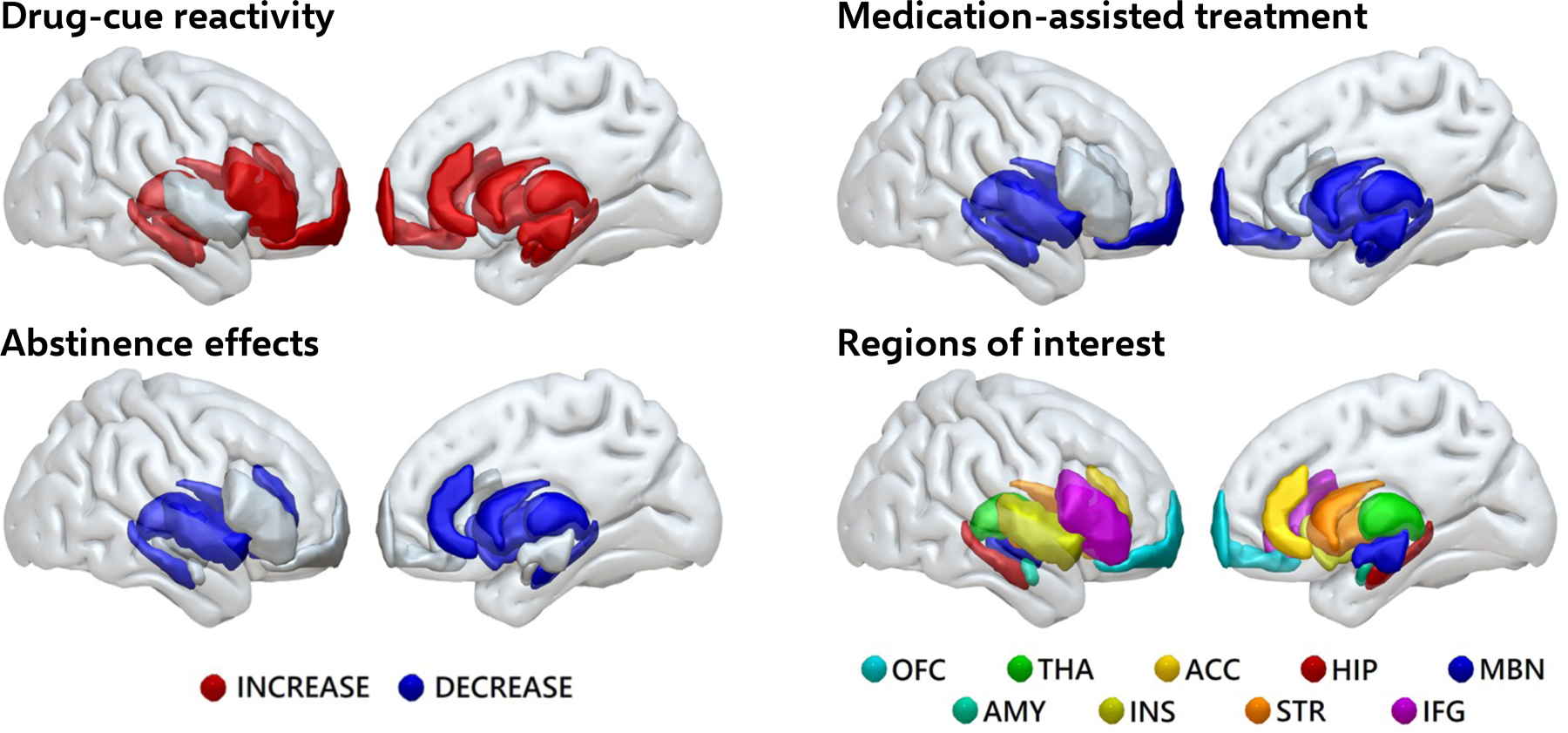
Summary of fMRI findings from drug cue reactivity studies in opioid-use disorder
Individuals with opioid-use disorder exhibit heightened brain responses to drug cues. These are reduced with medication assisted treatments and following prolonged abstinence. Based on data reviewed in: Moningka H, Lichenstein S, Worhunsky PD, DeVito EE, Scheinost D, & Yip SW. Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacology, 2018.
Abbreviations: OFC, orbitofrontal cortex; THA, thalamus; ACC, anterior cingulate cortex; HIP, hippocampus; MBN, midbrain; AMY, amygdala; INS, insula; STR, striatum; IFG, inferior frontal gyrus.
According to Volkow and colleagues (2016), addiction involves the desensitization of reward systems, which diminishes the pleasure derived from natural, non-drug-related rewards (65). Two fMRI studies evaluating neural responses to affectively positive stimuli relative to opioid-related stimuli in recently detoxified and abstinent OUD individuals found attenuated activations in amygdalar and posterior cortical regions (66) along with the anterior cortical regions (67). However, no fMRI study has directly compared neural responses during exposure to opioid-related cues and naturally appetitive reinforcers (e.g. food, sex and social interactions). Studies investigating non-drug-related reward-based learning processes (e.g. anticipation vs. outcome of rewards vs. loss) also found diminished neural responses in the insula and inferior frontal gyrus in methadone-maintained individuals compared to controls (68, 69).
Two recent pharmacotherapy studies suggest that XRNTX treatment may modulate neural responses to drug- and non-drug-related stimuli in individuals with OUD. During XRNTX treatment, individuals with OUD exhibited reduced NAcc and mOFC cue-reactivity to opioid-related (vs. control) stimuli, which was correlated with reductions in withdrawal severity (70). However, this study was not placebo-controlled. The second study showed that after two weeks of XRNTX, ventral striatal activations to baby-related schema increased, which was correlated with reduced opioid-craving (71). This may suggest a return of non-drug-related rewards associated with caregiving, which may be otherwise dampened when addicted to opioids. Hence, taken together, these task-based findings suggest that OUD individuals may demonstrate heightened neural responses towards opioid-related (vs. non-drug-related) stimuli, which may normalize after sustained abstinence or pharmacotherapy.
Emotion processing/regulation.
Individuals with OUD show dampened amygdala and insula activation relative to controls when viewing negative images (66). Similarly, when exposed to neutral distractor images during an emotional oddball task, individuals with comorbid borderline personality and OUD demonstrated less amygdala activation relative to individuals without either disorder (72). However, in contrast, more recent studies of OUD individuals undergoing heroin maintenance therapy reported heightened amygdala responses to negative emotional faces compared to controls, which were attenuated following heroin administration (73). These results converge with predominant neurobiological theories of addiction that propose emotional dysregulation as a key component of the addiction cycle. However, the precise nature of emotion regulation difficulties and their neural underpinnings in OUD remain to be fully elucidated.
Cognitive and inhibitory control.
Task-based fMRI studies indicate that individuals with OUD exhibit greater activations to heroin-related cues in neural regions underpinning executive functioning, which are correlated with subjective reports of craving (62). Moreover, altered PFC activations have been reported in individuals with OUD during inhibitory control tasks (e.g., Go/No-Go, Stroop) (74–76), which may relate to difficulties in exerting control over drug cravings. Nevertheless, the directionality of these findings is inconsistent: two studies reported decreased lateral PFC recruitment following heroin administration (74, 76) whereas one reported the reverse pattern (75). Such inconsistencies could arise from methodological differences or participant characteristics including variability in abstinence duration. Further longitudinal studies are needed to obtain reliable neural characterization of cognitive control in OUD after extended abstinence.
It should be noted that a large proportion of the above mentioned task-based fMRI studies were conducted approximately a decade ago. In light of this, further research investigating task-related neural responses underlying reward, emotion and cognitive control processes along with their relation to treatment outcomes is warranted. For a systematic review of fMRI studies in OUD, see (61).
Neuroimaging research: Resting-state functional connectivity (RSFC)
As described above, studies assessing neural activity during task performance have facilitated the identification of brain regions and complex processes related to OUD. Complimentary insights may be yielded by investigating the dynamics of system-level neural circuits while the brain is ‘at-rest’, as in RSFC analyses (77). RSFC reflects the degree to which intrinsic fluctuations of hemodynamic signals in different neural regions are temporally correlated in the absence of an explicit task (78), thereby providing a measure of the brain’s intrinsic functional organization (79). Similar to task-based fMRI studies, RSFC research has identified alterations in reward circuits (e.g., the MCL system), emotion and stress circuits (e.g., the extended amygdala) and cognitive control circuits in OUD individuals. Moreover, alterations in large-scale ICN interactions, especially among the DMN, SN and FPN have been reported in SUDs and are implicated in OUD as well (77, 80).
RSFC studies have reported enhanced connectivity within MCL circuitry and between MCL and other subcortical and cortical areas in OUD individuals. For instance, Ma et al. (2010) reported increased RSFC in the MCL system between the NAcc and ventromedial PFC in a sample of abstinent, methadone-maintained individuals (81). In contrast, decreased RSFC between the MCL system and other subcortical and cortical areas have also been reported. For example, prescription-opioid-dependent individuals exhibited RSFC reductions between the NAcc and right anterior insula and ventromedial PFC, which were associated with longer periods of opioid dependence (82). Similarly, another study reported widespread RSFC reductions between the insula and inferior OFC, putamen and caudate areas (83). Given between-study differences in clinical populations and analysis methods, further work directly comparing OUD subgroups (e.g., methadone maintained individuals versus prescription opioid users) is needed.
Some research suggests that individuals with addictions also exhibit RSFC alterations within large-scale, ‘canonical’ ICNs. These include the 1) DMN, comprising the posterior cingulate cortex, MPFC, and precuneus; 2) SN, comprising the dorsal ACC and the insula (84) and 3) frontoparietal network (FPN), comprising lateral prefrontal and posterior parietal cortical areas (85). The DMN is implicated in task-independent processing including self-referential thinking (86), whereas the FPN supports a range of cognitive functions (e.g. working memory and attention) (87). Evidence indicates that the DMN and FPN are ‘anticorrelated’, such that the DMN tends to deactivate when the FPN is activated, for instance, during cognitive tasks (88). The SN is implicated in salience detection and processing and is posited to mediate interactions between DMN and FPN (89).
Sutherland et al. (2012) proposed a triple-network model of SUDs in which the SN induces aberrant salience towards rewarding drug effects and internal withdrawal symptoms during abstinence, thereby biasing processing towards the DMN instead of the ECN. This model is thus consistent with the binge-intoxication and negative emotionality stages of Koob and Volkow’s three-stage model (2010). This shift in network dynamics is exemplified by findings of increased DMN-SN and decreased FPN-SN RSFC in acutely abstinent smokers, which was correlated with subjective craving reports and working-memory task performance (90).
Nevertheless, few studies have investigated the functioning of these large-scale networks in OUD individuals. Among the RSFC studies conducted, DMN alterations have been consistently reported across different stages of opioid dependency. For instance, reduced anterior DMN RSFC has been demonstrated in recently detoxified OUD individuals compared to controls (91), in OUD relapsers relative to abstainers (92), and in active heroin users compared to controls (93). Moreover, reduced DMN RSFC has been associated with longer lifetime heroin use (94). Findings for other resting-state networks have been more mixed. Decreased RSFC within the SN (insula) and FPN (dorsolateral PFC and parietal areas) have been reported in methadone-maintained heroin users relative to controls (83, 94, 95), with the former finding being associated with a lower risk of an opiate-positive urine test (83). Nevertheless, other studies have also reported the reverse finding of increased insula and ACC RSFC (96, 97). Finally, one recent prospective study has investigated between-network interactions among these three large-scale ICNs specifically in methadone-maintained individuals (80). Findings suggest that relative to controls, methadone-maintained individuals showed increased DMN-SN RSFC, which was associated with a greater likelihood of relapse (80).
Taken together, these findings lend support to the triple-network model’s hypothesis of network alterations among the DMN, SN and FPN, albeit the directionality of findings is inconsistent. Moreover, existing findings raise the possibility that altered network interactions may confer risk of future relapse in OUD. Therefore, these studies suggest that OUD is characterized by systems-level alterations, extending beyond a single brain region or network. However, more studies need to be conducted to further ascertain these systems-level predictors of OUD treatment outcome and whether these networks could serve as potential treatment targets.
Neuroimaging research: Structural MRI
Studies using voxel-based morphometry indicate that, compared to healthy controls, individuals with OUD exhibit significant reductions in gray matter within the PFC, ACC, insular and temporal regions (95, 98–100). These reductions have further been associated with duration of heroin use (95, 99, 100) and impulsivity (100). Reduced gray matter volumes for other regions involved in reward and emotion processing (e.g. NAcc and amygdala) have also been noted (82, 101). In addition, a recent meta-analysis concluded that OUD involves structural alterations within two separate, but largely overlapping, circuits: (i) a fronto-cerebellar system underlying impulsivity, compulsivity and emotion processing and (ii) a fronto-insular system implicated in cognitive and decision-making processes (102).
Preliminary data raise the possibility of some structural recovery with sustained abstinence. Wang et al. (2012) found significantly reduced gray matter densities in frontal cortical, cingulate and occipital regions in heroin-dependent individuals following three days of abstinence compared to controls. However, the differences in some regions (i.e., superior frontal gyrus) no longer reached significance following 30 days of abstinence (103). Moreover, another study found that currently methadone-maintained individuals exhibit more widespread decreases in corticostriatal gray matter compared to an abstinent group, which only had midbrain-thalamic gray matter reductions (104). Longitudinal studies, however, are needed to determine whether sustained abstinence reduces abnormalities or whether fewer structural abnormalities precede successful abstinence.
Diffusion-weighted MRI (dMRI) has also been used to assess white matter microstructures in OUD individuals. A number of dMRI studies have yielded findings of altered white matter ‘integrity’ in frontal, temporal and parietal areas in OUD individuals relative to controls (105–107). Disruptions in specific tracts connecting fronto-parietal, occipital and temporal regions (e.g., superior longitudinal fasciculus) (105, 108, 109) and amygdala connections with subocortical and limbic areas (e.g. stria terminalis, ventral amygdalofugal, uncinate fasciculus) have also been reported (82). Consistent with neurobiological theories of SUDs, these findings reveal microstructural white matter alterations in regions implicated in cognitive control and emotional processing. However, between-group differences have not been consistently reported across studies (110). The heterogeneity in dMRI findings could be attributed to different methodologies (e.g. whole-brain voxel-based vs. region-of-interest vs. tract-based spatial statistic analyses) (105) or differences in clinical characteristics (e.g., abstinence durations) between studies. As dMRI is particularly sensitive to motion, seemingly contrasting findings may also be related to uncontrolled motion effects within and across studies.
Notably, white matter alterations have also been linked to opioid use duration (105, 107) and shown to decrease following prolonged abstinence (109). Furthermore, a recent study demonstrated an association between reduced white matter and rates of opioid-positive urine tests at follow-up (111). These data raise the possibility that white matter disruptions may be associated with OUD relapse. As white matter and RSFC alterations may be linked (112), future work combining these modalities might inform understanding of OUD vulnerability, disease progression and treatment-related changes.
Conclusion
The evidence presented above suggests that genetic as well as functional and structural neural features are implicated in OUD. These neurobiological alterations, together with other psychological and socioenvironmental factors, may contribute to the multifaceted phenotype of opioid addiction. Prior studies have shown that dopaminergic and endogenous opioidergic systems are implicated in the potent euphoric and analgesic effects of opioids. Genetics research has identified several candidate genes (e.g., DRD2 and OPRM1) as implicated in OUD vulnerability and treatment response. FMRI evidence from both task-based (i.e., drug cue reactivity, emotional processing, Stroop and Go/No-Go tasks) and resting-state studies suggest alterations in brain regions and networks (e.g., MCL, DMN, SN and FPN circuitry) subserving reward, emotion/stress and cognitive control processes. This lends support to neurobiological theories of addiction (e.g., three-stage and triple-network models) in which prolonged substance use is theorized to result in neuroadaptations including heightened salience of drug cues, difficulties in emotion/stress regulation and impairments in cognitive control (14, 77). Finally, widespread structural alterations in gray and white matter in prefrontal, parietal, temporal, occipital, insular, and amygdalar regions have been reported in OUD. These neurobiological alterations have also been associated with neurocognitive and behavioral indices as well as measures of treatment outcome.
However, it should be noted that the studies presented above report heterogeneous findings and possess a number of limitations. For instance, the above genetics studies reported either inconsistent or little to no associations between specific SNPs in candidate genes (e.g., OPRM1) and OUD, highlighting the need for further large studies in independent samples. Similarly, the functional and structural neuroimaging studies report inconsistencies in relation to the anatomical specificity and directionality of findings. Such heterogeneity could be attributed to differences including (but not limited to) the stage of OUD (e.g., recently detoxified vs. abstinent), medication status (e.g., methadone- vs. buprenorphine) and abstinence durations. Moreover, there is a lack of neuroimaging studies including women and those that conduct sex-specific analyses, which should be addressed in future studies. Most of the genetic and neuroimaging studies also focus on heroin-dependent individuals. Given the changing face of the opioid epidemic in which prescription opioid misuse has increased substantially (113), further studies should examine this population and examine whether shared versus specific neurobiological mechanisms underlie different OUD subgroups.
It is still unclear whether identified neurobiological alterations confer risk for OUD or manifest as a result of chronic opioid use. Prospectively investigating these putative neurobiological markers in opioid naïve and occasional users versus those who transition to dependency may provide valuable insights as to why some individuals are conferred with resilience (114). Similarly, investigating OUD individuals as they progress through early remission and longer term abstinence may provide insight into the neurobiological mechanisms underlying different stages of recovery.
Finally, it is important to keep in mind that genetics and neuroimaging represent only a small part of a larger effort to understand the etiology and treatment of opioid addiction. Addiction is a complex multifaceted construct that warrants investigation from different perspectives and multiple levels of analyses. Integrating socioenvironmental factors into addictions neuroscience research (115) and employing a multi-modal, longitudinal approach may further yield a superior characterization of OUD across different stages of addiction and recovery. Moreover, further research identifying reliable neurobiological markers underlying successful MAT outcomes may one day provide a basis to tailor treatments at the individual level (i.e., precision medicine). Recently, there has been increasing interest in employing data-driven approaches (e.g., machine-learning) to probe the neural basis of addictive behavior and treatment response using a wide range of measures including whole-brain functional connectivity data, genetics, psychosocial, and behavioral data (60, 116, 117). Such studies have promise for comprehensively mapping the developmental trajectory of OUD, identifying clinically relevant markers to improve prevention and treatment efforts and for informing the development of individualized, optimized interventions.
Funding:
This work was supported by NIDA grants T32 DA022975, K01DA039299 and R21DA045969.
Footnotes
Conflict of Interest:
Hestia Moningka, and Drs. Sarah Lichenstein and Sarah Yip declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
References:
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Diagnostic and statistical manual of mental disorders : DSM-5: Fifth edition. Arlington, VA: : American Psychiatric Publishing, [2013] ©2013; 2013. [Google Scholar]
- 2.Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in Receipt of Buprenorphine and Naltrexone for Opioid Use Disorder Among Adolescents and Young Adults, 2001–2014. JAMA Pediatrics. 2017;171(8):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Donnell JK, Gladden RM, Seth P. Trends in Deaths Involving Heroin and Synthetic Opioids Excluding Methadone, and Law Enforcement Drug Product Reports, by Census Region - United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(34):897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuckit MA. Treatment of opioid-use disorders. New England Journal of Medicine. 2016;375(4):357–68. [DOI] [PubMed] [Google Scholar]
- 5.Bart G Maintenance Medication for Opiate Addiction: The Foundation of Recovery. J Addict Dis. 2012;31(3):207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D. A Systematic Review on the Use of Psychosocial Interventions in Conjunction With Medications for the Treatment of Opioid Addiction. J Addict Med. 2016;10(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kouimtsidis C, Reynolds M, Coulton S, Drummond C. How does cognitive behaviour therapy work with opioid-dependent clients? Results of the UKCBTMM study. Drugs: education, prevention and policy. 2012;19(3):253–8. [Google Scholar]
- 8.Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, et al. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am J Psychiatry. 2008;165(7):881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hser YI, Mooney LJ, Saxon AJ, Miotto K, Bell DS, Zhu Y, et al. High Mortality Among Patients With Opioid Use Disorder in a Large Healthcare System. J Addict Med. 2017;11(4):315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendershot CS, Witkiewitz K, George WH, Marlatt GA. Relapse prevention for addictive behaviors. Subst Abuse Treat Prev Policy. 2011;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertschy G Methadone maintenance treatment: an update. European archives of psychiatry and clinical neuroscience. 1995;245(2):114–24. [DOI] [PubMed] [Google Scholar]
- 14.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abi-Dargham A, Horga G. The search for imaging biomarkers in psychiatric disorders. Nat Med. 2016;22(11):1248–55. [DOI] [PubMed] [Google Scholar]
- 16••.Darcq E, Kieffer BL. Opioid receptors: drivers to addiction? Nature Reviews Neuroscience. 2018;19(8):499–514. [DOI] [PubMed] [Google Scholar]; This review discusses the role of the opioid receptors in addiction and the translational potential of genetic, pharmacological and neuroimaging research in OUD.
- 17.Pert CB, Snyder SH. Properties of opiate-receptor binding in rat brain. Proceedings of the National Academy of Sciences. 1973;70(8):2243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267(5611):495. [DOI] [PubMed] [Google Scholar]
- 19.Benarroch EE. Endogenous opioid systems: current concepts and clinical correlations. Neurology. 2012;79(8):807–14. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115(6):1363–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toll L, Bruchas MR, Cox BM, Zaveri NT. Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacological reviews. 2016;68(2):419–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700(1–2):89–98. [DOI] [PubMed] [Google Scholar]
- 23.Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012;124(3):223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, et al. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45(2):330–4. [PubMed] [Google Scholar]
- 25.Fields HL, Margolis EB. Understanding Opioid Reward. Trends in neurosciences. 2015;38(4):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89(4):1379–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards EM, Mathews DC, Luckenbaugh DA, Ionescu DF, Machado-Vieira R, Niciu MJ, et al. A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology (Berl). 2016;233(6):1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayanga D, Shorter D, Kosten TR. Update on pharmacotherapy for treatment of opioid use disorder. Expert Opin Pharmacother. 2016;17(17):2307–18. [DOI] [PubMed] [Google Scholar]
- 29.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63–75. [DOI] [PubMed] [Google Scholar]
- 30.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009(3):CD002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014(2):CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Onofrio G, O’Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Jarvis BP, Holtyn AF, Subramaniam, Tompkins DA, Oga EA, Bigelow GE, et al. Extended-release injectable naltrexone for opioid use disorder: a systematic review. Addiction. 2018;113(7):1188–209. [DOI] [PMC free article] [PubMed] [Google Scholar]; This systematic review examined extended-release injectable naltrexone (XR-NTX) for opioid use disorder, with regards to induction and adherence rates to XR-NTX as well as its effect on opioid use outcomes.
- 34.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011(4):CD001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–13. [DOI] [PubMed] [Google Scholar]
- 36•.Tanum L, Solli KK, Latif ZE, Benth JS, Opheim A, Sharma-Haase K, et al. Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence: A Randomized Clinical Noninferiority Trial. JAMA Psychiatry. 2017;74(12):1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]; This randomized clinical trial demonstrates that injectable extended-release naltrexone is as effective as buprenorphine-naloxone in maintaining short-term abstinence from opioids.
- 37.Carroll KM, Nich C, Frankforter TL, Yip SW, Kiluk BD, DeVito EE, et al. Accounting for the uncounted: Physical and affective distress in individuals dropping out of oral naltrexone treatment for opioid use disorder. Drug Alcohol Depend. 2018;192:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samples H, Williams AR, Olfson M, Crystal S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abuse Treat. 2018;95:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berrettini W A brief review of the genetics and pharmacogenetics of opioid use disorders. Dialogues in Clinical Neuroscience. 2017;19(3):229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke TK, Weiss AR, Ferarro TN, Kampman KM, Dackis CA, Pettinati HM, et al. The dopamine receptor D2 (DRD2) SNP rs1076560 is associated with opioid addiction. Annals of human genetics. 2014;78(1):33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bart G, Heilig M, LaForge K, Pollak L, Leal S, Ott J, et al. Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Molecular psychiatry. 2004;9(6):547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drakenberg K, Nikoshkov A, Horváth MC, Fagergren P, Gharibyan A, Saarelainen K, et al. μ Opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proceedings of the National Academy of Sciences. 2006;103(20):7883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coller JK, Beardsley J, Bignold J, Li Y, Merg F, Sullivan T, et al. Lack of association between the A118G polymorphism of the mu opioid receptor gene (OPRM1) and opioid dependence: A meta-analysis. Pharmacogenomics and personalized medicine. 2009;2:9–19. [PMC free article] [PubMed] [Google Scholar]
- 44.Haerian BS, Haerian MS. OPRM1 rs1799971 polymorphism and opioid dependence: evidence from a meta-analysis. Pharmacogenomics. 2013;14(7):813–24. [DOI] [PubMed] [Google Scholar]
- 45.Woodcock EA, Lundahl LH, Burmeister M, Greenwald MK. Functional mu opioid receptor polymorphism (OPRM1 A118G) associated with heroin use outcomes in Caucasian males: a pilot study. The American journal on addictions. 2015;24(4):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crist RC, Clarke TK, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ, et al. An intronic variant in OPRD1 predicts treatment outcome for opioid dependence in African-Americans. Neuropsychopharmacology. 2013;38(10):2003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke TK, Crist RC, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ, et al. Genetic variation in OPRD1 and the response to treatment for opioid dependence with buprenorphine in European-American females. Pharmacogenomics J. 2014;14(3):303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crist RC, Doyle GA, Nelson EC, Degenhardt L, Martin NG, Montgomery GW, et al. A polymorphism in the OPRM1 3′-untranslated region is associated with methadone efficacy in treating opioid dependence. The pharmacogenomics journal. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Smith AH, Jensen KP, Li J, Nunez Y, Farrer LA, Hakonarson H, et al. Genome-wide association study of therapeutic opioid dosing identifies a novel locus upstream of OPRM1. Mol Psychiatry. 2017;22(3):346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]; This genome-wide association study identified a significant association between methadone dose and a single nucleotide polymorphism closely located to the OPRM1 gene in African Americans, but not European Americans.
- 50.Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39(2):254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9. [DOI] [PubMed] [Google Scholar]
- 52.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–91. [DOI] [PubMed] [Google Scholar]
- 53.Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nature Reviews Neuroscience. 2011;12:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78(3):204–9. [DOI] [PubMed] [Google Scholar]
- 55.Avvisati R, Contu L, Stendardo E, Michetti C, Montanari C, Scattoni ML, et al. Ultrasonic vocalization in rats self-administering heroin and cocaine in different settings: evidence of substance-specific interactions between drug and setting. Psychopharmacology. 2016;233(8):1501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Pirro S, Galati G, Pizzamiglio L, Badiani A. The affective and neural correlates of heroin vs. cocaine use in addiction are influenced by environmental setting but in opposite directions. Journal of Neuroscience. 2018:0019–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartwell KJ, Back SE, McRae-Clark AL, Shaftman SR, Brady KT. Motives for Using: A Comparison of Prescription Opioid, Marijuana and Cocaine Dependent Individuals. Addictive Behaviors. 2012;37(4):373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Ahn W-Y, Vassileva J Machine-learning identifies substance-specific behavioral markers for opiate and stimulant dependence. Drug and alcohol dependence. 2016;161:247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used a machine-learning approach to identify multivariate substance-specific markers that classify heroin and amphetamine dependence respectively.
- 60.Ahn WY, Ramesh D, Moeller FG, Vassileva J. Utility of Machine-Learning Approaches to Identify Behavioral Markers for Substance Use Disorders: Impulsivity Dimensions as Predictors of Current Cocaine Dependence. Front Psychiatry. 2016;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61••.Moningka H, Lichenstein S, Worhunsky PD, DeVito EE, Scheinost D, Yip SW. Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a systematic review of fMRI studies in opioid use disorder, including studies comparing opioid-dependent and healthy control participants as well as studies on opioid medications, treatment and abstinence effects.
- 62.Li Q, Li W, Wang H, Wang Y, Zhang Y, Zhu J, et al. Predicting subsequent relapse by drug‐related cue‐induced brain activation in heroin addiction: an event‐related functional magnetic resonance imaging study. Addiction biology. 2015;20(5):968–78. [DOI] [PubMed] [Google Scholar]
- 63.Li Q, Yang WC, Wang YR, Huang YF, Li W, Zhu J, et al. Abnormal function of the posterior cingulate cortex in heroin addicted users during resting-state and drug-cue stimulation task. Chin Med J (Engl). 2013;126(4):734–9. [PubMed] [Google Scholar]
- 64.Lou M, Wang E, Shen Y, Wang J. Cue-elicited craving in heroin addicts at different abstinent time: an fMRI pilot study. Subst Use Misuse. 2012;47(6):631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65••.Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374(4):363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article briefly summarizes current knowledge on neuroscience addiction research, including conceptual frameworks and neural circuitry underlying addiction.
- 66.Wang ZX, Zhang JX, Wu QL, Liu N, Hu XP, Chan RC, et al. Alterations in the processing of non-drug-related affective stimuli in abstinent heroin addicts. Neuroimage. 2010;49(1):971–6. [DOI] [PubMed] [Google Scholar]
- 67.Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IH. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2009;99(1–3):183–92. [DOI] [PubMed] [Google Scholar]
- 68.Yip SW, DeVito EE, Kober H, Worhunsky PD, Carroll KM, Potenza MN. Anticipatory reward processing among cocaine-dependent individuals with and without concurrent methadone-maintenance treatment: Relationship to treatment response(). Drug and alcohol dependence. 2016;166:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gradin VB, Baldacchino A, Balfour D, Matthews K, Steele JD. Abnormal Brain Activity During a Reward and Loss Task in Opiate-Dependent Patients Receiving Methadone Maintenance Therapy. Neuropsychopharmacology. 2014;39(4):885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi Z, Wang A-L, Jagannathan K, Fairchild VP, O’Brien CP, Childress AR, et al. Effects of extended-release naltrexone on the brain response to drug-related stimuli in patients with opioid use disorder. Journal of Psychiatry & Neuroscience : JPN. 2018;43(4):254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang A-L, Lowen SB, Elman I, Shi Z, Fairchild VP, Bouril A, et al. Sustained opioid antagonism modulates striatal sensitivity to baby schema in opioid use disorder. Journal of substance abuse treatment. 2018;85:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smoski MJ, Salsman N, Wang L, Smith V, Lynch TR, Dager SR, et al. Functional imaging of emotion reactivity in opiate-dependent borderline personality disorder. Personal Disord. 2011;2(3):230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rossler A, et al. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biol Psychiatry. 2014;76(4):289–96. [DOI] [PubMed] [Google Scholar]
- 74.Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L, et al. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett. 2008;438(3):322–6. [DOI] [PubMed] [Google Scholar]
- 75.Yucel M, Lubman DI, Harrison BJ, Fornito A, Allen NB, Wellard RM, et al. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry. 2007;12(7):611, 91–702. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt A, Walter M, Gerber H, Schmid O, Smieskova R, Bendfeldt K, et al. Inferior frontal cortex modulation with an acute dose of heroin during cognitive control. Neuropsychopharmacology. 2013;38(11):2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62(4):2281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. [DOI] [PubMed] [Google Scholar]
- 79.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–11. [DOI] [PubMed] [Google Scholar]
- 80•.Li Q, Liu J, Wang W, Wang Y, Li W, Chen J, et al. Disrupted coupling of large-scale networks is associated with relapse behaviour in heroin-dependent men. Journal of Psychiatry & Neuroscience : JPN. 2018;43(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study investigated how resting-state functional connectivity among the salience, default mode and executive control networks correlate with heroin relapse behaviour.
- 81.Ma N, Liu Y, Li N, Wang C-X, Zhang H, Jiang X-F, et al. Addiction related alteration in resting-state brain connectivity. NeuroImage. 2010;49(1):738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133(Pt 7):2098–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang PW, Lin HC, Liu GC, Yang YH, Ko CH, Yen CF. Abnormal interhemispheric resting state functional connectivity of the insula in heroin users under methadone maintenance treatment. Psychiatry Res Neuroimaging. 2016;255:9–14. [DOI] [PubMed] [Google Scholar]
- 84.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214(5–6):655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–15. [DOI] [PubMed] [Google Scholar]
- 88.Fox MD, Zhang D, Snyder AZ, Raichle ME. The Global Signal and Observed Anticorrelated Resting State Brain Networks. Journal of Neurophysiology. 2009;101(6):3270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71(5):523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Q, Li Z, Li W, Zhang Y, Wang Y, Zhu J, et al. Disrupted Default Mode Network and Basal Craving in Male Heroin-Dependent Individuals: A Resting-State fMRI Study. J Clin Psychiatry. 2016;77(10):e1211–e7. [DOI] [PubMed] [Google Scholar]
- 92.Li W, Li Q, Wang D, Xiao W, Liu K, Shi L, et al. Dysfunctional Default Mode Network in Methadone Treated Patients Who Have a Higher Heroin Relapse Risk. Sci Rep. 2015;5:15181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W, Wang YR, Qin W, Yuan K, Tian J, Li Q, et al. Changes in functional connectivity of ventral anterior cingulate cortex in heroin abusers. Chin Med J (Engl). 2010;123(12):1582–8. [PubMed] [Google Scholar]
- 94.Ma X, Qiu Y, Tian J, Wang J, Li S, Zhan W, et al. Aberrant default-mode functional and structural connectivity in heroin-dependent individuals. PloS one. 2015;10(4):e0120861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yuan K, Qin W, Dong M, Liu J, Sun J, Liu P, et al. Gray matter deficits and resting-state abnormalities in abstinent heroin-dependent individuals. Neuroscience Letters. 2010;482(2):101–5. [DOI] [PubMed] [Google Scholar]
- 96.Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, et al. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett. 2009;460(1):72–7. [DOI] [PubMed] [Google Scholar]
- 97.Xie C, Shao Y, Fu L, Goveas J, Ye E, Li W, et al. Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behav Brain Res. 2011;216(2):639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lyoo IK, Pollack MH, Silveri MM, Ahn KH, Diaz CI, Hwang J, et al. Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology (Berl). 2006;184(2):139–44. [DOI] [PubMed] [Google Scholar]
- 99.Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y, et al. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn. 2009;71(3):223–8. [DOI] [PubMed] [Google Scholar]
- 100.Qiu Y-w, Jiang G-h, Su H-h, Lv X-f, Tian J-z, Li L-m, et al. The impulsivity behavior is correlated with prefrontal cortex gray matter volume reduction in heroin-dependent individuals. Neuroscience Letters. 2013;538:43–8. [DOI] [PubMed] [Google Scholar]
- 101.Seifert CL, Magon S, Sprenger T, Lang UE, Huber CG, Denier N, et al. Reduced volume of the nucleus accumbens in heroin addiction. Eur Arch Psychiatry Clin Neurosci. 2015;265(8):637–45. [DOI] [PubMed] [Google Scholar]
- 102•.Wollman SC, Alhassoon OM, Hall MG, Stern MJ, Connors EJ, Kimmel CL, et al. Gray matter abnormalities in opioid-dependent patients: A neuroimaging meta-analysis. The American Journal of Drug and Alcohol Abuse. 2017;43(5):505–17. [DOI] [PubMed] [Google Scholar]; This meta-analysis demonstrates that opioid-dependent individuals exhibited significantly decreased grey matter in fronto-cerebellar and fronto-insular regions compared to healthy individuals.
- 103.Wang X, Li B, Zhou X, Liao Y, Tang J, Liu T, et al. Changes in brain gray matter in abstinent heroin addicts. Drug and Alcohol Dependence. 2012;126(3):304–8. [DOI] [PubMed] [Google Scholar]
- 104.Tolomeo S, Gray S, Matthews K, Steele J, Baldacchino A. Multifaceted impairments in impulsivity and brain structural abnormalities in opioid dependence and abstinence. Psychological medicine. 2016;46(13):2841–53. [DOI] [PubMed] [Google Scholar]
- 105.Qiu Y, Jiang G, Su H, Lv X, Zhang X, Tian J, et al. Progressive White Matter Microstructure Damage in Male Chronic Heroin Dependent Individuals: A DTI and TBSS Study. PLoS ONE. 2013;8(5):e63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bora E, Yücel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, et al. White matter microstructure in opiate addiction. Addiction Biology. 2012;17(1):141–8. [DOI] [PubMed] [Google Scholar]
- 107.Liu H, Li L, Hao Y, Cao D, Xu L, Rohrbaugh R, et al. Disrupted white matter integrity in heroin dependence: a controlled study utilizing diffusion tensor imaging. Am J Drug Alcohol Abuse. 2008;34(5):562–75. [DOI] [PubMed] [Google Scholar]
- 108.Ivers JH, Fitzgerald J, Whelan C, Sweeney B, Keenan E, Fagan A, et al. Progressive white matter impairment as a predictor of outcome in a cohort of opioid‐dependent patient’s post‐detoxification. Addiction biology. 2018;23(1):304–12. [DOI] [PubMed] [Google Scholar]
- 109.Wollman SC, Alhassoon OM, Stern MJ, Hall MG, Rompogren J, Kimmel CL, et al. White matter abnormalities in long-term heroin users: a preliminary neuroimaging meta-analysis. Am J Drug Alcohol Abuse. 2015;41(2):133–8. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Li W, Li Q, Yang W, Zhu J, Wang W. White matter impairment in heroin addicts undergoing methadone maintenance treatment and prolonged abstinence: a preliminary DTI study. Neuroscience letters. 2011;494(1):49–53. [DOI] [PubMed] [Google Scholar]
- 111.Li W, Zhu J, Li Q, Ye J, Chen J, Liu J, et al. Brain white matter integrity in heroin addicts during methadone maintenance treatment is related to relapse propensity. Brain and Behavior. 2016;6(2):e00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral cortex. 2009;19(1):72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, et al. Trends in opioid analgesic abuse and mortality in the United States. New England Journal of Medicine. 2015;372(3):241–8. [DOI] [PubMed] [Google Scholar]
- 114.Pariyadath V, Gowin JL, Stein EA. Resting state functional connectivity analysis for addiction medicine: From individual loci to complex networks. Prog Brain Res. 2016;224:155–73. [DOI] [PubMed] [Google Scholar]
- 115.Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: bringing social context into addiction neuroscience. Nature reviews Neuroscience. 2016;17(9):592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512(7513):185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yip SW, Scheinost D, Potenza MN, Carroll KM. Connectome-based prediction of cocaine abstinence. American Journal of Psychiatry. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]


