Abstract
Background
Most current evidence on risk factors for hospitalization because of coronavirus disease 2019 (COVID-19) comes from studies using data abstracted primarily from electronic health records, limited to specific populations, or that fail to capture over-the-counter medications and adjust for potential confounding factors. Properly understanding risk factors for hospitalization will help improve clinical management and facilitate targeted prevention messaging and forecasting and prioritization of clinical and public health resource needs.
Objectives
To identify risk factors for hospitalization using patient questionnaires and chart abstraction.
Methods
We randomly selected 600 of 1,738 laboratory-confirmed Colorado COVID-19 cases with known hospitalization status and illness onset during March 9–31, 2020. In April 2020, we collected demographics, social history, and medications taken in the 30 days before illness onset via telephone questionnaire and collected underlying medical conditions in patient questionnaires and medical record abstraction.
Results
Overall, 364 patients participated; 128 were hospitalized and 236 were non-hospitalized. In multivariable analysis, chronic hypoxemic respiratory failure with oxygen requirement (adjusted odds ratio [aOR] 14.64; 95% confidence interval [CI] 1.45–147.93), taking opioids (aOR 8.05; CI 1.16–55.77), metabolic syndrome (aOR 5.71; CI 1.18–27.54), obesity (aOR 3.35; CI 1.58–7.09), age ≥65 years (aOR 3.22; CI 1.20–7.97), hypertension (aOR 3.14; CI 1.47–6.71), arrhythmia (aOR 2.95; CI 1.00–8.68), and male sex (aOR 2.65; CI 1.44–4.88), were significantly associated with hospitalization.
Conclusion
We identified patient characteristics, medications, and medical conditions, including some novel ones, associated with hospitalization. These data can be used to inform clinical and public health resource needs.
Introduction
Since the first cases of coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), were reported from China in late December 2019, the subsequent pandemic has resulted in millions of cases worldwide, including over 33.9 million cases and 600,000 deaths in the United States as of July 18, 2021 [1]. Early descriptions of hospitalized COVID-19 patients from China, Italy, and the United States found that large proportions of patients had underlying medical conditions [2–6]. As the pandemic progressed, many underlying medical conditions have been implicated as potential risk factors for severe COVID-19 illness (e.g., hospitalization, intensive care unit [ICU] admission, intubation, death) including cardiovascular disease, chronic kidney disease, chronic respiratory disease, diabetes mellitus (DM), hypertension, and obesity [7–13]. Patient characteristics, specifically older age, male sex, certain racial or ethnic groups, and smoking history had also been associated with increased risk of severe COVID-19 [11, 12, 14–16]. Additionally, early in the pandemic it was proposed that certain medications, including angiotensin-converting enzyme inhibitors (ACE inhibitors), angiotensin-receptor blockers (ARBs), and non-steroidal anti-inflammatory drugs (NSAIDs), could enhance SARS-CoV-2 binding and thus increase pathogenicity [17–20]. However, more recent reports have not found this association [21–25].
Early in the pandemic, most evidence on risk factors for hospitalization due to COVID-19 came from retrospective cohort studies and case series that used data abstracted solely from electronic health records [7, 14, 26–35], were limited to specific populations and types of data collected [30–32, 36–42], or failed to adjust for potential confounding factors such as age, sex, or other comorbidities [2, 34, 35, 43]. An improved understanding of factors driving healthcare utilization will inform clinical and public health guidance, facilitate messaging to high-risk groups, and allow for better estimates of clinical and public health resource needs, including preventive (i.e., vaccines), diagnostic, and therapeutic resource allocations. In this case-control study, we use data from interviews and medical record review to identify patient characteristics, underlying medical conditions, and medications that increase the risk of hospitalization among persons with laboratory-confirmed COVID-19.
Methods
Sample
Hospitalized and non-hospitalized patients were identified from laboratory-confirmed COVID-19 cases reported to the Colorado Electronic Disease Reporting System (CEDRS) as of April 5, 2020. Based on data available in CEDRS, patients were considered eligible if they resided in one of nine contiguous counties accounting for ~80% of Colorado’s population (Adams, Arapahoe, Boulder, Denver, Douglas, El Paso, Jefferson, Larimer, and Weld), had known hospitalization status, and self-reported illness onset during March 9–31, 2020 when there was ongoing community transmission and wider availability of SARS-CoV-2 testing in Colorado. To obtain at least 300 patient interviews, including 200 non-hospitalized and 100 hospitalized, we stratified by hospitalization status and used simple random sampling to select 600 patients from 1,738 COVID-19 cases meeting inclusion criteria.
Data collection
At least three attempts were made to contact selected patients on at least two separate days at different times of day during April 10–30, 2020. A standardized questionnaire was administered by telephone to patients who agreed to participate by providing oral consent. Demographic information, social history, underlying medical conditions, and medications and supplements taken in the 30 days prior to illness onset were obtained and hospitalization status and illness onset date from CEDRS were verified. Proxy (e.g., relative or caregiver) interviews were carried out for deceased patients, minors, and persons unable to be interviewed (e.g., those with dementia). Once an interview was complete, medical record abstraction was performed for all patients with records related to their COVID-19 illness available in three electronic medical record repositories covering the major medical systems in the selected counties. A standardized medical record abstraction form was used to collect information on underlying medical conditions and course of illness.
This activity was reviewed by the Centers for Disease Control and Prevention’s (CDC) Human Research Protection Office and determined to be exempt from human participants’ research regulations, including the need for documented written consent, as the activities involved identification, control or prevention of disease in response to an immediate public health threat. It was conducted consistent with applicable federal law and CDC policy (See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.).
Statistical analysis
Data were entered into a Research Electronic Data Capture database [44, 45]. Prescribed and over-the-counter (OTC) medications and supplements taken in the 30 days prior to illness onset were collected as free text during interviews and were categorized into general drug classes by three clinicians (EM, HC, and ES). Participants were considered to have an underlying medical condition if it was reported in either their interview or medical record as the latter has been shown to be a more comprehensive approach to capture these data [46]. Body Mass Index (BMI) was calculated in kg/m2 using height and weight reported in interviews.
Frequencies and percentages were calculated stratified by hospitalization status. Univariable logistic regression was performed to investigate the association of individual patient characteristics, underlying medical conditions, and medications with the outcome of hospitalization; crude odds ratios (OR) and 95% confidence intervals (CI) were calculated. A multivariable logistic regression model for hospitalization was performed to calculate adjusted ORs (aOR) for factors previously reported to be associated with hospitalization or severe disease including age, sex, race, ethnicity, insurance status, smoking status, alcohol use, BMI, hypertension, DM, cardiovascular disease (excluding hypertension), chronic renal disease, and chronic respiratory disease [7–12]. A series of multivariable logistic regression models were then conducted to calculate aORs for individual patient characteristics, medications, and underlying medical conditions adjusted for the previously reported risk factors listed above. When evaluating the association of individual medical conditions of interest within organ system or disease categories with hospitalization, the disease category variable excluded the individual medical condition being evaluated as a risk factor. For example, when calculating the aOR for asthma, the chronic respiratory disease variable included all chronic respiratory diseases except asthma. We assessed collinearity among all variables that were adjusted for in multivariable analysis; no significant collinearity was identified. Univariable and multivariable analyses were only performed for variables reported by 10 or more patients. Statistical analyses were conducted using SAS 9.4 (SAS Institute Cary, NC) and R version 3.6.3 [47].
Results
Of 600 randomly selected patients, 364 (61%) completed the interview, 46 (8%) were ineligible (i.e., illness onset date prior to March 9 or asymptomatic), 57 (10%) declined to participate, and 133 (22%) were unreachable. Median age of the 364 participating patients was 50 years (range 2 months–94 years), 187 (51%) were male, 279 (77%) identified as White, and 75 (21%) as Hispanic. Almost all (345; 95%) reported having health insurance, and 128 (35%) were hospitalized. Eighteen (5%) patients died, including 15 who were hospitalized and 3 who were not. Compared with patients who declined to participate or were unreachable, participating patients resided proportionately in the same counties, and had similar hospitalization rates (35% versus 31%) and case-fatality ratios (5% vs 8%) but were older than non-participating patients (median age 50 vs 43 years).
Hospitalized patients were older than non-hospitalized patients, with median ages of 61 years (interquartile range [IQR] 48–72 years) and 44 years (IQR 31–57 years), respectively. On univariable analysis when compared to non-hospitalized patients, hospitalized patients more frequently reported being male, having only public health insurance, and having a history of smoking (Table 1). Hospitalized patients less frequently reported current marijuana use or any alcohol consumption within the last year when compared to non-hospitalized patients. Hospitalization status did not differ by race or ethnicity.
Table 1. Demographic characteristics and social behaviors reported in interview among persons with laboratory-confirmed COVID-19, by hospitalization status (n = 364)—Colorado, March 2020.
| Hospitalized (n = 128) | Non-hospitalized (n = 236) | Crude OR1 (95%CI) | Adjusted OR2 (95%CI) | ||||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | ||||||
| Age group, y | |||||||
| <18 | 3 (2) | 1 (0) | -- | -- | |||
| 19–44 | 23 (18) | 118 (50) | Reference | Reference | |||
| 45–64 | 50 (39) | 84 (36) | 3.05 (1.73–5.39) | 1.97 (0.99–3.95) | |||
| ≥65 | 52 (41) | 33 (14) | 8.08 (4.33–15.09) | 3.22 (1.20–7.97) | |||
| Sex | |||||||
| Male | 79 (62) | 108 (46) | 1.91 (1.23–2.96) | 2.65 (1.44–4.88) | |||
| Female | 49 (38) | 127 (54) | Reference | Reference | |||
| Other | 0 (0) | 1 (0) | -- | -- | |||
| Race | |||||||
| White | 90 (70) | 189 (80) | Reference | Reference | |||
| Black | 13 (10) | 12 (5) | 2.28 (0.99–5.19) | 1.26 (0.39–4.02) | |||
| Asian3 | 8 (6) | 8 (3) | 2.1 (0.76–5.78) | -- | |||
| Pacific Islander3 | 1 (1) | 1 (0) | -- | -- | |||
| American Indian3 | 2 (2) | 1 (0) | -- | -- | |||
| Other3 | 5 (4) | 17 (7) | 0.62 (0.22–1.73) | 1.22 (0.55–2.69) | |||
| Unknown | 4 (3) | 4 (2) | -- | -- | |||
| Multiracial3 | 5 (4) | 4 (2) | -- | -- | |||
| Ethnicity | |||||||
| Non-Hispanic | 86 (67) | 163 (69) | Reference | Reference | |||
| Hispanic | 29 (23) | 46 (19) | 1.2 (0.70–2.04) | 1.1 (0.53–2.31) | |||
| Unknown | 13 (10) | 27 (11) | 0.91 (0.45–1.86) | 0.69 (0.24–1.96) | |||
| Health insurance | |||||||
| Private Insurance | 68 (53) | 198 (84) | Reference | Reference | |||
| Public Insurance4 | 50 (39) | 29 (12) | 5.02 (2.94–8.56) | 1.8 (0.81–4.00) | |||
| Uninsured | 8 (6) | 6 (3) | 3.88 (1.30–11.59) | 3.43 (0.80–14.71) | |||
| Unknown | 2 (2) | 3 (1) | -- | -- | |||
| Smoking history | |||||||
| Ever Smoker | 60 (47) | 67 (28) | 2.23 (1.42–3.48) | 1.31 (0.72–2.38) | |||
| Current Smoker | 3 (2) | 5 (2) | -- | -- | |||
| Currently Vape | 3 (2) | 9 (4) | 0.61 (0.16–2.28) | 0.4 (0.05–2.99) | |||
| Current recreational drug use | |||||||
| Marijuana (THC) | 6 (5) | 32 (14) | 0.31 (0.13–0.77) | 1.49 (0.47–4.75) | |||
| Inhale | 4 (3) | 18 (8) | 0.39 (0.13–1.18) | 1.48 (0.35–6.27) | |||
| Consume | 3 (2) | 22 (9) | 0.23 (0.07–0.80) | 1.64 (0.39–6.97) | |||
| Cocaine | 0 (0) | 2 (1) | -- | -- | |||
| Methamphetamine | 1 (1) | 0 (0) | -- | -- | |||
| Heroin | 0 (0) | 0 (0) | -- | -- | |||
| Alcohol consumption in the past year | |||||||
| Never | 48 (38) | 39 (17) | Reference | Reference | |||
| ≤ Once per month | 31 (24) | 53 (22) | 0.48 (0.26–0.88) | 0.92 (0.40–2.09) | |||
| 2–4 times per month | 27 (21) | 53 (22) | 0.41 (0.22–0.78) | 1.37 (0.57–3.32) | |||
| 2–3 times per week | 10 (8) | 52 (22) | 0.16 (0.07–0.35) | 0.42 (0.14–1.23) | |||
| ≥4 times per week | 12 (9) | 39 (17) | 0.25 (0.12–0.54) | 0.68 (0.23–1.97) | |||
Abbreviations: CI–confidence interval; OR–odds ratio; y–years.
1Exact methods were used in crude analysis if there was one or more expected cell count less than 5.
2Multivariable model used for adjustment included age, sex, race, ethnicity, insurance status, smoking history, alcohol use, BMI, hypertension, diabetes, cardiovascular disease, chronic renal disease, and chronic respiratory disease.
3For multivariable analysis, all races except White or Black were combined into one category.
4Reported only having Medicaid or Medicare.
Medications that were reportedly taken in the 30 days prior to illness onset among hospitalized patients and non-hospitalized patients are shown in Table 2. Anticoagulants, antihyperglycemics, antihypertensives, cholesterol medications, neuropathic pain treatments, opioids, and pain/fever reducing medications were significantly associated with hospitalization in crude univariable analysis.
Table 2. Medications reported in interview to have been taken by persons with laboratory-confirmed COVID-19 in the 30 days prior to illness onset, by hospitalization status (n = 364)—Colorado, March 2020.
| Medication category | Hospitalized (n = 128) | Non-hospitalized (n = 236) | Crude OR1 (95%CI) | Adjusted OR2 (95%CI) | |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Allergy and antihistamine | 13 (10) | 19 (8) | 1.29 (0.62–2.71) | 1.01 (0.37–2.75) | |
| Antacid | 13 (10) | 13 (6) | 1.93 (0.87–4.32) | 0.65 (0.21–2.01) | |
| Antiarrhythmic | 2 (2) | 0 (0) | -- | -- | |
| Antibiotic | 1 (1) | 5 (2) | -- | -- | |
| Anticoagulant/antiplatelet | 25 (20) | 14 (6) | 3.85 (1.92–7.71) | 1.04 (0.40–2.65) | |
| Antiemetic | 0 (0) | 0 (0) | -- | -- | |
| Antiepileptic | 3 (2) | 1 (0) | -- | -- | |
| Antihyperglycemic | 21 (16) | 17 (7) | 2.53 (1.28–4.99) | 0.8 (0.22–2.84) | |
| Antihypertensives | 52 (41) | 27 (11) | 5.29 (3.11–9.03) | 0.95 (0.37–2.44) | |
| ACE inhibitor | 19 (15) | 8 (3) | 4.97 (2.11–11.70) | 0.8 (0.27–2.37) | |
| Angiotensin receptor blocker | 14 (11) | 6 (3) | 4.71 (1.76–12.57) | 1.5 (0.40–5.62) | |
| Beta blocker | 19 (15) | 8 (3) | 4.96 (2.11–11.70) | 1.24 (0.42–3.69) | |
| Calcium channel blocker | 15 (12) | 8 (3) | 3.78 (1.56–9.19) | 1.02 (0.32–3.22) | |
| Thiazide | 6 (5) | 5 (2) | 2.27 (0.68–7.60) | 0.58 (0.13–2.52) | |
| Other diuretic | 11 (9) | 4 (2) | 5.45 (1.70–17.50) | 2.57 (0.53–12.49) | |
| Other | 1 (1) | 0 (0) | -- | -- | |
| Antitussive | 1 (1) | 2 (1) | -- | -- | |
| Antiviral | 0 (0) | 6 (3) | -- | -- | |
| Asthma treatment (oral) | 6 (5) | 6 (3) | 1.89 (0.60–5.97) | 1.02 (0.21–4.92) | |
| Cancer treatment | 5 (4) | 1 (0) | -- | -- | |
| Cholesterol treatment | 28 (22) | 23 (10) | 2.59 (1.42–4.73) | 0.81 (0.34–1.96) | |
| Decongestant | 1 (1) | 5 (2) | -- | -- | |
| Hormone replacement | 2 (2) | 5 (2) | -- | -- | |
| Hypothyroid treatment | 10 (8) | 12 (5) | 1.58 (0.66–3.77) | 0.57 (0.17–1.94) | |
| Immunosuppressant | 1 (1) | 1 (0) | -- | -- | |
| Inhaler (including inhaled steroids) | 16 (13) | 19 (8) | 1.63 (0.81–3.30) | 0.83 (0.29–2.42) | |
| Mental health treatment | 25 (20) | 36 (15) | 1.34 (0.77–2.37) | 1.41 (0.65–3.08) | |
| Migraine treatment | 0 (0) | 5 (2) | -- | -- | |
| Muscle relaxant | 6 (5) | 3 (1) | -- | -- | |
| Neuropathic pain treatment | 10 (8) | 3 (1) | 6.58 (1.78–24.37) | 1.31 (0.26–6.68) | |
| Nitrates (cardiac) | 2 (2) | 0 (0) | -- | -- | |
| NSAID | 27 (21) | 48 (20) | 1.05 (0.62–1.78) | 0.51 (0.25–1.05) | |
| Opioid | 8 (6) | 2 (1) | 7.8 (1.63–37.31) | 8.05 (1.16–55.77) | |
| Oral Contraceptive | 0 (0) | 14 (6) | -- | -- | |
| Pain medication/fever reducer | 26 (20) | 26 (11) | 2.06 (1.14–3.72) | 1.63 (0.71–3.70) | |
| Phosphodiesterase-5 enzyme inhibitor | 0 (0) | 0 (0) | -- | -- | |
| Steroids | 4 (3) | 3 (1) | -- | -- | |
| Topical | 0 (0) | 1 (0) | -- | -- | |
| Vitamins and supplements | 54 (42) | 107 (45) | 0.88 (0.57–1.36) | 0.72 (0.40–1.30) | |
Abbreviations: CI–confidence interval; OR–odds ratio.
1Exact methods were used in crude analysis if there was one or more expected cell count less than 5.
2Multivariable model used for adjustment included age, sex, race, ethnicity, insurance status, smoking history, alcohol use, BMI, hypertension, diabetes, cardiovascular disease, chronic renal disease, and chronic respiratory disease.
Hospitalized patients had a higher median BMI (30; IQR 26–35) than non-hospitalized patients (26; IQR 23–30) and reported more individual underlying medical conditions among all organ systems and disease categories when compared with non-hospitalized patients (Table 3). Chronic lung disease, cardiovascular disease, endocrine disorders, renal disease, liver disease, autoimmune disorders, hematologic disorders, cancer, neurologic or neurodevelopmental disorders, and psychiatric diagnoses were all significantly associated with hospitalization in crude univariable analysis. Immunocompromising conditions were the only broad category not associated with hospitalization status on univariable analysis.
Table 3. Underlying medical conditions reported in interviews or medical records among persons with laboratory-confirmed COVID-19, by hospitalization status (n = 364)—Colorado, March 2020.
| Hospitalized (n = 128) | Non-hospitalized (n = 236) | Crude OR1 (95%CI) | Adjusted OR2 (95%CI) | |||
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| Cardiovascular disease | 75 (59) | 49 (21) | 5.4 (3.37–8.66) | 1.11 (0.51–2.43) | ||
| Hypertension | 68 (53) | 29 (12) | 8.09 (4.80–13.62) | 3.14 (1.47–6.71) | ||
| Coronary artery disease, heart attack | 23 (18) | 4 (2) | 12.7 (4.29–37.66) | 3.33 (0.87–12.77) | ||
| Heart failure, congestive heart failure | 9 (7) | 1 (0) | 17.77 (2.23–141.94) | 2.47 (0.25–24.85) | ||
| Cerebrovascular accident, stroke | 3 (2) | 3 (1) | -- | -- | ||
| Congenital heart disease | 0 (0) | 1 (0) | -- | -- | ||
| Valvular heart disease | 2 (2) | 1 (0) | -- | -- | ||
| Arrhythmia | 17 (13) | 10 (4) | 3.46 (1.53–7.81) | 2.95 (1.00–8.68) | ||
| Hyperlipidemia3 | 27 (21) | 8 (3) | -- | -- | ||
| Chronic lung disease | 56 (44) | 51 (22) | 2.82 (1.77–4.50) | 1.82 (0.97–3.39) | ||
| Asthma or reactive airway disease | 24 (19) | 36 (15) | 1.28 (0.73–2.26) | 1.17 (0.54–2.54) | ||
| Emphysema, COPD, or chronic bronchitis | 18 (14) | 6 (3) | 6.27 (2.42–16.24) | 3.01 (0.80–11.31) | ||
| Interstitial lung disease | 0 (0) | 0 (0) | -- | -- | ||
| Pulmonary fibrosis | 1 (1) | 0 (0) | -- | -- | ||
| Restrictive lung disease | 3 (2) | 1 (0) | -- | -- | ||
| Sarcoidosis | 0 (0) | 0 (0) | -- | -- | ||
| Cystic fibrosis | 0 (0) | 0 (0) | -- | -- | ||
| Chronic hypoxemic respiratory failure with oxygen requirement | 13 (10) | 1 (0) | 26.57 (3.43–205.56) | 14.64 (1.45–147.93) | ||
| Obstructive sleep apnea | 18 (14) | 10 (4) | 3.7 (1.65–8.28) | 0.67 (0.23–1.97) | ||
| Active tuberculosis | 1 (1) | 2 (1) | -- | -- | ||
| Endocrine disorder | 65 (51) | 54 (23) | 3.48 (2.19–5.51) | 1.81 (0.91–3.59) | ||
| Diabetes Mellitus | 34 (27) | 20 (8) | 3.9 (2.14–7.14) | 1.08 (0.45–2.61) | ||
| Pre-diabetes | 10 (8) | 13 (6) | 1.45 (0.62–3.42) | 0.9 (0.31–2.57) | ||
| Hypothyroidism3 | 18 (14) | 15 (6) | -- | -- | ||
| Renal disease | 25 (20) | 11 (5) | 4.96 (2.35–10.47) | 1.71 (0.62–4.70) | ||
| Chronic kidney disease or insufficiency | 13 (10) | 5 (2) | 5.22 (1.82–15.00) | 0.66 (0.15–2.91) | ||
| End-stage renal disease | 5 (4) | 0 (0) | -- | -- | ||
| Dialysis | 4 (3) | 0 (0) | -- | -- | ||
| Hemodialysis | 3 (2) | 0 (0) | -- | -- | ||
| Peritoneal | 1 (1) | 0 (0) | -- | -- | ||
| Liver disease | 9 (7) | 4 (2) | 4.39 (1.32–14.54) | 2.21 (0.49–10.04) | ||
| Alcoholic hepatitis | 0 (0) | 0 (0) | -- | -- | ||
| Chronic liver disease | 0 (0) | 0 (0) | -- | -- | ||
| Cirrhosis or end stage liver disease | 1 (1) | 0 (0) | -- | -- | ||
| Hepatitis B, chronic | 1 (1) | 0 (0) | -- | -- | ||
| Hepatitis C, chronic | 1 (1) | 0 (0) | -- | -- | ||
| Non-alcoholic fatty liver disease | 2 (2) | 3 (1) | -- | -- | ||
| Autoimmune disorder | 13 (10) | 11 (5) | 2.31 (1.00–5.32) | 2.58 (0.82–8.16) | ||
| Rheumatoid arthritis | 4 (3) | 2 (1) | -- | -- | ||
| Systemic lupus | 1 (1) | 1 (0) | -- | -- | ||
| Hematologic disorder | 21 (16) | 14 (6) | 3.11 (1.52–6.36) | 2.18 (0.88–5.43) | ||
| Anemia | 13 (10) | 7 (3) | 3.7 (1.43–9.52) | 2.04 (0.56–7.44) | ||
| Sickle cell disease | 0 (0) | 0 (0) | -- | -- | ||
| Sickle cell trait | 0 (0) | 0 (0) | -- | -- | ||
| Bleeding or clotting disorder | 5 (4) | 2 (1) | -- | -- | ||
| Immunocompromised condition | 9 (7) | 10 (4) | 1.71 (0.68–4.32) | 1.42 (0.39–5.13) | ||
| HIV infection | 0 (0) | 1 (0) | -- | -- | ||
| AIDS | 0 (0) | 0 (0) | -- | -- | ||
| Solid organ transplant | 3 (2) | 0 (0) | -- | -- | ||
| Stem cell transplant | 0 (0) | 0 (0) | -- | -- | ||
| Leukemia | 1 (1) | 1 (0) | -- | -- | ||
| Lymphoma | 0 (0) | 1 (0) | -- | -- | ||
| Multiple myeloma | 1 (1) | 0 (0) | -- | -- | ||
| Splenectomy or asplenia | 0 (0) | 2 (1) | -- | -- | ||
| Cancer | 22 (17) | 18 (8) | 2.51 (1.29–4.89) | 1.6 (0.66–3.86) | ||
| IV chemotherapy | 6 (5) | 5 (2) | 2.27 (0.68–7.60) | 2.79 (0.57–13.65) | ||
| Oral chemotherapy | 1 (1) | 2 (1) | -- | -- | ||
| Radiation | 6 (5) | 6 (3) | 1.89 (0.60–5.97) | 1.51 (0.36–6.35) | ||
| Other | 12 (9) | 9 (4) | 2.61 (1.07–6.37) | 1.86 (0.55–6.31) | ||
| Neurologic or neurodevelopmental disorder | 27 (21) | 16 (7) | 3.68 (1.90–7.12) | 1.61 (0.66–3.92) | ||
| Migraines3 | 3 (2) | 11 (5) | -- | -- | ||
| Dementia3 | 4 (3) | 5 (2) | -- | -- | ||
| Psychiatric diagnosis | 40 (31) | 49 (21) | 1.73 (1.06–2.83) | 1.17 (0.58–2.36) | ||
| Depression3 | 27 (21) | 30 (13) | -- | -- | ||
| Anxiety3 | 21 (16) | 32 (14) | -- | -- | ||
| Other chronic diseases | 78 (61) | 51 (22) | -- | -- | ||
| Gastroesophageal reflux disease3 | 20 (16) | 5 (2) | -- | -- | ||
| Allergic rhinitis3 | 8 (6) | 11 (5) | -- | -- | ||
| Arthritis3 | 14 (11) | 5 (2) | -- | -- | ||
| Chronic pain3 | 7 (5) | 1 (0) | -- | -- | ||
| Benign prostatic hyperplasia3 | 7 (5) | 0 (0) | -- | -- | ||
| Bone density abnormality3 | 2 (2) | 4 (2) | -- | -- | ||
| BMI 4 (kg/m 2 ) | ||||||
| Underweight (<18.5) | 3 (2) | 1 (0) | -- | -- | ||
| Normal (18.5 to <25) | 23 (18) | 93 (39) | Reference | Reference | ||
| Overweight (25 to <30) | 31 (24) | 85 (36) | 1.48 (0.80–2.73) | 0.66 (0.29–1.49) | ||
| Obese (30+) | 67 (52) | 54 (23) | 5.02 (2.81–8.96) | 3.35 (1.58–7.09) | ||
| Class 1 (30 to <35) | 38 (30) | 34 (14) | 4.52 (2.36–8.66) | -- | ||
| Class 2 (35 to <40) | 20 (16) | 15 (6) | 5.39 (2.40–12.12) | -- | ||
| Class 3 (40+) | 9 (7) | 5 (2) | 7.28 (2.23–23.80) | -- | ||
| Unknown | 4 (3) | 3 (1) | -- | -- | ||
| Metabolic Syndrome 5 | 19 (15) | 2 (1) | 20.39 (4.67–89.11) | 5.71 (1.18–27.54) | ||
Abbreviations: CI–confidence interval; OR–odds ratio.
1Exact methods were used in crude analysis if there was one or more expected cell count less than 5.
2Multivariable model used for adjustment included age, sex, race, ethnicity, insurance status, ever smoking, alcohol use, BMI, hypertension, diabetes, cardiovascular disease, chronic renal disease, and chronic respiratory disease.
3These variables were categorized from free text responses to “Other” chronic disease answers. Because they were not collected systematically like the other underlying medical conditions, descriptive statistics alone are reported.
4Body mass index (BMI) was calculated from height and weight reported during interviews.
5Multivariable model when investigating metabolic syndrome did not include individual hypertension, diabetes, and BMI variables.
In multivariable analysis, age ≥65 years (aOR 3.22; 95% CI 1.20–7.97) and male sex (aOR 2.65; 95% CI 1.44–4.88) were the only patient characteristics significantly associated with hospitalization (Table 1). Additionally, history of taking opioids (aOR 8.05; 95% CI 1.16–55.77) was significantly associated with hospitalization. Opioids that patients noted taking in the 30 days before their illness onset included buprenorphine, hydrocodone, hydromorphone, morphine, oxycodone, and tramadol. Among underlying medical conditions, chronic hypoxemic respiratory failure with oxygen requirement (aOR 14.64; 95% CI 1.45–147.93), hypertension (aOR 3.14; 95% CI 1.47–6.71), having an arrhythmia (aOR 2.95; 95% CI 1.00–8.68), and obesity (BMI ≥30 kg/m2) (aOR 3.35; 95% CI 1.58–7.09) were significantly associated with hospitalization.
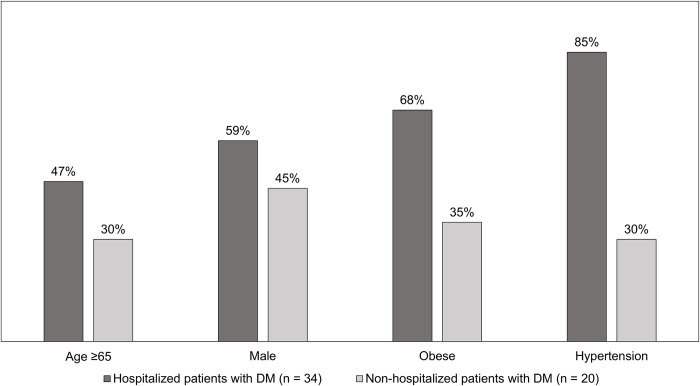
DM was reported as an underlying medical condition for 34 (27%) hospitalized patients and 20 (8%) non-hospitalized patients but was not significantly associated with hospitalization in multivariable analysis. However, when compared to non-hospitalized patients with DM, hospitalized patients with DM were more often ≥65 years old, male, obese, hypertensive, and had at least one other underlying condition adjusted for in our multivariable analysis (32 [94%] versus 11 [55%]) (Fig 1). Finally, patients with metabolic syndrome (the coexistence of DM, hypertension, and obesity) had significantly higher odds of hospitalization in multivariable analysis (aOR 5.71; CI 1.18–27.54).
Fig 1. Percentage of select characteristics among laboratory-confirmed COVID-19 patients with diabetes mellitus (DM), by hospitalization status (n = 54)—Colorado, March 2020.
Discussion
Among patients with laboratory-confirmed COVID-19 in Colorado, our analysis found many patient characteristics, underlying medical conditions, and medications to be associated with hospitalization in crude analysis. However, after adjusting for previously described risk factors for severe disease, nine factors were found to be associated with hospitalization due to COVID-19, some that have been previously described (i.e., age, sex, obesity, hypertension, arrhythmia, metabolic syndrome) and others appear to be newly identified (i.e., chronic hypoxemic respiratory failure with oxygen requirement and opioid use). In addition, we did not detect a significant association between hospitalization and some factors that have been previously associated with hospitalization or other indicators of severe COVID-19, including race and ethnicity. Some differences in findings of this analysis and previous reports examining risk factors for severe COVID-19 are expected, due to differences in data collection methods (e.g., interview versus medical record abstraction), measures of disease severity used as outcomes (e.g., hospitalization, ICU admission, mechanical ventilation, or death), and how underlying medical conditions are distributed among different populations and categorized in analyses.
Older age and male sex have been consistently identified as risk factors for hospitalization and severe COVID-19 illness in many studies [7, 8, 13, 14, 27, 29, 48]. The increased risk of poor outcomes among older patients is likely, in part, related to waning immune function that comes with aging [49]. The biologic mechanism causing more severe COVID-19 in males compared to females is unknown but likely multifactorial [12]. The finding of older patients, and in some cases older male patients, having more severe disease is similar to many other viral respiratory diseases (e.g., influenza, SARS, MERS, respiratory syncytial virus) [50–52].
There is increasing evidence of the association between obesity and worse outcomes among persons with COVID-19 [7, 13, 27–30, 36–38, 53]. A dose-dependent effect among classes of obesity was present in our univariable analysis, but because of a large amount of overlap in CIs between classes, they were collapsed to a single “obese” category for multivariable analysis. Obesity has been linked to the development of several chronic conditions, including sleep apnea, coronary artery disease, type 2 DM, and hypertension [54]. Once we adjusted for obesity in multivariable analysis, several of these related conditions were no longer significantly associated with hospitalization. Since we also adjusted for age in multivariable analysis, the association between hospitalization and obesity suggests that patients with obesity are more likely to be hospitalized, regardless of age, which has been found by others [32, 36].
While cardiovascular disease, a category inclusive of multiple individual conditions, has been identified as a risk factor for hospitalization and severe COVID-19 [2, 10, 15], it was not statistically significant in our multivariable analysis. Previous reports have found individual cardiac conditions (e.g., congestive heart failure, coronary artery disease, atrial fibrillation/arrythmia, and hypertension) to be associated with hospitalization [7, 26, 27, 33, 41–43, 55]. Within this disease category, we found that history of hypertension or arrhythmia was associated with increased risk of hospitalization in multivariable analysis. However, we did not capture information regarding the severity (e.g., stage) or control of hypertension so we do not know how much of our observed effect might be because of uncontrolled hypertension. Despite an observed higher frequency of hyperlipidemia in hospitalized participants, we did not evaluate its association with hospitalization given the condition was reported in a free text field, reported less frequently during interviews, and many non-hospitalized patients had limited or no medical records available for review. However, in the few studies that systematically collected hyperlipidemia through chart review, patients with hyperlipidemia were less or similarly likely to be hospitalized with COVID-19 [6, 56].
Similar to cardiovascular disease, chronic respiratory disease as a category was not significantly associated with hospitalization in multivariable analysis. There is mixed evidence in the literature regarding chronic respiratory disease as a risk factor for hospitalization and severe COVID-19 [7, 8], which could be related to chronic respiratory disease being a diverse group of conditions that occur in different demographics groups and individual diseases might have different associations with COVID-19 outcomes. For instance, some evaluations found chronic obstructive pulmonary disease (COPD) associated with worse COVID-19 outcomes, while asthma was often found to have no or a protective association with worse COVID-19 outcomes [31, 38, 41, 42]. Although we did not find COPD to be associated with hospitalization, our analysis identified 13/14 individuals with chronic hypoxemic respiratory failure with an oxygen requirement in this investigation were hospitalized. This finding is not surprising but suggests that the severity of underlying medical conditions might further influence their association with COVID-19 hospitalization. While smoking was associated with hospitalization in crude analysis, it was not significant in multivariable analysis. Findings of previous studies of smoking and COVID-19 outcomes have been mixed, including increased, decreased, or no impact on risk as was found in this analysis [16, 39, 57–59]. Smoking has also been found to causes variable risk for SARS-CoV-2 infection suggesting a potential complex affect [60].
In our multivariable analysis, DM was not significantly associated with hospitalization despite being significantly associated in crude analysis. However, when DM was evaluated as part of metabolic syndrome, the syndrome was significantly associated with hospitalization suggesting that DM might only be a risk factor for hospitalization when in combination with other underlying conditions. Metabolic syndrome has been found, in at least one other study, to be associated with multiple negative outcomes among hospitalized COVID-19 patients [61]. We did not differentiate between type 1 and type 2 DM in our analysis, so we are unable to determine if the relationship between DM and COVID-19 hospitalization varies by type. Additionally, we had a lower number of patients with DM, which might have reduced our power to detect an association in multivariable analysis, as was reported in at least one other study [35] compared to studies where DM was reported to occur at a higher frequency and was found to be a risk factor for hospitalization [7, 27, 29, 30, 34, 48, 55, 56]. Compared to other U.S. states, Colorado’s population is relatively healthy having the lowest proportion of adults with obesity and the fourth lowest percentage of residents with at least one of six underlying medical conditions found to be associated with an increased COVID-19 case fatality ratio in China [62, 63].
Race and ethnicity were not found to be significantly associated with hospitalization in this analysis, in contrast to other reports that found persons of Black race [13–15, 26, 29–31, 40, 64] or Hispanic ethnicity [7, 33] had worse COVID-19 outcomes. Overall, we had a small number of Black participants in this analysis, which is consistent with the racial makeup of Colorado [65], and likely substantially limited our ability to identify an association between Black persons and hospitalization. Our cohort had a notable proportion (20%) of participants who identified as Hispanic, which is also consistent with the Colorado population [65]. Of at least five previously published U.S. cohort studies that reported ethnicity, two found an association between persons of Hispanic ethnicity and hospitalization due to COVID-19 [7, 33]. Both studies included >5,000 participants of whom 25–49% were Hispanic compared to the three other studies and our own that had <2,000 participants of whom 15–28% were Hispanic [26, 30, 31]. This suggests that the association between persons of Hispanic ethnicity and hospitalization due to COVID-19 might only be detected in large cohort studies.
One of the unique attributes of this evaluation is the collection of medication and supplement use from patient interviews, which allowed for exploration of the use of prescribed and OTC medications taken in the 30 days prior to COVID-19 symptom onset and their association with hospitalization. It has been hypothesized that patients taking ACE inhibitors or ARBs might be at increased risk for more severe COVID-19 based on SARS-CoV-2 binding to ACE2 receptors found on epithelial cells in the respiratory tract as well as in intestine, kidney, and blood vessels [17, 18, 66, 67]. In our analysis, a larger proportion of hospitalized patients reported taking ACE inhibitors and ARBs than non-hospitalized patients, but the difference was not significant after adjusting for several factors, including age and hypertension, in multivariable analysis. This finding is consistent with several other analyses examining ACE inhibitor and/or ARB use as risk factors for worse COVID-19 outcomes, including at least one that suggests a protective effect against adverse COVID-19 outcomes [21–25, 68]. We also did not detect an association with NSAID use in the 30 days prior to illness onset and hospitalization due to COVID-19, despite previous concerns regarding NSAIDs use and increased risk of worse COVID-19 outcomes [18, 20].
Although no association was seen with ACE inhibitor, ARB, or NSAID use, an association between hospitalization due to COVID-19 and reported opioid use in the 30 days prior to illness onset was observed. Several mechanisms have been proposed for how opioids might cause worse COVID-19 outcomes, including respiratory depression, suppression of immune function, and drug interactions [69]. Given the small number of participants reporting taking these medications and resulting wide confidence intervals, the actual magnitude of the association cannot be predicted with certainty. However, this potential association between COVID-19 and medications that might interfere with respiratory and immune function deserves further evaluation, particularly given the current opioid epidemic in the United States [70].
This investigation is subject to multiple limitations. First, interviews were conducted several weeks after illness onset, which allowed for accurate classification of patients by hospitalization status but might have led to recall bias. However, time between illness onset and interview did not differ by hospitalization status so any existing recall bias should not significantly influence associations with hospitalization. Second, non-hospitalized participants were less likely to have medical records related to their COVID-19 illness available for abstraction (n = 88 or 37% of non-hospitalized patients had records available for review) biasing the number of underlying medical conditions reported towards those who were hospitalized. Third, BMI was calculated using self-reported height and weight. Given the tendency for people to under-report their weight, and the higher degree of under-reporting among those in higher BMI categories, the actual association between obesity and severe disease might be underestimated [71]. Fourth, our findings are specific to this population and might not be generalizable to all populations due to the evolution of testing practices and characteristics of infected persons during the pandemic, socioeconomic status, or underlying health status of participants [42]. Fifth, because many demographic or social characteristics, specific underlying medical conditions, and medications were reported by a small number of participants, our ability to make conclusions about their associations with hospitalization was limited and confidence intervals for some estimates were wide. This also prevented adequate exploration and adjustment for interaction between potential risk factors. Lastly, because data were partly self-reported, there is a possibility of response bias.
In this analysis, age ≥65 years, male sex, obesity, hypertension, chronic hypoxemic respiratory failure with an oxygen requirement, arrhythmia, metabolic syndrome, and opioid use were determined to be independent risk factors for hospitalization among COVID-19 patients in Colorado. Understanding risk factors for hospitalization can inform strategic planning and resource allocation at multiple levels including prevention (e.g., vaccine allocation), diagnosis, and treatment. Given the unique findings of this analysis as well as conflicting findings among published studies, further analyses with larger sample sizes of persons from diverse backgrounds throughout the U.S. and worldwide could help build consensus in our understanding of what patient characteristics, medications, and underlying medical conditions are associated with hospitalization and worse outcomes in persons with COVID-19. Persons in high risk groups should be targeted for tailored public health messaging, prioritized for preventive measures, and should receive appropriate clinical management as soon as possible after developing symptoms compatible with COVID-19. It is important to remember that all persons, regardless of demographics, medication use, and underlying medical conditions are at risk for severe COVID-19 illness and should take all recommended precautions to prevent infection and transmission including mask wearing, social distancing, and hand hygiene.
Acknowledgments
Colorado investigations team: Alison J. Basile; Alyssa R. Beck; Karen L. Boroughs; Anna Bowen; Paul L. Burns; Cathy L. Buschmeier; Nathaniel M. Byers; Amanda E. Calvert; Trudy V. Chambers; David T. Dennis; Mary Ellen Fernandez; Katherine T. Ficalora; Kelly A. Fitzpatrick; Shannon Fleck-Derderian; Erik S. Foster; Christin H. Goodman; Carolyn V. Gould; Garrett Heck; Claire Y.-H. Huang; Amy J. Lambert; Aine Lehane; Jennifer A. Lehman; Kristine Lindell; Nicole P. Lindsey*; Sarah E. Maes; Courtney C. Nawrocki; Nancy H. Nay; Kathleen A. Orloski; Lynn Osikowicz; Christina Parise; Lara C. Perinet; Mark A. Pilgard; Jordan A. Powers; María F. Rizzo; Brandy J. Russell; Tracey M. Semcer; Benjamin Skinner; Melanie Spillane; Julie Thwing
*Author group lead. Email: nplindsey@cdc.gov
The authors would like to thank Sarabeth Mathis for her assistance with database development and CDC Emergency Operation Center staff for facilitating deployment of personnel and equipment for this investigation.
Disclaimer: The findings and conclusion in this report are those of the authors and do not necessarily represent the official position of the CDC.
Data Availability
All relevant data are within the manuscript.
Funding Statement
There was no specific funding for this work. The case investigations, analysis, and manuscript preparation were completed as part of official duties at CDC and the Colorado Department of Public Health and Environment (CDPHE).
References
- 1.Centers for Disease Control and Prevention. CDC Coronavirus Disease 2019 (COVID-19) Cases in the U.S. 2020. Accessed at https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html on 20 November 2020. [Google Scholar]
- 2.CDC COVID-19 Response Team. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019—United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020Apr3;69(13):382–386. doi: 10.15585/mmwr.mm6913e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020Apr6;323(16):1574–81. doi: 10.1001/jama.2020.5394 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020Apr30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 Epub 2020 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020Apr17;69(15):458–464. doi: 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polverino F, Stern DA, Ruocco G, et al. ItaliCO study group. Comorbidities, Cardiovascular Therapies, and COVID-19 Mortality: A Nationwide, Italian Observational Study (ItaliCO). Front Cardiovasc Med. 2020Oct9;7:585866. doi: 10.3389/fcvm.2020.585866 Erratum in: Front Cardiovasc Med. 2020 Dec 09;7:631602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020May22;369:m1966. doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R, Liang W, Jiang M, et al. Medical Treatment Expert Group for COVID-19. Risk Factors of Fatal Outcome in Hospitalized Subjects With Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest. 2020Jul;158(1):97–105. doi: 10.1016/j.chest.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020Aug;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021 Epub 2020 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020May;94:91–95. doi: 10.1016/j.ijid.2020.03.017 Epub 2020 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold JAW, Wong KK, Szablewski CM, et al. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19—Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020May8;69(18):545–550. doi: 10.15585/mmwr.mm6918e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutzeler CR, Bourguignon L, Weis CV, et al. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020Aug4;37:101825. doi: 10.1016/j.tmaid.2020.101825 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko JY, Danielson ML, Town M, et al. COVID-NET Surveillance Team. Risk Factors for Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2021Jun1;72(11):e695–e703. doi: 10.1093/cid/ciaa1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price-Haywood EG, Burton J, Fort D, et al. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med. 2020Jun25;382(26):2534–2543. doi: 10.1056/NEJMsa2011686 Epub 2020 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020Aug;584(7821):430–436. doi: 10.1038/s41586-020-2521-4 Epub 2020 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy RK, Charles WN, Sklavounos A, et al. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J Med Virol. 2020Aug4:10.1002/jmv.26389. doi: 10.1002/jmv.26389 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liabeuf S, Moragny J, Bennis Y, et al. Association between renin-angiotensin system inhibitors and COVID-19 complications. Eur Heart J Cardiovasc Pharmacother. 2020Jun12:pvaa062. doi: 10.1093/ehjcvp/pvaa062 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020Apr;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8 Epub 2020 Mar 11. Erratum in: Lancet Respir Med. 2020 Jun;8(6):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020Mar27;368:m1185. doi: 10.1136/bmj.m1185 [DOI] [PubMed] [Google Scholar]
- 20.Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020Mar17;368:m1086. doi: 10.1136/bmj.m1086 [DOI] [PubMed] [Google Scholar]
- 21.Fosbøl EL, Butt JH, Østergaard L, et al. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use With COVID-19 Diagnosis and Mortality. JAMA. 2020Jun19;324(2):168–77. doi: 10.1001/jama.2020.11301 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N Engl J Med. 2020Jun18;382(25):2441–2448. doi: 10.1056/NEJMoa2008975 Epub 2020 May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raisi-Estabragh Z, McCracken C, Ardissino M, et al. Renin-Angiotensin-Aldosterone System Blockers Are Not Associated With Coronavirus Disease 2019 (COVID-19) Hospitalization: Study of 1,439 UK Biobank Cases. Front Cardiovasc Med. 2020Jul14;7:138. doi: 10.3389/fcvm.2020.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hippisley-Cox J, Young D, Coupland C, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020Jul31:heartjnl-2020-317393. doi: 10.1136/heartjnl-2020-317393 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen R, Yang J, Gao X, et al. Influence of blood pressure control and application of renin-angiotensin-aldosterone system inhibitors on the outcomes in COVID-19 patients with hypertension. J Clin Hypertens (Greenwich). 2020Oct2:10.1111/jch.14038. doi: 10.1111/jch.14038 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities In Outcomes Among COVID-19 Patients In A Large Health Care System In California. Health Aff (Millwood). 2020Jul;39(7):1253–1262. doi: 10.1377/hlthaff.2020.00598 Epub 2020 May 21. [DOI] [PubMed] [Google Scholar]
- 27.van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2020Jul24:10.1002/jmv.26337. doi: 10.1002/jmv.26337 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imam Z, Odish F, Armstrong J, et al. Independent Correlates of Hospitalization in 2040 Patients with COVID-19 at a Large Hospital System in Michigan, United States. J Gen Intern Med. 2020Aug;35(8):2516–2517. doi: 10.1007/s11606-020-05937-5 Epub 2020 Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killerby ME, Link-Gelles R, Haight SC, et al. CDC COVID-19 Response Clinical Team. Characteristics Associated with Hospitalization Among Patients with COVID-19—Metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb Mortal Wkly Rep. 2020Jun26;69(25):790–794. doi: 10.15585/mmwr.mm6925e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebinger JE, Achamallah N, Ji H, et al. Pre-existing traits associated with Covid-19 illness severity. PLoS One. 2020Jul23;15(7):e0236240. doi: 10.1371/journal.pone.0236240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Foer D, Bates DW, et al. Risk factors for hospitalization, intensive care, and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol. 2020Jul29:S0091-6749(20)31039-3. doi: 10.1016/j.jaci.2020.07.018 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinberg E, Wright E, Kushner B. In Young Adults with COVID-19, Obesity Is Associated with Adverse Outcomes. West J Emerg Med. 2020Jun15;21(4):752–755. doi: 10.5811/westjem.2020.5.47972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottlieb M, Sansom S, Frankenberger C, Ward E, Hota B. Clinical Course and Factors Associated With Hospitalization and Critical Illness Among COVID-19 Patients in Chicago, Illinois. Acad Emerg Med. 2020Aug6:doi: 10.1111/acem.14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suleyman G, Fadel RA, Malette KM, et al. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw Open. 2020Jun1;3(6):e2012270. doi: 10.1001/jamanetworkopen.2020.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sisó-Almirall A, Kostov B, Mas-Heredia M, et al. Prognostic factors in Spanish COVID-19 patients: A case series from Barcelona. PLoS One. 2020Aug21;15(8):e0237960. doi: 10.1371/journal.pone.0237960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lighter J, Phillips M, Hochman S, et al. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin Infect Dis. 2020Jul28;71(15):896–897. doi: 10.1093/cid/ciaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamer M, Gale CR, Kivimäki M, et al. Overweight, obesity, and risk of hospitalization for COVID-19: A community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci U S A. 2020Sep1;117(35):21011–21013. doi: 10.1073/pnas.2011086117 Epub 2020 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannouchos TV, Sussman RA, Mier JM, et al. Characteristics and risk factors for COVID-19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory-confirmed COVID-19 cases. Eur Respir J. 2020Jul30:2002144. doi: Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernández-Galdamez DR, González-Block MÁ, Romo-Dueñas DK, et al. Increased Risk of Hospitalization and Death in Patients with COVID-19 and Pre-existing Noncommunicable Diseases and Modifiable Risk Factors in Mexico. Arch Med Res. 2020Jul22:S0188-4409(20)30722-0. doi: 10.1016/j.arcmed.2020.07.003 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel AP, Paranjpe MD, Kathiresan NP, et al. Race, socioeconomic deprivation, and hospitalization for COVID-19 in English participants of a national biobank. Int J Equity Health. 2020Jul6;19(1):114. doi: 10.1186/s12939-020-01227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkins JL, Masoli JAH, Delgado J, et al. Preexisting Comorbidities Predicting COVID-19 and Mortality in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci. 2020Jul20:glaa183. doi: 10.1093/gerona/glaa183 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giorgi Rossi P, Marino M, Formisano D, et al. Reggio Emilia COVID-19 Working Group. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS One. 2020Aug27;15(8):e0238281. doi: 10.1371/journal.pone.0238281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenforde MW, Billig Rose E, Lindsell CJ, et al. CDC COVID-19 Response Team. Characteristics of Adult Outpatients and Inpatients with COVID-19–11 Academic Medical Centers, United States, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020Jul3;69(26):841–846. doi: 10.15585/mmwr.mm6926e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris PA, Taylor R, Minor BL, et al. REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019Jul;95:103208. doi: 10.1016/j.jbi.2019.103208 Epub 2019 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 Epub 2008 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiskopf NG, Cohen AM, Hannan J, et al. Towards augmenting structured EHR data: a comparison of manual chart review and patient self-report. AMIA Annu Symp Proc. 2020Mar4;2019:903–912. [PMC free article] [PubMed] [Google Scholar]
- 47.R Core Team. R: A language and environment for statistical computing. Version 3.6.3 [software]. R Foundation for Statistical Computing. 2020. [cited 2020 August 18]. Available from: http://www.R-project.org/ [Google Scholar]
- 48.Bergman J, Ballin M, Nordström A, Nordström P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol. 2021Mar;36(3):287–298. doi: 10.1007/s10654-021-00732-w Epub 2021 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013Mar;123(3):958–65. doi: 10.1172/JCI64096 Epub 2013 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. Flu & People 65 Years and Older. Accessed at https://www.cdc.gov/flu/highrisk/65over.htm on 31 August 2020.
- 51.Channappanavar R, Fett C, Mack M, et al. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J Immunol. 2017May15;198(10):4046–4053. doi: 10.4049/jimmunol.1601896 Epub 2017 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee N, Lui GC, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013Oct;57(8):1069–77. doi: 10.1093/cid/cit471 Epub 2013 Jul 21. [DOI] [PubMed] [Google Scholar]
- 53.Kalligeros M, Shehadeh F, Mylona EK, et al. Association of Obesity with Disease Severity Among Patients with Coronavirus Disease 2019. Obesity (Silver Spring). 2020Jul;28(7):1200–1204. doi: 10.1002/oby.22859 Epub 2020 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. National Institutes of Health. 1998September. https://www.nhlbi.nih.gov/files/docs/guidelines/ob_gdlns.pdf [PubMed] [Google Scholar]
- 55.Alamri F, Alsofayan Y, AlRuthia Y, et al. Predictors of Hospitalization Among Older Adults with COVID-19 in Saudi Arabia: A Cross-Sectional Study of a Nationally Representative Sample. Risk Manag Healthc Policy. 2021Mar3;14:875–886. doi: 10.2147/RMHP.S294786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alali AS, Alshehri AO, Assiri A, et al. Demographics, comorbidities, and outcomes among young and middle-aged COVID-19 patients in Saudi Arabia. Saudi Pharm J. 2021Jun19. doi: 10.1016/j.jsps.2021.06.005 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7). Addiction. 2021Jun;116(6):1319–1368. doi: 10.1111/add.15276 Epub 2020 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saurabh S, Verma MK, Gautam V, et al. Tobacco, alcohol use and other risk factors for developing symptomatic COVID-19 vs asymptomatic SARS-CoV-2 infection: a case-control study from western Rajasthan, India. Trans R Soc Trop Med Hyg. 2021Jul1;115(7):820–831. doi: 10.1093/trstmh/traa172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meini S, Fortini A, Andreini R, Sechi LA, Tascini C. The Paradox of the Low Prevalence of Current Smokers Among Covid-19 Patients Hospitalized in Non-Intensive Care Wards: Results From an Italian Multicenter Case-Control Study. Nicotine Tob Res. 2020Sep23:ntaa188. doi: 10.1093/ntr/ntaa188 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colaneri M, Novelli V, Cutti S, et al. The experience of the health care workers of a severely hit SARS-CoV-2 referral Hospital in Italy: incidence, clinical course and modifiable risk factors for COVID-19 infection. J Public Health (Oxf). 2021Apr12;43(1):26–34. doi: 10.1093/pubmed/fdaa195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie J, Zu Y, Alkhatib A, et al. Metabolic Syndrome and COVID-19 Mortality Among Adult Black Patients in New Orleans. Diabetes Care. 2020Aug25:dc201714. doi: 10.2337/dc20-1714 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adams ML, Katz DL, Grandpre J. Population-Based Estimates of Chronic Conditions Affecting Risk for Complications from Coronavirus Disease, United States. Emerg Infect Dis. 2020Aug;26(8):1831–1833. doi: 10.3201/eid2608.200679 Epub 2020 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention. Overweight & Obesity: Adult Obesity Maps. 2009. Accessed at https://www.cdc.gov/obesity/data/prevalence-maps.html on 17 August 2020. [Google Scholar]
- 64.Yancy CW. COVID-19 and African Americans. JAMA. 2020Apr15. doi: 10.1001/jama.2020.6548 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 65.United States Census Bureau. QuickFacts Colorado. 2019. Accessed at https://www.census.gov/quickfacts/CO on 17 August 2020. [Google Scholar]
- 66.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020Apr16;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052 Epub 2020 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaduganathan M, Vardeny O, Michel T, et al. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020Apr23;382(17):1653–1659. doi: 10.1056/NEJMsr2005760 Epub 2020 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golpe R, Pérez-de-Llano LA, Dacal D, et al. Lugo Covid-19 team. Risk of severe COVID-19 in hypertensive patients treated with renin-angiotensin-aldosterone system inhibitors. Med Clin (Barc). 2020Dec11;155(11):488–490. doi: 10.1016/j.medcli.2020.06.013 Epub 2020 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schimmel J, Manini AF. Opioid Use Disorder and COVID-19: Biological Plausibility for Worsened Outcomes. Subst Use Misuse. 2020;55(11):1900–1901. doi: 10.1080/10826084.2020.1791184 Epub 2020 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Donnell J, Gladden RM, Mattson CL, et al. Vital Signs: Characteristics of Drug Overdose Deaths Involving Opioids and Stimulants—24 States and the District of Columbia, January-June 2019. MMWR Morb Mortal Wkly Rep. 2020Sep4;69(35):1189–1197. doi: 10.15585/mmwr.mm6935a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sahyoun NR, Maynard LM, Zhang XL, et al. Factors associated with errors in self-reported height and weight in older adults. J Nutr Health Aging. 2008Feb;12(2):108–15. doi: 10.1007/BF02982562 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.