Abstract
A novel label-free surface plasmon resonance (SPR) aptasensor has been constructed for the detection of N-gene of SARS-CoV-2 by using thiol-modified niobium carbide MXene quantum dots (Nb2C-SH QDs) as the bioplatform for anchoring N-gene-targeted aptamer. In the presence of SARS-CoV-2 N-gene, the immobilized aptamer strands changed their conformation to specifically bind with N-gene. It thus increased the contact area or enlarged the distance between aptamer and the SPR chip, resulting in a change of the SPR signal irradiated by the laser (He-Ne) with the wavelength (λ) of 633 nm. Nb2C QDs were derived from Nb2C MXene nanosheets via a solvothermal method, followed by functionalization with octadecanethiol through a self-assembling method. Subsequently, the gold chip for SPR measurements was modified with Nb2C-SH QDs via covalent binding of the Au-S bond also by self-assembling interaction. Nb2C-SH QDs not only resulted in high bioaffinity toward aptamer but also enhanced the SPR response. Thus, the Nb2C-SH QD-based SPR aptasensor had low limit of detection (LOD) of 4.9 pg mL−1 toward N-gene within the concentration range 0.05 to 100 ng mL−1. The sensor also showed excellent selectivity in the presence of various respiratory viruses and proteins in human serum and high stability. Moreover, the Nb2C-SH QD-based SPR aptasensor displayed a vast practical application for the qualitative analysis of N-gene from different samples, including seawater, seafood, and human serum. Thus, this work can provide a deep insight into the construction of the aptasensor for detecting SARS-CoV-2 in complex environments.
Graphical abstract
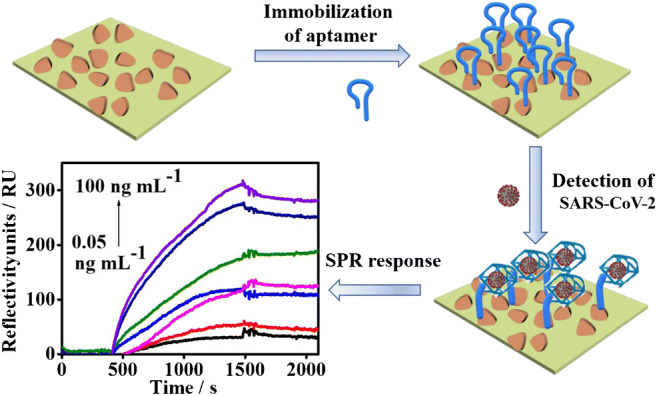
A novel label-free surface plasmon resonance aptasensor has been constructed to detect sensitively and selectively the N-gene of SARS-CoV-2 by using thiol-modified niobium carbide MXene quantum dots as the scaffold to anchor the N-gene-targeted aptamer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00604-021-04974-z.
Keywords: Nb2C MXene quantum dot, Surface plasmon resonance biosensor, Aptasensor, Detection of N-gene, SARS-CoV-2
Introduction
Since the end of 2019, the world has been suffering from the COVID-19 pandemic caused by novel SARS-CoV-2 coronavirus. This disease has spread across 205 countries and territories, infected more than one hundred million individuals, and resulted in 2 million mortalities [1]. The early clinical manifestations of COVID-19 are similar to the symptoms of the severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus disease, namely, fever, headache, myalgia, arthralgia, and lymph node enlargement. SARS-CoV-2 is characterized with moderate mortality rate, high infection rate, and longer incubation period, leading to long-term infection [2]. Thus, COVID-19 threatens the safety of people and causes serious financial crises. SARS-CoV-2 has been identified in diverse environments, such as water systems, frozen foods, or food packaging [3, 4]. Thereby, the rapid diagnosis of the COVID-19 virus is highly essential to control efficiently the spread of the virus and treat patients. Similar to other coronaviruses, SARS-CoV-2 is composed mainly of four structural proteins, namely, the spike (S), membrane (M), envelop (E), and nucleocapsid (N) proteins [5]. The proteins (e.g., S protein) [6] and viral RNA [7] can be used as targets for the qualitative and quantitative analyses of COVID-19. Alternatively, antibodies, such as IgM and IgG, from patient samples can also be detected to probe the infection history [8]. Diverse techniques have been developed to analyze SARS-CoV2, such as real-time polymerase chain reaction (PCR) [9], colorimetric assay [10], surface plasmon resonance (SPR) and localized SPR [11], electrochemical approaches [9, 12–15], optics methods [16, 17], optical/chemiluminescence immunosensors [18], fluorescent technique [19], wearable sensor [20], and isothermal rolling circle amplification (RCA) [21]. Although many studies have focused on the sensitive determination methods of SARS-CoV-2 [22–25], COVID-19 has resulted in a heavy burden for health systems and human society. Consequently, advanced and sensitive determination methods should be developed to assess SARS-CoV-2 in different environments and populations.
Most techniques have been formulated based on the specific recognition between antibodies and diverse proteins and RNAs in the SARS-CoV-2 virus. Compared with antibodies, as a powerful class of probes, DNA aptamers have some advantages, such as high specificity, strong affinity, rapid and reliable synthesis, ease of conjugation, and high feasibility to bind with other DNA-based reactions for efficient signal amplification. Thus, aptamers can be used in different applications for in vitro and in vivo diagnoses. Diverse techniques, such as PCR [26], electrochemical methods [27], and fluorescence approach [28], have been combined with aptasensors for analyzing biomarkers of SARS-CoV-2. However, the aforementioned techniques have some disadvantages. For example, PCR is more complex with need to achieve appropriate test conditions, often leading to low accuracy [29]; detection kits often exhibit low sensitivity for the analysis of SARS-CoV2 [30]. As for SPR sensing strategy, it is often applied for the construction of diverse immunoassays, for multiple detection of biomolecules, and in situ determination interactions of multiplexed chemical and biological analytes, such as interactions between antibodies and antigens, RNAs, DNAs, or proteins, and a wide variety of ligands or targets [31]. Although different SPR immunosensors have been constructed for detecting antibodies against SARS-CoV-2 [32–34], no SPR aptasensor for analyzing the RNA of N-gene has been reported yet.
Various nanomaterials, such as functional polymers [35], nanoparticles [36], graphene [37, 38], MXene nanosheets [39, 40], and MoS2 [41], have been utilized as sensing platforms for the fabrication of SPR aptasensors. MXenes, which are known as two-dimensional materials, have attracted extensive attention due to their similar structure and analogous performances to graphene. The versatile chemical structure, compositions, and tunable surface functionalization of MXenes facilitate the diverse applications of MXene, such as in solar cells [42], electronic devices [43], catalysts [44, 45], gas sensors or biosensors [46, 47], and cancer therapy [48]. MXenes have nanosheet-like structure, unique surface chemistry, high conductive properties, and excellent biocompatibility. These characteristics allow MXenes to efficiently act as platforms for the development of diverse biosensors [49, 50]. MXene nanosheets have been explored as a sensitive layer for immobilizing probes and developing SPR biosensors [40, 51]. However, the ultrathin MXene nanosheets are prone to aggregate to bulk size due to the π-π* stacking interaction. Optimal MXene nanosheets for the development of the SPR biosensor are difficult to obtain because of the limitation in the thickness of SPR biosensors, in which the sensitive layer is ~200 nm [52]. By contrast, zero-dimensional Ti3C2 quantum dots (QDs) from 2D bulk MXene demonstrate novel properties relative to their 2D counterparts because of a combination of quantum confinement, edge effect, and surface functionality [53]. Thus, MXene QDs can be utilized as sensitive nanomaterials to construct SPR aptasensors for detecting N-gene of SARS-CoV-2.
In this work, we have designed and constructed a novel SPR biosensor by using niobium carbide MXene QDs functionalized with the thiol group (denoted as Nb2C-SH QDs) as the bioplatform for adsorbing N-gene-targeted N58 aptamer. The biosensor was explored for the sensitive detection of N-gene from diverse complex environments, such as human serum, seawater, and seafood (Fig. 1). Nb2C QDs were derived from Nb2C MXene nanosheets by a solvothermal method, followed by the modification of Nb2C QDs with the thiol group (Fig. 1a). Given the self-assembling effect between the Nb2C-SH QDs and the Au chip of SPR, Nb2C-SH QDs were homogeneously coated over the chip surface, and the N58 aptamer was stably immobilized through π-π* stacking, electrostatic adsorption, and hydrogen bond [54]. In the presence of SARS-CoV-2, the G-quadruplex between the N58 aptamer and N-gene of SARS-CoV-2 was formed [55]. Thus, the aptamer strands changed its conformation [56] when binding with N-gene, leading to the enlargement of the contact area or distance between the probe molecule and the chip. These modifications were then converted to change in the SPR signal to detect N-gene of SARS-CoV-2 (Fig. 1b). Compared with previously reported SPR biosensors for the same detection [11], the SPR aptasensor based on the Nb2C-SH QDs exhibited superior sensing performances, such as high sensitivity, fast response, renewability, and availability in complex environments. These advantages are mainly ascribed to several factors. The multifunctional properties of Nb2C-SH QDs not only can ensure homogeneously deposition onto the SPR chip but also can tightly adsorb a large amount of aptamers, further resulting in high sensitivity. The highly specific recognition between N58 aptamer and N-gene endows the SPR aptasensor with a fast response toward N-gene. In addition, the Nb2C-SH QD-based SPR biosensor can be widely applied for detecting N-gene from diverse environments, such as human serum, seawater, and seafood, because of its easy construction, high stability, and good renewability. Thereby, the present work provides a new insight into the development of the SPR aptasensor for the fast and sensitive analysis of N-gene of SARS-CoV-2 from different real samples.
Fig. 1.
Schematic diagram of a the synthesis of Nb2C-SH QDs and b construction of the Nb2C-SH QD-based SPR aptasensor for detecting N-gene of SARS-CoV-2
Experimental section
Chemicals and materials
Bulk Nb2AlC powder, hydrofluoric acid (HF), dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), and n-octadecyl mercaptan were purchased from Aladdin Reagent Co. Ltd. Biologicals including influenza A virus (Flu A), influenza B virus (Flu B), M. pneumonia (P1), chlamydia pneumoniae (CPN), immunoglobulin (IgG), prostate-specific antigen (PSA), bovine albumin (BSA), and SARS-CoV-2 and SARS-CoV-2-N58 aptamer sequence: 5′-GCT GGA TGT CAC CGG ATT GTC GGA CAT CGG ATT GTC TGA GTC ATA TGA CAC ATC CAG C-3′ were ordered from Solarbio Life Sciences Co. Ltd. Additionally, human serum was obtained from the First Affiliated Hospital of Zhengzhou University after informed consent of the patient. This research protocol was in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent revisions and was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University.
Preparation of Nb2C QDs and Nb2C-SH QDs
The preparation of the Nb2C QDs was realized through a solvothermal method according to the reported literature [57]. The detailed preparation methods of the Nb2C QDs and Nb2C-SH QDs were described in the Electronic Supplementary Material.
SPR measurements
All SPR measurements were performed by a Biacore™ X100 device (GE-Healthcare Bio-Sciences AB, Sweden, the wavelength of the laser (He-Ne) of 633 nm) at 25 °C with flowing 0.01 M phosphate buffer saline (PBS) (pH = 7.4) containing 2.7 mM KCl, 137 mM NaCl, and 0.5% DMSO. In prior to use, the work chip, a standard 10 × 12 × 0.3 mm glass chip coated with gold film (~50 nm), was cleaned with H2SO4/H2O2 (70/30 v/v) for 1 min, following by washing with Milli-Q water and drying in N2 stream. Afterwards, 10 μL of Nb2C-SH QDs suspension (0.1 mg mL−1) was dropped on the gold chip surface, forming the homogeneous Nb2C QDs layer via the covalent bond of Au-S between thiol group bearing on Nb2C-SH QDs and gold atom by the self-assembling interaction [58]. Equilibration of the baseline was completed by a continuous flow (5 μL min−1) of running buffer through the chip for 0.5 h. Afterward, the Nb2C-SH QDs-modified gold chip was incubated with the aptamer immobilization (100 nM) at a flow rate of 5 μL min−1 for 0.5 h. After obtaining a stable baseline, the SPR flow cell was washed with running buffer to remove the unbounded aptamer. Each binding experiment for the detection of N-gene was dissolved in PBS at predetermined concentrations (0.05, 0.1, 1, 5, 10, 50, and 100 ng mL−1) and introduced into the flow cell at a flow rate of 5 μL min−1. The specificity analysis of the SPR aptasensor was performed by injecting several kinds of interferents, which may be coexisted with N-gene of SARS-CoV-2, including Flu A, Flu B, P1, CPN, IgG, PSA, and BSA, respectively, at a flow rate of 5 μL min−1. The loading of the SPR chip was calculated considering one reflectivity unit (RU) corresponding to a change in refractive of 10−6 and approximately 1 pg mm−2 of bound protein.
Real samples
For the analysis of real samples, human serum without pre-treatment was firstly spiked with different amounts of SARS-CoV-2 N-gene. Afterward, human serum was diluted 10 times with PBS. Sea water was obtained from the Yellow Sea. Different amounts of N-gene of SARS-CoV-2 were added to sea water. In addition, seafood was purchased from seafood market in Zhengzhou and treated according to a previous report [59]. After being spiked with different amounts N-gene of SARS-CoV-2, 2 g of shrimp was added to 4 mL of 3% trichloroacetic acid (TA) under stirring for 10 min. Next, the resulting extract was centrifuged at 12,000 rpm for 10 min. Moreover, the pH value of supernatant was then adjusted to 7 with 1.0 M NaOH, following by diluted 100-fold with deionized water.
Results and discussion
Basic characterizations of Nb2C-SH QDs
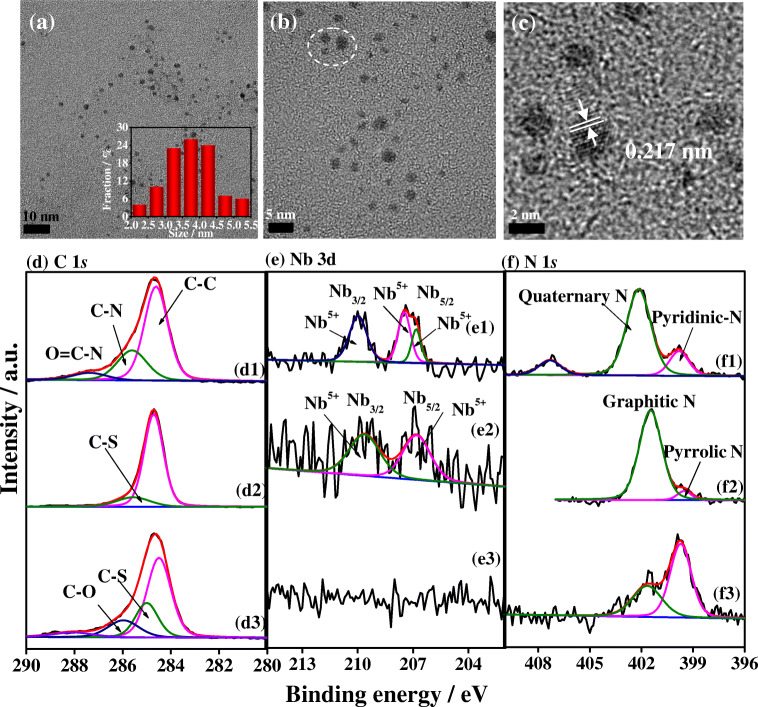
Transmission electron microscopy (TEM) was performed to characterize the microstructure and morphological characteristics of Nb2C-SH QDs (Fig. 2a–c). The QDs are clearly observed in the low-magnification TEM image of Nb2C-SH QDs (Fig. 2a), which have average lateral sizes from 2.3 to 5.4 nm (Fig. 2b). The inset image in Fig. 2a depicts the corresponding size distribution curve. A clear fringe is obtained in the high-resolution TEM image of the Nb2C-SH QDs (Fig. 2c), indicating the single-crystalline feature of the Nb2C-SH QDs. The distance between adjacent lattice fringes is 0.217 nm, which well matches with the [001] direction plane of graphene carbon [60].
Fig. 2.
a Low-magnification, b high-magnification, and c high-resolution TEM images (the inset images show the corresponding size distribution curves) of the Nb2C QDs. High-resolution C 1s, Nb 3d, and N 1s XPS spectra of (d1, e1, and f1) Nb2C QDs, (d2, e2, and f2) Nb2C-SH QDs and (d3, e3, and f3) Apt/Nb2C-SH QDs
Fourier transform infrared spectra (FT-IR) of the Nb2C QDs and Nb2C-SH QDs were shown in Fig. S1 (see the Electronic Supplementary Material), indicating that a sufficient number of oxygen-bearing functional groups are decorated on both QDs. To further evaluate the chemical structure and component of the Nb2C QDs and Nb2C-SH QDs, X-ray photoelectron spectroscopy (XPS) characterization was performed. The XPS survey spectrum of the Nb2C QDs (curve i, Fig. S2) indicates four peaks at the binding energies (BEs) of 286.1, 532.1, 407.1, and 232.1 eV, which are assigned to C1s, O1s, N1s, and Nb 3d, respectively. For Nb2C-SH QDs, an additional signal due to S 2p at the BE of 168.1 eV is obtained (curve ii, Fig. S2). To further probe the chemical environments and valence states of each element, the high-resolution C 1s, Nb 3d, and N 1s XPS spectrum of the Nb2C QDs and Nb2C-SH QDs were analyzed (Fig. 2d–f). The high-resolution C 1s spectrum (Fig. 2 (d1)) of Nb2C QDs can be deconvoluted into three peaks at BEs of 284.5, 285.5, and 287.4 eV, which are attributed to graphitic C–C, C–N, and O=C-N, respectively. The presence of O=C-N indicates that the Nb2C QDs are slightly oxidized during the preparation. The high-resolution Nb 3d XPS spectrum of Nb2C QDs (Fig. 2 (e1)) is fitted by two peaks at 207.3 and 210 eV, which are assigned to the BEs of Nb 3d5/2 and Nb 3d3/2 of Nb5+, respectively. This finding reveals that the Nb2C phase is substantially oxidized [61, 62]. The high-resolution N 1s spectrum of the Nb2C QDs (Fig. 2 (f1)) has been commonly simulated with two peaks at the BEs of 399.8 and 402.1 eV, corresponding to pyridinic N and quaternary N [63]. Compared with the C 1s X-ray photoelectron spectrum of Nb2C QDs, an additional peak at the BE of 285 eV due to C-S bond appears in the C 1s X-ray photoelectron spectrum of the Nb2C QDs-SH (Fig. 2 (d2)). This peak is caused by the modified thiol group. No clear change is observed for the Nb 3d and N 1 s X-ray photoelectron spectra of the Nb2C-SH QDs compared with that of Nb2C QDs but with lower peak intensity. A discernable observation of the N-species is that the conversion from pyrrolic N to graphitic N occurs upon the additional doping of S into the Nb2C QDs [64]. Moreover, the S 2p XPS spectrum of Nb2C-SH QDs (Fig. S3) is analyzed in Section S3 of the Electronic Supplementary Material. These results verify the coverage of the self-assembling layer over the Nb2C QDs.
In prior to the SPR measurements, XPS was used to investigate whether the N-58-targeted aptamer can be immobilized over the Nb2C QDs-modified Au chip. For the C 1s X-ray photoelectron spectrum of the AptN-58 aptamer/Nb2C-SH QDs (Fig. 2 (d3)), the two peaks at the BEs of 285.0 and 285.9 eV, which are due to C-S and C-O, respectively, are deconvoluted. The C–O bond is caused by the immobilized aptamer strands. In addition, no clear Nd 3d XPS signal is present in the AptN-58 aptamer/Nb2C-SH QDs (Fig. 2 (e3)), indicating that the full coverage of the aptamer strands over the modified layer. The N 1s X-ray photoelectron spectrum of the AptN-58 aptamer/Nb2C QDs can be fitted to two peaks at the BEs of 399.7 and 401.7 eV, which are attributed to pyridinic-N and graphitic N, respectively [65]. As an indicator of the aptamer strands, the P 2p XPS signal was obtained in the XPS survey scan spectrum of the AptN-58 aptamer/Nb2C-SH QDs. The high-resolution P 2p XPS spectrum (Fig. S4) can be separated into two peaks at the BEs of 132.9 and 133.9 eV, which are due to P 2p3/2 and P 2p1/2, respectively. Apparently, the clear P 2p XPS signal is from the phosphate backbone of the N 58 aptamer [66].
The optical properties of Nb2C-SH QDs were studied by UV-vis spectrum and fluorescence spectroscopy (Fig. S5). The results and analysis are supplied in Section S4 of the Electronic Supplementary Material.
Sensing performance of the Nb2C-SH QD-based aptasensor toward N-gene
Ahead of investigating the optimization of the determination conditions, the electrochemical surface area (ECSA) of the aptasensor was calculated from corresponding CVs (Fig. S6). The ECSA of the aptasensor is 10.5 cm2, revealing good electrochemical activity. In prior to the analysis of N-gene by using the Nb2C-SH QD-based aptasensor, the determination conditions or parameters, including the concentration of aptamer, pH value of PBS, and the concentration of Nb2C-SH QDs, were investigated to obtain the optimal sensing performance of the Nb2C-SH QD-based SPR aptasensor. The results (Fig. S7) and analysis are supplied in Section S6 of the Electronic Supplementary Material. The optimal conditions for the construction of the Nb2C-SH QD-based aptasensor are aptamer solution concentration of 100 nM, 0.01 M PBS with pH 7.4, and the Nb2C-SH QDs concentration of 1 mg mL−1.
Under the optimal detection conditions, the sensing performance of the Nb2C-SH QD-based aptasensor toward N-gene was probed. The Nb2C-SH QD-coated Au chip was incubated with different concentrations of N-gene and measured by the SPR instrument (Fig. 3a). The SPR response gradually increases from 40 to 270 RU with increasing N-gene concentration from 0.05 to 100 ng mL−1 within 1000 s (Fig. 3b). As aforementioned, the N58 aptamer strands would bind with N-gene of SARS-CoV-2, specifically forming the G-quadruplex between the aptamer and N-gene. This characteristic further increases the thickness of the Au chip for the SPR biosensor, which can vary the refractive index of the overlayer [67]. After 1000 s, the Nb2C-SH QD-based SPR aptasensor was rinsed with 0.01 M PBS. The resulting decrease in the SPR response is ~15 RU, which is considerably low. This finding indicates the strong binding interaction between N-gene and aptamer strands. When ΔRU of the Nb2C-SH QD-based SPR aptasensor before and after the detection of N-gene (ΔRU = RUN-gene-RUApt) is defined as the detected signal, the ΔRU value is proportional to the logarithm value of N-gene concentration from 0.05 to 100 ng mL−1. The linear regression equation was ΔRU = 70.26logCN-gene + 121.09, with the correlation coefficient (R2) of 0.9923 (the inset of Fig. 3b). The detection limit (LOD) was estimated to be as low as 4.9 pg mL−1 at a signal-to-noise ratio of 3 [68]. The LOD was calculated with the equation (LOD = 3.3 SB/m) derived from the criterion of the IUPAC recommendation, where SB refers to the standard deviation of the blank (n = 3), and m refers to the slope of the linear calibration curve [69]. Compared with the reported SPR, optical, or electrochemical aptasensors for analyzing N-gene of SARS-CoV-2 (Table S1), the SPR aptasensor based on Nb2C-SH QDs demonstrates lower LOD and faster response. The low LOD and fast response toward N-gene by using the Nb2C-SH QDs-based aptasensor is mainly due to several aspects. (i) The strong self-assembling interaction between thiol bearing on the Nb2C-SH QDs and Au chip surface provides the outstanding stability of the aptasensor in the aqueous solution [70]. (ii) The strong bioaffinity between the aptamer and highly conjugated Nb2C-SH QDs via π-π* stacking, hydrogen bonds, and Van der Waal force facilitates the full coverage of the aptamer onto the Nb2C-SH QD-modified Au chip, leading to the high detection efficiency toward N-gene [71]. (iii) The highly specific recognition between aptamer and N-gene can reduce the nonspecific adsorption of other interferents on the Nb2C-SH QD-based biosensor [72]. No substantial ΔRU change is found for the BSA adsorption, hinting the absence of a strong non-specific binding (Fig. S8).
Fig. 3.
a SPR aptasensor responses of the AptN58/Nb2CQDs/Au with different concentrations of N-gene (0.05, 0.1, 1, 5, 10, 50, and 100 ng mL−1). b Relationship between the ΔRU values (ΔRU = RUN-gene - RUN58 aptamer) and N-gene concentration. Inset: Linear relationship between ΔRU and logarithm of Nb2C-SH QDs concentration. c Selectivity of the Nb2C-SH QD-based SPR aptasensor for N-gene detection (1 ng mL−1) in the presence of CPN, flu A, Flu B, P1, IgG, PSA, and BSA (100 ng mL−1)
The selectivity of the constructed Nb2C-SH QD-based aptasensor was also examined by analyzing different interferents, including other kinds of respiratory viruses (i.e., Flu A, Flu B, P1, and CPN) and proteins in the human serum (i.e., IgG, PSA, and BSA), with the concentration of 100 ng mL−1, which was 100-fold of the used N-gene concentration. The ΔRU values for each interferent and the detection of N-gene are shown in Fig. 3c. The result indicates that the ΔRU value obtained from the analysis of the interferents is negligible, greatly lower than that of the Nb2C-SH QD-based SPR response of N-gene. This finding suggests that the Nb2C-SH QD-based aptasensor has the outstanding selectivity for analyzing N-gene.
Determination of N-gene in different environments and human serum
Given that the Nb2C-SH QD-based aptasensor displays high sensitivity and good selectivity, the aptasensor can be employed to detect N-gene in diverse environments, such as in seawater, seafood (frozen shrimp), and human serum, to verify the suitability of the aptasensor. The pretreatment procedure of each sample is provided in the Real samples of the Experimental section. The original concentration of the target analyte (before the sample being spiked) in the real samples was also investigated. The result (Fig. S9) shows that the ΔRU of the Nb2C-SH QD-based SPR aptasensor before and after the detection of N-gene in seawater, seafood, and human serum is negligible. This finding verifies that no N-gene exists in real samples. Consequently, the applicability of the developed aptasensor can be assessed by spiking a series of N-gene solutions with different concentrations into real samples. Different concentrations of N-gene were spiked into diverse samples. Then, the determination using the Nb2C-SH QD-based aptasensor was carried out as described in previous sections. According to the calibration curve, the real concentrations of N-gene were deduced. All results are summarized in Tables 1, 2, and 3. As indicated in Table 1, the recoveries for detecting N-gene in seawater range from 97.70 to 111.30%, along with low RSD values of 1.04–2.79%. For N-gene in seafood (Table 2), the found recoveries are from 91.80 to 95.86%, while they are ranging from 98.67 to 104.86% in the human serum (Table 3). The corresponding RSD values are 1.99–4.21% and 1.76–4.12%. These results verify that the Nb2C-SH QD-based aptasensor can be utilized to analyze N-gene from different environments.
Table 1.
Analysis of N-gene in seawater using the Nb2C-SH QD-based aptasensor
| Added before processing (ng mL−1) | Actual added (ng mL−1) | ∆RU | Found (ng mL−1) | Apparent recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 0.05 | 0.05 | 29.50 | 0.0497 | 99.40 | 2.13 |
| 0.1 | 0.1 | 54.10 | 0.1113 | 111.30 | 1.98 |
| 1 | 1 | 121.00 | 0.9970 | 99.70 | 2.26 |
| 10 | 10 | 192.00 | 10.216 | 102.16 | 1.04 |
| 100 | 100 | 260.90 | 97.700 | 97.70 | 2.79 |
Table 2.
Analysis of N-gene in seafood using the Nb2C-SH QD-based aptasensor
| Added before processing (ng mL−1) | Actual added (ng mL−1) | ∆RU | Found (ng mL−1) | Apparent recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 5 | 0.05 | 27.10 | 0.0459 | 91.80 | 3.34 |
| 10 | 0.1 | 48.90 | 0.0938 | 93.80 | 2.79 |
| 100 | 1 | 119.60 | 0.9523 | 95.23 | 2.07 |
| 1000 | 10 | 190.06 | 9.5860 | 95.86 | 1.99 |
| 10,000 | 100 | 259.30 | 92.709 | 92.71 | 4.21 |
Table 3.
Analysis of N-gene in human serum using the Nb2C-SH QD-based aptasensor
| Added before processing (ng mL−1) | Actual added (ng mL−1) | ∆RU | Found (ng mL−1) | Apparent recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 0.5 | 0.05 | 29.60 | 0.0498 | 99.60 | 4.12 |
| 1 | 0.1 | 52.00 | 0.1039 | 103.90 | 2.37 |
| 10 | 1 | 120.90 | 0.9937 | 99.37 | 2.49 |
| 100 | 10 | 192.80 | 10.486 | 104.86 | 1.76 |
| 1000 | 100 | 261.20 | 98.665 | 98.67 | 3.22 |
Conclusion
A novel SPR aptasensor was designed and constructed by using Nb2C-SH QDs as the sensitive layer for anchoring N-gene-targeted aptamer strands, which were used for the specific determination of N-gene of SARS-CoV-2. Given the thiol-functionalized, highly conjugated structure, and graphene-like MXene phase, the prepared Nb2C-SH QDs not only displayed high binding interaction with the Au chip via the self-assembling force by forming Au−S bond but also demonstrated strong bioaffinity toward the aptamer strands, and the amplified SPR effect. Apart from low detection limit within a wide linear range of N-gene concentration, high selectivity, excellent repeatability exhibits, and good applicability, the provided SPR aptasensor based on Nb2C-SH QDs apparently demonstrates the advantage of feasible construction, fast response, and in situ analysis. This work paves the way for the fabrication of N-gene aptasensor and provides a new sensing strategy for the early and sensitive analysis of N-gene. Nonetheless, the Nb2C QDs-based SPR aptasensor still exhibits some unsatisfied features such as poor repeatability and regenerability when detecting N-gene.
Supplementary information
(DOCX 931 kb)
Funding
This work was supported by the Excellent Youth Science Foundation of Henan Province (202300410494), the Distinguished Youth Science Foundation of Henan Province (No. 202300410492), and Innovative Technology Team of Henan Province (CXTD2014042).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhihong Zhang, Email: mainzhh@163.com.
Zhonghou Zhang, Email: gxncl272@163.com.
References
- 1.Parashar NC, Poddar J, Chakrabarti S, Parashar G. Repurposing of SARS-CoV nucleocapsid protein specific nuclease resistant RNA aptamer for therapeutics against SARS-CoV-2. Infect Genet Evol. 2020;85:104497. doi: 10.1016/j.meegid.2020.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolodny O, Berger M, Feldman MW, Ram Y. A new perspective for mitigation of SARS-CoV-2 infection: priming the innate immune system for viral attack. Open Biol. 2020;10:200138. doi: 10.1098/rsob.200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu P, Yang M, Zhao X, Guo Y, Wang L, Zhang J, Lei W, Han W, Jiang F, Liu WJ, Gao GF, Wu G. Cold-chain transportation in the frozen food industry may have caused a recurrence of COVID-19 cases in destination: successful isolation of SARS-CoV-2 virus from the imported frozen cod package surface. Biosafety Health. 2020;2:199–201. doi: 10.1016/j.bsheal.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohapatra S, Menon NG, Mohapatra G, Pisharody L, Pattnaik A, Menon NG, Bhukya PL, Srivastava M, Singh M, Barman MK, Gin KY, Mukherji S. The novel SARS-CoV-2 pandemic: possible environmental transmission, detection, persistence and fate during wastewater and water treatment. Sci Total Environ. 2021;765:142746. doi: 10.1016/j.scitotenv.2020.142746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkhansa A, Lakkis G, El Zein L. Mutational analysis of SARS-CoV-2 ORF8 during six months of COVID-19 pandemic. Gene Rep. 2021;23:101024. doi: 10.1016/j.genrep.2021.101024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabiani L, Saroglia M, Galatà G, De Santis R, Fillo S, Luca V, Faggioni G, D’Amore N, Regalbuto E, Salvatori P, Terova G, Moscone D, Lista F, Arduini F. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: a reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens Bioelectron. 2021;171:112686. doi: 10.1016/j.bios.2020.112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan Z, Yao B, Ding Y, Zhao J, Xie M, Zhang K. Entropy-driven amplified electrochemiluminescence biosensor for RdRp gene of SARS-CoV-2 detection with self-assembled DNA tetrahedron scaffolds. Biosens Bioelectron. 2021;178:113015. doi: 10.1016/j.bios.2021.113015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayin Q, Huang L, Ren C, Fu Y, Ma X, Guo J. Anti-SARS-CoV-2 IgG and IgM detection with a GMR based LFIA system. Talanta. 2021;227:122207. doi: 10.1016/j.talanta.2021.122207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karami A, Hasani M, Azizi Jalilian F, Ezati R. Conventional PCR assisted single-component assembly of spherical nucleic acids for simple colorimetric detection of SARS-CoV-2. Sens Actuat B-Chem. 2021;328:128971. doi: 10.1016/j.snb.2020.128971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moitra P, Alafeef M, Dighe K, Frieman MB, Pan D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano. 2020;14:7617–7627. doi: 10.1021/acsnano.0c03822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funari R, Chu K, Shen AQ. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens Bioelectron. 2020;169:112578. doi: 10.1016/j.bios.2020.112578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukkitgar SD, Shetti NP, Aminabhavi TM (2020) Electrochemical investigations for COVID-19 detection-a comparison with other viral detection methods. Chem Eng J 127575 [DOI] [PMC free article] [PubMed]
- 13.Rashed MZ, Kopechek JA, Priddy MC, Hamorsky KT, Palmer KE, Mittal N, Valdez J, Flynn J, Williams SJ. Rapid detection of SARS-CoV-2 antibodies using electrochemical impedance-based detector. Biosens Bioelectron. 2021;171:112709. doi: 10.1016/j.bios.2020.112709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alafeef M, Dighe K, Moitra P, Pan D. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano. 2020;14:17028–17045. doi: 10.1021/acsnano.0c06392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eissa S, Alhadrami HA, Al-Mozaini M, Hassan AM, Zourob M. Voltammetric-based immunosensor for the detection of SARS-CoV-2 nucleocapsid antigen. Microchim Acta. 2021;188:199. doi: 10.1007/s00604-021-04867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das CM, Guo Y, Yang G, Kang L, Xu G, Ho H, Yong K. Gold nanorod assisted enhanced plasmonic detection scheme of COVID-19 SARS-CoV-2 spike protein. AdvTheor Simul. 2020;3:2000185. doi: 10.1002/adts.202000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soler M, Estevez MC, Cardenosa-Rubio M, Astua A, Lechuga LM. How nanophotonic label-free biosensors can contribute to rapid and massive diagnostics of respiratory virus infections: COVID-19 case. ACS Sens. 2020;5:2663–2678. doi: 10.1021/acssensors.0c01180. [DOI] [PubMed] [Google Scholar]
- 18.Roda A, Cavalera S, Di Nardo F, Calabria D, Rosati S, Simoni P, Colitti B, Baggiani C, Roda M, Anfossi L. Dual lateral flow optical/chemiluminescence immunosensors for the rapid detection of salivary and serum IgA in patients with COVID-19 disease. Biosens Bioelectron. 2021;172:112765. doi: 10.1016/j.bios.2020.112765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cady NC, Tokranova N, Minor A, Nikvand N, Strle K, Lee WT, Page W, Guignon E, Pilar A, Gibson GN. Multiplexed detection and quantification of human antibody response to COVID-19 infection using a plasmon enhanced biosensor platform. Biosens Bioelectron. 2021;171:112679. doi: 10.1016/j.bios.2020.112679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quer G, Radin JM, Gadaleta M, Baca-Motes K, Ariniello L, Ramos E, Kheterpal V, Topol EJ, Steinhubl SR. Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat Med. 2021;27:73–77. doi: 10.1038/s41591-020-1123-x. [DOI] [PubMed] [Google Scholar]
- 21.Chaibun T, Puenpa J, Ngamdee T, Boonapatcharoen N, Athamanolap P, Mullane OAP, Vongpunsawad S, Poovorawan Y, Lee SY, Lertanantawong B. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nat Commun. 2021;12:802. doi: 10.1038/s41467-021-21121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg M, Sharma AL, Singh S. Advancement in biosensors for inflammatory biomarkers of SARS-CoV-2 during 2019-2020. Biosens Bioelectron. 2021;171:112703. doi: 10.1016/j.bios.2020.112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan DS, Prasad R, Srivastava R, Jaggi M, Chauhan SC, Yallapu MM. Comprehensive review on current interventions, diagnostics, and nanotechnology perspectives against SARS-CoV-2. Bioconjug Chem. 2020;31:2021–2045. doi: 10.1021/acs.bioconjchem.0c00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui F, Zhou HS. Diagnostic methods and potential portable biosensors for coronavirus disease 2019. Biosens Bioelectron. 2020;165:112349. doi: 10.1016/j.bios.2020.112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L, Li D, Ramadan S, Li Y, Klein N. Facile biosensors for rapid detection of COVID-19. Biosens Bioelectron. 2020;170:112673. doi: 10.1016/j.bios.2020.112673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, He L, Hu Y, Luo Z, Zhang J. A serological aptamer-assisted proximity ligation assay for COVID-19 diagnosis and seeking neutralizing aptamers. Chem Sci. 2020;11:12157–12164. doi: 10.1039/D0SC03920A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zakashansky JA, Imamura AH, Salgado DF, Romero Mercieca HC, Aguas RFL, Lao AM, Pariser J, Arroyo-Currás N, Khine M (2020) Detection of the SARS-CoV-2 spike protein in saliva with Shrinky-Dink© electrodes. medRxiv2011-2020 [DOI] [PubMed]
- 28.Woo CH, Jang S, Shin G, Jung GY, Lee JW. Sensitive fluorescence detection of SARS-CoV-2 RNA in clinical samples via one-pot isothermal ligation and transcription. Nat Biomed Eng. 2020;4:1168–1179. doi: 10.1038/s41551-020-00617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cennamo N, Pasquardini L, Arcadio F, Lunelli L, Vanzetti L, Carafa V, Altucci L, Zeni L. SARS-CoV-2 spike protein detection through a plasmonic D-shaped plastic optical fiber aptasensor. Talanta. 2021;233:122532. doi: 10.1016/j.talanta.2021.122532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akib TBA, Mou SF, Rahman MM, Rana MM, Islam MR, Mehedi IM, Mahmud MAP, Kouzani AZ. Design and numerical analysis of a graphene-coated SPR biosensor for rapid detection of the novel coronavirus. Sensors. 2021;21:3491. doi: 10.3390/s21103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rezabakhsh A, Rahbarghazi R, Fathi F. Surface plasmon resonance biosensors for detection of Alzheimer’s biomarkers; an effective step in early and accurate diagnosis. Biosens Bioelectron. 2020;167:112511. doi: 10.1016/j.bios.2020.112511. [DOI] [PubMed] [Google Scholar]
- 32.Tripathi PK, Upadhyay S, Singh M, Raghavendhar S, Bhardwaj M, Sharma P, Patel AK. Screening and evaluation of approved drugs as inhibitors of main protease of SARS-CoV-2. Int J Biol Macromol. 2020;164:2622–2631. doi: 10.1016/j.ijbiomac.2020.08.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye M, Luo G, Ye D, She M, Sun N, Lu Y, Zheng J (2020) Network pharmacology, molecular docking integrated surface plasmon resonance technology reveals the mechanism of Toujie Quwen Granules against coronavirus disease 2019 pneumonia. Phytomedicine153401 [DOI] [PMC free article] [PubMed]
- 34.Bong J, Kim T, Jung J, Lee SJ, Sung JS, Lee CK, Kang M, Kim HO, Pyun J. Pig sera-derived anti-SARS-CoV-2 antibodies in surface plasmon resonance biosensors. Bio Chip J. 2020;14:358–368. doi: 10.1007/s13206-020-4404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldoneschi V, Palladino P, Banchini M, Minunni M, Scarano S. Norepinephrine as new functional monomer for molecular imprinting: an applicative study for the optical sensing of cardiac biomarkers. Biosens Bioelectron. 2020;157:112161. doi: 10.1016/j.bios.2020.112161. [DOI] [PubMed] [Google Scholar]
- 36.Jain S, Paliwal A, Gupta V, Tomar M. Refractive index tuning of SiO2 for long range surface plasmon resonance based biosensor. Biosens Bioelectron. 2020;168:112508. doi: 10.1016/j.bios.2020.112508. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Liu Y, Yu Q, Peng W. Self-referencing SPR biosensing with an ultralow limit-of-detection using long-wavelength excitation. Sens Actuat B-Chem. 2021;327:128935. doi: 10.1016/j.snb.2020.128935. [DOI] [Google Scholar]
- 38.Qu L, Bai J, Peng Y, Han D, Ning B, Zhou H, Li S, Gao Z. Detection of three different estrogens in milk employing SPR sensors based on double signal amplification using graphene. Food Anal Methods. 2021;14:54–65. doi: 10.1007/s12161-020-01852-x. [DOI] [Google Scholar]
- 39.Wu Q, Li N, Wang Y, Liu Y, Xu Y, Wei S, Wu J, Jia G, Fang X, Chen F, Cui X. A 2D transition metal carbide MXene-based SPR biosensor for ultrasensitive carcinoembryonic antigen detection. Biosens Bioelectron. 2019;144:111697. doi: 10.1016/j.bios.2019.111697. [DOI] [PubMed] [Google Scholar]
- 40.Wu L, You Q, Shan Y, Gan S, Zhao Y, Dai X, Xiang Y. Few-layer Ti3C2Tx MXene: a promising surface plasmon resonance biosensing material to enhance the sensitivity. Sens Actuat B-Chem. 2018;277:210–215. doi: 10.1016/j.snb.2018.08.154. [DOI] [Google Scholar]
- 41.Md BH, Md AK, Md SH, Khondoker ZI, Md SH, M IP, Nibir M, Lway FA, Md AH, Md MR. Numerical modeling of MoS2-graphene bilayer-based high-performance surface plasmon resonance sensor: structure optimization for DNA hybridization. Opt Eng. 2020;59:1–21. [Google Scholar]
- 42.Chen X, Xu W, Ding N, Ji Y, Pan G, Zhu J, Zhou D, Wu Y, Chen C, Song H. Dual interfacial modification engineering with 2D MXene quantum dots and copper sulphide nanocrystals enabled high-performance perovskite solar cells. Adv Funct Mater. 2020;30:2003295. doi: 10.1002/adfm.202003295. [DOI] [Google Scholar]
- 43.Zhou B, Su M, Yang D, Han G, Feng Y, Wang B, Ma J, Ma J, Liu C, Shen C. Flexible MXene/silver nanowire-based transparent conductive film with electromagnetic interference shielding and electro-photo-thermal performance. ACS Appl Mater Inter. 2020;12:40859–40869. doi: 10.1021/acsami.0c09020. [DOI] [PubMed] [Google Scholar]
- 44.Yan L, Chen X, Liu X, Chen L, Zhang B. In situ formed VOOH nanosheet arrays anchored on a Ti3C2Tx MXene as a highly efficient and robust synergistic electrocatalyst for boosting water oxidation and reduction. J Mater Chem A. 2020;8:23637–23644. doi: 10.1039/D0TA09410B. [DOI] [Google Scholar]
- 45.Li G, Li N, Peng S, He B, Wang J, Du Y, Zhang W, Han K, Dang F. Highly efficient Nb2C MXene cathode catalyst with uniform O-terminated surface for lithium-oxygen batteries. Adv Energy Mater. 2021;11:2002721. doi: 10.1002/aenm.202002721. [DOI] [Google Scholar]
- 46.Deshmukh K, Kovářík T, Khadheer Pasha SK. State of the art recent progress in two dimensional MXenes based gas sensors and biosensors: a comprehensive review. Coordin Chem Rev. 2020;424:213514. doi: 10.1016/j.ccr.2020.213514. [DOI] [Google Scholar]
- 47.Song M, Pang S, Guo F, Wong M, Hao J. Fluoride-free 2D niobium carbide MXenes as stable and biocompatible nanoplatforms for electrochemical biosensors with ultrahigh sensitivity. Adv Sci. 2020;7:2001546. doi: 10.1002/advs.202001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Liu F, Huang D, Xue N, Dang Y, Zhang M, Zhang L, Li B, Liu D, Wang L, Liu H, Tao X. Nonoxidized MXene quantum dots prepared by microexplosion method for cancer catalytic therapy. Adv Funct Mater. 2020;30:2000308. doi: 10.1002/adfm.202000308. [DOI] [Google Scholar]
- 49.Mohammadniaei M, Koyappayil A, Sun Y, Min J, Lee M. Gold nanoparticle/MXene for multiple and sensitive detection of oncomiRs based on synergetic signal amplification. Biosens Bioelectron. 2020;159:112208. doi: 10.1016/j.bios.2020.112208. [DOI] [PubMed] [Google Scholar]
- 50.Chia HL, Mayorga-Martinez CC, Antonatos N, Sofer Z, Gonzalez-Julian JJ, Webster RD, Pumera M. MXene titanium carbide-based biosensor: strong dependence of exfoliation method on performance. Anal Chem. 2020;92:2452–2459. doi: 10.1021/acs.analchem.9b03634. [DOI] [PubMed] [Google Scholar]
- 51.Wu Q, Li N, Wang Y, Xu Y, Wu J, Jia G, Ji F, Fang X, Chen F, Cui X. Ultrasensitive and selective determination of carcinoembryonic antigen using multifunctional ultrathin amino-functionalized Ti3C2-MXene nanosheets. Anal Chem. 2020;92:3354–3360. doi: 10.1021/acs.analchem.9b05372. [DOI] [PubMed] [Google Scholar]
- 52.He L, Duan F, Song Y, Guo C, Zhao H, Tian J, Zhang Z, Liu C, Zhang X, Wang P, Du M, Fang S (2017) 2D zirconium-based metal-organic framework nanosheets for highly sensitive detection of mucin 1: consistency between electrochemical and surface plasmon resonance methods. 2D Mater 4: 25098
- 53.Shao B, Liu Z, Zeng G, Wang H, Liang Q, He Q, Cheng M, Zhou C, Jiang L, Song B. Two-dimensional transition metal carbide and nitride (MXene) derived quantum dots (QDs): synthesis, properties, applications and prospects. J Mater Chem A. 2020;8:7508–7535. doi: 10.1039/D0TA01552K. [DOI] [Google Scholar]
- 54.Hughes ZE, Walsh TR. Structural disruption of an adenosine-binding DNA aptamer on graphene: implications for aptasensor design. ACS Sensors. 2017;2:1602–1611. doi: 10.1021/acssensors.7b00435. [DOI] [PubMed] [Google Scholar]
- 55.Xi H, Juhas M, Zhang Y. G-quadruplex based biosensor: a potential tool for SARS-CoV-2 detection. Biosens Bioelectron. 2020;167:112494. doi: 10.1016/j.bios.2020.112494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X, Chen J, Wu M, Zhao JX. Aptamers: active targeting ligands for cancer diagnosis and therapy. Theranostics. 2015;5:322–344. doi: 10.7150/thno.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xue Q, Zhang H, Zhu M, Pei Z, Li H, Wang Z, Huang Y, Huang Y, Deng Q, Zhou J, Du S, Huang Q, Zhi C. Photoluminescent Ti3C2 MXene quantum dots for multicolor cellular imaging. Adv Mater. 2017;29:1604847. doi: 10.1002/adma.201604847. [DOI] [PubMed] [Google Scholar]
- 58.Li S, Qiu W, Zhang X, Ni J, Gao F, Wang Q. A high-performance DNA biosensor based on the assembly of gold nanoparticles on the terminal of hairpin-structured probe DNA. Sensor Actuat B-Chem. 2016;223:861–867. doi: 10.1016/j.snb.2015.09.121. [DOI] [Google Scholar]
- 59.Tian Y, Bu T, Zhang M, Sun X, Jia P, Wang Q, Liu Y, Bai F, Zhao S, Wang L. Metal-polydopamine framework based lateral flow assay for high sensitive detection of tetracycline in food samples. Food Chem. 2021;339:127854. doi: 10.1016/j.foodchem.2020.127854. [DOI] [PubMed] [Google Scholar]
- 60.Hu Y, Xie X, Wang X, Wang Y, Zeng Y, Pui DYH, Sun J. Visible-light upconversion carbon quantum dots decorated TiO2 for the photodegradation of flowing gaseous acetaldehyde. Appl Surf Sci. 2018;440:266–274. doi: 10.1016/j.apsusc.2018.01.104. [DOI] [Google Scholar]
- 61.Hu W, Liu Y, Withers RL, Frankcombe TJ, Norén L, Snashall A, Kitchin M, Smith P, Gong B, Chen H, Schiemer J, Brink F, Wong-Leung J. Electron-pinned defect-dipoles for high-performance colossal permittivity materials. Nat Mater. 2013;12:821–826. doi: 10.1038/nmat3691. [DOI] [PubMed] [Google Scholar]
- 62.Han J, Liu Z, Ma Y, Cui G, Xie F, Wang F, Wu Y, Gao S, Xu Y, Sun X. Ambient N2 fixation to NH3 at ambient conditions: using Nb2O5 nanofiber as a high-performance electrocatalyst. Nano Energy. 2018;52:264–270. doi: 10.1016/j.nanoen.2018.07.045. [DOI] [Google Scholar]
- 63.Wu F, Yue L, Su H, Wang K, Yang L, Zhu X. Carbon dots@platinum porphyrin composite as theranostic nanoagent for efficient photodynamic cancer therapy. Nanoscale Res Lett. 2018;13:357. doi: 10.1186/s11671-018-2761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng X, Wu J, Cao X, Abbott J, Jin C, Wang H, Strasser P, Yang R, Chen X, Wu G. N-, P-, and S-doped graphene-like carbon catalysts derived from onium salts with enhanced oxygen chemisorption for Zn-air battery cathodes. Appl Catal B-Environ. 2019;241:442–451. doi: 10.1016/j.apcatb.2018.09.054. [DOI] [Google Scholar]
- 65.Luo X, Ren H, Ma H, Yin C, Wang Y, Li X, Shen Z, Wang Y, Cui L. In situ integration of Co5.47N and Co0.72Fe0.28 alloy nanoparticles into intertwined carbon network for efficient oxygen reduction. J Colloid Interf Sci. 2020;569:267–276. doi: 10.1016/j.jcis.2020.02.086. [DOI] [PubMed] [Google Scholar]
- 66.Yu Z, Luan Y, Li H, Wang W, Wang X, Zhang Q. A disposable electrochemical aptasensor using single-stranded DNA-methylene blue complex as signal-amplification platform for sensitive sensing of bisphenol A. Sensor Actuat B-Chem. 2019;284:73–80. doi: 10.1016/j.snb.2018.12.126. [DOI] [Google Scholar]
- 67.Wijaya E, Lenaerts C, Maricot S, Hastanin J, Habraken S, Vilcot J, Boukherroub R, Szunerits S. Surface plasmon resonance-based biosensors: from the development of different SPR structures to novel surface functionalization strategies. Curr Opin Solid St M. 2011;15:208–224. doi: 10.1016/j.cossms.2011.05.001. [DOI] [Google Scholar]
- 68.Li C, Li J, Yang X, Gao L, Jing L, Ma X. A label-free electrochemical aptasensor for sensitive myoglobin detection in meat. Sensor Actuat B-Chem. 2017;242:1239–1245. doi: 10.1016/j.snb.2016.09.087. [DOI] [Google Scholar]
- 69.Chen X, Yamaguchi A, Namekawa M, Kamijo T, Teramae N, Tong A. Functionalization of mesoporous silica membrane with a Schiff base fluorophore for Cu(II) ion sensing. Anal Chim Acta. 2011;696:94–100. doi: 10.1016/j.aca.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Huang C, Yao Y, Montes García V, Stoeckel M, von Holst M, Ciesielski A, Samorì P (2021) Highly sensitive strain sensors based on molecules-gold nanoparticles networks for high-resolution human pulse analysis Small 2007593 [DOI] [PubMed]
- 71.Jing M, Bowser M. Methods for measuring aptamer-protein equilibria: a review. Anal Chim Acta. 2011;686:9–18. doi: 10.1016/j.aca.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duan F, Zhang S, Yang L, Zhang Z, He L, Wang M. Bifunctional aptasensor based on novel two-dimensional nanocomposite of MoS2 quantum dots and g-C3N4 nanosheets decorated with chitosan-stabilized Au nanoparticles for selectively detecting prostate specific antigen. Anal Chim Acta. 2018;1036:121–132. doi: 10.1016/j.aca.2018.06.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 931 kb)