Abstract
Recent work suggests a participation of mitochondria in apoptotic cell death. This role includes the release of apoptogenic molecules into the cytosol preceding or after a loss of mitochondrial membrane potential ΔΨm. The two uncouplers of oxidative phosphorylation carbonyl cyanide m-chlorophenylhydrazone (CCCP) and 2,4-dinitrophenol (DNP) reduce ΔΨm by direct attack of the proton gradient across the inner mitochondrial membrane. Here we show that both compounds enhance the apoptosis-inducing capacity of Fas/APO-1/CD95 signaling in Jurkat and CEM cells without causing apoptotic changes on their own account. This amplification occurred upstream or at the level of caspases and was not inhibited by Bcl-2. The effect could be blocked by the cowpox protein CrmA and is thus likely to require caspase 8 activity. Apoptosis induction by staurosporine in Jurkat cells as well as by Fas in SKW6 cells was unaffected by CCCP and DNP. The role of cytochrome c during Fas-DNP signaling was investigated. No early cytochrome c release from mitochondria was detected by Western blotting. Functional assays with cytoplasmic preparations from Fas-DNP-treated cells also indicated that there was no major contribution by cytochrome c or caspase 9 to the activation of effector caspases. Furthermore, an increase of rhodamine-123 uptake into intact cells, which has been explained by mitochondrial swelling, occurred considerably later than the caspase activation and was blocked by Z-VAD-fmk. These data show that uncouplers of oxidative phosphorylation can presensitize some but not all cells for a Fas death signal and provide information about the existence of separate pathways in the induction of apoptosis.
Apoptotic cell death is the result of the activation of a specialized intracellular pathway. Apoptosis can be induced by a wide variety of agents of very different natures including, for example, signaling through cell surface molecules or treatment with chemicals and viral infection (reviewed in reference 35). Despite this difference in apoptotic triggers, a cell induced to undergo apoptosis in probably all cases activates components of the same apoptosis system. Although the molecular events in the activation of the intracellular apoptosis pathway are still largely unknown, some steps of the actual execution of cell death have been unraveled. The most clearly and unequivocally defined step is the activation of members of the caspase family of cysteine proteases whose proteolytic activity either destroys the cell or signals destruction by activating further downstream components (for a review, see reference 26). Caspase activity appears to be essential for the appearance of the morphological signs of apoptosis such as nuclear condensation, DNA degradation, and cell membrane changes (26).
Recent data suggest that effector molecules localized in the mitochondria of the cell may contribute to the initiation of apoptosis. During apoptosis, several changes in the mitochondria are observed. Cytochrome c is normally physically associated with the inner mitochondrial membrane facing the intermembrane space. The addition of cytochrome c to cytosolic extracts has been shown to be a determining factor in the activation of caspase 3 via a complex of caspase 9 and apaf-1, a molecule with homology to the Caenorhabditis elegans cell death protein CED-4 (17, 18, 43). Since at least in some cases cytochrome c is also released in intact cells undergoing apoptosis prior to caspase activation (4, 32), this release may be one trigger of the execution system.
However, recent work points out that the initiation of apoptosis is more complex and that different agents might act via different molecular triggers. Studies in genetically modified mice suggest a model where so-called death receptors, such as tumor necrosis factor receptor I and Fas/APO-1/CD95 use a pathway independent of caspase 9 and apaf-1, whereas other stimuli, such as dexamethasone or UV irradiation, appear at least to some extent to rely on this signal chain (5, 10, 12, 39). Moreover, different cell lines and possibly different tissue types may react differently to the same stimulus.
A second mitochondrial parameter that has been recognized to change during apoptosis is the mitochondrial membrane potential (ΔΨm). A decrease in ΔΨm has been observed in several forms of apoptosis (for a review, see reference 11) and is assumed to be mediated by the opening of a mitochondrial multicomplex pore in a process termed permeability transition. This process has been suggested to be necessary to release apoptogenic molecules into the cytosol (31, 41). The reduction in ΔΨm has been found by some authors to be an event early in apoptosis committing the cell to death (40), but others have found it to be a later step, namely, to occur after the activation of caspases (4). Moreover, a recent report suggests that an increase in ΔΨm occurs early in apoptosis, preceding the later final reduction (34).
After electron transport through the respiratory chain, protons are pumped from the mitochondrial matrix into the intermembrane space. ΔΨm is the result of this asymmetrical distribution of protons (and other ions) between the mitochondria and the cytosol (for a review, see reference 11). Coupling of electron transport through the respiratory chain and ATP generation are disrupted by some acidic aromatic substances such as carbonyl cyanide m-chlorophenylhydrazone (CCCP) and 2,4-dinitrophenol (DNP). These so-called uncouplers of oxidative phosphorylation carry protons across the inner mitochondrial membrane. This specific attack of oxidative phosphorylation leads both to a reduction of ΔΨm and to the cessation of ATP generation in the mitochondrion.
In order to investigate the role of ΔΨm in apoptosis, we examined the effect of CCCP and DNP on apoptotic cell death. Apoptosis was induced in various cell lines with anti-Fas or staurosporine in the presence of these compounds. Both CCCP and DNP were able to enhance a Fas death signal in Jurkat and CEM cells but not SKW6 cells, and they had no effect on apoptosis induced by staurosporine. It was investigated at which level in the apoptotic pathway this cooperative effect took place. The effects of high-level expression of the antiapoptosis protein Bcl-2 and the expression of the cowpox caspase inhibitor CrmA on this enhancement were investigated. Changes in ΔΨm during apoptosis induced either by anti-Fas-DNP treatment or by staurosporine treatment were monitored. The role of cytochrome c release from the mitochondria was studied.
MATERIALS AND METHODS
Cell culture and induction of apoptosis.
The following cell lines were used in this study: Jurkat human T-cell leukemia (ATCC) cells, Jurkat cells overexpressing human Bcl-2 under the control of the EF1α promoter (36), the T-cell line CEM (provided by Marcus Peter, DKFZ, Heidelberg, Germany), and CEM CrmA (10a) and the lymphoblastoid cell line SKW6 (both provided by Andreas Strasser, WEHI, Melbourne, Australia). All cells were grown in Click’s RPMI supplemented with glutamine, 10% fetal calf serum, and antibiotics. For the apoptosis assays, cells were cultured at 106/ml (2 × 106/ml for the preparation of cytoplasmic extracts) for the times indicated at 37°C and 5% CO2. Death stimuli were the anti-human Fas monoclonal antibody (MAb) (Upstate Biotechnologies [distributed by Biozol, Munich, Germany]), staurosporine, CCCP, and DNP (from Sigma). Anti-Fas MAb was kept constant at 100 ng/ml in all of the experiments shown here. Staurosporine was normally used at 1 μM. In some experiments its concentration was titrated as indicated in the figure legends. CCCP was used at concentrations between 2.5 and 200 μM; DNP was used at concentrations between 125 μM and 2 mM as indicated.
Assays for apoptosis.
For assays of nuclear fragmentation, cells were stained with acridine orange, and the chromatin structure was analyzed visually under a fluorescence microscope. At least 200 nuclei were scored for each sample. The DNA content of the nuclei was measured as described previously (21). Cells were washed once in phosphate-buffered saline (PBS) and resuspended in staining buffer (0.1% sodium citrate, 0.1% Triton X-100, 50 μg of propidium iodide per ml). Samples were stored at 4°C in the dark and analyzed on the following day with a Becton Dickinson FACScalibur apparatus as described previously (21). To assess the morphology, cells were photographed at a 40-fold magnification.
Assay for caspase activity.
Extracts were prepared from cells stimulated as indicated. Cells were collected by centrifugation and washed once in PBS. Lysis was performed by incubation of the cells at a density of 2 × 107/ml in lysis buffer (150 mM NaCl; 50 mM Tris-HCl, pH 8.0; 1% Nonidet P-40) for 10 min on ice followed by vigorous vortexing. Extracts were then cleared by centrifugation for 5 min at 10,000 × g at 4°C, transferred to fresh vials, and stored at −70°C. For the assay of DEVD-cleaving activity, extracts were diluted 1:10 in reaction buffer (mitotic dilution buffer [14] containing 10 mM HEPES-KOH, pH 7.0; 40 mM β-glycerophosphate; 50 mM NaCl; 2 mM MgCl2; 5 mM EGTA; and 1 mM dithiothreitol [DTT]) supplemented with 0.1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} and 100 μg of bovine serum albumin and containing acetyl-DEVD-7-amino-4-methylcoumarin (DEVD-AMC) at a final concentration of 10 μM. Reactions were performed in triplicate in flat-bottomed 96-well plates at 37°C for 1 h. Free AMC was then measured by determining the fluorescence at 390 nm (excitation) and at 460 nm (emission) in a Millipore Cytofluor 96 reader. Values were calculated by subtracting the background fluorescence (buffer and substrate alone) and are presented as the mean and three times the standard error of the mean (mean/3 × the SEM).
Staining for ΔΨm.
Cells were cultured as described above and stimulated as indicated in the figure legends. Rhodamine-123 (Sigma) was added 30 min before the end of the stimulation period to 10 μg/ml. The culture was continued, and cells were washed twice in PBS and then analyzed by flow cytometry. JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbozyanine iodide; Molecular Probes) was added 15 min before the end of the stimulation period to 5 μg/ml, and the cells were incubated at room temperature in the dark (27). Cells were then washed with PBS and analyzed as described above.
Subcellular fractionation and Western blotting.
Jurkat cells (2 × 107 to 4 × 107 per sample) were treated as indicated in the figure legends. Fractions containing mitochondria and cytosol were prepared essentially as described earlier (37). Briefly, cells were washed once in cold PBS, resuspended in mitochondrion buffer (20 mM HEPES-KOH, pH 7.4; 10 mM KCl; 1.5 mM MgCl2; 1 mM EDTA; 1 mM EGTA; 250 mM sucrose) and lysed with 20 strokes in a Dounce homogenizer (the extent of lysis was checked by eosin exclusion staining and was found to be about 30%). More-extensive Dounce homogenization led to the release of cytochrome c from the mitochondria also in the untreated cells (not shown). Lysates were centrifuged twice for 10 min at 750 × g and once for 10 min at 1,000 × g at 4°C. The resulting supernatants were spun at 10,000 × g for 10 min. The supernatants from this step are designated cytosolic fractions (containing cytosol and light membrane fraction); the pellets contained mitochondria. The protein concentration was measured, and equal amounts were loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with monoclonal anti-cytochrome c (R & D Systems) or anti-cytochrome oxidase (subunit I; Molecular Probes) antibody. Blots were developed by using an enhanced chemiluminescence system (NEN).
Caspase-9 processing and caspase activation in extracts.
Extracts were prepared as described above. Radiolabelled human caspase 9 was generated by using the TNT-rabbit reticulocyte system (Promega) according to the manufacturer’s instructions. For the determination of caspase 9 processing activity, 1 μl of this reaction mixture was incubated with 100 μg of the corresponding cytosolic fraction in a final volume of 60 μl (the volume was made up by using S-100 buffer [20 mM HEPES-KOH, pH 7.4; 10 mM KCl; 1.5 mM MgCl2; 1 mM EDTA; 1 mM EGTA; 1 mM DTT; and the protease inhibitors phenylmethylsulfonyl fluoride, pepstatin, leupeptin, and aprotinin]) for 3 h at 37°C. Some samples also contained 1 mM dATP (Sigma) and 1 μg of cytochrome c (from bovine heart; Sigma) as indicated in the figures. After 3 h, samples were heated to 95°C in Laemmli buffer and separated on an SDS–14% polyacrylamide gel. Gels were dried and exposed to a PhosphorImager screen (Molecular Dynamics, Bad Homburg, Germany) for 1 to 3 days.
To measure the caspase-activating activity of cytosolic fractions, reaction mixtures (final volume, 40 μl) were set up to contain cytosolic fractions (50 μg) in S-100 buffer. As indicated, dATP (1 mM) and cytochrome c (0.5 μg) were added. Reaction mixtures were incubated at 37°C for 90 min, 10-μl aliquots were then taken, and DEVD-cleaving activity was measured for 60 min as described above.
RESULTS
Uncouplers of oxidative phosphorylation enhance an anti-Fas death signal in Jurkat and CEM cells but not in SKW6 cells.
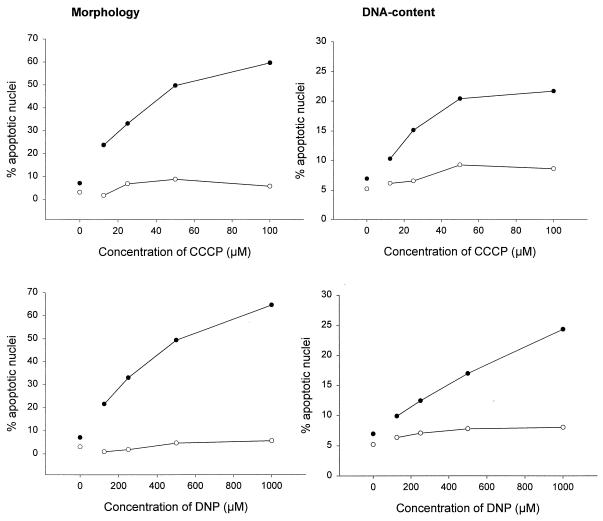
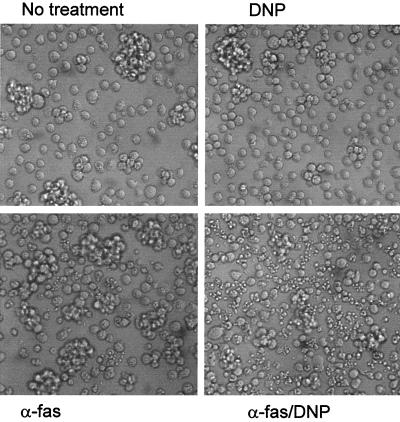
Signaling via the Fas surface receptor (also called CD95 and APO-1) induced apoptosis in a variety of cell lines. We first investigated whether CCCP and DNP affected Fas-triggered cell death in Jurkat cells. Cells were stimulated with an MAb against human Fas either alone or in the presence of various concentrations of CCCP or DNP. Apoptosis was measured by assessing the nuclear fragmentation morphologically and by determining the loss of DNA from nuclei as described previously (21). As shown in Fig. 1, there was no significant nuclear fragmentation or loss of genomic DNA after 6 h in cells treated with either CCCP or DNP alone at the concentrations used here. However, both agents increased the number of cells with apoptotic nuclei after Fas stimulation when added at the time of Fas cross-linking (Fig. 1) in both assays. Fas signaling induces further signs of apoptosis, such as vigorous blebbing and shedding of apoptotic bodies in Jurkat cells. As shown in Fig. 2, these signs of apoptosis were also markedly enhanced by DNP.
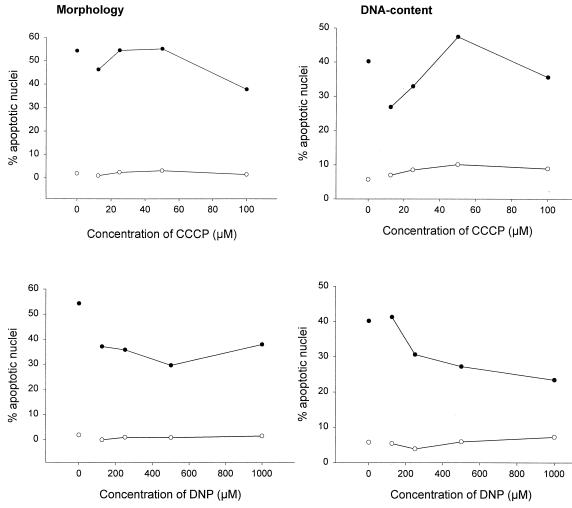
FIG. 1.
CCCP and DNP enhance the Fas apoptosis signal in Jurkat cells. Jurkat cells (2 × 105 per well) were incubated for 6 h in 96-well plates in 200 μl of complete medium or with various concentrations of CCCP (top panels) or DNP (bottom panels) as indicated either without (○) or with (●) anti-Fas MAb (100 ng/ml). Aliquots of the cultures were then assayed for apoptotic changes either morphologically (left panels) or after staining with propidium iodide for DNA content by flow cytometry (right panels). The experiments for these titration curves were performed three times with very similar results.
FIG. 2.
Morphology of Jurkat cells treated with anti-Fas–DNP. Jurkat cells (106 in 1 ml of complete culture medium in a 6-well plate) were incubated with the indicated stimuli (DNP was used at 1 mM, and anti-Fas [α-fas] was used at 100 ng/ml). Pictures were obtained after 6 h. A very similar effect was observed more than 20 times.
Cell membrane integrity was measured by propidium iodide exclusion to determine total cell death, and a similar enhancing effect of CCCP and DNP was observed when that criterion was used (data not shown). In some experiments, cells were stained simultaneously with acridine orange and ethidium bromide to assess membrane integrity and nuclear changes simultaneously. With these two dyes, necrotic cells can be defined as cells with a nonapoptotic nucleus but with defective plasma membrane. CCCP or DNP did not increase the percentage of such necrotic cells either when used alone or when used in combination with anti-Fas antibody (data not shown).
A similar effect was observed when genomic DNA was extracted and analyzed after 18 h of treatment: while CCCP and DNP alone did not induce DNA fragmentation in Jurkat cells, both increased the extent of DNA fragmentation after Fas ligation (data not shown).
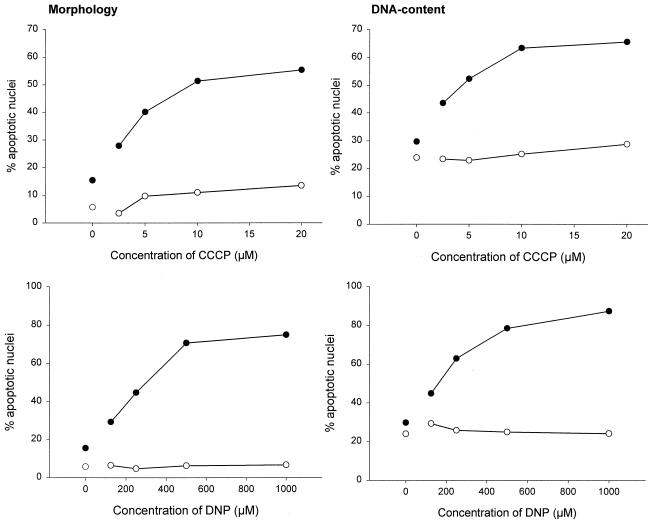
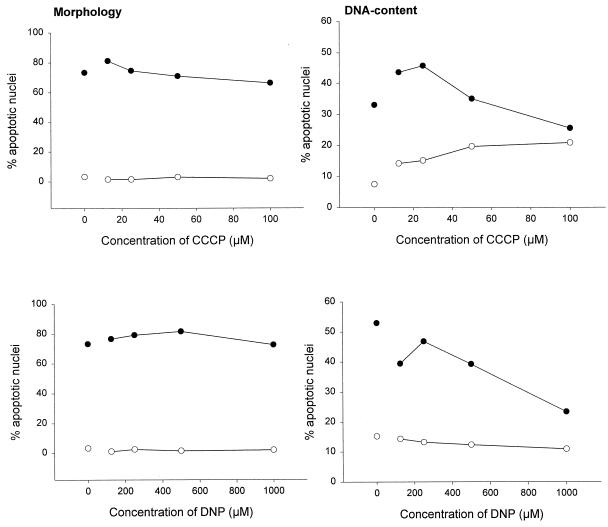
Different cell lines have recently been demonstrated to display different reactions to Fas engagement by a stimulating MAb, and a division into two types of cells has been proposed (28). According to this nomenclature, Jurkat cells belong to the group of type II cells in which Bcl-2 inhibits Fas-induced cell death and in which caspase activation may, at least initially, not be mediated directly via recruitment to the Fas molecule (28). We tested another type II cell line, CEM, and one type I cell line, SKW6, for their susceptibility towards CCCP and DNP effects. CEM cells reacted essentially like Jurkat cells in this system, i.e., CCCP and DNP enhanced Fas-triggered apoptosis (Fig. 3), whereas they lacked this capacity in SKW6 cells (Fig. 4). Therefore, these two agents act specifically in the Fas pathway operative in type II cells and offer a novel approach for distinguishing between the two types of cells.
FIG. 3.
CCCP and DNP enhance the Fas apoptosis signal in CEM cells. CEM cells (2 × 105 per well) were incubated for 3 h in 96-well plates in 200 μl of complete medium or with various concentrations of CCCP (top panels) or DNP (bottom panels) as indicated either without (○) or with (●) Anti-Fas MAb (100 ng/ml). Aliquots of the cultures were then assayed for apoptotic changes either morphologically (left panels) or after staining with propidium iodide for DNA content by flow cytometry (right panels). Note that the concentrations of CCCP required are lower than for Jurkat cells. Experiments for these titration curves were performed three times with very similar results.
FIG. 4.
CCCP and DNP do not alter the susceptibility of SKW6 cells to Fas-induced apoptosis. SKW6 cells (2 × 105 per well) were incubated for 3 h in 96-well plates in 200 μl of complete medium or with various concentrations of CCCP (top panels) or DNP (bottom panels) as indicated either without (○) or with (●) anti-Fas MAb (100 ng/ml). Aliquots of the cultures were then assayed for apoptotic changes either morphologically (left panels) or after staining with propidium iodide for DNA content by flow cytometry (right panels). The experiments for these titration curves were performed three times with similar results.
Dissipation of the proton gradient across the mitochondrial membrane by CCCP and DNP not only reduces ΔΨm but also will eventually inhibit ATP generation. We measured ATP levels in Jurkat cells after 2 and 6 h of treatment. After 2 h, there was no decrease in intracellular ATP abundance. After 6 h, ATP levels had not significantly dropped (<10%) in cells incubated with CCCP (100 μM) or DNP (1 mM) alone. ATP levels decreased to about 20 to 30% of the control level in cells treated with anti-Fas–CCCP and about 50% in cells treated with anti-Fas–DNP (data not shown). However, it has been shown that when ATP levels drop to below a certain threshold level, nuclear apoptosis is blocked (8, 15). Since nuclear apoptosis occurred in the described model, it is unlikely that this drop in ATP availability had a significant impact on the progress of apoptosis. Also, at higher concentrations, CCCP and DNP alone did induce nuclear fragmentation and DNA cleavage in Jurkat cells (not shown). Antimycin A and rotenone, which inhibit oxidative phosphorylation at earlier steps in the respiratory chain without directly reducing ΔΨm, did not enhance the death-inducing effect of anti-Fas stimulation (data not shown).
Recent data suggest that signaling after Fas triggering differs from apoptosis induction by other stimuli (10, 39). Apoptosis was therefore induced with the kinase inhibitor staurosporine in the presence of CCCP or DNP. As shown in Fig. 5, CCCP and DNP had no effect on the apoptosis-inducing activity of staurosporine.
FIG. 5.
CCCP and DNP do not alter the susceptibility of Jurkat cells to apoptosis induced by staurosporine. Jurkat cells (2 × 105 per well) were incubated for 6 h in 96-well plates in 200 μl of complete medium or with various concentrations of CCCP (top panels) or DNP (bottom panels) as indicated either without (○) or with (●) staurosporine (1 μM). Aliquots of the cultures were then assayed for apoptotic changes either morphologically (left panels) or after staining with propidium iodide for DNA content by flow cytometry (right panels). The experiments for these titration curves were performed three times with very similar results.
Effect of Bcl-2 and CrmA on the inhibition of Fas-CCCP- and Fas-DNP-induced apoptosis.
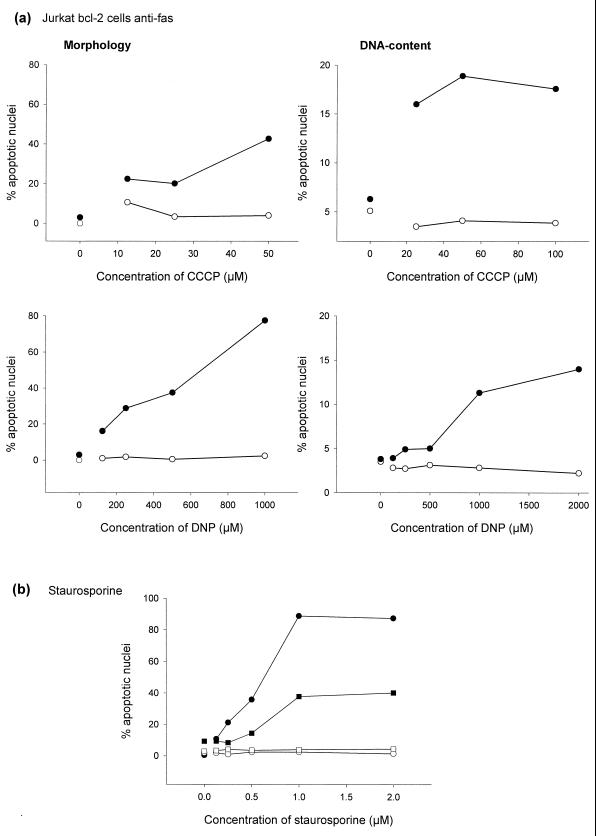
Although the cellular protein Bcl-2 does not inhibit Fas-induced cell death in all cell types, it does inhibit apoptosis induced by a wide variety of stimuli, including a Fas signal in type II cells such as Jurkat cells (2, 28). A subclone of Jurkat cells overexpressing human Bcl-2 under the control of the elongation factor 1α promoter (Jurkat Bcl-2 cells [36]) was treated with anti-Fas and anti-fas–CCCP or anti-fas–DNP. CCCP and DNP still clearly enhanced the Fas death signal in these cells (Fig. 6a). To confirm the functional expression of Bcl-2 in these cells, cells were treated with various concentrations of staurosporine. As shown in Fig. 6b, Bcl-2 protected cells very efficiently against this apoptosis-inducing agent.
FIG. 6.
(a) CCCP and DNP enhance the Fas apoptosis signal in Jurkat Bcl-2 cells. Jurkat Bcl-2 cells (2 × 105 per well) were incubated for 7 h in 96-well plates in 200 μl of complete medium or with various concentrations of CCCP (top panels) or DNP (bottom panels) as indicated either without (○) or with (●) anti-Fas MAb (100 ng/ml). Aliquots of the cultures were then assayed for apoptotic changes either morphologically (left panels) or after staining with propidium iodide for DNA content by flow cytometry (right panels). In some experiments the titration ranges were different (as shown). The experiments for these titration curves were performed twice with similar results. More than three additional experiments with CCCP at 100 μM and DNP at 1 mM were also done; in these experiments CCCP and DNP showed a similar enhancing effect. (b) High-level Bcl-2 protects Jurkat cells against staurosporine-induced apoptosis. A total of 2 × 105 Jurkat cells (solid symbols) or Jurkat Bcl-2 cells (open symbols) per well were incubated for 7 h in 96-well plates in 200 μl of complete medium or with various concentrations of staurosporine as indicated. Aliquots of the cultures were then assayed for apoptotic changes either morphologically (circles) or after staining with propidium iodide for DNA content by flow cytometry (squares).
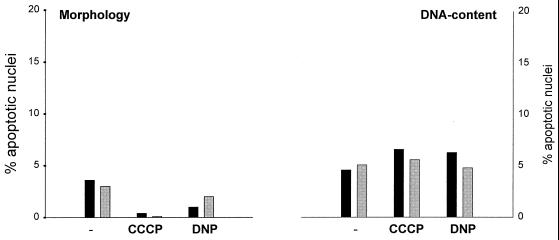
The signaling of Fas via the adapter molecule FADD/MORT-1 to caspase 8 is well defined. Recent work, however, suggested an alternative pathway for apoptosis induction by Fas which required the protein Daxx but not FADD (6, 38). A possible way to determine caspase 8 involvement in apoptosis is to measure the inhibitory potential of the cowpox protein CrmA. CrmA is a serpin-like molecule which inhibits several but not all caspases. Caspase 8 activity is blocked efficiently by CrmA, whereas CrmA has very little effect on other caspases, such as caspase 3 (42). CEM cells expressing CrmA were treated with anti-Fas in the presence of CCCP or DNP. As shown in Fig. 7, Fas did not induce apoptosis to a detectable extent in these cells either alone or in the presence of CCCP and DNP (the time and concentrations of agents were as described in the experiments for Fig. 3). The apoptosis-enhancing effect of CCCP and DNP is therefore likely to involve caspase 8 activation via FADD.
FIG. 7.
CEM CrmA cells are resistant to apoptosis induced by anti-Fas, anti-Fas–CCCP, or anti-fas–DNP. CEM CrmA cells (2 × 105 per well) were incubated for 3 h in 96-well plates in 200 μl of complete medium or with CCCP (20 μM) or DNP (1 mM) as indicated either without (solid columns) or with (shaded columns) anti-Fas MAb (100 ng/ml). Aliquots of the cultures were then assayed for apoptotic changes either morphologically (left panel) or after staining with propidium iodide for DNA content by flow cytometry (right panel). Concentrations of CCCP and DNP were the same as the highest concentrations used for CEM maternal cells in the experiment shown in Fig. 3. This experiment was performed three times with very similar results.
Cooperation between apoptotic signals from Fas and CCCP-DNP occurs upstream or at the level of caspase activation.
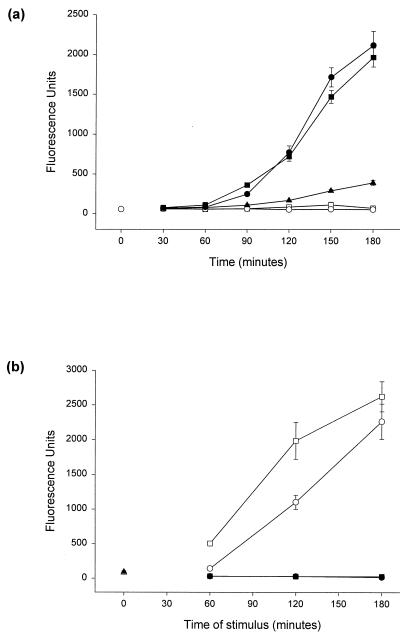
Intracellular proteolysis by caspases with the substrate specificity Asp-Glu-Val-Asp (DEVD) appears to be essential for the implementation of apoptosis (26). We analyzed whether the apoptosis-enhancing effect of CCCP and DNP involved caspase activation. DEVD-cleaving activity was measured in extracts from Jurkat cells treated with anti-Fas, CCCP, or DNP or the combinations anti-Fas–CCCP and anti-Fas–DNP. As shown in Fig. 8a, CCCP and DNP induced little if any detectable DEVD-cleaving activity on their own, but they strongly enhanced this caspase activity when induced by a Fas signal. DEVD-cleaving activity was most likely due to caspase-mediated proteolysis since the broad-spectrum inhibitor of caspases Z-VAD-fmk completely inhibited the activity when added to extracts from both staurosporine-treated and Fas-DNP-treated cells (Fig. 8b). Thus, the point in the apoptotic pathway where the two signals merge lies upstream of or at the level of caspase activation.
FIG. 8.
(a) CCCP and DNP enhance DEVD-cleaving activity in extracts from cells treated with anti-Fas. Jurkat cells (106 per time point) were incubated in 1 ml of complete medium containing no stimulus (○, at left), CCCP (200 μM; ○), DNP (2 mM; □), anti-Fas MAb (100 ng/ml; ▴), anti-Fas MAb and CCCP (●), or anti-Fas MAb and DNP (■). The agents were added at the times indicated prior to extraction, and all samples were harvested at the same time. Extracts were assayed in triplicate to determine the DEVD-AMC cleaving activity, and values are given as the mean/3 × the SEM. (For details of the extraction and assay for enzyme activity, see Materials and Methods). This kinetics experiment was done at least four times with all of the stimuli used at various concentrations of CCCP and DNP. A similar enhancing effect was seen in all experiments. (b) DEVD-cleaving activity is blocked by Z-VAD-fmk. Extracts were prepared from 106 unstimulated Jurkat cells (triangle) or Jurkat cells stimulated either with anti-Fas (100 ng/ml) and DNP (1 mM; circles) or with staurosporine (1 μM; squares) for the times indicated. Cells were extracted, and the DEVD-cleaving activity was measured as described for panel a (open symbols). Z-VAD-fmk (final concentration, 25 μM) was added 5 min before the addition of substrate to one aliquot of extract (colosed symbols). This experiment was performed twice with very similar results.
When extracts from cells treated with anti-Fas and from cells treated with DNP were mixed in vitro, no overadditive caspase activity was detected (data not shown). This indicates that intact organization and compartmentalization of the cell are required for the enhancing effect.
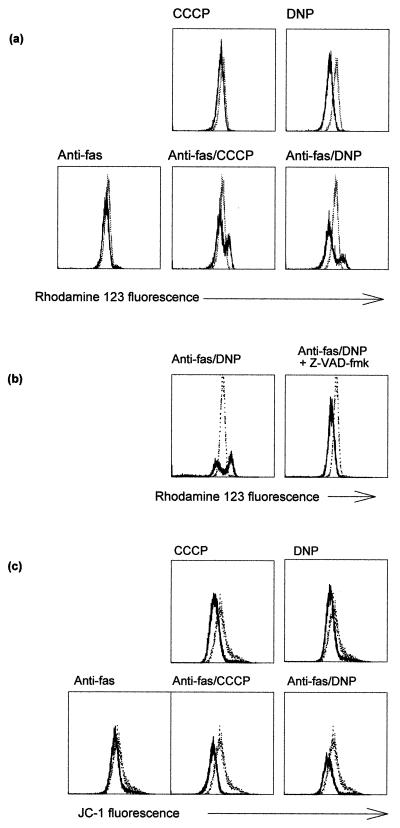
CCCP and DNP lead to a reduction of ΔΨm but enhance the size of the population with high rhodamine-123 uptake induced by anti-Fas.
Changes in the mitochondrial membrane potential ΔΨm have been reported to be a general feature of apoptotic cell death (11). A decrease in ΔΨm was in some cases found early in the apoptotic process, whereas other studies suggested that this process occurs relatively late, downstream of caspase activation. An increase in ΔΨm has also been suggested to take place at an earlier stage of apoptosis (34). We assessed changes in ΔΨm after stimulation of Jurkat cells with anti-Fas and either CCCP or DNP. ΔΨm was measured as the uptake of the cationic dyes rhodamine-123 or JC-1 as described earlier (27, 34). As predicted, both CCCP and DNP led to a reduction in uptake of these dyes (Fig. 9a and c), although it was noted that the apparent reduction in ΔΨm was somewhat different dependent on which dye was used (Fig. 9a and c). A signal through the Fas receptor induced an increase in rhodamine-123 staining in a small portion of cells over a period of 6 h (Fig. 9a and data not shown) which was not visible when cells were stained with JC-1 (Fig. 9c). Surprisingly, the presence of CCCP or DNP markedly increased the proportion of cells with a higher rhodamine-123 fluorescence (Fig. 9a; for all the results shown here, only cells with forward scatter-side scatter criteria for living cells are given). These cells with high rhodamine-123 fluorescence (rhodamine-123-high cells) had still intact plasma membranes, as determined by propidium iodide staining (data not shown).
FIG. 9.
Staining for ΔΨm in Jurkat cells after treatment with anti-Fas and uncouplers of oxidative phosphorylation. (a) Appearance of a rhodamine-123-high-staining population. Jurkat cells (106 per sample) were treated for 6 h with the agents indicated (anti-Fas, 100 ng/ml; CCCP, 100 μM; DNP, 1 mM). Cells were then stained and analyzed as described in Materials and Methods. The curves show untreated (control) cells as dotted lines and treated cells as solid lines. The mean fluorescence values for cells in the top panel were as follows: untreated, 25.1; CCCP treated, 21.6; and DNP treated, 17.8. This experiment was done at least five times with each agent with very similar results. (b) The appearance of a rhodamine-123-high-staining population is blocked by Z-VAD-fmk. Jurkat cells were treated as for panel a. Cells were treated with anti-Fas–DNP with or without Z-VAD-fmk (50 μM) as indicated. Cells were stained and analyzed as described above. Results are shown for control (untreated) cells (dotted lines) and treated cells (solid lines) plotted in each diagram. Treatment with Z-VAD-fmk alone did not change the rhodamine-123 staining (not shown). These experiments were done four times with very similar results. (c) Changes in JC-1 staining after treatment. Cells were treated as for panel a and were stained and analyzed as described in Materials and Methods. Results are shown for untreated cells (dotted lines) or cells treated with the indicated agents (solid lines). The treatment lasted 6 h as for panel a. This experiment was done three times with similar results.
A final loss of rhodamine-123 fluorescence occurred at about the same time as the changes in scatter signals, since almost all the dead cells (by forward scatter-side scatter criteria) but very few cells in the live cell gate exhibited low rhodamine-123 staining (data not shown). It should be noted that the intensity of rhodamine-123 staining in the population of rhodamine-123-high-staining cells was the same whether the cells were treated with anti-Fas or with anti-Fas–CCCP or –DNP, i.e., regardless of the presence of CCCP or DNP (Fig. 9a and data not shown). This indicates that the increase in staining does not depend on the proton gradient across the inner mitochondrial membrane.
In cells expressing high levels of Bcl-2, the increase in rhodamine-123 staining after anti-Fas or anti-Fas–DNP treatment was reduced. This is consistent with earlier data showing that Bcl-x can inhibit the increase in rhodamine-123 staining during Fas- or staurosporine-induced apoptosis (34). The decrease in ΔΨm induced by DNP, however, was the same as in normal Jurkat cells (not shown). These findings further support the interpretation that Bcl-2 can reduce the apoptosis-inducing capacity of a Fas signal but not the mechanism of amplification by DNP. Thus, a chemically induced loss of ΔΨm does not necessarily lead to apoptosis, whereas the increase in rhodamine-123 uptake is an event of apoptosis downstream of the checkpoint controlled by Bcl-2.
The suitability of rhodamine-123 for measurement of ΔΨm has been questioned (20). We therefore included a second dye, JC-1, in these studies. In comparative studies, JC-1 uptake has been found to be a more reliable parameter for monitoring ΔΨm in the presence of, for example, plasma membrane depolarization (27). As shown in Fig. 9c, JC-1 staining indeed gave a different result, i.e., only the expected decrease in staining upon treatment with CCCP or DNP but a complete absence of the high-staining population visible when rhodamine-123 was used.
The increase in rhodamine-123 staining has been interpreted as the result of an early swelling of mitochondria during apoptosis that is blocked by Bcl-x in a mitochondrion-autonomous manner and that precedes caspase activation (34). In the system described here, the increase in rhodamine-123 staining became noticeable first after between 1 and 2 h of treatment with anti-Fas–DNP and was evident somewhat later when cells were treated with anti-Fas alone. The proportion of cells in this rhodamine-123-high-staining population increased gradually over the period of 6 h investigated (data not shown). Since the maximum of caspase activity was reached after ca. 3 h of anti-Fas–DNP stimulation, we considered the possibility that the increase in rhodamine-123 uptake was a later event in this model of apoptosis. When cells were treated with anti-Fas–DNP in the presence of the caspase inhibitor Z-VAD-fmk, no increase in rhodamine-123 staining was observed (Fig. 9b) and the cells did not display any of the morphological signs of cell death, such as nuclear fragmentation and loss of cell membrane integrity (not shown). We conclude that the increase in rhodamine-123 uptake is a sign of apoptosis occurring downstream of caspase proteolysis. Caspase activity was not required directly for high levels of rhodamine-123 uptake, however, since the addition of the caspase inhibitor 30 min to 2 h prior to staining with rhodamine-123 had only little effect on the increase (data not shown). Thus, the inhibitory effect of Bcl-2 on the increase in rhodamine-123 staining is likely the result of a block in the activation of the apoptosis machinery rather than of a direct action by Bcl-2 on mitochondrial parameters.
When Jurkat cells were treated with staurosporine, a rhodamine-123-high-staining population could also be detected, but not when Z-VAD-fmk was present (not shown). These results suggest that an increase in rhodamine-123 uptake into dying cells is an event common to at least several forms of apoptosis. Since it could be prevented by the inhibition of caspase proteolysis and since caspase activity is a necessary feature of apoptosis, this increase in rhodamine-123 staining is likely to be an event common to all forms of apoptosis occurring downstream of caspases.
A further indication that the increase in rhodamine-123 staining is a late event in apoptosis came from the two-parameter analysis of flow cytometry data, where forward scatter signal (as a measure for the size of the cells) was plotted against rhodamine-123 fluorescence. This representation showed that cells with increased rhodamine-123 uptake tend to give a smaller forward scatter signal, suggesting that they have already started to shrink, even when gated only on cells in the normal scatter gate for live cells and more so when all cells were gated (not shown). Together, these results suggest that an increase in rhodamine-123 fluorescence probably occurs independently of ΔΨm and is a late event in apoptosis, i.e., at a stage immediately before or concurrent with other morphological signs of apoptosis.
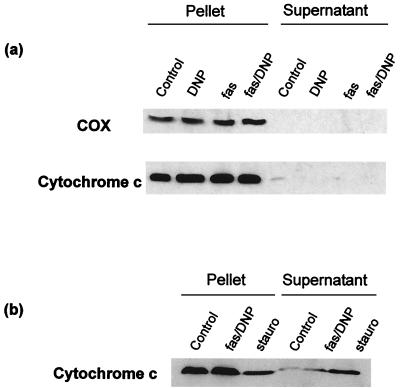
Induction of caspase activity by anti-Fas–DNP is likely to occur independently of cytochrome c release.
In several models of apoptosis, translocation of cytochrome c from mitochondria into the cytosol has been observed. In cytosolic extracts in the presence of dATP, free cytochrome c can trigger caspase activation, which raises the possibility that cytochrome c release in intact cells may be a mechanism to trigger apoptosis. Whether cytochrome c release plays a role in apoptosis induction after Fas ligation is still uncertain. We investigated whether cytochrome c release into the cytosol contributed to the activation of caspases after treatment with anti-Fas–DNP in Jurkat cells. As shown in Fig. 10a, neither anti-Fas nor DNP treatment alone nor the combination of both led to detectable cytochrome c redistribution after 2 h of stimulation; almost all immunoreactivity was detected in the pellet of the centrifugation at 10,000 × g (containing mitochondria), and no cytochrome c exceeding the amount found in the control sample of untreated cells was detectable in the supernatant. When cells were treated with staurosporine, cytochrome c was detectable in the cytosol and disappeared from the mitochondria; the results of an experiment when Jurkat cells were treated with either anti-Fas–DNP or staurosporine for 2.5 h are shown in Fig. 10b. When fractions were prepared, the amount of cytochrome c in the 10,000 × g pellet had dropped significantly in the sample of staurosporine-treated cells, while very little if any redistribution was visible in the samples from cells treated with anti-Fas–DNP (Fig. 10b). In time course experiments, no significant cytochrome c release was detectable during 4 h of treatment with anti-Fas–DNP (data not shown).
FIG. 10.
Enhancement of the anti-Fas signal by DNP occurs in the absence of detectable cytochrome c redistribution. (a) Jurkat cells (4 × 107/sample) were treated with the agents indicated as described above. After 2 h, cells were collected by centrifugation and homogenized as described in Materials and Methods. Supernatants (containing cytosol and light membranes) and pellets (containing mitochondria) from the 10,000 × g centrifugation step were separated by SDS-PAGE and analyzed by Western blotting. Separate blots were probed with MAb to cytochrome oxidase subunit I (COX, top) and cytochrome c (bottom). These results are representative of five independent experiments. (b) Jurkat cells (2 × 107/sample) were treated for 2.5 h as indicated (fas/DNP, anti-Fas MAb [100 ng/ml] and DNP [1 mM]; stauro, staurosporine [1 μM]). Fractions were prepared and analyzed as described above.
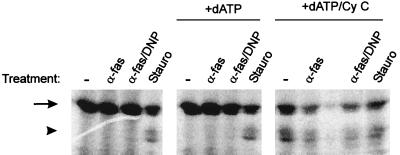
The reported chain of events after cytochrome c release into the cytosol is the binding of cytochrome c to apaf-1 followed by recruitment of caspase 9 into the complex and the subsequent activation of this protease. Active caspase 9 then probably activates other caspases (17, 43). To obtain additional evidence about the role of cytochrome c in Fas-DNP-induced apoptosis, we assayed cytosolic extracts for caspase 9-processing activity. Jurkat cells either were left untreated or were treated with anti-Fas, anti-Fas–DNP, or staurosporine, and cytosolic extracts were prepared after 2 h as described in Materials and Methods. Aliquots (100 μg) of these extracts were incubated with radiolabelled human pro-caspase 9 for 3 h, and the products of these reactions were analyzed by SDS-PAGE and autoradiography. As shown in Fig. 11, extracts from untreated cells or from cells treated with anti-Fas or anti-Fas–DNP contained no activity which would have been able to process pro-caspase 9. Incubation with extract from staurosporine-treated cells, however, led to the appearance of two fragments of about 35 kDa (Fig. 11, left panel). The processing of pro-caspase 9 to these two smaller fragments has been shown earlier to be induced by cytochrome c in a cell-free system and to require an active site in caspase 9 (22).
FIG. 11.
Addition of cytochrome c is required for the appearance of pro-caspase 9-processing activity in cytosolic extracts from Jurkat cells treated with anti-Fas or anti-Fas–DNP. Cytosolic extracts were prepared from Jurkat cells which either had been left untreated or had been treated with anti-Fas (100 ng/ml), anti-Fas–DNP (1 mM), or staurosporine (1 μM) for 2 h as described in Materials and Methods. Aliquots (100 μg) were incubated in a final volume of 60 μl of S-100 buffer with 1 μl of in vitro-translated pro-caspase 9 for 3 h at 37°C either alone (left), in the presence of 1 mM dATP (middle), or in the presence of 1 mM dATP and 1 μg of cytochrome c (right). Reactions were then boiled in Laemmli buffer and subjected to SDS-PAGE. Gels were dried, and caspase-9 was visualized by autoradiography. Arrow, intact pro-caspase 9; arrowhead, processed fragments (ca. 35 kDa). Very similar results were obtained in four experiments with three independent sets of extracts.
In intact cells, cytochrome c appears to be sufficient to process caspase 9, whereas in extracts cytochrome c requires dATP to initiate this activation (4, 18). We added dATP alone or with cytochrome c to the extracts and determined the activation of pro-caspase 9 as described above. Figure 11 (middle and right panels) shows that for extracts from normal cells and from cells treated with anti-Fas or anti-Fas–DNP the addition of cytochrome c was required in order to trigger caspase 9 processing, while it had no detectable effect on extracts from staurosporine-treated cells.
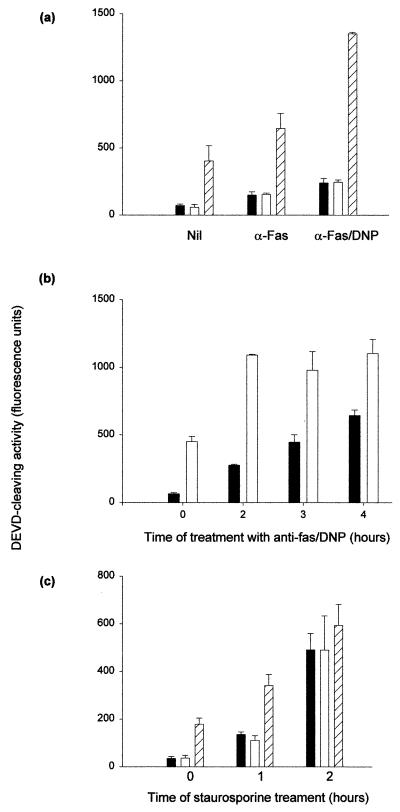
We continued investigation of this pathway by determining DEVD-cleaving activity in a similar system. Extracts (50 μg) were incubated for 90 min at 37°C either alone or together with dATP or a combination of dATP and externally added cytochrome c. Then the DEVD-cleaving activity was measured in the products of these reactions. Figure 12a shows the results of a typical experiment with extracts from cells either left untreated or treated for 2 h with anti-Fas or anti-Fas–DNP. As expected, DEVD-cleaving activity was highest in extracts from cells treated with anti-Fas–DNP when extracts were incubated alone (Fig. 12a, solid columns). In all three settings, the addition of dATP alone had no effect on the activity (Fig. 12a, open columns). When dATP and cytochrome c were both added, significant DEVD-cleaving activity was induced (Fig. 12a, hatched bars). Figure 12b and c give the results of time course experiments with either the combination anti-Fas–DNP (b) or treatment with staurosporine (c). In extracts from staurosporine-treated cells, after 2 h cytochrome c addition did not induce any additional DEVD-cleaving activity (Fig. 12c). In extracts from cells treated with anti-Fas–DNP, however, such activity was induced by cytochrome c even when extracts were prepared after 4 h of treatment (Fig. 12b).
FIG. 12.
Induction of DEVD-cleaving activity by cytochrome c in extracts from normal and apoptotic cells. Jurkat cells were treated as indicated (anti-Fas MAb, 100 ng/ml; DNP, 1 mM; staurosporine, 1 μM), and cytosolic extracts were prepared as described in Materials and Methods. Extracts (50 μg) were incubated in 40-μl reaction mixtures for 90 min at 37°C. DEVD-cleaving activity was then measured in triplicates of 10 μl from each mixture as described in the text. Results are given as the mean/3 × the SEM. (a) Extracts were prepared after 2 h of the indicated treatment and incubated either alone (solid columns), with 1 mM dATP (open columns), or with 1 mM dATP and 0.5 μg of cytochrome c (hatched columns). (b) Cells were either left untreated (time zero) or were treated with a combination of anti-Fas MAb and DNP for the time periods indicated. Extracts were prepared and incubated either alone (solid columns) or with 1 mM dATP and 0.5 μg of cytochrome c (open columns). (c) Cells were either left untreated (time zero) or were treated with staurosporine for 1 or 2 h as indicated. Extracts were prepared and incubated alone (solid columns), with 1 mM dATP (open columns), or with 1 mM dATP and 0.5 μg of cytochrome c (hatched columns).
Although it is possible that small quantities of cytochrome c did play a role in activating DEVD-cleaving activity in intact cells, its importance appears to be not as great in Fas-DNP-triggered apoptosis as in staurosporine-induced cell death. The result that cytochrome c in all cases was able to add to the DEVD-cleaving activity in extracts from cells treated with anti-Fas–DNP suggests that this pathway had at least not completely been triggered in the intact cell. This set of experiments also provides evidence that cytochrome c is not the agent responsible for the amplification of a Fas death signal by DNP.
DISCUSSION
In this report we show that uncouplers of oxidative phosphorylation are capable of enhancing a Fas death signal in so-called type II but not in type I cells. This enhancing effect requires caspase 8 activity, involves enhanced caspase activation, and is not blocked by Bcl-2. Enhanced caspase activation is likely to occur in the absence of cytochrome c release into the cytosol. Using this model, we demonstrate that increased rhodamine-123 uptake is a cellular response to apoptosis-inducing stimuli downstream of caspase proteolysis.
CCCP and DNP are thought to act specifically to dissipate the proton gradient across the inner mitochondrial membrane. Although this attack will eventually lead to ATP depletion of the cell, several points of evidence support the view that this disruption is not the relevant mechanism that enhances the Fas signal. First, ATP levels did not drop significantly over the period investigated. Second, other inhibitors of oxidative phosphorylation that block ATP generation at an earlier step, such as antimycin A, do not have this effect. Third, efficient disruption of ATP generation leads to a cellular condition where apoptosis seems to be impossible and where the cell responds to an otherwise apoptotic signal by undergoing necrosis without, for example, nuclear fragmentation (8, 15, 29). Since CCCP and DNP clearly enhance the apoptosis-inducing capacity of Fas leading to nuclear fragmentation, we consider it unlikely that a block in ATP generation underlies the enhancing effect.
The appearance of a population with a high level of rhodamine-123 staining was noted when cells were treated with anti-Fas. Although both CCCP and DNP reduced the intensity of rhodamine-123 staining, the fluorescence intensity of the cells in this rhodamine-123-high-staining population was not affected by either CCCP or DNP. The size of this population even increased in Jurkat cells in the presence of CCCP or DNP, and this population was also found when staurosporine was used to induce apoptosis. The caspase inhibitor Z-VAD-fmk prevented the appearance of this population in all cases. When cells were stained with JC-1, no population with increased fluorescence was observed.
Most earlier work regarding changes in ΔΨm has focused on the description of the final loss of ΔΨm that is indicated by a reduction in staining with several dyes, including rhodamine-123. One study has described an early increase in rhodamine-123-staining during apoptosis. This increase was interpreted to be mainly the result of mitochondrial swelling (34). Our results demonstrate that high sequestration of rhodamine-123 is inhibited by the caspase inhibitor Z-VAD-fmk, suggesting that caspase proteolysis is necessary for this change. Accordingly, DNP, which enhances caspase activity induced by Fas, also accelerated the appearance and increased the size of the rhodamine-123-high-staining population.
The principal possibilities for the mechanism of increased rhodamine-123 uptake include an increase in ΔΨm, a swelling of mitochondria, a proliferation of mitochondria, and uptake of rhodamine-123 that occurs independently of ΔΨm. The staining with the mitochondrion selective dye MitoTracker Green (Molecular Probes), which is taken up into mitochondria independently of ΔΨm, did not increase during anti-Fas–DNP treatment (data not shown). This makes it unlikely that proliferation of mitochondria is responsible for the increase in rhodamine-123 staining. Also, when stained with the cationic dye JC-1, which, like rhodamine-123, has been found to stain selectively mitochondria in proportion to their ΔΨm, no increase in staining was seen in cells treated with anti-Fas or anti-Fas–DNP. It is therefore unlikely that the effect was a true reflection of increased ΔΨm.
It has been suggested that the quenching of rhodamine-123 might be the reason for its inconsistent behavior in staining cells (20). However, the authors of that study detected quenching only in solution but not in titration curves in intact cells, so this explanation is not satisfactory.
The suggestion that the enhanced staining is due to an increase of the volume in individual mitochondria with an unaltered ΔΨm (34) does not explain why JC-1 staining is not also increased. The appearance of a rhodamine-123-high-staining population during apoptosis has been demonstrated to be accompanied by mitochondrial swelling (34). Since early studies of apoptosis had found that mitochondria remained intact until late stages of the process, this contradiction has been puzzling (24). Our data now show that the increase in rhodamine-123 does not occur during the initiation of apoptosis but rather during the execution phase, i.e., downstream of caspase activity. This result reconciles the apparently conflicting data and defines the increased rhodamine-123 uptake as a relatively late event in apoptosis, when other morphological changes, such as nuclear condensation and cell shrinkage, can be also detected.
A study published by Salvioli et al. (27) has suggested that several ΔΨm-sensitive dyes including rhodamine-123 may also be sensitive to changes in plasma membrane potential. In fact, depolarization of the plasma membrane by KCl led to an increase in rhodamine-123 staining (27). It is therefore possible that plasma membrane changes during apoptosis provoke a similar pattern. Another possibility is that the efflux of rhodamine-123 from the cell, which appears to be achieved actively by the P glycoprotein (3), is disturbed as a consequence of caspase activity, which might in turn lead to an accumulation of the dye inside the cell.
Whether cytochrome c release from mitochondria plays a role in Fas-induced cell death is still a contentious issue. It is generally assumed that the activation of the caspase-mediated proteolytic activity after the recognition sequence DEVD is required for and generated during all forms of apoptosis. The activation of these caspases, however, appears to be accomplished by separate pathways. The demonstration that Bcl-2 and the loss of Fas can act in a cooperative fashion during lymphocyte apoptosis suggested that there were different pathways of apoptosis induction which could or which could not be blocked by Bcl-2 (30). The clearest demonstration of such independent pathways comes from the recently published studies of caspase 9- and apaf-1-deficient mice. Both types of modified mice display reduced apoptosis in response to many commonly used in vitro stimuli (e.g., staurosporine) but not in response to Fas signaling (5, 10, 12, 39). The one reported exception, i.e., that embryonic fibroblasts from apaf-1 knockout mice were less sensitive to Fas-induced death (5), may be a peculiarity of this cell type.
Staurosporine appears to act via cytochrome c release (4). Whether Fas signaling causes cytochrome c release is less clear. Some authors have reported a release of cytochrome c from mitochondria during Fas-induced apoptosis (9, 28), whereas others have found apoptosis to occur in the absence of detectable cytochrome c in the cytoplasm (1, 7). This has at least in part been attributed to differences in the preparation of the samples (1). In our experiments, we found no detectable cytochrome c release during at least the early stages of Fas-induced apoptosis. It is conceivable that different cells or even subclones of established cell lines such as Jurkat display a somewhat different sensitivity to Fas signaling. It is also difficult to assess the significance of any such results since we do not know what amount of cytochrome c would be required for the induction of caspase activity in intact cells. Since the cytochrome c-initiated pathway involves the activation of caspase 9, we examined cytosolic extracts for caspase 9-processing activity. Such activity was found in extracts from cells treated with staurosporine but not, at least for the early stages investigated (i.e., in the first 2 h), from cells treated with anti-Fas or anti-Fas–DNP. However, when cytochrome c was added to these extracts, caspase 9-processing activity could be evoked, suggesting that the cytochrome c-triggered pathway of apoptosis had not been initiated prior to the preparation of the extracts.
Similarly, in extracts from cells treated with Fas–DNP it was possible to increase DEVD-cleaving activity by the addition of cytochrome c. Although it is conceivable that small amounts of cytochrome c had already been liberated into the cytosol, though not enough to activate all of the caspase 9 molecules, it should be noted that during staurosporine-induced apoptosis cytochrome c addition failed to enhance DEVD-cleaving activity after 2 h. This observation is in accordance with the lack of detectable caspase 9-processing activity in the extracts and is compatible with the hypothesis that cytochrome c plays a less-important role in Fas-triggered apoptosis.
Caspase 8 has been reported to cleave the protein Bid which then, at least in a cell-free system, translocates to mitochondria and induces release of cytochrome c (16, 19). Since the only known molecular effect of cytochrome c in apoptosis is the activation of caspase 9 and since Fas-triggered apoptosis is normal in caspase 9 knockout mice, it is unlikely that this mechanism plays an important role in vivo. Also, in this model Bcl-x can completely inhibit cytochrome c release, whereas Bcl-x inhibits anti-Fas-induced cell death only in some cell types (28, 30). In a cell-free system, cytochrome c release was able to enhance caspase 8-induced apoptosis (13), so cytochrome c release could be a mechanism for enhancing apoptosis without being essential for the process.
In addition to this distinction, different cell lines have been reported to react in a different fashion to a Fas signal (28). In so-called type I cells such as cells from the SKW6 cell line, Fas signaling leads to rapid caspase activation and cell death cannot be blocked by Bcl-2 overexpression. In type II cells such as Jurkat and CEM cells, caspase activation follows slower kinetics and can be inhibited to some extent by Bcl-2 (28). In the experiments reported here we observed a similarly different response to a Fas signal: in the two cell lines formerly termed type II cells CCCP and DNP were able to enhance a Fas signal, whereas in a type I cell line this effect was not seen. Therefore, such sensitivity to CCCP and DNP may be an additional way of allocating cells to either of these groups.
The Bcl-2 protein is well known for its ability to inhibit apoptosis under most circumstances (25). It is therefore remarkable that in the presence of high levels of Bcl-2 CCCP and DNP still are able to exert their apoptosis-enhancing effect. In accordance with this, Bcl-2 has no influence on the reduction in ΔΨm by DNP. One possible interpretation is that CCCP and DNP revert type II cells into type I cells in which Bcl-2 is unable to inhibit the Fas death signal (28).
How do Fas and uncouplers of oxidative phosphorylation cooperate? Since the enhanced caspase activity was seen only in intact cells but not when extracts were mixed, we think it unlikely that an apoptosis-enhancing molecule was liberated from the mitochondria by CCCP and DNP. Uncoupling of oxidative phosphorylation will, in addition to the drop in ΔΨm, also have other effects. As the cell attempts to compensate, the uncoupling will lead to an increase in respiration rate and energy metabolism and to an enhanced availability of free electrons (which are generated at the same rate as protons and which normally react with these molecules). Since mere blockade of the respiratory chain with rotenone and antimycin A did not convey the enhancing effect reported here, the hypothesis that one of these changes presensitizes the cell to a Fas signal is attractive. Free electrons may permit increased generation of free oxygen radicals. It is therefore conceivable that such oxygen intermediates participate in the process of Fas-induced apoptosis. It should be kept in mind, however, that CCCP and DNP on their own have almost no effect on apoptosis over a wide range of concentrations. For example, while DNP at 1 mM did not cause significant apoptosis it was still capable of strongly enhancing a Fas signal at 125 μM.
When ATP levels in treated cells were measured, it was found that these levels did not change within the first 2 h (ruling out the possibility that a lack of energy was responsible for the enhancement). After 6 h there was still no change when cells were treated with either anti-Fas or with CCCP or DNP alone, but there was a significant drop when the anti-Fas–CCCP or anti-Fas–DNP combinations were used. It is possible that this decrease was the immediate result of cell death (for example, loss via shedding of apoptotic bodies, leakage, or increased ATP consumption by components of the cell death pathway). However, the drop might at least in part be a consequence of the increased respiration rate caused by uncoupling oxidative phosphorylation. It also indicates that Fas signaling significantly increases ATP consumption. Since Fas has, in addition to apoptosis, a variety of options of pathways to trigger, such as the activation of JNK (33, 38) or NF-κB (23), the quality of a Fas signal might therefore, at least in some cells, depend on the respiration rate and possibly on determinants such as the differentiation state of the cell.
In less-complex organisms (e.g., C. elegans and Drosophila spp.) there is still no indication that mitochondria play a role in triggering apoptosis. In mammals, the cell death system has been put to several other uses, such as defense and homeostasis. Such a diversification is likely to require molecular adaptations of the ancient system, and the use of mitochondrial triggers or intermediates of apoptosis may be one of these adaptations.
ACKNOWLEDGMENTS
We thank Juliane Vier for technical assistance.
This work was supported by grant Ha 2128/2 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Adachi S, Gottlieb R A, Babior B M. Lack of release of cytochrome C from mitochondria into cytosol early in the course of Fas-mediated apoptosis of Jurkat cells. J Biol Chem. 1998;273:19892–19894. doi: 10.1074/jbc.273.31.19892. [DOI] [PubMed] [Google Scholar]
- 2.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 3.Altenberg G A, Vanoye C G, Han E S, Deitmer J W, Reuss L. Relationships between rhodamine 123 transport, cell volume, and ion-channel function of P-glycoprotein. J Biol Chem. 1994;269:7145–7149. [PubMed] [Google Scholar]
- 4.Bossy-Wetzel E, Newmeyer D D, Green D R. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cecconi F, Alvarez-Bolado A, Meyer B I, Roth K A, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- 6.Chang H Y, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- 7.Chauhan D, Pandey P, Ogata A, Teoh G, Krett N, Halgren R, Rosen S, Kufe D, Kharbanda S, Anderson K. Cytochrome c-dependent and -independent induction of apoptosis in multiple myeloma cells. J Biol Chem. 1997;272:29995–29997. doi: 10.1074/jbc.272.48.29995. [DOI] [PubMed] [Google Scholar]
- 8.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 9.Ferrari D, Stepczynska A, Los M, Wesselborg S, Schulze-Osthoff K. Differential regulation and ATP requirement for caspase-8 and caspase-3 activation during CD95- and anticancer drug-induced apoptosis. J Exp Med. 1998;188:979–984. doi: 10.1084/jem.188.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman S A, Lowe S W, Penninger J M, Mak T W. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 10a.Huang, D. C. S., and A. Strasser. Submitted for publication.
- 11.Kroemer G, Zamzami N, Susin S A. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 12.Kuida K, Haydar T F, Kuan C Y, Gu Y, Taya C, Karasuyama H, Su M S, Rakic P, Flavell R A. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 13.Kuwana T, Smith J J, Muzio M, Dixit V, Newmeyer D D, Kornbluth S. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem. 1998;273:16589–16594. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]
- 14.Lazebnik Y A, Cole S, Cooke C A, Nelson W G, Earnshaw W C. Nuclear events of apoptosis in vitro in cell-free mitotic extracts: a model system for analysis of the active phase of apoptosis. J Cell Biol. 1993;123:7–22. doi: 10.1083/jcb.123.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leist M, Single B, Castoldi A F, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Zhu H, Xu C J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 20.Metivier D, Dallaporta B, Zamzami N, Larochette N, Susin S A, Marzo I, Kroemer G. Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1-triggered apoptosis of Jurkat T lymphoma cells. Comparison of seven mitochondrion-specific fluorochromes. Immunol Lett. 1998;61:157–163. doi: 10.1016/s0165-2478(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 21.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 22.Pan G, Humke E W, Dixit V M. Activation of caspases triggered by cytochrome c in vitro. FEBS Lett. 1998;426:151–154. doi: 10.1016/s0014-5793(98)00330-5. [DOI] [PubMed] [Google Scholar]
- 23.Ponton A, Clement M V, Stamenkovic I. The CD95 (APO-1/Fas) receptor activates NF-kappaB independently of its cytotoxic function. J Biol Chem. 1996;271:8991–8995. doi: 10.1074/jbc.271.15.8991. [DOI] [PubMed] [Google Scholar]
- 24.Reed J C. Cytochrome c: can’t live with it—can’t live without it. Cell. 1997;91:559–562. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- 25.Reed J C. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 26.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 27.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiO6(3) or rhodamine 123, is a reliable fluorescent probe to assess DY changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- 28.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefanelli C, Bonavita F, Stanic I, Farruggia G, Falcieri E, Robuffo I, Pignatti C, Muscari C, Rossoni C, Guarnieri C, Caldarera C M. ATP depletion inhibits glucocorticoid-induced thymocyte apoptosis. Biochem J. 1997;322:909–917. doi: 10.1042/bj3220909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strasser A, Harris A H, Huang D C S, Krammer P H, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1996;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Susin S A, Zamzami N, Castedo M, Daugas E, Wang H G, Geley S, Fassy F, Reed J C, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang D G, Li L, Zhu Z, Joshi B. Apoptosis in the absence of cytochrome c accumulation in the cytosol. Biochem Biophys Res Commun. 1998;242:380–384. doi: 10.1006/bbrc.1997.7969. [DOI] [PubMed] [Google Scholar]
- 33.Toyoshima F, Moriguchi T, Nishida E. Fas induces cytoplasmic apoptotic responses and activation of the MKK7-JNK/SAPK and MKK6-p38 pathways independent of CPP32-like proteases. J Cell Biol. 1997;139:1005–1015. doi: 10.1083/jcb.139.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 35.Vaux D L, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilhelm S, Roloff S, Hacker G. Inhibition of etoposide-induced apoptotic events by azide. Immunol Lett. 1997;59:53–59. doi: 10.1016/s0165-2478(97)00099-0. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 38.Yang X, Khosravi-Far R, Chang H Y, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida H, Kong Y-Y, Yoshida R, Elia J E, Hakem A, Hakem R, Penninger J M, Mak T W. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 40.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J L, Petit P X, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Q, Snipas S, Orth K, Muzio M, Dixit V M, Salvesen G S. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 43.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]