Abstract
Background
We aimed to identify the affecting features of persistent acute kidney injury (pAKI) for patients in intensive care units (ICU).
Methods
The Medical Information Mart for Intensive Care IV (MIMIC-IV) database and eICU Collaborative Research Database (eICU-CRD) were used to identify AKI patients with and without duration of more than 48 hours. Least absolute shrinkage and selection operator (LASSO) regression and support vector machine (SVM-RFE) were utilized to screen for the significant clinical indexes associated with pAKI. Predictive nomogram was created based on the above informative parameters to predict the probability of pAKI.
Results
LASSO regression and SVM-RFE revealed that serum albumin, chronic kidney disease, AKI stage, sequential organ failure assessment score, lactate and renal replacement therapy during the first day were significantly associated with pAKI in the training cohort. The predictive nomogram based on the six predictors exhibited good predictive performance as calculated by C-index 0.730 (95% CI 0.710–0.749) in the training group, 0.702 (95% CI 0.672–0.722) in the internal validation set and 0.704 (0.677–0.731) in the external validation cohort for the prediction of pAKI. Moreover, the predictive nomogram exhibited not only encouraging calibration ability, but also great clinical utility in the training group, in the internal validation group as well as in the external validation cohort.
Conclusion
Serum albumin, CKD, AKI stage, SOFA score, lactate, RRT during the first day were closely associated with pAKI in patients in ICU. The predictive nomogram for pAKI manifested good predictive ability for the identification of ICU patients with pAKI.
Keywords: persistent, acute kidney injury, intensive care units, prognosis, nomogram
Introduction
Acute kidney injury (AKI), as one of the most frequent complications in patients in intensive care units (ICU), is still a global problem with high morbidity, mortality and increased risk of chronic kidney disease (CKD), CKD progression and end-stage kidney disease (ESKD).1–5 Despite a great amount of literature dedicated to its clinical features and subsequent consequences, AKI remains a frustrating disease without any effective treatments and increased length of stays and healthcare costs.6–8 Moreover, recent studies have demonstrated that timely renal recovery is associated with better short-term risk of mortality and long-term risk of ESKD.9,10 In contrast, persistence of AKI is of great importance in that it aggrandizes patients’ risk of CKD, and specific recommendations for the management of AKI patients have been proposed so as to avoidfurther kidney damage and associated mortality.11,12 Thus, identifying patients at high risk of AKI or in the early phase of AKI may result in earlier intervention, shorter AKI duration and better prognosis.
Several biomarkers have been shown to be associated with the duration of AKI. A recent study using the data from RUBY, a multi-center, international, prospective observational study, demonstrated that urinary C-C motif chemokine ligand 14 was the most predictive biomarker for persistent AKI (pAKI) in critically ill patients with severe AKI.13 What’s more, Jeremiah et al. constructed and externally validated a tool for predicting AKI duration and subsequent short- and long-term survival in patients after cardiac surgery. However, this tool was so complicated that it might be difficult for clinicians to use in clinical practice and the predictive accuracy was also relatively low (C-index = 0.66).14 Moreover, several nomograms had been established in previous studies in patients with sepsis or in patients in ICU,15,16 nevertheless, limited data are available for predicting pAKI for critical care unit patients until now. Hence, in the current study, we tested novel common variables to develop and validate a useful nomogram for predicting pAKI in two large critical care databases.
Methods
Data Source
The data were collected from two large US-based critical care databases named Medical Information Mart for Intensive Care IV (MIMIC-IV version 1.0) (https://mimic.mit.edu/iv/) and the eICU Collaborative Research Database (eICU-CRD version 2.0)17 in accordance with the ethical standards of the Institutional Review Board (IRB) of the Massachusetts Institute of Technology (MIT). eICU-CRD covers 200,859 ICU admissions in 2014 and 2015 of 139,367 patients at 208 US hospitals. MIMIC-IV contains information of more than 70,000 patients admitted to the ICUs of Beth Israel Deaconess Medical Center in Boston, MA, from 2008 to 2019. Given that all patients in this database were de-identified, informed consent was waived and data were extracted by structured query language with PostgreSQL 9.6.
Selection of Participants
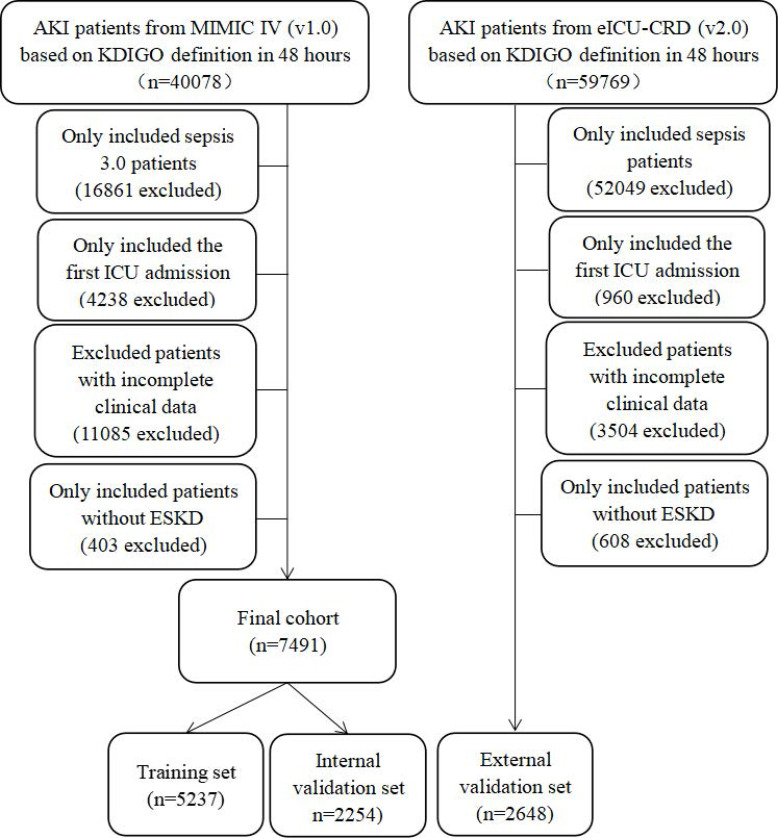
The inclusion criteria in this study were as follows: (1) sepsis 3.0 criteria; (2) KDIGO-AKI criteria based on serum creatinine in the first 48 hours of their ICU admission.18 We further excluded patients with repeat ICU stays, under the age of 18 years old, with incomplete clinical data (variables with >20% missing values), and had a history of ESKD. Patients without serum creatinine measures between 48 to 72 hours after the diagnosis of AKI were also excluded from this study. A total of 7491 patients in the MIMIC-IV database and 2648 patients in the eICU database were finally included in this study. Then, these participants in MIMIC-IV database were randomly assigned into the training cohort (N = 5237) or internal validation cohort (N = 2254) based on the ratio of 7:3 while the patients in the eICU database were assigned to external validation (N = 2648).
Variable Extraction
Baseline characteristics and admission information: age, gender, weight, and severity score measured by the sequential organ failure assessment (SOFA) score, the systemic inflammatory response syndrome (SIRS) score, the simplified acute physiology score II (SAPSII) were calculated as described in previous studies.19–22 Comorbidities including hypertension, diabetes, chronic kidney disease (CKD), coronary artery disease (CAD), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), liver disease and malignant cancer were also collected for analysis based on the recorded ICD codes in the two databases. Use of mechanical ventilation (MV) and renal replacement therapy (RRT) at the first day of their ICU admission were also recorded in this study. Moreover, initial vital signs and laboratory results were also measured during the first 24 hours of ICU admission.
Definitions
Baseline creatinine was the minimum values on the first day of their hospital admissions. Recovery of AKI was defined as greater than or equal to 50% decrease in serum creatinine after the diagnosis of AKI and/or return of serum creatinine to the baseline value. Persistent AKI was defined as renal dysfunction without recovery within 2 days or before death.11
The primary outcome in this study was the occurrence of pAKI.
Construction of the Predictive Nomogram
The recurrent nomogram was built using a three-step approach. First, we employed LASSO regression to identify the potentially advantageous differential indexes which were closely associated with pAKI in the training cohort. Then, we also adopted recursive feature elimination for a support vector machines (SVM-RFE) regression model to rank the informative features on the basis of their permutation importance in the training cohort. In order to avoid the bias caused by a single regression model, we only selected the overlapping features of the two models to construct the predictive nomogram which could provide the clinicians with an intuitive and quantitative prediction tool to identify the patients with high risk of pAKI. Finally, we validated the predictive efficiency and clinical ability of the nomogram in the internal and external validation cohort.
Statistical Analysis
X-tile version 3.6.1 and R software (version 4.1.0, http://www.r-project.org) were used for all analyses. Continuous variables were expressed as mean (standard deviation), categorical covariates were reported as number and percentage. We compared the continuous variables using the independent sample t-test and Chi-square test was used to compare the categorical covariates. X-tile software was utilized to determine the optimal cut-off value of all selected variables. Kaplan-Meier curves and Log rank tests were exploited to compare the differences in survival rate between the pAKI and tAKI groups in the training, internal validation and external validation cohort. P<0.05 was considered statistically significant.
Results
Patients’ Characteristics
A total of 10,139 patients were finally analyzed in this study (5237 patients in the training cohort, 2254 cases in the internal validation cohort and 2648 participants in the external validation) (Figure 1). Among them, 1891 (36.1%) patients in the training set, 812 (36.0%) cases in the internal validation cohort and 755 (26.6%) patients in the external validation developed pAKI during their ICU admission. As described in Table 1, compared with patients in the transient AKI (tAKI, defined as AKI of less than 48-hour duration) group, patients in the pAKI group were older, with a higher proportion of advanced AKI stage, hypertension, coronary artery disease, chronic kidney disease, mechanical ventilation and renal replacement therapy on first day of their ICU admission, higher level of GCS, SOFA score, red cell distribution width, aspartate aminotransferase, alanine aminotransferase, total bilirubin, anion gap, blood urea nitrogen, lactate, potassium, international normalized ratio, activated partial thromboplastin time, and lower level of hemoglobin, red blood cell, albumin.
Figure 1.
The flow chart of this study.
Table 1.
Clinicopathological Characteristics of All Patients
| Characteristics | Training Set (n=5237) | Internal Validation Set (n=2254) | External Validation Set (n=2648) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Persistent AKI | Transient AKI | P value | Persistent AKI | Transient AKI | P value | Persistent AKI | Transient AKI | P value | |
| N | 1891 | 3346 | 812 | 1442 | 705 | 1943 | |||
| Age, years | 64.8±16.1 | 66.4±15.3 | 0.001 | 69.1±15.4 | 64.2±15.9 | 0.004 | 62.3±15.0 | 65.6±15.1 | <0.001 |
| Gender, male, n(%) | 1166(61.7) | 2024(60.5) | 0.404 | 466(57.4) | 852(59.1) | 0.433 | 393(55.7) | 1037(53.4) | 0.279 |
| Weight, kg | 86.0±26.1 | 86.0±24.6 | 0.953 | 85.2±23.0 | 84.6±23.9 | 0.502 | 87.0±29.8 | 87.6±29.3 | 0.633 |
| Ethnicity, n(%) | 0.404 | 0.442 | 0.377 | ||||||
| White | 1171(61.9) | 2130(63.7) | 524(64.6) | 955(66.2) | 556(78.9) | 1553(79.9) | |||
| Black | 260(13.7) | 453(13.5) | 105(12.9) | 196(13.6) | 94(13.3) | 223(11.5) | |||
| Other | 460(24.3) | 763(22.8) | 182(22.4) | 291(20.2) | 55(7.8) | 167(8.6) | |||
| AKI stage | <0.001 | 0.004 | <0.001 | ||||||
| Stage I | 1363(72.1) | 2670(79.8) | 599(73.8) | 1150(79.8) | 472(67.0) | 1627(83.7) | |||
| Stage II | 257(13.6) | 395(11.8) | 115(14.2) | 153(10.6) | 82(11.6) | 103(5.3) | |||
| Stage III | 271(14.3) | 281(8.4) | 98(12.1) | 139(9.6) | 151(21.4) | 213(11.0) | |||
| Comorbidities, n(%) | |||||||||
| Hypertension | 814 (43.0) | 1615 (48.3) | <0.001 | 329 (59.5) | 659 (45.7) | <0.001 | 387 (54.9) | 1115 (57.4) | 0.039 |
| Diabetes | 677 (35.8) | 1232 (36.8) | 0.137 | 316 (38.9) | 479 (33.2) | <0.001 | 277 (39.3) | 741 (38.1) | 0.293 |
| CAD | 434 (23.0) | 621 (18.6) | <0.001 | 163 (20.1) | 282 (19.6) | 0.554 | 58 (8.2) | 191 (9.1) | 0.012 |
| Cerebrovascular disease | 290 (15.3) | 521 (15.6) | 0.651 | 117 (14.4) | 197 (13.7) | 0.327 | 75 (10.6) | 224 (11.5) | 0.198 |
| COPD | 128 (6.8) | 216 (6.5) | 0.380 | 57 (7.0) | 111 (7.7) | 0.238 | 101 (14.3) | 344 (17.7) | <0.001 |
| CKD | 689 (36.4) | 982 (29.3) | <0.001 | 316 (38.9) | 427 (29.6) | <0.001 | 224 (31.8) | 427 (22.0) | <0.001 |
| Cancer | 283 (15.0) | 533 (15.9) | 0.064 | 118 (14.5) | 211 (14.6) | 0.897 | 84 (11.9) | 280 (14.4) | 0.001 |
| Severity of illness, points | |||||||||
| GCS score | 14.1±2.3 | 14.3±1.8 | 0.001 | 14.0±2.1 | 14.6±1.9 | 0.034 | 12.6±3.5 | 13.1±3.0 | 0.002 |
| SOFA score | 4.5±2.5 | 4.0±2.2 | <0.001 | 5.3±2.5 | 4.2±2.5 | 0.025 | 8.1±7.2 | 6.9±6.3 | <0.001 |
| OASIS score | 38.3±9.6 | 38.3±9.5 | 0.736 | 37.9±9.7 | 38.3±9.6 | 0.348 | 30.3±11.8 | 29.5±10.7 | 0.144 |
| APSIII score | 69.7±28.0 | 68.3±27.2 | 0.088 | 66.5±26.0 | 68.8±28.5 | 0.053 | 71.2±27.7 | 63.4±26.1 | <0.001 |
| Vital signs | |||||||||
| Heart rate, bpm | 107.8±22.9 | 107.8±21.8 | 0.960 | 108.4±22.8 | 106.6±22.0 | 0.061 | 109.1±28.4 | 107.1±27.2 | 0.094 |
| SBP, mmHg | 148.2±24.2 | 148.6±24.4 | 0.574 | 150.3±25.8 | 147.8±24.8 | 0.026 | 129.0±34.4 | 127.5±33.2 | 0.300 |
| DBP, mmHg | 86.4±20.8 | 86.5±20.9 | 0.862 | 88.6±22.9 | 86.4±20.2 | 0.018 | 67.1±22.2 | 67.0±21.1 | 0.952 |
| MAP, mmHg | 107.1±30.8 | 106.7±30.0 | 0.640 | 108.3±30.1 | 107.0±30.6 | 0.330 | 86.1±26.7 | 85.3±26.4 | 0.503 |
| Respiratory rate, bpm | 28.9±6.7 | 29.0±6.9 | 0.696 | 29.1±6.6 | 28.8±6.8 | 0.264 | 26.0±9.7 | 25.8±9.1 | 0.576 |
| Temperature, °C | 36.1±0.9 | 36.2±0.8 | 0.072 | 36.1±1.0 | 36.2±0.8 | 0.101 | 37.6±1.3 | 37.5±1.3 | 0.175 |
| SpO2, % | 99.6±1.4 | 99.6±0.9 | 0.072 | 99.6±1.0 | 99.6±1.0 | 0.787 | 96.7±5.5 | 96.8±3.9 | 0.540 |
| Laboratory results | |||||||||
| WBC, × 109/L | 16.6±5.7 | 16.5±5.5 | 0.800 | 16.6±6.5 | 16.4±4.6 | 0.595 | 18.1±6.1 | 18.3±5.4 | 0.697 |
| Hemoglobin, g/dL | 9.4±2.2 | 9.7±2.2 | <0.001 | 9.2±2.2 | 9.8±2.2 | 0.028 | 9.4±2.2 | 9.8±2.3 | <0.001 |
| Platelets, × 109/L | 212.5±80.6 | 224.7±84.8 | 0.001 | 218.8±89.4 | 220.2±89.2 | 0.798 | 228.6±88.2 | 243.8±91.1 | 0.008 |
| RBC, × 1012/L | 3.1±0.8 | 3.2±0.8 | <0.001 | 3.0±0.8 | 3.7±0.8 | 0.021 | 3.2±0.7 | 3.4±0.7 | <0.001 |
| Hematocrit, % | 34.2±6.5 | 34.5±6.5 | 0.003 | 34.1±6.4 | 34.9±6.6 | 0.036 | 34.2±7.3 | 36.1±7.0 | <0.001 |
| Neutrophils, × 109/L | 12.4±7.0 | 12.3±6.2 | 0.867 | 12.7±7.6 | 12.4±6.9 | 0.533 | 13.6±5.2 | 13.0±6.5 | 0.574 |
| Lymphocytes, × 109/L | 7.1±3.7 | 7.5±3.4 | 0.578 | 5.7±2.5 | 6.8±2.5 | 0.281 | 8.3±2.5 | 9.1±3.1 | 0.091 |
| Monocytes, × 109/L | 2.6±2.0 | 2.3±2.0 | 0.284 | 2.4±2.1 | 2.0±2.2 | 0.665 | 3.4±2.0 | 3.0±2.1 | 0.119 |
| MCH, pg | 29.8±2.7 | 29.7±2.6 | 0.202 | 29.7±2.8 | 30.0±2.7 | 0.170 | 29.6±2.5 | 29.4±2.8 | 0.261 |
| MCHC, g/L | 32.3±1.7 | 32.4±1.6 | 0.224 | 32.2±1.7 | 32.4±1.7 | 0.011 | 32.7±1.5 | 32.6±1.5 | 0.484 |
| RDW, % | 15.4±2.4 | 15.1±2.3 | 0.001 | 15.4±2.4 | 15.1±2.2 | 0.010 | 17.3±4.0 | 16.5±2.7 | <0.001 |
| AST, U/L | 548.9±78.0 | 281.4±50.7 | <0.001 | 450.5±57.3 | 335.3±71.6 | 0.100 | 104.2±45.9 | 93.1±38.8 | 0.071 |
| ALT, U/L | 311.2±62.1 | 184.4±56.4 | <0.001 | 235.0±82.1 | 198.1±79.0 | 0.294 | 74.4±31.3 | 74.1±27.5 | 0.967 |
| Albumin, g/dL | 3.1±0.5 | 3.2±0.5 | <0.001 | 3.1±0.5 | 3.2±0.5 | <0.001 | 2.7±0.7 | 3.0±0.7 | <0.001 |
| Total bilirubin, mmol/L | 3.5±1.3 | 2.5±1.5 | <0.001 | 3.0±1.0 | 2.5±1.2 | 0.016 | 2.1±1.8 | 1.6±1.3 | <0.001 |
| Anion gap, mEq/L | 18.6±6.1 | 18.0±5.3 | <0.001 | 18.5±6.0 | 17.7±5.3 | 0.001 | 14.8±5.3 | 14.9±5.3 | 0.626 |
| Bicarbonate, mEq/L | 23.5±4.6 | 23.8±4.6 | 0.011 | 23.6±4.7 | 24.0±4.4 | 0.024 | 24.7±5.1 | 25.0±5.2 | 0.138 |
| BUN, mg/dL | 39.8±8.2 | 36.1±6.4 | <0.001 | 40.0±8.0 | 36.9±7.6 | 0.010 | 48.0±7.2 | 43.5±6.0 | 0.001 |
| Baseline creatinine, mg/dL | 1.6±1.6 | 1.6±1.8 | 0.088 | 1.7±1.5 | 1.6±1.7 | 0.404 | 1.7±1.5 | 1.5±1.3 | <0.001 |
| Glucose, mg/dL | 187.3±62.1 | 189.2±61.5 | 0.559 | 191.7±66.1 | 184.2±63.5 | 0.147 | 208.2±58.9 | 208.5±55.9 | 0.958 |
| ALP, U/L | 110.1±45.1 | 100.1±45.7 | 0.002 | 110.0±55.2 | 102.3±45.6 | 0.105 | 143.8±58.4 | 124.1±45.1 | 0.001 |
| Lactate, mmol/L | 3.9±1.0 | 3.3±1.5 | <0.001 | 4.0±1.1 | 3.2±1.3 | <0.001 | 3.7±1.8 | 3.4±1.5 | 0.005 |
| Sodium, mmol/L | 139.8±5.5 | 139.8±5.4 | 0.956 | 140.0±5.4 | 139.7±5.6 | 0.440 | 139.7±5.6 | 140.1±6.3 | 0.095 |
| Potassium, mmol/L | 4.9±1.0 | 4.8±0.9 | 0.009 | 4.8±0.9 | 4.8±0.9 | 0.158 | 4.7±0.9 | 4.7±0.9 | 0.995 |
| Calcium, mg/dL | 8.6±1.1 | 8.6±0.9 | 0.295 | 8.6±1.0 | 8.6±1.0 | 0.226 | 8.2±0.9 | 8.2±0.8 | 0.396 |
| Chloride, mmol/L | 105.9±7.3 | 106.0±7.0 | 0.485 | 105.6±6.9 | 105.6±7.2 | 0.996 | 106.2±7.3 | 106.9±7.7 | 0.029 |
| INR | 1.9±1.6 | 1.7±1.2 | <0.001 | 1.8±1.3 | 1.7±1.1 | 0.077 | 1.8±1.1 | 1.9±1.4 | 0.367 |
| Prothrombin time, s | 20.7±5.3 | 18.6±4.7 | <0.001 | 19.6±5.2 | 18.7±6.0 | 0.099 | 20.0±7.4 | 20.4±8.3 | 0.427 |
| APTT, s | 51.6±15.9 | 49.0±16.7 | 0.007 | 52.2±14.4 | 48.3±13.0 | 0.009 | 42.4±16.3 | 41.3±15.5 | 0.116 |
| PH | 7.4±0.1 | 7.4±0.1 | 0.461 | 7.4±0.1 | 7.4±0.1 | 0.222 | 7.3±0.1 | 7.3±0.1 | 0.982 |
| PO2, mmHg | 242.1±71.0 | 236.6±68.6 | 0.054 | 238.5±67.3 | 236.5±70.0 | 0.723 | 108.2±63.9 | 106.9±66.1 | 0.685 |
| PCO2, mmHg | 47.9±13.4 | 47.6±13.0 | 0.383 | 49.1±13.3 | 47.8±12.0 | 0.021 | 40.2±11.9 | 40.2±13.3 | 0.990 |
| PaO2/FiO2 ratio | 214.7±103.7 | 218.9±95.0 | 0.147 | 208.9±98.0 | 222.8±99.8 | 0.001 | 205.7±24.4 | 204.8±13.7 | 0.214 |
| Base excess, mmol/L | −0.1±1.5 | 0.1±1.3 | 0.060 | 0.1±1.7 | 0.2±1.1 | 0.519 | 1.0±1.2 | 1.4±1.8 | 0.055 |
| Interventions first day | |||||||||
| RRT first day, n(%) | 269 (14.2) | 291 (8.7) | <0.001 | 126 (15.5) | 129 (8.9) | <0.001 | 176 (25.0) | 125 (6.4) | <0.001 |
| MV first day, n(%) | 1271 (67.2) | 2156 (64.4) | 0.042 | 538 (66.3) | 900 (62.4) | <0.001 | 334 (47.4) | 715 (36.8) | <0.001 |
| Input first day, mL | 10950(5470, 18,910) | 10,427(5100, 17,419) | 0.003 | 11,045(4646, 19,247) | 9982(4698, 16,844) | 0.001 | 2154(0, 9043) | 1906(0, 9917) | |
| Output first day, mL | 1070(450, 1935) | 1298(731, 2095) | <0.001 | 1078(470, 1899) | 1356(737, 2221) | <0.001 | 1200(106, 3687) | 1550(200, 4350) | |
| Length of hospital, days | 14.9(8.7, 23.7) | 13.1(8.2, 22.2) | 0.026 | 14.7(8.5, 23.3) | 12.8(7.7, 21.1) | 0.033 | 18.5(12.5, 27.4) | 14.1(9.0, 21.4) | <0.001 |
| Hospital mortality, n (%) | 463 (24.5) | 440 (12.8) | <0.001 | 181 (22.3) | 202 (14.0) | <0.001 | 178 (25.2) | 272 (14.0) | <0.001 |
| Length of ICU, days | 6.2(3.0, 12.1) | 5.3(2.7, 10.8) | <0.001 | 5.9 (2.9, 12.0) | 5.3 (2.9, 10.1) | 0.045 | 9.8 (5.3, 14.8) | 5.3 (2.8, 10.4) | <0.001 |
| ICU mortality, n (%) | 329 (17.4) | 261 (7.8) | <0.001 | 128 (15.8) | 117 (8.1) | <0.001 | 131 (18.6) | 143 (15.2) | <0.001 |
Notes: For all continuous covariates except for input and output first day, length of hospital and length of ICU, the mean values and standard deviations are reported.
Abbreviations: AKI, acute kidney injury; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; GCS, Glasgow; SOFA, sequential organ failure assessment; OASIS, Oxford acute severity of illness score; APSIII, acute physiology score III; SBP, systolic pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; WBC, white blood cell; RBC, red blood cell; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; ALP, alkaline phosphatase; INR, international normalized ratio; APTT, activated partial thromboplastin time; PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; MV, mechanical ventilation; RRT, renal replacement therapy; ICU, intensive care unit.
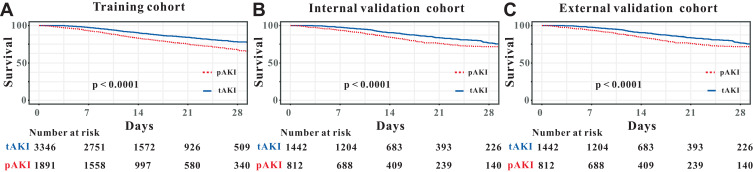
Moreover, compared with patients in the tAKI group, patients in the pAKI group had relatively worse survival rate in the training cohort, in the internal validation cohort as well as in the external validation cohort (Figure 2A–C).
Figure 2.
Survival analyses comparing between persistent and transient acute kidney injury patients in the training cohort (A), internal validation cohort (B) and external validation cohort (C).
Identification of Significant Features
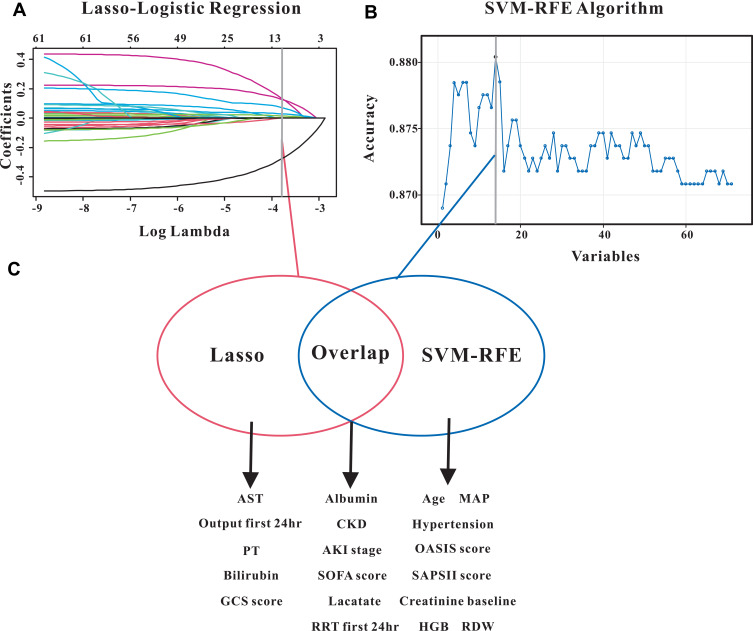
LASSO regression was performed to identify factors that were significantly associated with pAKI in the training group. As graphically demonstrated in Figure 3A, serum albumin, CKD, AKI stage, SOFA score, lactate, renal replacement therapy (RRT) during the first day, aspartate aminotransferase, output during first day, prothrombin time, total bilirubin and Glasgow score were risk factors for predicting pAKI. For the purpose of constructing an easy-to-use predictive model with relatively high accuracy, we also applied the SVM-RFE model to screen for the significant indexes associated with early recurrence of CRC. Results from SVM-RFE algorithm showed that 14 clinical parameters were screened out by this regression model, including age, mean arterial pressure, hypertension, OASIS score, SAPSII score, baseline serum creatinine, hemoglobin, red cell distribution width, serum albumin, CKD, AKI stage, SOFA score, lactate and RRT during the first day (Figure 3B).
Figure 3.
Selection of significant indexes associated with persistent acute kidney injury patients. (A) LASSO Cox regression model. (B) Support vector machine model. (C) The overlapping features identified by the two models.
Construction and Validation of the Predictive Nomogram
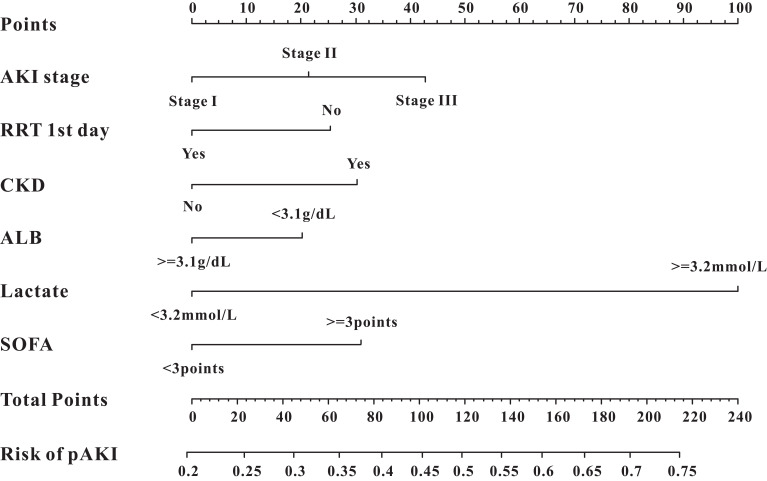
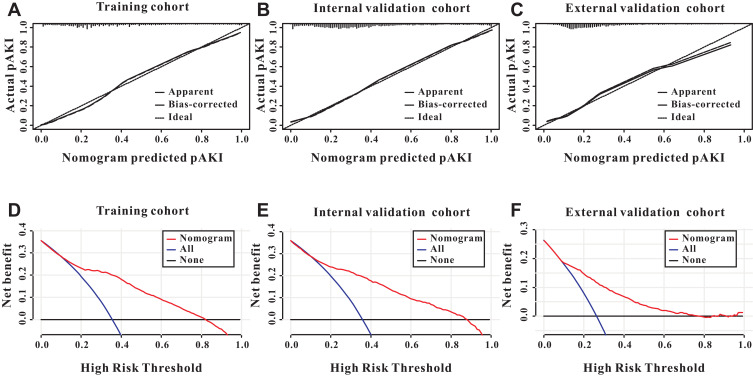
We only included the overlapping features selected by the LASSO regression model and SVM-RFE algorithm into the constitution of the predictive nomogram (Figure 3C). Based on the results of LASSO and SVM-RFE, six features were finally included in the predictive nomogram for pAKI (serum albumin, CKD, AKI stage, SOFA score, lactate, RRT during the first day) (Figure 4). The predictive performance of the predictive nomogram as measured by C-index was 0.730 (95% CI 0.710–0.749) in the training group, 0.702 (95% CI 0.672–0.722) in the internal validation group and 0.704 (0.677–0.731) in the external validation group for the prediction of pAKI, indicating that the nomogram had a relatively good model discriminative capacity. The calibration curve for the predictive nomogram exhibited a high agreement between the actual probability and predicted probability of pAKI in the training set, internal validation set, and in the external validation set (Figure 5A–C).
Figure 4.
The predictive nomogram for persistent acute kidney injury.
Figure 5.
Calibration and clinical utility of the predictive nomogram. The predictive nomogram exhibited a high correlation between the actual probability and predicted probability in the training cohort (A), internal validation cohort (B) and external validation cohort (C). Decision curves analysis for the predictive nomogram to predict the persistent acute kidney injury in the training cohort (D), internal validation cohort (E) and external validation cohort (F).
Finally, we utilized decision curve analysis (DCA) to determine the clinical utilities of the predictive nomogram. The DCA curve also demonstrated that the survival nomogram derived from the training set was clinically useful in the training set, internal validation set as well as in the external validation set (Figure 5D–F).
Discussion
In the current study, we utilized LASSO and SVM-RFE models to select the overlapped affecting features of pAKI to firstly build a predicting nomogram based on serum albumin, CKD, AKI stage, SOFA score, lactate, RRT during the first day. This nomogram possessed good predictive ability for the identification of ICU patients with pAKI. To further validate the feasibility of the predictive value of the nomogram, we independently verified this conclusion in patients in another public database. Therefore, these data suggest that the nomogram may be a good tool for identifying patients at high risk of pAKI among ICU patients.
Although numerous studies have investigated the development and prognosis of AKI patients, renal recovery after AKI was largely neglected and their criteria was still poorly defined or validated until now.11,23 In fact, timing of renal recovery is associated with end-stage renal failure risk,10 long-term prognosis24–26 and has been identified as an important endpoint for clinical trials.27 Joana et al. demonstrated that pAKI was an independent predictor of in-hospital mortality in contrast to tAKI in a retrospective study of 450 patients who underwent major abdominal surgery.28 Similar to this, we also found that pAKI patients had a relatively higher in-hospital mortality compared with tAKI patients in the training cohort, internal validation cohort as well as in the external validation cohort.
A considerable number of clinical studies have investigated the independent predictors of AKI and prognosis in different populations, however, predictors of pAKI were limited. Coca et al. first described urinary injury markers as predictors for AKI duration in a prospective cohort study of 1199 adult patients who underwent cardiac surgery and found that all urinary injury markers including urine neutrophil gelatinase associated lipocalin (uNGAL) were independently associated with AKI duration.29 Using the data of 1322 AKI patients’ registry at King Chulalongkorn Memorial Hospital, Nuttha et al. also demonstrated that uNGAL was associated with pAKI as well as prognosis of AKI patients.30 In addition, several factors have been shown to be associated with pAKI in previous studies. Firstly, comorbidities, especially for patients with pre-existing renal dysfunction, were associated with longer AKI duration.10 Our study added the evidence that patients with pre-existing CKD were associated with higher risk of pAKI. Secondly, the severity of AKI, both assessed by oliguria and increased serum creatinine concentrations, was also a strong predictor for pAKI.31 In the current study, AKI stage defined by creatinine concentrations increases was also an overlapped index for pAKI both in the LASSO regression model and in the SVM-RFE model. Finally, the severity of illness, and need for additional organ support were also associated with higher risk of pAKI.32 Consistent with these results, our study also concluded that SOFA score and need for RRT support at first 24 hours after ICU admission were also associated with AKI duration.
Considering that the clinical usefulness of a single biomarker is more or less limited in clinical practice by its low predictive efficiency, we utilized a nomogram, an easy-to-use predictive model which had been widely applied in the prediction of the prognosis of cancer patients,33,34 to combine different clinical indexes to achieve an excellent predictive performance for predicting pAKI. Fortunately, as we described in the aforementioned, the predicted nomogram possessed excellent predictive value for patients in ICU with pAKI. Moreover, we further independently verified our results in another ICU database, and this nomogram also possessed good predictive ability in patients in ICU. Hence, our predictive nomogram was an efficient tool for clinicians to improve AKI risk stratification.
Several limitations should be considered in this study. First of all, this was a retrospective study based on two large electronic public databases, which may result in limited generalizability. Secondly, the definition of AKI was based on the serum creatinine concentrations, thus patients with AKI by oliguria may not be included in this study. Finally, some other clinical and imaging indexes might be correlated with the pAKI. Unfortunately, they were unavailable in the public database. Hence, prospective clinical trials from multicenters are needed to verify the predictive nomogram in the near future.
Conclusions
Serum albumin, CKD, AKI stage, SOFA score, lactate, RRT during the first day were closely associated with pAKI in patients in ICU. The predictive nomogram for pAKI manifested good predictive ability for the identification of ICU patients with pAKI. This nomogram may be a good tool for identifying patients at high risk of pAKI among ICU patients.
Funding Statement
There is no funding to report.
Statement of Ethics
The study has been approved by the Institutional Review Board (IRB) of the Massachusetts Institute of Technology (MIT). After successfully accomplishing the National Institutes of Health’s (NIH) online training course and the Protection of Human Research Participants Examination (certification number 37474354), we had the access to extract data from MIMIC IV and eICU databases. Given that all patients in this database were de-identified, informed consent was waived.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared that there is no conflict of interest.
References
- 1.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–1964. doi: 10.1016/S0140-6736(19)32563-2 [DOI] [PubMed] [Google Scholar]
- 2.Negi S, Koreeda D, Kobayashi S, et al. Acute kidney injury: epidemiology, outcomes, complications, and therapeutic strategies. Semin Dial. 2018;31(5):519–527. doi: 10.1111/sdi.12705 [DOI] [PubMed] [Google Scholar]
- 3.Hoste E, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–625. doi: 10.1038/s41581-018-0052-0 [DOI] [PubMed] [Google Scholar]
- 4.Mehta RL, Cerda J, Burdmann EA, et al. International society of nephrology’s 0 by 25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987):2616–2643. doi: 10.1016/S0140-6736(15)60126-X [DOI] [PubMed] [Google Scholar]
- 5.Harrois A, Soyer B, Gauss T, Hamada S, Raux M, Duranteau J. Prevalence and risk factors for acute kidney injury among trauma patients: a Multicenter Cohort Study. Crit Care. 2018;22(1):344. doi: 10.1186/s13054-018-2265-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015;261(6):1207–1214. doi: 10.1097/SLA.0000000000000732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collister D, Pannu N, Ye F, et al. Health care costs associated with AKI. Clin J Am Soc Nephrol. 2017;12(11):1733–1743. doi: 10.2215/CJN.00950117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, Liu H, Fu S, Wan J, Li X. Red blood cell distribution width is an independent predictor of AKI and mortality in patients in the coronary care unit. Kidney Blood Press Res. 2017;42(6):1193–1204. doi: 10.1159/000485866 [DOI] [PubMed] [Google Scholar]
- 9.Darmon M, Truche AS, Abdel-Nabey M, Schnell D, Souweine B. Early recognition of persistent acute kidney injury. Semin Nephrol. 2019;39(5):431–441. doi: 10.1016/j.semnephrol.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 10.Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi: 10.1164/rccm.201604-0799OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi: 10.1038/nrneph.2017.2 [DOI] [PubMed] [Google Scholar]
- 12.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66. doi: 10.1056/NEJMra1214243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoste E, Bihorac A, Al-Khafaji A, et al. Identification and validation of biomarkers of persistent acute kidney injury: the RUBY Study. Intensive Care Med. 2020;46(5):943–953. doi: 10.1007/s00134-019-05919-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeremiah BR, Kramer RS, MacKenzie TA, Coca SG, Sint K, Parikh CR. Determinants of acute kidney injury duration after cardiac surgery: an externally validated tool. Ann Thorac Surg. 2012;93(2):570–576. doi: 10.1016/j.athoracsur.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Deng Y, Lao H, et al. A nomogram incorporating functional and tubular damage biomarkers to predict the risk of acute kidney injury for septic patients. Bmc Nephrol. 2021;22(1):176. doi: 10.1186/s12882-021-02388-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan J, Zou G, He B, et al. Development and external validation a novel inflammation-based score for acute kidney injury and prognosis in intensive care unit patients. Int J Gen Med. 2021;14:2215–2226. doi: 10.2147/IJGM.S311021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollard TJ, Johnson A, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU collaborative research database, a freely available multi-center database for critical care research. Sci Data. 2018;5(1):180178. doi: 10.1038/sdata.2018.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Gall JR, Loirat P, Alperovitch A, et al. A simplified acute physiology score for ICU patients. Crit Care Med. 1984;12(11):975–977. doi: 10.1097/00003246-198411000-00012 [DOI] [PubMed] [Google Scholar]
- 20.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5 [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the working group on sepsis-related problems of the European society of intensive care. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 22.Rass V, Gaasch M, Kofler M, et al. Systemic inflammatory response syndrome as predictor of poor outcome in nontraumatic subarachnoid hemorrhage patients. Crit Care Med. 2018;46(12):e1152–9. doi: 10.1097/CCM.0000000000003429 [DOI] [PubMed] [Google Scholar]
- 23.Ronco C, Ferrari F, Ricci Z. Recovery after acute kidney injury: a new prognostic dimension of the syndrome. Am J Respir Crit Care Med. 2017;195(6):711–714. doi: 10.1164/rccm.201610-1971ED [DOI] [PubMed] [Google Scholar]
- 24.Bhatraju PK, Zelnick LR, Chinchilli VM, et al. Association between early recovery of kidney function after acute kidney injury and long-term clinical outcomes. JAMA Netw Open. 2020;3(4):e202682. doi: 10.1001/jamanetworkopen.2020.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coelho S, Fonseca JN, Gameiro J, Jorge S, Velosa J, Lopes JA. Transient and persistent acute kidney injury in acute liver failure. J Nephrol. 2019;32(2):289–296. doi: 10.1007/s40620-018-00568-w [DOI] [PubMed] [Google Scholar]
- 26.Korenkevych D, Ozrazgat-Baslanti T, Thottakkara P, et al. The pattern of longitudinal change in serum creatinine and 90-day mortality after major surgery. Ann Surg. 2016;263(6):1219–1227. doi: 10.1097/SLA.0000000000001362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palevsky PM, Molitoris BA, Okusa MD, et al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol. 2012;7(5):844–850. doi: 10.2215/CJN.12791211 [DOI] [PubMed] [Google Scholar]
- 28.Joana G, Duarte I, Marques F, et al. Transient and persistent AKI and outcomes in patients undergoing major abdominal surgery. Nephron. 2020;144(5):236–244. doi: 10.1159/000506397 [DOI] [PubMed] [Google Scholar]
- 29.Coca SG, Nadkarni GN, Garg AX, et al. First post-operative urinary kidney injury biomarkers and association with the duration of AKI in the TRIBE-AKI cohort. PLoS One. 2016;11(8):e161098. doi: 10.1371/journal.pone.0161098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuttha L, Amprai M, Tachaboon S, et al. Urine neutrophil gelatinase-associated lipocalin (NGAL) for prediction of persistent AKI and major adverse kidney events. Sci Rep. 2020;10(1):8718. doi: 10.1038/s41598-020-65764-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perinel S, Vincent F, Lautrette A, et al. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a Multicenter Cohort Study. Crit Care Med. 2015;43(8):e269–75. doi: 10.1097/CCM.0000000000001077 [DOI] [PubMed] [Google Scholar]
- 32.Truche AS, Ragey SP, Souweine B, et al. ICU survival and need of renal replacement therapy with respect to AKI duration in critically ill patients. Ann Intensive Care. 2018;8(1):127. doi: 10.1186/s13613-018-0467-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeong SH, Kim RB, Park SY, et al. Nomogram for predicting gastric cancer recurrence using biomarker gene expression. Eur J Surg Oncol. 2020;46(1):195–201. doi: 10.1016/j.ejso.2019.09.143 [DOI] [PubMed] [Google Scholar]
- 34.Zheng P, Lai C, Yang W, Guo J, Xiao S, Chen Z. Nomogram predicting cancer-specific survival in elderly patients with stages I–III colon cancer. Scand J Gastroenterol. 2020;55(2):202–208. doi: 10.1080/00365521.2020.1720280 [DOI] [PubMed] [Google Scholar]