Abstract
BACKGROUND
Inferior vena cava (IVC) filters are widely used for prevention of pulmonary embolism (PE). However, uncertainty persists about their efficacy and safety.
OBJECTIVES
The authors conducted a systematic review and meta-analysis of the published reports on the efficacy and safety of IVC filters.
METHODS
The authors searched PubMed, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov through October 3, 2016, for randomized controlled trials (RCTs) or prospective controlled observational studies of IVC filters versus none in patients at risk of PE. Inverse variance fixed-effects models with odds ratio (OR) as the effect measure were used for primary analyses. Main outcomes included subsequent PE, PE-related mortality, all-cause mortality, and subsequent deep vein thrombosis (DVT).
RESULTS
The authors' search retrieved 1,986 studies, of which 11 met criteria for inclusion (6 RCTs and 5 prospective observational studies). Quality of evidence for RCTs was low to moderate. Overall, patients receiving IVC filters had lower risk for subsequent PE (OR: 0.50; 95% confidence interval [CI]: 0.33 to 0.75); increased risk for DVT (OR: 1.70; 95% CI: 1.17 to 2.48); nonsignificantly lower PE-related mortality (OR: 0.51; 95% CI: 0.25 to 1.05); and no change in all-cause mortality (OR: 0.91; 95% CI: 0.70 to 1.19). Limiting the results to RCTs showed similar results. Findings were substantively similar across a wide range of sensitivity analyses.
CONCLUSIONS
Very few prospective controlled studies, with limited quality of evidence, exist regarding the efficacy and safety of IVC filters. Overall, filters appear to reduce the risk of subsequent PE, increase the risk for DVT, and have no significant effect on overall mortality.
Keywords: bleed, mortality, prevention, risk, venous thromboembolism
Venous thromboembolic disease (VTE) is the third most common vascular disease after myocardial infarction and stroke (1,2). Annually, approximately 1 million new cases of fatal or nonfatal pulmonary embolism (PE), the most serious presentation of VTE, occur in the United States and Europe combined (3-5). Inferior vena cava (IVC) filters have been available as a preventive option for patients at risk for PE since the 1970s and are widely used as a therapeutic option in patients with VTE or to prevent PE without current VTE. Nearly 1 in 6 Medicare beneficiaries with PE receives an IVC filter, and the global estimated market of IVC filters exceeded $430 million in 2016 (6-10).
Despite the frequent utilization of IVC filters, evidence for their efficacy is limited, resulting in conflicting recommendations by experts and guidelines, and wide variations in utilization (7,10-16). In view of continued uncertainty, regulatory concerns, and publication of a few recent controlled studies (17-25), we conducted a systematic review of trials of IVC filter use versus no use for preventing PE to determine their efficacy and safety as well as to explore the results in major clinical subgroups.
METHODS
DATA COLLECTION AND EXTRACTION.
We searched PubMed and the Cochrane Central Register of Controlled Trials for randomized controlled trials (RCTs) and prospective controlled observational studies of patients at risk of PE who received IVC filters versus those who did not (last search date October 3, 2016), with no time or language limits. We searched prior systematic reviews to ascertain evaluation of all potentially eligible studies, and we searched ClinicalTrials.gov to identify any ongoing RCTs (Online Table 1).
We included RCTs and nonrandomized studies that prospectively enrolled and compared patients who received an IVC filter to those who did not receive IVC filters. We excluded retrospective studies, noncontrolled studies, studies that included historical controls, and studies that did not have an a priori plan for enrolling patients and prospectively capturing the study information regarding the efficacy and safety of IVC filters. The study protocol was drafted by 2 of the authors (B.B. and H.M.K.) and revised by all coauthors. One author (M.M.) provided additional original data from 3 cohorts related to 2 publications (21,24). One author (B.B.) extracted the data, which were independently verified by another author (S.C.). Discrepancies were discussed and resolved by consensus.
OUTCOMES.
The primary outcomes were subsequent PE, PE-related mortality, and all-cause mortality. Secondary outcomes included subsequent deep vein thrombosis (DVT), hospital readmission, and bleeding. IVC filter complications, such as filter thrombosis and migration, were also captured from the original studies.
We used the Cochrane risk of bias table to report risk of bias in each study and subsequently used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system to determine the methodological quality of major study outcomes assessed in the included studies (26).
STATISTICAL ANALYSIS.
For primary analyses, data were pooled using inverse variance fixed-effects models (26). We reported the effect measure for each outcome as the odds ratio (OR) with the related 95% confidence interval (CI). The fixed-effects approach has fewer assumptions and weights the size of studies more accurately; inverse variance controls for confounding in the individual studies by allowing use of the adjusted risk estimates. For the primary analysis, we separately reported the results for RCTs and observational studies, as well as the overall results.
Additional analyses were conducted with pooling the data using Mantel-Haenszel random-effects models with risk difference as the effect measure with related 95% CIs. We calculated number needed to treat and number needed to harm for risk estimates where risk difference was significant. The risk difference analysis allowed the inclusion of studies with zero events. We ran supplemental sensitivity analyses with Mantel-Haenszel random-effects models with OR as the effect measure to ascertain the robustness of results.
Among the included studies, we identified 1 quasi-randomized trial (27). While acknowledging differences between RCTs and quasi-randomized trials, we included that study among RCTs. One identified study, the FILTER-PEVI (Filter Implantation to Lower Thromboembolic Risk in Percutaneous Endovenous Intervention) study, used IVC filter implantation during percutaneous endovenous intervention for DVT (22). Before the overall data were extracted or pooled, the study group made an a priori plan to run a sensitivity analysis by excluding the FILTER-PEVI study because of the fundamentally different cohort of patients studied in that trial.
To avoid duplicate data, where there were updated analyses or corrections for the same cohort of patients in multiple publications, we used the most accurately reported results with the longest follow-up duration, where available. We made a priori plans to investigate the robustness of results across demographic subgroups, for primary versus secondary prevention for PE, for use of permanent versus retrievable filters, for patients with cancer, and for those receiving thrombolytic therapy.
We quantified heterogeneity using I2 (I2 <25% considered as low; I2 >75% considered as high) (28), with I2 representing the percentage of variability in the effect risk estimate owed to heterogeneity rather than due to chance.
All tests were 2-sided. and a p value <0.05 was considered statistically significant (except for heterogeneity, for which a p value <0.10 was used because of the conservative nature of the test). We used RevMan version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) for all analyses.
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (Online Appendix).
RESULTS
Our PubMed search identified 1,986 study reports, of which 30 full texts were relevant for evaluation. After exclusion of retrospective studies and those with historical controls, we identified 11 publications related to 11 studies (1 study with 2 separate cohorts, 2 related to the same study with different follow-ups, and 8 related to 8 other distinct studies) (Table 1). Searches of the Cochrane databases and ClinicalTrials.gov did not identify any additional RCTs.
TABLE 1.
Baseline Characteristics of Included Studies
| First Author (Ref. #) |
Year | Design | Filter Type |
Average Age (yrs) |
Patients With IVC Filters (n) |
Controls (n) |
IVC Filter Placement Setting |
|---|---|---|---|---|---|---|---|
| Fullen et al. (27) | 1973 | Quasi-RCT | Permanent | 68.0 | 41 | 59 | Prophylactically for patients with acute traumatic proximal femur fracture. |
| PREPIC (29,30) | 1998 2005 |
RCT | Permanent | 72.5 | 200 | 200 | Patients with acute proximal DVT who were receiving anticoagulation with heparin or enoxaparin. The study was terminated early because of slow recruitment. The study excluded patients who had indications for thrombolysis (n = 52). |
| Gargiulo et al. (31) | 2006 | Observational | Mix of both | NA | 17 | 18 | A study with multiple subcohorts undergoing open gastric bypass surgery. We included 35 cases and controls with prospective data. All patients received sequential compression devices and prophylactic heparin. |
| FILTER-PEVI (22) | 2012 | RCT | Retrievable | 55.0 | 70 | 71 | Patients with acute proximal DVT undergoing percutaneous endovenous intervention. All received anticoagulation and compression stockings. |
| Rajasekhar et al. (17) | 2011 | RCT | Retrievable | 47.0 | 18 | 16 | Prophylactically in high-risk trauma patients. |
| Barginear et al. (18) | 2012 | RCT | Permanent | 65.0 | 33 | 31 | Patients with cancer, and acute DVT treated with fondaparinux. |
| Birkmeyer et al. (19) | 2013 | Observational | Mix of both | 48.5 | 1,077 | 1,077 | Patients undergoing bariatric surgery in Michigan. |
| Jimenez et al. (20,21) | 2014 | Observational | NA | 69.5 | 336 | 336 | Patients with acute VTE and absolute or relative contraindications to anticoagulation in the first 30 days after VTE diagnosis (controls had similar baseline risk of bleeding and other covariates, but did not receive an IVC filter). |
| PREPIC-II (23) | 2015 | RCT | Retrievable | 73.5 | 200 | 199 | Patients with acute PE and associated coexisting lower-extremity thrombosis, and high risk of recurrence who were receiving anticoagulation. |
| Mellado et al. (24) | 2016 | Observational | NA | 61.0 | 48 | 91 | Patients with VTE and evidence of recurrence in the form of PE during the first 3 months of anticoagulation initial VTE event. Results were separately presented for the cohort with initial DVT and with initial PE. |
| Mellado et al. (24) | 2016 | Observational | NA | 60.5 | 17 | 49 | Patients with VTE and evidence of recurrence in the form of DVT during the first 3 months of anticoagulation initial VTE event. |
DVT = deep vein thrombosis; FILTER-PEVI = Filter Implantation to Lower Thromboembolic Risk in Percutaneous Endovenous Intervention; IVC = inferior vena cava; NA = not available; PE = pulmonary embolism; PREPIC = Prevention du Risque d'Embolie Pulmonaire par Interruption Cave; RCT = randomized controlled trial; VTE = venous thromboembolism.
Four registries continue to recruit patients, including RIETE (Registry of Patients with Venous Thromboembolism), GARFIELD-VTE (Global Anticoagulant Registry in the FIELD-Venous Thromboembolic Events), VTEval (which evaluates 3 prospective cohorts of individuals with suspected and incidental VTE), and PREFER in VTE (Prevention of Thromboembolic Events-European Registry in Venous Thromboembolism) registries, although we did not find any additional published controlled studies to include in the systematic review (Online Figure 1). The final list included 5 RCTs (17,18,22,29,30), 1 quasi-randomized controlled trial (27), and 5 controlled prospective observational studies (19-21,24,31). The studies were published between 1973 and 2016 across a wide array of indications for IVC filter use, such as primary prevention (after orthopedic or bariatric surgical procedures) or secondary prevention of PE. A total of 2,055 patients and 2,149 controls were included.
CLINICAL OUTCOMES.
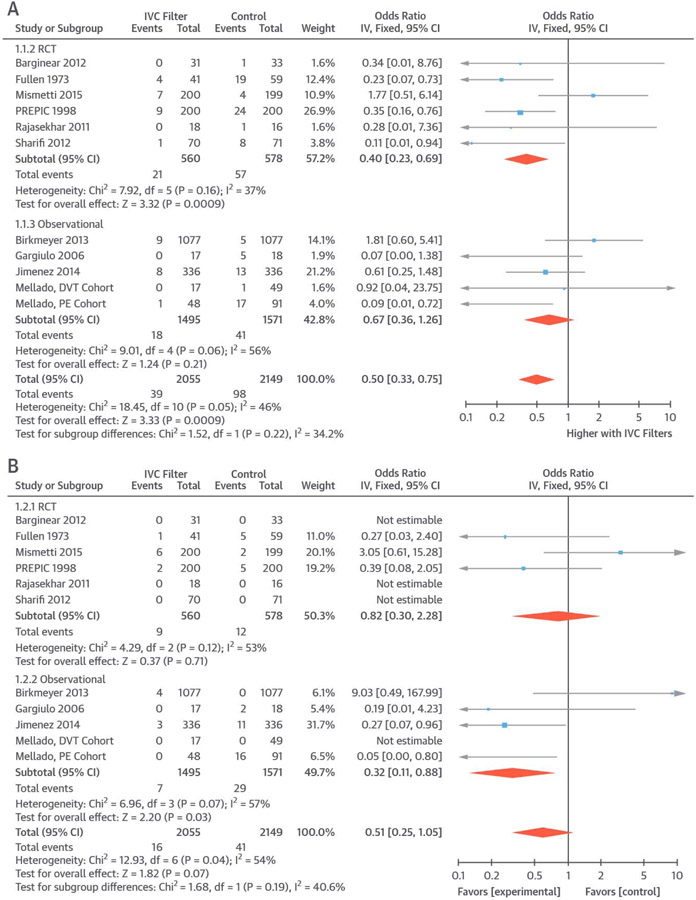
All 11 studies reported subsequent PE as an outcome (39 PEs in 2,055 patients receiving IVC filters and 98 PEs in 2,149 controls). Use of IVC filters was associated with reduced risk of subsequent PE (OR: 0.50; 95% CI: 0.33 to 0.75; I2 = 48%) (Figure 1A). Limiting the analyses to RCTs, the results were similar (OR: 0.40; 95% CI: 0.23 to 0.69; I2 = 37%). Repeating the results in all 11 trials using risk difference yielded similar results (absolute risk difference: −0.05; 95% CI: −0.08 to −0.02; number needed to treat = 20).
FIGURE 1. PE and PE-Related Mortality.
Pooled results from included studies indicated a lower rate of subsequent PE (A) and a nonsignificantly lower rate of PE-related mortality (B) in patients receiving IVC filters. CI = confidence interval; df = degree of freedom; DVT = deep vein thrombosis; IV = inverse variance; IVC = inferior vena cava; PE = pulmonary embolism; PREPIC = Prevention du Risque d'Embolie Pulmonaire par Interruption Cave; RCT = randomized controlled trial.
Information on PE-related mortality was available across all 11 studies (16 PE-related deaths in 2,055 patients receiving IVC filters and 41 among 2,149 controls). PE-related mortality was nonsignificantly lower in patients who had an IVC filter compared with controls (OR: 0.51; 95% CI: 0.25 to 1.05; I2 = 54%) (Figure 1B). Limiting the results to RCTs, there was no significant difference between the 2 groups (OR: 0.82; 95% CI: 0.30 to 2.28; I2 = 53%). Results were consistent when risk difference was used (risk difference: −0.01; 95% CI: −0.03 to 0.01).
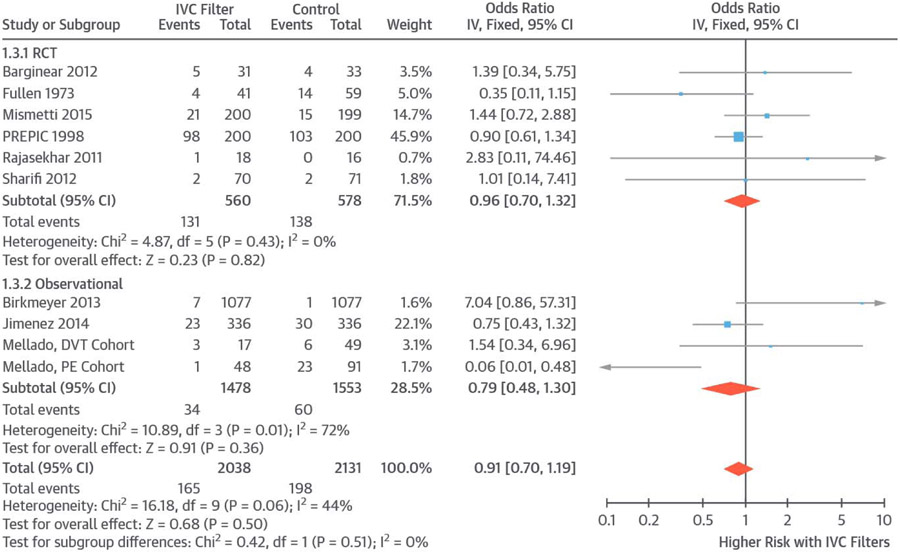
Overall, 10 studies reported information about all-cause mortality (165 deaths in 2,038 patients receiving IVC filters and 198 in 2,131 controls). Use of IVC filters was not associated with a significant change in all-cause mortality (OR: 0.91; 95% CI: O.70 to 1.19; I2 = 44%) (Figure 2). When limiting the results to RCTs, findings were similar (OR: 0.96; 95% CI: 0.70 to 1.32; I2 = 0%) and remained similar when risk difference was used (risk difference: −0.02; 95% CI: −0.06 to 0.02; I2 = 70%).
FIGURE 2. All-Cause Mortality.
Pooled results indicated no significant difference in all-cause mortality between patients who received IVC filters versus controls. Abbreviations as in Figure 1.
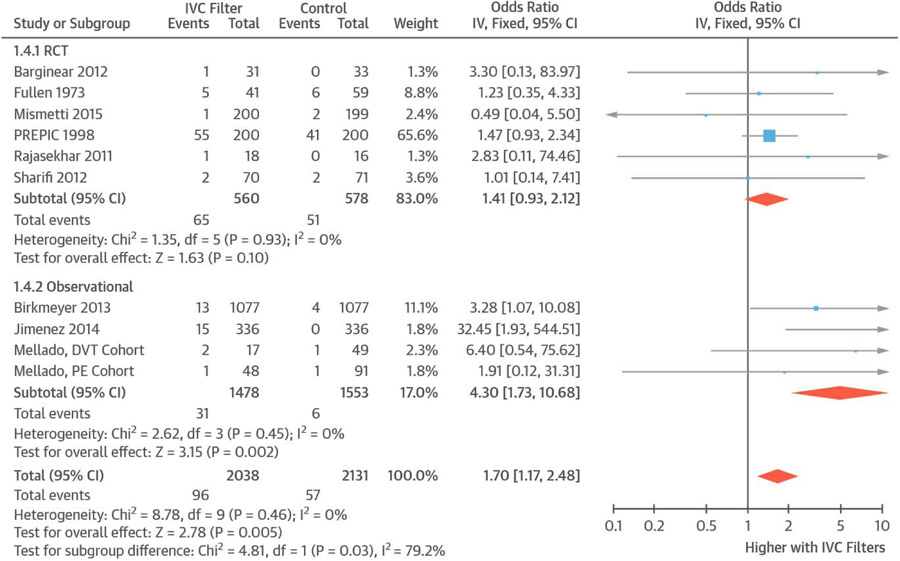
Ten studies reported information about subsequent DVT (96 events in 2,038 patients receiving IVC filters and 57 in 2,131 controls). Compared with controls, patients receiving an IVC filter had an increased risk of subsequent DVT (OR: 1.70; 95% CI: 1.17 to 2.48; I2 = 0%) (Figure 3). Restricting the results to RCTs, there was a similar, but nonsignificant, increase in the risk of DVT in those receiving IVC filters compared with controls (OR: 1.41; 95% CI: 0.93 to 2.12; I2 = 0%). Repeating the results with risk difference across 10 included studies showed similar results (risk difference: 0.02; 95% CI: 0.00 to 0.03; number needed to harm 50).
FIGURE 3. Subsequent DVT.
Pooled results showed increased risk of subsequent DVT in patients receiving IVC filters versus controls. Abbreviations as in Figure 1.
Excluding the study by Jimenez et al. (21) (conducted in patients with recent major bleeding or at high risk of bleeding), pre-enrollment bleeding, reported in 7 studies, was rare and had occurred in 2 patients receiving IVC filters and 3 patients in the control group. Bleeding events during the follow-up period were reported in 8 studies, with a total of 1,981 patients. Patients receiving IVC filters had a similar risk of bleeding compared with those who did not receive IVC filters (OR: 0.90; 95% CI: 0.58 to 1.38; I2 = 0%). Overall, 5 studies including a total of 1,578 patients reported 32 cases of filter thrombosis. None of the studies reported on organ injury due to IVC filters. Filter migration was reported in 1 of the patients in the study by Birkmeyer et al. (19). None of the included studies reported subsequent hospital readmission rates.
RISKS OF BIAS AND QUALITY ASSESSMENT.
For all the RCTs, there were limitations in methodology and in outcomes assessment (per Cochrane and GRADE criteria). All RCTs were open label, and blinded outcome assessment was reported in only 1 trial (23). One study had high risk of bias for lack of random sequence generation. The same study also had limitations for PE ascertainment in some cases (27) (Online Table 2).
Confidence in the outcomes estimates derived from pooled data from the RCTs for all outcomes was low (based on GRADE criteria). Study populations for included studies were varied (e.g., post-DVT on anticoagulation, post-trauma with no known DVT and no prophylactic anticoagulation, cancer-associated DVT). As such, pooled data were indirect for specific indications for IVC filters. Quality of evidence was downgraded for imprecision for outcomes of PE-related mortality and total mortality (Table 2). Due to the small number of studies, we generated a funnel plot for only subsequent PE, which was suggestive of publication bias (Online Figure 2).
TABLE 2.
GRADE Assessment*
| Outcome | Risk of Bias | Consistency | Directness | Precision | Publication Bias | Quality of Evidence |
Relative Effect (95% CI) |
|---|---|---|---|---|---|---|---|
| PE | No serious limitations† | No serious limitations‡ | Serious limitations§ | No serious limitations | Serious limitation§ | Low | 0.40 (0.23–0.69) |
| PE-related mortality | No serious limitations† | No serious limitations | Serious limitations§ | Serious limitations∥ | No serious limitation | Low | 0.82 (0.30–2.28) |
| All-cause mortality | No serious limitations | No serious limitations | Serious limitations§ | Serious limitations∥ | No serious limitation | Low | 0.96 (0.70–1.32) |
| DVT | No serious limitations | No serious limitations | Serious limitations§ | No serious limitations∥ | No serious limitation | Mod | 1.41 (0.93–2.12) |
Included controlled trials for major outcomes (6 studies with 1,138 participants). We also planned to assess other IVC filter complications (including organ injury and IVC filter migration), but such data were not available from the vast majority of included studies.
In 1 study, cases with likely diagnosis of PE were included. This was not felt to impart significant bias.
I2 = 30%. Did not downgrade for mild heterogeneity.
Study populations for included studies were varied (e.g., post-DVT on therapy, post-trauma with no known DVT, cancer-associated DVT). As such pooled data were indirect for specific indications for IVC filter.
95% confidence interval (CI) include important harm and benefit. Low number of outcome events.
GRADE = Grading of Recommendations, Assessment, Development and Evaluation; other abbreviations as in Table 1.
MAJOR SUBGROUP AND SENSITIVITY ANALYSES.
A pre-specified analysis of 4 primary prevention studies before surgical procedures showed nonsignificant trends toward reduced risk of PE (OR: 0.56; 95% CI: 0.27 to 1.20; I2 = 66%) and increased risk of DVT (OR: 2.16; 95% CI: 0.96 to 4.86; I2 = 0%). There was no significant difference in PE-related mortality (OR: 0.64; 95% CI: 0.14 to 2.96; I2 = 54%) or all-cause mortality (OR: 0.91; 95% CI: 0.70 to 1.19; I2 = 44%).
Pre-specified results for the 3 studies that used retrievable filters showed no difference in risk of PE (OR: 0.78; 95% CI: 0.28 to 2.16; I2 = 62%), DVT (OR: 0.96; 95% CI: 0.24 to 3.85; I2 = 0%), PE-related mortality (OR: 3.05; 95% CI: 0.61 to 15.28; I2 = 0%), or all-cause mortality (OR: 1.42; 95% CI: 0.75 to 2.71; I2 = 0%).
A post hoc analysis of 3 studies with cohorts similar to guideline-recommended indications (i.e., contraindications to anticoagulation or recurrent VTE despite adequate anticoagulation) showed trends toward reduced risk of recurrent PE (OR: 0.47; 95% CI: 0.21 to 1.04; I2 = 31%), increased risk of subsequent DVT (OR: 7.21; 95% CI: 1.53 to 33.85; I2 = 0%), reduced rates of PE-related mortality (OR: 0.20; 95% CI: 0.06 to 0.64; I2 = 16%), and no change in all-cause mortality (OR: 0.70; 95% CI: 0.42 to 1.16; I2 = 69%).
Exclusion of the FILTER-PEVI trial from the 11 studies did not substantively change any of the major findings (data not shown). A substudy from the ICOPER registry (International Cooperative Pulmonary Embolism Registry) reported outcomes in patients with massive PE who received an IVC filter versus those who did not (32). This study was not included in the primary analyses because of the unmatched nature of comparisons. A sensitivity analysis including ICOPER substudy results (for the 2 reported outcomes of recurrent PE and all-cause mortality) did not substantially change the meta-analysis results (Online Figures 3 and 4). Use of 2-year results for the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) trial (29), instead of the 8-year results (30), yielded fundamentally similar findings (data not shown). A post hoc analysis that divided the studies based on maximum follow-up (<3 or >3 months) showed similar findings (Online Figures 5A to 5D). Finally, repeating all the analyses using Mantel-Haenszel random effects models with OR as the effect measure yielded similar results (Online Figures 6A to 6D).
DISCUSSION
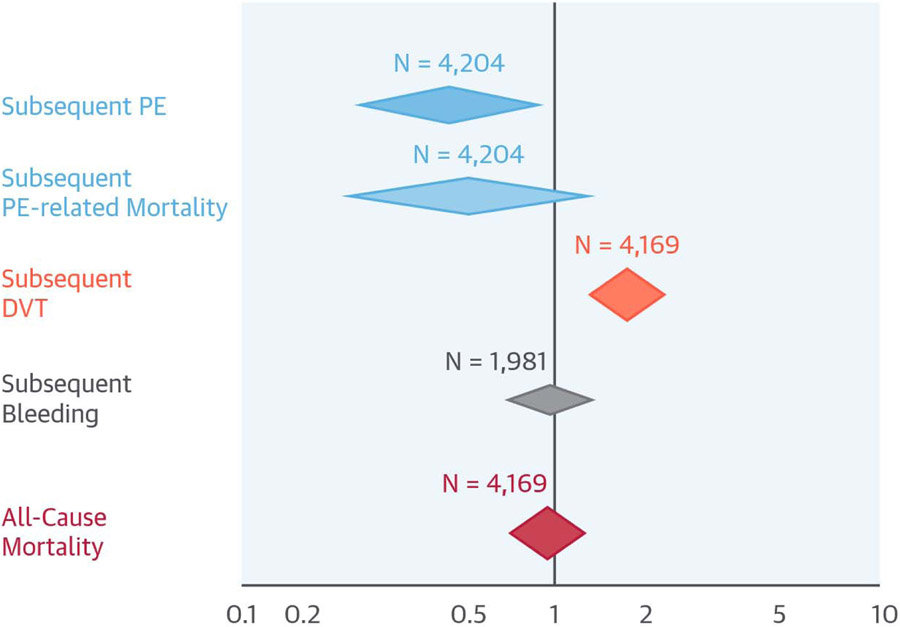
Our systematic review of safety and efficacy of IVC filters was notable for existence of only a few prospective and controlled studies (RCT or observational) with a total of 4,204 patients and limitations in methodology for included studies. Summary evidence from included studies shows that use of IVC filters across various indications is associated with reduced risk of subsequent PE, increased risk of DVT, and nonsignificantly lower PE-related mortality, but no difference in all-cause mortality (Central Illustration). Of note, the nonsignificant reduction in PE-related mortality reached statistical significance in the study subgroup most similar to guideline-recommended indications (i.e., those with contraindication to anticoagulation and recurrent events despite adequate anticoagulation) (11,12,16). Limiting the analyses to RCTs yielded similar findings, as did a wide array of additional sensitivity analyses and subgroup analyses.
CENTRAL ILLUSTRATION. Use of IVC Filters Compared With Controls.
In this review and analysis of studies of IVC filters versus none in patients at risk of PE, the results were limited by the small number of patients (total N = 4,204 patients, but fewer for some outcomes), methodological limitations with the included studies, and clinical heterogeneity across the studies. Summary results suggested that for every 100 patients, there would be 5 fewer subsequent PEs, 2 excess DVTs, and no change in all-cause mortality. The results appeared more favorable in limited scenarios that resembled guidelines indications, although no randomized trials existed. DVT = deep vein thrombosis; IVC = inferior vena cava; PE = pulmonary embolism.
Prior studies have shown frequent use of IVC filters, with some indicating they are being used more widely than supported by recommendations from existing expert guidelines (6-9,33-35). Our findings for this commonly used device indicated need for subsequent high-quality investigations and for optimizing use of the device so it is focused on subgroups where benefits would be clear and outweigh the potential harms.
Pooled results of our analyses in subgroups similar to guideline recommendations appeared encouraging, although the included cohorts still had some differences with those of guidelines. A single small trial (22) suggested better outcomes for IVC filter placement in patients receiving thrombolytic therapy with endovenous intervention. Enthusiasm by some experts, at least until more evidence is available, also exists for use of IVC filters in patients with VTE who receive other forms of thrombolytic therapy, including after a massive PE (36). For several other indications, physicians and patients should be informed of the limited evidence for efficacy (Table 3).
TABLE 3.
Evidence Base for Use of IVC Filters Across Clinical Scenarios
| Clinical Scenario | Evidence Base* |
|---|---|
| Acute VTE in patients with contraindication to anticoagulation | Guidelines recommendations were primarily based on expert recommendation. A controlled observational study suggested significantly reduced PE-related mortality rates and a trend towards lower all-cause mortality in patients at high-risk of bleeding, who received IVC filters compared with those who did not (21). |
| Recurrent VTE despite adequate anticoagulation | Guidelines recommendations were primarily based on expert recommendation. A recent controlled study of patients with recurrent VTE (all of whom were on anticoagulant therapy) showed that in patients with recurrence in the form of a PE, there were reduced rates of PE, and mortality. This was not the case for patients with recurrence in the form of a DVT (27). |
| Massive (hemodynamically unstable) PE | Guidelines recommendations were primarily based on expert recommendations or unmatched observational studies. No adequately controlled studies available. |
| Acute VTE and concurrent poor cardiopulmonary reserve | Guidelines recommendations were primarily based on expert recommendation. |
| Acute VTE being treated with thrombolytic therapy | A randomized trial of patients receiving endovenous interventions for lower extremity DVT showed lower rate of PE in patients receiving IVC filters compared with controls. No controlled data are available for other settings (22). |
| Acute VTE in patients with active cancer | A small, underpowered RCT of 64 patients with active cancer and VTE did not show a significant difference in recurrent PE or mortality (18). |
| Acute proximal DVT without contraindication for antithrombotic therapy | The PREPIC trial showed reduced rates of PE but increased rates of recurrent DVT, without a change in mortality rates in patients receiving an IVC filter compared with those who received anticoagulation alone (29,30). |
| Acute PE without contraindication for antithrombotic therapy | The PREPIC-II trial did not show a decline in the rates of recurrent PE or mortality in patients with acute PE with high risk of recurrence who received a retrievable IVC filter in addition to anticoagulation compared with those receiving anticoagulation alone (23). |
| Prophylactic use in patients with acute traumatic femur fracture | A small quasi-randomized trial suggested reduced rates of PE and mortality in patients receiving IVC filters compared with controls. Of note, in all patients, no anticoagulation was used pre-operatively or post-operatively (27). |
| Prophylactic use in high-risk patients with acute major trauma | Guidelines recommendations were based on expert recommendations or lower-quality evidence. A small, underpowered feasibility trial did not show a difference in the rates of PE or mortality. The vast majority of patients had received some form of pharmacological VTE prophylaxis (17). |
| Prophylactic use in patients undergoing bariatric surgery | A small, controlled sub-study from an observational study suggested numerically lower PEs and PE deaths in patients receiving IVC filters compared with controls. All patients had received pharmacological and mechanical VTE prophylaxis (31). |
| A larger, controlled observational study of patients undergoing bariatric surgery did not find a difference in the rates of PE or mortality. The vast majority of patients had received some form of pharmacological VTE prophylaxis (19). |
Only controlled prospective observational studies and controlled trials are discussed.
Abbreviations as in Table 1.
In addition to including RCTs, we chose to include prospective controlled observational studies. This decision was made in part because there is consensus that for certain indications there are no available RCTs, and most likely there will be no RCTs in future (Table 3) (10,20). We, however, excluded retrospective studies using administrative data, as they frequently miss important clinical variables, and have had no or limited validation of their strategy (37). The small list of our included studies illustrates the great need for additional high-quality studies evaluating IVC filters in different populations (e.g., primary prevention, after massive PE, with ileofemoral DVT).
STUDY LIMITATIONS.
This study and the available evidence have several limitations. First, our study was notable for inclusion of only 4,204 patients from 11 relatively small studies. In addition to clinical heterogeneity across the included studies, the small size of studies brings potential limitations to the summary results, with prior examples in the cardiovascular literature suggesting different results from larger studies compared with summary results of smaller ones (38). We hope that our study will motivate the design of subsequent larger prospective controlled studies to better inform the evidence base across clinical scenarios (Table 3).
Second, lack of a sham procedure could have potentially biased the results of the individual studies and thereby the pooled estimates (39). Third, our study might have underestimated the rates of IVC filter-related complications, such as filter migration, penetration, perforation, organ injury, and IVC thrombosis and stenosis (40-42), either because these findings were not systematically reported in detail across all studies (especially the observational studies) or because standards of real-world practice might be different from (riskier than) those of controlled trials. In this sense, we believe the ongoing PRESERVE (Predicting the Safety and Effectiveness of Inferior Vena Cava Filters) study could provide useful information about the safety of this device. PRESERVE (NCT02381509) is a collaborative effort between the Society for Vascular Surgery, Society for Interventional Radiology, and IVC filter manufacturing companies. This study was specifically designed to address some of the concerns by regulatory agencies such as the Food and Drug Administration with regard to the safety of IVC filters and has been enrolling patients who receive IVC filters for >1 year. Despite providing valuable information related to safety, however, PRESERVE is not a randomized trial and does not have a control arm. As such, the unmet needs for investigations related to the efficacy of IVC filters should be addressed by studies other than PRESERVE.
Fourth, the major outcomes had different methods of identification and were reported in varying time intervals. Our multiple subgroup analyses and sensitivity analyses detailed earlier, however, showed very similar results, supporting the robustness of our findings. Fifth, none of the included studies reported hospital readmission rates related to IVC filter use. Finally, we would have preferred to present pooled results across other major clinical subgroups (such as by age groups or in those with heart failure or cancer). However, such data were not available in any of the included studies.
CONCLUSIONS
Our study identified only a few prospective controlled studies reporting the safety and efficacy of IVC filters. The quality of the data had serious limitations for some of the outcomes. Overall, the existing evidence was indicative of reduced risk of subsequent PE, increased risk of subsequent DVT, nonsignificantly reduced risk of PE-related mortality (that reached significance in studies most similar to those in existing guideline recommendations), and no change in all-cause mortality for patients who received IVC filters compared with controls. On the basis of the existing evidence, it would be reasonable to consider IVC filters for limited scenarios, such as contraindication to antithrombotic therapy or recurrent PE despite adequate anticoagulation. For the majority of remaining indications, the data are limited or conflicting. Additional studies are required to better inform the benefits and harms of this procedure; until then, practitioners should be mindful about indiscriminate use of IVC filters.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
IVC filters are associated with a lower rate of subsequent PE, increased risk of subsequent DVT, and no significant change in mortality.
TRANSLATIONAL OUTLOOK:
Further studies are required to elucidate the safety and efficacy of IVC filters across various indications and patient subgroups.
ACKNOWLEDGMENTS
The authors thank Dr. David Jimenez from the Ramón y Cajal Hospital and Alcalá de Henares University in Madrid, Spain, and Dr. Jose Ignacio Pijoan from the Clinical Epidemiology Unit, BioCruces Health Research Institute, Hospital Universitario Cruces, Bizkaia, Spain, for providing additional data about a RIETE substudy included in this paper.
Dr. Kirtane has received institutional research grants through Columbia University/Cardiovascular Research Foundation from Boston Scientific, Abbott Vascular, Medtronic, Abiomed, CathWorks, and Siemens. Dr. Goldhaber has received research support from BiO2 Medical, Boehringer Ingelheim, Bristol-Myers Squibb, BTG EKOS, Daiichi-Sankyo, and Janssen; and has been a consultant for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, Portola, and Zafgen. Dr. Krumholz receives support through Yale University from Medtronic and Johnson & Johnson to develop methods of clinical trial data sharing, from Medtronic and the Food and Drug Administration to develop methods for post-market surveillance of medical devices, and from the Centers of Medicare & Medicaid Services to develop and maintain performance measures that are used for public reporting; chairs a cardiac scientific advisory board for UnitedHealth; is on the advisory board for Element Science; is a participant/participant representative of the IBM Watson health life sciences board; is on the physician advisory board for Aetna; and is the founder of Hugo, a personal health information platform. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CI
confidence interval
- DVT
deep vein thrombosis
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- IVC
inferior vena cava
- OR
odds ratio
- PE
pulmonary embolism
- RCT
randomized controlled trial
- VTE
venous thromboembolism
Footnotes
APPENDIX For the PRISMA checklist as well as supplemental figures and tables, please see the online version of this article.
REFERENCES
- 1.Bikdeli B, Gupta A, Mody P, Lampropulos JF, Dharmarajan K. Most important outcomes research papers on anticoagulation for cardiovascular disease. Circ Cardiovasc Qual Outcomes 2012;5:e65–74. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heit JA, Cohen AT, Anderson FJ. Estimated annual number of incident and recurrent, nonfatal and fatal venous thromboembolism (VTE) events in the US. Blood 2005;106:1. [Google Scholar]
- 4.Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756–64. [DOI] [PubMed] [Google Scholar]
- 5.Bikdeli B, Bikdeli B. Updates on advanced therapies for acute pulmonary embolism. Int J Cardiovasc Pract 2016;1:47–50. [Google Scholar]
- 6.Duszak R Jr., Parker L, Levin DC, Rao VM. Placement and removal of inferior vena cava filters: national trends in the Medicare population. J Am Coll Radiol 2011;8:483–9. [DOI] [PubMed] [Google Scholar]
- 7.Bikdeli B, Wang Y, Minges KE, et al. Vena caval filter utilization and outcomes in pulmonary embolism: Medicare hospitalizations from 1999 to 2010. J Am Coll Cardiol 2016;67:1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimenez D, de Miguel-Diez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J Am Coll Cardiol 2016;67:162–70. [DOI] [PubMed] [Google Scholar]
- 9.Stein PD, Matta F, Hull RD. Increasing use of vena cava filters for prevention of pulmonary embolism. Am J Med 2011;124:655–61. [DOI] [PubMed] [Google Scholar]
- 10.Bikdeli B, Ross JS, Krumholz HM. Data desert for inferior vena caval filters: limited evidence, supervision, and research. JAMA Cardiol 2017;2:3–4. [DOI] [PubMed] [Google Scholar]
- 11.Kearon C, AkL EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–52. [DOI] [PubMed] [Google Scholar]
- 12.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788–830. [DOI] [PubMed] [Google Scholar]
- 13.Caplin DM, Nikolic B, Kalva SP, et al. Quality improvement guidelines for the performance of inferior vena cava filter placement for the prevention of pulmonary embolism. J Vasc Interv Radiol 2011;22:1499–506. [DOI] [PubMed] [Google Scholar]
- 14.Jaff MR, Kaufman J. A measured approach to vena cava filter use-respect rather than regret. JAMA Cardiol 2017;2:5–6. [DOI] [PubMed] [Google Scholar]
- 15.White RH, Geraghty EM, Brunson A, et al. High variation between hospitals in vena cava filter use for venous thromboembolism. JAMA Intern Med 2013;173:506–12. [DOI] [PubMed] [Google Scholar]
- 16.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033–69, 3069a-k. [DOI] [PubMed] [Google Scholar]
- 17.Rajasekhar A, Lottenberg L, Lottenberg R, et al. A pilot study on the randomization of inferior vena cava filter placement for venous thromboembolism prophylaxis in high-risk trauma patients. J Trauma 2011;71:323–8; discussion 328-9. [DOI] [PubMed] [Google Scholar]
- 18.Barginear MF, Gralla RJ, Bradley TP, et al. Investigating the benefit of adding a vena cava filter to anticoagulation with fondaparinux sodium in patients with cancer and venous thromboembolism in a prospective randomized clinical trial. Support Care Cancer 2012;20: 2865–72. [DOI] [PubMed] [Google Scholar]
- 19.Birkmeyer NJ, Finks JF, English WJ, et al. Risks and benefits of prophylactic inferior vena cava filters in patients undergoing bariatric surgery. J Hosp Med 2013;8:173–7. [DOI] [PubMed] [Google Scholar]
- 20.Muriel A, Jimenez D, Aujesky D, et al. Survival effects of inferior vena cava filter in patients with acute symptomatic venous thromboembolism and a significant bleeding risk. J Am Coll Cardiol 2014; 63:1675–83. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez D, Muriel A, Monreal M, Yusen RD. Reply: Immortal time bias and the use of IVC filters. J Am Coll Cardiol 2014;64:955–6. [DOI] [PubMed] [Google Scholar]
- 22.Sharifi M, Bay C, Skrocki L, Lawson D, Mazdeh S. Role of IVC filters in endovenous therapy for deep venous thrombosis: the FILTER-PEVI (Filter Implantation to Lower Thromboembolic Risk in Percutaneous Endovenous Intervention) trial. Cardiovasc Intervent Radiol 2012;35:1408–13. [DOI] [PubMed] [Google Scholar]
- 23.Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA 2015;313:1627–35. [DOI] [PubMed] [Google Scholar]
- 24.Mellado M, Pijoan JI, Jimenez D, et al. Outcomes associated with inferior vena cava filters among patients with thromboembolic recurrence during anticoagulant therapy. J Am Coll Cardiol Intv 2016;9:2440–8. [DOI] [PubMed] [Google Scholar]
- 25.Removing Retrievable Inferior Vena Cava Filters: Initial Communications. Available at: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm396377.htm.Accessed May 22, 2016.
- 26.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: www.handbook.cochrane.org. Accessed March 1, 2017. [Google Scholar]
- 27.Fullen WD, Miller EH, Steele WF, McDonough JJ. Prophylactic vena caval interruption in hip fractures. J Trauma 1973;13:403–10. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 29.Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med 1998;338: 409–15. [DOI] [PubMed] [Google Scholar]
- 30.PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study. Circulation 2005;112:416–22. [DOI] [PubMed] [Google Scholar]
- 31.Gargiulo NJ 3rd, Veith FJ, Lipsitz EC, Suggs WD, Ohki T, Goodman E. Experience with inferior vena cava filter placement in patients undergoing open gastric bypass procedures. J Vasc Surg 2006;44:1301–5. [DOI] [PubMed] [Google Scholar]
- 32.Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–82. [DOI] [PubMed] [Google Scholar]
- 33.Spencer FA, Bates SM, Goldberg RJ, et al. A population-based study of inferior vena cava filters in patients with acute venous thromboembolism. Arch Intern Med 2010;170: 1456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel G, Panikkath R, Fenire M, Gadwala S, Nugent K. Indications and appropriateness of inferior vena cava filter placement. Am J Med Sci 2015;349:212–6. [DOI] [PubMed] [Google Scholar]
- 35.Sader RB, Friedman A, Berkowitz E, Martin E. Inferior vena cava filters and their varying compliance with the ACCP and the SIR guidelines. South Med J 2014;107:585–90. [DOI] [PubMed] [Google Scholar]
- 36.Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med 2012;125: 465–70. [DOI] [PubMed] [Google Scholar]
- 37.Curtis JP, Krumholz HM. The predicament of comparative effectiveness research using observational data. Ann Intern Med 2015;163: 799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusuf S, Flather M. Magnesium in acute myocardial infarction. BMJ 1995;310:751–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messerli FH, Bangalore S. Renal denervation for resistant hypertension? N Engl J Med 2014; 370:1454–7. [DOI] [PubMed] [Google Scholar]
- 40.Jia Z, Wu A, Tam M, Spain J, McKinney JM, Wang W. Caval penetration by inferior vena cava filters: a systematic literature review of clinical significance and management. Circulation 2015; 132:944–52. [DOI] [PubMed] [Google Scholar]
- 41.Malgor RD, Labropoulos N. A systematic review of symptomatic duodenal perforation by inferior vena cava filters. J Vase Surg 2012;55: 856–61.e3. [DOI] [PubMed] [Google Scholar]
- 42.Angel LF, Tapson V, Galgon RE, Restrepo MI, Kaufman J. Systematic review of the use of retrievable inferior vena cava filters. J Vasc Interv Radiol 2011;22:1522–30.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.