The coronavirus disease 2019 (COVID-19) is an acute respiratory disease caused by the novel coronavirus SARS-CoV-2. Despite the second vaccination for SARS-CoV-2, the number of individuals infected with SARS-CoV-2 variants (i.e., delta and lambda) has markedly increased worldwide. Although approximately 80% of individuals infected with SARS-CoV-2 is mild to moderate, a part of them may convert to severe clinical stages in about 1 week, ultimately resulting in the intubation or death. Using drug repurposing, it is, therefore, necessary to discover drugs that can prevent clinical deterioration [1]. Here, we discuss the emergent use of the old antidepressant fluvoxamine which may block clinical deterioration in mild to moderate patients infected with SARS-CoV-2.
In November 2020, Dr. Lenze and his colleagues reported that fluvoxamine could prevent clinical deterioration in adult outpatients infected with SARS-CoV-2. In the study, clinical deterioration occurred in 0 of the fluvoxamine group (n = 80) and in 6 of placebo group (n = 72) [2]. Although sample size of this study was small, this study strongly encouraged further trials using a large sample size. In February 2021, Dr. Seftel and his colleague reported a prospective, non-randomized observational cohort study of fluvoxamine in outpatients (n = 113) infected with SARS-CoV-2 at the Golden Gate Fields horse racing track in Berkeley, California [3]. Incidence of hospitalization was 0 of the fluvoxamine-treated group (n = 65) and 6 of the observation alone group (n = 48). Two patients required intensive care unit stay with mechanical ventilation, one of them died. On April 23, 2021, fluvoxamine was added in the US National Institutes of Health (NIH) COVID-19 Guidelines Panel although there is insufficient evidence for the efficacy of fluvoxamine.
On August 6, 2021, the interim results of TOGETHER trial (NCT04727424) by a multinational group in Canada and Brazil were presented at the NIH symposium. They compared three compounds, fluvoxamine, the antidiabetic drug metformin, and the antiparasitic drug ivermectin. Although metformin and ivermectin did not show beneficial effects, fluvoxamine was much more promising. Among the randomized participants (n = 1,480), fluvoxamine significantly reduced the risk of disease progression by 29% (95% confidence interval 0.54–0.93) [4].
Detailed mechanisms of action of fluvoxamine for COVID-19 are currently unknown. In 1996, we reported that fluvoxamine binds to endoplasmic reticulum (ER) protein sigma-1 receptor with high affinity, suggesting a role of sigma-1 receptor in the mechanisms of its action [5]. Subsequent studies suggest that fluvoxamine is a potent agonist at sigma-1 receptor which plays a key role in inflammation [1, 5, 6]. Among the antidepressants, fluvoxamine was the most potent at sigma-1 receptor [1, 5, 6]. Furthermore, fluvoxamine has several beneficial effects, including reduction in platelet aggregation by serotonin transporter inhibition, decreased mast cell degranulation, interference with lysosomal trafficking of virus, inhibition of acid sphingomyelinase (ASM), and increased levels of metatonin by cytochrome P450 inhibition [7].
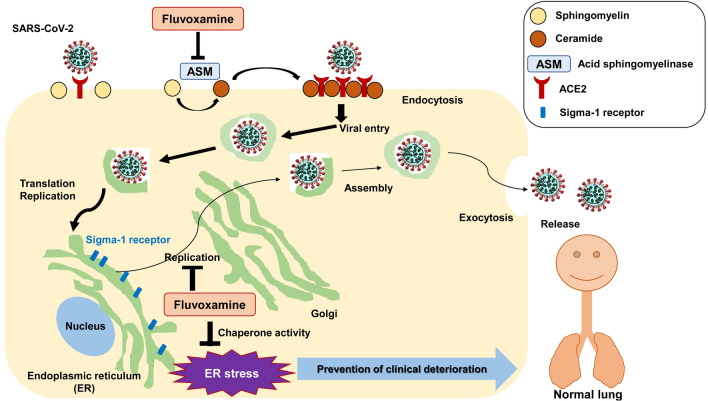
In October 2020, Gordon et al. [8] identified the sigma-1 receptor (encoded by SIGMAR1) as a functional host-dependency factor for SARS-CoV-2. Knockout or knockdown of SIGMAR1 produced robust reductions in SARS-CoV-2 replication, indicating a key role of the sigma-1 receptor in SARS-CoV-2 replication (Fig. 1). In 2019, Rosen et al. [9] demonstrated that the sigma-1 receptor is essential for the cytokine production in a mouse model of septic shock, and that fluvoxamine could protect against inflammatory response and lethal septic shock. Taken together, it is likely that the potent sigma-1 receptor agonists, such as fluvoxamine, might ameliorate inflammatory events (i.e., cytokine storm) associated with ER stress due to SARS-CoV-2 replication (Fig. 1) [1].
Fig. 1.
Proposed biological mechanisms of fluvoxamine in the treatment of SARS-CoV-2-infected patients. SARS-CoV-2 binds to ACE2 receptor on the cells, resulting in activation of the acid sphingomyelinase (ASM) which converts sphingomyelin to ceramide. ASM/ceramide system can facilitate viral entry. Antidepressants such as fluvoxamine inhibit ASM and formation of ceramide-enriched membrane domains, resulting in decreased viral entry. Recent study shows that sigma-1-receptor ligands can attenuate SARS-CoV-2 replication [8]. Through sigma-1 receptor chaperone activity [1], the sigma-1-receptor agonist fluvoxamine may attenuate ER stress due to SARS-CoV-2 replication in cells, thus resulting in a blockade against inflammatory events (i.e., cytokine storm). Thus, early intervention using fluvoxamine may block or delay clinical deterioration in individuals with SARS-CoV-2 infection. A slight modification with Fig. 1 in the reference [10] and Fig. 3 in the reference [1]
A recent observational multicenter study (n = 2846) showed association between the use of functional inhibitors of ASM and reduced risk of intubation or death in hospitalized patients with severe COVID-19 [10]. The functional inhibitors of ASM include the antidepressants such as fluvoxamine, fluoxetine, and escitalopram. Interestingly, fluoxetine and escitalopram are also sigma-1 receptor agonists although they are less potent than fluvoxamine [1]. Considering the role of sigma-1 receptor and ASM in biological actions of SARS-CoV-2 in cells (Fig. 1), both fluoxetine and escitalopram may be prophylactic drugs for mild to moderate patients infected with SARS-CoV-2 although further clinical study is needed.
The advantages of fluvoxamine are favorable safety profiles, widespread availability, very low cost, oral administration and use for children and adolescents. If fluvoxamine is used in individuals with COVID-19 as quickly as possible after confirmation of SARS-CoV-2 infection, clinical deterioration might be prevented [1]. Importantly, fluvoxamine could be a prophylactic drug for COVID-19 in countries with low vaccination rates or low health system.
Author contributions
The authors did the reference search and wrote the commentary.
Declarations
Conflict of interest
Dr. Y. Hashimoto and Dr. Suzuki have no conflict of interest. Dr. K. Hashimoto has received speakers’ honoraria from Abbott and Meiji Seika.
References
- 1.Hashimoto K. Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor. Eur Arch psychiatry Clin Neurosci. 2021;271(1):249–258. doi: 10.1007/s00406-020-01231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenze E, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE, Miller JP, Yang L, Yingling M, Avidan MS, Reiersen AM. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19. A randomized clinical trial. JAMA. 2020;324(22):2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seftel D, Boulware DR. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis. 2021;8(2):ofab050. doi: 10.1093/ofid/ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sax PE (2021) Could this be our first effective, inexpensive, widely available outpatient treatment for COVID-19? NEJM J Watch. https://blogs.jwatch.org/hiv-id-observations/index.php/could-this-be-our-first-effective-inexpensive-widely-available-outpatient-treatment-for-covid-19/2021/08/12/
- 5.Narita N, Hashimoto K, Tomitaka S, Minabe Y. Interaction of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur J Pharmacol. 1996;307(1):117–119. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto K. Activation of sigma-1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. J Pharmacol Sci. 2015;127(1):6–9. doi: 10.1016/j.jphs.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme V. Fluvoxamine: a review of its mechanisms of actions and its role in COVID-19. Front Pharmacol. 2021;12:652688. doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon DE, Hiatt J, Bouhaddou M, Rezelj VV, Ulferts S, Braberg H, Jureka AS, Obernier K, Guo JZ, Batra J, Kaake RM, Weckstein AR, Owens TW, Gupta M, Pourmal S, Titus EW, Cakir M, Soucheray M, McGregor M, Cakir Z, Jang G, O'Meara MJ, Tummino TA, Zhang Z, Foussard H, Rojc A, Zhou Y, Kuchenov D, Hüttenhain R, Xu J, Eckhardt M, Swaney DL, Fabius JM, Ummadi M, Tutuncuoglu B, Rathore U, Modak M, Haas P, Haas KM, Naing ZZC, Pulido EH, Shi Y, Barrio-Hernandez I, Memon D, Petsalaki E, Dunham A, Marrero MC, Burke D, Koh C, Vallet T, Silvas JA, Azumaya CM, Billesbølle C, Brilot AF, Campbell MG, Diallo A, Dickinson MS, Diwanji D, Herrera N, Hoppe N, Kratochvil HT, Liu Y, Merz GE, Moritz M, Nguyen HC, Nowotny C, Puchades C, Rizo AN, Schulze-Gahmen U, Smith AM, Sun M, Young ID, Zhao J, Asarnow D, Biel J, Bowen A, Braxton JR, Chen J, Chio CM, Chio US, Deshpande I, Doan L, Faust B, Flores S, Jin M, Kim K, Lam VL, Li F, Li J, Li YL, Li Y, Liu X, Lo M, Lopez KE, Melo AA, Moss FR, 3rd, Nguyen P, Paulino J, Pawar KI, Peters JK, Pospiech TH, Jr, Safari M, Sangwan S, Schaefer K, Thomas PV, Thwin AC, Trenker R, Tse E, Tsui TKM, Wang F, Whitis N, Yu Z, Zhang K, Zhang Y, Zhou F, Saltzberg D, QCRG Structural Biology Consortium. Hodder AJ, Shun-Shion AS, Williams DM, White KM, Rosales R, Kehrer T, Miorin L, Moreno E, Patel AH, Rihn S, Khalid MM, Vallejo-Gracia A, Fozouni P, Simoneau CR, Roth TL, Wu D, Karim MA, Ghoussaini M, Dunham I, Berardi F, Weigang S, Chazal M, Park J, Logue J, McGrath M, Weston S, Haupt R, Hastie CJ, Elliott M, Brown F, Burness KA, Reid E, Dorward M, Johnson C, Wilkinson SG, Geyer A, Giesel DM, Baillie C, Raggett S, Leech H, Toth R, Goodman N, Keough KC, Lind AL, Zoonomia Consortium. Klesh RJ, Hemphill KR, Carlson-Stevermer J, Oki J, Holden K, Maures T, Pollard KS, Sali A, Agard DA, Cheng Y, Fraser JS, Frost A, Jura N, Kortemme T, Manglik A, Southworth DR, Stroud RM, Alessi DR, Davies P, Frieman MB, Ideker T, Abate C, Jouvenet N, Kochs G, Shoichet B, Ott M, Palmarini M, Shokat KM, García-Sastre A, Rassen JA, Grosse R, Rosenberg OS, Verba KA, Basler CF, Vignuzzi M, Peden AA, Beltrao P, Krogan NJ. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020;370(6521):eabe9403. doi: 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen DA, Seki SM, Fernández-Castañeda A, Beiter RM, Eccles JD, Woodfolk JA, Gaultier A. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11(478):eaau5266. doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoertel N, Sánchez-Rico M, Gulbins E, Kornhuber J, Carpinteiro A, Lenze EJ, Reiersen AM, Abellán M, de la Muela P, Vernet R, Blanco C, Cougoule C, Beeker N, Neuraz A, Gorwood P, Alvarado JM, Meneton P, Limosin F. AP-HP / Université de Paris / INSERM COVID-19 research collaboration, AP-HP COVID CDR Initiative, “Entrepôt de Données de Santé” AP-HP Consortium (in press) Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID-19: an observational multicenter study. Clin Pharmacol Ther. 2021 doi: 10.1002/cpt.2317.10.1002/cpt.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]