Abstract

The effect of Er3+ and Y3+ ion-co-substituted Mn0.5Zn0.5ErxYxFe2–2xO4 (MZErYF) (x ≤ 0.10) spinel nanoferrites (SNFs) prepared by a sonochemical approach was investigated. Surface and phase analyses were carried out using SEM, TEM, and XRD. Hyperfine parameters were determined by fitting room-temperature (RT) Mossbauer spectra. Magnetic field-dependent magnetization data unveiled the superparamagnetic nature at RT and ferrimagnetic nature at 10 K. RT saturation magnetization (MS) and calculated magnetic moments (nB) are 34.84 emu/g and 1.47 μB, respectively, and have indirect proportionalities with increasing ion content. MS and nB data have a similar trend at 10 K including remanent magnetizations (Mr). The measured coercivities (HC) are between 250 and 415 Oe. The calculated squareness ratios are in the range of 0.152–0.321 for NPs and assign the multidomain nature for NPs at 10 K. The extracted effective magnetocrystalline constants (Keff) have an order of 104 erg/g except for Mn0.5Zn0.5Er0.10Y0.10Fe1.80O4 SNFs that has 3.37 × 105 erg/g. This sample exhibits the greatest magnetic hardness with the largest magnitude of HC = 415 Oe and an internal anisotropy field Ha = 1288 Oe among all magnetically soft NPs.
1. Introduction
Spinel ferrite with formula AB2O4 has a cubic structure, where A and B indicate the tetrahedral and octahedral sites, respectively.1 Spinel ferrite contains 56 atoms in each unit cell distributed among A and B sites.2,3 The significance of spinel ferrite is because of its wide range of industrial and scientific applications; chemical stability; and the engineering feasibility of tuning its electrical, magnetic, and optical properties. MnZn nanoferrites are a significant category of soft magnetic materials, and their importance has been increasing because of their good electrical, magnetic, and optical properties; high magnetization, resistivity, and initial permeability; permittivity; low power losses; and coercivity. Due to these advantages, MnZn ferrites are fit for use in transformers/transducers, information storage systems, multilayered chip inductors, sensors, biosensor magnetic fluids, refrigerators, mobile chargers, TVs, desktops, printers, microwaves, ovens, LED bulbs, laptops, juicer mixers, washing machines, mobile phones, drug delivery, MRI, etc.4−9
The doping of Mn–Zn ferrites, which have a mixed spinel structure, by transition metal ions remarkably influences the structure and optical, electrical, and magnetic attributes. These features depend on the method of preparation, chemical composition, distribution of cations between two interstitial sites, the additives or substitutions, and grain size.10−13 The inhomogeneous cation distribution and rare earth (RE) substitution can produce a lattice enlargement due to the differences in the Fe3+–Fe2+ superexchange interactions and maintain the symmetry of the spinel crystal lattice.14 The larger ionic radii and the stable 3+ charge of RE ions can give rise to distortions in the crystal structure and the magnetic coercivity and enhance the structural, magnetic, and electrical features.15 When spinel ferrites are doped by RE ions, 4f–3d coupling determines the magnetocrystalline anisotropy of the products. The Mn–Zn ferrites doped by RE ions show high resistivity and enhanced magnetic features. However, special attention must be paid to the procedure of synthesis. It is essential that the spinel crystal structure suffers major deformation because of the solubility limit of the large amount of RE substitution; accordingly, specific ratios of dopant were chosen. The effect of neodymium (Nd+3) on the magnetic and electrical features of MnZn ferrite was studied by Naik et al.16,17 Angadi et al.18 studied the magnetic properties of Sm- and Gd-doped MnZn nanoparticles. Jadhav et al.19 studied Gd-doped MnZn NPs as magnetic carriers for magnetic fluid hyperthermia (MFH). A comparison study of ultrasonic and sol–gel synthesis of Dy-Y-co-substituted MnZn ferrites was performed by Almessiere et al.20 There are many synthesis methods for the synthesis of MnZn ferrites, such as solid-state, sol–gel, hydrothermal, co-precipitation, microwave, ultrasonic synthesis, etc. During the ultrasonic synthesis of MnZn ferrites, the following advantages are obtained: the decrease of the reaction time, enhanced reaction rate, the crystal size distribution control, avoidance of agglomeration of nanoparticles, and so on.21
Accordingly, no reports have been found on erbium- and yttrium-co-doped Mn–Zn ferrites to date. Therefore, in this study, the structural, magnetic, and Mossbauer parameters of nanocrystalline RE Er3+- and Y3+-co-substituted manganese zinc nanospinel ferrites were deeply investigated.
2. Results and Discussion
2.1. Structure
XRD patterns of MZErYF (x ≤ 0.10) spinel nanoferrites (SNFs) are represented in Figure 1. All patterns indicated the formation a pure phase of cubic Mn–Zn spinel ferrites according to ICDD card no. 00-010-0319, devoid of any impurity. Broad peaks were noticed in all XRD patterns. Due to the nature of the sonochemical synthesis approach, all XRD patterns presented broad XRD peaks, which confirmed the small crystallite sizes of all products. The cell constants are determined via refinement with Match3! and Full-proof, as listed in Table 1. Moreover, the XRD patterns showed the effective incorporation of Er3+ (∼0.103 nm) and Y3+ (∼0.104 nm) ions into the spinal lattice. The average crystallite sizes were computed by means of the Debye–Scherrer relation and were found to be in the range of 7–13 nm. The cell constant “ao” increases with increasing “x” value. This increase in the lattice constant of “ao” is due to the nonconformity in the ionic radii of the Er3+ (∼0.103 nm) and Y3+ (∼0.104 nm) ions and the host Fe3+ (∼0.064 nm) ions in the octahedral sites, which caused the expansion of the unit cell due to the induced strain in the lattice.22−24
Figure 1.
XRD powder patterns of MZErYF (x ≤ 0.10) SNFs.
Table 1. Refined Cell Parameters for MZErYF (x ≤ 0.10) SNFs.
| x | a (Å) | V (Å3) | DXRD (nm) ±0.04 | χ2 (chi2) | RBragg |
|---|---|---|---|---|---|
| 0.00 | 8.390(1) | 590.62 | 7.23 | 1.19 | 1.06 |
| 0.02 | 8.394(6) | 591.57 | 9.67 | 1.36 | 0.63 |
| 0.04 | 8.396(7) | 592.00 | 11.14 | 1.36 | 2.21 |
| 0.06 | 8.396(8) | 592.02 | 11.33 | 1.22 | 4.78 |
| 0.08 | 8.401(1) | 592.93 | 12.59 | 1.20 | 1.18 |
| 0.10 | 8.407(6) | 594.31 | 10.87 | 1.07 | 1.59 |
The cation distribution in spinel ferrite can be obtained from the analysis of the X-ray diffraction pattern. In the present work, the Bertaut method is used to determine the cation distribution.25,26 This method selects a few pairs of reflections according to the expression
| 1 |
where 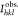 and
and  are the observed and calculated intensities
for reflection (hkl), respectively. The best information
on cation distribution is achieved when comparing experimental and
calculated intensity ratios for reflections whose intensities (i)
are nearly independent of the oxygen parameter, (ii) vary with the
cation distribution in opposite ways, and (iii) do not differ significantly.
are the observed and calculated intensities
for reflection (hkl), respectively. The best information
on cation distribution is achieved when comparing experimental and
calculated intensity ratios for reflections whose intensities (i)
are nearly independent of the oxygen parameter, (ii) vary with the
cation distribution in opposite ways, and (iii) do not differ significantly.
In the present work, the (220), (400), and (440) planes were used to calculate the intensity ratio. These planes are assumed to be sensitive to the cation distribution. The temperature and absorption factors are not taken into account in our calculations as they do not affect the intensity calculation. If an agreement factor (R) is defined as in eq 2, the best-simulated structure that matches the actual structure of the sample leads to a minimum value of R and the corresponding cation distribution is obtained for each hkl and h′k′l′ reflection pair considered
| 2 |
For the calculation of the relative integrated intensity (Ihkl) of a given diffraction line from powder specimens as observed using a diffractometer with a flat-plate sample holder, the following formula is valid
| 3 |
where F is the structure factor, P is the multiplicity factor, and LP is the Lorentz-polarization factor, and
| 4 |
The obtained cation distributions of the MZErYF (x ≤ 0.10) SNFs are tabulated in Table 2. Mn preferred the Td (tetrahedral) site as compared to the Oh (octahedral) site, which is in accordance with the preference to Td sites.27 As anticipated, Zn ions occupy the Td site only. As expected, Fe3+ ions occupy the Oh site, and its occupancy linearly decreased with Er and Y substitution. Er and Y ions occupy the Oh site because of their higher ionic radii.
Table 2. Cation Distribution of MZErYF (x ≤ 0.10) SNFs.
| x | Td site | Oh site |
|---|---|---|
| 0.00 | Mn0.35Zn0.5Fe0.15 | Mn0.15Fe1.85 |
| 0.02 | Mn0.3Zn0.5Fe0.2 | Mn0.2Er0.02Y0.02Fe1.76 |
| 0.04 | Mn0.3Zn0.5Fe0.2 | Mn0.2Er0.04Y0.04Fe1.72 |
| 0.06 | Mn0.3Zn0.5Fe0.2 | Mn0.2Er0.06Y0.06Fe1.68 |
| 0.08 | Mn0.3Zn0.5Fe0.2 | Mn0.2Er0.08Y0.08Fe1.64 |
| 0.10 | Mn0.3Zn0.5Fe0.2 | Mn0.2Er0.1Y0.1Fe1.60 |
2.2. Morphology
Figure 2 displays a high-magnification SEM analysis of MZErYF (x ≤ 0.10) SNFs. The SEM images demonstrated nonuniform clusters of fine spherical particles. It is observed that the aggregation changed with increasing dopant ions as a result of the behavior of nanosized ferrites. The EDX spectra exhibited the weight percentage of the elements in MZErYF (x ≤ 0.10) SNFs in the absence of undesired elements, as shown in Figure 3. TEM images of MZErYF (x ≤ 0.10) SNFs are shown in Figure 4. The particles exhibited a cubical structure and appeared aggregated. The histograms show the size distribution between 6 and 24 nm, and the average particle size was estimated to be in the range of 12–20 nm, respectively. The SAED patterns exhibited six well-divided bright rings. These rings are the counterpart of the hkl values of (220), (311), (400), (422), (511), and (440) planes, in agreement with the XRD patterns of Mn–Zn spinel ferrite.
Figure 2.

SEM micrographs of MZErYF (x ≤ 0.10) SNFs.
Figure 3.
EDX spectra of MZErYF (x ≤ 0.10) SNFs.
Figure 4.
TEM images, SAED patterns, and particle size histograms (x ≤ 0.10) of SNFs.
2.3. Magnetic Properties
2.3.1. Mossbauer Study
The room-temperature (RT) Mossbauer spectra of MZErYF (x ≤ 0.10) SNFs are depicted in Figure 5, which were fitted to obtain various parameters included in Table 3. The variation of some Mössbauer parameters by x is given in Figure 6. The spectra of all samples were fitted using two doublets. The doublet, which has a smaller isomer shift value, is due to Fe3+ ions in the Td site, while the others are due to Fe3+ ions in the Oh site.
Figure 5.
RT Mossbauer spectra of MZErYF (x ≤ 0.10) SNFs.
Table 3. Mossbauer Parameters of MZErYF (x ≤ 0.10) SNFsa.
| x | assignment of sites | IS (±0.001) (mm/s) | QS (±0.002) (mm/s) | Γ (±0.01) (mm/s) | RA (%) |
|---|---|---|---|---|---|
| 0.00 | Db-A:Fe3+ | 0.340 | 0.754 | 0.464 | 59.529 |
| Db-B:Fe3+ | 0.347 | 0.416 | 0.361 | 40.471 | |
| 0.02 | Db-A:Fe3+ | 0.318 | 0.726 | 0.778 | 60.375 |
| Db-B:Fe3+ | 0.349 | 0.520 | 0.373 | 39.625 | |
| 0.04 | Db-A:Fe3+ | 0.328 | 0.681 | 0.705 | 77.478 |
| Db-B:Fe3+ | 0.348 | 0.487 | 0.284 | 22.522 | |
| 0.06 | Db-A:Fe3+ | 0.329 | 0.687 | 0.592 | 79.290 |
| Db-B:Fe3+ | 0.346 | 0.452 | 0.268 | 20.710 | |
| 0.08 | Db-A:Fe3+ | 0.331 | 0.688 | 0.595 | 81.619 |
| Db-B:Fe3+ | 0.342 | 0.455 | 0.268 | 18.381 | |
| 0.10 | Db-A:Fe3+ | 0.331 | 0.822 | 0.467 | 51.502 |
| Db-B:Fe3+ | 0.336 | 0.464 | 0.323 | 48.498 |
IS, isomer shift; QS, quadrupole splitting; Γ, line width; and RA, relative area.
Figure 6.

Variations in (a) isomer shift, (b) quadrupole splitting, and (c) relative area with Er–Y substitution in MZErYF (x ≤ 0.10) SNFs.
The doublets in the structure show that the samples exhibit a superparamagnetic (SPM) nature.28 The isomer shift is caused by the electron interaction among the nucleus and the density of s electron. The isomer shift (IS) of the Oh site is more than that of the Td site as a result of a larger bond length between Fe3+ and O in cubic spinel ferrites as compared with tetrahedral sides. According to the reported data, the IS of Fe2+ varies between 0.6 and 1.7 mm/s, while that of Fe3+ lies in the range of 0.1–0.5 mm/s at RT.29,30 Our results show that the IS values belong to the Fe3+ charge state. The variation of some Mössbauer parameters by x is given in Figure 6, which shows that the IS value of the Td site increases while that of the Oh site decreases with substitution. The increase of the IS value illustrates the decrease of the s-electron density at the iron nucleus or vice versa. Therefore, the s-electron density of the iron nucleus at the Td site increases, while that of the Oh site decreases. The isomer shift is also affected by the site volume.31 The information on the symmetry of the crystal lattice and its local distortions can be gathered from the value of QS. Chemical disorder in the crystal structure is provoked by nonzero quadrupole splitting (QS). The variations of QS reflect distortion of symmetry at Td and Oh sites. According to Table 3, the B/A ratio decreases with increasing Er+3 and Y+3 content up to x = 0.08 and then increases at x = 0.1. These results are in agreement with the XRD results. These results also show that the Er+3 and Y+3 ions occupy the Oh site.
2.3.2. Magnetization Investigation
Magnetic investigations were performed for MZErYF (x ≤ 0.10) SNFs using a vibrating sample magnetometer (VSM) device by recording the field (M–H)- and the temperature (M–T)-dependent specific magnetizations. M–H data were alsoobtained at 300 and 10 K to collect information about the temperature-dependent dominating magnetic phases. Figure 7 presents the M–H curves recorded at 300 K and under an externally acting DC field of (a) ±70 kOe and (b) enlarged views for the range of ±5 kOe. The typical S-shape spectra, which have a negligible amount of coercivities (HC) or remnant magnetizations (Mr), are not observed even in enlarged views. This case identifies the presence of a SPM phase for all NPs at 300 K. The single-domain nature is the characteristic feature of nanoparticles that exhibit the SPM phase. All magnetic moments align in a preferential direction determined by the leading type of anisotropy in the structure. That is, a single-domain structured nanoparticle behaves like a single giant magnetic moment having a net spontaneous magnetization MS. The existence of the SPM phase not only necessitates a critically small dimension of nanoparticles (or grains) but also requires the absence of an adequate magnitude of exchange interactions needed for internal spontaneous magnetization. Further, the absence of interparticle interactions, which stimulate different magnetic phases, is another obligation for a conventional SPM phase. The critical size for Mn0.5Zn0.5Fe2O4 SNFs to exhibit a SPM nature is reported to be ∼25 nm in the literature.32,33 Some recorded TEM images reveal the dimension of fabricated SNFs to be smaller than this maximum limit.
Figure 7.
(a) M–H curves of MZErYF (x ≤ 0.10) SNFs recorded at RT and (b) enlarged view of the magnetization curves.
Although a 70 kOe applied external field is a rather strong field, spectra do not depict a horizontal alignment assigning the obtained saturation magnetizations (MS) for NPs. This is why MS magnitudes were estimated from linear fits to M vs 1/H2 plots, as shown in Figure 8. Undoped Mn0.5Zn0.5Fe2O4 SNFs among ultrasonically fabricated samples have an MS,max value of 34.84 emu/g. The co-doping process with Er3+ and Y3+ ions decreases the saturation magnetizations, exhibiting almost an indirect proportionality with the increasing ratio of two ions. Hence, it is estimated that Mn0.5Zn0.5Er0.10Y0.10Fe1.80O4 SNFs that have a maximum doping content have a MS,min value of 19.35 emu/g. The magnetic moment per chemical formula (nB) was calculated in units of Bohr magneton using the molecular weight (MW)- and MS-dependent equation as given below22,34,35
| 5 |
Figure 8.
M vs 1/H2 plots for MZErYF (x ≤ 0.10) SNFs at RT.
The maximum and minimum magnetic moments of 1.47 and 0.87 μB belong to Mn0.5Zn0.5Fe2O4 NPs and Mn0.5Zn0.5Er0.10Y0.10Fe1.80O4 SNFs, respectively. Due to co-doped ions, the changing content of cations at both tetrahedral and octahedral sites plays a significant role in the resultant magnetic moments. A similar indirect proportionality as observed for the magnetization data is also valid for the experimentally evaluated magnetic moments as a function of the increasing content (x) of co-doped Er3+ and Y3+ ions. In the literature, the experimentally observed magnetic moment values of Zn2+, Mn2+, Fe3+, Er3+, and Y3+ ions are reported to be 0, 4.6, 4.1, 9.5, and 0 μB, respectively.36−38 The increasing content of Er3+ ions that are located at the octahedral B site in the reverse direction with a large experimentally observed nB of 9.5 μB might be the main reason for the decreasing magnetic moment and magnetization of the doped samples. All derived magnetic parameters from the M–H spectra that were obtained at 300 K are presented in Table 4.
Table 4. Magnetic Parameters of MZErYF (x ≤ 0.10) SNFs.
| 300
K |
10
K |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | MW (g/mol) | MS (emu/g) | nB (μB) | MS (emu/g) | nB (μB) | Mr (emu/g) | HC (Oe) | SQR (Mr/MS) | Keff (×104 erg/g) | Ha (Oe) |
| 0.00 | 235.854 | 34.84 | 1.47 | 62.35 | 2.63 | 20.00 | 361 | 0.321 | 3.52 | 1128 |
| 0.02 | 238.744 | 33.86 | 1.45 | 66.14 | 2.83 | 17.20 | 284 | 0.260 | 2.93 | 888 |
| 0.04 | 241.633 | 26.88 | 1.16 | 55.37 | 2.40 | 14.88 | 250 | 0.269 | 2.16 | 781 |
| 0.06 | 244.523 | 24.76 | 1.08 | 57.95 | 2.54 | 13.00 | 409 | 0.224 | 3.72 | 1284 |
| 0.08 | 247.412 | 20.84 | 0.92 | 51.99 | 2.30 | 11.75 | 378 | 0.226 | 3.07 | 1181 |
| 0.10 | 250.302 | 19.35 | 0.87 | 56.88 | 2.55 | 8.63 | 415 | 0.152 | 33.70 | 1288 |
The M–H hysteresis loops that were recorded at 10 K and their enlarged views for the field range of ±4 kOe are presented in Figure 9a,b, respectively. Figure 9a clearly illustrates that Mn0.5Zn0.5Er0.02Y0.02Fe1.96O4 SNFs have the largest magnetization ability among all samples at 10 K. However, Figure 9b reveals that magnetic NPs do not perpetuate their SPM characteristics at 10 K, and, instead, a ferrimagnetic phase as expected from the ferrite NPs is observed. Low-temperature conditions exclude the demagnetizing effect of heat energy on the magnetization process and enable magnetic materials to exhibit their magnetic phases that originate due to moment alignment in the host crystal structure. Undoped Mn0.5Zn0.5Fe2O4 NPs have the largest remnant magnetization (Mr) of 20 emu/g and a remarkable magnitude of coercivity (HC) of 361 Oe. Co-doped samples have a decreasing magnitude of Mr values on increasing the ratio of Er3+ and Y3+ ion contents.
Figure 9.
(a) M–H hysteresis loops of MZErYF (x ≤ 0.10) SNFs recorded at 10 K and (b) enlarged view of these loops.
The coercivity field range has a narrow band with HC,min = 250 Oe (belonging to Mn0.5Zn0.5Er0.04Y0.04Fe1.92O4 SNFs) and HC,max = 415 Oe (belonging to Mn0.5Zn0.5Er0.10Y0.10Fe1.80O4 SNFs). However, one can easily claim that the order of the measured coercivities categorizes all pristine or co-doped samples as a member of magnetically soft spinel ferrites. Although ferrimagnetic phases were observed, none of the samples could reach saturated magnetization even under a 70 kOe acting field. Therefore, the order of MS magnitudes was specified by the intercept of the fitting lines at the magnetization axis as for 1/H2 equal to zero, as depicted in Figure 10. Pristine Mn0.5Zn0.5Fe2O4 NPs have the second largest saturation magnetization of 62.35 emu/g among the 10 K data despite being first among the 300 K saturation data. This determination stimulates the interpretation of the ratio of estimated saturation magnetizations (MS,10 K/MS,300 K) for all samples by assuming that cationic distributions or lattice defects due to co-doped ions are stable at both 300 and 10 K. Disordered surface spin layers are other mainly observed defects that affect negatively the total magnetic moment and magnetization of Mn0.5Zn0.5Fe2O4 NPs. The magnitude of the ratio for pristine ferrite is 1.79. On the other hand, the determined ratios are 1.95, 2.06, 2.34, 2.50, and 2.94 corresponding to co-doped ion contents of x = 0.02, 0.04, 0.06, 0.08, and 0.10, respectively. The steadily increasing ratios might be attributed to thicker disordered surface layers with increasing co-doped ion content. One can expect better ordering of these disordered surface spins at 10 K when the demagnetizing effect of heat is excluded at a high ratio. In the literature, strengthening of superexchange interactions is also reported as a reason for increasing magnetization parameters.22 Another kind of division, that is, Mr/MS, is described as the dimensionless squareness ratio (SQR), which gives information particularly about the domain feature of the host magnetic material. SQRs start from 0.321 and steadily decrease to 0.152. This range of SQRs is attributed to the multidomain nature of NPs according to the Stoner–Wohlfarth theory.22,39
Figure 10.
M vs 1/H2 plots for MZErYF (x ≤ 0.10) SNFs.
The internal magnetocrystalline anisotropy field, Ha, is directly responsible for the detection of the coercivity and the degree of magnetic hardness of the material. Once the effective magnetocrystalline anisotropy constant (Keff) is specified, then the order of the anisotropy field for the fabricated nanoparticle samples can be easily extracted. The following equation relates three magnetic parameters: Keff, measured HC values, and estimated saturation magnetizations40
| 6 |
Therefore, we determined Keff values for all samples by substituting the already determined 10 K magnetic data in eq 2. Keff values are in the range of 2.16–3.72 × 104 erg/g, except for the Mn0.5Zn0.5Er0.10Y0.10Fe1.80O4 SNFs. Since this sample has the largest coercivity, the determined magnitude of Keff concludes the largest magnitude to be 3.37 × 105 erg/g among all NPs too. The internal anisotropy field can be calculated from the following equation:
| 7 |
Among calculated internal magnetocrystalline anisotropy fields, Ha,min = 781 Oe belongs to Mn0.5Zn0.5Er0.04Y0.04Fe1.92O4 SNFs and Ha,max = 1288 Oe belongs to Mn0.5Zn0.5Er0.10Y0.10Fe1.80O4 SNFs due to their minimum and maximum coercivity magnitudes as expected. Other samples have internal anisotropies between these two limiting values. Hence, the presence of disordered spins on the surface of nanoparticles and an internal magnetocrystalline anisotropy field (causing coercivity and magnetic hardness) are the main perpetrators in the magnetic host that prevent the observation of saturation magnetization even under high acting fields and at low-temperature conditions. All magnetic parameters derived from 10 K hysteresis loops are reported in Table 4 with 300 K magnetization data.
Figure 11 presents the zero-field cooling (ZFC) and field cooling (FC) magnetization spectra of some previously determined MZErYF (x ≤ 0.10) SNFs. For ZFC measurements, samples were first cooled in the zero field until 10 K, and then magnetization data were recorded on increasing the temperature up to 325 K by simultaneously exposing them to a static dc field of 100 Oe. For FC measurements, the same selected samples were cooled in the same field until 10 K and then the FC data were recorded by increasing the temperature up to 325 K without removing the acting static field. The maxima observed in the ZFC spectra provide the magnitude of blocking temperature (TB) for magnetic NPs. Ferrimagnetic phases are observed below TB, and above TB, NPs exhibit a SPM phase consistent with the Cure–Weiss law.5 In the SPM phase, the coercivity and remnant magnetizations disappear.
Figure 11.
Temperature dependence of ZFC and FC magnetizations for MZErYF (x = 0.00, 0.02, 0.06, and 0.10) SNFs.
The corresponding blocking temperatures for our NPs are 68.6, 66.1, 57.5, and 53.4 K. We observe a slight red shift in TB values with increasing co-doped ion concentrations. When the temperature decreases below TB, the antiferromagnetic interactions become stronger in octahedral B sites of spinel ferrite. This case concludes collective spin freezing and an intensive decrease in the magnitude of magnetization. On the other hand, the FC magnetization curve is expected to keep its increasing trend and maximum magnetization should be recorded at a minimum cooling temperature. However, our recorded FC magnetization curves exhibit some decreases below their peak temperature positions. This type of behavior is attributed to the presence of spin-glass-like phases in temperature ranges below TB.41 According to XRD and TEM investigations, the particle/crystallite sizes increase with increasing Er3+ and Y3+ ion ratio. However, a reverse correlation between the increasing particle/crystallite size and the decreasing blocking temperature is observed.
3. Conclusions
The sonochemical technique was applied for fabrication of MZErYF (x ≤ 0.10) SNFs. The pure structure of MZErYF SNFs was confirmed via the analyses of XRD patterns. The crystal size was found to be within the range of 7–12 nm. It was found that the cell parameters increased with increased dopant ions due to the enlargement the lattice. The cubic morphology and chemical compositions were revealed using SEM, TEM, EDX, and elemental mappings. According to Mössbauer results, all of the samples exhibit superparamagnetism at RT. The s-electron density of iron nucleus at A and B sites is affected by substitution. The doped ions occupy the octahedral B site. According to M–H investigations, MZErYF (x ≤ 0.10) SNFs exhibit a single-domain nature at 300 K, and in the ferrimagnetic phase, they exhibit a multidomain nature at 10 K. The co-doping process with Er3+ and Y3+ ions tunes magnetic data remarkably and mainly results in reduced Mr and MS values. An indirect proportionality is observed for experimentally detected magnetization parameters as a function of the increasing content (x) of co-doped Er3+ and Y3+ ions. Mn0.5Zn0.5Er0.02Y0.02Fe1.96O4 SNFs have the largest magnetization among all samples according to 10 K M–H investigations. However, all samples are members of magnetically soft ferrite materials, although Mn0.5Zn0.5Er0.10Y0.10Fe1.80O4 SNFs are the magnetically hardest among them. The fraction between 300 and 10 K saturation magnetizations indictaes the presence of disordered surface spin layers that become thicker with increasing ion concentration. The characteristics of ZFC and FC spectra revealed the presence of collective spin freezing and spin-glass-like behavior below the blocking temperature in M–T investigations. The blocking temperature has indirect proportionalities with increasing co-doped ion content and particle/crystallite size.
4. Experimental Section
4.1. Instrumentation
The structure of the products was analyzed using a Benchtop Rigaku Miniflex X-ray diffractometer. Mossbauer spectra were recorded using a spectrometer with a constant acceleration mode of a 10mCi 57Co (Rh matrix) radiation source. The nanoparticles’ morphology was obtained using FEI Titan ST SEM and TEM linked with EDX spectroscopy for elemental analysis. The SAED (selected area electron diffraction) patterns were obtained to study the crystalline structure. The magnetic measurements of samples were performed using a Quantum Design North America PPMS DynaCool-9 system coupled with a VSM.
4.2. Nanomaterial Synthesis
A series of MZErYF (x ≤ 0.10) SNFs were synthesized using the sonochemical approach. All chemicals were obtained from commercial sources (Merck and US Research Nanomaterials, Inc.). For the typical synthesis, iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O), manganese(II) nitrate hexahydrate (Mn(NO3)2·6H2O), zinc nitrate hexahydrate (Zn(NO3)2·6H2O), erbium nitrate pentahydrate (Er(NO3)3·5H2O), and yttrium nitrate hexahydrate (Y(NO3)3·6H2O) were used as the source material for Fe, Mn, Zn, Er, and Y, respectively. Stoichiometric amounts of metal nitrates Mn(NO3)2·6H2O, Fe(NO3)3·9H2O, Zn(NO3)2·6H2O, Er(NO3)3·5H2O, and Y(NO3)3·6H2O were thawed in 50 mL of deionized (DI) H2O, and the pH was set at 11 using a 2 M NaOH solution; then, they were irradiated using a UZ SONOPULS HD 2070 ultrasonic homogenizer at 20 kHz and 70 W for 40 min (Table 5). At the end of ultrasonication, the reaction temperature was measured to be 90 °C owing to the high number of collisions between the reactants. The obtained product was washed several times with DI water. The magnetic solid product was separated from the liquid using a simple external magnet and was then dried at 60 °C for 12 h.21,22 No calcination process was applied to the products. This was the main advantage of this synthesis procedure.
Table 5. Number of Moles of Each Element Used during the Synthesis of Each Product.
| x | Mn | Zn | Er | Y | Fe |
|---|---|---|---|---|---|
| 0.00 | 0.5 | 0.5 | 2 | ||
| 0.02 | 0.5 | 0.5 | 0.02 | 0.02 | 1.96 |
| 0.04 | 0.5 | 0.5 | 0.04 | 0.04 | 1.92 |
| 0.06 | 0.5 | 0.5 | 0.06 | 0.06 | 1.88 |
| 0.08 | 0.5 | 0.5 | 0.08 | 0.08 | 1.84 |
| 0.1 | 0.5 | 0.5 | 0.1 | 0.1 | 1.80 |
Acknowledgments
The authors are thankful to the Institute for Research and Medical Consultations (IMRC) of Imam Abdulrahman BinFaisal University (IAU, Saudi Arabia) for providing lab facilities. This work was financially supported by the Deanship for Scientific Research (project application no. 2020-164-IRMC) of Imam Abdulrahman Bin Faisal University (IAU, Saudi Arabia).
The authors declare no competing financial interest.
References
- Karimunnesa S.; Ullah A. K. M. A.; Hasan M. R.; Shanta F. S.; Islam R.; Khan M. N. I. Effect of holmium substitution on the structural, magnetic and transport properties of CoFe2-xHoxO4 ferrites. J. Magn. Magn. Mater. 2018, 457, 57–63. 10.1016/j.jmmm.2018.02.077. [DOI] [Google Scholar]
- Snelling E. C.Soft Ferrites: Properties and Applications, 1st ed.; Iliffe: London, 1969; pp 1–385. [Google Scholar]
- Smit J.Magnetic Properties of Materials; McGraw-Hill: New York, 1971; pp 1–448. [Google Scholar]
- Thakur P.; Chahar D.; Taneja S.; Bhalla N.; Thakur A. A review on MnZn ferrites: Synthesis, characterization and applications. Ceram. Int. 2020, 46, 15740–15763. 10.1016/j.ceramint.2020.03.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almessiere M. A.; Slimani Y.; Rehman S.; Khan F. A.; Güngüneş Ç. D.; Güner S.; Shirsath S. E.; Baykal A. Magnetic properties, anticancer and antibacterial effectiveness of sonochemically produced Ce3+/Dy3+ co-activated Mn-Zn nanospinel ferrites. Arabian J. Chem. 2020, 13, 7403–7417. 10.1016/j.arabjc.2020.08.017. [DOI] [Google Scholar]
- Angadi V. J.; Choudhury L.; Sadhana K.; Lin Liu H.; Sandhya R.; Matteppanavar S.; Rudraswamy B.; Pattar V.; Anavekar R. V.; Praveena K. Structural, electrical and magnetic properties of Sc3+ doped Mn-Zn ferrite nanoparticles. J. Magn. Magn. Mater. 2017, 424, 1–11. 10.1016/j.jmmm.2016.10.050. [DOI] [Google Scholar]
- Praveena K.; Sadhana K.; Bharadwaj S.; Murthy S. R. Development of nanocrystalline Mn–Zn ferrites for high frequency transformer applications. J. Magn. Magn. Mater. 2009, 321, 2433–2437. 10.1016/j.jmmm.2009.02.138. [DOI] [Google Scholar]
- Praveena K.; Sadhana K.; Murthy S. R. Elastic behaviour of microwave hydrothermally synthesized nanocrystalline Mn1–xZnx ferrites. Mater. Res. Bull. 2012, 47, 1096–1103. 10.1016/j.materresbull.2011.11.054. [DOI] [Google Scholar]
- Praveena K.; Murthty S. R. Magneto acoustical emission in nanocrystalline Mn–Zn ferrites. Mater. Res. Bull. 2013, 48, 4826–4833. 10.1016/j.materresbull.2013.08.042. [DOI] [Google Scholar]
- Praveena K.; Sadhana K.; Bharadwaj S.; Murthy S. R. Fabrication of dc–dc converter using nanocrystalline Mn–Zn ferrites. Mater. Res. Innovations 2010, 14, 102–106. 10.1179/143307510X12599329343448. [DOI] [Google Scholar]
- Praveena K.; Sadhana K.; Bharadwaj S.; Murthy S. R. Development of nanocrystalline Mn–Zn ferrites for forward type DC–DC converter for switching mode power supplies. Mater. Res. Innovations 2010, 14, 56–61. 10.1179/143307510X12599329343727. [DOI] [Google Scholar]
- Praveena K.; Sadhana K.; Virk H. S. Structural and magnetic properties of Mn-Zn ferrites synthesized by microwave-hydrothermal process. Solid State Phenom. 2015, 232, 45–64. 10.4028/www.scientific.net/SSP.232.45. [DOI] [Google Scholar]
- Islam R.; Hakim M. A.; Rahman M. O.; Narayan Das H.; Mamun M. A. Study of the structural, magnetic and electrical properties of Gd-substituted Mn–Zn mixed ferrites. J. Alloys Compd. 2013, 559, 174–180. 10.1016/j.jallcom.2012.12.080. [DOI] [Google Scholar]
- Abdellatif M. H.; Azab A. A.; Salerno M. Effect of rare earth doping on the vibrational spectra of spinel Mn-Cr ferrite. Mater. Res. Bull. 2018, 97, 260–264. 10.1016/j.materresbull.2017.09.012. [DOI] [Google Scholar]
- Zhao L.; Yang H.; Yu L.; Cui Y.; Zhao X.; Feng S. Magnetic properties of resubstituted Ni–Mn ferrite nanocrystallites. J. Mater. Sci. 2007, 42, 686–691. 10.1007/s10853-006-0273-7. [DOI] [Google Scholar]
- Naik P. P.; Hasolkar S. S.; Kothawale M. M.; Keluskar S. H. P. Altering saturation magnetization of manganese zinc ferrite nanoparticles by doping with rare earth Nd+3 ions. Phys. B 2020, 584, 412111 10.1016/j.physb.2020.412111. [DOI] [Google Scholar]
- Naik P. P.; Tangsali R. B. Enduring effect of rare earth (Nd3+) doping and γ-radiation on electrical properties of nanoparticle manganese zinc ferrite. J. Alloys Compd. 2017, 723, 266–275. 10.1016/j.jallcom.2017.06.207. [DOI] [Google Scholar]
- Angadi V. J.; Manjunatha K.; Praveena K.; Pattar V. K.; Fernandes B. J.; Manjunatha S. O.; Husain J.; Angadi S. V.; Horakeri L. D.; Ramesh K. P. Magnetic properties of larger ionic radii samarium and gadalonium doped manganese zinc ferrite nanoparticles prepared by solution combustion method. J. Magn. Magn. Mater. 2021, 529, 167889 10.1016/j.jmmm.2021.167899. [DOI] [Google Scholar]
- Jadhav S. V.; Shewale P. S.; Shin B. C.; Patil M. P.; Kim G. D.; Rokade A. A.; Park S. S.; Bohara R. A.; Yu Y. S. Study of structural and magnetic properties and heat induction of gadolinium-substituted manganese zinc ferrite nanoparticles for in vitro magnetic fluid hyperthermia. J. Colloid Interface Sci. 2019, 541, 192–203. 10.1016/j.jcis.2019.01.063. [DOI] [PubMed] [Google Scholar]
- Almessiere M. A.; Slimani Y.; Rehman S.; Khan F. A.; Gökçe Polat E.; Sadaqat A.; Shirsath S. E.; Baykal A. Synthesis of Dy-Y co-substituted manganese-zinc spinel nanoferrites induced anti-bacterial and anti-cancer activities: Comparison between sonochemical and sol-gel auto-combustion methods. Mater. Sci. Eng., C 2020, 116, 111186 10.1016/j.msec.2020.111186. [DOI] [PubMed] [Google Scholar]
- Almessiere M. A.; Slimani Y.; Shirsath S. E.; Wudil Y. S.; Baykal A.; Ercan I. Customized magnetic properties of (Mn0.5Zn0.5)[EuxNdxFe2-2x]O4 nanospinel ferrites synthesized via ultrasonic irradiation approach. Results Phys. 2020, 19, 103350 10.1016/j.rinp.2020.103350. [DOI] [Google Scholar]
- Almessiere M. A.; Slimani Y.; Demir Korkmaz A.; Güner S.; Baykal A.; Shirsath S. E.; Ercan I.; Kögerler P. Sonochemical synthesis of Dy3+ substituted Mn0.5Zn0.5Fe2–xO4 nanoparticles: Structural, magnetic and optical characterizations. Ultrason. Sonochem. 2020, 61, 1048362 10.1016/j.ultsonch.2019.104836. [DOI] [PubMed] [Google Scholar]
- Almessiere M. A.; Trukhanov A. V.; Khan F. A.; Slimani Y.; Tashkandi N.; Turchenko V. A.; Zubar T. I.; Tishkevich D. I.; Trukhanov S. V.; Panina L. V.; Baykal A. Correlation between microstructure parameters and anti-cancer activity of the [Mn0.5Zn0.5](EuxNdxFe2-2x)O4 nanoferrites produced by modified sol-gel and ultrasonic methods. Ceram. Int. 2020, 46, 7346–7354. 10.1016/j.ceramint.2019.11.230. [DOI] [Google Scholar]
- Rehman S.; Almessiere M. A.; Khan F. A.; Demir Korkmaz A.; Tashkandi N.; Slimani Y.; Baykal A. Synthesis and biological characterization of Mn0.5Zn0.5EuxDyxFe1.8-2xO4 nanoparticles by sonochemical approach. Mater. Sci. Eng., C 2020, 109, 110534 10.1016/j.msec.2019.110534. [DOI] [PubMed] [Google Scholar]
- Weil L.; Bertaut E. F.; Bochirol L. Magnetic properties and structure of the quadratic phase of copper ferrite. J. Phys. Radium 1950, 11, 208 10.1051/jphysrad:01950001105020800. [DOI] [Google Scholar]
- Cullity B. D.; Stock S. R.. Elements of X-ray Diffraction, 3rd ed.; Pearson: USA, 1956; pp 696. [Google Scholar]
- Klein L.; Aparicio M.; Jitianu A.. Handbook of Sol-Gel Science and Technology, 2nd ed.; Springer International Publishing: 2018; pp XXXIX, 3789. [Google Scholar]
- Ata-Allah S. S.; Hashhash A. Jahn–Teller effect and superparamagnetism in Zn substituted copper-gallate ferrite. J. Magn. Magn. Mater. 2006, 307, 191–197. 10.1016/j.jmmm.2006.04.002. [DOI] [Google Scholar]
- Kumar H.; Srivastava R. C.; Singh J. P.; Negi P.; Agrawal H. M.; Das D.; Chae K. H. Structural and magnetic study of dysprosium substituted cobalt ferrite nanoparticles. J. Magn. Magn. Mater. 2016, 401, 6–21. 10.1016/j.jmmm.2015.09.077. [DOI] [Google Scholar]
- Naik P. P.; Tangsali R. B.; Meena S. S.; Bhatt P.; Sonaye B.; Sugur S. Gamma radiation roused lattice contraction effects investigated by Mossbauer spectroscopy in nanoparticle Mn–Zn ferrite. Radiat. Phys. Chem. 2014, 102, 147–152. 10.1016/j.radphyschem.2014.04.038. [DOI] [Google Scholar]
- Drickamer H. G.; Frank C. W.. Electronic Transitions and the High Pressure Chemistry and Physics of Solids, 1st ed.; Chapman and Hall: London, 1973; pp ix–185. [Google Scholar]
- Zhang C. F.; Zhong X. C.; Yu H. Y.; Liu Z. W.; Zeng D. C. Effects of cobalt doping on the microstructure and magnetic properties of Mn–Zn ferrites prepared by the co-precipitation method. Phys. B 2009, 404, 2327–2331. 10.1016/j.physb.2008.12.044. [DOI] [Google Scholar]
- Joseyphus R. J.; Narayanasamy A.; Shinoda K.; Jeyadevan B.; Tohji K. Synthesis and magnetic properties of the size-controlled Mn–Zn ferrite nanoparticles by oxidation method. J. Phys. Chem. Solids 2006, 67, 1510–1517. 10.1016/j.jpcs.2005.11.015. [DOI] [Google Scholar]
- Baykal A.; Güner S.; Demir A.; Esir S.; Genç F. Effect of Zinc substitution on magneto-optical properties of Mn1-xZnxFe2O4/SiO2 nanocomposites. Ceram. Int. 2014, 40, 13401–13408. 10.1016/j.ceramint.2014.05.059. [DOI] [Google Scholar]
- Baykal A.; Güner S.; Demir A. Synthesis and magneto-optical properties of triethylene glycol stabilized Mn1-xZnxFe2O4 nanoparticles. J. Alloys Compd. 2015, 619, 5–11. 10.1016/j.jallcom.2014.08.237. [DOI] [Google Scholar]
- Cullity B. D.; Graham C. D.. Introduction to Magnetic Materials, 2nd ed.; Wiley-IEEE Press: 2009; pp 568. [Google Scholar]
- Dalal M.A Textbook of Inorganic Chemistry, 1st ed.; Dalal Institute: India, 2017; pp 11–471. [Google Scholar]
- Alves T. E. P.; Pessoni H. V. S.; Franco A. Jr. The effect of Y3+ substitution on the structural, optical band-gap, and magnetic properties of cobalt ferrite nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 16395–16405. 10.1039/C7CP02167D. [DOI] [PubMed] [Google Scholar]
- Stoner E. C.; Wohlfarth E. P. A mechanism of magnetic hysteresis in heterogeneous alloys. Philos. Trans. R. Soc. A 1948, 240, 599–642. 10.1098/rsta.1948.0007. [DOI] [Google Scholar]
- Slimani Y.; Almessiere M. A.; Güner S.; Kurtan U.; Shirsath S. E.; Baykal A.; Ercan I. Magnetic and microstructural features of Dy3+ substituted NiFe2O4 nanoparticles derived by sol–gel approach. J. Sol-Gel Sci. Technol. 2020, 95, 202–210. 10.1007/s10971-020-05292-1. [DOI] [Google Scholar]
- Khurshid H.; Kelley P. L.; Iglesias O.; Alonso J.; Phan M. H.; Sun C. J.; Saboungi M. L.; Srikanth H. Spin-glass-like freezing of inner and outer surface layers in hollow γ-Fe2O3 nanoparticles. Sci. Rep. 2015, 5, 15054 10.1038/srep15054. [DOI] [PMC free article] [PubMed] [Google Scholar]











