Abstract
Acetone and n-heptane are common solvents in the pharmaceutical industry and they have been found in wastewater. Under atmospheric conditions, the mixture of these compounds creates a minimum-boiling azeotrope. The extractive distillation process with a high boiling solvent is commonly utilized to separate the azeotropes in the industry to minimize waste, reuse resources, achieve clean production, and preserve the environment. In this work, extractive distillation was applied to separate the binary azeotropic system of acetone and n-heptane in wastewater using butyl propionate as a solvent. The characteristics of the process are designed and simulated via Aspen Plus. The simulation results showed that to get a distillate containing at least 99.5 mass% acetone, a solvent-to-feed ratio of 1.4, a reflux ratio of 1.5, a number of stages of 30, a feed stage of 26, a solvent stage of 10, and a solvent temperature of 298.15 K were required. The optimum operating parameters of the process were also obtained using the NLP optimization method, with the minimum total annual cost as the objective function. While the process was operating in optimal mode, CO2 emissions were calculated to be 0.0780 kg CO2/kg feed.
1. Introduction
In recent years, reducing or removing wastes from chemical processes has become a significant concern.1,2 The solvents used to purify intermediates and products are the most considerable waste produced by the industry’s organic compound synthesis.3
Acetone (ACT) and n-heptane (HEP) are essential chemicals used in the pharmaceutical industries as safe solvents for organic synthesis.4,5 These two compounds can also be present in various industrial wastewaters.6−8 If wastewater is discharged directly, it would boost the total annual cost and pollute the environment. As a result, separating the mixture of these two substances is crucial and attractive. However, conventional separation methods for recovering ACT and HEP from wastewater with high purity are ineffective because these substances can create a minimum-boiling azeotrope.
Among the various separation methods of azeotropes,9−11 azeotropic distillation (AD),12−14 extractive distillation (ED),15−19 and pressure-swing distillation (PSD)20−22 are the most common methods. Wang et al.23 investigated the influence of solvent flow rates on controlling extractive distillation for separation of a minimum-boiling azeotrope mixture (HEP/isobutanol). Yuan et al.24 utilized extractive distillation to investigate the separation of four binary azeotrope mixtures (ACT/tetrahydrofuran (THF), n-C6/THF, n-C6/ethyl acetate (EtOAc), and EtOAc/ethanol). Simulating the separation process was done with Aspen Plus. According to their findings, the suggested methodology could become a practical and widely used method for selecting solvents to develop new processes that save money and time. Zhu et al.5 surveyed the effect of four different ionic liquids as extractant agents for the separation of the HEP/ACT azeotrope mixture. The results revealed that [BMIM][OTF] extracted ACT from HEP more efficiently than [HMIM][OTF], and [OTF]− had a higher extraction performance than [PF6]− and [NTf2]−. Zhang et al.25 used extractive distillation to separate an ACT/HEP binary azeotropic mixture experimentally. They employed 1-chloro-butane (1-CB) and butyl propanoate (BP) as intermediate and heavy boiling entrainer agents. They proved that the VLE values are thermodynamically consistent according to the van Ness26 and Herington tests,27 and the excess Gibbs energy was determined as well. The UNIQUAC, NRTL, and Wilson equations were also exploited to match the determined VLE data.
Despite extensive studies in the explained research field, to our knowledge, the separation of ACT and HEP has rarely been reported so far, and it has not been simulated yet. Because of the vast number of parameters involved, laboratory experiments are time-consuming and costly. It would be beneficial to use existing simulation tools to predict experimental data. Computer simulations for process design are a well-established best practice in the chemical and petrochemical industries for process development and optimization. In the current research, for the first time, the continuous extractive distillation to separate the ACT/HEP azeotrope mixture was simulated using ASPEN Plus V10. This work aims to investigate and establish industrial operating conditions and column configuration for the extractive distillation of ACT/HEP with butyl propionate (BP) as a solvent. Finally, the NLP optimization approach with the minimum total annual cost was applied as the objective function to achieve the process’s optimum operating parameters.
2. Simulation Procedure and Methods
In this study, the UNIQUAC thermodynamic model was utilized as an appropriate fluid package to simulate the ED process. The UNIQUAC model predicted the azeotropic mixture’s pseudo-binary vapor-liquid equilibrium data. The mixtures’ VLE on a solvent-free basis is shown in Figure 1 to observe the azeotrope point and compared with experimental data reported by Maripuri and Ratcliff.28 This figure demonstrates that the UNIQUAC model is appropriate and comparable to that of the experimental data.
Figure 1.
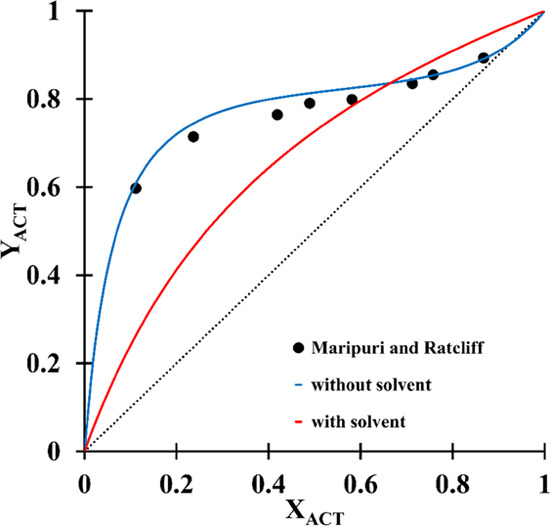
Pseudo-binary Y–X diagram for the ACT/HEP system with and without a solvent, compared to the experimental data reported by Maripuri and Ratcliff.28 Reproduced from ref (28). Copyright 1972 American Chemical Society.
Adding a solvent capable of significantly altering the mixture’s relative volatility is essential in extractive distillation (ED). In ED design, choosing one or more candidate solvents and column configurations is the first step. BP from all possible solvents was selected to separate the azeotrope mixture under study based on the solvent screening guidelines.29,30 This solvent has been considered a green and environmentally friendly solvent because of its low vapor pressure, good mixing ability, high electrical resistance, and tolerable odor.31 Residue curve maps are extremely useful in the design and analysis of separation processes, mainly distillation processes. The residue curve map with a univolatility line has the optimal configuration for extractive distillation, as shown in Figure 2, indicating that BP is a viable solvent for the ACT/HEP separation. The point where the univolatility curve intersects with the binary side ACT/BP can be utilized to assess the solvent’s capacity.16 Intersection close to ACT indicates low solvent usage, higher separation efficiency, and lower energy costs.32Table 1 shows the physical characteristics of ACT, HEP, and BP.
Figure 2.
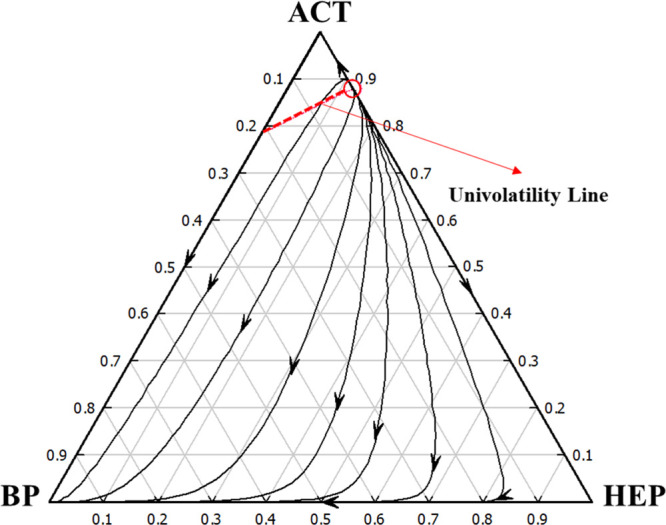
Residue curve map with a univolatility line for the ACT/HEP/BP system at 101.325 kPa, calculated using the UNIQUAC thermodynamic model.
Table 1. Physical Properties of the System’s Compounds (ACT, HEP, and BP).
| component | molecular weight (kg/kmol) | boiling point (K) | liquid density (kg/m3) |
|---|---|---|---|
| acetone (ACT) | 58.08 | 329.2 | 790.0 |
| n-heptane (HEP) | 100.2 | 371.6 | 686.8 |
| butyl propionate (BP) | 130.2 | 419.8 | 880.7 |
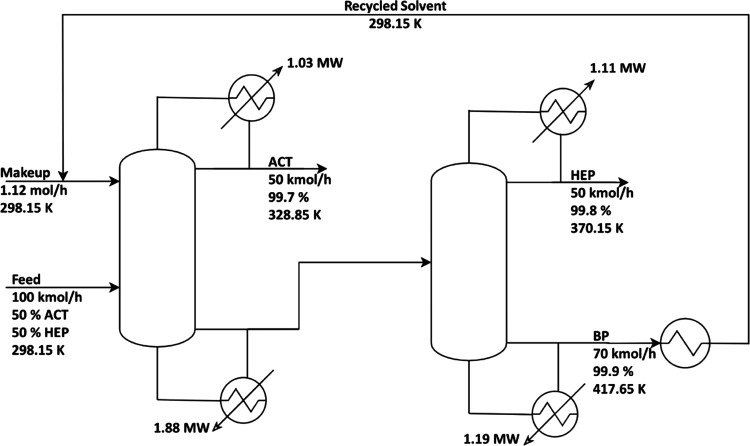
The binary interaction parameters of the UNIQUAC model for ACT, HEP, with BP are listed in Table 2. Detailed information on these parameters can be found in the report of Zhang et al.25 Our study specified the temperature, pressure, flow rate, and composition of binary feed. The reported literature is used to determine the setpoints for the fresh feed flow rate and stipulated composition.11,15 An equimolar ACT/HEP binary mixture is fed to the process at a feed flow rate of 100 kmol/h (∼7914.2 kg/h) at 298.15 K. The azeotropic compositions under atmospheric conditions are 87.81 mass% ACT and 12.19 mass% HEP. Figure 3 depicts the ED process flow diagram in which the solvent enters the column through the top trays. The second column was used to recover the solvent by removing heptane from BP. After cooling, the lean solvent is recycled back into the first column. To maintain a constant solvent-to-feed ratio, a solvent make-up stream is needed. This was accomplished using the calculator block.
Table 2. Binary Interaction Parameters of the UNIQUAC Model for ACT and HEP with BP.
| aij | aji | bij/K | bji/K | |
|---|---|---|---|---|
| ACT–BP | 12.5547 | –7.9191 | –1645.91 | –2390.13 |
| HEP–BP | 23.4640 | –7.0087 | –2713.31 | 2498.76 |
Reprinted from ref (25). Copyright 2021, with permission from Elsevier.
Figure 3.
Process flow diagram of the extractive distillation process for ACT/HEP separation.
3. Result and Discussion
3.1. Sensitivity Analysis
A sensitivity analysis was performed to obtain the ED column. The number of stages, feed and solvent stages, reflux ratio, solvent-to-feed ratio, and solvent temperature were the parameters studied as follows.
Figure 4 depicts the variations in the mass fraction of ACT in the distilled and bottom products of the ED column to the reflux ratio. According to the ASTM purity standard, the acceptable purity of acetone is ∼99.5 mass%.33 As can be viewed, the highest and lowest acetone mass fractions in the top and bottom streams, respectively, were found at refluxes of 1–2. At a reflux ratio of 1.5, the maximum XACT was achieved. The acetone mass fraction in the distillate decreases at reflux ratios greater than 1.5. This is due to the fact that the liquid phase, which must be BP-rich, is diluted by high reflux.33,34 In other words, the higher reflux dilutes the solvent concentration in the column’s liquid phase, necessitating additional stages for light component extraction. Figure 5 illustrates how the reflux ratio affects distillate composition as well as condenser and reboiler duties. The duty of the condenser and reboiler increases as the reflux ratios rise. So, the optimum point is selected to have the highest acetone purity while using the least amount of energy.
Figure 4.
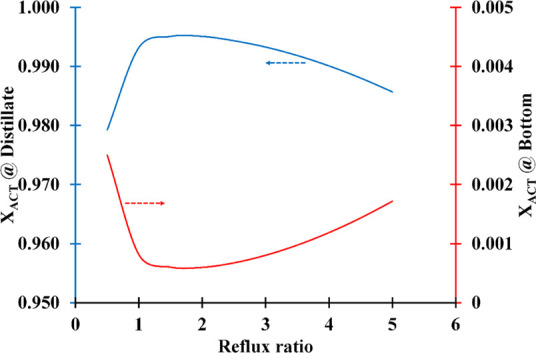
Influence of reflux ratio on the distillate and bottom ACT mass fraction (number of stage: 30; solvent feed stage: 10; feed stage: 26; solvent-to-feed ratio: 1.4; solvent temperature: 298.15 K).
Figure 5.
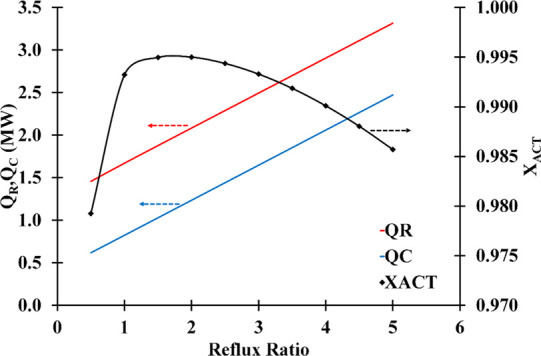
Effect of the reflux ratio on distillate composition and energy duty (condenser and reboiler) (number of stage: 30; solvent feed stage: 10; feed stage: 26; solvent-to-feed ratio: 1.4; solvent temperature: 298.15 K).
Figure 6 illustrates how the acetone mass fraction (XACT) changes with the number of stages at various reflux ratios. It is possible to see that the distillate composition does not change substantially when the number of stages is greater than 30. According to the findings, the column can achieve maximum acetone purity by operating at a reflux rate of 1.5 with 30 stages.
Figure 6.
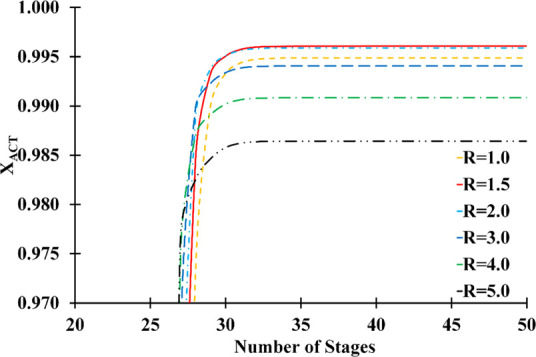
Acetone mass fraction variation distillate vs number of stages and reflux ratio (solvent feed stage: 10; feed stage: 26; solvent-to-feed ratio: 1.4; solvent temperature: 298.15 K).
The reboiler (QR) and condenser (QC) heat duty variations as a function of the number of stages and reflux ratio are represented in Figures 7 and 8, respectively. The reflux rate significantly influenced the column energy consumption, while the number of stages had little effect on the duties in either situation. The reflux ratio was directly proportional to the heating and cooling requirements. Based on the sensitivity analysis, the composition of distillate and energy consumption criteria are met at a reflux ratio of 1.5.
Figure 7.
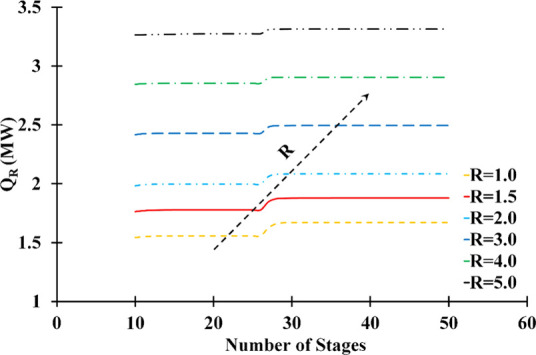
Influence of number of stages and reflux ratio on the reboiler heat duty (solvent feed stage: 10; feed stage: 26; solvent-to-feed ratio: 1.4; solvent temperature: 298.15 K).
Figure 8.
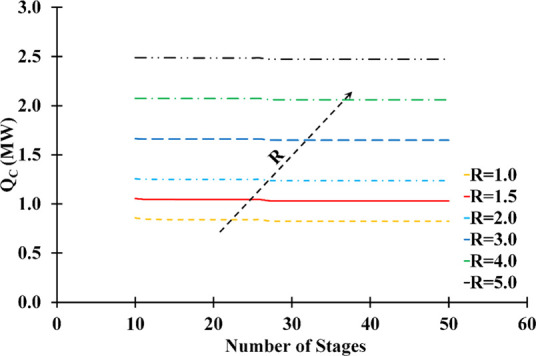
Influence of number of stages and reflux ratio on the condenser heat duty (solvent feed stage: 10; feed stage: 26; solvent-to-feed ratio: 1.4; solvent temperature: 298.15 K).
Figure 9 explains the feed stage’s effect on the ACT mass composition in distillate (XACT) in different reflux ratios. As can be seen, the feed mixture should be fed between the 26th and 28th stages at reflux rates between 1.0 and 2.0 to achieve high-purity acetone in the distillate. Therefore, near the bottom of the column, the azeotropic feed mixture was fed since it had a longer contact time with the solvent and had higher acetone purity in the distillate.
Figure 9.
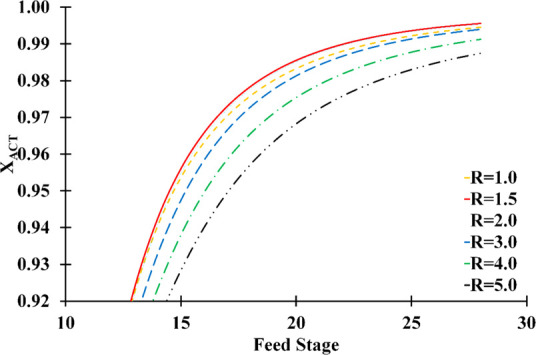
Feed stage and reflux ratio effect on acetone mass fraction in the distillate (number of stage: 30; solvent feed stage: 10; solvent-to-feed ratio: 1.4; solvent temperature: 298.15 K).
At various reflux ratios, the effect of the solvent stage on the acetone mass composition in the distillate is illustrated in Figure 10. It was revealed that reflux ratios of 1.0 and 2.0 caused the maximum acetone in the distilled product. It is also possible to observe that the acetone purity was lower at the higher solvent stage. It can be ensured that the solvent in the liquid phase is present in all of the tower’s lower trays as the solvent is fed into the upper stages. These findings revealed that the 10th stage is the most feasible for solvent feeding to get the maximum acetone concentration.
Figure 10.
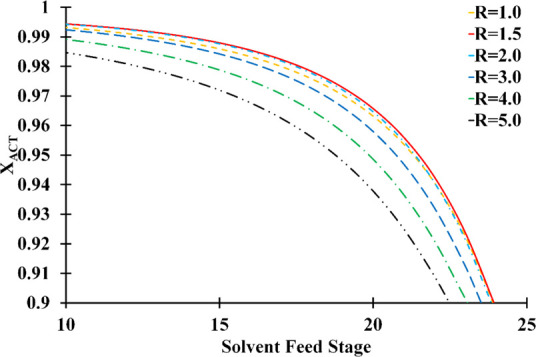
Solvent feed stage and reflux ratio influence on the distillate acetone mass composition (number of stage: 30; feed stage: 26; solvent-to-feed ratio: 1.4; solvent temperature: 298.15 K).
Figure 11 presents the influence of solvent-to-feed ratio (S/F) on ACT’s mass composition at a fixed reflux ratio (1.5). The mass composition of acetone was greater at high S/F ratios. Since the S/F ratios are higher, the solvent dilution produced by the reflux is reduced, and the distillate purity improves. However, the most cost-effective solvent-to-feed ratio should be chosen based on the appropriate acetone concentration.
Figure 11.
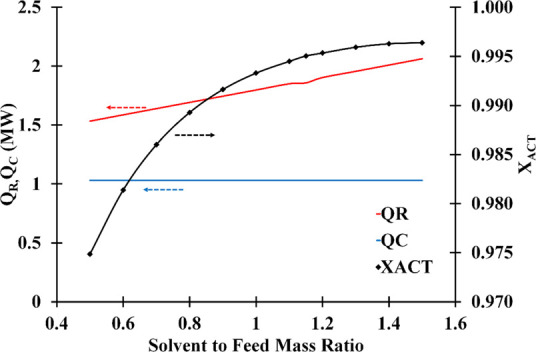
Solvent-to-feed mass ratio and reflux ratio influence on the distillate acetone mass fraction (number of stage: 30; solvent feed stage: 10; feed stage: 26; solvent temperature: 298.15 K).
It is also possible to find in Figure 11 that with the increased S/F, the reboiler duty changed significantly, whereas the condenser duty was constant over all S/F ranges analyzed. At high solvent-to-feed ratios, a large volume of liquid needs to evaporate; therefore, the reboiler’s energy consumption is raised. The S/F value of 1.4 could be optimum since it allowed high acetone purity while using less energy.
The solvent temperature significantly affects the reboiler duty and the composition of distillate; the reflux ratio influences this effect. As can be seen in Figure 12, high solvent temperatures necessitate high reflux ratios to achieve the desired separation. The purity of acetone decreases as the solvent temperature rises at low reflux ratios (1–2). This happens due to the fact that a portion of HEP evaporates as the solvent’s temperature rises and lowers acetone purity in the distillate. The reflux ratio must be increased to compensate for this effect; however, as previously stated in Figure 4, a high reflux dilutes the liquid phase and reduces acetone purity. As a result, low reflux operations require a solvent fed at temperatures of 298.15 K to maintain distillate purity.
Figure 12.
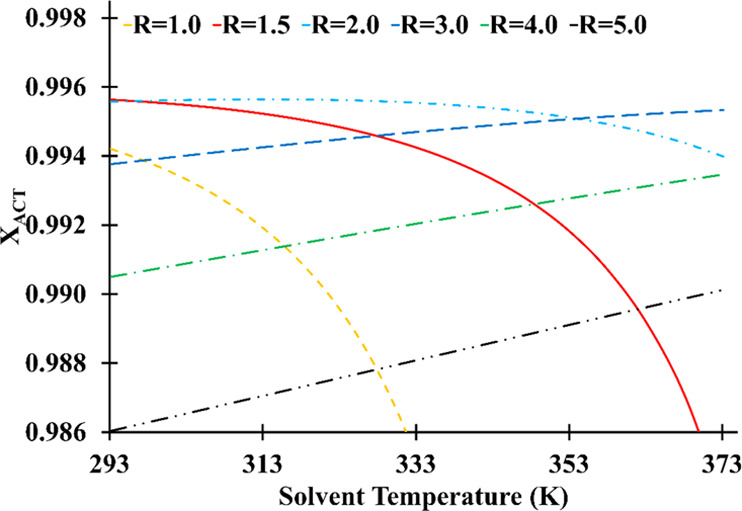
Solvent feed temperature and reflux ratio influence on the distillate acetone mass fraction (number of stage: 30; solvent feed stage: 10; feed stage: 26; solvent-to-feed ratio: 1.4).
There was no effect on the condenser duty when the solvent temperature was varied from 293.15 to 373.15 K (Figure 13). Nonetheless, the inlet solvent temperature had a substantial impact on the reboiler energy consumption. Since feeding a solvent at low temperatures needs extra energy to evaporate the liquids at the bottom, the reboiler duty reduces as the solvent temperature increases.
Figure 13.
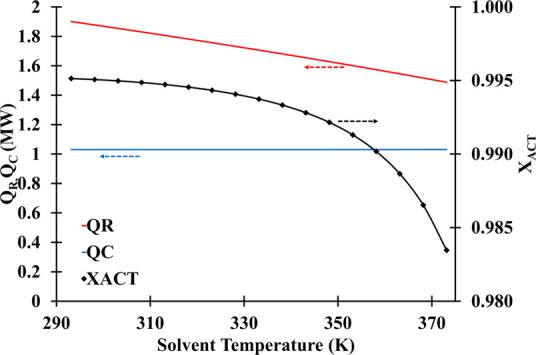
Solvent temperature and reflux ratio influence on the distillate acetone mass fraction (number of stage: 30; solvent feed stage: 10; feed stage: 26; solvent-to-feed ratio: 1.4; reflux ratio: 1.5).
The primary column’s configuration and operating conditions based on the sensitivity analysis results are given in Table 3. The solvent is fed above the feed mixture in the optimal design, resulting in higher acetone purity. Similar findings have been published in the literature,35,36 implying that the proposed design is suitable for separating azeotrope mixtures with a minimum boiling point.
Table 3. Extractive and Recovery Column Design Parameters.
| column | parameter | value |
|---|---|---|
| extractive | feed flow rate (kmol/h) | 100 |
| solvent flow rate (kmol/h) | 70 | |
| feed temperature (K) | 298.15 | |
| solvent temperature (K) | 298.15 | |
| number of stages | 30 | |
| feed stage | 26 | |
| solvent stage | 10 | |
| reflux ratio | 1.5 | |
| pressure (kPa) | 101.325 | |
| recovery | number of stages | 29 |
| feed stage | 15 | |
| reflux ratio | 1.5 | |
| pressure (kPa) | 101.325 |
3.2. Optimization
Based on the nonlinear programming (NLP) approach, process optimization is implemented to achieve the best economic efficiency of the process. The total annual cost (TAC) (as eq 1) with 3 year payback periods15,37,38 was used as the objective function39 to achieve high-purity ACT and HEP in distillates and maximum solvent recovery. The procedure was followed to determine the optimum operating conditions and the most cost-effective process using the solvent-to-feed ratio, reflux ratios, and reboiler duties as decision variables.
| 1 |
The costs of the reboiler, condenser, column shells, and trays are included in the capital cost. In addition, condenser, and reboiler energy costs are put in the operating cost. Details of cost relationships can be found in ref (15). Table 4 summarizes the optimization results.
Table 4. Optimization Results of the Extractive Distillation Process.
| parameter | extractive column | recovery column | system |
|---|---|---|---|
| reflux ratio | 1.5 | 1.5 | |
| condenser duty (MW) | 1.03 | 1.11 | |
| reboiler duty (MW) | 1.88 | 1.19 | |
| diameter (m) | 1.4 | 1.3 | |
| AReboiler (m2) | 96.20 | 60.21 | |
| ACondenser (m2) | 86.94 | 93.94 | |
| cooler (m2) | 27.12 | ||
| capital | |||
| column shell (106 $) | 0.286 | 0.250 | |
| heat exchanger (106 $) | 0.275 | 0.244 | |
| total capital (106 $) | 1.077 | ||
| energy cost (106 $/year) | 0.925 | ||
| TAC (106 $/year) | 1.284 |
CO2 emissions are measured to analyze the process from an environmental perspective. Assuming that natural gas was used to meet the heating requirement (CO2 emission factor from US-EPA-Rule-E9-5711 is 5.589 × 10–8 kg/J),14 the CO2 emissions were 0.0780 kg CO2/kg feed.
4. Conclusions
In this study, the ED process was simulated and designed to investigate the separation of an acetone and n-heptane azeotrope mixture. Butyl propionate was a feasible solvent. A sensitivity analysis was performed to survey the effect of essential parameters. According to the simulation results, the reflux ratio has the most significant impact on energy consumption. It needs to be set to low rates. Similarly, the S/F ratio is a valuable variable to receive high-purity acetone in the top stream. The findings revealed that a solvent-to-feed ratio of 1.4, a reflux ratio of 1.5, a number of stages of 30, a feed stage of 26, a solvent stage of 10, and a solvent temperature of 298.15 K were necessary to obtain high-purity acetone. To achieve the optimum operating parameters of the ED process, the NLP optimization method was used to minimize the total annual cost as the objective function. Finally, while the process was working optimally, CO2 emissions were computed as 0.0780 kg CO2/kg feed from an environmental standpoint.
The authors declare no competing financial interest.
References
- Laird T. Green Chemistry Is Good Process Chemistry. Org. Process Res. Dev. 2012, 16, 1–2. 10.1021/op200366y. [DOI] [Google Scholar]
- Anastas P. T.; Warner J. C.. Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998. [Google Scholar]
- Drueckhammer D. G.; Gao S. Q.; Liang X.; Liao J. Acetone-Heptane as a Solvent System for Combining Chromatography on Silica Gel with Solvent Recycling. ACS Sustainable Chem. Eng. 2013, 1, 87–90. 10.1021/sc300044c. [DOI] [Google Scholar]
- Berje J.; Schedemann A.; Gmehling J. Liquid Densities of Acetone and N-Heptane and Excess Volumes of the Binary System in a Wide Temperature and Pressure Range. Fluid Phase Equilib. 2011, 300, 110–115. 10.1016/j.fluid.2010.10.028. [DOI] [Google Scholar]
- Zhu Z.; Bai W.; Qi P.; Dai Y.; Wang Y.; Cui P.; Gao J. Liquid Liquid Equilibrium Data for the Separation of Acetone from n -Heptane Using Four Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2019, 64, 1202–1208. 10.1021/acs.jced.8b01105. [DOI] [Google Scholar]
- Liu J.-L.; Wang X.-Y.; Zhang L.-L.; Fang M.-J.; Wu Y.-L.; Wu Z.; Qiu Y.-K. Two-Dimensional Countercurrent Chromatography × High Performance Liquid Chromatography with Heart-Cutting and Stop-and-Go Techniques for Preparative Isolation of Coumarin Derivatives from Peucedanum Praeruptorum Dunn. J. Chromatogr. A 2014, 1374, 156–163. 10.1016/j.chroma.2014.11.053. [DOI] [PubMed] [Google Scholar]
- Tuzimski T. A New Procedure for Separation of Complex Mixtures of Pesticides by Multidimensional Planar Chromatography. J. Sep. Sci. 2007, 30, 964–970. 10.1002/jssc.200600446. [DOI] [PubMed] [Google Scholar]
- Marlot L.; Faure K. Preparative Two Dimensional Separations Involving Liquid–Liquid Chromatography. J. Chromatogr. A 2017, 1494, 1–17. 10.1016/j.chroma.2017.03.031. [DOI] [PubMed] [Google Scholar]
- Li G.-B.; Song B.-Q.; Wang S.-Q.; Pei L.-M.; Liu S.-G.; Song J.-L.; Yang Q.-Y. Selective Adsorption of Water, Methanol, and Ethanol by Naphthalene Diimide-Based Coordination Polymers with Constructed Open Cu 2+ Metal Sites and Separation of Ethanol/Acetonitrile. ACS Omega 2019, 4, 1995–2000. 10.1021/acsomega.8b03229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam A.; Gurunathan R. K.; Chanda Nagarajan P. Investigation of Potential Azeotrope Breakers Using DFT and COSMO Approach. ACS Omega 2020, 5, 16885–16900. 10.1021/acsomega.0c02086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayevskiy M.; Frolkova A.; Frolkova A. Separation and Purification of Methyl Isobutyl Ketone from Acetone + Isopropanol + Water + Methyl Isobutyl Ketone + Methyl Isobutyl Carbinol + Diisobutyl Ketone Mixture. ACS Omega 2020, 5, 25365–25370. 10.1021/acsomega.0c03718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Zhong L.; He Y.; Meng J.; Yao F.; Guo Y.; Xu C. Multiple Steady-States Analysis and Unstable Operating Point Stabilization in Homogeneous Azeotropic Distillation with Intermediate Entrainer. Ind. Eng. Chem. Res. 2015, 54, 7668–7686. 10.1021/acs.iecr.5b00572. [DOI] [Google Scholar]
- Huang X.; Li Z.; Tian Y. Process Optimization of an Industrial Acetic Acid Dehydration Progress via Heterogeneous Azeotropic Distillation. Chin. J. Chem. Eng. 2018, 26, 1631–1643. 10.1016/j.cjche.2017.10.030. [DOI] [Google Scholar]
- Pla-Franco J.; Lladosa E.; Loras S.; Montón J. B. Azeotropic Distillation for 1-Propanol Dehydration with Diisopropyl Ether as Entrainer: Equilibrium Data and Process Simulation. Sep. Purif. Technol. 2019, 212, 692–698. 10.1016/j.seppur.2018.11.082. [DOI] [Google Scholar]
- Luyben W. L. Comparison of Extractive Distillation and Pressure-Swing Distillation for Acetone/Chloroform Separation. Comput. Chem. Eng. 2013, 50, 1–7. 10.1016/j.compchemeng.2012.10.014. [DOI] [Google Scholar]
- Shen W.; Benyounes H.; Gerbaud V. Extension of Thermodynamic Insights on Batch Extractive Distillation to Continuous Operation. 1. Azeotropic Mixtures with a Heavy Entrainer. Ind. Eng. Chem. Res. 2013, 52, 4606–4622. 10.1021/ie3011148. [DOI] [Google Scholar]
- Li G.; Liu S.; Yu G.; Dai C.; Lei Z. Extractive Distillation Using Ionic Liquids-Based Mixed Solvents Combined with Dividing Wall Column. Sep. Purif. Technol. 2021, 269, 118713. 10.1016/j.seppur.2021.118713. [DOI] [Google Scholar]
- Zhang H.; Zhao F.; Ma Z.; Liu X.; Cui P.; Gao J.; Wang Y.; Zheng S. Design and Optimization for the Separation of Cyclohexane-Isopropanol-Water Using Mixed Extractants with Thermal Integration Based on Molecular Mechanism. Sep. Purif. Technol. 2021, 266, 118541. 10.1016/j.seppur.2021.118541. [DOI] [Google Scholar]
- Wang C.; Zhuang Y.; Dong Y.; Zhou C.; Zhang L.; Du J. Conceptual Design of the Triple-Column Extractive Distillation Processes with Single Entrainer and Double Entrainer for Separating the N-Hexane/Acetone/Chloroform Ternary Multi-Azeotropic Mixture. Chem. Eng. Sci. 2021, 237, 116578. 10.1016/j.ces.2021.116578. [DOI] [Google Scholar]
- Wang Y.; Zhang H.; Yang X.; Shen Y.; Chen Z.; Cui P.; Wang L.; Meng F.; Ma Y.; Gao J. Insight into Separation of Azeotrope in Wastewater to Achieve Cleaner Production by Extractive Distillation and Pressure-Swing Distillation Based on Phase Equilibrium. J. Cleaner Prod. 2020, 276, 124213. 10.1016/j.jclepro.2020.124213. [DOI] [Google Scholar]
- Vaz Mangili P.; Prata D. M. Exergoenvironmental Analysis of Tetrahydrofuran/Ethanol Separation through Extractive and Pressure-Swing Distillation. Chem. Prod. Process Model. 2020, 10.1515/cppm-2019-0114. [DOI] [Google Scholar]
- Wang Y.; Yang X.; Zhao J.; Liu X.; Yao D.; Cui P.; Wang L.; Zhu Z.; Li X.; Xu D. Design and Comprehensive Analysis of a Novel Pressure-Swing Batch Distillation Process for the Separation of a Binary Azeotrope with Various Boiling Behaviors. Sep. Purif. Technol. 2020, 251, 117329. 10.1016/j.seppur.2020.117329. [DOI] [Google Scholar]
- Wang Y.; Liang S.; Bu G.; Liu W.; Zhang Z.; Zhu Z. Effect of Solvent Flow Rates on Controllability of Extractive Distillation for Separating Binary Azeotropic Mixture. Ind. Eng. Chem. Res. 2015, 54, 12908–12919. 10.1021/acs.iecr.5b03666. [DOI] [Google Scholar]
- Yuan S.; Zou C.; Yin H.; Chen Z.; Yang W. Study on the Separation of Binary Azeotropic Mixtures by Continuous Extractive Distillation. Chem. Eng. Res. Des. 2015, 93, 113–119. 10.1016/j.cherd.2014.05.005. [DOI] [Google Scholar]
- Zhang T.; Li A.; Xu X.; Ma Y.; Xu D.; Zhang L.; Gao J.; Wang Y. Separation of Azeotropic Mixture (Acetone + n-Heptane) by Extractive Distillation with Intermediate and Heavy Boiling Entrainers: Vapour-Liquid Equilibrium Measurements and Correlation. J. Chem. Thermodyn. 2021, 152, 106284. 10.1016/j.jct.2020.106284. [DOI] [Google Scholar]
- Van Ness H. C.; Byer S. M.; Gibbs R. E. Vapor-Liquid Equilibrium: Part I. An Appraisal of Data Reduction Methods. AIChE J. 1973, 19, 238–244. 10.1002/aic.690190206. [DOI] [Google Scholar]
- Herington G. M. Tests for Consistency of Experimental Isobaric Vapour-Liquid Equilibrium Data. J. Inst. Pet. 1951, 37, 457–470. [Google Scholar]
- Maripuri V. O.; Ratcliff G. A. Measurement of Isothermal Vapor-Liquid Equilibria for Acetone-n-Heptane Mixtures Using Modified Gillespie Still. J. Chem. Eng. Data 1972, 17, 366–369. 10.1021/je60054a031. [DOI] [Google Scholar]
- Van Winkle M.Distillation; McGraw Hill: New York, 1967. [Google Scholar]
- Lladosa E.; Montón J. B.; Burguet M. C.; Muñoz R. Phase Equilibria Involved in Extractive Distillation of Dipropyl Ether+1-Propyl Alcohol Using 2-Ethoxyethanol as Entrainer. Fluid Phase Equilib. 2007, 255, 62–69. 10.1016/j.fluid.2007.03.030. [DOI] [Google Scholar]
- Liu W.-T.; Tan C.-S. Vapor–Liquid Equilibrium for Propionic Acid + n -Butyl Propionate from 60 to 101.3 KPa. J. Chem. Eng. Data 2002, 47, 1367–1371. 10.1021/je0255168. [DOI] [Google Scholar]
- Sun S.; Chun W.; Yang A.; Shen W.; Cui P.; Ren J. The Separation of Ternary Azeotropic Mixture: Thermodynamic Insight and Improved Multi-Objective Optimization. Energy 2020, 206, 118117. 10.1016/j.energy.2020.118117. [DOI] [Google Scholar]
- Gil I. D.; Botía D. C.; Ortiz P.; Sánchez O. F. Extractive Distillation of Acetone/Methanol Mixture Using Water as Entrainer. Ind. Eng. Chem. Res. 2009, 48, 4858–4865. 10.1021/ie801637h. [DOI] [Google Scholar]
- Yuan S.; Yang W.; Yin H.; Chen Z. Separation of Acetone-Tetrahydrofuran Azeotropic Mixture by Continuous Extractive Distillation. J. Chem. Technol. Biotechnol. 2013, 88, 1523–1528. 10.1002/jctb.3997. [DOI] [Google Scholar]
- Luyben W. L. Comparison of Extractive Distillation and Pressure-Swing Distillation for Acetone–Methanol Separation. Ind. Eng. Chem. Res. 2008, 47, 2696–2707. 10.1021/ie701695u. [DOI] [Google Scholar]
- Yao J.-Y.; Lin S.-Y.; Chien I.-L. Operation and Control of Batch Extractive Distillation for the Separation of Mixtures with Minimum-Boiling Azeotrope. J. Chinese Inst. Chem. Eng. 2007, 38, 371–383. 10.1016/j.jcice.2007.08.004. [DOI] [Google Scholar]
- Yang A.; Sun S.; Shi T.; Xu D.; Ren J.; Shen W. Energy-Efficient Extractive Pressure-Swing Distillation for Separating Binary Minimum Azeotropic Mixture Dimethyl Carbonate and Ethanol. Sep. Purif. Technol. 2019, 229, 115817. 10.1016/j.seppur.2019.115817. [DOI] [Google Scholar]
- Yang A.; Sun S.; Eslamimanesh A.; Wei S.; Shen W. Energy-Saving Investigation for Diethyl Carbonate Synthesis through the Reactive Dividing Wall Column Combining the Vapor Recompression Heat Pump or Different Pressure Thermally Coupled Technique. Energy 2019, 172, 320–332. 10.1016/j.energy.2019.01.126. [DOI] [Google Scholar]
- Sun S.; Yang A.; Chien I.-L.; Shen W.; Wei S.; Ren J.; Zhang X. Intensification and Performance Assessment for Synthesis of 2-Methoxy-2-Methyl-Heptane through the Combined Use of Different Pressure Thermally Coupled Reactive Distillation and Heat Integration Technique. Chem. Eng. Process. - Process Intensif. 2019, 142, 107561. 10.1016/j.cep.2019.107561. [DOI] [Google Scholar]