Abstract
The incidence of simultaneous kidney heart transplant (SHK) has increased markedly in the last 15 years. There are no universally agreed upon indications for SHK vs. heart alone (HA) transplant, and center evaluation processes vary widely. We utilized Scientific Registry of Transplant Recipients data from 2003–2017 to quantify changes in the practice of SHK, examine the survival of SHK vs. HA, and identify patients with marginal benefit from SHK. We used Kaplan-Meier curves and Cox proportional hazards to assess differences in survival. The incidence of SHK increased more than four-fold between 2003 and 2017 from 1.6% to 6.6% of total hearts transplanted, while the proportion of dialysis-dependent patients undergoing SHK has remained constant. SHK was associated with increased survival in dialysis-dependent patients (Median Survival SHK: 12.6 vs. HA: 7.1 years p<0.0001) but not non-dialysis-dependent patients (Median Survival SHK: 12.5 vs. HA 12.3, p=0.24). The marginal effect of SHK in decreasing the hazard of death diminished with increasing eGFR. Delayed graft function occurred in 26% of SHK recipients. Post-transplant chronic dialysis was similar for both operations (6.4% of HA and 6.0% of SHK). Further study is needed to define patients that benefit from SHK.
Introduction
Heart transplantation is definitive therapy for patients with end stage heart disease (ESHD)1. Renal disease often occurs concomitantly with ESHD, and ranges in severity between acute kidney injury as a result of cardiac dysfunction and chronic kidney disease (CKD) as a sequela of comorbid conditions2. Similarly, renal injury is a common complication of heart transplantation, due to the necessity of cardiopulmonary bypass, or as a consequence of subsequent immunosuppressant regimens that include nephrotoxic agents such as tacrolimus3,4. Rates of renal dysfunction after heart transplantation range from 14–76% for acute kidney injury and 2–28% for dialysis requirement after transplantation 5–7. Additionally, the incidence of renal dysfunction after heart transplantation has been increasing since 2002, likely attributable to an increase in the comorbidities of heart transplant patients8. Importantly, the need for dialysis after heart transplantation has been independently associated with poor outcomes after transplantation6,9.
Simultaneous heart kidney transplant (SHK) has become significantly more common since it was first described in 1978, with a seven fold increase in incidence from 2000 to 2015 in the US 10,11; much of this increase has occurred in the contemporary period with the incidence doubling from 2013–2017 12,13. Improved patient survival with SHK vs heart transplant alone has been described in the dialysis-dependent population via several retrospective studies; however, the benefit of SHK in non-dialysis-dependent patients is not completely clear 14–18. Prior studies investigating the benefit in this population are limited by inconsistent renal function measurement, comparison using a range of estimated glomerular filtration rate (eGFR) thresholds, and analyses that do not distinguish the need for dialysis within the study cohort. As such, there are no clinical practice guidelines that define a maximum eGFR or minimum duration of renal injury, beyond which SHK should not be pursued.
In the present study, we utilized Scientific Registry of Transplant Recipients (SRTR) data from 2003–2017 to quantify changes in the practice of SHK, examine the relative survival benefit of SHK vs. HA, and identify patients with marginal benefit from SHK.
Methods
Data Source and Study Population
This study was a secondary analysis of deidentified data and was granted approval by the Duke University Institutional Review Board to be performed without patient consent. We used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the US. These data are submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. We identified all heart and simultaneous heart-kidney transplant candidates and recipients aged 18 years or older in the United States from January 1, 2003 to December 31, 2017. Candidates were excluded if they underwent a multiorgan transplant other than SHK or if they underwent kidney after heart transplant (KAHT) within 1 year of heart transplant. Our final sample size was 30,697 patients.
Primary and Secondary Outcomes
Our primary outcome of interest was patient survival. Secondary outcomes included delayed graft function, defined as hemodialysis within one week of undergoing kidney transplant, and chronic dialysis, both as reported in the SRTR recipient follow up data. Of note, need for chronic post-transplant dialysis was determined using the heart transplant follow-up file for consistency of definitions between HA and SHK groups. Dialysis-dependent status was determined using variables from both the heart and kidney transplant records for maximum ascertainment of pre-transplant dialysis.
Statistical Analysis
Donor and recipient demographic variables were summarized by both SHK and dialysis-dependent status and compared using the appropriate categorical (chi-squared or Fisher’s Exact) and continuous (Student T or Wilcoxon-Rank Sum) tests. Patients missing data were excluded from multivariable analyses and the degree of missingness reported.
To examine temporal trends in SHK, we plotted the absolute number of SHK and the proportion of heart transplant recipients that were dialysis-dependent at the time of transplant according to the SRTR data. We also examined post-transplant renal function using variables that indicated both delayed graft function (DGF) and need for chronic dialysis.
We used Kaplan-Meier curves to examine the relative survival of SHK vs. HA patients among dialysis-dependent and non-dialysis-dependent patients. In addition, we derived eGFR at transplant using the CKD-EPI formula19 based on creatinine at the time of transplant as reported to the SRTR, though we note a single creatinine value cannot generally be used to define an eGFR. We stratified the non-dialysis-dependent patients by eGFR. We also examined survival of patients that were intended for SHK—as defined by an active kidney listing at the time of heart transplant or listing for kidney transplant up to one year prior to heart transplant with inactivation on the kidney waiting list within the 30 days immediately prior to heart transplant. We performed a sensitivity analysis excluding these data and found no substantive differences in our results. All survival curves were censored at 15 years due to a loss of density of data. The log rank test was used to compare curves.
Next, we calculated Cox-proportional hazards to evaluate the association of SHK with survival of non-dialysis-dependent patients. Models were adjusted for independent variables previously thought to be important in post-transplant survival20: recipient age (stratified 18–40, and then by decade thereafter), gender, insurance status (private vs. public), black race, body mass index, VAD usage, inotrope usage, center volume (total heart transplants per year), transplant era(2003–2007, 2008–2012, and 2013–2017), diabetes status, patient status, SHK, donor age (stratified 18–40, and then by decade thereafter). We evaluated the interaction between SHK and eGFR in both an unadjusted model where only SHK and eGFR were covariates and an adjusted model with all above terms. We then determined the exponentiated marginal effect (which resembles a hazard ratio) of SHK at all levels of eGFR from 0–100 mL/min/1.73m2 using previously described methods21. The assumption of proportional hazards was tested in all models by plotting individual Kaplan-Meier plots and Log-Log plots of survival for all categorical variables and by computing Schoenfeld residuals for continuous variables. Given the large number of observations, only large deviations from proportionality were adjusted for. Ultimately diabetes status and age at transplant were included as time varying co-variates.
Of note, the two most recent creatinine values available for SHK patients, one from the heart transplant record and one from the kidney transplant record, varied in 68% of cases and changed the eGFR decile in which a patient would be categorized 41% of the time. A sensitivity analysis showed that this did not influence the results of our study (data not shown). Additionally, we determined the CKD stage for those patients for whom a measurement was available in both groups, with the assumption that the two creatinine measurements were at least 90 days apart.
All statistical analysis was performed in Stata v15 (Stata Corp, College Station, TX) and R v4.0.2 (Foundation for Statistical Computing, Vienna, Austria).
Results
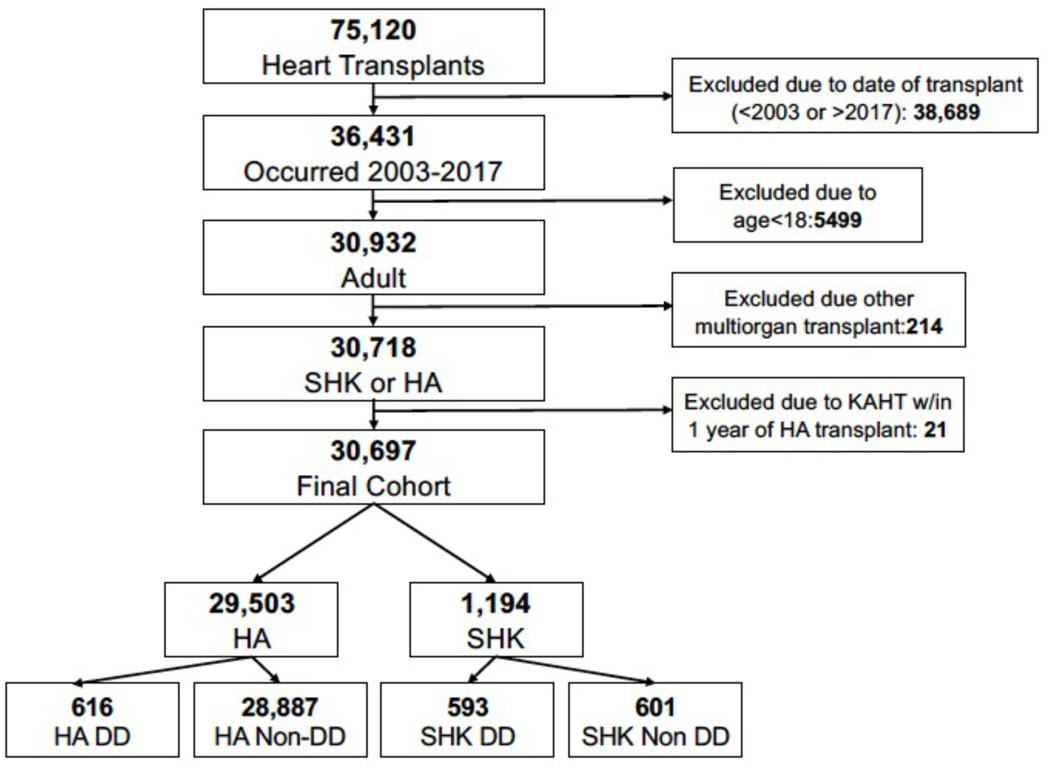
As shown in Figure 1, we identified a total of 30,697 adult heart transplants that were performed during the study period that were SHK or HA. Among SHK recipients, 593 were dialysis-dependent and 601 were non-dialysis-dependent. Of the HA recipients, 616 were dialysis-dependent and 28,887 were non-dialysis-dependent.
Figure 1:
Flow diagram describing cohort creation for the study.
SHK is increasing overall, but the proportion of SHK recipients on dialysis has not changed.
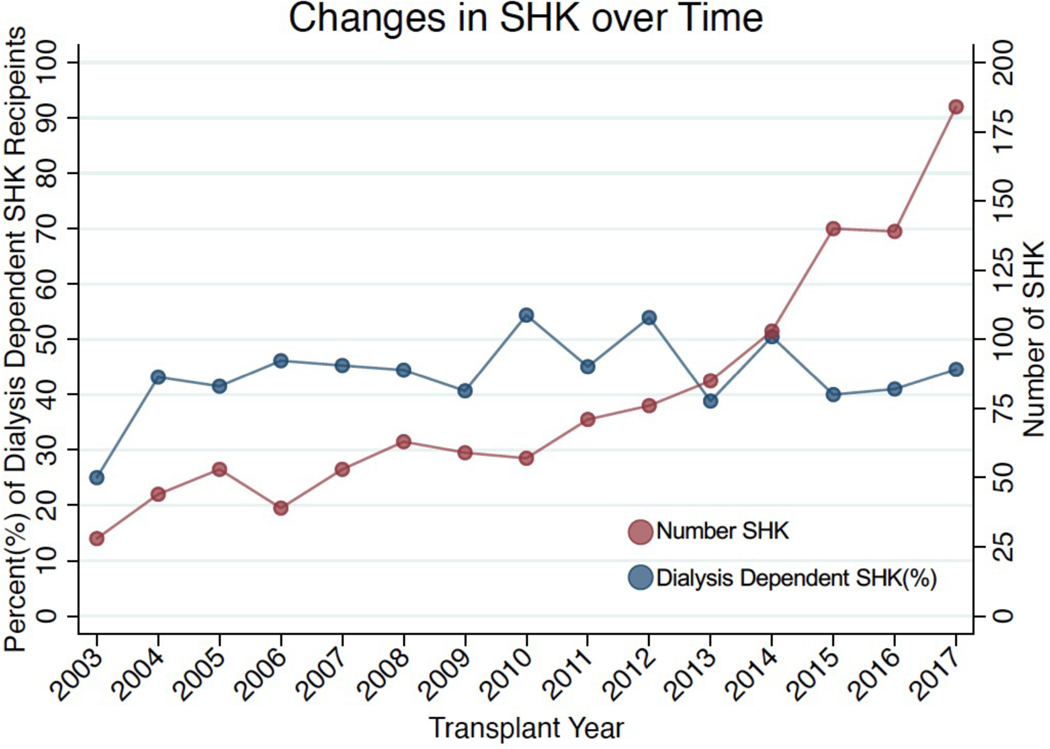
From 2003–2017, the absolute number of SHK increased 6.5 times from 28 in 2003 to 184 in 2017 while the proportion increased greater than four times (from 1.6% of all heart transplants in 2003 to 6.6% in 2017). There was an increase in the total number of non-dialysis-dependent SHK performed from 19 in 2003 to 90 in 2017, but the proportion of patients who were dialysis-dependent remained the same at approximately 50% (Figure 1).
SHK recipients differ from HA recipients but receive similar organs
SHK and HA recipient and donor characteristics were compared in a pairwise fashion. Among non-dialysis-dependent patients (Tables 1 & 2) SHK recipients were less often female (20% vs. 25%, p=0.006) and older (median age 58 versus 56 years; p<0.001). Diabetes was more common (40% vs. 26%, p<0.001) and heart function at the time of transplant was better among SHK recipients as reflected by higher cardiac index (2.4 vs. 2.2 L/min/m2, p<0.001) and less frequent VAD usage (28% vs. 39%, p<0.001). Among these patients, eGFR was significantly greater in the HA group (Median 68 IQR [51–89] vs. 29 [23–39], p<0.001); most were CKD stage IV (57%), though 27% were CKD stage III. Dialysis-dependent patients showed the same trends age was similar between groups (Supplemental Tables 1&2).
Table 1:
Recipient Characteristics by Transplant Type (Non-dialysis-dependent Only)
| HA Non-dialysis-dependent | SHK Non-dialysis-dependent | p-value | |
|---|---|---|---|
| N=28,887 | N=601 | ||
| Gender(Female)-n(%) | 7,329 (25%) | 123 (20%) | 0.006 |
| Age-Med (IQR) | 55.0 (46.0–62.0) | 58.0 (51.0–64.0) | <0.001 |
| Race-n(%) | <0.001 | ||
| Asian | 879 ( 3%) | 17 ( 3%) | |
| Black | 5,647 (20%) | 165 (27%) | |
| Multiethnic | 122 ( 0%) | 2 ( 0%) | |
| Native American | 93 ( 0%) | 1 ( 0%) | |
| Pacific Islander | 94 ( 0%) | 3 ( 0%) | |
| White | 22,052 (76%) | 413 (69%) | |
| Insurance Status-n(%) | 0.76 | ||
| Public | 13,783 (48%) | 292 (49%) | |
| Private | 14,840 (51%) | 305 (51%) | |
| Self | 264 ( 1%) | 4 ( 1%) | |
| HTx Center Volume-Med(IQR) | 22.6 (15.6–41.9) | 25.3 (16.1–41.9) | 0.072 |
| SHK Center Volume-Med(IQR) | 0.9 (0.3–1.5) | 1.4 (0.9–2.4) | <0.001 |
| eGFR (CKD EPI, mL/min/1.73m2) -Med(IQR) | 68 (51–89) * | 29 (23–39)* | <0.001 |
| Serum Albumin(g/dL) -Med(IQR) | 3.8 (3.3–4.2)† | 3.7 (3.2–4.1) & | 0.030 |
| Primary Cause of Heart Failure-n(%) | 0.27 | ||
| Autoimmune | 456 ( 2%)* | 14 ( 2%)* | |
| Congenital | 1,769 ( 6%) | 29 ( 5%) | |
| Idiopathic | 11,618 (40%) | 231 (39%) | |
| Infectious | 618 ( 2%) | 13 ( 2%) | |
| Ischemic | 11,309 (39%) | 241 (41%) | |
| Other | 2,575 ( 9%) | 48 ( 8%) | |
| Valvular | 507 ( 2%) | 16 ( 3%) | |
| Last Status Before Transplant-n(%) | 0.166 | ||
| Status 1A | 15,383(53%)* | 337(56) | |
| Status 1B | 10,471(36%) | 214(36) | |
| Status 2 | 3,029(11%) | 50(8) | |
| Diabetes-n(%) | 7,509 (26%)* | 240 (40%)* | <0.001 |
| Hypertension-n(%) | 10,596 (50%)† | 291 (76%)† | <0.001 |
| Body Mass Index (BMI)-Med(IQR) | 26.8 (23.6–30.4)* | 26.4 (23.2–29.9)* | 0.038 |
| Cardiac Index (L/min/m^2)-Med(IQR) | 2.2 (1.8–2.7)^ | 2.4 (2.0–2.9)& | <0.001 |
| Systolic PAP (mmHg)-Med(IQR) | 39 (30–50)^ | 42 (33–52)^ | <0.001 |
| Mean PAP (mmHg)-Med(IQR) | 27 (20–34)^ | 28 (22–36)^ | <0.001 |
| PCWP (mmHg)-Med(IQR) | 18 (12–24)& | 19 (14–25)& | <0.001 |
| Any Life Support (Mechanical Support or Inotropes) -n(%) | 22,128 (77%) | 452 (75%) | 0.42 |
| Ventricular Assist Device (VAD)-n(%) | 11,323 (39%)* | 167 (28%) | <0.001 |
| Inotropes-n(%) | 11,402 (39%) | 285 (47%) | <0.001 |
| Ventilator-n(%) | 486 ( 2%) | 5 ( 1%) | 0.11 |
| IABP-n(%) | 1,684 ( 6%) | 40 ( 7%) | 0.39 |
| ECMO-n(%) | 176 ( 1%) | 1 ( 0%) | 0.16 |
0–5% Missing
5–10% Missing
10–20% Missing
>20% Missing
Table 2:
Donor Characteristics by Transplant Type (Non-dialysis-dependent Only)
| HA Non-dialysis-dependent | SHK Non-dialysis-dependent | p-value | |
|---|---|---|---|
| N=28,887 | N=601 | ||
| Gender(Female)-n(%) | 8,408 (29%) | 153 (25%) | 0.051 |
| Age-Med(IQR) | 30.0 (22.0–41.0) | 29.0 (22.0–41.0) | 0.73 |
| Race-n(%) | 0.30 | ||
| Asian | 478 ( 2%) | 13 ( 2%) | |
| Black | 4,566 (16%) | 91 (15%) | |
| Multiracial | 65 ( 0%) | 0 ( 0%) | |
| Native American | 172 ( 1%) | 0 ( 0%) | |
| Pacific Islander | 45 ( 0%) | 1 ( 0%) | |
| White | 23,567 (82%) | 496 (83%) | |
| Body Mass Index(BMI)-Med(IQR) | 26.1 (23.1–30.0) | 26.1 (23.1–29.4) | 0.64 |
| Inotrope Support-n(%) | 14,223 (49%) | 285 (47%) | 0.33 |
| Ejection Fraction-Med(IQR) | 60.0 (55.0–65.0)* | 60.0 (55.0–65.0)* | 0.93 |
| Cause of Death-n(%) | 0.056 | ||
| Anoxia | 6,046 (21%) | 145 (24%) | |
| CVA | 6,091 (21%) | 105 (17%) | |
| Head Trauma | 15,933 (55%) | 338 (56%) | |
| CNS Tumor | 208 ( 1%) | 1 ( 0%) | |
| Other | 602 ( 2%) | 12 ( 2%) | |
| Diabetes-n(%) | 907 ( 3%)* | 15 ( 3%)* | 0.37 |
| Cigarette Smoking-n(%) | 4,522 (16%)* | 90 (15%)* | 0.67 |
| Cocaine Use-n(%) | 4,679 (16%)* | 104 (18%)* | 0.49 |
| Kidney Cold Ischemia Time(Hours)-Med(IQR) | N/A | 12 (8–18)^ | |
| Heart Total Ischemia Time (Minutes)-Med(IQR) | 191 (146–230)* | 182 (142–225)* | 0.011 |
0–5% Missing
5–10% Missing
10–20% Missing
>20% Missing
We also examined donor characteristics by recipient transplant and dialysis status (SHK-dialysis dependent, SHK non-dialysis dependent, HA-dialysis dependent, HA non-dialysis dependent). There were no differences in donor gender, age, race, body mass index, need for inotrope support, ejection fraction, cause of death, diabetes status, history of smoking or cocaine use. However, donor heart cold ischemic times were shorter in the SHK groups for both non-dialysis-dependent (p=0.01) and dialysis-dependent recipients (p<0.001). Of note, all patients who received an SHK received their kidney from the same deceased donor as their heart.
SHK is associated with survival among dialysis dependent but not non-dialysis dependent patients
We next examined the unadjusted survival of SHK and HA recipients based on dialysis status at the time of transplant. Among dialysis-dependent patients, SHK is associated with a significant increase in survival with SHK (Logrank p<0.0001, Supplemental Figure 1a). Median patient survival in the SHK group was 12.6 (95% CI: 10.3−∞) years while survival in the HA group was 7.1 (95% CI 6.0–9.0) years. In contrast, there were no differences in survival between SHK and HA recipients not on dialysis (Supplemental Figure 1b, Logrank Test: p=0. 39). Median survival in the SHK group was 12.5 (95% CI 11.1–13. 6) years and 12.3 (95% CI 12.1–12. 6) years in the HA group.
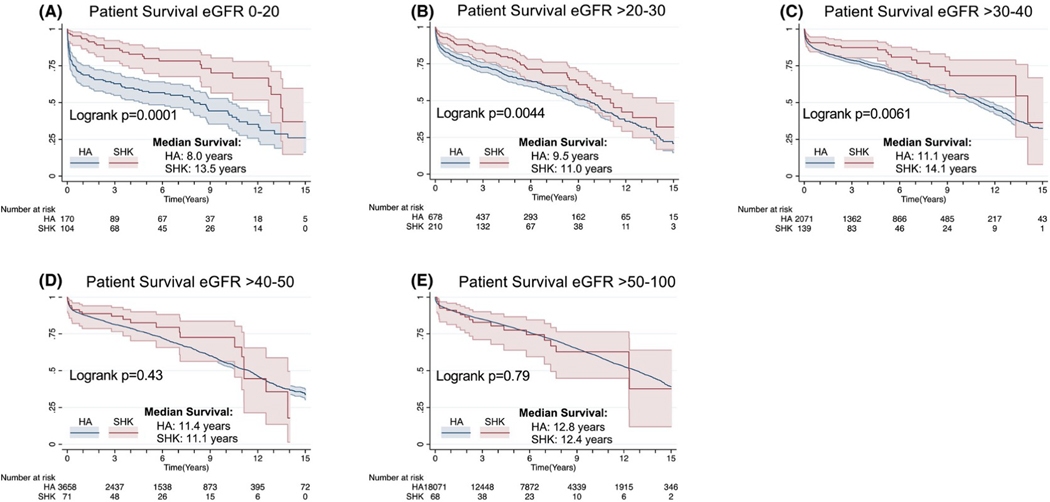
The association of SHK with survival is inversely correlated with eGFR
We next calculated differences in survival among SHK and HA recipients as a function of eGFR starting with 20 mL/min/1.73m2 (as there were only 4 SHK non-dialysis-dependent transplants with a GFR of < 10 mL/min/1.73m2) and then tested for different eGFR groups (20–30, 30–40, 40–50, and 50–100 mL/min/1.73m2 to ensure sufficient patient number). We observed a large, statistically significant association of SHK and survival in the lower eGFR groups. There was a stepwise decrease in the association of SHK and survival in the non-dialysis-dependent population as eGFR increased. The association between SHK and differential survival was statistically insignificant starting with the eGFR 40–50 mL/min/1.73m2 group (Figure 3d; Logrank P=0.27). Of note, 25% of all SHK (n=148) were performed at eGFR >40 mL/min/1.73m2, 10% of all HA (n=2076) were performed at eGFR<40, and 68 patients underwent SHK with eGFR>50 mL/min/1.73m2. When examining the survival of patients with an eGFR>30–40 mL/min/1.73m2, one-year survival was 85.2% in the HA group and 90.7% in the SHK group, a 5.5% absolute survival difference. Excluding patients intended for SHK who received HA did not substantively change this analysis: the absolute survival at 1 year is 91.3 % for SHK and 87.3% for HA for a difference of 4%. There were no differences in SHK survival when stratified by CKD stage (data not shown).
Figure 3:
The survival benefit associated with SHK is inversely proportional to pre-transplant eGFR. Overall patient survival among non-DD patients is plot stratified by eGFR with all patients with an eGFR 0–20 (a), >20–30 (b), >30–40 (c), >40–50 (d), and >50–100 mL/min/1.73m2 (e) plotted.
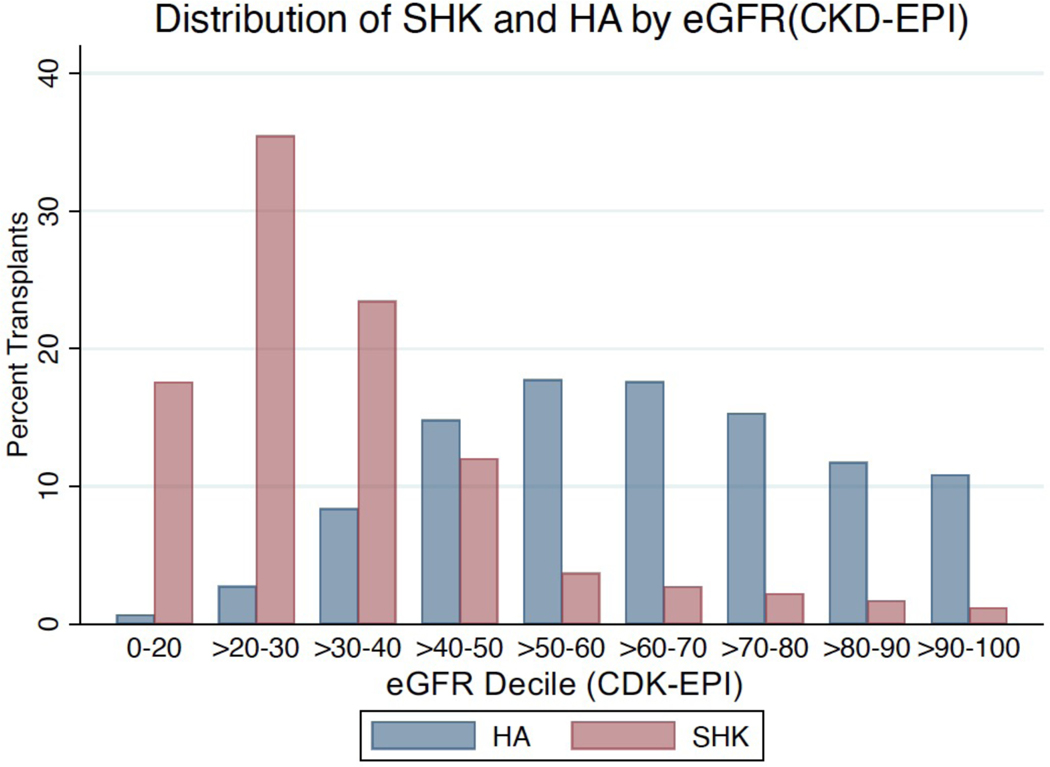
Additionally, we plotted the proportion of transplants (either HA or SHK) by eGFR decile. We observed overlap, especially in the deciles of eGFR from 20–50 mL/min/1.73m2 (Figure 4).
Figure 4:
There is overlap in the eGFR at which SHK and HA are practiced. Distribution of eGFR stratified by SHK/HA. Y-axis is percentage of transplants within the specified category among non-dialysis dependent patients.
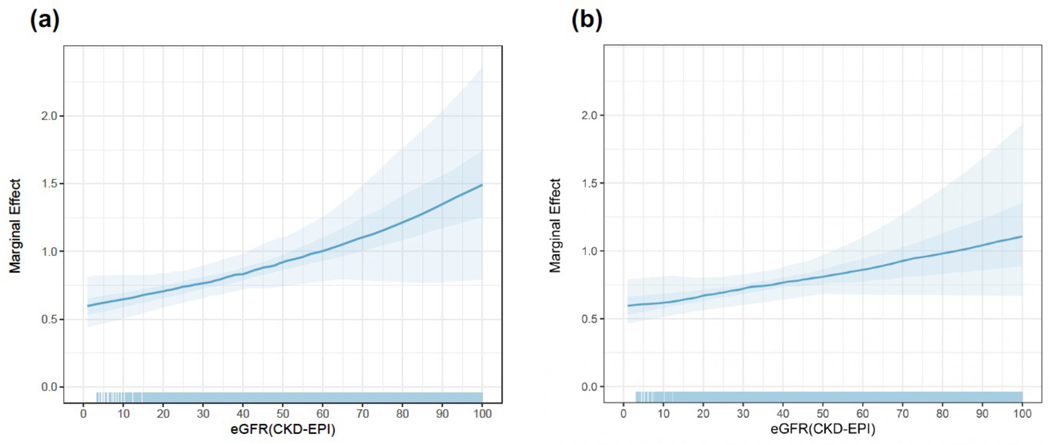
We also calculated the association of SHK vs. HA with survival across eGFR as a continuous variable using a multivariable Cox proportional hazard model in an unadjusted model using only SHK and eGFR as covariates or using an adjusted model including other potential mediators of survival (Figure 5; Supplemental Table 3). We plotted the marginal effect of SHK on the hazard of death and saw that it decreased as eGFR increased, with the 95% confidence interval crossing 1 in the interval of eGFR 40–60mL/min/1.73m2. Other variables in our multivariable model associated with an increased hazard of death are diabetes, previous heart transplant, increasing age, black race, VAD usage, and increasing donor age while private insurance and increasing center volume were associated with a decreased hazard of death.
Figure 5:
SHK benefit is inversely proportional to eGFR in a multivariable Cox regression analysis. Marginal effect of SHK on hazard of death across values of eGFR in (a) unadjusted model and (b) adjusted model. The line plotted is the median of simulations performed to obtain marginal effects with the inner darker band the 50% CI and outer lighter band the 95% CI. The rug represents the distribution of values used to perform the underlying Cox regression.
Patients intended for SHK who receive HA have very poor survival
We next evaluated the survival of patients that were intended for SHK (i.e. had an active waitlisting for a kidney transplant at the time of heart transplant or were listed for kidney transplant up to 1 year prior to transplant and were inactivated within 30 days of heart transplant; N=178, 15% of all patients intended for SHK). The survival of SHK intended patients who received a heart alone transplant was significantly worse than heart alone intended patients (Logrank test, p<0.0001), with a median survival of only 2.9 years vs. 12.3 years (Supplemental Figure 2). This increases to 4.1 years (95%CI 1.6–5.2 years) after excluding patients who died within 3 days of transplant. Of note, the median eGFR of patients intended for SHK who received HA was 38 (IQR CI 27–52) mL/min/1.73m2.
Post-transplant rates of delayed kidney graft function in SHK recipients are high and need for long term dialysis is equivalent in both groups.
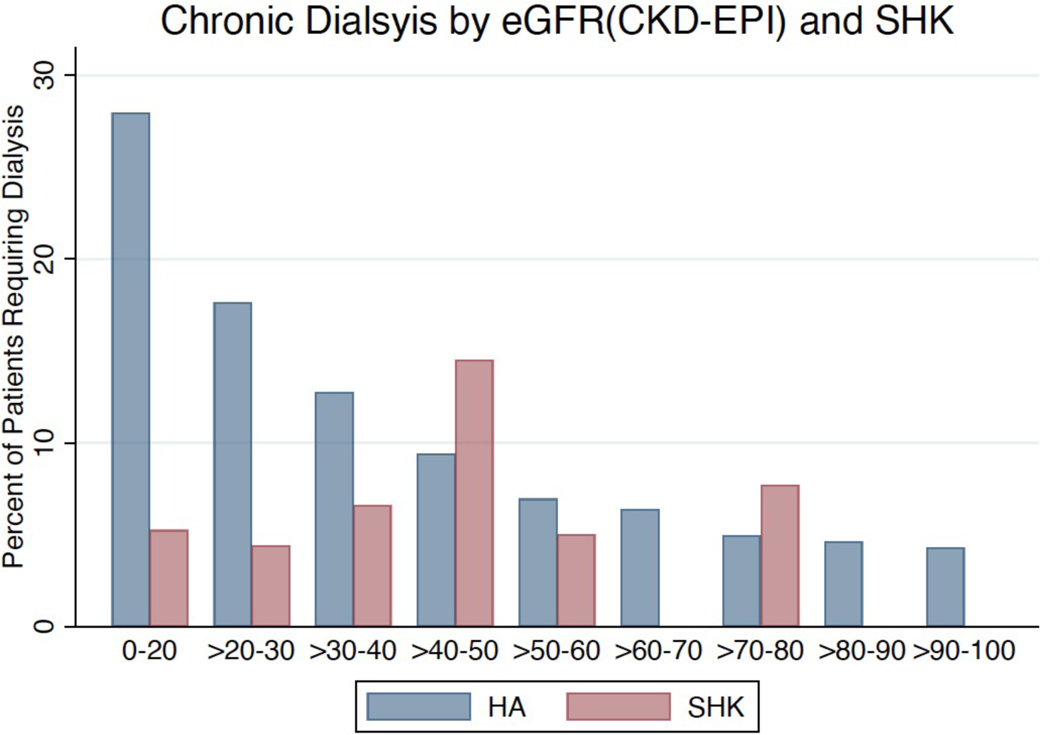
We also evaluated the post-transplant kidney function of SHK and HA recipients. DGF (defined as the need for dialysis within 1 week of transplant) occurred in 309 (26%) SHK recipients, 14% of patients who were non-dialysis-dependent prior to transplant and 38% of those who were dialysis-dependent. Of those patients who did not require pre-transplant dialysis, 6.4% of HA recipients and 6.0% of SHK recipients with at least 90 days of follow-up eventually required chronic dialysis. Additionally, there was an inverse relationship between pre-transplant eGFR and need for chronic dialysis among HA recipients, but an irregular relationship between eGFR and chronic dialysis among SHK recipients (Figure 6).
Figure 6:
Need for chronic dialysis is inversely proportional to eGFR among pre-transplant non-dialysis dependent patients among HA but inconsistently correlated with eGFR among SHK.
Discussion
In this observational study of 15 years of SRTR data, we found that the incidence of SHK has increased 4-fold, but the proportion of SHK recipients who are dialysis-dependent at the time of transplant has not changed. The association of improved survival with SHK compared to HA was most pronounced in the dialysis-dependent population and decreased as eGFR at transplant increased. These findings provide important insight on the increased use of SHK, which may be driven by practice and not an increase in dialysis need among heart transplant candidates. Additionally, these data demonstrate persistent challenges in candidate selection: 25% of SHK were performed in patients with an eGFR >40 with no gross survival difference observed, 15% of patients intended for SHK by our definition did not receive a kidney graft and had a median survival of only 2.9 years, 26% of SHK recipients experienced DGF despite the high quality of kidney grafts, and eventual chronic dialysis rates were similar among SHK and HA recipients irrespective of dialysis status prior to transplant.
The diagnostic conundrum underlying consideration of heart transplant candidates for simultaneous kidney transplant is that of native renal recovery. Several authors have sought to answer this question using national registry data and varying measures of renal function over the past 10 years. A 2008 study by Gill et al. showed that there was no benefit to SHK among non-dialysis-dependent patients.14 Although Gill et al did not stratify their analyses by recipient eGFR at transplant, multiple subsequent studies identified subgroups at eGFR ranging from 30–60 at which patients derive survival benefit from SHK compared to HA16–18,20. Lui combined dialysis-dependent and non-dialysis-dependent patients and showed a benefit to SHK among patients with an eGFR <20 (by CKD-EPI).16 Schaffer showed a benefit to SHK among non-dialysis-dependent patients when examining a propensity matched cohort. However, the benefit was less pronounced than dialysis-dependent patients, and overall survival among non-dialysis-dependent HA recipients was 69% implying a large prevalence of cardiorenal syndrome amenable to reversal with improved cardiac function.20 Karamlou et al examined outcomes after SHK vs HA by eGFR quintile and found 155 (26%) of SHK recipients required dialysis within 30 days, compared with 2181 (9%) of HA recipients. The patients in the lowest eGFR quintile had a significantly increased risk of needing dialysis and the worst median survival, although the survival advantage with SHK vs HA was only six months17. Our findings are consistent with prior work and that the benefit of SHK is clearly most pronounced in dialysis-dependent patients.
The present study expands upon this previous work in several aspects. First, we demonstrated a large increase in the number of SHK among non-dialysis-dependent patients during the study period despite the fact that SHK is not, when examining the whole group, associated with improved survival. Additionally, we examined multiple thresholds at which SHK might be performed and studied the continuous interaction of eGFR and receipt of SHK. We found that SHK was associated with survival among dialysis dependent patients, with a median survival 5 years longer than HA. This is an important finding because, whereas previous studies utilized the UNOS STAR files14,16–18,20, we used the SRTR, which contains more complete death data drawn from the Center for Medicare/Medicaid services and the National Technical Information Services death master file22. The reduction in the association of SHK with a decreased hazard of mortality as eGFR increases is generally in line with practice guidelines which state that an eGFR<40 is a relative contraindication to heart transplant alone23.
Our finding of a decreased marginal effect of SHK on the hazard of death as eGFR increases without increased hazard of death at very high eGFRs has multiple implications. Though one would postulate that adding a second surgery adds morbidity and mortality to the heart transplant, there have been great improvements in early post-operative survival after heart transplant25. Given that SHK has increased in frequency over time, we may not detect the potential early mortality given this improvement in perioperative survival. There is also some evidence to suggest implantation of a kidney at the time of heart transplant offers immunoprotection, with patients who undergo SHK having fewer rejection episodes and improved cardiac allograft survival, though the mechanisms are not well defined26.
Approximately 15% of the SHK waitlisted cohort did not go on to receive a simultaneous kidney transplant and--even when excluding immediate perioperative mortality—the median survival for these patients is dismal at only 4.1 years. This result is in line with but appears more pronounced than results from a similar study of simultaneous liver kidney transplants24. In light of these results, it is important to consider that a subset of patients listed for SHK have a poor post-transplant prognosis that cannot be altered with the addition of a kidney transplant. It also suggests that the association of SHK with improved survival may in part be due to factors unrelated to the transplant kidney, but rather other perioperative factors not accounted for in our analysis. Examining these patients who are listed for but do not undergo kidney implantation concomitant with heart transplantation, along with the subset of SHK recipients with early kidney graft failure, has the potential to improve allocation as many of these patients derive little utility from their grafts.
Currently, SHK recipients move to the top of the kidney wait list at allocation. Despite this prioritization, 26% of SHK recipients suffered DGF. We noted that DGF was more common among patients who were dialysis-dependent prior to transplant. This may be because some non-dialysis-dependent patients have residual native renal function—as has been previously observed in simultaneous liver kidney recipients27—or due to other perioperative factors known to increase the incidence of DGF28. Moreover, multi organ transplant recipients generally have been previously shown to obtain kidney allografts that are of far superior quality (as measured by the Kidney Donor Profile Index, KDPI) compared to kidney alone recipients29. As kidney transplantation is a life-saving procedure, with greatly improved survival after transplant compared to dialysis30, it is imperative to ensure that kidneys allocated to SHK provide the intended benefit. That is, kidneys allocated to multi organ transplant generally and SHK specifically may not generate the maximum utility. As we have previously written, though concerns of beneficence, or ensuring that the sickest patients who would most immediately derive value from an organ, are important, long term claims on utility must also be considered given that kidneys are truly scarce resources13.
It may be of use to utilize the experience of simultaneous liver kidney transplantation (SLK) to inform how best to reconcile practice patterns with known data in order to ensure best use of kidney grafts for all waitlisted patients. In SLK, liberal thresholds for kidney dysfunction (either chronic or acute) at which kidney allocation is allowable are defined. Additionally, a “safety net” system allows for the allocation of a kidney after liver transplantation for up to 12 months if a patient subsequently develops renal failure (defined as an eGFR<20, 90 days after transplant)31. A similar system may be of benefit in heart transplantation13. To guide this, Cheng and colleagues have posited a “willingness to transplant” threshold in SLK which allows the definition of a minimum number of Quality Adjusted Life Years (QALYs) gained per organ transplanted to inform allocation policy32. They have recently also proposed that this framework be applied to SHK33. Our study begins to answer the question of the population of heart transplant patients appropriate for SHK and the magnitude of benefit at varying degrees of renal dysfunction.
We acknowledge several important limitations in this study. First, we lack sufficiently detailed data both on pre-transplant and post-transplant dialysis, and our analysis showed a high degree of discordance between two different creatinine values reported for the same patients undergoing the same transplant event. Moreover, our estimation of kidney function was limited to a single creatinine recorded at the time of listing which cannot reliably differentiate between acute kidney injury and chronic kidney disease and may not reflect steady state renal function. Linkage of transplant registry data with other datasets to definitively describe these trends (including whether dialysis was acute inpatient dialysis or chronic outpatient dialysis) would be helpful. Additionally, we utilize the full CKD-EPI formula, which includes adjustment for race. These adjustments have recently been shown to be problematic34, however, we chose to keep this adjustment as this is a retrospective study and wanted to utilize a value that would have been representative of the eGFR used by the transplant team in decision making. Additionally, a sensitivity analysis removing the race term showed did not change our results (data not shown). In dialysis-dependent patients who undergo SHK, we possess data on dialysis vintage but we do not have these data in the HA-dialysis-dependent group and therefore cannot determine the impact of chronic vs. acute dialysis, which has been shown to be important in SLK35. We also lack important clinical data including traditional risk factors for CKD and ESRD such as duration and manifestations of diabetes (e.g. retinopathy, neuropathy, nephropathy), nonsteroidal anti-inflammatory drug use, duration of CKD and clinical markers of underlying kidney disease such as proteinuria or kidney size and echogenicity on imaging which are used at the patient level to predict renal recovery. These data would help to inform the appropriate use of kidneys in heart transplantation and potentially more accurately predict the need for post-transplant dialysis. Finally, though we lack extensive co-morbidity data, we do note that we have tried to appropriately determine the relationship of eGFR, SHK, and post-transplant mortality by controlling for comorbidities, including metabolic syndrome, by including BMI, age, and diabetes status in our multivariable models.
Conclusion
In summary, we have shown that the incidence of SHK is rising rapidly in both the dialysis-dependent and non-dialysis-dependent population despite evidence that the association of SHK with decreased mortality is weaker among patients with higher eGFRs. These data argue for increasing standardization in SHK practice. As an intermediate step, more detailed data on pre-transplant renal function, predictors of ESRD and perioperative management of heart transplant candidates is needed. These efforts would improve our ability to predict post-transplant renal function In the heart failure population and inform guidelines for SHK.
Supplementary Material
Figure 2:
While the number of SHK performed has increased significantly (red line) the proportion that are non-dialysis dependent has remained constant (blue line).
Acknowledgements
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government. We would also like to thank the Duke Transplant Center for Supporting this work and the Duke Group for Abdominal Transplantation Outcomes Research (GATOR) for providing critical feedback and improving upon this analysis.
Abbreviations
- CKD
Chronic Kidney Disease
- DGF
Delayed Graft Function
- eGFR
Estimated Glomerular Filtration Rate
- ESRD
End Stage Renal Disease
- ESHD
End Stage Heard Disease
- HA
Heart Alone
- KAHT
Kidney After Heart Transplant
- OPTN
Organ Procurement and Transplantation Network
- SHK
Simultaneous Heart Kidney
- SLK
Simultaneous Liver Kidney
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Disclosures
The authors have no relevant financial disclosures.
Data Availability Statement
Primary data are available upon request from the corresponding author.
References
- 1.Guglin M, Zucker MJ, Borlaug BA, et al. Evaluation for Heart Transplantation and LVAD Implantation: JACC Council Perspectives. J Am Coll Cardiol. 2020;75(12):1471–1487. doi: 10.1016/j.jacc.2020.01.034. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Zhao X, Hammill BG, et al. Trends in Noncardiovascular Comorbidities Among Patients Hospitalized for Heart Failure: Insights From the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2018;11(6):e004646. doi: 10.1161/CIRCHEARTFAILURE.117.004646. [DOI] [PubMed] [Google Scholar]
- 3.Sikma MA, Hunault CC, Kirkels JH, Verhaar MC, Kesecioglu J, de Lange DW. Association of Whole Blood Tacrolimus Concentrations with Kidney Injury in Heart Transplantation Patients. Eur J Drug Metab Pharmacokinet. 2018;43(3):311–320. doi: 10.1007/s13318-017-0453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortrie G, Manintveld OC, Caliskan K, Bekkers JA, Betjes MGH. Acute Kidney Injury as a Complication of Cardiac Transplantation. Transplantation. 2016;100(8):1740–1749. doi: 10.1097/TP.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 5.Escoresca Ortega AM, Ruíz de Azúa López Z, Hinojosa Pérez R, et al. Kidney Failure After Heart Transplantation. Transplant Proc. 2010;42(8):3193–3195. doi: 10.1016/j.transproceed.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 6.García-Gigorro R, Renes-Carreño E, Corres Peiretti MA, et al. Incidence, Risk Factors and Outcomes of Early Acute Kidney Injury After Heart Transplantation. Transplantation. 2018;102(11):1901–1908. doi: 10.1097/TP.0000000000002293. [DOI] [PubMed] [Google Scholar]
- 7.Thongprayoon C, Lertjitbanjong P, Hansrivijit P, et al. Acute Kidney Injury in Patients Undergoing Cardiac Transplantation: A Meta-Analysis. Medicines 2019;6(4):108. doi: 10.3390/medicines6040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadkarni GN, Chauhan K, Patel A, et al. Temporal trends of dialysis requiring acute kidney injury after orthotopic cardiac and liver transplant hospitalizations. BMC Nephrol. 2017;18(1):819. doi: 10.1186/s12882-017-0657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolsrud O, Karason K, Holmberg E, et al. Renal function and outcome after heart transplantation. J Thorac Cardiovasc Surg. 2018;155(4):1593–1604.e1. doi: 10.1016/j.jtcvs.2017.11.087. [DOI] [PubMed] [Google Scholar]
- 10.Givens RC, Topkara VK, Restaino SW, et al. Trends in Simultaneous Heart-Kidney Transplantation_ An Analysis of UNOS/OPTN Data from 2000 to 2015. Journal of Heart and Lung Transplantation. 2017;36(Supplement):S128–S129. doi: 10.1016/j.healun.2017.01.332. [DOI] [Google Scholar]
- 11.Norman JC, Cooley DA, Kahan BD, et al. Total Support Of The Circulation Of A Patient With Post-Cardiotomy Stone-Heart Syndrome By A Partial Artificial Heart (ALVAD) For 5 Days Followed By Heart And Kidney Transplantation. Lancet. 1978;311(8074):1125–1127. doi: 10.1016/S0140-6736(78)90301-X. [DOI] [PubMed] [Google Scholar]
- 12.OPTN. Ethical Implications of Multi-Organ Transplants. June2019:1–38. [Google Scholar]
- 13.Shaw BI, Sudan DL, Boulware LE, McElroy LM. Striking a Balance in Simultaneous Heart Kidney Transplant: Optimizing Outcomes for All Wait-Listed Patients. J Am Soc Nephrol. June2020. doi: 10.1681/ASN.2020030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill J, Shah T, Hristea I, et al. Outcomes of Simultaneous Heart-Kidney Transplant in the US: A Retrospective Analysis Using OPTN/UNOS Data. Am J Transplant. 2009;9(4):844–852. doi: 10.1111/j.1600-6143.2009.02588.x. [DOI] [PubMed] [Google Scholar]
- 15.Schaffer JM, Chiu P, Singh SK, Oyer PE, Reitz BA, Mallidi HR. Heart and Combined Heart-Kidney Transplantation in Patients With Concomitant Renal Insufficiency and End-Stage Heart Failure. Am J Transplant. 2013;14(2):384–396. doi: 10.1111/ajt.12522. [DOI] [PubMed] [Google Scholar]
- 16.Lui C, Fraser CD, Zhou X, et al. Increased Use of Multiorgan Transplantation in Heart Transplantation: Only Time Will Tell. Ann Thorac Surg. 2020. doi: 10.1016/j.athoracsur.2019.12.081. [DOI] [PubMed] [Google Scholar]
- 17.Karamlou T, Welke KF, McMullan DM, et al. Combined heart-kidney transplant improves post-transplant survival compared with isolated heart transplant in recipients with reduced glomerular filtration rate: Analysis of 593 combined heart-kidney transplants from the United Network Organ Sharing Database. Ann Thorac Surg. 2014;147(1):456–461.e1. doi: 10.1016/j.jtcvs.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Kilic A, Grimm JC, Whitman GJR, et al. The Survival Benefit of Simultaneous Heart-Kidney Transplantation Extends Beyond Dialysis-Dependent Patients. J Thorac Cardiovasc Surg. 2015;99(4):1321–1327. doi: 10.1016/j.athoracsur.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaffer JM, Chiu P, Singh SK, Oyer PE, Reitz BA, Mallidi HR. Heart and Combined Heart-Kidney Transplantation in Patients With Concomitant Renal Insufficiency and End-Stage Heart Failure. American Journal of Transplantation. 2013;14(2):384–396. doi: 10.1111/ajt.12522. [DOI] [PubMed] [Google Scholar]
- 21.Gandrud C. simPH: An R package for ullustrating Estimates from Cox Proportional Hazard Models Including for Interactive and Nonlinear Effects. J Stat Softw. 2015; 65(3). doi: 10.18637/jss.v065.i03 [DOI] [Google Scholar]
- 22.Massie AB, Kucirka LM, Kuricka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723–1730. doi: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016;35(1):1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Hmoud B, Kuo YF, Wiesner RH, Singal AK. Outcomes of liver transplantation alone after listing for simultaneous kidney: comparison to simultaneous liver kidney transplantation. Transplantation. 2015; 99(4), 823–828. 10.1097/TP.0000000000000438 [DOI] [PubMed] [Google Scholar]
- 25.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report - 2019; focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1056–1066. doi: 10.1016/j.healun.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou AS, Habertheuer A, Chin AL, Sultan I, Vallabhoajosyula P. Heart-Kidney and Heart-Liver Transplantation Provide Immunoprotection to the Cardiac Allograft. Ann Thorac Surg. 2019;108(2):458–466. doi: 10.1016/j.athoracsur.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Aparici CM, Bains SN, Carlson D, et al. Recovery of Native Renal Function in Patients with Hepatorenal Syndrome Following Combined Liver and Kidney Transplant with Mercaptoacetyltriglycine-3 Renogram: Developing a Methodology. World J Nucl Med. 2016; 15(1), 44–49. 10.4103/1450-1147.172140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nashan B, Abbud-Filho M, Citterio F. Prediction, prevention, and management of delayed graft function: where are we now? Clin Transplant. 2016; 30(10), 1198–1208. 10.1111/ctr.12832 [DOI] [PubMed] [Google Scholar]
- 29.Reese PP, Veatch RM, Abt PL, Amaral S. Revisiting multi-organ transplantation in the setting of scarcity. Am J Transplant. 2014;14(1):21–26. doi: 10.1111/ajt.12557. [DOI] [PubMed] [Google Scholar]
- 30.Axelrod DA, Schnitzler MA, Xiao H, et al. An economic assessment of contemporary kidney transplant practice. Am J Transplant. 2018;18(5):1168–1176. doi: 10.1111/ajt.14702. [DOI] [PubMed] [Google Scholar]
- 31.Formica RN, Aeder M, Boyle G, et al. Simultaneous Liver-Kidney Allocation Policy: A Proposal to Optimize Appropriate Utilization of Scarce Resources. Am J Transplant. 2016;16(3):758–766. doi: 10.1111/ajt.13631. [DOI] [PubMed] [Google Scholar]
- 32.Cheng XS, Goldhaber-Fiebert J, Tan JC, Chertow GM, Kim WR, Wall AE. Defining a Willingness-to-transplant Threshold in an Era of Organ Scarcity: Simultaneous Liver-kidney Transplant as a Case Example. Transplantation. 2020;104(2):387–394. doi: 10.1097/TP.0000000000002788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng XS, Khush KK, Wiseman A, Teuteberg J, Tan JC. To Kidney or Not to Kidney: Applying Lessons Learned from the Simultaneous Liver-Kidney Transplant Policy to Simultaneous Heart-Kidney Transplantation. Clin Transpalnt. 2020:1–27. doi: 10.1111/ctr.13878. [DOI] [PubMed] [Google Scholar]
- 34.Powe NR. Black Kidney Function Matters: Use or Misuse of Race? JAMA. 2020: 324(8), 737–738. 10.1001/jama.2020.13378 [DOI] [PubMed] [Google Scholar]
- 35.Northup PG, Argo CK, Bakhru MR, Schmitt TM, Berg CL, Rosner MH. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl. 2010;349:NA–NA. doi: 10.1002/lt.22008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.