Abstract
Objectives
This study aimed at estimating the real-life impact of vaccination on COVID-19 mortality, with adjustment for SARS-CoV-2 variants spread and other factors across Europe and Israel.
Study design
Time series analysis.
Methods
Time series analysis of the daily number of COVID-19 deaths was performed using non-linear Poisson mixed regression models. Variables such as variants’ frequency, demographics, climate, health, and mobility characteristics of thirty-two countries between January 2020 and April 2021 were considered as potentially relevant adjustment factors.
Results
The analysis revealed that vaccination efficacy in terms of protection against deaths was 72%, with a lower reduction of the number of deaths for B.1.1.7 vs non-B.1.1.7 variants (70% and 78%, respectively). Other factors significantly related to mortality were arrivals at airports, mobility change from the prepandemic level, and temperature.
Conclusions
Our study confirms a strong effectiveness of COVID-19 vaccination based on real-life public data, although lower than expected from clinical trials. This suggests the absence of indirect protection for non-vaccinated individuals. Results also show that vaccination effectiveness against mortality associated with the B.1.1.7 variant is slightly lower than that with other variants. Lastly, this analysis confirms the role of mobility reduction, within and between countries, as an effective way to reduce COVID-19 mortality and suggests the possibility of seasonal variations in COVID-19 incidence.
Keywords: COVID-19 mortality, SARS-CoV-2 variants, B.1.1.7 variant, VOC, Vaccination, Mobility
Introduction
The pandemic of the coronavirus infectious disease 2019 (COVID-19) is continuously evolving, driven by the spread of new variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). During the second half of 2020 and early 2021, a variety of new SARS-CoV-2 variants emerged. EU2 variant (mutation S:447N), first observed in July 2020 in Western Europe, was found to be capable of increasing virus infectivity.1 , 2 Then, several variants of concern (VOCs) have been identified, including B.1.1.7 developed first in the UK in September 2020,3 B.1.351 in South Africa in December 2020,4 P.1 in Brazil in January 2021,5 and the ‘Indian’ variant B.1.617 reported first in Maharashtra in January 2021.6 The disease mortality has been increased in these countries after new variants were developed.7, 8, 9, 10 An increased risk of transmissibility, hospitalization, and death associated with the B.1.1.7 variant was reported by a number of authors.8 , 11, 12, 13, 14, 15, 16 The B.1.351 variant was found to have an increased transmissibility and immune escape17 and was estimated to be 50% more transmissible than pre-existing variants.18 Higher incidence of COVID-19 cases in younger age groups was observed in the Amazonas state, suggesting changes in pathogenicity of the P.1 variant.19 Preliminary findings suggest also a significant increase in case fatality rate in young and middle-aged population for the P.1 mutant.20 The region of Maharashtra, where the B.1.617 variant emerged, experienced a significant rise in daily infection rate after the new variant appeared.10
To control the SARS-CoV-2 spread, a number of different vaccines have been developed and analyzed in clinical trials, including eight vaccines having emergency use or conditional marketing authorizations worldwide or across regions, as of May 2021.21 The worldwide vaccination campaign started in December 2020 aiming to provide herd immunity across societies. The threshold for COVID-19 ‘herd immunity’ was placed between 60 and 70% of the population gaining immunity through vaccinations or past disease exposure; however, scientists warn that herd immunity is unlikely to be achieved owing to factors such as vaccine hesitancy and the spread of new variants.22 , 23 Israel was far ahead of other countries in terms of the proportion of vaccinated inhabitants, exceeding 62% at the end of April 2021, with the UK reaching 50% and the USA 42% at the same time.24
Results of clinical trials on vaccine efficacy revealed that Pfizer-BioNTech had 95% efficacy at preventing symptomatic COVID-19 infection in people without prior infection.25 Efficacy of 94.1% was reported for Moderna,26 70.4% for Oxford-AstraZeneca,27 66.5% for Johnson & Johnson,28 and 96.4% for Novavax,29 with the latter being still under the investigation before authorization. For the prevention against a severe disease course, Pfizer, Moderna, AstraZeneca, and Novavax reported a 100% efficacy, whereas 84% was observed for Johnson & Johnson; however, the latter was tested on a broader range of countries, including the USA, South Africa, and Brazil, after the new VOCs spread.
Clinical evidence suggests that newly developed virus variants may affect the protective efficacy of both naturally acquired immunity and vaccinations. Studies on neutralization of convalescent sera against distinct strains showed that VOCs were harder to neutralize than the original strain, an early Wuhan-related strain of SARS-CoV-2. Neutralization titers against the B.1.1.7 variant showed a threefold reduction,30 a 3.4-fold reduction was observed for the P.1 variant,31 and a 13.3-fold reduction for the B.1.531 variant.32 Johnson & Johnson vaccine was found to have 64% efficacy against infection in South Africa and 68% in Brazil after the spread of B.1.135 and P.1 variants, whereas the efficacy against severe-critical disease was 82% and 88% in both countries.28 Late March 2021, AstraZeneca was observed as having 70.4% efficacy against the B.1.1.7 variant;33 however, the vaccine did not protect as well against the B.1.351 variant.34 For Novavax, the initial evidence suggests 86.3% efficacy against the B.1.1.7 variant35 and 49.4% against the B.1.351 variant.36 Early May 2021, Pfizer vaccine was found to be 87% effective against infection with the B.1.1.7 variant and 72% against B.1.351 variant, whereas 97.4% efficacy against severe disease course was observed for any of these mutations.37 On the other hand, Wang et al.38 observed a reduced neutralization of Moderna and Pfizer vaccine-immune sera against the B.1.351 variant (12.4-fold for Moderna; 10.3-fold for Pfizer), but no significant impact was observed for the B.1.1.7 variant. Next, a 3.8- to 4.8-fold reduced neutralization of Moderna vaccine was observed against the P.1 variant.31 Preliminary evidence suggests a significant drop in neutralization of B.1.617 compared with other variants, including B.1.1.7 and P.1, with sera of Indian's vaccine, Covaxine.10
The other concern is the probability of reinfection after recovery or vaccination. Hansen et al.39 observed an 80.5% protection against reinfection in a population-level observational study on Danish patients previously tested positive for SARS-CoV-2; however, the study was performed before VOCs spread. The probability of reinfection after vaccination is also a big concern. As reported by the US Centers for Disease Control and Prevention (CDC), there were around 9200 infections among vaccinated inhabitants among 95 million of those who have already been vaccinated in the USA (0.01%) as of 26 April 2021.40 Despite these optimistic preliminary data, experts alarm that additional data are needed to assess the potential impact of VOCs on future vaccine efficacy.41 Considering all the concerns associated with new VOC spread, the real vaccination effectiveness becomes hard to assess and judge but can be expected to decrease over time. Also, it is likely that vaccination may favor the emergence of new variants by selection of new, better fitted mutants. Some scientists suggest that, similarly as for seasonal flu vaccines, COVID-19 vaccines will need to be redesigned or even updated periodically to protect against new variants.42 , 43
Vaccination efficacy and distinct variants spread are the only two factors among numerous other variables affecting COVID-19 infection and death rates across the world. A variety of potential predictors were assessed in the literature, including demographic characteristics, mobility and social-distancing measures, environmental and climate variables, as well as health characteristics.44, 45, 46, 47, 48, 49, 50, 51, 52, 53
This study aims at estimating the real-life impact of vaccination on COVID-19 mortality based on publicly available data from Europe and Israel, using time series analysis with non-linear mixed regression models. Variants frequency, including B.1.1.7 and other variants, as well as country-specific demographic and meteorological characteristics, health indicators, and mobility factors were considered as potentially relevant adjustment factors. Results of the current study should inform policy decision-makers, scientists, and the general public about the role of vaccination and social-distancing strategies in controlling the COVID-19 pandemic in the face of new VOCs spread.
Methods
Data collection
A total of 32 countries were considered in the analysis, including European countries and Israel. The daily number of COVID-19 deaths was the primary outcome of interest. Values were smoothed using 7-day moving average, divided by the number of inhabitants of a given country and reported as daily numbers of deaths per 1 million inhabitants.
The main explanatory variables of interest were proportion of vaccinated inhabitants (vaccination coverage), as well as average proportions of SARS-CoV-2 variants calculated across strains forming 12 Nextstrain clades. The focus was on 20A (EU2), 20E (EU1), and 20I (B.1.1.7) variants, with the two formers being dominant in Europe during the summer 2020 and the latter VOC being most frequent early 2021. Other time-varying covariates were maximum daily temperature, mean daily wind speed, the number of arrivals at two biggest airports of a country, and change in mobility from the prepandemic level (considering the average across retail/recreation, transit stations, and groceries/pharmacies). Additional fixed covariates were proportion of population aged 65 years or older, prevalence of diabetes, and rate of cardiovascular deaths.
Data on COVID-19 deaths and vaccination were obtained from Our World in Data on 15 April 2021.24 Metadata on SARS-CoV-2 virus variants (clades) identified up to mid-April 2021 were downloaded from the Nextstrain platform.54, 55, 56 We assumed that if a strain was observed on a given date, it could be observed in a range of ±14 days from the observation date. Because the data were not reported daily, linear interpolation was used to impute missing observations, assuming zeros a month before the first and after the last (if up to 1 March 2021) reported occurrence of a variant. Finally, data were smoothed with the use of 14-day moving average.
Countries’ characteristics were obtained from Our World in Data, Eurostat, the National Centers for Environmental Information, Aviation Intelligence Portal, and Google COVID-19 Community Mobility Reports.24 , 57, 58, 59, 60 Data on arrival flights and mobility were smoothed using 7-day moving average.
Statistical analysis
Regression analysis was used to investigate the association between COVID-19 mortality and daily reported time-varying variables and fixed covariates.
The primary analysis of the daily number of COVID-19 deaths was performed with the use of non-linear Poisson mixed model with random country-level intercept and mobility effect. The considered period was from the date of the first reported death in Europe, 29 January 2020, up to 15 April 2021.
Owing to the presence of autocorrelations, and to consider the fact that the number of infections on a given day is dependent on the number of infectious cases in the population over previous days which translates into the respective number of deaths, the model was adjusted for the logarithm of the daily number of COVID-19 deaths reported 7 days earlier. To capture the fact that increasing or decreasing trends in COVID-19 mortality over time are generally stable over several weeks or months, the logarithm of quotient of COVID-19 deaths 7 days before divided by deaths 14 days before the actual date was added as a covariate. All other time-varying variables were considered with a 21-day lag, to account for the virus incubation period, assuming 7 days from contact to symptoms onset, and a delay between symptoms onset and death due to the disease, assuming another 14 days. In addition, heterogeneity between countries was considered with random intercepts and mobility effects varying between countries.
Assuming M indicates mortality with vaccination coverage ‘c’, M o is the mortality without vaccination, and ‘VE’ represents the vaccine efficacy, we have:
After applying the logarithmic transformation and considering a set of covariates and random effects on intercept and selected , this equation was extended as shown in the following to specify the non-linear model:
For the exploratory analysis, vaccine efficacy against B.1.1.7 and non-B.1.1.7 variants was analyzed using a similar approach. Assuming that there are two classes of virus variants with known proportions equaled and , the vaccine efficacy could be considered as the average efficacy weighted by variants proportions:
The formula for the non-linear model is then as follows:
Additionally, three scenarios were tested as sensitivity analyses, varying either the time to symptoms onset or the time between symptoms onset and death. A detailed methodology is presented in Supplementary Materials.
A P-value lower than 0.05 was considered as statistically significant. Akaike's information criterion (AIC) was provided to inform about models' fit statistic. Analyses were performed using SAS, version 9.4, software.
Results
Descriptive statistics
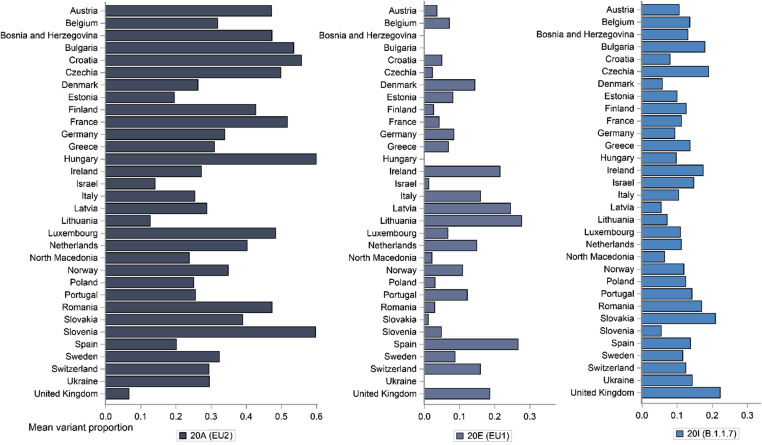
Descriptive statistics of outcomes and covariates across 32 countries included in the analysis, for the period between January 2020 and April 2021, are presented in Table 1 . Mean proportions of SARS-CoV-2 variants, EU2, EU1, and B.1.1.7, for each country are presented in Fig. 1 . Until mid-April 2021, the variant EU2 was the most frequently spread for vast majority of countries, except Israel and the UK for which B.1.1.7 was more frequent, as well as Spain and Lithuania with EU1 being more commonly observed.
Table 1.
Descriptive statistics of outcomes and covariates across 32 countries (January 2020–April 2021).
| Variable | N | Mean | Standard deviation | Median | Lower quartile | Upper quartile | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|
| Number of daily COVID-19 deaths | 13,229 | 3.355 | 4.618 | 1.159 | 0.206 | 4.933 | 0 | 28.770 |
| Proportion of vaccinated inhabitants | 13,181 | 0.018 | 0.058 | 0 | 0 | 0 | 0 | 0.616 |
| Proportion of 20A (EU2) variant | 13,106 | 0.349 | 0.250 | 0.313 | 0.154 | 0.509 | 0 | 1.000 |
| Proportion of 20E (EU1) variant | 12,754 | 0.090 | 0.159 | 0 | 0 | 0.114 | 0 | 0.741 |
| Proportion of 20I (B.1.1.7) variant | 13,099 | 0.124 | 0.256 | 0 | 0 | 0.065 | 0 | 1.000 |
| Max daily temperature | 13,008 | 15.597 | 9.347 | 15.833 | 8.750 | 22.389 | −17.111 | 43.111 |
| Mean daily wind speed | 13,010 | 6.227 | 3.353 | 5.600 | 3.700 | 8.100 | 0.100 | 25.800 |
| Arrivals at two biggest airports | 12,748 | 140.609 | 195.648 | 66.429 | 27.714 | 177.929 | 1.857 | 1780.143 |
| Mobility change from prepandemica | 12,918 | −0.217 | 0.170 | −0.201 | −0.322 | −0.091 | −0.792 | 0.199 |
| Proportion of inhabitants aged ≥65 years | 13,229 | 0.184 | 0.025 | 0.190 | 0.168 | 0.198 | 0.117 | 0.230 |
| Diabetes prevalence | 13,229 | 0.062 | 0.019 | 0.058 | 0.048 | 0.072 | 0.033 | 0.101 |
| Cardiovascular death rate | 13,229 | 2.037 | 1.124 | 1.535 | 1.148 | 2.783 | 0.861 | 5.398 |
Average mobility change from prepandemic level calculated across retail/recreation, transit stations, and groceries/pharmacies [0.01]. Descriptive statistics were calculated for 32 countries based on daily data between 29 January 2020 and 15 April 2021.
Fig. 1.
Mean SARS-CoV-2 variants proportions across countries, between January 2020 and April 2021. Mean proportions of variants were calculated based on daily data on variants proportions across observed strains used to form Nextstrain clades (variants), between 29 January 2020 and 15 April 2021.
Primary analysis
Analysis of the non-linear Poisson mixed model of the number of COVID-19 deaths revealed that the effect of vaccination effectiveness against mortality was assessed as significant and equaled to 0.720 (P < 0.001; Table 2 ). Other covariates that were found significant in the model were temperature (−0.005, P < 0.001), arrivals at airports (0.709, P < 0.001), and mobility change from the prepandemic level (0.753, P < 0.001). Variables used to account for autocorrelation and minimize the effect of trend were assessed as significant (Log of the number of daily COVID-19 deaths 7 days before: 0.926, P < 0.001; Log of the number of COVID-19 deaths 7 days before/14 days before: 0.158, P < 0.010). The random intercept variance was statistically significant, which indicated significant unexplained variability between countries (0.014, P = 0.023).
Table 2.
Results of the non-linear Poisson mixed model of the number of daily COVID-19 deaths.
| Variable | Estimate | Standard error | P value |
|---|---|---|---|
| Intercept | 0.467 | 0.202 | 0.028 |
| Logarithm of the number of daily COVID-19 deaths 7 days beforea | 0.926 | 0.007 | <0.001 |
| Logarithm of the number of COVID-19 deaths 7 days before/14 days before | 0.158 | 0.015 | <0.001 |
| Proportion of 20A (EU2) variantb | 0.012 | 0.036 | 0.746 |
| Proportion of 20E (EU1) variantb | −0.032 | 0.046 | 0.501 |
| Proportion of 20I (B.1.1.7) variantb | 0.059 | 0.033 | 0.084 |
| Max daily temperatureb | −0.005 | 0.001 | <0.001 |
| Mean daily wind speedb | −0.002 | 0.002 | 0.351 |
| Arrivals at two biggest airports [in thousands]b | 0.709 | 0.085 | <0.001 |
| Mobility change from prepandemicb,d | 0.753 | 0.064 | <0.001 |
| Proportion of inhabitants aged ≥65 yearsc | −1.238 | 0.930 | 0.193 |
| Diabetes prevalencec | 0.544 | 1.293 | 0.677 |
| Cardiovascular death ratec | 0.023 | 0.023 | 0.322 |
| Vaccination effectivenessb | 0.720 | 0.132 | <0.001 |
| Variance for RE on intercept | 0.014 | 0.006 | 0.023 |
| Variance for RE on mobility | 0.036 | 0.023 | 0.126 |
AIC = 30,184.
Abbreviations: AIC = Akaike's information criterion; mln = million; RE = random effects.
Non-linear Poisson mixed model of the daily number of COVID-19 deaths per 1 mln inhabitants, with country-specific random effects on intercept and mobility. Daily data for 32 countries were included, from 29 January 2020 to 15 April 2021. Assumed time to symptoms onset is 7 days; assumed time between symptoms onset and death is 14 days.
Time-varying variable with a 7-days lag.
Time-varying variable with a 21-days lag.
Fixed variable.
Average mobility change from prepandemic level calculated across retail/recreation, transit stations, and groceries/pharmacies [0.01].
Exploratory analysis
Results of the analysis of the exploratory model revealed numerically lower vaccine effectiveness against B.1.1.7 than against non-B.1.1.7 variants, although the difference was not statistically significant (0.697, P = 0.002 and 0.778, P = 0.049, respectively; Table 3 ). The same set of covariates was found significant in the exploratory model as in the primary analysis: temperature (−0.005, P < 0.001), arrivals at airports (0.703, P < 0.001), and mobility change from the prepandemic level (0.753, P < 0.001). Variables used to account for autocorrelation and minimize the effect of trend were assessed as significant (Log of the number of daily COVID-19 deaths 7 days before: 0.926, P < 0.001; Log of the number of COVID-19 deaths 7 days before/14 days before: 0.158, P < 0.010), as was the variance for random intercept (0.013, P = 0.025).
Table 3.
Results of the non-linear Poisson mixed model of the number of daily COVID-19 deaths with interactions between variants proportions and vaccination effectiveness.
| Variable | Estimate | Standard error | P value |
|---|---|---|---|
| Intercept | 0.409 | 0.204 | 0.054 |
| Logarithm of the number of daily COVID-19 deaths 7 days beforea | 0.926 | 0.007 | <0.001 |
| Logarithm of the number of COVID-19 deaths 7 days before/14 days before | 0.158 | 0.015 | <0.001 |
| Proportion of 20A (EU2) variantb | 0.012 | 0.036 | 0.747 |
| Proportion of 20E (EU1) variantb | −0.034 | 0.047 | 0.467 |
| Proportion of 20I (B.1.1.7) variantb | 0.059 | 0.033 | 0.085 |
| Max daily temperatureb | −0.005 | 0.001 | <0.001 |
| Mean daily wind speedb | −0.002 | 0.002 | 0.346 |
| Arrivals at two biggest airports [in thousands]b | 0.703 | 0.085 | <0.001 |
| Mobility change from prepandemicb,d | 0.753 | 0.064 | <0.001 |
| Proportion of inhabitants aged ≥65 yearsc | −0.805 | 0.925 | 0.391 |
| Diabetes prevalencec | 0.124 | 1.285 | 0.924 |
| Cardiovascular death ratec | 0.025 | 0.023 | 0.278 |
| Vaccination effectiveness against 20I (B.1.1.7)b | 0.697 | 0.201 | 0.002 |
| Vaccination effectiveness against variants other than 20I (B.1.1.7)b | 0.778 | 0.379 | 0.049 |
| Variance for RE on intercept | 0.013 | 0.006 | 0.025 |
| Variance for RE on mobility | 0.035 | 0.023 | 0.132 |
AIC = 30,186.
Abbreviations: AIC = Akaike's information criterion; mln = million; RE = random effects.
Non-linear Poisson mixed model of the daily number of COVID-19 deaths per 1 mln inhabitants, with country-specific random effects on intercept and mobility, including interactions between variants proportions and vaccination effectiveness. Daily data for 32 countries were included, from 29 January 2020 to 15 April 2021. Assumed time to symptoms onset is 7 days; assumed time between symptoms onset and death is 14 days.
Time-varying variable with a 7-days lag.
Time-varying variable with a 21-days lag.
Fixed variable.
Average mobility change from prepandemic level calculated across retail/recreation, transit stations, and groceries/pharmacies [0.01].
Sensitivity analysis
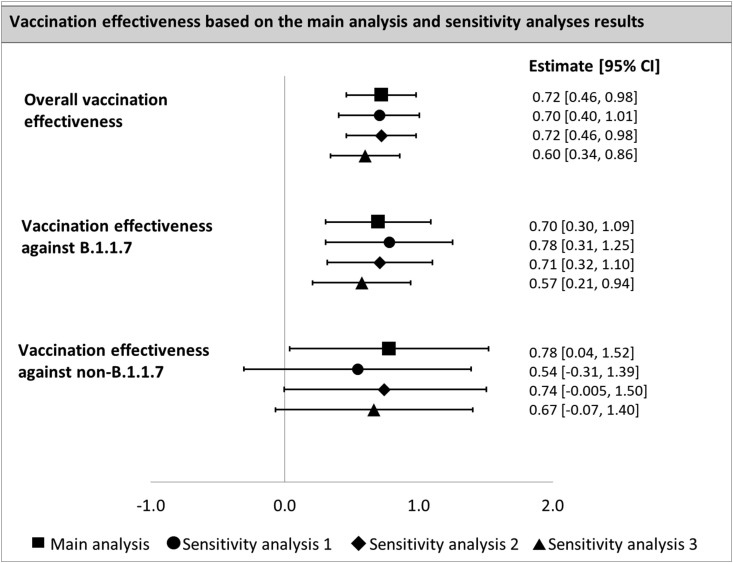
Sensitivity analyses yielded overall vaccination effectiveness estimates against mortality between 0.60 and 0.72 (Fig. 2 ). Regarding vaccination effectiveness associated with variants, two out of three scenarios provided consistent results with the main analysis, i.e., a trend towards lower effectiveness against the B.1.1.7 variant, and the opposite trend was observed in the remaining scenario (Fig. 2). Detailed results are provided in Supplementary Materials.
Fig. 2.
Estimates of the vaccination effectiveness against mortality – main analysis and sensitivity analyses results. Abbreviations: 95% CI = 95% Confidence Interval. Assumptions of the analyses: Main analysis: time to onset = 7 days, time between symptoms onset and death = 14 days. Sensitivity analysis 1: time to onset = 14 days, time between symptoms onset and death = 14 days. Sensitivity analysis 2: time to onset = 14 days, time between symptoms onset and death = 7 days. Sensitivity analysis 3: time to onset = 7 days, time between symptoms onset and death = 7 days.
Discussion
In this study, we investigated the association between daily mortality due to COVID-19 and vaccination coverage, proportions of SARS-CoV-2 variants, and additional factors, such as demographics, health, mobility, and meteorological variables, analyzing country-level data across Europe and Israel. Results of the analysis suggest that vaccination effectiveness against deaths is equal to 72% and that it is slightly lower against the B.1.1.7 variant than against non-B.1.1.7 variants (difference not statistically significant). These findings suggest lower effectiveness against death than reported efficacy against severe or critical disease course in clinical trials of vaccines (84–100%).25, 26, 27, 28, 29
This lower-than-expected effectiveness might be explained by the difference in considered populations: clinical trials included restrictive populations, and our study covers general populations, irrespective of age, concomitant therapies, medical condition, and general condition. In particular, vaccinated people in real life are older on average than subjects enrolled in clinical trials (12.2% aged ≥55 years in the AstraZeneca trial; 24.7% aged ≥65 years in the Moderna trial; 33.5% aged ≥60 years in the Johnson & Johnson trial; 42.3% aged ≥55 years in the Pfizer trial). However, our results suggesting lower protection against the B.1.1.7 variant are consistent with reported data so far from in vivo experiments and patient-level studies, providing an external validation of these findings. Laboratory evidence revealed a slight reduction in neutralization against the B.1.1.7 variant compared with the original strain. Neutralization titers against this VOC were threefold lower when analyzing convalescent sera and 3.3-fold and 2.5-fold lower for Pfizer and AstraZeneca vaccinees, respectively.30 Real-world studies on the B.1.1.7 VOC suggested that it caused increased mortality compared with non-B.1.1.7 variants,15 , 61 which, therefore, might not have been contained with similar effectiveness by vaccination. Our results evoke the question of variants evading vaccine antibodies in the future and the need to adapt such vaccine for each new season, which was earlier suggested by experts.42 , 43
While the utilization of individual-level data, collected in real-world setting, could provide more precise estimates of vaccine effectiveness, the use of aggregate data at country level also has a major advantage: the vaccination impact estimated in this analysis should capture the indirect protection provided by vaccination. If the vaccine protects against infection, the number of infectious cases would decrease as more people are vaccinated. The lower number of infectious cases in the population would lead to a reduced probability for susceptible individuals to get in contact with infectious cases, thus leading to a reduction in incidence among all people, including non-vaccinated people. This indirect protection can be captured when comparing different populations with different rates of vaccination coverage, but could not be captured when comparing vaccinated and non-vaccinated individuals from the same population. Interestingly, the fact that our estimated vaccine effectiveness is relatively low compared with vaccine efficacy reported in clinical trials suggests that there is no or little indirect protection provided by vaccination. This could indicate that the vaccine protects against disease but not against infection or that vaccinated groups of population are not those that contribute to the propagation of the virus.
A positive relationship between the number of arrivals at airports and mortality has been observed in this analysis, similarly as between mobility change and mortality. It suggests that both increased long-distance travel and increased mobility are strong predictors of growth in the daily number of COVID-19 deaths. These findings highlight the role of mobility reduction, both within and between countries, as an effective way to reduce COVID-19 mortality, especially when new virus variants spread across the world. Our results are in line with previous study by Jabłońska et al.50 suggesting that countries with lower reduction in mobility at the beginning of the pandemic experienced a higher COVID-19 daily deaths peak. The role of social distancing was also underlined by Badr et al.62 who observed a significant impact of mobility on COVID-19 transmission in the USA.
The daily temperature was found as a significant predictor of COVID-19 mortality in this study, with increasing temperature associated with the reduction in the number of deaths. Kerr et al.63 found no consensus on the impact of meteorological factors on COVID-19 spread in their literature review; however, they suggested existence of environmental sensitivity of COVID-19, but not as significant as non-pharmaceutical interventions and human behavior. Several authors underlined that disease seasonality may exist,64, 65, 66, 67 including Liu et al.66 who found that COVID-19 infection and mortality rates were higher in colder climates and that the cold season caused an increase in total infections, while the warm season contributed to the opposite effect. Because our analysis covered a full annual cycle of COVID-19, our result suggests the possibility of seasonal variations in COVID-19 incidence. Such seasonality has been well established in temperate climate for other respiratory viruses.68 , 69
Limitations
Our study has several limitations. First, our analysis was conducted on a country-level basis to estimate the vaccination efficacy, which should be seen as a less precise method than analysis of individual-level data, as previously noted. However, given the range of included countries, our results shed light on the problem of vaccination effectiveness from a broader perspective and investigate the effect of vaccination across societies, considering variability of vaccination coverage through time and between countries. Second, the quality of data on variants distribution varied between countries and was low for some of them; therefore, results of the exploratory analysis should be treated with cautious. To limit bias and avoid fluctuations, we used methods of interpolation and smoothing. Countries with limited data were excluded. Third, the set of covariates used in the multivariate analysis can be assessed as non-exhaustive. We decided to consider factors that were previously assessed as significantly impacting the risk of severe illness or mortality from COVID-19 in the literature.44, 45, 46, 47, 48, 49, 50, 51, 52, 53 Also, the significant random intercept observed in our models reflects unexplained between-countries variability resulting from the omission of influential variables. It was previously shown, for example, that COVID-19 mortality may be influenced by economic factors, which were not considered in this analysis.46 Finally, we were not able to consider other new SARS-CoV-2 VOCs, except B.1.1.7, in the current analysis, which was due to their limited spread in Europe as of April 2021. It rises a need for further research on this topic in the future.
Conclusions
This study confirms a strong effectiveness of COVID-19 vaccination based on real-life public data, in terms of protection against deaths being around 72%, although it appears to be slightly lower than could be expected from clinical trial results. This suggests the absence of indirect protection for non-vaccinated individuals. Results also suggest that vaccination effectiveness against mortality associated with the B.1.1.7 variant is high but slightly lower than other variants (70% and 78%, respectively). Finally, this analysis confirms the role of mobility reduction, both within and between countries, as an effective way to reduce COVID-19 mortality and supports the possibility of seasonal variations in COVID-19 incidence.
Author statements
Ethical approval
Not required. This study does not require ethical approval as it was conducted on country-level data and involved information freely available in the public domain.
Funding
None. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Competing interests
None declared.
Contributors
MT conceptualized the study. KJ and SA validated the study concept. KJ and SA collected and analyzed the data. KJ, SA, and MT interpreted the results. KJ wrote the first draft of the manuscript. KJ, SA, and MT revised the manuscript and approved the final version.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2021.07.037.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Liu Z., VanBlargan L.A., Rothlauf P.W., Bloyet L.-M., Chen R.E., Stumpf S., et al. Landscape analysis of escape variants identifies SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. bioRxiv. 2020 doi: 10.1016/j.chom.2021.01.014. 2020.11.06.372037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodcroft E.B. 2021. CoVariants: SARS-CoV-2 mutations and variants of interest.https://covariants.org/ [Google Scholar]
- 3.Rambaut A, Loman N, Pybus O. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 (accessed 15th March 2021).
- 4.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 2020:2020. 12.21.20248640. [Google Scholar]
- 5.Faria N., Claro I., Candido D., Franco L., Andrade P., Coletti T., et al. 2021. Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings.https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586 [Google Scholar]
- 6.Barnagarwala T., Mascarenhas A. 2021. Explained: B.1.617 variant and the Covid-19 surge in India.https://indianexpress.com/article/explained/maharashtra-double-mutant-found-b-1-617-variant-and-the-surge-7274080/ [Google Scholar]
- 7.Naveca F., da Costa C., Nascimento V. 2021. SARS-CoV-2 reinfection by the new variant of concern (VOC) P.1 in Amazonas, Brazil.https://virological.org/t/sars-cov-2-reinfection-by-the-new-variant-of-concern-voc-p-1-in-amazonas-brazil/596 [Google Scholar]
- 8.Iacobucci G. Covid-19: new UK variant may be linked to increased death rate, early data indicate. BMJ. 2021;372:n230. doi: 10.1136/bmj.n230. [DOI] [PubMed] [Google Scholar]
- 9.Jassat W., Mudara C., Ozougwu L., Tempia S., Blumberg L., Davies M.-A., et al. Increased mortality among individuals hospitalised with COVID-19 during the second wave in South Africa. Lancet Glob Health. 2021;9(9):e1216–e1225. doi: 10.1016/S2214-109X(21)00289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yadav P.D., Sapkal G.N., Abraham P., Ella R., Deshpande G., Patil D., et al. Neutralization of variant under investigation B.1.617 with sera of BBV152 vaccinees. Clin Infect Dis. 2021 May 7 doi: 10.1093/cid/ciab411. ciab411. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Wallace D.J., Ackland G.J. Abrupt increase in the UK coronavirus death-case ratio in December 2020. medRxiv. 2021 2021.01.21.21250264. [Google Scholar]
- 12.Challen R., Brooks-Pollock E., Read J.M., Dyson L., Tsaneva-Atanasova K., Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) 2021. Resport to SAGE: increased disease severity in people infected with variant of concern (VOC) B.1.1.7 compared to people infected with non-VOC virus variants.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/961042/S1095_NERVTAG_update_note_on_B.1.1.7_severity_20210211.pdf [Google Scholar]
- 14.Public Health England . 2021. SARS-CoV-2 variants of concern and variants under investigation in England.https://www.gov.uk/government/publications/investigation-of-novel-sars-cov-2-variant-variant-of-concern-20201201https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/968581/Variants_of_Concern_VOC_Technical_Briefing_7_England.pdf Technical briefing 7. 11th March. [Google Scholar]
- 15.Davies N.G., Jarvis C.I., van Zandvoort K., CMMID COVID-19 Working Group. Edmunds W.J., Jewell N.P., et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593:270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538) doi: 10.1126/science.abg3055. eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592(7854):438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 18.Pearson C., Russell T., Davies N., Kucharski A., CMMID COVID-19 working group. Edmunds W., et al. Centre for Mathematical Modelling of Infectious Diseases; 2021. Estimates of severity and transmissibility of novel SARS-CoV-2 variant 501Y.V2 in South Africa.https://cmmid.github.io/topics/covid19/sa-novel-variant.html [Google Scholar]
- 19.Freitas A.R.R., Beckedorff O.A., de Góes Cavalcanti L.P., Siqueira A.M., Castro D.B., Costa C.F.D., et al. 2021. The emergence of novel SARS-CoV-2 variant P.1 in Amazonas (Brazil) was temporally associated with a change in the age and gender profile of COVID-19 mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Oliveira M.H.S., Lippi G., Henry B.M. Sudden rise in COVID-19 case fatality among young and middle-aged adults in the south of Brazil after identification of the novel B.1.1.28.1 (P.1) SARS-CoV-2 strain: analysis of data from the state of Parana. medRxiv. 2021 2021.03.24.21254046. [Google Scholar]
- 21.The New York Times . 2021. Coronavirus vaccine tracker.https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html [Google Scholar]
- 22.Aschwanden C. 2021. Five reasons why COVID herd immunity is probably impossible.https://www.nature.com/articles/d41586-021-00728-2 [DOI] [PubMed] [Google Scholar]
- 23.Gu Y. 2021. Path to normality: 2021 outlook of COVID-19 in the US.https://covid19-projections.com/path-to-herd-immunity/ [Google Scholar]
- 24.Our world in data. Coronavirus (COVID-19) vaccinations. 2021. https://ourworldindata.org/covid-vaccinations [Google Scholar]
- 25.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novavax Inc . 2021. Novavax confirms high levels of efficacy against original and variant COVID-19 strains in United Kingdom and South Africa trials.https://ir.novavax.com/news-releases/news-release-details/novavax-confirms-high-levels-efficacy-against-original-and-0 11 March 2021. [Google Scholar]
- 30.Supasa P., Zhou D., Dejnirattisai W., Liu C., Mentzer A.J., Ginn H.M., et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184(8):2201–2211. doi: 10.1016/j.cell.2021.02.033. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang P., Casner R.G., Nair M.S., Wang M., Yu J., Cerutti G., et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29(5):747–751. doi: 10.1016/j.chom.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou D., Dejnirattisai W., Supasa P., Liu C., Mentzer A., Ginnet H., et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184(9):2348–2361. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emary K.R.W., Golubchik T., Aley P.K., Ariani C.V., Angus B., Bibi S., et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397(10282):1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.University of Oxford . 2021. ChAdOx1 nCov-19 provides minimal protection against mild-moderate COVID-19 infection from B.1.351 coronavirus variant in young South African adults.https://www.research.ox.ac.uk/Article/2021-02-07-chadox1-ncov-19-minimal-protection-against-mild-covid-19-from-b-1-351-variant-in-young-sa-adults [Google Scholar]
- 35.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Efficacy of the NVX-CoV2373 Covid-19 vaccine against the B.1.1.7 variant. N Engl J Med. 2021 Jun 30 doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinde V., Bhikha S., Hoosain Z., Archary M., Bhorat Q., Fairlie L., et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1899–1909. doi: 10.1056/NEJMoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 39.Hansen C.H., Michlmayr D., Gubbels S.M., Mølbak K., Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397(10280):1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention . 2021. COVID-19 breakthrough case investigations and reporting.https://www.cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html [Google Scholar]
- 41.Sanders R.W., de Jong M.D. Pandemic moves and countermoves: vaccines and viral variants. Lancet. 2021;397(10282):1326–1327. doi: 10.1016/S0140-6736(21)00730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Callaway E., Ledford H. How to redesign COVID vaccines so they protect against variants. Nature. 2021;590(7844):15–16. doi: 10.1038/d41586-021-00241-6. [DOI] [PubMed] [Google Scholar]
- 43.Bian L., Gao F., Zhang J., He Q., Mao Q., Xu M., et al. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expet Rev Vaccine. 2021:1–9. doi: 10.1080/14760584.2021.1903879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aldibasi O.S., Alharbi N.K., Alkelya M., Zowawi H., Alghnam S. The association of country-level factors with outcomes of COVID-19: analysis of the pandemic after one million cases. Res Square. 2020 [Google Scholar]
- 45.Burden S.J., Rademaker J., Weedon B.D., Whaymand L., Dawes H., Jones A. Associations of global country profiles and modifiable risk factors with COVID-19 cases and deaths. medRxiv. 2020 2020.06.17.20133454. [Google Scholar]
- 46.Sorci G., Faivre B., Morand S. Explaining among-country variation in COVID-19 case fatality rate. Sci Rep. 2020;10:18909. doi: 10.1038/s41598-020-75848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia de Alcaniz J.G., Romero-Lopez J., Martinez R.P., Lopez-Rodas V., Costas E. What variables can better predict the number of infections and deaths worldwide by SARS-CoV-2? Variation through time. medRxiv. 2020 2020.06.04.20122176. [Google Scholar]
- 48.Woody S., Tec M., Dahan M., Gaither K., Lachmann M., Fox S.J., et al. Projections for first-wave COVID-19 deaths across the US using social-distancing measures derived from mobile phones. medRxiv. 2020:2020. 04.16.20068163. [Google Scholar]
- 49.Courtemanche C., Garuccio J., Le A., Pinkston J., Yelowitz A. Strong social distancing measures in the United States reduced the COVID-19 growth rate. Health Affairs. 2020;39(7):1237–1246. doi: 10.1377/hlthaff.2020.00608. [DOI] [PubMed] [Google Scholar]
- 50.Jabłońska K., Aballéa S., Toumi M. Factors influencing the COVID-19 daily deaths' peak across European countries. Public Health. 2021;194:135–142. doi: 10.1016/j.puhe.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020:1–8. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention . 2021. COVID-19: people at increased risk with certain medical conditions.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html [Google Scholar]
- 53.Omar S.A., Naif K.A., Mohamed A., Hosam Z., Suliman A. The association of country-level factors with outcomes of COVID-19: analysis of the pandemic after one million cases. Res Square. 2021 [Google Scholar]
- 54.Hadfield J., Megill C., Bell S.M., Huddleston J., Potter B., Callender C., et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018 doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bedford T., Neher R., Hadfield J., Hodcroft E., Sibley T., Huddleston J., et al. 2021. Nextstrain - real-time tracking of pathogen evolution.https://nextstrain.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Global Initiative on Sharing All Influenza Data (GISAID). The GISAID Initiative. https://www.gisaid.org/(accessed 14th March 2021).
- 57.Eurostat . 2021. Eurostat database.https://ec.europa.eu/eurostat/data/database [Google Scholar]
- 58.National Centers for Environmental Information . 2021. Global surface summary of the day.https://www.ncei.noaa.gov/access/search/data-search/global-summary-of-the-day [Google Scholar]
- 59.EUROCONTROL . 2021. Aviation intelligence portal.https://ansperformance.eu/data/ [Google Scholar]
- 60.Google . 2021. COVID-19 community mobility Reports.https://www.google.com/covid19/mobility/ [Google Scholar]
- 61.Jabłońska K., Aballéa S., Auquier P., Toumi M. On the association between SARS-COV-2 variants and COVID-19 mortality during the second wave of the pandemic in Europe. medRxiv. 2021 doi: 10.1080/20016689.2021.2002008. 2021.03.25.21254289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Badr H.S., Du H., Marshall M., Dong E., Squire M.M., Gardner L.M. Association between mobility patterns and COVID-19 transmission in the USA: a mathematical modelling study. Lancet Infect Dis. 2020;20(11):1247–1254. doi: 10.1016/S1473-3099(20)30553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kerr G.H., Badr H.S., Gardner L.M., Perez-Saez J., Zaitchik B.F. Associations between meteorology and COVID-19 in early studies: inconsistencies, uncertainties, and recommendations. One Health. 2021;12:100225. doi: 10.1016/j.onehlt.2021.100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merow C., Urban M.C. Seasonality and uncertainty in global COVID-19 growth rates. Proc Natl Acad Sci Unit States Am. 2020;117(44):27456–27464. doi: 10.1073/pnas.2008590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engelbrecht F.A., Scholes R.J. Test for Covid-19 seasonality and the risk of second waves. One Health. 2021;12:100202. doi: 10.1016/j.onehlt.2020.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X., Huang J., Li C., Zhao Y., Wang D., Huang Z., et al. The role of seasonality in the spread of COVID-19 pandemic. Environ Res. 2021;195:110874. doi: 10.1016/j.envres.2021.110874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Audi A., AlIbrahim M., Kaddoura M., Hijazi G., Yassine H.M., Zaraket H. Seasonality of respiratory viral infections: will COVID-19 follow suit? Front Public Health. 2020;8(576) doi: 10.3389/fpubh.2020.567184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of respiratory viral infections. Annu Rev Virol. 2020;7(1):83–101. doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- 69.Martinez M.E. The calendar of epidemics: seasonal cycles of infectious diseases. PLoS Pathog. 2018;14(11) doi: 10.1371/journal.ppat.1007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.