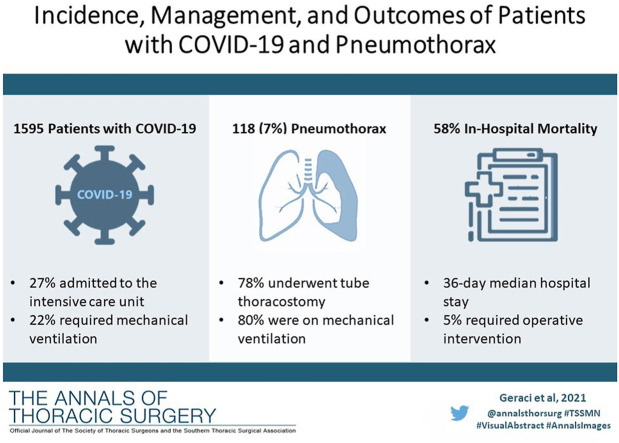
Abstract
Background
Our objective was to report the incidence, management, and outcomes of patients who developed a secondary pneumothorax while admitted for coronavirus disease 2019 (COVID-19).
Methods
A single-institution, retrospective review of patients admitted for COVID-19 with a diagnosis of pneumothorax between March 1, 2020, and April 30, 2020, was performed. The primary assessment was the incidence of pneumothorax. Secondarily, we analyzed clinical outcomes of patients requiring tube thoracostomy, including those requiring operative intervention.
Results
From March 1, 2020, to April 30, 2020, 118 of 1595 patients (7.4%) admitted for COVID-19 developed a pneumothorax. Of these, 92 (5.8%) required tube thoracostomy drainage for a median of 12 days (interquartile range 5-25 days). The majority of patients (95 of 118, 80.5%) were on mechanical ventilation at the time of pneumothorax, 17 (14.4%) were iatrogenic, and 25 patients (21.2%) demonstrated tension physiology. Placement of a large-bore chest tube (20 F or greater) was associated with fewer tube-related complications than a small-bore tube (14 F or less) (14 vs 26 events, P = .011). Six patients with pneumothorax (5.1%) required operative management for a persistent alveolar-pleural fistula. In patients with pneumothorax, median hospital stay was 36 days (interquartile range 20-63 days) and in-hospital mortality was significantly higher than for those without pneumothorax (58% vs 13%, P < .001).
Conclusions
The incidence of secondary pneumothorax in patients admitted for COVID-19 is 7.4%, most commonly occurring in patients requiring mechanical ventilation, and is associated with an in-hospital mortality rate of 58%. Placement of large-bore chest tubes is associated with fewer complications than small-bore tubes.
Visual Abstract
Dr Cerfolio discloses a financial relationship with AstraZeneca, Bard Davol, Bovie Medical Corporation, C-SATS, ConMed, Covidien/Medtronic, Ethicon, Fruit Street Health, Google/Verb Surgical, Intuitive Surgical, KCI/Acelity, Myriad Genetics, Neomend, Pinnacle Biologics, ROLO-7, Tego, and TransEnterix; Dr Bizekis with CSA Medical; Dr. Zervos with Intuitive Surgical.
Patients with severe coronavirus disease 2019 (COVID-19) from infection with acute respiratory syndrome coronavirus 2 often develop hypoxia and hypercarbia as a result of profound parenchymal inflammation and alveolar injury. In an estimated 20% of patients admitted with COVID-19, respiratory insufficiency progresses to acute respiratory distress syndrome (ARDS) mandating mechanical ventilation.1 With decreased pulmonary compliance and inflamed alveoli, high levels of airway pressure and fraction of inspired oxygen (FiO2) are often required to maintain adequate ventilation and gas exchange. In this context, a number of pulmonary complications may occur, including secondary spontaneous pneumothorax. Equally, secondary pneumothorax often results after the development of cystic lung degeneration with or without secondary infection, pathophysiology that mirrors the destructive lung processes observed in patients with severe COVID-19.2
To determine the incidence of secondary pneumothorax in patients admitted with COVID-19, we performed a retrospective review of patients admitted to our hospital during the initial wave of the COVID-19 pandemic. Additionally, we evaluated the care of patients with pneumothorax, including management with tube thoracostomy and select patients requiring operative intervention. Lastly, we assessed clinical outcomes in patients with pneumothorax, including tube associated complications and in-hospital mortality.
Patients and Methods
A retrospective analysis was performed including patients age 18 years or greater, admitted to the New York University Langone Health Tisch Hospital from March 1, 2020, to April 30, 2020, with a diagnosis of COVID-19 and pneumothorax. Data collection was performed to August 15, 2020, to allow for 30-day perioperative outcomes in all patients. The diagnosis of COVID-19 was established by nasopharyngeal swab for reverse transcriptase polymerase chain reaction. The diagnosis of pneumothorax was established by radiology reports of chest radiographs and computed tomography scans. A tension pneumothorax was defined as the development of new onset hypotension in the setting of suspected or documented pneumothorax that resolved after needle decompression and/or placement of a chest tube. Patients with isolated pleural effusion (8 patients), traumatic pneumothorax (2 patients), or those requiring an emergent tube thoracostomy during active cardiopulmonary resuscitation (3 patients) were excluded. Patients hospitalized for a thoracic surgical procedure and/or an alternative pathology resulting in pneumothorax, who subsequently tested positive for COVID-19, were also excluded (4 patients).
Clinical data were obtained through retrospective review of the electronic medical record. Descriptive statistics were used to summarize the data and results are reported as median values and interquartile range (IQR) for all nonparametric data. Patients were stratified into 2 groups based on size of initial chest tube placed for pneumothorax. Categorical variables were compared using Fischer's exact test. A 2-sided P value less than .05 was predetermined to be statistically significant. Statistical analyses were performed with SPSS Statistics for Windows, version 25.0 (IBM Corp, Armonk, NY). Data collection and management were approved by the institutional review board at New York University Langone Health (# i20-00655).
Results
From March 1, 2020, to April 30, 2020, 1595 patients were admitted with COVID-19, of which 439 (27.5%) were admitted to an intensive care unit, and 344 (21.6%) required mechanical ventilation. Of all patients admitted for COVID-19, 118 (7.4%) developed a pneumothorax and 92 required tube thoracostomy drainage (5.8% overall, 78% of patients with pneumothorax) for a median of 12 days (IQR 5-25 days) with a range of 2 to 86 days. In patients with a pneumothorax, median hospital stay was 36 days (IQR 18-66 days) with a median of 10 days (6.8-25 days) between chest tube removal and discharge in survivors.
Among patients with pneumothorax, the median patient age was 67 years (IQR 59-74 years), and the majority were male (76%) never smokers (51%). Pneumothorax occurred as the presenting pathology of COVID-19 (present on the patient’s initial chest radiograph) in 2 patients (1.7%) and no patients who developed a pneumothorax had evidence of pulmonary blebs or bullous emphysema on initial chest radiograph. Overall, patients were admitted for a median of 14 days (IQR 6-22 dayS) prior to developing a pneumothorax. On univariate analysis, the development of pneumothorax was associated with the need for mechanical ventilation (95 patients, 80.5%; P < .001) and venovenous extracorporeal membrane oxygenation for COVID-19 respiratory failure (10 patients, 8.5%; P < .001). Patients requiring mechanical ventilation were supported for a median of 8 days (IQR 1-18 days) prior to pneumothorax, with median positive inspiratory pressure (PIP) of 33 cmH2O (IQR 27-42), median positive end-expiratory pressure of 10 cm H2O (IQR 8-12 cm H2O), and median FiO2 of 75% (IQR 50%-100%). At the time of pneumothorax, patients had a median arterial partial pressure of oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio (P:F ratio) of 100 (IQR 70-178). Patient characteristics are reported in Table 1 .
Table 1.
Characteristics of Patients Admitted for COVID-19 Who Developed a Secondary Pneumothorax vs Patients Without Pneumothorax
| Variable | Patients With Pneumothorax (n = 118) | Patients Without pneumothorax (n = 1477) | P Value |
|---|---|---|---|
| Age, y | 67 (59-74) | 65 (55-73) | .315 |
| Sex | .004 | ||
| Male | 90 (76) | 931 (63) | |
| Female | 28 (24) | 546 (37) | |
| Body mass index, kg/m2 | 28 (24-32) | 29 (24-34) | .121 |
| Smoking history | |||
| Never smoker | 60 (51) | 796 (54) | .565 |
| Yes, current smoker | 5 (4.3) | 77 (5.2) | .829 |
| Yes, former smoker | 25 (21) | 295 (20) | .721 |
| Not assessed/unknown | 28 (24) | 309 (21) | .482 |
| Comorbid conditions | |||
| Hypertension | 61 (52) | 915 (62) | .031 |
| Hyperlipidemia | 48 (41) | 635 (43) | .699 |
| Diabetes mellitus | 42 (36) | 517 (35) | .920 |
| Coronary artery disease | 18 (15) | 295 (20) | .231 |
| Chronic kidney disease | 14 (12) | 266 (18) | .532 |
| Asthma, chronic obstructive pulmonary disease | 11 (9.3) | 162 (11) | .879 |
| Heart failure | 12 (10) | 177 (12) | .460 |
| Malignancy | 6 (5.1) | 118 (8) | .369 |
| Respiratory support | |||
| Mechanical ventilation | 95 (80.5) | 310 (21) | <.001 |
| At time of pneumothorax | 33 (27-42) | ||
| PIP, cm H2O | 10 (8-12) | ||
| PEEP, cm H2O | 75 (50-100) | ||
| FiO2, % | 100 (70-178) | ||
| P:F ratio | 10 (8.5) | 17 (1.2) | <.001 |
| ECMO | |||
| Admitted to the intensive care unit | 110 (93) | 329 (22) | <.001 |
| Hospital days prior to PTX | 14 (6-22) | N/A | |
| Hospital days requiring mechanical ventilation prior to PTX | 8 (1-18) | N/A |
Values are presented as median (interquartile range) or n (%).
ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen, PEEP, positive end-expiratory pressure; P:F partial pressure of arterial oxygen / fraction of inspired oxygen; PIP, peak inspiratory pressure; PTX, pneumothorax.
Pneumothorax was right-sided in 59 patients (50%) and left-sided in 55 patients (47%) and 4 patients (3.4%) had bilateral pneumothoraces. Pneumothorax was iatrogenic in 17 patients (14%), most commonly after placement of a central venous catheter (65%). Twenty-five patients (21%) demonstrated tension physiology, of which 2 patients suffered cardiopulmonary arrest. Ten patients (8.4%) developed concomitant subcutaneous emphysema (distant from or prior to tube thoracostomy) and 6 patients (5.1%) had pneumomediastinum. Subcutaneous emphysema and pneumomediastinum were diagnosed independent of a pneumothorax in 14 patients (0.8%) and 34 patients (2.1%), respectively. Pneumothorax data are reported in Table 2 .
Table 2.
Clinical Data and Outcomes of Patients Admitted for COVID-19 With Secondary Pneumothorax (N = 118)
| Variable | Value |
|---|---|
| Pneumothorax | |
| Left side | 55 (47) |
| Right side | 59 (50) |
| Bilateral | 4 (3.4) |
| Pneumothorax requiring chest tube | 92 (78) |
| Placement of second chest tube on ipsilateral side | 15 (16) |
| Placement of second chest tube on contralateral side | 10 (11) |
| Number of chest tubes, median (interquartile range; range) | 1 (1-2; 1-5) |
| Iatrogenic pneumothorax | 17 (14) |
| Post central venous access | 11 (65) |
| Post percutaneous tracheostomy | 2 (2.2) |
| Post cardiopulmonary resuscitation | 2 (2.2) |
| Post pleural drainage procedure | 1 (1.1) |
| Post nasogastric feeding tube | 1 (1.1) |
| Associated pathology | |
| Tension physiology | 25 (21) |
| Cardiopulmonary arrest attributed to tension physiology | 2 (8) |
| Subcutaneous emphysema | 10 (8.4) |
| Pneumomediastinum | 6 (5.1) |
| Operative intervention | 6 (5.1) |
| Length of stay, d | 36 (18-66) |
Values are presented as median (interquartile range) or n (%), unless otherwise marked.
In patients who underwent tube thoracostomy, a median of 1 chest tube was required (range 1-5), for a median of 15 days (IQR 5-25 days) until removal or death. Fourteen patients (15%) required interval placement of a second ipsilateral tube and 10 patients (11%) required a second contralateral tube. A small-bore (14 F or less) tube was placed in 47 patients (51%) and a large-bore tube (20 F or greater) in 45 patients (49%). A total of 40 complications in 40 unique patients were attributed to tube thoracostomy, most commonly from incomplete lung expansion requiring a second tube or upsizing of a small-bore tube. Placement of a large-bore chest tube was associated with fewer tube-related complications than a small-bore tube (14 vs 26 events, P = .011). Tube thoracostomy data and complications are reported in Table 3 .
Table 3.
Data and Outcomes of Tube Thoracostomy for Pneumothorax in Patients With COVID-19 (N = 92)
| Variable | Value | P Value |
|---|---|---|
| Chest tube type | … | |
| Small-bore tube (14 F or less) | 47 (51) | |
| 8.5 F | 15 (16) | |
| 12 F | 5 (5.4) | |
| 14 F | 27 (29) | |
| Large-bore tube (20 F or greater) | 45 (49) | |
| 20 F | 1 (1.1) | |
| 24 F | 3 (3.3) | |
| 28 F | 36 (39) | |
| 32 F | 5 (5.4) | |
| Chest tube complications | 40 events in 40 patients (43%) | … |
| Incomplete lung re-expansion due to air-leak | 26 (28) | … |
| Placement of second chest tube on ipsilateral side | 14 (15) | |
| Required upsizing of chest tube | 12 (13) | |
| Tube dislodged, retracted, or fell out, requiring replacement | 6 (6.5) | |
| Tube clotted/ obstructed requiring replacement | 5 (5.4) | |
| Bleeding attributed to chest tube/ placement | 3 (3.3) | |
| Chest tube complications per tube type | .011 | |
| Small-bore tube (14 Fr or less) | 26 events in 47 patients (55%) | |
| Large-bore tube (20 Fr or greater) | 14 events in 45 patients (31%) | |
| Chest tube duration, median (IQR; range), d | 12 (5-25; 2-86) | … |
| Mortality with chest tube in place | 38 (41) | |
| If discharged, days from tube removal to discharge | 10 (6.8-25) |
Values are presented as median (interquartile range) or n (%), unless otherwise marked.
Six patients (5.1%) required operative management after developing a pneumothorax. Patients were taken to surgery after a median of 47 days (IQR 27-57 days) with a chest tube. Four patients developed cystic lung destruction with a pneumatocele resulting in a persistent air leak from an alveolar-pleural fistula. A single patient with cystic lung destruction was taken to the operating room as a rescue effort for acute hypercapnic respiratory failure. One patient required lysis of adhesions and tracheostomy exchange for a persistent air leak with pneumomediastinum.
The in-hospital mortality rate was significantly higher in patients who developed a pneumothorax, than those who did not (58% vs 13%, P < .001). Specifically, the mortality rate among patients admitted to the intensive care unit requiring mechanical ventilation was significantly higher in those that developed a pneumothorax (66% vs 43%, P < .001). In-hospital mortality data are reported in Table 4 .
Table 4.
In-Hospital Mortality of Patients Admitted for COVID-19 With and Without Pneumothorax Based on Hospital Level of Care and Respiratory Support
| Variable | Patients with pneumothorax (N = 118) | Patients without pneumothorax (N = 1477) | P Value |
|---|---|---|---|
| In-hospital mortality | 69 (58) | 198 (13) | <.001 |
| Hospitalized, not in an ICU | 8 (6.8) | 1148 (78) | .425 |
| Mortality rate | 1 (0.8) | 76 (5.1) | |
| ICU, not requiring mechanical ventilation | 15 (13) | 80 (5.4) | .297 |
| Mortality rate | 5 (4.2) | 15 (1.0) | |
| ICU, requiring mechanical ventilation | 95 (81) | 249 (17) | <.001 |
| Mortality rate | 63 (53) | 107 (7.2) |
Values are presented as n (%).
ICU, intensive care unit.
Comment
Patients with COVID-19 disease are often admitted with respiratory symptoms and are at risk for progressive respiratory failure and ARDS. In an autopsy study of patients who died from COVID-19, the pulmonary parenchyma revealed diffuse alveolar damage with perivascular T-cell infiltration and concomitant widespread thrombosis with microangiopathy of the pulmonary vessels.3 In this context of noncompliant diseased lung subjected to high levels of mechanical ventilator support, a number of pulmonary complications may occur, including secondary spontaneous pneumothorax.
In this study, we report a 7.4% incidence of secondary pneumothorax in patients admitted for COVID-19. The majority of patients (78%) admitted for COVID-19 who developed a pneumothorax required tube thoracostomy. The in-hospital mortality rate for patients who developed a pneumothorax was 58%, primarily driven by a high mortality rate (66%) of mechanically ventilated patients in the intensive care unit. Similar to our results, in a review of 49 patients, Want and associates4 reported a 10% incidence of secondary pneumothorax in critically ill patients with COVID-19, which was associated with high mortality (4 of 5, 80%). For context, in non–COVID-19 patients requiring mechanical ventilation, the incidence of secondary pneumothorax is approximately 3%, but is dependent on the underlying lung pathology: The incidence ranges from 2.9% in patients with chronic obstructive pulmonary disease, to 6.3% in patients with asthma, and 10% in patients with interstitial lung disease.5 , 6 The incidence of secondary pneumothorax in patients with non–COVID-19 ARDS is approximately 6.5% and was relatively infrequent (1.7%) in patients who had severe acute respiratory syndrome in 2003.7 , 8 Data reported over the past decade estimate an approximate 30% 30-day mortality from non–COVID-19 ARDS.9
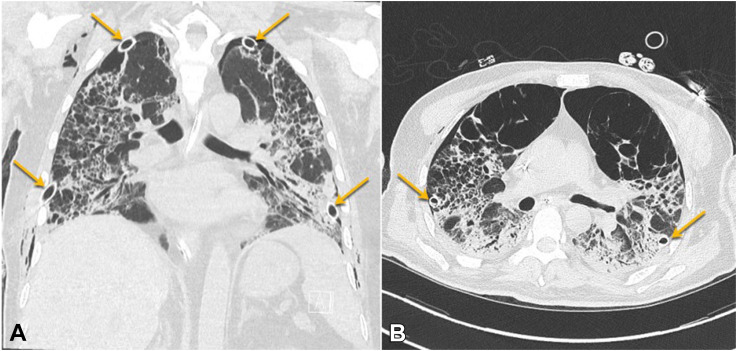
The majority of patients who developed a pneumothorax were supported with mechanical ventilation with high positive inspiratory pressure, positive end-expiratory pressure and had a low P:F ratio, suggesting barotrauma and/or hyperinflation as a potential mechanism of injury in the setting of inflamed lung parenchyma with cystic degeneration from COVID-19 (Figure 1 ). Equally, a secondary mechanism of disease is suggested by the fact that only a minority of patients who developed a pneumothorax had a preadmission diagnosis of COPD (11 patients, 9.3%) and no patients who developed a pneumothorax had evidence of pulmonary blebs or bullous emphysema on initial chest radiograph. Barotrauma in patients on mechanical ventilation for COVID-19 has been associated with prolonged hospitalization and higher mortality.10 Furthermore, critically ill patients with COVID-19 required invasive procedures, which resulted in a 14% rate of iatrogenic pneumothorax, most commonly occurring after placement of a central venous catheter (65%).
Figure 1.
Cystic parenchymal destruction and bilateral tube thoracostomy drainage (arrows) in a 38-year-old patient with severe COVID-19 disease. (A) Coronal computed tomography image. (B) Axial computed tomography image.
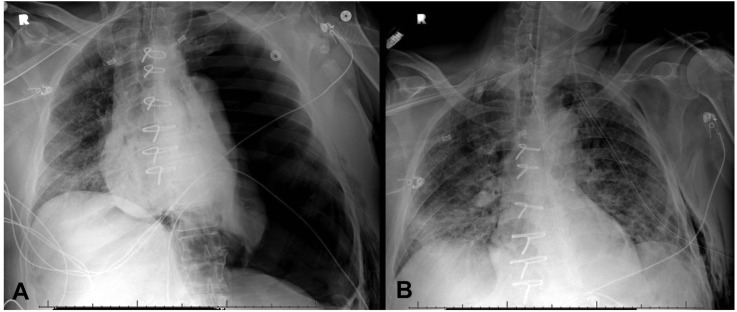
In the setting of high ventilator pressures and volumes, a significant number of patients with pneumothorax developed tension physiology (21%) and 2 patients subsequently sustained cardiopulmonary arrest (Figure 2 ). The incidence of tension pneumothorax is unknown, but is relatively rare, most often reported after visceral pleural injury from chest trauma and is more common in ventilated than awake patients.11 Given that patients with COVID-19 often manifested symptoms similar to those with pneumothorax—hypoxia, tachycardia, tachypnea, and decreased breath sounds by auscultation—the high incidence of tension physiology may have reflected a delay in diagnosis, allowing continued egress of air through a pleural defect leading to more severe respiratory and hemodynamic compromise. In ventilated patients, this process was likely accelerated by high driving pressures, tidal volumes, and/or positive end-expiratory pressure.
Figure 2.
Anterior-posterior chest radiographs of a 58-year-old patient with COVID-19 disease. (A) Left pneumothorax after placement of a left internal jugular central venous catheter. The patient developed tension physiology which resolved after (B) placement of a left pleural 28 F tube thoracostomy.
Pneumothorax in patients with COVID-19 was associated with subcutaneous emphysema (8.4%) and pneumomediastinum (5.1%), although these pathologies also developed independent of pneumothorax (0.8% and 2.1%, respectively). The majority of subcutaneous emphysema occurred in patients with a pneumothorax and resolved with tube thoracostomy alone, although a decompressing “blow-hole” incision was required in 2 patients with massive subcutaneous emphysema. Other pleural complications were relatively rare, such as secondary bacterial infection causing empyema (2 patients) or reactive and/or parapneumonic pleural effusion requiring drainage (8 patients).
The majority of COVID-19 patients (78%) who developed a pneumothorax required tube thoracostomy with a median of 1 tube (range, 1-5 tubes) for a median of 12 days (IQR 5-25 days; range, 2-86 days). Patients who did not require a chest tube most commonly had either a small volume of extrapleural air that did not progress with observation, or goals of care that did not include invasive procedures. The thoracic surgery service placed the majority of chest tubes (68 of 92, 74%), recommending 20 mm Hg of suction drainage until resolution of air leak. We did not apply viral filters or other devices to the chest drains. Due to the high volume of care, however, the management of chest tubes varied substantially; the titration of suction to water seal and the timing of removal were most frequently at the discretion of the primary treatment team.
Patients treated with large-bore chest tubes (20 F or greater) sustained fewer complications than patients with small bore chest tubes (14 F or less) (14 vs 26 events, P = .011). Small-bore chest tubes more often failed to achieve full lung reexpansion and more often became obstructed with fibrinous material or blood clot, and therefore required upsizing or replacement with a large-bore tube. A second ipsilateral chest tube was placed in 14 patients for failure of lung expansion with air leak, 6 after an initial tube fell out or was dislodged, and 5 for obstruction with fibrinous material or clot. In all cases, the indication for another tube was for an expanding pneumothorax and/or uncontrolled air leak, often clinically manifesting as hypoxia, hypercarbia, or difficulty maintaining adequate ventilation. Similarly, 12 patients with a small-bore chest tube had an expanding pneumothorax and/or uncontrolled air leak requiring up-sizing to a large-bore tube. The severity of air leak was not objectively recorded in this study, although anecdotally we observed that the volume of air-leak from COVID-19 disease was often greater than non-COVID patients. We conjecture that this was due to high ventilator settings in the context of severely diseased lung tissue.
Bleeding complications associated with chest tube placement were difficult to determine given a lack of defined clinically relevant volume of loss. Three patients, however, had greater than 500 mL of blood loss attributed to tube thoracostomy (1 small bore, 2 large-bore tubes). All patients were anticoagulated on a heparin infusion at time of chest tube placement. In all 3 patients, bleeding resolved upon cessation of anticoagulation and none required transfusion or operative intervention. None of the patients had recurrent bleeding upon continuation of anticoagulation 12-24 hours after the event.
In a minority of patients, progressive destruction of the lung parenchyma resulted in volume loss, cystic degeneration, and/or pneumatocele, which resulted in a persistent alveolar-pleural fistula. In 6 patients who had initially developed a pneumothorax from COVID-19, a surgical intervention was eventually required to treat the underlying disease process. Successful surgical interventions for COVID-19–related pulmonary disease have been published in limited case reports.12 , 13 The primary indication for surgery in these patients was for persistent pneumothorax with an alveolar-pleural fistula that did not resolve with tube thoracostomy. We did not operate on patients with cystic disease and/or pneumatocele in the absence of recurrent pneumothorax or air-leak (2 patients). In 4 patients, resection of a pneumatocele was performed. In cases of a discrete anatomic location of air leak, surgical stapling was successful in sealing the pleural fistula. Two patients underwent emergent surgical intervention as a rescue effort for severe respiratory failure due to an uncontrolled air-leak which impaired ventilation resulting in progressive hypoxia and hypercarbia.
The development of a pneumothorax due to COVID-19 was associated with a median 36-day (IQR 20-63 days) length of stay. The duration of hospitalization is likely a reflection of critical illness and not pneumothorax alone. To this point, in survivors, there was a median of 10 days (IQR 6.8-25 days) between chest tube removal and discharge, suggesting that pneumothorax and tube thoracostomy were rarely the limiting factor in hospital discharge. The mortality rate in patients with COVID-19 who developed a pneumothorax was significantly higher than patients without pneumothorax (58% vs 13%, P < .001) primarily driven by a high mortality rate (66%) of patients with a pneumothorax who were mechanically ventilated patients in the intensive care unit. Equally, a substantial number of patients requiring tube thoracostomy died with the chest tube in place (41%). These data suggest that the development of a pneumothorax may be a marker of more severe COVID-19 infection and greater associated pulmonary disease.
This study is supported by a relatively large sample size of patients with follow-up through hospital discharge in the majority of patients. Data were complete in all patients without missing variables. Of note, throughout the operative and perioperative care of COVID-19 patients with pneumothorax, no health care provider or staff tested positive for the virus. The primary limitation of this study is its retrospective design. Furthermore, a more robust comparison group that included ventilator settings would have potentially identified factors associated with the development of pneumothorax in mechanically ventilated patients. Regarding tube thoracostomy, there was considerable variability in the placement and management of chest tubes, which confounds conclusions drawn from the data. Equally, we did not assess the post insertion location of chest tubes, which may have affected their overall function more than size alone.
In conclusion, in our institutional experience, the incidence of secondary pneumothorax in patients admitted with COVID-19 was 7.4%. The development of a pneumothorax was associated with the need for mechanical ventilation and/or extracorporeal membrane oxygenation. Placement of a large-bore chest tube for pneumothorax was associated with fewer complications than a small-bore tube. Patients with COVID-19–associated pneumothorax had a significantly higher in-hospital mortality rate than COVID-19 patients who did not develop a pneumothorax, which was primarily driven by mortality in patients requiring mechanical ventilation.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020:3231239–3231242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X.H., Duan J., Han X., et al. High incidence and mortality of pneumothorax in critically Ill patients with COVID-19. Heart Lung. 2021;50:37–43. doi: 10.1016/j.hrtlng.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anzueto A., Frutos-Vivar F., Esteban A., et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004;30:612–619. doi: 10.1007/s00134-004-2187-7. [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L., Bombino M., Pelosi P., et al. ARDS: lung structure and function in different stages of severe adult respiratory distress syndrome. JAMA. 1994;271:1772–1779. [PubMed] [Google Scholar]
- 7.Woodside K.J., vanSonnenberg E., Chon K.S., Loran D.B., Tocino I.M., Zwischenberger J.B. Pneumothorax in patients with acute respiratory distress syndrome: pathophysiology, detection, and treatment. J Intensive Care Med. 2003;18:9–20. doi: 10.1177/0885066602239120. [DOI] [PubMed] [Google Scholar]
- 8.Sihoe A.D.L., Wong R.H.L., Lee A.T.H., et al. Severe acute respiratory syndrome complicated by spontaneous pneumothorax. Chest. 2004;125:2345–2351. doi: 10.1378/chest.125.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Máca J., Jor O., Holub M., et al. Past and present ARDS mortality rates: a systematic review. Respir Care. 2017;62:113–122. doi: 10.4187/respcare.04716. [DOI] [PubMed] [Google Scholar]
- 10.Jones E., Gould A., Pillay T.D., et al. Subcutaneous emphysema, pneumomediastinum, and pneumothorax in critically ill patients with coronavirus disease 2019: a retrospective cohort study. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leigh-Smith S., Harris T. Tension pneumothorax—time for a re-think? Emerg Med J. 2005;22:8–16. doi: 10.1136/emj.2003.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castiglioni M., Pelosi G., Meroni A., et al. Surgical resections of superinfected pneumatoceles in a COVID-19 patient. Ann Thorac Surg. 2021;111:e23–e25. doi: 10.1016/j.athoracsur.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geraci T.C., Narula N., Smith D.E., et al. Lobectomy for hemorrhagic lobar infarction in a patient with COVID-19. Ann Thorac Surg. 2021;111:e183–e184. doi: 10.1016/j.athoracsur.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]