Abstract
Objectives
To assess the time to resolution of respiratory and systemic symptoms and their associated factors in outpatients during the coronavirus disease 2019 (COVID-19) pandemic.
Methods
Cohort study including adult outpatients, managed with Covidom, a telesurveillance solution, with RT-PCR-confirmed diagnosis, from 9 March 2020 until 23 February 2021. Follow up was 30 days after symptom onset.
Results
Among the 9667 patients included, mean age was 43.2 ± 14.0 years, and 67.5% were female (n = 6522). Median body mass index (BMI) was 25.0 kg/m2 (interquartile range 22.1–28.8 kg/m2). Main co-morbidities were: hypertension (12.9%; n = 1247), asthma (11.0%; n = 1063) and diabetes mellitus (5.5%; n = 527). The most frequent symptom during follow up was dyspnoea (65.1%; n = 6296), followed by tachypnoea (49.9%; n = 4821), shivers (45.6%; n = 4410) and fever (36.7%; n = 3550). Median times to resolution of systemic and respiratory symptoms were 3 days (95% CI 2−4 days) and 7 days (95% CI 6−8 days), respectively. Ultimately, 17.2% (95% CI 15.7%−18.8%) still presented respiratory symptoms at day 30. Longer time to respiratory symptom resolution was associated with older age, increased BMI, chronic obstructive pulmonary disease, coronary artery disease, asthma and heart failure. Regarding systemic symptoms, coronary artery disease, asthma, age above 40 years and elevated BMI were associated with longer time to resolution.
Conclusions
Time to symptom resolution among outpatients with COVID-19 seemed shorter for systemic than respiratory symptoms. Prolonged respiratory symptoms were common at day 30. Risk factors associated with later resolution included age, and cardiovascular and pulmonary diseases.
Keywords: Community setting, Coronavirus disease 2019, Dyspnoea, Sequelae, Symptom duration, Telemedicine
Introduction
The clinical manifestations in individuals with coronavirus disease 2019 (COVID-19) range from asymptomatic to severe forms requiring critical care, and the duration of symptoms can vary widely [1,2]. Most studies described the clinical course of hospitalized severe cases [[3], [4], [5]]. However, most patients present with milder forms of COVID-19 [6], and are therefore managed as outpatients. Data on this population are scarce, but describing the symptom course of outpatients with COVID-19 and factors associated with symptom duration may help provide adequate follow up [2,[7], [8], [9]].
Our study aimed to evaluate the time to resolution of COVID-19 respiratory and systemic symptoms, and factors associated with a longer duration in a large cohort of outpatients.
Materials and methods
This study included adults (≥18 years), initially managed as outpatients, from 9 March 2020 to 23 February 2021 by Covidom, a telesurveillance solution for home monitoring of patients with COVID-19 in the greater Paris area [10]. Covidom outpatients were registered by a physician after consulting for COVID-related symptoms and agreeing to the monitoring. We included patients with an RT-PCR-confirmed diagnosis, having answered at least one monitoring questionnaire, and who provided medical background at inclusion. Available data for each patient included age, gender, date of first symptoms, weight, height and co-morbidities.
When included in Covidom, the patients completed, on a daily basis, one or two self-administered questionnaires on symptoms until 30 days after symptom onset. Self-reported data were: respiratory rate (tachypnoea defined as >20 breaths per minute), heart rate (tachycardia defined as >100 beats per minute), temperature (fever and hypothermia defined as temperatures >38.5°C and <35.5°C, respectively), dyspnoea (on a 1-to-5 modified Borg scale [11], slight dyspnoea defined as a rating ≥2, and moderate dyspnea by ≥ 3), oxygen saturation (desaturation defined as <95%), dizziness and shivers.
Two main groups of symptoms were defined:
-
-
Systemic symptoms: fever or hypothermia, dizziness, tachycardia, shivers.
-
-
Respiratory symptoms: dyspnea, tachypnea, low oxygen saturation.
Kaplan–Meier estimators were used to evaluate time to resolution of symptoms. Patients were considered at risk from the date of first symptom onset (declared at inclusion) until the last occurrence of each symptom in daily questionnaires. Regarding symptom groups, patients were considered at risk until the last day of any symptom occurrence of the group. Patients were censored at the end of their follow up (30 days or earlier in case of premature ending). Factors independently associated with longer time to resolution of systemic and respiratory symptoms were evaluated separately on complete cases using multivariate Cox models and inverse hazard ratios as the event of interest was initially resolution of symptoms. α risk was set at 5%.
Patients provided electronic consent for the Covidom telesurveillance program and were informed of the use of their anonymized data for research. This study was approved by the scientific and ethical committee of the Assistance Publique-Hôpitaux de Paris (IRB00011591).
Results
Among the 62 993 patients included in the Covidom cohort as outpatients, 15 086 (23.9%) had a COVID-19 RT-PCR-confirmed diagnosis, 14 965 (23.8%) were adults, 11 984 (19.0%) answered at least one monitoring questionnaire and 9667 (15.3%) provided medical background at inclusion and so were included in this study (patients characteristics are presented in Table S1 in the Supplementary material).
Mean age was 43.2 years old (SD 14.0) and 67.5% were female (n = 6522). Median body mass index (BMI) was 25.0 kg/m2 (interquartile range (IQR) 22.1–28.8 kg/m2). Main co-morbidities were hypertension (12.9%; n = 1247), asthma (11.0%; n = 1063) and diabetes mellitus (5.5%; n = 527). Other co-morbidities were reported by less than 1.5% of patients. Data on weight (and BMI) was missing for 121 patients, and on gender for 18 patients.
The last answer to a daily questionnaire occurred at a median time of 28 days after symptom onset (IQR 17−30 days), with a median Covidom monitoring duration of 19 days (IQR 11−24 days). There were 65.1% of patients (n = 7805) who were missing at least one daily monitoring questionnaire, and the median number of skipped questionnaires per patient was 2 (IQR 0−5).
The most common symptom was dyspnoea in 65.1% of patients (n = 6296), with 2802 patients (29.0%) rating their dyspnoea as moderate or higher. Tachypnoea, shivers and fever were reported by 49.9% (n = 4821), 45.6% (n = 4410) and 36.7% (n = 3550) of patients, respectively. A total of 2590 (26.8%) patients reported tachycardia, 1197 (12.4%) reported dizziness and 582 (25.1%) of 2319 patients with an oximeter reported desaturation at least once.
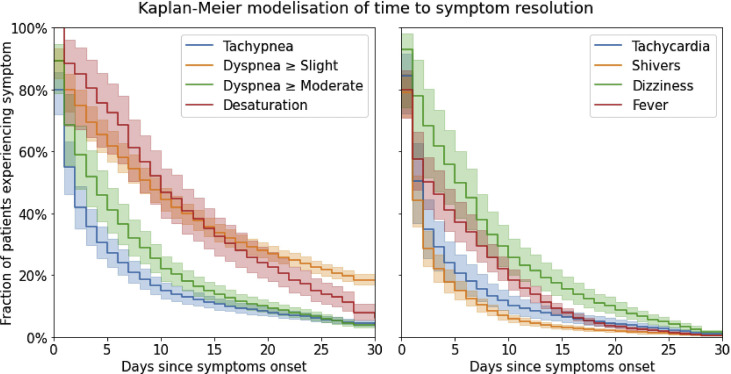
Considering time to symptom resolution (Fig. 1 ), the longest symptoms were desaturation with a median (95% CI) of 10 days (8−12 days), and slight dyspnoea with a median of 9 days (7−10 days). Median time to resolution of moderate dyspnoea was 4 days (2−5 days). Symptoms with shorter time to resolution included shivers, tachypnoea and tachycardia, with median durations (95% CI) respectively of 1 day (1−2 days), 2 days (1−2 days) and 2 days (1−2 days). The probability of slight dyspnoea lasting until 30 days after onset of first symptoms was 18.3% (95% CI 16.6%−20.0%), whereas the systemic symptom with highest probability at day 30 was dizziness with 1.1% (0.5%−2.2%).
Fig. 1.
Time to resolution of respiratory and systemic symptoms (in days) modeled using Kaplan–Meier estimators.
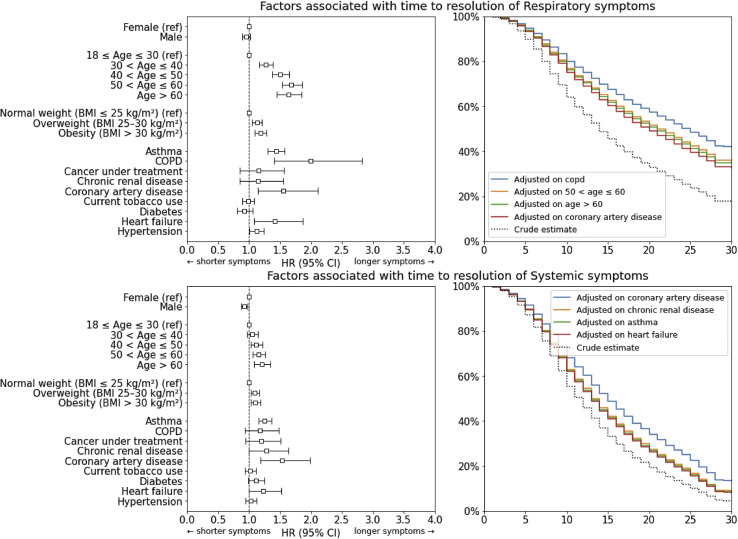
Age above 30 years (hazard ratio (HR) 1.27; 95% CI 1.16−1.38), 40 years (HR 1.50; 95% CI 1.37−1.65), 50 years (HR 1.68; 95% CI 1.53−1.85) and 60 years (HR 1.64; 95% CI 1.45−1.84), as well as increased BMI (both obesity and overweight, HR 1.13; 95% CI 1.15−1.21 and HR 1.18; 95% CI 1.09−1.28, respectively), chronic obstructive pulmonary disease (HR 2.00; 95% CI 1.41−2.83), coronary artery disease (HR 1.55; 95% CI 1.14−2.11), asthma (HR 1.43; 95% CI 1.30−1.58), heart failure (HR 1.42; 95% CI 1.08−1.86) and hypertension (HR 1.12; 95% CI 1.01−1.23) were associated with longer time to resolution of respiratory symptoms (Fig. 2 ). No variable appeared to be associated with a shorter time to resolution.
Fig. 2.
Risk factors associated with longer time to resolution of clinical symptoms.
For systemic symptoms, coronary artery disease (HR 1.53; 95% CI 1.18−1.98), as well as age above 40 years (HR 1.12; 95% CI 1.02−1.22, HR 1.16; 95% CI 1.06−1.26 and HR 1.20; 95% CI 1.08−1.34, respectively for ages >40, >50 and > 60 years) and increased BMI (HR 1.09; 95% CI 1.03−1.16 for BMI >25 kg/m2, HR 1.10; 95% CI 1.03−1.18 for BMI >30 kg/m2) were associated with longer time to resolution (Fig. 2). Male sex was associated with shorter time to resolution (HR 0.92; 95% CI 0.87−0.97). Complete analysis results are presented in Table S2 in the Supplementary material.
Lastly, the number and proportion of patients per monitoring question, answer and day are described in Fig. S1 in the Supplementary material.
Discussion
Our study provides important inputs regarding time to resolution of respiratory and systemic symptoms over 1 month in ambulatory patients, which can help direct care [5,[12], [13], [14]]. Median time to resolution of symptoms was up to 10 days with wide variability, and 18.3% of slight dizziness symptoms still present at end of follow up. Systemic symptoms were slightly less frequent than respiratory symptoms, and had a shorter time to resolution. Factors associated with prolonged symptoms included age over 30 years, elevated BMI, and cardiovascular and pulmonary diseases.
The strength of our study includes the size of the cohort and the standardized questionnaire during the entire 30-day follow up. Several studies showed, among hospitalized patients, that fever, cough, dyspnoea and gastrointestinal symptoms were the main clinical manifestations of COVID-19 [15]. In our cohort that included only non-hospitalized patients, those symptoms had a lower prevalence.
Our results corroborate that COVID-19 may cause prolonged symptoms, even among outpatients with mild disease [2], or in patients with or without few co-morbidities.
Limits
Our study relies on patients' self-reporting, resulting in possible recall bias. Furthermore, we focused on general and respiratory symptoms and did not evaluate the duration of other symptoms like smell and taste alteration. Second, non-respondents might have differed from respondents regarding their clinical course. Finally, our models do not take into account each symptom's onset delay, only the date until which each symptom lasts, starting from the onset of the first symptom. This describes the course of symptoms relative to the illness globally, but different factors may affect symptom onset delay and symptom duration.
Conclusion
Time to symptom resolution in outpatients with COVID-19 varies widely with shorter systemic symptoms and longer respiratory symptoms. Prolonged respiratory symptoms, especially dyspnoea, were common at day 30. Factors associated with later resolution were age over 30 years, elevated BMI, and cardiovascular and pulmonary diseases.
Transparency declaration
The authors declare that they have no conflicts of interest. All authors have full control of all primary data and they agree to allow review of their data upon request.
Fundings
This study received a funding by the Programme Hospitalier de Recherche Clinique 2020 of the French Ministry of Health, by a research fund by APHP-Fondation de France. The Covidom platform received a funding from EIT Health specific COVID-19 fund.
Authors' contributions
ADi, LJ, ADe, XL and YY conceptualized the study. ADi, LJ, ADe, CD, HM and YY collected, analysed and interpreted the data. LH conducted the statistical analysis. ADi, LJ, ADe, CD and YY wrote the first draft of the manuscript. All authors revised the manuscript and approved the final version. ADi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.08.021.
Contributor Information
Data-sciences committee:
Apra Caroline (AC), Jaulmes Luc (JL), and Mensch Arthur (MA)
Scientific committee:
Aime-Eusebi Amélie, Apra Caroline, Bleibtreu Alexandre, Debuc Erwan, Dechartres Agnes, Deconinck Laurène, Dinh Aurélien, Jourdain Patrick, Katlama Christine, Lebel Josselin, Lescure François-Xavier, and Yordanov Youri
Covidom regional centre steering commitee:
Artigou Yves, Banzet Amélie, Boucheron Elodie, Boudier Christiane, Buzenac Edouard, Chapron Marie-Claire, Chekaoui Dalhia, De Bastard Laurent, Debuc Erwan, Aurélien Dinh, Grenier Alexandre, Haas Pierre-Etienne, Hody Julien, Jarraya Michèle, Jourdain Patrick, Lacaille Louis, Le Guern Aurélie, Leclert Jeremy, Male Fanny, Marchand-Arvier Jerôme, Martin-Blondet Emmanuel, Nassour Apolinne, Ourahou Oussama, Thomas Penn, Ribardiere Ambre, Robin Nicolas, Rouge Camille, Nicolas Schmidt, and Villie Pascaline
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizrahi B., Shilo S., Rossman H., Kalkstein N., Marcus K., Barer Y., et al. Longitudinal symptom dynamics of COVID-19 infection. Nat Commun. 2020;11:6208. doi: 10.1038/s41467-020-20053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S., et al. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pullen M.F., Skipper C.P., Hullsiek K.H., Bangdiwala A.S., Pastick K.A., Okafor E.C., et al. Symptoms of COVID-19 outpatients in the United States. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapostolle F., Schneider E., Vianu I., Dollet G., Roche B., Berdah J., et al. Clinical features of 1487 COVID-19 patients with outpatient management in the Greater Paris: the COVID-call study. Intern Emerg Med. 2020;15:813–817. doi: 10.1007/s11739-020-02379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim G.-U., Kim M.-J., Ra S.H., Lee J., Bae S., Jung J., et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020;26:948. doi: 10.1016/j.cmi.2020.04.040. e1–948.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yordanov Y., Dechartres A., Lescure X., Apra C., Villie P., Marchand-Arvier J., et al. Covidom, a telesurveillance solution for home monitoring patients with COVID-19. J Med Internet Res. 2020;22 doi: 10.2196/20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muza S.R., Silverman M.T., Gilmore G.C., Hellerstein H.K., Kelsen S.G. Comparison of scales used to quantitate the sense of effort to breathe in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141:909–913. doi: 10.1164/ajrccm/141.4_Pt_1.909. [DOI] [PubMed] [Google Scholar]
- 12.The Lancet Facing up to long COVID. Lancet. 2020;396:1861. doi: 10.1016/S0140-6736(20)32662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davido B., Seang S., Tubiana R., de Truchis P. Post-COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect. 2020;26:1448–1449. doi: 10.1016/j.cmi.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nehme M., Braillard O., Alcoba G., Aebischer Perone S., Courvoisier D., Chappuis F., et al. COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med. 2021;174:723–725. doi: 10.7326/M20-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.