Abstract
Some members of nuclear hormone receptors, such as the thyroid hormone receptor (TR), silence gene expression in the absence of the hormone. Corepressors, which bind to the receptor’s silencing domain, are involved in this repression. Hormone binding leads to dissociation of corepressors and binding of coactivators, which in turn mediate gene activation. Here, we describe the characteristics of Alien, a novel corepressor. Alien interacts with TR only in the absence of hormone. Addition of thyroid hormone leads to dissociation of Alien from the receptor, as shown by the yeast two-hybrid system, glutathione S-transferase pull-down, and coimmunoprecipitation experiments. Reporter assays indicate that Alien increases receptor-mediated silencing and that it harbors an autonomous silencing function. Immune staining shows that Alien is localized in the cell nucleus. Alien is a highly conserved protein showing 90% identity between human and Drosophila. Drosophila Alien shows similar activities in that it interacts in a hormone-sensitive manner with TR and harbors an autonomous silencing function. Specific interaction of Alien is seen with Drosophila nuclear hormone receptors, such as the ecdysone receptor and Seven-up, the Drosophila homologue of COUP-TF1, but not with retinoic acid receptor, RXR/USP, DHR 3, DHR 38, DHR 78, or DHR 96. These properties, taken together, show that Alien has the characteristics of a corepressor. Thus, Alien represents a member of a novel class of corepressors specific for selected members of the nuclear hormone receptor superfamily.
Corepressors are involved in gene silencing by various transcriptional repressor proteins such as MAD/MAX and MxiI (2), YY1 (74), KRAB domain proteins (27), NGF1-A, KROX 20 (59, 63) and some members of the nuclear hormone receptor (NHR) superfamily, such as thyroid hormone receptor (TR) and retinoic acid receptor (RAR) (8, 19, 38, 50). Both TR and RAR repress gene activity in the absence of hormone in vivo (3, 4, 21, 73) and in vitro (28, 67, 68). This repression is mediated by a silencing domain in the carboxy terminus, encompassing about 250 amino acids (aa) (3, 33, 46, 58). In addition to the silencing function, TR and RAR harbor several other functions C-terminal to their DNA binding domain (DBD) including dimerization, hormone binding and hormone-dependent transactivation. These activities can be transferred to heterologous proteins and therefore represent functional domains (for reviews, see references 6, 50, and 65).
Gene silencing by NHRs is relieved by addition of the cognate ligand, which induces a conformational change and transforms the receptor into a transcriptional activator. In this way, both hormone binding and the small conserved receptor activation domain, AF2/AF2-AD/τ4/τc (8, 11, 12, 22, 49, 50), representing helix 12 (14, 38, 55, 57, 71), are required to dissociate corepressors from the receptors (8, 9, 19, 38).
For the liganded (holo)receptors, the activation domain AF2/AF2-AD/τ4/τc is also essential for binding of coactivators (36, 40, 71) that mediate gene activation. Interestingly, a large number of coactivators for NHRs have been cloned, including SRC1 (54), TIF1 (44), TIF2 (71)/GRIP1 (37)/TRAM-1 (64), RIP140 (16), RIP160 (42), TRIP230 (18), ARA70 (76), p/CIP (69), CREB binding protein (19, 42), PGC-1 (56), and additional TR-associated proteins (30). Thus, multiple classes of coactivators are involved in NHR gene activation. It is yet unknown why so many different coactivators are involved in transcriptional activation by NHRs.
Gene silencing by TR, RAR, Rev-erbAα, and COUP-TF is mediated, at least in part, by corepressors in vivo (8, 10, 19, 25, 38, 61) and in vitro (68), which bind to the unliganded (apo)receptors. Only one class of nuclear receptor corepressors has been identified, which exhibit hormone-sensitive interaction. This class contains two related members, SMRT and N-CoR (19, 38). These corepressors were isolated by the yeast two-hybrid system and bind to the silencing domains of TR and RAR only in the absence of ligand. Hormone binding by the receptor leads to dissociation of these corepressors. Furthermore, SMRT and N-CoR are localized in the cell nucleus and harbor an autonomous silencing function when bound to DNA (19, 38). The mechanism of repression by the SMRT/N-CoR class involves interaction with SIN3 and a histone deacetylase function (1, 35, 52).
Here, we describe a novel corepressor, Alien, which is unrelated to SMRT and N-CoR and is highly conserved from humans to Drosophila. Conserved sequences are even found in Ricinus communis and Caenorhabditis elegans. Alien interacts with TR only in the absence of hormone and does not interact with RAR, retinoid X receptor (RXR), or glucocorticoid receptor (GR). Addition of thyroid hormone leads to dissociation of Alien from TR as shown by yeast two-hybrid, glutathione S-transferase (GST) pull-down, and coimmunoprecipitation experiments. Alien is able to enhance receptor-mediated silencing, is localized in the cell nucleus, and harbors an autonomous silencing function. Taking these results together, Alien fulfills the characteristics of a corepressor, is specific for some members of the NHR superfamily, and represents a novel class of corepressors.
(This work contains part of the Ph.D. thesis of U. Dressel, Justus Liebig University of Giessen, Giessen, Germany.)
MATERIALS AND METHODS
Plasmids. (i) GST fusion expression vectors.
GST–d-Alien aa 1 to 360 was constructed by insertion of the EcoRI-EcoRI fragment from pABgal94–d-Alien aa 1 to 360 into the EcoRI site of pGST-linker (5). GST–h-Alien aa 1 to 305 was cloned by inserting the EcoRI-XhoI fragment (partial digest) from pABgal94–h-Alien into the EcoRI-SalI site of pGST-linker.
(ii) In vitro translation vectors.
Ecdysone receptor (EcR) aa 330 to 878 was excised from pABgal94 (8)-EcR fusion with EcoRI-HincII, filled in with Klenow enzyme, and ligated to the pT7βSal (53) HincII site. Full-length DHR 38, DHR 78, and DHR 96 cDNAs and the P9 FTZ-F1 cDNA were gifts from C. S. Thummel; pBSK− SVP cDNA was a gift from M. Mlodzik; COUP-TF1, hRXRβ, and hRARα were obtained from M.-J. Tsai and B. W. O’Malley; and cDNAs E75B and DHR3 were a gift from S. Munroe. pT7βSal hTRβ 5-461 was described previously (5).
(iii) Expression vectors.
Generation of reporter constructs was described previously (3, 4). pABgal94-d-Alien was generated by insertion of the cDNA (31) in frame with the Gal4-DBD coding sequence in the vector pABgal94 (8). PCR cloning of human Alien (h-Alien) was performed with HeLa cells as the RNA source. Oligo(dT) primers were used to generate cDNA. h-Alien-specific primers were used in accordance with the known 5′-end sequence of TRIP15 (45). pBS-SK−–h-Alien was cloned by insertion of the EcoRI-XhoI 2-kb fragment of pJG-TRIP15/h-Alien into the EcoRI-XhoI site of pBluescript II SK− (Stratagene). pAB–h-Alien was cloned by insertion of the SmaI-XhoI (Klenow) fragment of pBS-SK–h-Alien into the PvuII site of pABΔgal (3). pHA-TRα c.t. was generated by replacing the coding sequence of the Gal4 DBD in the vector pABgal94-TRα by that of hemagglutinin epitope tag through the use of synthetic oligonucleotides. Full-length pHA-TRα is a fusion of the entire TRα coding region from full-length pEG-TRα into the pHA vector.
(iv) Yeast two-hybrid expression vectors.
pJG-EcR 330–878 was constructed by excision of the coding sequence of EcR (aa 330 to 878) from pABgal94-EcR HindIII (Klenow)-EcoRI and insertion into the XhoI (Klenow)-EcoRI site of pJG4-5 (24). pJG-d-Alien was created by insertion of the EcoRI fragment of pABgal–d-Alien into EcoRI sites of pJG4-5. The lex fusions pEG–v-erbA, pEG-TRα, and pEG-TRβ, point mutants, and deletions were generated by insertion of the receptor C termini from the corresponding pABgal fusions (4, 15) with EcoRI and filled-in HindIII sites into blunted XhoI-EcoRI sites of pEG202. Insertion of the entire SMRT cDNA from Gal-SMRT (19) into the vector pJG4-5 created pJG-SMRT-f.l (9). pJG-SMRT and pJG-N-CoR were described previously (9). pEG-SIN3A was generated by insertion of the ScaI fragment of pVZ-Sin3A into pEG202. pEG-RAR and deletions were described previously (9, 10), as was pEG-hGRα (60). Hormones were added at the following concentrations: 10−6 M for 3,3′,5-triiodothyroacetic acid (TRIAC), 105 M for retinoic acids, and 10−6 M for triamcinolone diacetate.
Cell culture.
HeLa, CV1, Ltk−, and COS1 cells were grown in Dulbecco modified Eagle medium plus 10% fetal calf serum (FCS) at 37°C under 5% CO2; HD3 cells were grown in Dulbecco modified Eagle medium plus 8% FCS and 2% chicken serum at 37°C under 5% CO2. For both HeLa and CV1 cells, cotransfections were carried out by the calcium phosphate method. A 0.5-pmol portion of expression vector was cotransfected with 1.0 pmol of indicated reporter plasmid. The DEAE-dextran method of transfection was used for COS1 and Ltk− cells essentially as described previously (21). In detail, for COS1 or Ltk− cells 2.5 × 106 or 1 × 106 cells, respectively, were trypsinized, washed once with Tris-buffered saline (TBS), and incubated with DNA transfection solution containing 30 μg (1 pmol of reporter and the indicated expression vectors) of expression plasmid in 100 μl (20 μl for Ltk− cells) Tris-EDTA, 900 μl (150 μl for Ltk− cells) TBS, and 1,200 μl (220 μl for Ltk− cells) DEAE-dextran (1% in TBS). After a 1-h incubation at room temperature, the cells were collected by centrifugation and cultured on 15-cm (6-cm for Ltk− cells) cell culture dishes in normal or (for hormonal studies) charcoal-treated 10% FCS and were grown for a further 2 days before harvest.
For augmentation of TR-mediated silencing on a natural TR response element (TRE), 1.5 pmol of reporter, 2 μg of Gal-fusion, and 3 μg of h-Alien or empty expression vector were used in CV1 cells. For the potentiation of silencing of Alien, 1 pmol of reporter (pUAS6× tkCAT), 1 pmol of Gal-fusion, and 2 pmol of rat TRα were cotransfected in CV1 cells. For detection of the silencing function of Alien, different cell lines were transfected with 3 pmol of Gal-fusion and 1 pmol of reporter. Trichostatin A (Biomol Research Labs) was added at a final concentration of 100 ng/ml 8 h before cell harvest.
GST pull-down experiments.
Bacterial expression of GST, GST-TR, GST–h-Alien or GST–d-Alien was performed by induction of gene expression with 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 25°C for GST-TR and 30°C for GST and GST-Alien in HB101 cells. The purification of recombinant protein and interaction studies with in vitro-translated, [35S]methionine-labeled h-Alien or NHR were as described previously (7). In each experiment, the amount used in the input lane was 10% of that incubated with the GST-beads. The GST fusion proteins were stained with Coomassie brilliant blue to ensure equal loading, and the bound proteins were visualized by autoradiography. For ligand-sensitive interaction studies, 5 × 10−8 mol of TRIAC was used.
Coimmunoprecipitation.
COS1 cells were transfected with HA-TRα expression vector coding for the hemagglutinin-tagged carboxy terminus (aa 120 to 410) of TR α (TRα c.t.) or the full-length TRα. Hormone (10−7 M T3) was added 3 h to 1 day prior to harvest. Cells were lysed on ice in 300 mM NaCl–0.1% Nonidet P-40–1 mM dithiothreitol–1 mM phenylmethylsulfonyl fluoride–50 mM Tris-HCl (pH 7.6). After cell lysis, water was added to dilute the solution to a final salt concentration of 80 mM. Cell debris were pelleted at 100,000 × g at 4°C for 30 min. HA antibody (1:5,000) coupled to 20 μl of protein A-Sepharose beads (Pharmacia) was incubated for 2 h with cell extract at 4°C. The beads were washed five times with 0.3× lysis buffer, and the samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Western analysis was performed by using anti-Alien peptide antibody (31) and the enhanced chemiluminescence detection method (Amersham). Anti-Alien peptide antibody was used for coimmunoprecipitations of SIN3A by the method of Eggert et al. (26) with 0.1% Nonidet P-40. Anti-SIN3A antibody (Santa Cruz) was used for detection of SIN3A in Western analysis.
Immunofluorescence.
Indirect immunofluorescence of cells was performed essentially as described by Eggert et al. (26). For detection, preimmune antiserum or rabbit anti-Alien peptide antibody (31) and tetramethylrhodamine-5-isothiocyanate (TRITC)-conjugated swine anti-rabbit antibody (Dakopatts) were used.
Yeast two-hybrid assay.
Yeast two-hybrid assays were performed as described previously (9, 32). The yeast strain EGY48 was transformed with three plasmids, the lex fusion as the bait, pJG or pVP vectors as the activator, and pSH18-34 as the reporter.
Nucleotide sequence accession number.
The sequences of h-Alien and TRIP15 have been assigned accession no. AF120268 and L40388, respectively.
RESULTS
Alien is a highly conserved protein which interacts in the absence of hormone with a subset of members of the NHR superfamily.
TR is a transcriptional silencer in the absence of hormone as well as a hormone-dependent trans-activator (3, 21, 22). The silencing domain is localized in the receptor C terminus, together with the hormone binding and the hormone-dependent trans-activation functions.
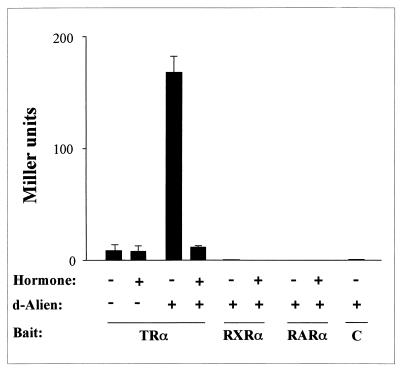
We previously isolated Drosophila Alien (d-Alien [31]), which has homologies to the partial sequence of a TR-interacting factor, TRIP15, isolated by a yeast two-hybrid screen (45). We were interested whether d-Alien was able to interact in a hormone-sensitive manner with TR. Therefore, yeast two-hybrid experiments were performed which revealed that d-Alien interacted with TR only in the absence of ligand (Fig. 1). Addition of hormone leads to the dissociation of the Alien-TR complex. In contrast, RXR and RAR failed to interact with d-Alien. Further characterization of d-Alien showed that it harbors an autonomous silencing function and that the d-Alien antibody cross-reacts with h-Alien in the cell nucleus of HeLa cells (see below). We then isolated and sequenced full-length h-Alien/TRIP15 from HeLa cells.
FIG. 1.
d-Alien interacts with TR in a hormone-sensitive manner. The C termini of TRα, RXRα, and RARα were used as bait (lex fusion) and d-Alien was used as the activator, with or without the cognate ligands, in yeast two-hybrid experiments as described by Gyuris et al. (32). Interaction leads to activation of the cotransfected reporter (lacZ gene). As controls, the bait alone (lex vector) or the empty activator vector (C) were used.
h-Alien bears very high homologies to its Drosophila homologue throughout the entire amino acid sequence (Fig. 2). h-Alien isolated from HeLa cells is composed of 305 aa (Fig. 2). The sequence was verified by several independent experiments involving reverse transcription-PCR cloning and subsequent sequencing. The cDNA is 2,001 bp long and is polyadenylated (data not shown). The GenBank-submitted sequence of TRIP15 (45) ends at bp 933 of the cDNA. The d-Alien protein has an extended C-terminal part (31) compared to h-Alien. Alignment of d- and h-Alien revealed high homologies (90% identity and 95% similarity at the amino acid level for the entire h-Alien protein). Further searches of the data banks yielded putative open reading frames from several species with quite strong similarities, such as R. communis (31) (accession no. T14894) and C. elegans (accession no. U97190). An anti-Alien-antibody was generated against the indicated peptide sequence (Fig. 2), which shows 100% identity between the human and Drosophila homologues. Western analysis from various mammalian cell lines including human (HeLa and C33A), mouse (NIH 3T3) and monkey (CV1 and COS1) cells showed an apparent molecular mass of about 41 kDa (not shown), which is similar to that of the in vitro-translated cDNA clone. Alien harbors an acidic region in the N terminus, a putative Zn finger in the C terminus, and a central hydrophobic region flanked by two putative α-helical structures and one putative nuclear localization signal.
FIG. 2.
Alien is highly conserved in evolution. h-Alien is aligned with the d-Alien homologue and translated open reading frames of C. elegans (C.e.) and Ricinus communis (R.c.). Identical amino acids to h-Alien are indicated by vertical lines, and related amino acids are shown in boldface type. Sequence comparison between h-Alien and d-Alien revealed 90% identity and about 95% similarity at the amino acid level. The peptide sequence for antibody generation is indicated by a box. Two incomplete open reading frames have been retrieved from C. elegans (Alien 1 and 2), and one partial open reading frame has been retrieved from R. communis. Missing amino acids are indicated by dots.
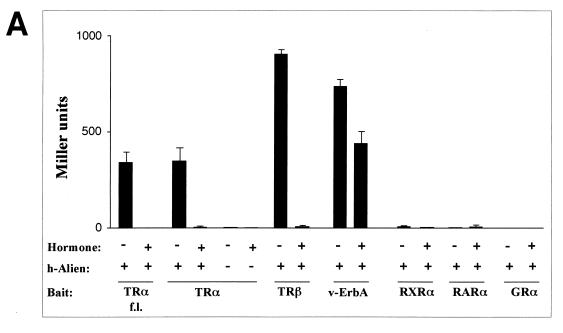
h-Alien interacts in a hormone-sensitive manner with full-length TRα as well as with both TRα and TRβ C termini (Fig. 3A). h-Alien also binds to the C terminus of the oncogene v-ErbA, which acts as a hormone-independent silencer in vertebrate cells. Accordingly, h-Alien interacts with v-ErbA in a hormone-independent manner. Interestingly, h-Alien does not interact with hGRα, hRXRα, or hRARα (Fig. 3A). In the absence of Alien, all nuclear hormone receptors tested did not show a significant effect; the results for TRα are shown as an example. We did not see an interaction of h-Alien with RXR in the presence of ligand, whereas Lee et al. (45) showed an interaction of TRIP15 with RXR only in the presence of hormone. These different observations may be because we used full-length h-Alien. As controls, we used established interaction partners and found hormone-sensitive interaction of the SMRT and N-CoR class of corepressors with hRXRα or hRARα and hormone-dependent interaction of GRIP1 with GR (reference 61 and data not shown). Thus, the lack of interaction of Alien with RAR is dissimilar to the situation for SMRT or N-CoR.
FIG. 3.
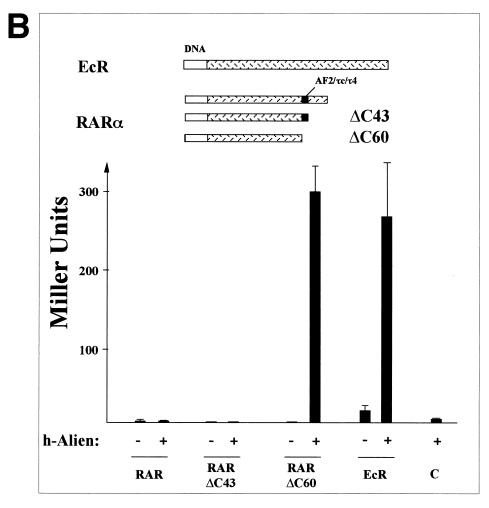
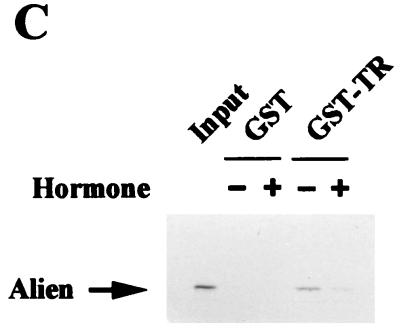
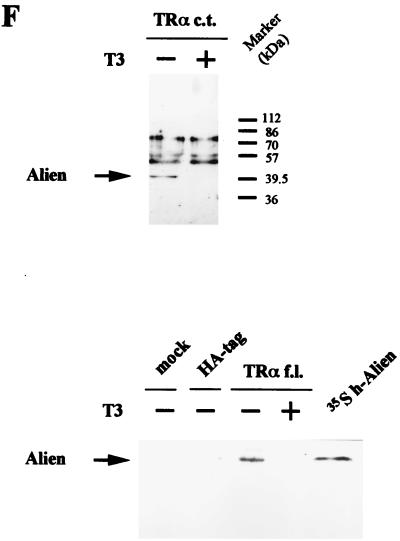
(A) Alien interacts specifically with TR but not with RAR, RXR, or GR. h-Alien was tested for interaction with TRα f.l., the C termini of the TRα, TRβ, the oncogene v-erbA, RARα, RXRα, and GRα in the presence or absence of the cognate hormones. Yeast two-hybrid experiments were performed as described for Fig. 1. As controls, the parental expression vectors were used. The hormones used were TRIAC (10−6 M) for TR and v-erbA; retinoic acids (10−5 M) for RAR and RXR; and triamcinolon diacetate (10−6 M) for GR. (B) Alien interacts with the silencing domains of both RAR (RARΔC60) and EcR. Yeast two-hybrid experiments were performed as described in the legend to Fig. 1 in the absence of hormone, with or without h-Alien, with the C terminus of EcR, with hRARα, and with C-terminal deletions of hRARα: a 43-aa deletion (RARΔC43), lacking the RAR F-region, and a 60-aa deletion (RARΔC60), lacking both the receptor F-region and the AF2-AD/τ4/τc. (C) Interaction of Alien with TR is hormone sensitive in vitro. GST pull-down experiments were performed with bacterially expressed GST or GST-hTRβ fusion and in vitro-translated, 35S-labeled h-Alien. The ligand TRIAC (5 × 10−8 M) was added to the reaction mixture in the indicated lanes. (D) Alien interacts with a subset of Drosophila NHRs. GST pull-down assays were performed with bacterially expressed GST, GST–d-Alien, or GST–h-Alien incubated with various in vitro-translated, 35S-labeled NHRs from mammals or Drosophila. The input lane shows 10% of total input. Luciferase served as a negative control. GST–h-Alien was used for the human receptors, and GST–d-Alien was used for the Drosophila receptors. (E) Schematic presentation of the Alien-receptor interaction of the GST pull-down experiments. Results obtained in the experiment in Fig. 3D were plotted as the percentage of bound receptor compared to the input of each nuclear receptor. Interaction was observed with TR, EcR, and FTZ-F1, weak interaction was observed with COUP-TF1 and SVP, and no significant interaction was observed with RXR, USP, DHR 3, DHR 38, DHR 78, and DHR 96. (F) Alien is complexed with TR in the absence of hormone in vivo. Coimmunoprecipitations were performed with HA-tagged TRα c.t. (top) expressed in COS1 cells and endogenous Alien, using anti-HA-antibody for coimmunoprecipitation and anti-Alien antibody for Western analysis. Transfected COS1 cells were treated with or without thyroid hormone (10−6 M) for one day prior to harvest. Nonspecific bands with a lower migration rate appear in both lanes with similar intensity, while Alien (arrow) is complexed only in the absence of ligand with TR. TRα f.l. complexed with Alien was used in coimmunoprecipitation experiments (bottom). HA-tagged full-length TRα from transfected COS cells coprecipitates Alien only in the absence of ligand. Extracts from HA-tag-transfected cells, untransfected cells, and added hormone in extracts transfected with full-length TRα did not coprecipitate Alien. In vitro-translated [35S]methionine-labeled h-Alien is shown as a migration control.
However, h-Alien interacts with the core silencing domain of hRARα (RARΔC60) (Fig. 3B). Interestingly, a larger RAR fragment containing both the silencing domain and the AF2-AD/τ4/τc domain (RARΔC43) did not interact with h-Alien, indicating that the presence of helix 12 inhibits the binding of Alien to RAR. Furthermore, Alien interacts with the Drosophila EcR (Fig. 3B), which is known to repress gene transcription (24, 70) and to harbor a silencing domain in its C terminus (data not shown). Treatment of yeast cells with the EcR ligand 20-hydroxy-ecdysone or muristerone A had only a marginal effect on the EcR-Alien interaction (data not shown). Possibly, penetration of the yeast cell wall by these ligands is impaired or they are rapidly degraded. Another possibility is that the affinity of EcR to the ligand is greatly impaired when ECR is not heterodimerized with ultraspiracle (75). Thus, since we have not seen an interaction of Alien with intact RAR, it suggests that Alien complexes with a different set of NHRs from that used by the SMRT/N-CoR class of corepressors.
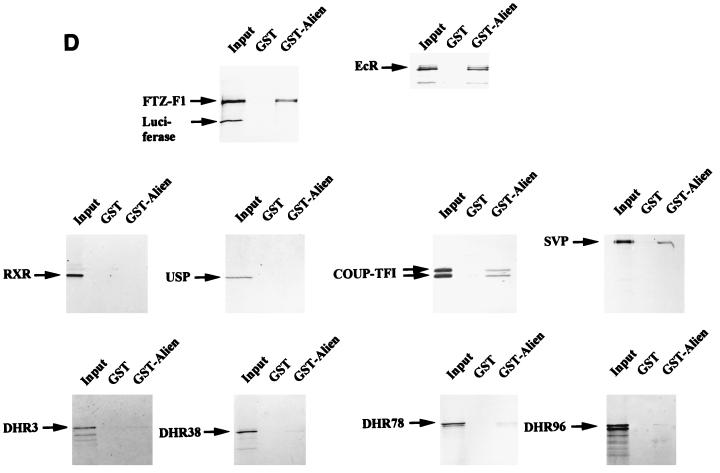
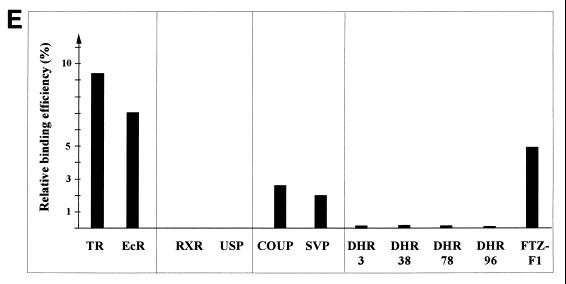
GST pull-down experiments were performed to test whether the binding of Alien to NHRs is direct. Bacterially expressed GST-TRα was incubated with in vitro-translated, 35S-labeled h-Alien. h-Alien, which migrates at about 41 kDa, was bound to TR in the absence of hormone (Fig. 3C). Furthermore, addition of thyroid hormone decreased the interaction of h-Alien with TR in vitro. We also performed interaction analysis with various members of the NHR superfamily, human COUP-TF1, its Drosophila homologue Seven-up (SVP), RXR, its Drosophila homologue USP, Fushi-tarazu-F1 (Ftz-F1), and Drosophila hormone receptors DHR 3, DHR 38, DHR 78, and DHR 96 (DHR 96 is the homologue of the vitamin D3 receptor). GST pull-down experiments were performed with bacterially expressed GST or GST-Alien and in vitro-translated, 35S-labeled NHRs (Fig. 3D). Human receptors were incubated with GST–h-Alien, and Drosophila receptors were incubated with GST–d-Alien. The relative binding efficiency of the tested NHR is summarized in Fig. 3E. We have observed strong interactions of Alien with EcR and TR, weaker interactions with COUP-TF1, SVP, and Ftz-F1, and almost no interactions of Alien with RXR, USP, DHR 3, DHR 38, DHR 78, and DHR 96. For most of the NHRs interacting with Alien, a silencing function has been shown (references 4, 20, 24, and 70 and data not shown).
To verify the hormone-sensitive interaction of Alien with TR in vivo, we performed coimmunoprecipitation experiments. For this purpose, we fused the full-length TR (TR f.l.) or its C terminus (TR c.t.) with the HA tag and transfected COS1 cells to test whether TR is complexed with endogenous Alien. Coimmunoprecipitation with the HA antibody shows that endogenous Alien is associated with TRα f.l. or with TRα c.t. in the cell only in the absence of hormone (Fig. 3F). Other slower-migrating, nonspecific bands appear with similar intensity independent of hormone treatment. No Alien was coimmunoprecipitated from untransfected or HA-tag-transfected cells. Addition of hormone (T3) to extracts containing full-length TRα does not lead to coimmunoprecipitation of Alien (Fig. 3F).
Thus, Alien is interacting with TR in the absence of hormone in vitro and in vivo. Furthermore, ligand binding leads to dissociation of the Alien-TR complex in vivo and in vitro. Interestingly, Alien is a highly conserved protein of higher eukaryotes, including animals and plants. Since we could not find any significant sequence homologies between Alien and the corepressors SMRT or N-CoR and observed distinct interaction properties for RAR, we suggest that Alien represents a member of a new class of corepressors.
Alien is localized in the cell nucleus and harbors an autonomous silencing function.
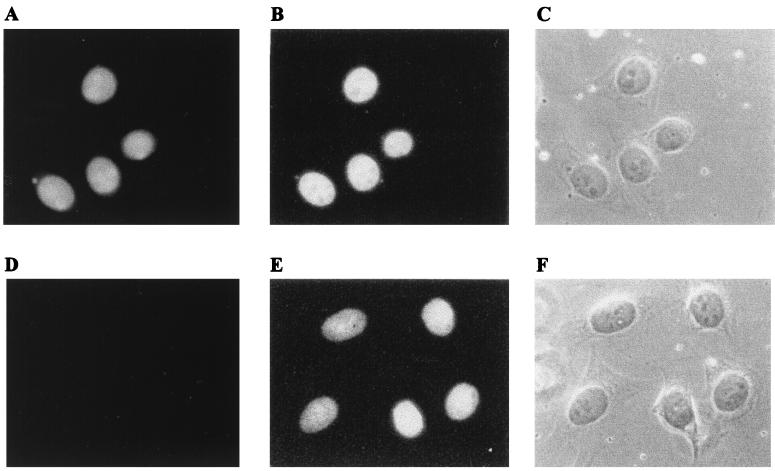
To be involved in transcriptional regulation, Alien would be expected to be localized in the cell nucleus. Therefore, we performed immunofluorescence experiments with the anti-Alien peptide antibody. As seen in Fig. 4A, Alien is localized predominantly within the nucleus of HeLa cells. The preimmune antiserum (Fig. 4D) showed only an extremely weak staining. For the same cells 4′,6-diamidino-2-phenylindole (DAPI) staining (Fig. 4B and E) and phase-contrast pictures (Fig. 4C and F) are also shown. We have also seen similar results with Ltk− and CV1 cells (results not shown).
FIG. 4.
Alien is localized predominantly in the cell nucleus. Rabbit anti-Alien peptide antibody (A to C) or preimmune antiserum (D to F) was used for indirect immunofluorescence analysis of HeLa cells with TRITC-conjugated anti-rabbit antibody. A triplicate picture was taken of the same cells: (A and D) immunofluorescence of immune and preimmune antisera, respectively; (B and E) DAPI staining; (C and F) phase-contrast pictures.
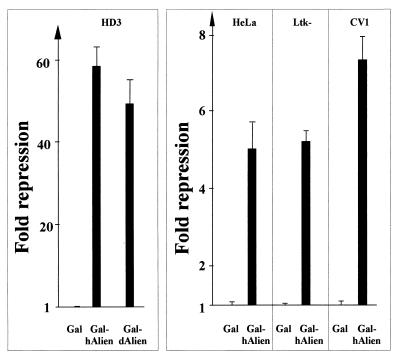
If Alien is involved in mediating silencing, we expected that Alien would harbor an autonomous silencing function. For that purpose, we fused the full-length Alien cDNA to the Gal4 DBD (aa 1 to 94) to tether Alien to the DNA and tested the ability of Alien to modulate transcription of a heterologous promoter in different cell lines via reporter assays. Both h-Alien and d-Alien strongly repress promoter activity in HD3 cells (Fig. 5). Furthermore, we found that Alien silences promoter activity in HeLa, CV1, and Ltk− cells (Fig. 5), albeit to a different extent from that in HD3 cells.
FIG. 5.
Alien harbors an autonomous silencing function. Both d-Alien and h-Alien were fused as full-length proteins to the DBD of Gal4 (aa 1 to 94) and were tested with a UAS-tkCAT reporter in chicken HD3 cells for their ability to repress promoter activity. In addition, h-Alien was tested in HeLa, mouse Ltk−, and monkey CV1 cells. For Gal fusions, 3 pmol of expression vectors was transfected. Values obtained with Gal4 DBD were set arbitrarily to 1.
Thus, both h-Alien and d-Alien harbor an autonomous silencing function.
Loss of the silencing function of TR correlates with loss of interaction with Alien.
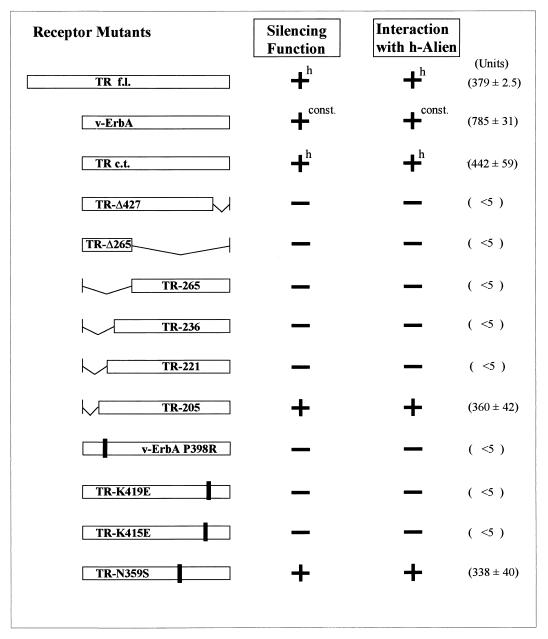
To fulfill criteria necessary to establish Alien as a corepressor of TR, binding of Alien to TR should correlate with the ability of TR to silence transcription. Therefore, a number of TR deletion and point mutants were generated and tested for interaction with Alien (Fig. 6) in the yeast two-hybrid assay. A summary of the silencing function of the mutant receptors and the corresponding results obtained by the interaction assay is shown in Fig. 6. The extent of the receptor-silencing domain and the effect of the mutations on receptor activity have been shown previously (14, 15, 50). As previously shown (Fig. 3A), TR interacts in a hormone-sensitive manner with h-Alien. Deletion of part of the hinge region up to aa 205 (TR-205), does not affect the silencing function (51) or interaction with Alien. A further deletion of only 16 aa (TR-221), which abolishes the silencing function, eliminates the interaction of TR with Alien simultaneously. Similarly, a receptor with a truncation of 34 aa from the C terminus of TR (TRΔ427) does not silence transcription (4) and also fails to interact with Alien. Lack of interaction with TRΔ427 and Alien has also been confirmed in GST pull-down experiments (the interaction is below 0.5% of the input [results not shown]).
FIG. 6.
Interaction of Alien with TR correlates with its silencing function. Yeast two-hybrid experiments with TR mutants as bait were tested for interaction with Alien as prey, as described in the legend to Fig. 1. This figure gives an overview of point mutants and deletions of TR used. Interaction with h-Alien is indicated as a plus, and the corresponding Miller units obtained are listed. The extent of the receptor-silencing domain and the silencing function of receptor mutants were described previously (4, 15, 51). Solid bars represent the single-amino-acid exchanges K419E, K415E, and N359S of TR and the naturally occurring P398R mutant of v-ErbA; numbers indicate the amino acid endpoints of the deletion of TR mutants. h: hormone-sensitive silencing function and interaction with Alien; const., constitutive silencing function and interaction with Alien.
Furthermore, we tested three point mutants of TR, for which the hormone-dependent transactivation properties are unaffected (15). Two of the point mutants (K419E and K415E) lack the silencing function, while the point mutant N359S silences transcription to a similar extent to the wild-type receptor (15). Western analysis of the transformed yeast cells showed that the TR mutants are expressed in yeast cells (data not shown). The TR mutants lacking the silencing function (K419E and K415E) also lacked interaction with Alien, while the interaction of Alien with TR-N359S remained unaffected by the mutation (Fig. 6). A naturally occurring CoR-box mutant, P398R of the v-erbA oncogene product, which has been shown to lack the silencing function (15, 22) also did not interact with Alien. This mutation occurs in the region of the v-erbA oncogene product which corresponds to the critical amino acids in TR required for binding of Alien, as shown by the deletions TR-205 and TR-221.
Thus, both the hinge region and the C-terminal end of the silencing domain of TR are required for interaction with Alien. Furthermore, the interaction of Alien with TR correlates with the ability of TR and TR mutants to mediate transcriptional silencing.
Alien and TR mutually enhance the ability to mediate silencing.
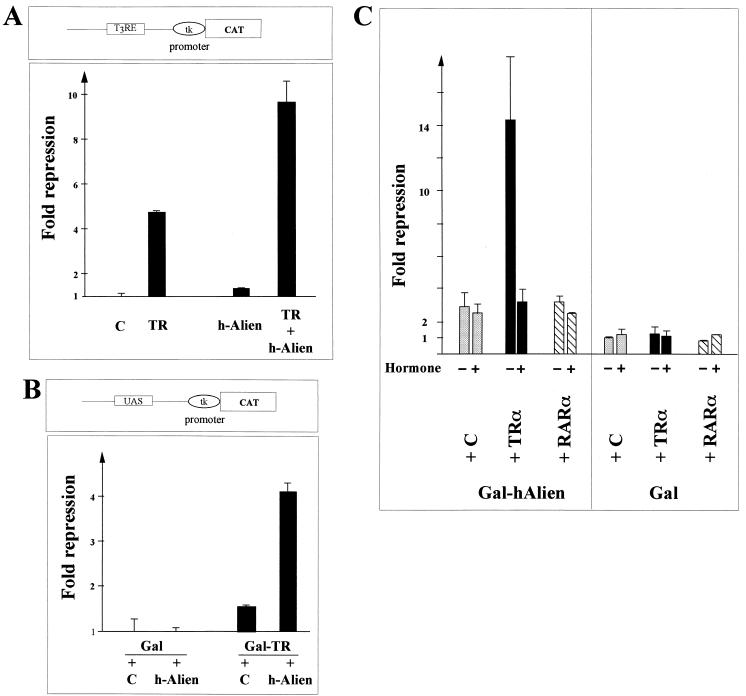
To test the ability of either Alien or TR to influence the transcriptional properties of its interacting partner, we used cotransfection experiments. In one set of experiments, we transfected full-length TR with the reporter containing the lysozyme TREs (F23×-tkCAT [3]) or a Gal-TR fusion with a reporter containing an upstream activation sequence (UAS) driven promoter (4) together with h-Alien in the absence of ligand. Since all cell lines analyzed contain Alien, we chose CV1 cells, which do not contain measurable amounts of functional TR. As seen in Fig. 7A, Alien moderately but significantly enhanced the silencing function of TR bound to a T3RE. Alien itself had only very weak effects on this reporter in this test system. The Gal-TR fusion, which encompasses the C terminus of TR fused to the DBD of Gal4, together with overexpressed Alien, results in significantly higher silencing than does Gal-TR alone (Fig. 7B). When we used Gal-RAR in CV1 cells, we did not see, as expected, an effect of Alien on RAR-mediated transcription (data not shown).
FIG. 7.
TR and Alien mutually enhance the ability to silence gene transcription. (A) The silencing function of TR is increased when Alien is coexpressed on a natural response element. The results of cotransfection of TRα with h-Alien and the reporter pTRElys3× tkCAT (4.4 μg) bearing the natural lysozyme TRE in CV1 cells are shown. As a control (lane C), an empty expression vector was used. (B) The silencing mediated by the C terminus of TR is enhanced by coexpression of h-Alien. Gal-TRβ (1 μg) encompassing the Gal4 DBD fused to the TRβ c.t. was coexpressed with h-Alien (10 μg) and the reporter pUAS6× tkCAT (3 μg) containing Gal4 binding sites. As controls, both the Gal4 DBD expression vector and an empty expression vector were used. (C) The silencing of Alien is enhanced by expression of TRα only in the absence of hormone. Expression vectors for Gal–h-Alien (1 pmol), a fusion of full-length h-Alien to the Gal4 DBD (aa 1 to 94), and TRα (2 pmol) were tested for hormone-dependent effects of the TR-Alien interaction with the reporter pUAS6× tkCAT in CV1 cells (1 pmol) (mammalian one-hybrid). As controls, an empty expression vector (lane C), the Gal4 DBD alone, and RARα were used.
HD3 cells express the oncogene v-erbA (13), and Alien represses promoter activity in this cell line more effectively than in other cell lines (Fig. 5). Therefore, we analyzed whether v-erbA/TR can strengthen Alien-mediated repression in CV1 cells. To be able to test the effect of ligand on the Alien-TR interaction, we tethered h-Alien to the Gal4 DBD and cotransfected the TR expression vector with a UAS-driven reporter. The silencing mediated by h-Alien is weaker than shown in Fig. 5 because smaller amounts of Gal-Alien expression vector were used. No effects on the reporter were observed when only the Gal4 DBD was expressed. However, expression of TRα potentiated the repression mediated by Gal–h-Alien in the absence of ligand. This potentiation of repression was completely abolished by addition of thyroid hormone. This result suggests a functional complex of TR with Alien in mammalian cells which is hormone sensitive. As controls, we used both the Gal DBD alone and RARα, which does not interact with Alien in yeast two-hybrid experiments (Fig. 3A). As seen in Fig. 7C, neither the hormone-dependent effect nor the potentiation of silencing was observed with the controls. This indicates that Alien and RAR do not form a functional complex, which is in accordance with our previous results.
Thus, our results suggest that Alien and TR mutually potentiate repression in mammalian cells.
Mechanisms of Alien-mediated silencing.
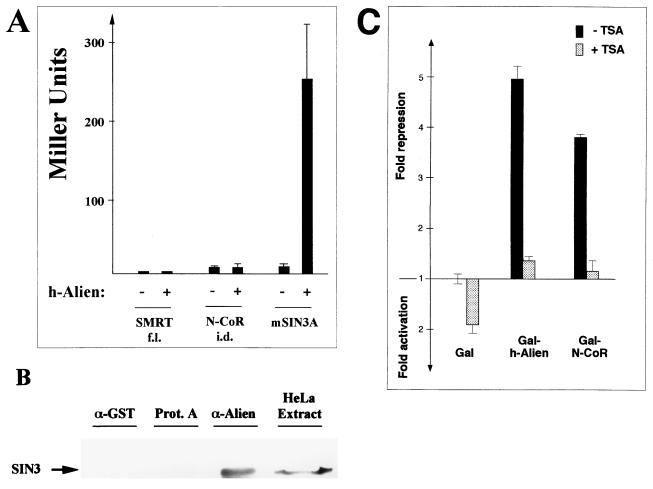
To gain insight into the mechanism of Alien-mediated gene repression, we tested whether the silencing function of Alien is based on complex formation with the known corepressors SMRT and N-CoR or with SIN3A (2, 35). We tested the ability of full-length h-Alien to interact with either full-length SMRT (9), the C terminus of N-CoR, or mouse SIN3A in the yeast two-hybrid system. We did not detect any interaction of h-Alien with the SMRT/N-CoR class of corepressors (Fig. 8A). Interestingly, we detected a strong interaction of Alien with SIN3A, a protein shown to be part of a deacetylase complex (33, 35, 41, 43, 80). We also observed a specific Alien-SIN3A interaction in GST pull-down experiments with GST–h-Alien and in vitro-translated SIN3A (data not shown). To verify this interaction, coimmunoprecipitations were performed. The anti-Alien antibody immunoprecipitated SIN3A from HeLa extracts (Fig. 8B). Protein A-Sepharose alone or anti-GST antibody, as controls, did not show immunoprecipitation of h-Alien. This indicates that Alien is mediating silencing, at least in part, by recruiting a factor known to be involved in deacetylase activity.
FIG. 8.
Alien interacts with SIN3A but not with the SMRT/N-CoR class of corepressors. (A) Full-length h-Alien was tested for interaction with full-length SMRT (SMRT f.l.), the receptor interaction domain of N-CoR (N-CoR i.d.), or SIN3A. Yeast two-hybrid assays were done as in the experiment in Fig. 1. (B) Coimmunoprecipitation of endogenous h-Alien with SIN3A. HeLa cells were used for immunoprecipitation with the anti-Alien peptide antibody. Western blotting was performed with the anti-SIN3A antibody. Protein A-Sepharose alone and anti-GST antibody were used as negative controls. As a migration control, HeLa extract was loaded. (C) The histone deacetylase inhibitor TSA decreases the silencing activity of Alien. Cotransfection experiments were performed with the Gal-Alien expression vector and the UAS6× tkCAT reporter in CV1 cells. TSA (100 ng/ml) was added 8 h prior to cell harvest. Gal-N-CoR was used as a positive control.
To further investigate the association of Alien with a deacetylase activity, we used trichostatin A (TSA), a specific inhibitor of histone deacetylases (77). CV1 cells were transfected with Gal–h-Alien or Gal–N-CoR and treated with TSA for 8 h. Addition of TSA reduces silencing by both Alien and N-CoR (Fig. 8C), a protein known to repress transcription by recruitment of deacetylase activity. Taken together, this supports a role for deacetylase activity in Alien-mediated silencing. Since the SMRT/N-CoR class of corepressor also interacts with SIN3A, the observed TR-Alien-SIN3A interaction may strengthen the recruitment of a deacetylase complex to genes regulated by selected NHRs.
Thus, our data indicate that one mechanism by which Alien confers silencing may, at least to some extent, be based on recruitment of deacetylase activity via interaction with SIN3A.
DISCUSSION
Alien represents a novel type of corepressor.
Alien shows the characteristics of a corepressor in that it interacts only in the absence of ligand with TR, dissociates from the receptor in the presence of ligand, is localized within the nucleus, and harbors an autonomous silencing function. Furthermore, we observed that Alien potentiates TR-mediated silencing and that the interaction of Alien with TR or TR mutants correlates with the ability of TR to silence transcription. Interestingly, RAR does not interact with Alien. This indicates that Alien has characteristics different from those of the SMRT/N-CoR class of corepressors. This correlates with the observation that there is no obvious sequence homology between Alien and SMRT or N-CoR. Taking these points together, we suggest that Alien represents a member of a new class of corepressors.
Corepressors exhibit differential interaction with RAR.
The SMRT/N-CoR class of corepressors exhibits interaction with NHRs such as TR, RAR, and RXR (19, 38, 48, 78). Interestingly, Alien shows different properties in its ability to interact with different NHRs. In contrast to the SMRT/ N-CoR class, Alien fails to bind RAR as shown by yeast two-hybrid experiments, mammalian one-hybrid experiments, and lack of influence of Alien on RAR transcriptional activity. However, by using RAR deletion mutants, we observed an interaction of Alien with the RAR silencing domain (RAR ΔC60) when it was separated from the activation domain AF2-AD/τ4/τc (helix 12) and the receptor F-region. However, a mutant with C-terminal truncation, RARΔC43, which harbors the AF2-AD/τ4/τc sequence but lacks the receptor F-region, fails to interact with Alien. These findings suggest that helix 12 of RAR prevents the binding of Alien to the RAR silencing domain. Thus, binding of Alien to RAR is inhibited by the AF2-AD/τ4/τc domain.
A functional silencing domain is required for interaction of NHRs with the SMRT/N-CoR class of corepressors. All the TR mutants we tested that have lost the silencing function also lacked interaction with SMRT and N-CoR (results not shown). While we observed a difference in interaction between Alien and the SMRT/N-CoR class for RAR, a large battery of TR mutants that have only partially lost the silencing function will be required to distinguish between the two corepressor classes for TR.
We found that the silencing mediated by DNA-bound Alien was enhanced by overexpression of TR. Hormone treatment abolished this enhancement. This suggests that the enhancement of silencing mediated by the Alien-TR complex is relieved because thyroid hormone binding is able to dissociate the Alien-receptor complex. We also saw an enhancement of Alien-mediated silencing by TR in the absence of ligand (Fig. 7C), indicating that Alien is not competing for the binding of the SMRT and N-CoR corepressors. Rather, we observed that TR can potentiate the Alien-mediated silencing. We hypothesize that TR is cocomplexed with Alien and the SMRT/ N-CoR class and is recruiting the SMRT/N-CoR class into the TR-Alien complex and hence enhancing silencing. In addition, there are no sequence homologies of SMRT or N-CoR to Alien, indicating that Alien represents a member of a novel class of corepressors. Taken together, these results imply that Alien interacts with nuclear receptors in a distinct manner from the SMRT/N-CoR class which we observed for RAR.
Since a large number of coactivators for NHRs have been found, it is quite possible that these oppose a similar number of counteracting corepressors. The biological role of multiple cofactors may be that they provide certain specificities. This specificity may be present at the level of development if some cofactors are expressed in temporarily distinct patterns, at the level of differentiation, or at the level of receptor-specific interaction.
TR-mediated silencing.
In vitro transcription experiments demonstrate that unliganded TR mediates repression in vitro (28, 67, 68), even with highly purified basal transcription factors (28, 29). This shows that one mechanism of TR-mediated silencing may exclude the involvement of chromatin and consequently histone deacetylation and may direct into a different mode of repression. This is indicated by interaction of TR with basal transcription factors (5, 28, 29). The newly identified pathways including histone deacetylase function represent an additional mechanism by which TR can repress promoter activity and suggest that NHRs use multiple pathways for gene silencing. Thus, analyzing single interaction partners in a cell containing multiple interacting partners may show that the impact of each leads to moderate actions in the cellular context. This may explain the moderate but significant effect of Alien on potentiation of the repression mediated by TR. The extent of potentiation is similar to that by other corepressors, such as SMRT (47), KAP-1 (27), RPD3 (74), and SUN-CoR (79) on transcriptional silencer proteins.
Mechanisms of Alien-mediated repression.
The mechanism by which Alien mediates repression is unknown. Our observation that Alien interacts with SIN3A provides a link to the SIN3A-associated deacetylase activity recently identified for MAD/MAX-Mxi- and N-CoR/SMRT-mediated repression (34, 41, 43, 63, 80). This suggests that Alien-mediated repression is, at least in part, due to histone deacetylation by recruitment of SIN3A. Interestingly, a 42-kDa protein (p42) has been reported to be associated with SIN3A in experiments involving coimmunoprecipitation with an anti-SIN3 antibody (34). Such a migration may correlate with our observed 41-kDa h-Alien. Since we have not seen interaction of Alien with the SMRT and N-CoR class of corepressors, Alien-mediated repression through SIN3A represents a SMRT- and N-CoR-independent pathway for SIN3 recruitment. It is therefore possible that both types of corepressors, Alien and SMRT/N-CoR, act synergistically to recruit SIN3A. An enhanced recruitment of SIN3A would augment chromatin-mediated repression of receptor target genes. This may be especially important when the expression of corepressors is tissue specific or developmentally regulated, leading to a differential silencing strength in different tissues. Another possibility is that there are several types of deacetylase complexes in a cell, which contain alternatively SMRT/N-CoR and Alien. However, other mechanisms of gene repression by Alien cannot be ruled out.
Alien is highly conserved in evolution.
Alien is highly conserved at the amino acid level among different species including vertebrates, Drosophila, C. elegans, and plants such as Ricinus (Fig. 2). The biological role of Alien in all these species is unknown. In Drosophila, Alien shows tissue-specific expression and is developmentally regulated (reference 31 and unpublished data). Since there is no Drosophila Alien mutant available, the developmental role of d-Alien is still unclear. In yeast, a putative protein predicted from an open reading frame shows only a very weak homology to h-Alien (20% at the amino acid level). Interestingly, a GenBank database search revealed that one Alien gene is located on human chromosome 21. Taken together, Alien appears to be highly conserved only in multicellular organisms.
Alien exhibits 90% identity between Drosophila and human. The high degree of conservation is striking but not unusual when comparing human COUP-TF1 and its Drosophila homologue SVP, which show only one amino acid difference in their DBD and also exhibit about 90% identity in their receptor E region. Similarly, USP and RXR can be functionally interchanged to confer EcR- or RAR-mediated transcriptional regulation in mammalian cells (66).
The high degree of conservation throughout the Alien protein indicates that it plays an important biological role. It is possible that Alien harbors multiple functions and may interact with additional, as yet unidentified proteins. Future mapping of additional Alien functions may shed more light on the role of Alien and the basis of its high degree of conservation in multicellular organisms.
ACKNOWLEDGMENTS
We thank C. S. Thummel for in vitro translation vectors for full-length DHR 38, DHR 78, DHR 96, and P9 αFTZ-F1; M. Mlodzic for SVP cDNA; D. D. Moore for pJG-TRIP15, pEG-TRα, and pEG-RXRα; M.-J. Tsai and B. W. O’Malley for in vitro translation vectors for COUP-TF1, hRXRα, and hRARα, S. Munroe for E75B and DHR 3 cDNAs, David Hogness for EcR cDNA, R. N. Eisenman for mSIN3, Urban Deutsch for anti-HA antibody, A. J. Hörlein and M. G. Rosenfeld for pEG-TRα and N-CoR cDNA, and Roger Brent for the yeast two-hybrid system. We are grateful to Kristine Krüger for excellent technical help. We thank L. J. Burke for critically reading the manuscript.
This work was supported by grants from the Sonderforschungsbereich SFB 397 and GRK 370 of the Deutsche Forschungsgemeinschaft and from the Fonds der Chemischen Industrie.
REFERENCES
- 1.Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho R A. Role of N-CoR and histone deacetylase in SIN3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 2.Ayer D E, Lawrence Q A, Eisenman R N. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell. 1995;80:767–776. doi: 10.1016/0092-8674(95)90355-0. [DOI] [PubMed] [Google Scholar]
- 3.Baniahmad A, Steiner C, Köhne A C, Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990;61:505–514. doi: 10.1016/0092-8674(90)90532-j. [DOI] [PubMed] [Google Scholar]
- 4.Baniahmad A, Köhne A C, Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-erbA oncogene product and in the retinoic acid receptor. EMBO J. 1992;11:1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baniahmad A, Ha I, Reinberg D, Tsai S Y, Tsai M-J, O’Malley B W. Interaction of human thyroid hormone receptor β with transcription factor TFIIB may mediate target gene derepression and activation by thyroid hormone. Proc Natl Acad Sci USA. 1993;90:8832–8836. doi: 10.1073/pnas.90.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baniahmad A, Burris T P, Tsai M-J. The nuclear hormone receptor superfamily. In: Tsai M-J, O’Malley B W, editors. Mechanism of steroid hormone regulation of gene transcription. Austin, Tex: Landes Co., CRC Press; 1994. pp. 1–24. [Google Scholar]
- 7.Baniahmad C, Baniahmad A, O’Malley B W. A rapid method combining a functional test of fusion proteins in vivo and their purification. BioTechniques. 1994;16:194–196. [PubMed] [Google Scholar]
- 8.Baniahmad A, Leng X, Burris T P, Tsai S Y, Tsai M-J, O’Malley B W. The τ4 activation domain of the thyroid hormone receptor is required for release of a putative corepressor(s) necessary for transcriptional silencing. Mol Cell Biol. 1995;15:76–86. doi: 10.1128/mcb.15.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baniahmad A, Thormeyer D, Renkawitz R. τ4/τc/AF-2 of the thyroid hormone receptor relieves silencing of the RAR silencer core independent of both τ4 activation function and full dissociation of corepressors. Mol Cell Biol. 1997;17:4259–4271. doi: 10.1128/mcb.17.8.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baniahmad A, Dressel U, Renkawitz R. Cell-specific inhibition of RARα silencing by the AF2/τc activation domain can be overcome by the corepressor SMRT, but not N-CoR. Mol Endocrinol. 1998;12:504–512. doi: 10.1210/mend.12.4.0093. [DOI] [PubMed] [Google Scholar]
- 11.Barettino D M, Ruiz M V, Stunnenberg H G. Characterization of the ligand-dependent transactivation domain of thyroid hormone receptor. EMBO J. 1994;13:3039–3049. doi: 10.1002/j.1460-2075.1994.tb06603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 13.Beug H, Döderlein C, Freudenstein C, Graf T. Erythroblast cell lines transformed by a temperature-sensitive mutant of avian erythroblast virus: a model system to study erythroid differentiation in vitro. J Physiol Suppl. 1982;1:195–207. doi: 10.1002/jcp.1041130427. [DOI] [PubMed] [Google Scholar]
- 14.Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-α. Nature. 1995;375:377–382. doi: 10.1038/375377a0. [DOI] [PubMed] [Google Scholar]
- 15.Busch K, Martin B, Baniahmad A, Renkawitz R, Muller M. At least three subdomains of v-erbA are involved in its silencing function. Mol Endocrinol. 1997;11:379–389. doi: 10.1210/mend.11.3.9903. [DOI] [PubMed] [Google Scholar]
- 16.Cavailles V, Davois S, L’Horset F, Lopez G, Hoare S, Kushner P J, Parker M G. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montminy M, Evans R M. Role of CBP/P300 in nuclear receptor signalling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 18.Chang K-H, Chen Y, Chen T-T, Chou W-H, Chen P-L, Ma Y-Y, Yang-Feng T L, Leng X, Tsai M-J, O’Malley B W, Lee W-H. A thyroid hormone receptor coactivator negatively regulated by the retinoblastoma protein. Proc Natl Acad Sci USA. 1997;94:9040–9045. doi: 10.1073/pnas.94.17.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J D, Evans R M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 20.Cooney A J, Leng X, Tsai S Y, O’Malley B W, Tsai M-Y. Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor-dependent repression of transactivation by the vitamin D, thyroid hormone, and retinoic acid receptors. J Biol Chem. 1993;268:4152–4160. [PubMed] [Google Scholar]
- 21.Damm K, Thompson C C, Evans R M. Protein encoded by v-erbA functions as a thyroid hormone receptor antagonist. Nature. 1989;339:593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- 22.Damm K, Evans R M. Identification of a domain required for oncogenic activity and transcriptional suppression by v-erbA and thyroid-hormone receptor α. Proc Natl Acad Sci USA. 1993;90:10668–10672. doi: 10.1073/pnas.90.22.10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danielian P S, White R, Lees J A, Parker M G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992;11:1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobens L, Rudolph K, Berger E M. Ecdysterone regulatory elements function as both transcriptional activators and repressors. Mol Cell Biol. 1991;11:1846–1853. doi: 10.1128/mcb.11.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Downes M, Burke L J, Bailey P J, Muscat G E O. Two receptor interaction domains in the corepressor N-CoR/RIP13, are required for an efficient interaction with Rev-erbA α and RVR: physiological association is dependent on the E-region of the orphan receptors. Nucleic Acids Res. 1996;24:4379–4387. doi: 10.1093/nar/24.22.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eggert M, Michel J, Schneider S, Bornfleth H, Baniahmad A, Fackelmayer F O, Schmidt S, Renkawitz R. The glucocorticoid receptor is associated with the RNA-binding nuclear matrix protein hnRNP U. J Biol Chem. 1997;272:28471–28478. doi: 10.1074/jbc.272.45.28471. [DOI] [PubMed] [Google Scholar]
- 27.Friedman J R, Fredericks W J, Jensen D E, Speicher D W, Huang X-P, Neilson E G, Rauscher F J., III KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996;10:2067–2078. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- 28.Fondell J D, Roy A L, Roeder R G. Unliganded thyroid hormone receptor inhibits formation of a functional preinitiation complex: implications for active repression. Genes Dev. 1993;7:1400–1410. doi: 10.1101/gad.7.7b.1400. [DOI] [PubMed] [Google Scholar]
- 29.Fondell J D, Brunel F, Hisatake K, Roeder R G. Unliganded thyroid hormone receptor alpha can target TATA-binding protein for transcriptional repression. Mol Cell Biol. 1996;16:281–287. doi: 10.1128/mcb.16.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fondell J D, Ge H, Roeder R G. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc Natl Acad Sci USA. 1996;93:8329–8333. doi: 10.1073/pnas.93.16.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goubeaud A, Knirr S, Renkawitz-Pohl R, Paululat A. The Drosophila gene alien is expressed in the muscle attachment sites during embryogenesis and encodes a protein highly conserved between plants, Drosophila and vertebrates. Mech Dev. 1996;57:59–68. doi: 10.1016/0925-4773(96)00532-1. [DOI] [PubMed] [Google Scholar]
- 32.Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 33.Hanna-Rose W, Hansen U. Active repression mechanisms of eukaryotic transcription repressors. Trends Genet. 1996;12:229–234. doi: 10.1016/0168-9525(96)10022-6. [DOI] [PubMed] [Google Scholar]
- 34.Hassig C A, Fleischer T C, Billin A N, Schreiber S L, Ayer D E. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 35.Heinzel T, Lavinsky R M, Mullen T-M, Söderström M, Laherty C D, Torchia J, Yang W-M, Brard G, Ngo S D, Davie J R, Seto E, Eisenman R N, Rose D W, Glass C K, Rosenfeld M G. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 36.Henttu P M A, Kalkhoven E, Parker M G. AF-2 activity and recruitment of steroid receptor coactivator 1 to the estrogen receptor depend on a lysine residue conserved in nuclear receptors. Mol Cell Biol. 1997;17:1832–1839. doi: 10.1128/mcb.17.4.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong H, Kohli K, Trivedi A, Johnson D L, Stallcup M R. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hörlein A, Näär A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass C K, Rosenfeld M G. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 39.Hörlein A J, Heinzel T, Rosenfeld M G. Gene regulation by thyroid hormone receptors. Endocrinol Diabetes. 1996;3:412–416. [Google Scholar]
- 40.Horwitz K B, Jackson T A, Bain D L, Richter J K, Takimoto G S, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 41.Kadosh D, Struhl K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell. 1997;89:365–371. doi: 10.1016/s0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- 42.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 43.Laherty C D, Yang W-M, Sun J-M, Davie J R, Seto E, Eisenman R N. Histone deacetylase associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 44.LeDouarin B, Zechel C, Garnier J M, Lutz Y, Tora L, Pierrat P, Heery D, Gronemeyer H, Chambon P, Losson R. The N-terminal part of TIF1, a putative mediator of the ligand-dependent activation function AF-2 of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J. 1995;14:2020–2033. doi: 10.1002/j.1460-2075.1995.tb07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J W, Choi H-S, Gyuris J, Brent R, Moore D D. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol. 1995;9:243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- 46.Levine M, Manley J L. Transcriptional repression of eukaryotic promoters. Cell. 1989;59:404–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Leo C, Schroen D J, Chen J D. Characterization of receptor interaction and transcriptional repression by the corepressor SMRT. Mol Endocrinol. 1997;11:2025–2037. doi: 10.1210/mend.11.13.0028. [DOI] [PubMed] [Google Scholar]
- 48.Lin B C, Hong S H, Krig S, Yoh S M, Privalsky M L. A conformational switch in nuclear hormone receptors is involved in coupling hormone binding to corepressor release. Mol Cell Biol. 1997;17:6131–6138. doi: 10.1128/mcb.17.10.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mangelsdorf D J, Evans R M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 51.Martin B, Renkawitz R, Muller M. Two silencing sub-domains of v-erbA synergize with each other, but not with RXR. Nucleic Acids Res. 1994;22:4899–4905. doi: 10.1093/nar/22.23.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagy L, Kao H-Y, Chakravarti D, Lin R J, Hassig C A, Ayer D E, Schreiber S L, Evans R M. Role of N-CoR and histone deacetylase in SIN3-mediated transcriptional repression mediated by a complex containing SMRT, mSIN3A and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 53.Norman C, Runswick M, Pollock R, Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988;55:989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 54.Oñate S A, Tsai S Y, Tsai M-J, O’Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 55.Parker M G, White R. Nuclear receptors spring into action. Nat Struct Biol. 1996;3:113–115. doi: 10.1038/nsb0296-113. [DOI] [PubMed] [Google Scholar]
- 56.Puigserver P, Wu Z, Park C W, Graves R, Wright M, Spiegelman B M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 57.Renaud J-P, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D. Crystal structure of the RAR-γ-ligand-binding domain bound to all-trans retinoic acid. Nature. 1995;378:681–689. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- 58.Renkawitz R. Transcriptional repression in eukaryotes. Trends Genet. 1990;6:192–197. doi: 10.1016/0168-9525(90)90176-7. [DOI] [PubMed] [Google Scholar]
- 59.Russo M W, Sevetson B R, Milbrandt J. Identification of NAB1, a repressor of NGFI-A and Krox20 mediated transcription. Proc Natl Acad Sci USA. 1995;92:6873–6877. doi: 10.1073/pnas.92.15.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt S, Baniahmad A, Eggert M, Schneider S, Renkawitz R. Multiple receptor interaction domains of GRIP1 function in synergy. Nucleic Acids Res. 1998;26:1191–1197. doi: 10.1093/nar/26.5.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shibata H, Nawaz Z, Tsai S Y, O’Malley B W, Tsai M-Y. Gene silencing by chicken ovalbumin upstream promoter factor I COUP-TFI is mediated by transcriptional corepressors, nuclear receptor corepressor N-CoR and silencing mediator for retinoic acid and thyroid hormone receptor SMRT. Mol Endocrinol. 1997;11:714–724. doi: 10.1210/mend.11.6.0002. [DOI] [PubMed] [Google Scholar]
- 62.Söderstrom M, Vo A, Heinzel T, Lavinsky R M, Yang W M, Seto E, Peterson D A, Rosenfeld M G, Glass C K. Differential effects of nuclear receptor corepressor (N-CoR) expression levels on retinoic acid receptor-mediated repression support the existence of dynamically regulated corepressor complexes. Mol Endocrinol. 1997;11:682–692. doi: 10.1210/mend.11.6.0018. [DOI] [PubMed] [Google Scholar]
- 63.Svaren J B, Sevetson R, Apel E B, Zimonjic B D, Popescu N C, Milbrandt J. NAB2, a corepressor of NGFI-A Egr-1 and Krox20, is induced by proliferative and differentiative stimuli. Mol Cell Biol. 1996;16:3545–3553. doi: 10.1128/mcb.16.7.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeshita A, Cardona G R, Koibuchi N, Suen C S, Chin W W. TRAM-1, a novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J Biol Chem. 1997;272:27778–27786. doi: 10.1074/jbc.272.44.27629. [DOI] [PubMed] [Google Scholar]
- 65.Tenbaum S P, Baniahmad A. Nuclear hormone receptors: structure, function and involvement in disease. Int J Biochem Cell Biol. 1997;29:1325–1341. doi: 10.1016/s1357-2725(97)00087-3. [DOI] [PubMed] [Google Scholar]
- 66.Thomas H E, Stunnenberg H G, Stewart A F. Heterodimerization of the Drosophila ecdysone receptor with retinoid X receptor and ultraspiracle. Nature. 1993;362:471–475. doi: 10.1038/362471a0. [DOI] [PubMed] [Google Scholar]
- 67.Tong G-X, Tanen M M R, Bagchi M K. Ligand modulates the interaction of thyroid hormone receptor β with the basal transcription machinery. J Biol Chem. 1995;270:10601–10611. doi: 10.1074/jbc.270.18.10601. [DOI] [PubMed] [Google Scholar]
- 68.Tong G-X, Jeyakumar M, Tanen M M R, Bagchi M K. Transcriptional silencing by unliganded thyroid hormone receptor β requires a soluble corepressor that interacts with the ligand-binding domain of the receptor Mol. Cell Biol. 1996;16:1909–1920. doi: 10.1128/mcb.16.5.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torchia J, Rosenfeld D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M G. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 70.Tourmente S, Chapel S, Dreau D, Drake M E, Bruhat A, Couderc J L, Dastugue B. Enhancer and silencer elements within the first intron mediate the transcriptional regulation of the beta 3 tubulin gene by 20-hydroxyecdysone in Drosophila Kc cells. Insect Biochem Mol Biol. 1993;23:137–143. doi: 10.1016/0965-1748(93)90092-7. [DOI] [PubMed] [Google Scholar]
- 71.Voegel J J, Heine M J S, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDA transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner R L, Apriletti J W, McGrath M E, West B L, Baxter J D, Fletterick R J. A structural role for hormone in the thyroid hormone receptor. Nature. 1995;378:690–697. doi: 10.1038/378690a0. [DOI] [PubMed] [Google Scholar]
- 73.Wong J, Shi Y-B, Wolffe A P. A role for nucleosome assembly in both silencing and activation of the Xenopus TRβA gene by the thyroid hormone receptor. Genes Dev. 1995;9:2696–2711. doi: 10.1101/gad.9.21.2696. [DOI] [PubMed] [Google Scholar]
- 74.Yang W-M, Inouye C, Zeng Y, Dears D, Seto E. Transcriptional repression by YY1 is mediated by interaction with a mammalian homolog of the yeast global regulator RPD3. Proc Natl Acad Sci USA. 1996;93:12845–12850. doi: 10.1073/pnas.93.23.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yao T-P, Forman B M, Jlang Z, Cherbas L, Chen J D, McKeown M, Cherbas P, Evans R M. Functional ecdysone receptor is the product of EcR and ultraspiracle. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- 76.Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostata cells. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshida M, Horinouchi S, Beppu T. Trichostatin A and Trapoxin: novel chemical probes for the role of histone acetylation in chromatin structure and function. Bioessays. 1995;17:423–430. doi: 10.1002/bies.950170510. [DOI] [PubMed] [Google Scholar]
- 78.Zamir I, Zhang J, Lazar M A. Stoichiometric and steric principles governing repression by nuclear hormone receptors. Genes Dev. 1997;11:835–846. doi: 10.1101/gad.11.7.835. [DOI] [PubMed] [Google Scholar]
- 79.Zamir I, Dawson J, Lavinsky R M, Glass C K, Rosenfeld M G, Lazar M A. Cloning and characterization of a corepressor and potential component of the nuclear hormone receptor repression complex. Proc Natl Acad Sci USA. 1997;94:14400–14405. doi: 10.1073/pnas.94.26.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D. Histone deacetylase and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357–364. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]