Abstract
Background
Endoscopic ultrasound (EUS)-guided transmural drainage for pancreatic fluid collections (PFCs) has become the first-line treatment with quicker recovery and more minor injury compared with surgery and percutaneous drainage. The efficacy of stents implantation and drainage for different PFCs remains controversial, especially lumen-apposing metal stents (LAMS). This study aimed to compare the efficacy and safety of LAMS drainage for pancreatic pseudocysts (PPC) and walled-off necrosis (WON).
Methods
A meta-analysis was performed for LAMS drainage for WON and PPC by systematically searching PubMed, Cochrane, and Embase databases from January 2010 to January 2020. From 2017 to 2019, 12 patients who were treated with LAMS drainage for PFCs in our medical center were also reviewed and included in this study.
Results
Combining 11 copies of documents with the data from our medical center, a total of 585 patients with PFCs were enrolled in this meta-analysis, including 343 patients with WON and 242 with PPC. The technical success rate in WON is not significantly different from that of PPC (P = 0.08 > 0.05). The clinical success of LAMS placement was achieved in 99% vs 89% in PPC and WON, respectively (RR = 0.92, 95% CI: 0.86–0.98, P = 0.01 < 0.05). The further intervention of direct endoscopic necrosectomy was required by 60% of patients in WON group. There was no significant difference in the incidence of adverse events, including infection, bleeding, stent migration and stent occlusion, after LAMS placement between WON and PPC.
Conclusions
Endoscopic ultrasound-guided LAMS for PFCs are feasible, effective with preferable technical and clinical success rates. The clinical effect of LAMS on PPC is slightly better than that of WON, but its adverse reactions still need to be verified in a large-sample prospective study.
Keywords: Pancreatic pseudocyst, Walled-off necrosis, Endoscopic treatment, Lumen-apposing metal stents
Introduction
Approximately 20%–40% of patients with severe acute pancreatitis will develop pancreatic and peripancreatic infection.1 Pancreatic fluid collections (PFCs) with amylase-rich collections usually occur after acute or chronic pancreatitis and pancreatic injury. According to the revised 2012 Atlanta Classification, PFCs were classified as peripancreatic fluid collections, pancreatic and peripancreatic necrosis (sterile or infected), pseudocyst and walled-off necrosis (sterile or infected). Pancreatic pseudocyst (PPC) is an encapsulated collection of fluid with a well -defined inflammatory wall with minimal or no necrosis. Walled-off necrosis (WON) is a mature, encapsulated collection of pancreatic and/or peripancreatic necrosis that has formed a well-defined inflammatory wall.2 It is reported that the majority of PFCs can resolve spontaneously. Still, the collections with a diameter of more than 5 cm may accompany a higher risk of complications, particularly those in absence of decreasing collection size over six weeks.3 Delaying intervention for at least four weeks after onset of acute pancreatitis is currently regarded as a favorable approach to drainage for PFCs.3, 4, 5
By establishing a channel between the digestive tract and the cyst to achieve the internal drainage of the cyst, endoscopic ultrasound (EUS)-guided stents placement for the drainage of PFCs has recently become a preferred therapeutic approach. Compared with surgery, it has a considerable curative effect, more minor trauma, faster recovery, and less cost.6 A novel lumen-apposing metal stent (LAMS) emerging after plastic stents and self-expanding metal stents, shaped like a saddle on both ends and covered with silicone, has been broadly used in recent years. These stents have a certain anchoring effect and expand to fit the space between the gastrointestinal tract wall and the cyst. Baron et al 7 firstly reported the treatment of WON with endoscopic debridement in 1996, which laid the foundation for the application of direct endoscopic necrosectomy (DEN). The larger luminal diameter of LAMS provides a convenient passage for a gastroscope into the cyst to observe the necrotic material and perform DEN when needed. Even if there is a large diameter drainage channel, solid necrotic tissue in WON cannot be completely drained out spontaneously with undesirable clinical effects if DEN is not performed.8
Research shows that the outcome of endoscopic drainage is closely related with the types of PFCs, and that the clinical success rate of organized necrosis was significantly lower than that of other collections.9,10 Hasan et al 4 proposed that it is crucial to distinguish PPC from WON due to the more additional interventions and lower success rate of definitive resolution in patients with WON than PPC. However, a meta-analysis from Hammad et al 11 published in 2018, aiming to compare the efficacy of LAMS drainage for PFCs with that of plastic stents, reported endoscopic LAMS drainage for WON has had no difference with that of PPC, meanwhile, another meta-analysis from Renelus et al 12 including metal and plastic stents published in 2019 also supported this finding. Concerning the controversial results of stents in the management of PFCs, we have carried out a comparative study centering on the efficacy of LAMS for PPC and WON through a meta-analysis combined with a series of medical records from our medical center to evaluate the outcomes of different types of PFCs.
Methods
Present series
Consecutive patients treated in Beijing Friendship Hospital and underwent EUS-guided LAMS placement for PPC or WON from January 2017 to December 2019 were retrospectively recruited in this present series. Inclusion criteria for the case series included:13,14 1. the age of patients with PPC or WON should stay between 18 and 75; 2. the collection occurs over 6 weeks after onset of acute pancreatitis and the diameter of cyst is larger than 6 cm, or there is persistent pain and complications, such as infection and mass effect. Patients who had gotten regional varices, suspected cystic neoplasms, coagulopathy (INR>1.5) were excluded from this study. The protocol was approved by the Medical Ethics Committees, and all patients gave their informed consent prior to LAMS placement.
Treatment and outcomes
All procedures were performed under general anesthesia or conscious sedation, depending on the patient's condition. Intravenous antibiotics were administered systematically before and after the procedure. CT or magnetic resonance imaging was performed to define quantification of solid debris in PFCs before the procedure, and the amount of solid debris was graded as minimal (<10%), moderate (10%–50%) and profound (>50%).15
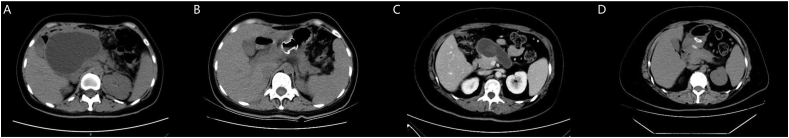
Firstly, the location and size of the collection were identified by endoscopic ultrasound (Supplementary Fig. 1A), then a 19-gauge needle was selected to puncture the cyst through the gastric and duodenal wall provided that interposed vessels were excluded at the puncture site. A 0.035-inch guidewire was passed through the needle in five patients and was coiled in the PFCs in necessity. Then AXIOS stents (Boston Scientific, United States) were released over a guidewire under fluoroscopic and EUS guidance (Supplementary Fig. 1B). In the remaining seven patients, the puncture site was further dilated by balloon and cystotome, followed by double mushroom head stents (DMHS, Micro-Tech Co., Jiangsu, China) over the guidewire. Additional endoscopic interventions, such as direct endoscopic necrosectomy, saline irrigation or placement of a nasocystic tube, were adopted at the discretion of endoscopists according to the clinical course of the patients (Supplementary Figs. 1C and 1D). Follow-up imaging with computed tomography was performed at one month after the initial LAMS placement to evaluate treatment outcome (Fig. 1). Patients with slow resolution on imaging were further followed based on medical judgment. The follow-up period of adverse events was three months after deployment.
Fig. 1.
The computed tomography scan shows the widespread fluid collection without solid necrotic tissue around pancreas before LAMS placement (A), resolution of the cyst after LAMS deployment (B), the walled off necrosis with non-liquid components in the head of pancreas before LAMS placement (C), and significantly shrinking of the cyst after LAMS deployment (D).
Clinical success was defined as at least a 60% decrease or the resolution of PFCs to <2 cm in the PFC size based on cross-sectional imaging at one month along with symptom relief. Technical success was defined as the successful insertion of the LAMS during the PFC drainage. Safety was evaluated according to the number of adverse events mainly consisting of postoperative infection, postoperative bleeding, stent occlusion and stent migration. Postoperative bleeding included immediate bleeding (<7 days) and delayed bleeding (>7 days).
Systematic review and meta-analysis
Search strategy
Relevant published studies from January 2010 to January 2020 were systematically searched and identified from 3 English databases, including PubMed, Cochrane Library, Embase database, and 3 Chinese databases, China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Journal Database (CQVIP), Wanfang Database. References of the searched studies and the bibliographies of recently published review articles were also screened. The search terms and details of the search strategy were showed in Supplementary Table 1.
Inclusion and exclusion criteria
The inclusion criteria of the studies were as follows: 1. the PPC and WON patients were simultaneously included, and all of these patients received endoscopic ultrasound-guided LAMS drainage; 2. the clinical success rates were reported or could be calculated; 3. the publication language should be either English or Chinese. The exclusion criteria were: 1. reviews, letters, and case reports; 2. lack of sufficient data.
Data extraction and quality assessment
The following variables were extracted with the agreement of the two investigators: the essential information and related data information, including the cyst type, stent type, technical success rate, clinical success rate, and incidences of adverse events. The Newcastle–Ottawa Scale (NOS; Ottawa Hospital, Ottawa, ON) assessed the quality of the included studies by two investigators. Discrepancies in data collection and quality assessment were referred to a senior methodologist for resolution. Studies with scores of 7 or higher were evaluated as high quality, and we assessed studies scored 5 or 6 as moderate quality.16
Statistical analysis
The primary outcome was the clinical success rates. The technical success rates and the adverse events rates constituted the secondary outcome. The relative risk (RR) with 95% confidence intervals (CI) were calculated, and summary statistics were estimated using meta-analysis models.17 Freeman-Tukey double arcsine transformation method was implemented to calculate an overall proportion. We added the number of 0.5 to each cell frequency for studies with a zero cell count. Publication bias was assessed by visual examination of the funnel plots and Egger's test of weighted linear regression. Statistical analysis was conducted with R software (version 3.5.1) with the “meta” package.
Results
Present series
A total of 12 patients with PFCs were included in this series as they met selection criteria, including two patients with PPC and 10 with WON. The details of the demographic characteristics of these 12 patients were shown in Supplementary Table 2. The average maximal diameter of PFCs was 12.5 ± 4.0 cm. Minimal concrete debris was noted in 4 cases (33.3%), moderate solid debris in 7 patients (58.3%), and profound solid debris in 1 patient (8.3%).
In terms of the efficacy of LAMS, stents placement was technically successful in 12 patients with a technical success rate of 100%. Endoscopic therapy using the LAMS was successful in 8 of 10 patients with WON compared with 2 of 2 patients with PPC, and the clinical success rate in patients with WON and PPC was 83.3% and 100%, respectively. Treatment failure occurred in two patients, in which one patient with moderate concrete debris required surgical intervention due to pseudoaneurysm. Another one with profound solid debris had poor drainage with cyst infection, followed by nasal cyst drainage as complementary endoscopic interventions, and in result the collection resolved partially across cross-sectional imaging at one month. Stent removal was performed after a median of 29.6 days (range 18–45). Procedural characteristics and clinical outcomes of PFCs are shown in Table 1.
Table 1.
Procedural characteristics and clinical outcomes of present series.
| Characteristics | PFCs (n = 12) | PPC (n = 2) | WON (n = 10) |
|---|---|---|---|
| Type of LAMS, n (%) | |||
| DMHS | 7 (58.3) | 1 (50.0) | 6 (60.0) |
| AXIOS | 5 (41.7) | 1 (50.0) | 4 (40.0) |
| Total No. of endoscopic reinterventions | |||
| 0 | 9 | 3 | 6 |
| 1 | 1 | 0 | 1 |
| >1 | 2 | 0 | 2 |
| Cyst irrigation, n (%) | 1 (8.3) | 0 | 1 (8.3) |
| Technical success, n (%) | |||
| YES | 12 (100) | 2 (100) | 10 (100) |
| Clinical success, n (%) | |||
| YES | 10 (83.3) | 2 (100) | 8 (80.0) |
| NO | 2 (16.7) | 0 | 2 (20.0) |
| Adverse events, n (%) | |||
| Infection | 6 (50) | 1 (50) | 5 (50) |
| Bleeding | 0 | 0 | 0 |
| Stent migration | 1 (8.3) | 0 | 1 (10.0) |
| Stent occlusion | 1 (8.3) | 0 | 1 (10.0) |
| Duration of procedure, min (mean ± SD) | 65.6 ± 24.3 | 51.5 ± 23.3 | 68.4 ± 24.7 |
| Hospital stay, days (mean ± SD) | 17.1 ± 8.6 | 16.5 ± 3.5 | 17.2 ± 9.4 |
| Days to stent removal, days (range) | 29.6 (18–45) | 27.5 (27–28]) | 30.1 (18–45) |
LAMS: lumen-apposing metal stents; PFCs: pancreatic fluid collections; PPC: pancreatic pseudocysts; WON: walled-off necrosis; SD: standard deviation.
Literature search
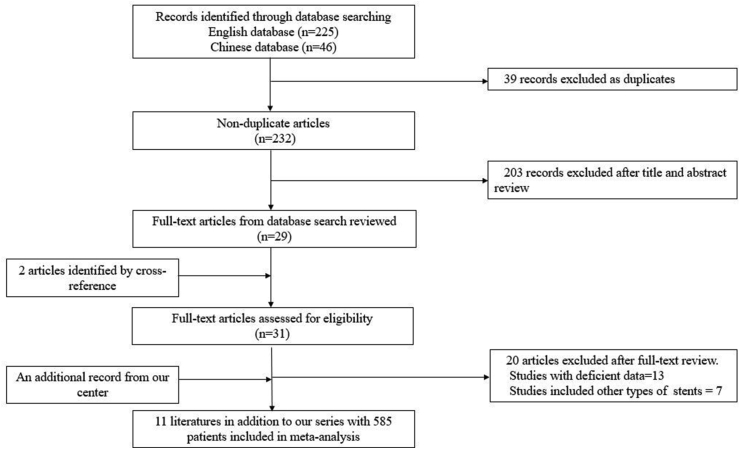
The flowchart of the literature search process is illustrated in Fig. 2. The retrieval time was from January 2010 to January 2020. According to the search strategy, 271 articles were enrolled during database searching, including 225 English articles and 46 Chinese articles. Among these articles, 39 articles were excluded due to duplicates, 203 articles were excluded after title and abstract review, 20 articles were excluded after full-text review for the following reason: 13 articles had no sufficient data, and seven reports targeted on other types of stents or mixed. In addition, we included two pieces from the reference of the screened articles and a record from our center. Finally, 585 patients from 12 studies were included in the meta-analysis.
Fig. 2.
PRISMA flowchart of studies included in the meta-analysis.
Study and population characteristics
The studies and population characteristics in the meta-analysis are shown in Table 2. Besides, we added the data from our series to the meta-analysis. All these treatments were implemented between the years 2011 to 2019. Most of the used LAMS subtypes were AXIOS stents or Nagi stents (Taewoong Medical, South Korea). The rest were DMHS and HANARO stents (MI-Tech, South Korea). The definitions of clinical success used in the included individual studies are shown in Supplementary Table 3.
Table 2.
The characteristics of the included studies and population.
| No | Author | Publication year | Country | Research period | No. of study institutes | Study design | Sample size | Study arm |
LAMS subtype | Mean age, years | Gender, Female/Male |
NOS Scores |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WON | PPC | ||||||||||||
| 1 | Natsuyo et al.8 | 2013 | Japan | 2011–2012 | 2 | Retrospective | 9 | 4 | 5 | Nagi | 49 | 3/6 | 7 |
| 2 | Daisy et al.18 | 2015 | European countries | 2011–2012 | 15 | Prospective | 61 | 46 | 15 | AXIOS | 55 | 23/38 | 7 |
| 3 | Chandran et al.19 | 2015 | Australia | 2013 | 13 | Retrospective | 47 | 9 | 38 | Nagi | 51a | 16/32b | 8 |
| 4 | Siddiqui et al.20 | 2016 | US | 2012–2014 | 4 | Retrospective | 82 | 68 | 14 | AXIOS | 51 | 33/48b | 8 |
| 5 | Josep et al.21 | 2017 | US | NA | 2 | Retrospective | 25 | 22 | 3 | AXIOS | 50 | 11/14 | 9 |
| 6 | Shuntaro et al.22 | 2017 | Japan | 2015–2016 | 1 | Prospective | 12 | 8 | 4 | HANARO | 66 | 2/10 | 7 |
| 7 | Suresh et al.23 | 2017 | UK and Ireland | 2015–2016 | 11 | Prospective | 116 | 70 | 46 | AXIOS | 53 | 38/78 | 8 |
| 8 | Dennis et al.24 | 2018 | US | 2010–2016 | Multi-center | Retrospective | 122 | 64 | 58 | AXIOS | 60 | 43/79 | 7 |
| 9 | Sundeep et al.25 | 2018 | Korea | NA | NA | Prospective | 5 | 4 | 1 | Nagi | 29 | 0/5 | 6 |
| 10 | Maria et al.26 | 2018 | Italy | 2013–2016 | 7 | Retrospective | 67 | 23 | 44 | Nagi | 59 | 21/46 | 8 |
| 11 | Rajat et al.27 | 2019 | US | 2014–2017 | 1 | Retrospective | 27 | 15 | 12 | AXIOS | 54 | 15/12 | 7 |
| 12 | This center | NA | China | 2016–2019 | 1 | Retrospective | 12 | 10 | 2 | AXIOS and DMHS | 47 | 4/8 | NA |
The age derived from Sujievvan et al was median age, and ages derived from other studies were mean ages.
The gender distribution was derived directly from the articles or calculated from gender ratio provided from the studies, which might bring the inconsistent with the total sample size.
A total of 585 patients with PFCs were included in the meta-analysis, in which 343 patients with WON and 242 with PPC. The age of these patients was around 50 years old except for the study from Sundeep et al. Due to missing data in some studies, 11 patients were finally excluded for calculating clinical success, adverse events (574/585), in which four patients were lost to follow-up in Dennis et al (1 and 3 in PPC and WON, respectively), two patients with PPC were lost to follow-up, and one patient with WON is being followed up in Rajat et al. Besides, in Daisy et al, data on four patients were limited to technical procedure and excluded from the further analysis (1 and 3 in PPC and WON, respectively). The specific scoring rules of the included trials according to NOS are shown in Supplementary Table 4. The quality assessment manifested an acceptable quality of the included studies.
Risk ratio of technical success between WON and PPC
A total of 10 studies provided data on the technical success rates of WON and PPC after LAMS placement. The overall risk ratio using the fixed-effect model (I2 = 0, P = 0.84) was 1.04 (95% CI: 1.00–1.08) (Supplementary Fig. 2). It showed no significance in technical success between WON and PPC (Z = 1.78, P = 0.08).
Clinical success rates of WON and PPC
Firstly, we summarized the overall clinical success rates of LAMS for patients with WON and PPC. As shown in Supplementary Fig. 3A, the clinical success rates of LAMS for WON ranged from 50% to 100%, with the overall clinical success rate of 89% (95% CI: 85%–93%) in fixed-effect model (I2 = 26%, P = 0.19). On the other hand, the clinical success rates of LAMS for PPC ranged from 83% to 100%, with the overall clinical success rate of 99% (95% CI: 95%–100%) in fixed effect model (I2 = 0, P = 0.80), showed in Supplementary Fig. 3B.
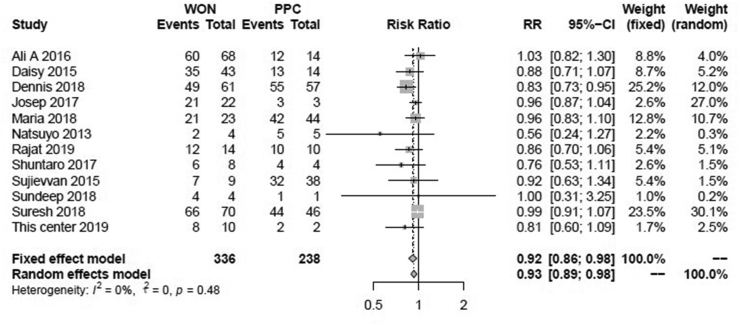
In order to clarify if the difference existed in the clinical success rates between LAMS in WON and PPC, the risk ratios of WON compared with PPC were calculated and showed in Fig. 3. The overall RR of WON compared with PPC was 0.92 (95%CI: 0.86–0.98) in the fixed effect model (I2 = 0, P = 0.48). The combined Z-test value was -2.73 (P = 0.01). The clinical effectiveness of LAMS in patients with WON was 0.92 times compared with LAMS in patients with PPC. Thus, LAMS drainage showed better clinical efficacy in PPC than that of WON.
Fig. 3.
The forest plot in risk ratio of clinical success rates of LAMS between WON and PPC.
The cumulative meta-analysis was also used on the clinical success rate (comparison of the efficacy of LAMS between PPC and WON) in the order of publication year and sample size, reflecting the dynamic trend in the included studies. In the cumulative meta-analysis of publication year, the risk ratio in the efficacy of LAMS between WON and PPC tended to be stable after the year 2017 (Supplementary Fig. 4A). Likewise, in the cumulative meta-analysis of sample size, the risk ratio between WON and PPC tended to be stable with a sample size larger than 30 patients (Supplementary Fig. 4B).
The studies were divided into three subgroups: Nagi group, AXIOS group, and other groups (including HANARO stents, AXIOS stents and DMHS mixed). Subgroup analysis of clinical success in different subtypes of LAMS showed the significant risk ratio in the AXIOS group (RR = 0.92, 95%CI: 0.86–0.99). In Nagi and other groups, the RR showed no significant success rates in different subtypes of LAMS. The clinical efficacy of AXIOS stents in PPC was better than WON (Supplementary Fig. 5).
Safety evaluation on postoperative complications
The most common adverse events in this review included postoperative infection, bleeding, stents migration and stent occlusion. There were at least five articles that provided data on each of these four complications. The summarized proportions of adverse events were shown in Supplementary Fig. 6 and Supplementary Fig. 7. All the four complications showed no significance on the complication rates between patients with WON and PPC in fixed-effect model (all the I2 < 50% and P > 0.05) (Supplementary Fig. 8). As shown in Supplementary Fig. 8A, the summary of RR on the postoperative infection was 1.57 (95% CI: 0.73–3.39) in the fixed-effect model, and there was no significance in the RR of WON cases compared with PPC cases (Z = 1.15, P = 0.25) on postoperative infection. The summary of RR on postoperative bleeding was 0.84 (95% CI: 0.34–2.09) (Supplementary Fig. 8B). We also found no significance in the RR of WON cases compared with PPC cases (Z = -0.37, P = 0.71) on postoperative bleeding. The summary of RR on the complication of migration was 1.04 (95% CI: 0.51–2.12) in the fixed effect model (Supplementary Fig. 8C). There was no significance in the RR of WON cases compared with PPC cases (Z = 0.11, P = 0.91) on stent migration. Supplementary Fig. 8D showed that the summary of RR on the complication of stents occlusion was 1.66 (95% CI: 0.92–2.98) in the fixed-effect model. We found no significance in the RR of WON cases compared with PPC cases (Z = 1.70, P = 0.09) on stent occlusion.
The proportion of patients undergoing DEN in WON cases
A total of 10 studies provided the information of DEN among WON cases. The summary proportion of patients undergoing DEN was 60% (95% CI: 34%–84%) in the random effect model (I2 = 94% > 50%, P < 0.01). The results showed 100% DEN proportion in Siddiqui et al20 and Chandran et al19 (Supplementary Fig. 9). Other studies with relatively more considerable weight showed of a low proportion of patients undergoing DEN in patients with WON ranging from 30% to 53%.
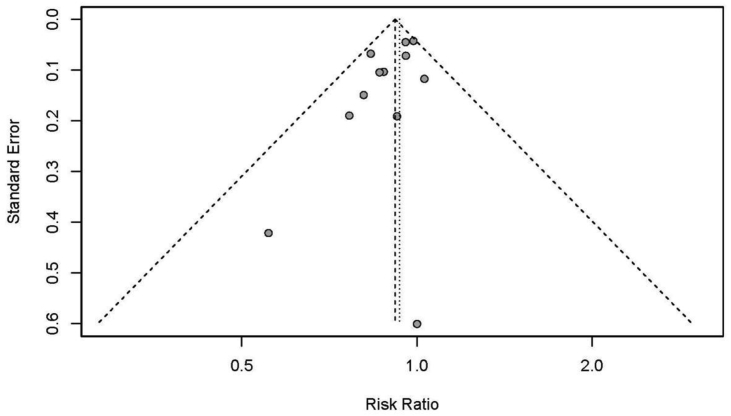
Publication bias
Finally, the publication bias was evaluated on the primary outcome of RR in clinical success rates. The results of the funnel plot showed that the studies distributed roughly symmetrical (Fig. 4). Furthermore, statistical analysis using Egger's test confirmed no evidence of publication bias in the clinical success risk ratio between WON and PPC (P = 0.07).
Fig. 4.
The funnel plot of publication bias.
Discussion
Although most of the individual studies have shown that endoscopic transmural LAMS in PPC have a higher clinical success rate than WON, many of which did not demonstrate statistical significance.19,28 Since the treatment success of LAMS drainage for PFCs is more than 80% in general, and it is difficult for studies with a relatively small sample to prove the superiority of PPC. As for the efficacy of LAMS in different types of PFCs, we found there was no significant difference in the technical success rate between PPC and WON. It can be comprehended that the existence of necrosis or the proportion of solid debris in the cyst would not affect the ease of stents placement.
Given most patients with PPC are prone to choose plastic stents for drainage with less cost and good efficacy,29 there is a small sample of patients with PPC undergoing LAMS drainage (n = 2) in our series. As for better treatment effect, some patients with the good economic conditions may choose LAMS with a larger diameter to drain cyst effectively. We found that the clinical success rate of LAMS drainage in WON and PPC was 83.3% and 100%, respectively. Further, our meta-analysis showed that the endoscopic transmural LAMS drainage in PPC showed a higher clinical success rate than in WON (99% vs. 89%), which was inconsistent with the two meta-analysis as previously mentioned. The main reason may lie in selection bias and inclusion criteria with different study aims. Studies of patients with either WON or PPC in absence of comparative analysis were enrolled in the meta-analysis of Hammad et al,11 they designated to evaluate the LAMS's cumulative efficacy and safety in the management of PFCs. While for our meta-analysis with a directly comparative aim, only studies that included WON and PPC simultaneously were enrolled with the study aim of comparing efficacy and safety of LAMS drainage for PPC and WON. On the other hand, the different techniques of LAMS deployment, treatment protocols, and endoscopists with varying levels of experience. Another previously published meta-analysis conducted by Renelus et al 12 included not merely mental stents but also plastic stents, which has a confounding impact on the outcome of PFCs. The study from Varadarajulu et al 30 in favor of our findings also showed clinical success was significantly higher for PPC and pancreatic abscess compared to WON. It may be explained by the fact that WON containing varying amounts of necrotic fluid and solid debris, comparing to PPC with minimal or no necrosis, has a lower response rate to EUS-guided drainage and a higher risk of complications, such as stent occlusion and stent migration.11,26
For the efficacy of different types of stent drainage of PFCs, there are no related reports about the comparative effects of different subtypes of LAMS on different types of PFCs. Our study mainly included AXIOS stents and Nagi stents made from different manufacturers with various specifications for clinical choosing. Subgroup analysis of LAMS showed that the clinical efficacy of AXIOS stents in PPC was better than in WON (RR = 0.92, 95% CI: 0.86–0.99). Similar trends of clinical success rates ratios between WON and PPC were also showed in the subgroups of Nagi (RR = 0.92, 95% CI: 0.79–1.07) and other stents (RR = 0.85, 95% CI: 0.58–1.25), despite no statistical significance. A large sample size of AXIOS stents (74.3%) might play a significant role in the summarized results. As we cannot obtain available data about the size of LAMS from the literature for analysis, the efficacy of different diameters and lengths of LAMS on PFCs may be more invaluable to clinical application.
A multivariate analysis of 304 patients about pooled adverse reactions of LAMS, published in 2020, showed that the risk of adverse events in patients with WON was 2.18 times higher than in patients with PPC. But the classification of adverse events was not further discussed.31 However, our subgroup analysis showed no significant difference in the incidence of the main adverse reactions between WON and PPC, including infection, bleeding, stent migration and stent occlusion. The results might owe to a multitude of reasons. Postoperative bleeding was not observed in our case series. Meanwhile, the rate of overall bleeding in PPC and WON from our meta-analysis were both 1%, which was in line with previously reported data. Because of the insufficient data about the occurrence time of bleeding provided by literature, the immediately bleeding and delayed bleeding cannot be further assessed discretely. On the other hand, a cumulative risk analysis from Garcia-Alonso et al 32 revealed that the incidences of bleeding after one week, 3 and 6 months were 3.4%, 4.4% and 5.4%, respectively, which reflected a low risk of delayed bleeding following LAMS placement. A newly published protocol also revealed half of LAMS-related bleeding occurs within the first week rather than stent dwell time impacts the risk of bleeding.33 In addition, the overall stent migration rates with the exclusion of asymptomatic displacement in PPC and WON were noted as 1% and 2%, respectively. Some research reported a higher rate of stent migration ranging from 19.0%–48.9%.32,34 The differences might lie in different definitions either asymptomatic migration or endoscopic interventions. Hence adverse events may be stratified for further analysis according to the severity grading system of the ASGE lexicon.35
Regrading to postoperative infection, 5/10 patients with WON had the disease in the case series from our center, of which four patients with postprocedural infection had moderate to profound solid debris evaluated by CT. Most of them were discharged after anti-infective therapy combined with endoscopic reinterventions (i.e., DEN or saline lavage). Our case series showed a higher rate of postoperative infection (50%) after LAMS placement compared with previously published studies,. We speculated this was related to the higher mean proportion of solid debris roughly calculated by CT in the infection group (32%) than the non-infection group (15%). Watanabe et al36 also proposed that patients with WON with a high proportion of necrotic tissue had a lower treatment success rate. Although we found no statistical difference in postoperative infection between WON and PPC, the summary of RR on the postoperative infection may indicate that the potential risk in WON (9%) was 1.57 times (95% CI: 0.73–3.39) higher than PPC (2%). Yang et al 24 proposed that assessing the proportion of necrotic tissue in WON cases can accurately predict the clinical outcome of LAMS drainage and provide a reference for selecting the best clinical protocol, such as LAMS with DEN, LAMS with double plastic stents, and only LAMS.
It has been reported that patients with PPC have stent blockage with food,23 and patients with WON containing large pieces of necrotic debris in cyst are more likely to stent occlusion resulting in secondary infection in the same way.18 In our series, one stent occlusion episode was present in a patient with WON containing 45% solid necrosis. Our meta-analysis showed no significance in the RR of patients with WON compared with patients with PPC on stent occlusion. Still, the results may potentially reflect a tendency of 1.66 times (95% CI: 0.92–2.98) higher risk of stent occlusion in WON (9%) than PPC (2%). A novel LAMS with an anti-reflux valve has recently, been developed to prevent food refluxing into the cyst cavity and causing secondary infection.37 Above all, more studies on a larger sample size may be needed to draw reliable conclusions about adverse events between WON and PPC.
Taking into account the existence of necrotic tissue in WON, more endoscopic procedures are needed. Our meta-analysis, including a total of 8 studies, showed that approximately 60% of patients with WON underwent DEN. Due to the paucity of data about the use of DEN in both arms provided, a good comparison for the benefit of DEN was not performed. The included literature also reported LAMS placement combining with other reasonable endoscopic treatments based on the available clinical expertise, such as the double pigtail plastic stent placed coaxially in LAMS,24 the double pigtail plastic stent replaced after the removal of LAMS,27 nasal cyst drainage tube placement, endoscopic retrograde pancreatic drainage, and percutaneous catheter drainage.20,23,25 All of them may have a contribution in the outcome of PFCs. Even with an inevitable effect of the above combination therapies, the clinical success rate of WON is still lower than that of PPC.
Although the development of DEN has improved the clinical outcomes of WON, several adverse events may be unavoidable during the endoscopic treatment course, such as ruptured pseudoaneurysm with bleeding.22 It has been in discussion whether DEN is burdened by an increased risk of adverse events. Fugazza et al30 thought endoscopic necrosectomy usually performed has not increased such risk. But two systematic reviews showed that the average sessions of DEN under endoscopy were 4–5, the clinical success rates were 81%–88%, and the incidences of complications were as high as 28%–36%.38,39 In their studies, the risks of stent displacement and bleeding after DEN were higher,39 and further endoscopic intervention was required to reset the stents to ensure adequate drainage. It had been reported that the stent was retrieved and redeployed under direct visualization after the stent migration.40 Considering the risks of the DEN itself, intervention in a sterile WON may carry with secondary iatrogenic infection. Not all patients with WON need endoscopic mechanical debridement. The research by Joan et al41 showed that saline lavage combined with LAMS drainage for WON could simplify the procedure of DEN with good efficacy. Siddiqui et al 42 also reported that necrotic tissue was rinsed with hydrogen peroxide solution under an endoscope. Hydrogen peroxide decomposes and releases oxygen to loosen the necrotic tissue through its foaming effect.
Therefore, the patients with PFCs should be carefully selected for DEN combined with endoscopic LAMS implantation. It is more appropriate that such complex procedures should be performed by skilled endoscopists, as well as the creation of multiple transluminal gateways in necessity.43 At the same time, a multidisciplinary approach involving specialized radiologists and surgeons can be carried out if necessary, such as angioembolization under interventional radiologic guidance, endoscopic and percutaneous drainage, or surgery. An open necrosectomy served as a maximally invasive salvage therapy offers the possibility to control critical complications such as bleeding and perform a further necrosectomy when other treatments have failed.44 On the other hand, although DEN has specific procedure-related adverse events, it is still a good choice compared to conventional surgery. It can also be used as a bridge for a successful surgery to stabilize the state of critical patients and optimize their clinical status before surgery.19
An extensive update of the literature published in the recent five years from various regions was embraced in our study, along with our case series from China. Most of the studies comparing WON, and PPC were multi-center studies. The complications were stratified and the effect of subtypes of LAMS on the drainage of PFCs was further analyzed. Meanwhile, we calculated the proportion of DEN in patients with WON to consider its possible impact on outcomes of LAMS. The main limitation of this meta-analysis was related to the study design of included studies. All these studies were retrospective or prospective longitudinal cohort studies. The lack of randomization in the treatment and varied duration of the follow-up time might cause the limitation of the evidence. Several variations in the technology of releasing LAMS and subsequent interventions without standardized treatment decision-making process, as well as inconformity of clinical success and adverse events in some studies, may result in overestimation or underestimation. However, the high homogeneity of this study in the statistics showed acceptable results and supported our views. Another limitation was the relatively smaller sample size in PPC cases than WON cases, which might reduce the statistical power.
In conclusion, the placement of the LAMS drainage through the intestinal wall has promising clinical efficacy and safety for PFCs. The clinical outcomes of LAMS drainage for pancreatic pseudocysts are slightly better than walled-off necrosis. Still, a larger sample size and high-quality follow-up studies are required to determine its adverse reactions. Meanwhile, more randomized controlled trials are needed to evaluate the role of DEN in walled-off necrosis.
Funding
This study was supported by National Natural Science Foundation of China (81570507), National Key Research and Development Program of China (2017YFC0113600), Research and application on clinical technology of diagnosis and treatment in Beijing (Z191100006619080, Z191100006619081) and The Digestive Medical Coordinated Development Center of Beijing Hospitals Authority (XXZ02).
Conflicts of interest
None.
Edited by Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cdtm.2021.07.001.
Appendix ASupplementary data
The following is the Supplementary data to this article:
Multimedia component 1
References
- 1.Leppaniemi A., Tolonen M., Tarasconi A. WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. doi: 10.1186/s13017-019-0247-0. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks P.A., Bollen T.L., Dervenis C. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 3.Bezmarevic M., van Dijk S.M., Voermans R.P., van Santvoort H.C., Besselink M.G. Management of (Peri)Pancreatic collections in acute pancreatitis. Visc Med. 2019;35:91–96. doi: 10.1159/000499631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasan M.K., Romagnuolo J. In: Diagnostic and Therapeutic Procedures in Gastroenterology: An Illustrated Guide. Sridhar S., Wu G.Y., editors. Springer International Publishing; Cham: 2018. Endoscopic management of pancreatic pseudocysts; pp. 429–445. [Google Scholar]
- 5.Baron T.H., DiMaio C.J., Wang A.Y., Morgan K.A. American gastroenterological association clinical practice update: management of pancreatic necrosis. Gastroenterology. 2020;158:67–75. doi: 10.1053/j.gastro.2019.07.064. e61. [DOI] [PubMed] [Google Scholar]
- 6.Varadarajulu S., Bang J.Y., Sutton B.S., Trevino J.M., Christein J.D., Wilcox C.M. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583–590. doi: 10.1053/j.gastro.2013.05.046. e581. [DOI] [PubMed] [Google Scholar]
- 7.Baron T.H., Thaggard W.G., Morgan D.E., Stanley R.J. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–764. doi: 10.1053/gast.1996.v111.pm8780582. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto N., Isayama H., Kawakami H. Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections. Gastrointest Endosc. 2013;77:809–814. doi: 10.1016/j.gie.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Baron T.H., Gc Fau - Morgan Harewood, DE, Morgan De Fau - Yates M.R. 2006. Outcome Differences after Endoscopic Drainage of Pancreatic Necrosis, Acute Pancreatic Pseudocysts, and Chronic Pancreatic Pseudocysts Endoscopic Drainage of Pancreatic-Fluid Collections in 116 Patients: A Comparison of Etiologies, Drainage Techniques, and Outcomes. (0016-5107 (Print)) [Google Scholar]
- 10.Hookey L.C., Debroux S., Delhaye M., Arvanitakis M., Le Moine O., Deviere J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635–643. doi: 10.1016/j.gie.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Hammad T., Khan M.A., Alastal Y. Efficacy and safety of lumen-apposing metal stents in management of pancreatic fluid collections: are they better than plastic stents? A systematic review and meta-analysis. Dig Dis Sci. 2018;63:289–301. doi: 10.1007/s10620-017-4851-0. [DOI] [PubMed] [Google Scholar]
- 12.Renelus B.D., Jamorabo D.S., Gurm H.K., Dave N., Briggs W.M., Arya M. Comparative outcomes of endoscopic ultrasound-guided cystogastrostomy for peripancreatic fluid collections: a systematic review and meta-analysis. Ther Adv Gastrointest Endosc. 2019;12 doi: 10.1177/2631774519843400. 2631774519843400 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pezzilli R., Zerbi A., Campra D. Consensus guidelines on severe acute pancreatitis. Dig Liver Dis. 2015;47:532–543. doi: 10.1016/j.dld.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Lihui S., Xin W., Xiaoyin Z. Progress in endoscopic treatment for pancreatic pseudocyst (in Chinese) J Pract Med. 2018;34:1743–1745. doi: 10.3969/j.issn.1006-5725.2018.10.042. 1748. [DOI] [Google Scholar]
- 15.Jahangeer B., Medarapalem S.A., Gulati Ajay, Manrai Manish. Mo1460 characterization of fluid collections using quantification of solid debris in acute pancreatitis - a comparative study of EUS vs. CT for prediction of intervention. Gastroenterology. 2014;79:AB445. [Google Scholar]
- 16.Wells G., Shea B., O'Connell J. vol. 7. Ottawa Health Research Institute Web site; 2014. (The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses). [Google Scholar]
- 17.Schmidt C.O., Kohlmann T. When to use the odds ratio or the relative risk? Int J Publ Health. 2008;53:165–167. doi: 10.1007/s00038-008-7068-3. [DOI] [PubMed] [Google Scholar]
- 18.Walter D., Will U., Sanchez-Yague A. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a prospective cohort study. Endoscopy. 2015;47:63–67. doi: 10.1055/s-0034-1378113. [DOI] [PubMed] [Google Scholar]
- 19.Chandran S., Efthymiou M., Kaffes A. Management of pancreatic collections with a novel endoscopically placed fully covered self-expandable metal stent: a national experience (with videos) Gastrointest Endosc. 2015;81:127–135. doi: 10.1016/j.gie.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui A.A., Adler D.G., Nieto J. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos) Gastrointest Endosc. 2016;83:699–707. doi: 10.1016/j.gie.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Yoo J., Yan L., Hasan R., Somalya S., Nieto J., Siddiqui A.A. Feasibility, safety, and outcomes of a single-step endoscopic ultrasonography-guided drainage of pancreatic fluid collections without fluoroscopy using a novel electrocautery-enhanced lumen-apposing, self-expanding metal stent. Endosc Ultrasound. 2017;6:131–135. doi: 10.4103/2303-9027.204814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukai S., Tsuchiya T., Itoi T. Prospective evaluation of a new biflanged metal stent for the treatment of pancreatic fluid collections (with videos) Gastrointest Endosc. Jul 2017;86:203–207. doi: 10.1016/j.gie.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Venkatachalapathy S.V., Bekkali N., Pereira S. Multicenter experience from the UK and Ireland of use of lumen-apposing metal stent for transluminal drainage of pancreatic fluid collections. Endosc Int Open. 2018;6:E259–E265. doi: 10.1055/s-0043-125362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang D., Perbtani Y.B., Mramba L.K. Safety and rate of delayed adverse events with lumen-apposing metal stents (LAMS) for pancreatic fluid collections: a multicenter study. Endosc Int Open. 2018;6:E1267–e1275. doi: 10.1055/a-0732-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakhtakia S., Nabi Z., Moon J.H. Endoscopic drainage of pancreatic fluid collections by use of a novel biflanged stent with electrocautery-enhanced delivery system. Video. 2018;3:284–288. doi: 10.1016/j.vgie.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrone M.C., Archibugi L., Forti E. Novel lumen-apposing metal stent for the drainage of pancreatic fluid collections: an Italian multicentre experience. United Eur Gastroenterol J. 2018;6:1363–1371. doi: 10.1177/2050640618785078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg R., Chaar A., Szpunar S., Mohan B.P., Barawi M. Efficacy and safety of lumen-apposing stents for management of pancreatic fluid collections in a community hospital setting. Clin Endosc. 2019 doi: 10.5946/ce.2019.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez-Sequeiros E., Baron T.H., Perez-Miranda M. Evaluation of the short- and long-term effectiveness and safety of fully covered self-expandable metal stents for drainage of pancreatic fluid collections: results of a Spanish nationwide registry. Gastrointest Endosc. 2016;84:450–457. doi: 10.1016/j.gie.2016.02.044. e452. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Yu Y., Li P., Zhang S.T. Advancements in the endoscopic treatment of pancreatic fluid collections. Chronic Dis Transl Med. 2020;6(3):158–164. doi: 10.1016/j.cdtm.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varadarajulu S., Bang J.Y., Phadnis M.A., Christein J.D., Wilcox C.M. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080–2088. doi: 10.1007/s11605-011-1621-8. [DOI] [PubMed] [Google Scholar]
- 31.Fugazza A., Sethi A., Trindade A.J. International multicenter comprehensive analysis of adverse events associated with lumen-apposing metal stent placement for pancreatic fluid collection drainage. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Alonso F.J., Sanchez-Ocana R., Penas-Herrero I. Cumulative risks of stent migration and gastrointestinal bleeding in patients with lumen-apposing metal stents. Endoscopy. Apr. 2018;50:386–395. doi: 10.1055/a-0581-9040. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad W., Fehmi S.A., Savides T.J., Anand G., Chang M.A., Kwong W.T. Protocol of early lumen apposing metal stent removal for pseudocysts and walled off necrosis avoids bleeding complications. Scand J Gastroenterol. 2020 doi: 10.1080/00365521.2019.1710246. [DOI] [PubMed] [Google Scholar]
- 34.Mukai S., Itoi T., Sofuni A., Tsuchiya T., Gotoda T., Moriyasu F. Clinical evaluation of endoscopic ultrasonography-guided drainage using a novel flared-type biflanged metal stent for pancreatic fluid collection. Endosc Ultrasound. 2015;4:120–125. doi: 10.4103/2303-9027.156738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cotton P.B., Eisen G.M., Aabakken L. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe Y., Mikata R., Yasui S. Short- and long-term results of endoscopic ultrasound-guided transmural drainage for pancreatic pseudocysts and walled-off necrosis. World J Gastroenterol. 2017;23:7110–7118. doi: 10.3748/wjg.v23.i39.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho I.R., Chung M.J., Jo J.H. A novel lumen-apposing metal stent with an anti-reflux valve for endoscopic ultrasound-guided drainage of pseudocysts and walled-off necrosis: a pilot study. PloS One. 2019;14 doi: 10.1371/journal.pone.0221812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabbri C., Luigiano C., Lisotti A. Endoscopic ultrasound-guided treatments: are we getting evidence based--a systematic review. World J Gastroenterol. 2014;20:8424–8448. doi: 10.3748/wjg.v20.i26.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Brunschot S., Fockens P., Bakker O.J. Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surg Endosc. 2014;28:1425–1438. doi: 10.1007/s00464-013-3382-9. [DOI] [PubMed] [Google Scholar]
- 40.Mistry T., Shah M., Javia S., Singhal S. Retrieval and redeployment of migrated lumen-apposing metal stent to facilitate endoscopic necrosectomy of walled-off necrosis. VideoGIE. May 2018;3:151–152. doi: 10.1016/j.vgie.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gornals J.B., Consiglieri C.F., Busquets J. Endoscopic necrosectomy of walled-off pancreatic necrosis using a lumen-apposing metal stent and irrigation technique. Surg Endosc. Jun 2016;30:2592–2602. doi: 10.1007/s00464-015-4505-2. [DOI] [PubMed] [Google Scholar]
- 42.Siddiqui A.A., Easler J., Strongin A. Hydrogen peroxide-assisted endoscopic necrosectomy for walled-off pancreatic necrosis: a dual center pilot experience. Dig Dis Sci. Mar. 2014;59:687–690. doi: 10.1007/s10620-013-2945-x. [DOI] [PubMed] [Google Scholar]
- 43.Varadarajulu S., Phadnis M.A., Christein J.D., Wilcox C.M. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011;74:74–80. doi: 10.1016/j.gie.2011.03.1122. [DOI] [PubMed] [Google Scholar]
- 44.Hackert T., Buchler M.W. Decision making in necrotizing pancreatitis. Dig Dis. 2016;vol. 34:517–524. doi: 10.1159/000445232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1