Abstract
Introduction
Delayed onset of immune‐related adverse events following immune‐checkpoint inhibitor discontinuation is underrecognized because of little available evidence.
Case presentation
A 50‐year‐old man with metastatic renal cell carcinoma (Case 1) and 58‐year‐old woman with renal cell carcinoma and retroperitoneal lymph node metastasis (Case 2) underwent nivolumab therapy. Case 1: Progressive disease forced nivolumab discontinuance after 18 months, and he underwent two courses of tyrosine kinase inhibitor therapy. immune‐related adverse events of pneumonitis, hepatitis, and renal dysfunction were diagnosed 142 days after nivolumab discontinuation, and he recovered with immunosuppressive treatment. Case 2: The immune‐related adverse event of pneumonitis forced nivolumab discontinuance after 14 months, and two courses of tyrosine kinase inhibitor therapy were administered. The immune‐related adverse event of hepatitis was diagnosed 436 days after nivolumab discontinuation, and she recovered with immunosuppressive treatment.
Conclusion
Two patients with delayed onset of immune‐related adverse events after nivolumab discontinuation were recovered with immunosuppressive treatment.
Keywords: delayed onset, immune‐checkpoint inhibitor, immune‐related adverse events, renal cell carcinoma
Abbreviations & Acronyms
- ALT
alanine aminotransferase
- Cr
creatinine
- CT
computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- DIRE
delayed immune‐related events
- ICI
immune‐checkpoint inhibitor
- irAE
immune‐related adverse event
- MMF
mycophenolate mofetil
- mPSL
methylprednisolone
- Paz
pazopanib
- PSL
prednisolone
- RCC
renal cell carcinoma
- ST
sulfamethoxazole/trimethoprim
- t‐bil
total bilirubin
- TKI
tyrosine kinase inhibitor
- γGTP
γ‐glutamyl transpeptidase
Keynote message.
Delayed onset of irAEs after discontinuation of ICI therapy is underrecognized because of little available evidence. IrAEs can occur even several months after the discontinuation of ICIs. Clinical vigilance is critical even after ICI cessation, and initiation of immunosuppressive therapy should not be delayed.
Introduction
ICI therapy is currently a standard option in the treatment of metastatic RCC. To realize a clinical benefit of ICI therapy, appropriate management of irAEs is mandatory. Although a few cases of delayed onset of irAEs after the discontinuation of ICIs have been reported, it remains underrecognized because of a paucity of evidence.1, 2, 3 We report here two cases of the delayed onset of irAEs occurring more than 4 months after the discontinuation of ICI therapy.
Case presentation
Case 1
A 50‐year‐old man underwent nephrectomy for left clear cell RCC in June 2015 and was treated with sunitinib for pulmonary and psoas muscle metastases. He was treated sequentially with everolimus and pazopanib, but at 19 months after the diagnosis, myocardial and ischial bone metastases developed. After radiation to the ischial bone, nivolumab was introduced. Nivolumab resulted in significant shrinkage of the myocardial metastasis, but 18 months later, brain, right scalene muscle, and left gluteal muscle metastases were discovered. Nivolumab was discontinued, and sunitinib was reintroduced. Three months of treatment with sunitinib failed to bring a clinical benefit, so sorafenib was introduced.
One month after the introduction of sorafenib, he began to intermittently run a fever of up to 38°C, and 8 days thereafter, slight but diffuse ground‐glass opacity appeared on CT scanning (Fig. 1a). Pneumocystis pneumonia was suspected and ST therapy was administered. At 14 days after ST administration, he developed liver dysfunction accompanied by a fever of up to 39.5°C. Because involvement of moniliasis and/or bacterial pneumonia and liver dysfunction caused by ST or sorafenib was suspected, the antimicrobial agent was changed to voriconazole, tazobactam/piperacillin, and garenoxacin and sorafenib was discontinued. At 2 days after the initiation of these drugs, his serum alanine aminotransferase (ALT) significantly increased to 606 IU/L (CTCAE grade 3), serum creatinine increased to 1.49 mg/dL (CTCAE grade 1), and 2 days later, CT revealed a significant exacerbation of the ground‐glass opacities (Fig. 1b). Because of the development of multiple organ dysfunction and exacerbation of pulmonary lesions even with extensive antimicrobial treatment, we thought he might be suffering from pneumonitis, hepatitis, and renal dysfunction related to irAEs caused by nivolumab, even though 142 days had passed since its last administration. Pulse corticosteroid therapy with 500 mg mPSL for 3 days and subsequent corticosteroid therapy with MMF resulted in recovery from the pneumonitis, hepatitis, and renal dysfunction (Fig. 1c).
Fig. 1.
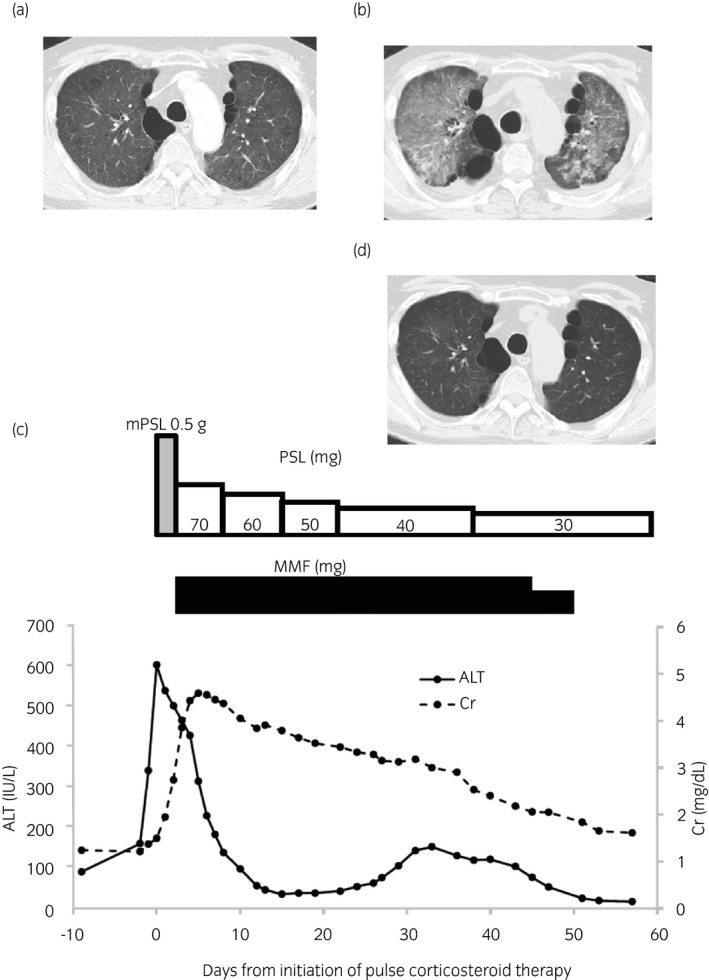
Time course of CT imaging, immunosuppressive therapy, and changes in serum levels of ALT and Cr. (a) Chest CT on day 124 after discontinuation of nivolumab. Slight but diffuse ground‐glass opacities in the bilateral lungs were observed. (b) Chest CT on day 142 after discontinuation of nivolumab. Worsening of ground‐glass opacities in the bilateral lung was observed. (c) Time course of immunosuppressive therapy and changes in serum levels of ALT and Cr. mPSL was started 142 days after discontinuation of nivolumab. After 3 doses of pulse corticosteroid therapy with 500 mg mPSL, PSL (1 mg/kg) was administered with MMF. (d) Chest CT at 43 days after initiation of pulse corticosteroid therapy showed improvement of ground‐glass opacities.
Case 2
A 58‐year‐old woman underwent nephrectomy for right chromophobe RCC with sarcomatoid change in December 2014 and was treated with sunitinib for retroperitoneal lymph node metastasis. She was treated sequentially with axitinib and nivolumab. After 14 months, nivolumab was discontinued due to the irAE of pneumonitis, which was relieved with steroid therapy for 5 months. Thereafter, axitinib was reintroduced. After 6 months, significant enlargement of her retroperitoneal lymph node metastases was observed, and pazopanib was introduced. Three months after the introduction of pazopanib, her serum ALT had increased to 535 IU/L (CTCAE grade 3). Hepatic toxicity caused by pazopanib was suspected, so it was discontinued. Although her serum ALT decreased to 200 IU/L (CTCAE grade 2), serum levels of γ‐glutamyl transpeptidase and total bilirubin further increased to 1490 IU/L (CTCAE grade 4) and 15.8 mg/dL (CTCAE grade 4), respectively. Because no organic change was seen on sonography and an autoimmune screen and hepatitis serology were negative, we thought she might have hepatitis related to an irAE caused by nivolumab, although 436 days had passed since its last administration. Pulse corticosteroid therapy with 1000 mg mPSL for 3 days and subsequent corticosteroid therapy with MMF resulted in recovery from hepatitis (Fig. 2). However, she died due to the development of systemic metastases 83 days after pulse corticosteroid therapy.
Fig. 2.
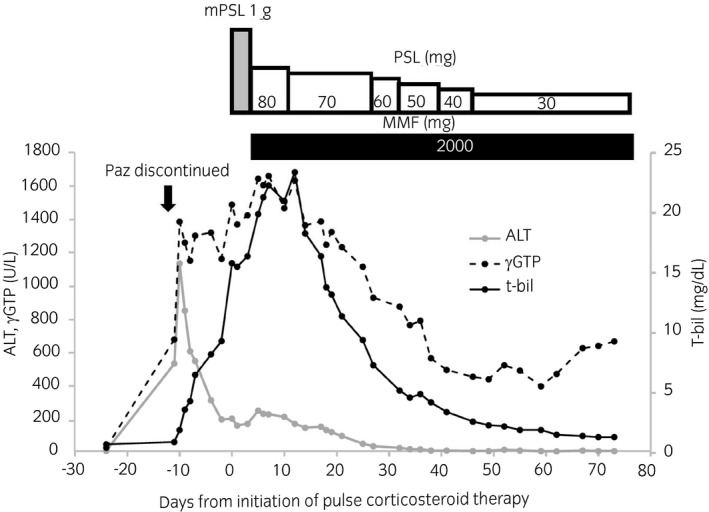
Time course of immunosuppressive therapy and changes in serum levels of ALT, γGTP, and t‐bil. ALT was increased to 535 IU/L (CTCAE grade 3) 425 days after discontinuation of nivolumab. Although ALT decreased to 205 IU/L (CTCAE grade 2) after discontinuation of Paz, serum levels of γGTP and t‐bil increased to 1490 IU/L (CTCAE grade 4) and 15.8 mg/dL (CTCAE grade 4), respectively. mPSL was started 436 days after discontinuation of nivolumab. After three doses of pulse corticosteroid therapy with 1000 mg mPSL, PSL (2 mg/kg) was administered with MMF.
Discussion
ICI therapy has been shown to improve overall survival of metastatic RCC and is currently a standard option in the treatment of metastatic RCC.4, 5, 6, 7 ICI therapy results in a high complete response rate compared with conventional TKI therapy, and in some cases, it brings about a durable response even after cessation of the ICI. A feature of ICI treatment is its potential to cause autoimmunity as a treatment side effect (irAE), and its management is mandatory to bring maximum clinical benefit.
In general, irAEs occur quite early, mostly within weeks to 3 months after the initiation of ICI therapy, but some cases of delayed‐onset irAEs are reported even several months after ICI discontinuation.1, 2 Couey et al. defined DIRE as irAEs occurring ≥90 days after withdrawal of immunotherapy, and they identified 23 cases of DIRE from a PubMed literature review. The median period to DIRE from cessation of ICI treatment was 6 (3–28) months, and more than half of the DIRE cases occurred following a brief course of immunotherapy of <4 doses.3 The frequency of DIRE is unclear because in more than 80% of ICI clinical trials, the duration of the serious adverse event reporting period following the last dose of ICI was <90 days, which resulted in the underrecognition of DIRE.3 In a retrospective study analyzing 470 patients with metastatic RCC and urothelial cancer treated with ICIs, 3 patients, respectively, developed DIRE at 3.8, 5.3, and 15.4 months after ICI discontinuation.8
The mechanism of delayed onset of irAEs might owe to the prolonged binding of ICI to T cells. Although the half‐life of nivolumab in blood is reported to be 2–3 weeks,9 Brahmer et al. showed that nivolumab occupies >70% of PD‐1 on circulating T cells more than 2 months following infusion.10 Osa et al. also reported prolonged binding of nivolumab to peripheral T cells more than 20 weeks after the last infusion.11 These findings might explain the mechanism of not only the durable response of ICI but also the delayed onset of irAEs.
Our first case was diagnosed as having irAEs (hepatitis, pneumonitis, and renal dysfunction) 142 days after the discontinuation of nivolumab, whereas the second case was diagnosed as having an irAE (hepatitis) 436 days after discontinuation. Because both patients were being treated with TKIs, we thought the events were related to drug toxicity of TKI, but each patient’s condition worsened even after TKI discontinuation. After excluding the possibility of infectious or autoimmune diseases, we considered these might be irAEs even though several months had passed since discontinuation of the nivolumab. The diagnosis of irAE is sometimes difficult because most irAEs lack pathognomonic symptoms. Biopsy of the target organ is not necessarily required to confirm the diagnosis or for subsequent patient management, but liver biopsy may be considered to assist in the differential diagnosis of more severe hepatic reactions.12 Most importantly, initiation of therapy should not be delayed while awaiting serological results if there is no other apparent cause.12 In clinical practice, delayed onset of irAEs is underrecognized because of a paucity of evidence,13 and it has possibility to be severe1, 2; thus, clinical vigilance is critical even after the cessation of ICI therapy.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an institutional reviewer board
Not applicable.
Informed consent
Not applicable.
Registry and the registration no. of the study/trial
Not applicable.
Nakai, Y, Otsuka T, Inoue T, et al. Two cases of delayed onset of immune‐related adverse events after discontinuation of nivolumab in patients with metastatic renal cell cancer. IJU Case Rep. 2021; 4: 326–329. 10.1002/iju5.12338.
References
- 1.Parakh S, Cebon J, Klein O. Delayed autoimmune toxicity occurring several months after cessation of anti‐PD‐1 therapy. Oncologist 2018; 23: 849–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phan T, Patwal K, Lipton L, Knight V, Aga A, Pianko S. Very delayed acute hepatitis after pembrolizumab therapy for advanced malignancy: How long should we watch? Curr. Oncol. 2021; 28: 898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couey MA, Bell RB, Patel AAet al. Delayed immune‐related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J. Immunother. Cancer 2019; 7: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, McDermott DFet al. Nivolumab versus everolimus in advanced renal cell carcinoma. N. Engl. J. Med. 2015; 373: 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Tannir NM, McDermott DFet al. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N. Engl. J. Med. 2018; 378: 1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Penkov K, Haanen Jet al. Avelumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N. Engl. J. Med. 2019; 380: 1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rini BI, Plimack ER, Stus Vet al. Pembrolizumab plus axitinib versus sunitinib for advanced renal cell carcinoma. N. Engl. J. Med. 2019; 380: 1116–27. [DOI] [PubMed] [Google Scholar]
- 8.Nuzzo PV, Pond GR, Abou Alaiwi Set al. Conditional immune toxicity rate in patients with metastatic renal and urothelial cancer treated with immune checkpoint inhibitors. J. Immunother. Cancer 2020; 8: e000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centanni M, Moes DJAR, Trocóniz IF, Ciccolini J, van Hasselt JGC . Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin. Pharmacokinet. 2019; 58: 835–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Drake CG, Wollner Iet al. Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010; 28: 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osa A, Uenami T, Koyama Set al. Clinical implication of monitoring nivolumab immunokinetics in non‐small cell lung cancer patients. JCI Insight 2018; 3: e59125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haanen J, Carbonnel F, Robert Cet al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann. Oncol. 2017; 28(Suppl 4): i119–i142. [DOI] [PubMed] [Google Scholar]
- 13.Chen TW, Razak AR, Bedard PL, Siu LL, Hansen AR. A systematic review of immune‐related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann. Oncol. 2015; 26: 1824–9. [DOI] [PubMed] [Google Scholar]


