Abstract
Background
Concentrations of C-reactive protein (CRP), interleukin 6 (IL-6) and other inflammatory markers are elevated in people with depression and anxiety compared to controls, but evidence for disorder-specificity, linearity and potential causality is sparse.
Methods
Using population-based data from up to 144,890 UK Biobank cohort participants, we tested associations of circulating CRP concentrations with depression and anxiety symptom scores and probable diagnosis, including tests for linearity, disorder-specificity and sex difference. We examined potential causality using 1-sample and 2-sample Mendelian randomisation (MR) analyses testing associations of genetically-predicted CRP concentration and IL-6 activity with depression and anxiety. The study was conducted from June 2019 to February 2021.
Findings
CRP concentration was associated with depressive and anxiety symptom scores and with probable diagnoses of depression and generalised anxiety disorder (GAD) in a dose-response fashion. These associations were stronger for depression than for anxiety, and for women than for men although less consistently. MR analyses provided consistent results suggesting that genetically predicted higher IL-6 activity was associated with increased risk for depressive symptoms, while genetically-predicted higher CRP concentration was associated with decreased risks of depressive and anxiety symptoms.
Interpretation
Altered activity of the IL-6/IL-6R pathway could be a risk factor for depression. The field now requires experimental studies of IL-6 modulation in humans and animal models to further examine causality, mechanisms and treatment potential. Such studies are also needed to elucidate mechanisms for divergent associations of genetically-predicted higher IL-6 activity (risk increasing) and higher CRP concentrations (protective) with depression/anxiety.
Funding
This research was funded in whole, or in part, by the Wellcome Trust (grant code: 201486/Z/16/Z). For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. This work was supported by a Data Science Award from the MQ: Transforming Mental Health (grant code: MQDS17/40) to GMK and PBJ, which also supported ZY. GMK also acknowledges funding support from the Wellcome Trust (grant code: 201486/Z/16/Z), the Medical Research Council UK (grant code: MC_PC_17213 and MR/S037675/1), and the BMA Foundation (J Moulton grant 2019). NK and SM are supported by the International Max Planck Research School of Translational Psychiatry (IMPRS-TP). GDS works in the Medical Research Council Integrative Epidemiology Unit at the University of Bristol, which is supported by the Medical Research Council (MC_UU_00011/1).
Keywords: Depression, Anxiety, Inflammation, CRP, Il-6, Mendelian randomisation
Research in context.
Evidence before this study
Systematic reviews and meta-analyses suggest concentrations of circulating CRP and other inflammatory markers are elevated in patients with depression and generalised anxiety disorder as compared to healthy controls. However, there are key outstanding questions: (1) Is inflammation a potentially causal factor for depression and anxiety disorders; (2) is inflammation a specific or common risk factor for depression and anxiety, which are highly comorbid; (3) is there a sex difference in the associations between inflammation and risks for affective symptoms/disorders, and are these associations linear or quadratic?
Added value of this study
We report that inflammation is associated with depression and anxiety in a linear dose-response fashion, and more strongly in women than in men, albeit less consistently. The associations were larger for depression than for anxiety and persist after controlling for current anxiety symptoms, but not vice versa, indicating disorder specificity. Furthermore, using Mendelian randomisation analysis we report that genetically-predicted higher IL-6 activity and genetically-predicted lower CRP concentrations are associated with increased risk of depressive symptoms, suggesting that inflammation, particularly altered activity of the IL-6/IL-6R pathway, could be a risk factor for depression.
Implications of all the available evidence
Evidence for a role of IL-6 in depression supports the need for experimental studies in humans and animals to further investigate causality, mechanisms and treatment potential. Evidence that inflammation could represent a relatively larger risk factor for depression than for anxiety could inform patient selection criteria in immunotherapy trials. Experimental studies are also required to elucidate mechanisms for divergent effects for CRP and IL-6 on illness risk, as these may help in devising more targeted interventions.
Alt-text: Unlabelled box
1. Introduction
Innate immune dysfunction represents a putative mechanism for depression and other psychiatric disorders opening up the possibility of new treatment approaches distinct from current monoaminergic drugs [1,2]. In depression, for instance, there is evidence of low-grade systemic inflammation as indexed by elevated concentrations of C-reactive protein (CRP >3 mg/L) in 21–34% of patients [3], along with increased concentrations of interleukin-6 (IL-6) and other inflammatory cytokines in blood and in cerebrospinal fluid (CSF) [[4], [5], [6], [7], [8]]. A number of randomised controlled trials (RCTs) are now testing the effects of anti-inflammatory drugs in patients with depression (e.g., Khandaker et al. [9], NCT02473289, NCT02362529). However, there are key outstanding questions, particularly regarding specificity and causality of association, that require addressing for a clearer understanding of the potential role of inflammation in illness pathogenesis and to inform future clinical trials.
Depressive disorders overlap with anxiety disorders both genetically and clinically [10,11]. Anxiety symptoms now form part of the diagnostic criteria for major depressive disorder (MDD) as “anxious distress specifier” in the diagnostic and statistical manual of mental disorders 5th edition (DSM-5) [12]. Preliminary evidence from case-control studies also indicates that inflammation could be implicated in generalised anxiety disorder (GAD), although findings from studies are mixed and prospective studies indicate that inflammation could increase subsequent to the development of an anxiety disorder [13,14]. Additionally, to our knowledge no studies have tested whether inflammation is a common or specific risk factor for depression and anxiety. This is an important issue as it may help to identify potentially unique or shared mechanisms for psychiatric disorders that commonly co-occur.
Regarding causality, longitudinal studies and meta-analyses have reported evidence for a temporal association between elevated CRP and IL-6 concentrations at baseline and risk of depressive symptoms subsequently [[15], [16], [17], [18]], but other studies have not fully replicated associations of these markers with subsequent depressive disorders [19,20] and residual confounding still remains a possibility. Mendelian randomisation (MR) is an epidemiological approach that uses genetic variants as instruments to untangle the problem of unmeasured confounding as genetic variants are randomly inherited from parents to offspring and fixed at conception [21]. Therefore, if genetically-predicted values of a risk factor are associated with a disease outcome, then it is likely the association between the risk factor and outcome has a causal basis.
Existing MR studies have provided mixed evidence on the association of inflammation with different psychiatric disorders. Hartwig et al. reported potential protective effects of elevated CRP for schizophrenia [22], contrasting with findings from observational studies [23,24]. For depression, one study did not find evidence for a potential causal role of inflammation [25], while more recent studies reported potential causal roles for increased IL-6 and CRP serum concentrations in depression [26], for increased IL-6 activity for suicidality specifically [27], and for increased soluble IL-6R levels for recurrent depressive symptoms [28]. While these findings may indicate disorder-specificity, further research is required to enable definite conclusions regarding causality of association. Furthermore, to our knowledge, MR studies of inflammation and anxiety have thus far only investigated individual anxiety symptoms [29].
We have used data from up to 144,890 individuals from the UK Biobank study, a large general population-based cohort, to test associations of circulating CRP concentrations with depression and anxiety. As outcomes, we have used symptom scores and categorical probable diagnosis in the total sample and in men and women separately to assess potential sex difference, strength and reproducibility of association. We have examined evidence for dose-response by testing linearity of association. We have examined specificity of association by testing whether the association of CRP with depression and anxiety is stronger for one outcome than the other, or is similar between outcomes. Furthermore, we have carried out MR analysis in the full sample, and in men and women separately, to test whether associations of CRP and IL-6 with depression and anxiety are consistent with potential causal roles for these biomarkers in these conditions.
2. Methods
2.1. Study population
The UK Biobank is a population-based cohort with a range of phenotyping assessments, biochemical assays and genome-wide genotyping from over 500,000 UK residents aged 40–69 years at baseline, recruited between 2006 and 2010 from 22 assessment centres throughout the UK [30]. Our primary outcomes were depressive and anxiety symptoms that were assessed online as part of a follow-up mental health survey completed by up to 157,115 individuals between July 2016 and July 2017 [31]. The current study used available data from the maximum number of UK Biobank participants for each analysis (N up to 144,890). The UK Biobank study was subject to ethics committee approval and participants gave their informed consent prior to participation; see details in Supplementary Methods.
2.2. Exposure
Using blood samples collected in the UK Biobank baseline visit between 2006 and 2010 or the first repeat assessment visit between 2012 and 2013, serum high-sensitivity CRP concentrations were measured by immunoturbidimetric assay on a Beckman Coulter AU5800. Minimum detection limit was 0·08 mg/L. CRP values in the entire sample (n = 486,424) ranged from 0·08 to 79·96 mg/L; mean=2·60 (SD=4·36) mg/L. The distribution of CRP concentrations for this study (n = 146,954) was divided into quintiles or deciles, which were used as categorical variables. We also carried out additional analyses using CRP as a continuous variable (natural log-transformed).
2.3. Outcomes
Our primary outcomes were depressive and anxiety symptoms occurring in the last 2 weeks as measured using the Patient Health Questionnaire (PHQ)−9 and the Generalised Anxiety Disorder (GAD)−7 questionnaire, respectively [32,33]. Symptoms were coded as 0–3 depending on self-reported severity. We created sum-scores for each scale, which were used as primary outcomes. Categorical diagnoses of probable depression and GAD were used as secondary outcomes, which were defined using commonly used cut-off criteria of PHQ-9 ≥ 10 and GAD-7 ≥ 10. See details in the Supplementary Methods.
2.4. Covariates
As covariates, we included age, sex, body mass index (BMI), smoking, alcohol use, physical activity, ethnicity, Townsend Deprivation Index (TDI), and diabetes and cardiovascular disease; see Supplementary Methods for details.
2.5. Statistical analyses
Analyses were performed using Stata/SE 16.0 (Stata, College Station, TX). Baseline characteristics of participants were examined across CRP quintiles.
2.5.1. Association of CRP with depression and anxiety, linearity and sex difference
Linear regression was used to estimate the associations between CRP concentrations (quintiles or deciles) and depressive and anxiety symptom scores. For the purpose of interpretation, coefficient estimates were anti-log transformed to odds ratio and 95% confidence interval (CI). We adjusted regression models for age, sex, BMI, smoking, alcohol use, physical activity, ethnicity, TDI, and diabetes and cardiovascular disease.
To investigate the nature of associations with depressive and anxiety symptoms and any dose-response effect in greater detail, CRP concentrations were divided into deciles with deciles 2–10 compared with the lowest decile group (decile 1). Floating absolute risks were estimated, which were then plotted against the median CRP concentrations in each decile. We computed ORs for trend by using quintile number as predictor. We assessed potential quadratic associations by including a quadratic term (CRP-squared). We performed sex-stratified analyses and also tested for interaction between sex and CRP by including interaction terms in regression models. Lastly, we evaluated the influence of selection/collider bias for participation in the optional mental health survey using inverse probability weighted regression of the fully adjusted regression models of depression and anxiety outcomes on CRP [34,35]; see Supplementary Methods for details.
2.5.2. Test for specificity vs commonality of association of CRP between depression and anxiety
We used bivariate probit regression to test for specificity of association of CRP between depression and anxiety using both continuous and categorical outcomes. Probit regression jointly modelled the outcomes of depression and anxiety with CRP, and then tested for equality of regression parameters expressing the effect of CRP on each outcome using the likelihood ratio test. We compared a model that allowed estimates to differ between outcomes with a model where estimates were constrained to be equal for both outcomes. Probit estimates were converted into ORs by multiplying probit parameters by 1·6 [36] In addition, we adjusted the regression models of depression for anxiety (along with other covariates) and vice versa as additional tests for disorder specificity.
2.6. Mendelian randomisation approach
2.6.1. Genotyping
We used genotyping data of 342,081 unrelated individuals of White ancestry; see Supplementary Methods for details on genotyping array, central and post-imputation quality control. We used a summary-based approach for MR analyses [37], so sample sizes differed for estimation of SNP-exposure and SNP-outcome associations. For estimation of SNP-outcome associations, sample sizes varied between 100,739 and 110,173 per outcome; see Supplementary Table 1 for sample sizes for SNP-exposure associations.
2.6.2. SNP selection
We selected genetic variants in the CRP and IL-6 receptor (IL6R) gene regions previously shown to be associated with CRP or IL-6 concentrations (Supplementary Table 1) [[38], [39], [40], [41]]. Genetic instruments differ in strength based on the precision with which they have been estimated in original GWAS studies. As instrument strength informs statistical power for MR analysis, we used genetic instruments from Georgakis et al. [38] for primary MR analysis, which have the largest strength (Supplementary Table 1), and report results from other instruments [39], [40], [41] as sensitivity analysis.
We extracted SNP-exposure estimates from previous reports to perform 2-sample MR analysis. Based on availability of CRP concentrations in the UK Biobank study, which can be used as downstream readout of IL-6 activity under the classic IL-6 signalling pathway [38]. we also estimated SNP-exposure associations (for 1-sample MR) and SNP-outcome associations, in the full sample and separately for men and women for sex-stratified MR; see details in Supplementary Methods and Supplementary Fig. 1.
2.6.3. Mendelian randomisation analyses
We performed MR analysis using inverse-variance weighted (IVW) regression of the genetic associations with the outcome on the genetic associations with the exposure [37]. To evaluate the potential impact of selection/collider bias for participation in the optional mental health survey, we repeated IVW MR analyses with SNP-outcome associations obtained using inverse probability weighted regression [34]. We also evaluated potential horizontal pleiotropy using Cochran's Q [37]. See details in Supplementary Methods.
2.7. Role of the funding source
The funding sources had no role in study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the paper for publication.
3. Results
3.1. Baseline characteristics
In 146,954 participants (43·6% men), mean age at recruitment was 56·5 (SD=7·8) years. Median CRP concentration was 1·15 mg/L (IQR=0·58–2·38 mg/L). Table 1 shows characteristics of study participants by CRP quintiles. Mean depressive symptom scores were 2·76 (SD=3·70, range: 0–27) and mean anxiety symptom scores 2·15 (SD=3·41, range: 0–21); these scores exhibited a moderate-to-large correlation (Pearson's r = 0·68). 5·5% of individuals qualified for a probable diagnosis of depression, 4·4% for a probable diagnosis of GAD, and 0·6% for both probable depression and probable GAD.
Table 1.
Baseline characteristics of study participants by quintiles of CRP levels in the UK Biobank cohort (n = 146,954).
| Study characteristics | Q1 (n = 34,787) | Q2 (n = 32,125) | Q3 (n = 29,113) | Q4 (n = 26,733) | Q5 (n = 24,196) | P value |
|---|---|---|---|---|---|---|
| CRP (mg/L) median (range) | 0·36 (0·08–0·55) | 0·77 (0·56–1·02) | 1·33 (1·03–1·75) | 2·33 (1·76–3·33) | 5·42 (3·34–78·22) | <0·001 |
| Age (years) | 54·3 (7·8) | 55·82 (7·7) | 56·5 (7·6) | 56·9 (7·6) | 56·6 (7·7) | <0·001 |
| Women (%) | 20,262 (58·3) | 17,255 (53·7) | 15,588 (53·5) | 14,867 (55·6) | 14,931 (61·7) | <0·001 |
| White ethnicity (%) | 33,601 (96·6) | 31,166 (97·0) | 28,228 (97·0) | 25,907 (96·9) | 23,399 (96·7) | <0·001 |
| TDI, median (SD) | −1·7 (2·8) | −1·8 (2·8) | −1·8 (2·8) | −1·7 (2·8) | −1·5 (2·9) | <0·001 |
| BMI (kg/m2) | 24·1 (3·1) | 25·8 (3·4) | 27·0 (3·9) | 28·2 (4·3) | 30·1 (5·8) | <0·001 |
| Smoking status (%) | ||||||
| Never | 21,603 (62·1) | 18,927 (58·9) | 16,509 (56·7) | 14,722 (55·1) | 12,555 (51·9) | |
| Current | 1965 (5·7) | 1981 (6·2) | 2057 (7·1) | 2162 (8·1) | 2418 (10·0) | |
| Ex-smokers | 11,157 (32·1) | 11,138 (34·7) | 10,484 (36·0) | 9783 (36·6) | 9163 (37·9) | <0·001 |
| Alcohol status (%) | ||||||
| Never/Ex | 1743 (5·0) | 1581 (4·9) | 1578 (5·4) | 1633 (6·1) | 1659 (6·9) | |
| Occasional (≤ 3 times per week) | 14,376 (41·3) | 13,856 (43·2) | 13,052 (44·8) | 12,719 (47·6) | 12,184 (50·4) | |
| Regular (> 3 times per week) | 18,657 (53·7) | 16,677 (51·9) | 14,475 (49·7) | 12,369 (46·3) | 10,342 (42·8) | <0·001 |
| Physical activity (%) | ||||||
| Inactivity | 27,490 (90·0) | 24,961 (80·1) | 22,180 (79·1) | 19,756 (77·7) | 16,816 (74·9) | |
| Moderately inactive | 1350 (4·0) | 1548 (5·0) | 1633 (5·8) | 1742 (6·9) | 1969 (8·8) | |
| Moderately active | 4342 (12·8) | 3881 (12·5) | 3443 (12·3) | 3206 (12·6) | 2967 (13·2) | |
| Active | 779 (2·3) | 778 (2·5) | 780 (2·8) | 722 (2·8) | 711 (3·2) | <0·001 |
| Diabetes (%) | 780 (2·2) | 881 (2·7) | 983 (3·4) | 1022 (3·8) | 1210 (5·0) | <0·001 |
| Cardiovascular disease (%) | 1029 (3·0) | 1093 (3·4) | 1076 (3·7) | 1035 (3·9) | 973 (4·0) | <0·001 |
Note: Differences were estimated using mean and SD for continuous variables, with p-values from ANOVA test, or using number and percent for categorical variables, with χ2 test.
3.2. Association of CRP concentration with depressive and anxiety symptom scores
Results for associations of CRP with depressive and anxiety symptoms are presented in Fig. 1 across different CRP deciles in the total sample, and for women and men separately in Supplementary Figs. 2 and 3. Overall, CRP was associated with depressive and anxiety symptoms after adjusting for all potential confounding factors, but adjustment for BMI attenuated these associations to some extent (Supplementary Tables 2 & 3).
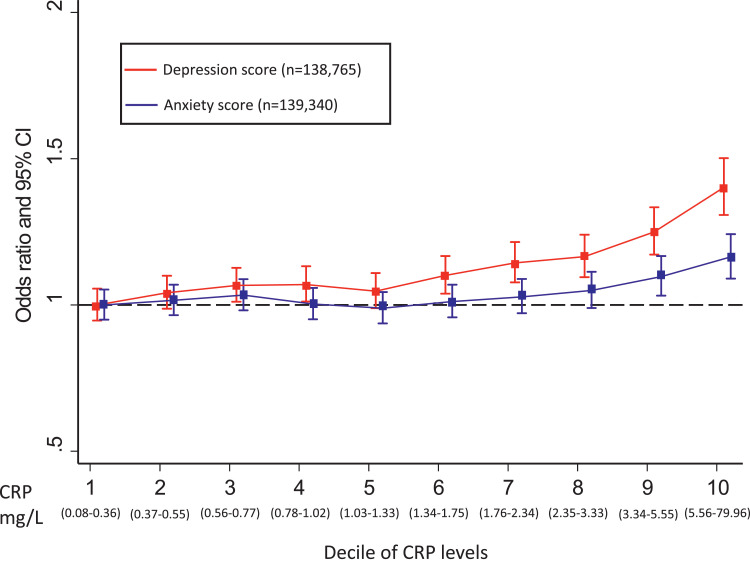
Fig. 1.
Odds ratios for higher depressive and anxiety symptom scores per decile of CRP levels in the UK Biobank cohort.
Error bars represent 95% confidence intervals (CIs), which were calculated using a floating absolute risk technique; CRP: C-reactive protein; Odds ratios were adjusted for age, sex, BMI, smoking status, alcohol intake, physical activity, TDI, ethnic group, diabetes and cardiovascular disease; red: depression score; blue: anxiety score (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Using CRP as a continuous variable, the adjusted OR for higher depressive symptom score per-unit increase in log CRP was 1·09 (95% CI, 1·06–1·11). Using CRP as a categorical variable, the adjusted OR for higher depressive symptom score for participants in the top, compared with bottom, quintile of CRP was 1·29 (95% CI, 1·21–1·38). Inverse probability weighted regression analyses of depressive symptoms did not suggest that results were affected by collider bias, as the adjusted OR=1·31 (95% CI, 1·22–1·41) for participants in the top, compared with bottom, quintile of CRP was similar.
Using CRP as a continuous variable, the adjusted OR for higher anxiety symptom score per-unit increase in log CRP was 1·03 (95% CI, 1·02–1·05). Using CRP as a categorical variable, the adjusted OR for higher anxiety symptom score for participants in the top, compared with bottom, quintile of CRP was 1·12 (95% CI, 1·05–1·19). Again, evidence did not suggest results were affected by collider bias with similar OR of 1·12 (95% CI, 1·05–1·20) in sensitivity analyses.
3.3. Association of CRP concentration with probable diagnoses of depression and GAD
CRP was associated with probable diagnosis of depression (Table 2). Using CRP as a continuous variable, the adjusted OR for depression per-unit increase in log CRP was 1·09 (95% CI, 1·06–1·11). Using CRP as a categorical variable, the adjusted OR for depression for participants in the top, compared with bottom, quintile of CRP was 1·29 (95% CI, 1·18–1·40). Evidence did not suggest results were affected by collider bias with similar OR of 1·29 (95% CI, 1·18–1·41) in sensitivity analyses.
Table 2.
Association of C-reactive protein levels with probable diagnosis of depression in the UK Biobank cohort.
| log CRP as continuous variable | CRP Q1 (n = 34,372) | CRP Q2 (n = 31,704) | CRP Q3 (n = 28,714) | CRP Q4 (n = 26,350) | CRP Q5 (n = 23,750) | Per-Q effect | P-value for trend | |
|---|---|---|---|---|---|---|---|---|
| All participants (cases = 8888; controls = 145,468) | ||||||||
| Model 1 (n = 144,890) | 1·27 (1·24–1·29) | 1 [reference] | 1·11 (1·03–1·19) | 1·19 (1·10–1·28) | 1·44 (1·34–1·54) | 2·05 (1·91–2·20) | 1·19 (1·17–1·21) | <0·001 |
| Model 2 (n = 144,600) | 1·12 (1·09–1·15) | 1 [reference] | 1·08 (1·00–1·16) | 1·10 (1·02–1·18) | 1·22 (1·13–1·31) | 1·41 (1·31–1·53) | 1·09 (1·07–1·10) | <0·001 |
| Model 3 (n = 138,766) | 1·09 (1·06–1·11) | 1 [reference] | 1·07 (0·99–1·15) | 1·08 (1·00–1·17) | 1·16 (1·07–1·26) | 1·28 (1·18–1·39) | 1·06 (1·04–1·08) | <0·001 |
| Model 4 (n = 138,765) | 1·09 (1·06–1·11) | 1 [reference] | 1·07 (0·99–1·16) | 1·08 (1·00–1·17) | 1·16 (1·07–1·26) | 1·29 (1·18–1·40) | 1·06 (1·04–1·08) | <0·001 |
| Women (cases = 5641; controls = 81,562) | ||||||||
| Model 1 (n = 81,610) | 1·28 (1·25–1·32) | 1 [reference] | 1·06 (0·96–1·16) | 1·22 (1·12–1·34) | 1·40 (1·28–1·53) | 2·11 (1·94–2·29) | 1·20 (1·18–1·23) | <0·001 |
| Model 2 (n = 81,454) | 1·12 (1·08–1·15) | 1 [reference] | 1·03 (0·94–1·13) | 1·13 (1·03–1·24) | 1·18 (1·07–1·30) | 1·41 (1·27–1·55) | 1·09 (1·06–1·11) | <0·001 |
| Model 3 (n = 77,818) | 1·10 (1·06–1·14) | 1 [reference] | 1·02 (0·93–1·13) | 1·13 (1·02–1·25) | 1·17 (1·06–1·30) | 1·33 (1·20–1·48) | 1·07 (1·05–1·10) | <0·001 |
| Model 4 (n = 77,818) | 1·10 (1·06–1·13) | 1 [reference] | 1·02 (0·93–1·13) | 1·13 (1·02–1·25) | 1·17 (1·06–1·30) | 1·33 (1·20–1·48) | 1·07 (1·05–1·10) | <0·001 |
| Men (cases = 3247; controls = 63,906) | ||||||||
| Model 1 (n = 63,280) | 1·22 (1·18–1·27) | 1 [reference] | 1·23 (1·09–1·38) | 1·17 (1·04–1·32) | 1·53 (1·36–1·72) | 1·87 (1·66–2·11) | 1·16 (1·13–1·19) | <0·001 |
| Model 2 (n = 63,146) | 1·13(1·08–1·17) | 1 [reference] | 1·16 (1·03–1·30) | 1·04 (0·92–1·18) | 1·27 (1·12–1·43) | 1·44 (1·27–1·64) | 1·08 (1·05–1·12) | <0·001 |
| Model 3 (n = 60,948) | 1·07 (1·02–1·11) | 1 [reference] | 1·12 (0·99–1·27) | 1·00 (0·88–1·14) | 1·14 (1·00–1·29) | 1·21 (1·06–1·39) | 1·04 (1·01–1·07) | 0·02 |
| Model 4 (n = 60,947) | 1·07 (1·03–1·12) | 1 [reference] | 1·13 (1·00–1·28) | 1·01 (0·89–1·15) | 1·15 (1·01–1·31) | 1·23 (1·07–1·41) | 1·04 (1·01–1·07) | 0·01 |
Data show OR and 95% CIs unless otherwise indicated. P for trend is from regression models with quintiles. Model 1, unadjusted; model 2, adjusted for age, sex, and BMI (body mass index); model 3, model 2 additionally adjusted for smoking, alcohol, physical activity, ethnicity, and TDI (Townsend deprivation index at recruitment); model 4, model 3 additionally adjusted for diabetes and cardiovascular disease; *: CRP concentration was log transformed; Median CRP level was 1·15 mg/L (range 0·08–78·22 mg/L).
CRP was associated with probable diagnosis of GAD (Table 3). Using CRP as a continuous variable, the adjusted OR for GAD per-unit increase in log CRP was 1·05 (95% CI, 1·02–1·08). Using CRP as a categorical variable, the adjusted OR for GAD for participants in the top, compared with bottom, quintile of CRP was 1·15 (95% CI, 1·05–1·26). Again, evidence did not support collider bias as likely explanation with similar OR of 1·13 (95% CI, 1·02–1·24) in sensitivity analyses.
Table 3.
Association of C-reactive protein levels with probable GAD diagnosis in the UK Biobank cohort.
| log CRP as continuous variable | CRP Q1 (n = 34,499) | CRP Q2 (n = 31,809) | CRP Q3 (n = 28,829) | CRP Q4 (n = 26,451) | CRP Q5 (n = 23,950) | Per-Q effect | P for trend | |
|---|---|---|---|---|---|---|---|---|
| All participants (cases = 6395; controls = 139,143) | ||||||||
| Model 1 (n = 145,538) | 1·11 (1·08–1·14) | 1 [reference] | 0·95 (0·88–1·03) | 0·95 (0·88–1·03) | 1·05 (0·97–1·13) | 1·38 (1·28–1·49) | 1·08 (1·06–1·10) | <0·001 |
| Model 2 (n = 145,239) | 1·07 (1·04–1·10) | 1 [reference] | 0·99 (0·91–1·07) | 0·99 (0·91–1·07) | 1·05 (0·97–1·14) | 1·24 (1·14–1·36) | 1·05 (1·03–1·07) | <0·001 |
| Model 3 (n = 139,341) | 1·05 (1·02–1·08) | 1 [reference] | 0·97 (0·90–1·06) | 0·99 (0·91–1·07) | 1·02 (0·94–1·12) | 1·15 (1·05–1·26) | 1·03 (1·01–1·05) | 0·004 |
| Model 4 (n = 139,340) | 1·05 (1·02–1·08) | 1 [reference] | 0·98 (0·90–1·06) | 0·98 (0·90–1·07) | 1·02 (0·94–1·11) | 1·15 (1·05–1·26) | 1·03 (1·01–1·05) | 0·005 |
| Women (cases = 4247; controls = 77,717) | ||||||||
| Model 1 (n = 81,964) | 1·10 (1·07–1·13) | 1 [reference] | 0·97 (0·88–1·07) | 0·95 (0·86–1·05) | 1·03 (0·93–1·13) | 1·38 (1·26–1·51) | 1·07 (1·05–1·10) | <0·001 |
| Model 2 (n = 81,799) | 1·08 (1·04–1·11) | 1 [reference] | 1·00 (0·91–1·10) | 0·98 (0·88–1·08) | 1·05 (0·94–1·16) | 1·29 (1·16–1·43) | 1·05 (1·03–1·08) | <0·001 |
| Model 3 (n = 78,110) | 1·07 (1·03–1·10) | 1 [reference] | 0·98 (0·89–1·09) | 0·99 (0·90–1·10) | 1·04 (0·94–1·16) | 1·23 (1·10–1·38) | 1·05 (1·02–1·07) | 0·001 |
| Model 4 (n = 78,110) | 1·06 (1·03–1·10) | 1 [reference] | 0·99 (0·89–1·09) | 0·99 (0·89–1·10) | 1·04 (0·93–1·16) | 1·23 (1·10–1·37) | 1·05 (1·02–1·07) | 0·001 |
| Men (cases = 2148; controls = 61,426) | ||||||||
| Model 1 (n = 63,574) | 1·10 (1·06–1·16) | 1 [reference] | 0·97 (0·85–1·11) | 1·02 (0·89–1·17) | 1·12 (0·98–1·28) | 1·33 (1·16–1·53) | 1·07 (1·04–1·11) | <0·001 |
| Model 2 (n = 63,440) | 1·07 (1·02–1·12) | 1 [reference] | 0·97 (0·85–1·11) | 1·02 (0·89–1·17) | 1·12 (0·98–1·28) | 1·33 (1·16–1·53) | 1·04 (1·01–1·08) | 0·018 |
| Model 3 (n = 61,231) | 1·02 (0·98–1·07) | 1 [reference] | 0·94 (0·82–1·08) | 0·95 (0·83–1·10) | 0·97 (0·84–1·13) | 1·02 (0·87–1·20) | 1·01 (0·97–1·04) | 0·74 |
| Model 4 (n = 61,230) | 1·02 (0·98–1·07) | 1 [reference] | 0·95 (0·82–1·08) | 0·95 (0·83–1·10) | 0·97 (0·84–1·13) | 1·02 (0·87–1·20) | 1·01 (0·97–1·04) | 0·74 |
Note: Data show ORs and 95% CIs unless otherwise indicated. P for trend is from regression models with quintiles. Model 1, unadjusted; model 2, adjusted for age, sex, and BMI (body mass index); model 3, model 2 additionally adjusted for smoking, alcohol, physical activity, ethnicity, and TDI (Townsend deprivation index at recruitment); model 4, model 3 additionally adjusted for diabetes and cardiovascular disease; *: CRP concentration was log transformed; Median CRP level was 1·33 mg/L (range 0·08–79·96 mg/L).
3.4. Test for specificity vs commonality of association of CRP with depression and anxiety
In bi-variate probit regression analysis, we found evidence for a stronger association of CRP with depressive symptoms (OR=1·014; 95% CI, 1·011–1·017) than anxiety symptoms (OR=1·004; 95% CI, 1·002–1·007). Results for probit regression using probable diagnoses of depression and GAD as outcomes were similar (see Supplementary Results).
In regression analyses, evidence for association of CRP with depression symptoms remained after adjusting for anxiety symptoms (OR=1·06; 95% CI, 1·05–1·08), but the association of CRP with anxiety symptoms switched its valence after adjusting for depressive symptoms (OR=0·98; 95% CI, 0·97–0·99).
3.5. Linearity of association
Evidence was compatible with linear associations of CRP with both depression and anxiety across all analyses using symptom scores and probable diagnoses as outcomes (P-value for all quadratic terms >0·05).
3.6. Examination of potential sex difference
In sex-stratified analyses, point estimates were larger for women than men for both depression and anxiety symptom outcomes (Supplementary Tables 2,3, Supplementary Figs. 2,3). However, evidence for an interaction between CRP and sex was present only for depressive symptoms (adjusted ORwomen=1·35; 95%CI, 1·23–1·48; adjusted ORmen=1·21; 95%CI, 1·10–1·33; P-value for interaction term=0·032). For categorical outcomes, point estimates were larger for women for probable GAD (Tables 2,3), but evidence did not support interaction for either outcomes (all P > 0·2).
3.7. Results for Mendelian randomisation analyses
Genetically-predicted concentration/activity of IL-6 and CRP were associated with both depression and anxiety. However, these associations differed with regards to direction of association (i.e., increased vs decreased risk), particular outcome definition, and sex. Table 4 shows results for IVW MR analyses based on Georgakis et al. [38] genetic instruments for CRP and IL-6.
Table 4.
IVW Mendelian randomisation analysis of association of IL-6 and CRP with depression and anxiety.
|
Depression Symptom Score |
Probable depression |
Anxiety Symptom Score |
Probable GAD |
|||||
|---|---|---|---|---|---|---|---|---|
| Model | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
|
CRP |
||||||||
| 2-Sample MR | 0·88 (0·80–0·98) | 0·020 | 0·95 (0·85–1·07) | 0·424 | 0·87 (0·80–0·95) | 0·003 | 0·82 (0·72–0·94) | 0·004 |
| 1-Sample MR | 0·89 (0·79–1·00) | 0·055 | 1·01 (0·88–1·14) | 0·939 | 0·88 (0·79–0·97) | 0·008 | 0·84 (0·73–0·98) | 0·027 |
| Women | 0·98 (0·85–1·12) | 0·754 | 1·12 (0·96–1·30) | 0·152 | 0·86 (0·76–0·98) | 0·023 | 0·85 (0·72–1·01) | 0·059 |
| Men | 0·78 (0·63–0·96) | 0·018 | 0·84 (0·66–1·06) | 0·138 | 0·91 (0·78–1·05) | 0·192 | 0·83 (0·62–1·11) | 0·209 |
| IL-6 | ||||||||
| 2-Sample MR | 1·34 (1·05–1·72) | 0·019 | 1·15 (0·86–1·54) | 0·340 | 1·13 (0·91–1·41) | 0·269 | 1·24 (0·89–1·73) | 0·194 |
| 1-Sample MR | 1·32 (1·03–1·67) | 0·025 | 1·18 (0·89–1·56) | 0·246 | 1·11 (0·90–1·37) | 0·313 | 1·18 (0·86–1·62) | 0·297 |
| Women | 1·42 (1·01–1·97) | 0·041 | 1·46 (1·00–2·13) | 0·048 | 1·15 (0·85–1·56) | 0·362 | 1·51 (1·01–2·25) | 0·044 |
| Men | 1·24 (0·88–1·74) | 0·218 | 0·86 (0·54–1·37) | 0·516 | 1·08 (0·79–1·47) | 0·636 | 0·79 (0·47–1·33) | 0·385 |
Note: Estimates for men and women are based on sex-stratified 1-sample MR analyses.
For CRP, per-unit increase in genetically-predicted concentrations of log-transformed CRP was associated with lower risk for depressive symptoms (1-sample MR: OR=0·89; 95% CI, 0·79–1·00; 2-sample MR: OR=0·88; 95% CI, 0·80–0·98), and lower risk for anxiety symptoms (1-sample MR: OR=0·88; 95% CI, 0·79–0·97; 2-sample MR: OR=0·87; 95% CI, 0·80–0·95). Using the categorical outcomes, MR analyses also showed that increased genetically-predicted CRP was associated with lower risk for probable GAD, but point estimates for probable depression were close to one (Table 4). In sex-stratified MR analyses, higher genetically predicted CRP concentrations were associated with relatively lower risk for depressive symptoms in men, and with relatively lower risk for anxiety symptoms in women.
For IL-6, per-unit increase in higher genetically-predicted IL-6 activity was associated with increased risk for depressive symptoms (1-sample MR: OR=1·32, 95% CI 1·03–1·67; 2-sample MR: OR=1·34, 95% CI 1·05–1·72), but not with probable depression or either anxiety outcome. In sex-stratified MR analyses, we found evidence that higher genetically-predicted IL-6 activity was associated with increased risk for depressive symptoms, probable depression, and probable GAD in women only.
MR analyses using alternative genetic instruments were directionally consistent with these results, albeit with larger confidence intervals possibly due to the lower statistical power for these instruments (Supplementary Table 4). Results for sensitivity analyses evaluating the impact of selection/collider bias were similar to main IVW analyses (Supplementary Table 5).
Evidence did not suggest directional horizontal pleiotropy was a likely explanation for any of the IVW MR results as assessed using Cochran's Q (Supplementary Table 6).
4. Discussion
Based on data from the UK Biobank cohort, a large general population cohort, we report that circulating CRP concentrations are associated with depressive and anxiety symptoms and with probable diagnoses of depression and GAD in a linear, dose-response fashion. At the same time, we show evidence for disorder-specificity suggesting that CRP is more strongly associated with depression compared to anxiety. We also found some evidence for sex-specificity. CRP was more strongly associated with depression in women than in men. Using MR analyses, we provide evidence that higher IL-6 activity could represent a potential causal factor increasing depression, while genetically predicted higher CRP concentrations appeared to potentially be protective for depression and anxiety, which contrasts findings for serum CRP.
Although inflammation was associated with both depression and anxiety, we report stronger associations for depression outcomes indicating disorder-specificity. This aligns with meta-analyses of case-control studies showing higher concentrations of CRP and other inflammatory markers in depression [3,4,[6], [7], [8]] ,while there are relatively fewer studies suggesting this for anxiety [13]. Cohort studies of affective symptoms also suggest that circulating IL-6 and CRP concentrations are predominantly associated with depressive rather than anxiety symptoms [29]. Together, current evidence is consistent with the idea that systemic inflammation may be particularly relevant for depression rather than anxiety disorders.
Our results also provide some evidence for sex-specificity. Associations of serum CRP concentrations with depression and anxiety were mostly stronger in women than men. Results for sex-stratified MR analyses suggested that higher IL-6 could be a risk factor for depressive symptoms specifically for women while higher CRP could be protective for depressive symptoms specifically for men and for anxiety symptoms specifically for women. It is important to note, however, that confidence intervals of sex-stratified MR estimates overlapped between sexes emphasising the tentative nature of these results. Existing evidence on potential sex-difference for associations between inflammatory makers and depression has also been mixed. A previous meta-analysis reported no sex-specificity of the association between CRP and depression [3]. In contrast, two recent studies reported that IL-6 was associated with depressive symptom chronicity and treatment response specifically in women [20,42]. Atypical depression, which is characterised by immuno-metabolic dysregulation, has also been reported to be more common in women [43]. Hitherto most studies have considered sex as a covariate. Further research is needed to replicate our findings regarding potential sex-specificity.
Our findings lend support to RCTs testing immunotherapies targeting the IL-6/IL-6R pathway for patients with depression. Anti-inflammatory treatments have been shown to exhibit antidepressant activity in chronic inflammatory illnesses [[44], [45], [46]]. In depression, initial results suggest that these drugs may be useful for patients with evidence of inflammation and inflammation-related risk factors [[47], [48], [49]]. This hypothesis is now being investigated in ongoing RCTs that are selecting patients based on evidence of inflammation and inflammation-related phenotypes [9,50]. The present study further highlights characteristics associated with inflammation, e.g., female sex, to inform stratified patient selection in future clinical trials.
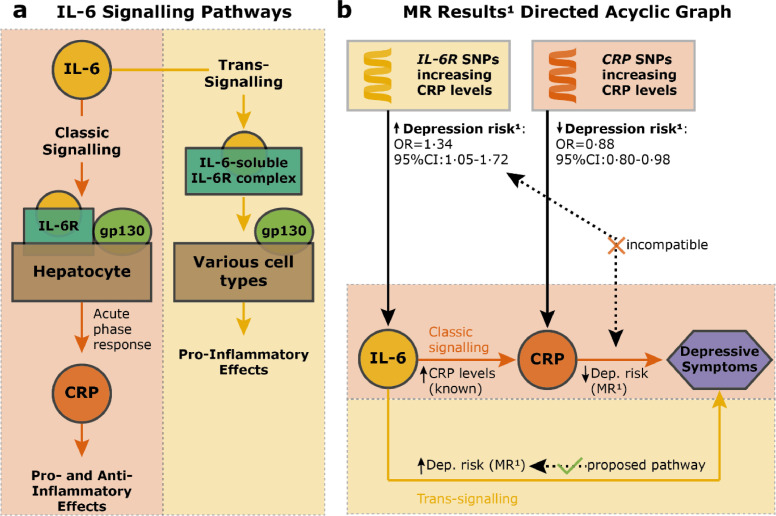
Using genetic variants in the IL6R and CRP gene loci, we have found that higher genetically predicted IL-6 activity was associated with increased risk of depression, but higher genetically predicted CRP levels were associated with decreased risk of depression. These findings are intriguing because IL-6 signalling is a key driver of CRP response [51,52], and so we would expect both to affect depression risk in a comparable way. One potential explanation could be that IL-6 classic and trans-signalling have divergent effects on depression risk. We have illustrated this hypothesis in Fig. 2, which describes IL-6 signalling pathways and a Directed Acyclic Graph of these pathways incorporating our MR results.
Fig. 2.
Potential divergent effects of specific IL-6 signalling pathways on depression risk.
Note: Fig. 2a shows IL-6 classic and trans-signalling pathways; see review by Hunter and Jones [51]. Fig. 2b displays our working hypothesis arising from MR results that IL-6 trans-signalling confers increased risk for depression. 1MR estimates are based on 2-sample MR analysis using Georgakis et al. [38] genetic instruments and continuous depressive symptoms as outcome (cf. Table 4). Abbreviations: gp130=glycoprotein 130; Dep.=depression; CRP=C-reactive protein; IL-6=interleukin-6.
In brief, IL-6 classic signalling occurs via its action on membrane-bound IL-6 receptors (IL-6Rs) expressed by limited cell types. IL-6 also binds with circulating soluble IL-6R (sIL-6R) to form an IL-6-sIL-6R complex, which then activates IL-6 signalling by binding with the ubiquitous glycoprotein 130 on other cells that naturally lack IL-6Rs. This is called IL-6 trans-signalling, which is thought to underlie pro-inflammatory effects of IL-6 in chronic inflammatory diseases [51].
Mechanistically, the observed increased depression risk conferred by IL6R SNPs that increase CRP levels [38] could happen as a result of either increased IL-6 classic or trans-signalling. Our results indicate that it may be due to increased trans-signalling, because we also see that SNPs in the CRP gene that increase CRP levels [38] are protective for depression. It is well-known that CRP is mainly produced by hepatocytes as a result of increased IL-6 classic signalling [51]. Taken together, these findings also align with a recent MR study on the effects of genetically predicted sIL-6R, sgp130 (an inhibitor of IL-6 trans-signalling [51]), and CRP on recurrent depression, which suggested that increased IL-6 trans-signalling or decreased IL-6 classic signalling could be responsible for a risk-increase in recurrent depressive symptoms [28].
While our findings suggest that altered activity of the IL-6/IL-6R pathway could be a risk factor for depression, disentangling the issue of IL-6 classic vs trans-signalling is beyond the scope of population genomics approaches as full effects of genetic variants used are unknown. The field now requires experimental studies of IL-6 modulation in humans and animals to further examine causality, pathogenic mechanisms, and therapeutic potential of anti-IL-6 and other immunotherapies for depression. Findings from these studies may help to devise more targeted IL-6 pathway-specific interventions.
Strengths of the work include use of a large population-based sample, a range of affective symptoms, and complementary analysis using protein levels and genetic variants. We assessed reproducibility and strength of association using different outcomes and sex-stratified analysis, evidence of linearity and potential causality of associations. Limitations of the work include focus on self-reported symptom score/probable diagnosis. Self-report measures of depression can capture different characteristics than observer-rated measures, so findings need to be replicated using the observer-rated modality [53]. Depression is also a phenotypically heterogeneous syndrome and previous studies have reported that inflammation may be associated with specific symptoms, such as fatigue, changes in appetite and sleep, and suicidality [27,29,43]. Aetiology of depressive symptoms could also vary across the lifespan, so findings from UK Biobank participants (mean age of 57 years) need to be replicated in other age groups. Second, although there was little evidence that associations of CRP with depression and anxiety could be due to selection/collider bias into the optional UK Biobank Mental Health Survey, selection/collider bias for participation in the UK Biobank cohort itself would likely be larger and remains a possible explanation for our findings that we could not explore. This is particularly relevant as the UK Biobank study includes individuals who are amongst others older, more likely to be women, healthier and of higher socioeconomic status compared to the general UK population [54]. Third, MR findings were based on a subgroup of individuals of European ancestry, which is a common issue in genetic studies, warranting replication in other ethnic groups. Finally, IL-6 was not measured in the UK Biobank cohort, so we were unable to assess associations of serum IL-6 concentrations with depression and anxiety.
In conclusion, we report evidence for associations of higher serum CRP concentrations with depressive and anxiety symptoms, which are stronger for depressive than for anxiety symptoms and, although less consistently, for women than for men. Findings from MR analyses are consistent with a role of altered activity of the IL-6/IL-6R pathway in depressive symptoms, suggesting that this pathway could be a promising, new therapeutic target for depression. Due to uncertainties regarding the full functional effects of genetic variants used as MR instruments, the field now requires human and animal experimental studies to elucidate mechanisms for divergent effects for CRP and IL-6 on illness risk. This may help to devise more targeted interventions.
Contribution
ZY, NK, and GMK were responsible for writing of the original draft; ZY, NK, SM, GDS, SB, PBJ and GMK for reviewing and editing the manuscript; ZY, NK, SM, GDS, SB, PBJ, and GMK for acquisition, analysis or interpretation of data; GMK, ZY, and NK for conceptualisation of the study; ZY and NK for visualisations; ZY and NK for statistical analysis; and GDS, SB, PBJ, and GMK for supervision. ZY and NK accessed the database and raw data.
Data sharing
UK Biobank data can be accessed through formal application to the cohort. GWAS summary data used as part of this report are freely available online or can be requested for CRP from the CHARGE inflammation working group. Genetic instrument estimates and scripts for MR processing and analysis are made available online for full reproducibility under https://osf.io/apme9/.
Funding
This research was funded in whole, or in part, by the Wellcome Trust (grant code: 201486/Z/16/Z). For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. This work was supported by a Data Science Award from the MQ: Transforming Mental Health (grant code: MQDS17/40) to GMK and PBJ, which also supported ZY. GMK also acknowledges funding support from the Wellcome Trust (grant code: 201486/Z/16/Z), the Medical Research Council UK (grant code: MC_PC_17213 and MR/S037675/1), and the BMA Foundation (J Moulton grant 2019). NK and SM are supported by the International Max Planck Research School of Translational Psychiatry (IMPRS-TP). GDS works in the Medical Research Council Integrative Epidemiology Unit at the University of Bristol, which is supported by the Medical Research Council (MC_UU_00011/1).
Declaration of Competing Interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100992.
Appendix. Supplementary materials
References
- 1.Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nusslock R., Miller G.E. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osimo E.F., Baxter L.J., Lewis G., Jones P.B., Khandaker G.M. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49:1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Köhler C.A., Freitas T.H., Maes M. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 5.Orlovska-Waast S., Köhler-Forsberg O., Brix S.W. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2018 doi: 10.1038/s41380-018-0220-4. published online Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 7.Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowlati Y., Herrmann N., Swardfager W. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Khandaker G.M., Oltean B.P., Kaser M. Protocol for the insight study: a randomised controlled trial of single-dose tocilizumab in patients with depression and low-grade inflammation. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendler K.S. Major depression and generalised anxiety disorder. Br J Psychiatry. 1996;168:68–75. [PubMed] [Google Scholar]
- 11.Wray N.R., Ripke S., Mattheisen M. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–681. doi: 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association . American Psychiatric Pub; Washington, DC: 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) [Google Scholar]
- 13.Costello H., Gould R.L., Abrol E., Howard R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-027925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glaus J., von Känel R., Lasserre A.M. The bidirectional relationship between anxiety disorders and circulating levels of inflammatory markers: results from a large longitudinal population-based study. Depress Anxiety. 2018;35:360–371. doi: 10.1002/da.22710. [DOI] [PubMed] [Google Scholar]
- 15.Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zalli A., Jovanova O., Hoogendijk W.J.G., Tiemeier H., Carvalho L.A. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology. 2016;233:1669–1678. doi: 10.1007/s00213-015-3919-9. (Berl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mac Giollabhui N., Ng T.H., Ellman L.M., Alloy L.B. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. 2020 doi: 10.1038/s41380-020-00867-4. published online Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valkanova V., Ebmeier K.P., Allan C.L. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Glaus J., von Känel R., Lasserre A.M. Mood disorders and circulating levels of inflammatory markers in a longitudinal population-based study. Psychol Med. 2017:1–13. doi: 10.1017/S0033291717002744. [DOI] [PubMed] [Google Scholar]
- 20.Lamers F., Milaneschi Y., Smit J.H., Schoevers R.A., Wittenberg G., Penninx B.W.J.H. Longitudinal association between depression and inflammatory markers: results from the Netherlands study of depression and anxiety. Biol Psychiatry. 2019;85:829–837. doi: 10.1016/j.biopsych.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Davey Smith G., Ebrahim S. Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 22.Hartwig F.P., Borges M.C., Horta B.L., Bowden J., Davey Smith G. Inflammatory biomarkers and risk of schizophrenia. JAMA Psychiatry. 2017;74:1226–1233. doi: 10.1001/jamapsychiatry.2017.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osimo E.F., Cardinal R.N., Jones P.B., Khandaker G.M. Prevalence and correlates of low-grade systemic inflammation in adult psychiatric inpatients: an electronic health record-based study. Psychoneuroendocrinology. 2018 doi: 10.1016/j.psyneuen.2018.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalf S.A., Jones P.B., Nordstrom T. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: a prospective birth cohort study. Brain Behav Immun. 2017;59:253–259. doi: 10.1016/j.bbi.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wium-Andersen M.K., Ørsted D.D., Nordestgaard B.G. Elevated C-reactive protein, depression, somatic diseases, and all-cause mortality: a mendelian randomization study. Biol Psychiatry. 2014;76:249–257. doi: 10.1016/j.biopsych.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Khandaker G.M., Zuber V., Rees J.M.B. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry. 2020;25:1477–1486. doi: 10.1038/s41380-019-0395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kappelmann N., Arloth J., Georgakis M.K. Dissecting the association between inflammation, metabolic dysregulation, and specific depressive symptoms. JAMA Psychiatry. 2021;78:161–170. doi: 10.1001/jamapsychiatry.2020.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly K., Smith J.A., Mezuk B. Depression and interleukin-6 signaling: a mendelian randomization study. Brain Behav Immun. 2021 doi: 10.1016/j.bbi.2021.02.019. published online Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milaneschi Y., Kappelmann N., Ye Z. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Molecular Psychiatry. 2021 doi: 10.1038/s41380-021-01188-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sudlow C., Gallacher J., Allen N. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis K.A.S., Coleman J.R.I., Adams M. Mental health in UK Biobank – development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open. 2020;6:e18. doi: 10.1192/bjo.2019.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Löwe B., Unützer J., Callahan C.M., Perkins A.J., Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42:1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 34.Griffith G.J., Morris T.T., Tudball M.J. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyrrell J., Zheng J., Beaumont R. Genetic predictors of participation in optional components of UK Biobank. Nat Commun. 2021;12:886. doi: 10.1038/s41467-021-21073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norton E.C., Dowd B.E. Log odds and the interpretation of logit models. Health Serv Res. 2018;53:859–878. doi: 10.1111/1475-6773.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess S., Davey Smith G., Davies N.M. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2020;4:186. doi: 10.12688/wellcomeopenres.15555.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Georgakis M.K., Malik R., Gill D. Interleukin-6 signaling effects on ischemic stroke and other cardiovascular outcomes. Circ Genomic Precis Med. 2020;13 doi: 10.1161/CIRCGEN.119.002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.C Reactive Protein Coronary Heart Disease Genetics Collaboration (CCGC) Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ. 2011;342:d548. doi: 10.1136/bmj.d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collaboration IGC and ERF Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jha M.K., Minhajuddin A., Chin-Fatt C., Greer T.L., Carmody T.J., Trivedi M.H. Sex differences in the association of baseline c-reactive protein (CRP) and acute-phase treatment outcomes in major depressive disorder: findings from the EMBARC study. J Psychiatr Res. 2019;113:165–171. doi: 10.1016/j.jpsychires.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milaneschi Y., Lamers F., Berk M., Penninx B.W.J.H. Depression heterogeneity and its biological underpinnings: toward immunometabolic depression. Biol Psychiatry. 2020;88:369–380. doi: 10.1016/j.biopsych.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Kappelmann N., Lewis G., Dantzer R., Jones P.B., Khandaker G.M. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23:335–343. doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Köhler-Forsberg O., Nicolaisen Lydholm C., Hjorthøj C., Nordentoft M., Mors O., Benros M.E. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019:0–2. doi: 10.1111/acps.13016. [DOI] [PubMed] [Google Scholar]
- 46.Wittenberg G.M., Stylianou A., Zhang Y. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry. 2020;25:1275–1285. doi: 10.1038/s41380-019-0471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raison C.L., Rutherford R.E., Woolwine B.J. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McIntyre R.S., Subramaniapillai M., Lee Y. Efficacy of adjunctive infliximab vs placebo in the treatment of adults with bipolar I/II depression. JAMA Psychiatry. 2019:1–8. doi: 10.1001/jamapsychiatry.2019.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nettis M.A., Lombardo G., Hastings C. Augmentation therapy with minocycline in treatment-resistant depression patients with low-grade peripheral inflammation: results from a double-blind randomised clinical trial. Neuropsychopharmacology. 2021:1–10. doi: 10.1038/s41386-020-00948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fourrier C., Sampson E., Mills N.T., Baune B.T. Anti-inflammatory treatment of depression: study protocol for a randomised controlled trial of vortioxetine augmented with celecoxib or placebo. Trials. 2018;19:447. doi: 10.1186/s13063-018-2829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 52.Del Giudice M., Gangestad S.W. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Enns M.W., Larsen D.K., Cox B.J. Discrepancies between self and observer ratings of depression. J Affect Disord. 2000;60:33–41. doi: 10.1016/s0165-0327(99)00156-1. [DOI] [PubMed] [Google Scholar]
- 54.Fry A., Littlejohns T.J., Sudlow C. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.