Abstract
Background
Percutaneous ventricular assist devices (pVADs) have been used to support patients who are in cardiogenic shock (CS). There is limited data on 30-day mortality predictors in patients supported by an Impella pVAD.
Methods
All CS patients requiring left-sided Impella implantation in Harefield Hospital (Greater London, United Kingdom) between 2017 and 2020 were included in the current study. Logistic regression analysis was used to identify predictors of 30-day mortality.
Results
A total of 92 patients were included. The mean age was 53.8 ± 14.9 years, and 78.3% were male. CS etiology was predominantly acute coronary syndromes (44.6%), followed by decompensated dilated cardiomyopathy (28.3%). Survival at 30 days was 63% (58 of 92). Deceased patients had a lower left ventricular ejection fraction (LVEF) (15.1 ± 9.6 vs 21.8 ± 14.2, P < 0.001), higher serum lactate levels (2.8[1.6 to 5.4] vs 1.45 [1.08 to 3.53], P = 0.012), a higher percentage of prolonged invasive ventilation (> 24 hours) (64.7% vs 13.8%, P < 0.001), and worse renal and liver function. Serum lactate, baseline LVEF, and prolonged ventilation (> 24 hours) were independent predictors of 30-day survival with an area under the curve of 0.85 (95% confidence interval 0.769 to 0.930), P < 0.001.
Conclusions
In the current retrospective registry of patients requiring Impella pVAD implantation, independent 30-day mortality predictors included serum lactate, baseline LVEF, and prolonged invasive ventilation (> 24 hours). These parameters could highlight patients who would benefit from earlier mechanical circulatory support escalation or neurologic assessment to inform withdrawal decisions.
Résumé
Contexte
Des dispositifs d'assistance ventriculaire percutanés (DAVp) sont utilisés chez des patients qui sont en choc cardiogénique (CC). Il existe peu de données sur les facteurs prédictifs de la mortalité à 30 jours chez des patients porteurs d'un DAVp Impella.
Méthodologie
Tous les patients en CC ayant eu besoin d'un dispositif implantable d'assistance gauche Impella à l'hôpital Harefield (région de Londres, Royaume-Uni) entre 2017 et 2020 ont été inclus dans la présente étude. Une analyse par régression logistique a servi à déterminer les facteurs prédictifs de la mortalité à 30 jours.
Résultats
Au total, 92 patients ont été inclus. L’âge moyen était de 53,8 ± 14,9 ans, et 78,3 % étaient des hommes. La cause du CC était principalement un syndrome coronarien aigu (44,6 %), suivi d'une cardiomyopathie dilatée décompensée (28,3 %). La survie à 30 jours était de 63 % (58 sur 92). Les patients décédés avaient une fraction d’éjection ventriculaire gauche (FEVG) plus faible (15,1 ± 9,6 contre 21,8 ± 14,2; p < 0,001) et un taux sérique de lactate plus élevé (2,8 [1,6 à 5,4] contre 1,45 [1,08 à 3,53]; p = 0,012), avaient été plus nombreux à avoir besoin d'une ventilation invasive prolongée (> 24 heures) (64,7 % contre 13,8 %; p < 0,001) et présentaient une altération plus importante des fonctions rénale et hépatique. Le taux sérique de lactate, la FEVG initiale et une ventilation prolongée (> 24 heures) ont été des facteurs prédictifs indépendants de la survie à 30 jours, l'aire sous la courbe étant de 0,85 (intervalle de confiance à 95 % : 0,769 à 0,930; p < 0,001).
Conclusions
Dans le présent registre rétrospectif de patients ayant dû recevoir un DAVp implantable Impella, les facteurs prédictifs indépendants de la mortalité à 30 jours ont été le taux sérique de lactate, la FEVG initiale et une ventilation invasive prolongée (> 24 heures). Ces paramètres pourraient faire ressortir les cas où il serait préférable d'intensifier plus tôt le soutien circulatoire mécanique ou d'effectuer une évaluation neurologique afin d’éclairer les décisions d'arrêt des soins.
Despite advances in medical knowledge and technological developments in mechanical circulatory support (MCS) devices, cardiogenic shock (CS) remains one of the most challenging clinical scenarios. Its mortality varies from 40% to 60%,1 and its successful outcome depends on timely diagnosis and treatment.
One of the most commonly used MCS devices is the microaxial percutaneous ventricular assist device (pVAD) Impella (Abiomed, Danvers, MA).2 The left-sided Impella family of devices directly unload the left ventricle, reducing the myocardial oxygen demand while increasing the myocardial blood flow as well as the coronary flow.3 As a result, the use of Impella in the treatment of CS has been adopted in several institutions around the world; however, its clinical benefit has yet to be shown in randomized trials.4 The only randomized trial to date5 failed to demonstrate any benefit, but the patients included in that particular trial were too far along the CS downward spiral, and the vast majority had undergone cardiac arrest.
Patient selection and optimal timing of MCS remain a matter of debate, but the use of well established shock classifications (such as that created by the Society for Cardiovascular Angiography and Interventions [SCAI]) could assist physicians in their decision-making6,7 and stratify outcomes,8 as the deeper the shock at presentation, the worse the patient outcomes.7
To date, little evidence has been gathered regarding predictors of short-term survival in patients established on MCS with the Impella device in heterogenous cohorts.9,10
Methods
This is a single-centre retrospective study including all patients that underwent implantation of a left Impella pVAD from April 2017 to October 2020 in Harefield Hospital (Royal Brompton and Harefield NHS Foundation Trust in London, United Kingdom). The decision to perform Impella implantation was made by the CS team according to local protocols. Echocardiography was performed in all patients prior to MCS initiation, as part of the initial CS assessment.
Ethics
Per consultation with our local research ethics committee, no informed consent was required, as the study was part of an ongoing audit and all data were pseudo-anonymized. Vital status was ascertained using the national Patient Demographic Service, which incorporates national death registry information as well as local notifications.
Impella implantation and explantation techniques
The Impella CP was implanted percutaneously in the catheterization laboratory using fluoroscopic guidance. Following ultrasound-guided common femoral puncture, the insertion of the 14F peel-away sheath was performed over an Amplatz super stiff wire Amplatz (Boston Scientific, Marlborough, MA) to avoid wire kinking and vascular injury. The Impella CP explantation was done either percutaeneously using x2 Proglides (Abbott Vascular, Abbott (Chicago, IL) Teleflex - Morrisville, NC) +/- 8F Angioseal (Terumo, NJ) or a 14F MANTA device (Teleflex, UK), or surgically following cut-down.
All Impella 5.0 pumps were implanted in the operating room under general anaesthesia and guided with either transoesophageal echocardiography or fluoroscopy. The mid-axillary artery was surgically exposed. A 10-mm Silver-coated Dacron graft (Braun, Melsungen, Germany) was anastomosed to the axillary artery using a running 4/0 Prolene suture. The graft was tunnelled through the skin.
All the Impella 5.0 pumps were explanted in the operating room under general anaesthesia and with transoesophageal echocardiography guidance.
The incision was reopened. The Impella was weaned and stopped. The device was removed. The graft was cut, leaving a 1-cm stump attached to the axillary artery. Clamps were removed to allow flow through the graft in order to flush out potential clots. Then, the graft was oversewn, and the wound was closed. We did not experience any explant complications.
The Impella CP was implanted in acute cardiogenic shock cases in SCAI shock classification D, E (sliding or in extremis) and in patients on extracorporeal membrane oxygentation (ECMO) for left ventricular unloading. The Impella 5.0 was implanted in more stable (or stabilized) shock patients (SCAI shock C) as a bridge to next therapy. An Impella CP was also implanted via axillary cut-down in some patients who did not have adequate-sized axillary vessels to accommodate a 5.0.
Blood samples
All patients had an arterial blood gas and serum blood sample taken prior to Impella implantation. Biochemical parameters were also recorded 24 hours post–Impella implantation, to assess the progress of the patients.
Statistical analysis
All continuous variables were tested for normality using the Kolmogorov–Smirnov test. Data are presented as percentages, mean ± standard deviation (SD), or median (interquartile range). Differences in proportions were tested with the χ2 test or the Fisher exact test, and differences in continuous variables were tested with either an independent t test or the Wilcoxon signed rank sum test for parametric and nonparametric variables, respectively. The Kaplan–Meier estimate was used to calculate survival. Survival comparisons were made using the log-rank test.
Binary logistic regression analysis was used to identify independent predictors of short-term survival in the 2 groups. The primary outcome was chosen to be 30-day mortality post Impella implantation. All variables with P < 0.05 in univariable analysis were included in the regression model. Serum creatinine and alanine aminotransferase were not included in the model, as often they are not available pre-MCS implantation in an emergency setting.
A separate binary logistic regression model was performed for patients on only Impella MCS (ECMO excluded). All the variables with P < 0.05 (Supplemental Table S1) were included in the forward stepwise binary logistic regression model. Data were analyzed using SPSS version 26 (IBM, Armonk, NY).
Results
From April 2017 to October 2020, a total of 92 left-side Impella devices were implanted in our institution. Of those, 70 were Impella CP, and 22 were Impella 5.0.
A total of 17 (18.5%) patients sustained at least one cardiac arrest prior to Impella implantation. A total of 17 (18.5%) patients were already established on ECMO prior to Impella implantation, and of those, 6 had sustained a cardiac arrest prior to MCS. Six patients with an Impella CP were upgraded to an Impella 5.0 as a bridge to next therapy.
With the exception of patients on ECMO, the right ventricular function of patients who underwent pVAD implantation was no more than moderately impaired. Our experience with Impella RP in patients with severe right ventricular impairment has been described elsewhere.11
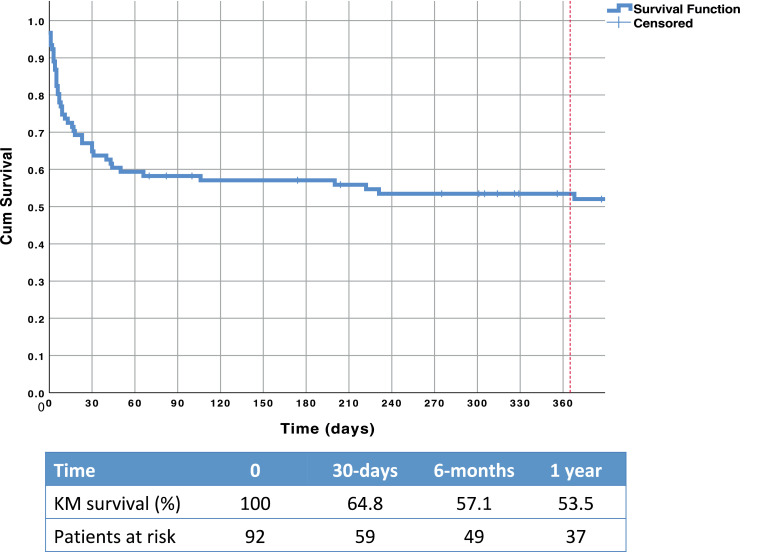
At 30-days post Impella implantation, 58 (63%) patients were still alive. At 1 year, an estimated 53.5% of patients were alive (Fig. 1). Baseline demographics of survivors vs those deceased are shown in Table 1. Supplemental Table S1 shows the baseline demographics for patients on only Impella MCS (ECMO patients excluded).
Figure 1.
Kaplan–Meier curve for long-term survival of cardiogenic shock patients on Impella (Abiomed, Danvers, MA) support. Cum, cumulative; KM, Kaplan–Meier.
Table 1.
Baseline demographics based on 30-day survival status
| Total(N = 92) | Alive(n = 58) | Deceased(n = 34) | P | |
|---|---|---|---|---|
| Age, y | 53.8 ± 14.9 | 53.6 ± 15.1 | 54.2 ± 14.7 | 0.853 |
| Male | 72 (78.3) | 42 (72.4) | 30 (88.2) | 0.076 |
| Diagnosis | 0.605 | |||
| ACS | 41 (44.6) | 26 (44.8) | 15 (44.1) | |
| Decompensated DCM | 26 (28.3) | 14 (24.1) | 12 (35.3) | |
| Decompensated ICM | 6 (6.5) | 5 (8.6) | 1 (2.9) | |
| ACS on top of end-stage CM | 7 (7.6) | 6 (10.3) | 1 (2.9) | |
| Fulminant myocarditis | 8 (8.7) | 4 (6.9) | 4 (11.8) | |
| Postcardiotomy | 2 (2.2) | 1 (1.7) | 1 (2.9) | |
| Arrhythmia on background TOF | 1 (1.1) | 1 (1.7) | 0 | |
| Sarcoidosis | 1 (1.1) | 1 (1.7) | 0 | |
| Hypertension | 26 (28.6) | 15 (25.9) | 33 (33.3) | 0.448 |
| Diabetes mellitus | 20 (22) | 12 (20.7) | 8 (24.2) | 0.694 |
| Current smoker | 14 (15.4) | 11 (19) | 3 (9.1) | 0.247 |
| Hypercholesterolemia | 15 (16.5) | 8 (13.8) | 7 (21.2) | 0.359 |
| Chronic kidney disease | 14 (15.4) | 7 (12.1) | 7 (21.2) | 0.245 |
| Previous stroke | 5 (5.5) | 3 (5.2) | 2 (6.1) | 0.858 |
| Previous myocardial infarction | 19 (20.7) | 11 (19) | 8 (23.5) | 0.602 |
| Previous CABG | 5 (5.4) | 5 (8.6) | 0 | 0.78 |
| Previous PCI | 16 (17.4) | 8 (13.8) | 8 (23.5) | 0.234 |
| ICD device | 0.249 | |||
| VVI-ICD | 2 (2.2) | 0 | 2 (5.9) | |
| DDD-ICD | 6 (6.5) | 3 (5.2) | 3 (8.8) | |
| CRT-D | 19 (20.7) | 12 (20.7) | 7 (20.6) | |
| Cardiac arrest | 17 (18.5) | 11 (19) | 6 (17.6) | 0.875 |
| Ventilation | 62 (67.4) | 36 (62.1) | 26 (76.5) | 0.155 |
| Ventilation for > 24 h | 30 (32.6) | 8 (13.8) | 22 (64.7) | < 0.001 |
| LVEF, % | 19 ± 13 | 21.8 ± 14.3 | 15.1 ± 9.6 | 0.009 |
| Previous ECMO | 17 (18.5) | 11 (19) | 6 (17.6) | 0.875 |
| Duration of support, d | 5 (1 to 12.8) | 7 (2 to 14) | 4.5 (1 to 7.3) | 0.04 |
| Impella-related complications | 0.075 | |||
| None | 62 (67.4) | 43 (74.1) | 19 (55.9) | |
| Ischemic limb | 2 (2.2) | 1 (1.7) | 1 (2.9) | |
| Bleeding (≥ BARC2) | 16 (17.4) | 8 (13.8) | 8 (23.5) | |
| Hemolysis | 7 (7.6) | 2 (3.4) | 5 (14.7) | |
| Malposition | 1 (1.1) | 0 | 1 (2.9) | |
| PCI during index admission | 40 (43.5) | 25 (43.1) | 15 (44.1) | 0.925 |
Values are n (%) or mean ± standard deviation, unless otherwise indicated. Bold indicates P < 0.05.
ACS, acute coronary syndrome; BARC, Bleeding Academic Research Consortium; CABG, coronary artery bypass graft; CM, cardiomyopathy; CRT-D, cardiac resynchronization therapy defibrillator; DCM, dilated cardiomyopathy; DDD, dual-chamber antibradycardia pacing; ECMO, extracorporeal membrane oxygenation; ICD: intra-cardiac defibrillator; ICM, ischemic cardiomyopathy; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; TOF, tetralogy of Fallot; VVI, single chamber ventricular pacemaker.
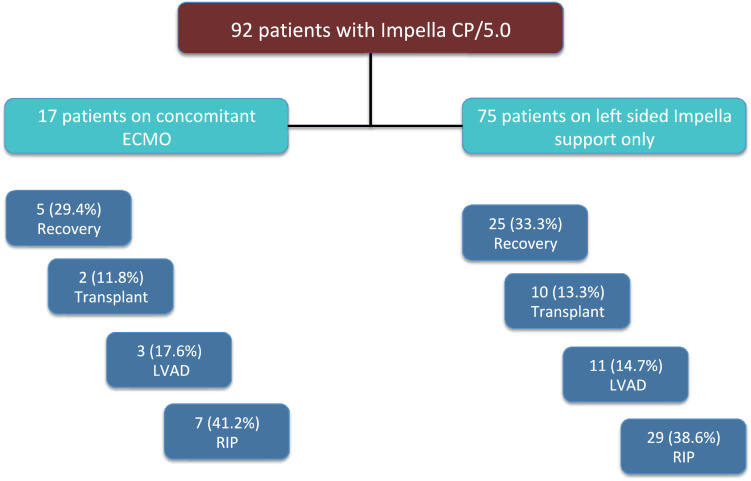
The Impella device bridged 30 patients to recovery (32.6%), 14 to a durable left ventricular assist device (15.2%), and 12 (13%) to heart transplants. One-year Kaplan–Meier estimated survival in this group who survived to next therapy was 80%. No significant differences in bridge to next therapy outcomes were observed between patients on concomitant ECMO and those on just left-sided Impella support (P = 0.978; Fig. 2)
Figure 2.
No significant differences in bridge-to-next-therapy outcomes were observed between patients on concomitant extracorporeal membrane oxygenation (ECMO) and those on just left-sided Impella (Abiomed, Danvers, MA) support. LVAD, left ventricular assist device; RIP, Rest in Peace (deceased).
The median duration of support in patients with Impella CP was 3 (range: 1 to 7) days, whereas in patients with an Impella 5.0, it was 16 (range: 8 to 30) days. The maximum duration of support with a 5.0 pump was 62 days as a bridge to heart transplantation. No difference was seen in the time from symptoms to support (5 [range: 1 to 12] vs 4 [range: 0.25 to 13]) days for 30-day survivors and the deceased, respectively, P = 0.568.
Deceased patients at 30 days had significantly worse left ventricular ejection fraction (LVEF) compared to survivors (Table 1). Seventeen (18.5%) patients had suffered cardiac arrest prior to Impella implantation, and the same number of patients were on ECMO. Around one third of patients (32.6%) were ventilated for over 24 hours. Prolonged ventilation was associated with increased 30-day mortality, P < 0.001 (Table 1; Fig. 3). There was a trend for males to predominate among deceased patients at 30 days. A similar trend was seen for patients who had an Impella-related complication (Table 1). Patients who underwent percutaneous coronary intervention did not demonstrate a survival benefit.
Figure 3.
Kaplan–Meier curves for patients on Impella (Abiomed, Danvers, MA) support with cardiogenic shock with and without prolonged invasive ventilation (> 24 hours). Comparisons were made using the log-rank test. Cum, cumulative.
Biochemical markers and their association with 30-day mortality is shown in Table 2. Pre-Impella implantation serum levels of lactate, creatinine, and alanine aminotransferase showed a significant association with 30-day mortality. There was no difference in lactate improvement within 24 hours in the 2 groups (survivors vs deceased).
Table 2.
Biochemical predictors of 30-day mortality
| Parameters | Timing pre- or 24-h post Impella* | Alive (n = 58) | Deceased (n = 34) | P |
|---|---|---|---|---|
| pH | Pre | 7.42 (7.31 to 7.44) | 7.36 (7.28 to 7.42) | 0.13 |
| Post | 7.44 (7.4 to 7.48) | 7.41 (7.37 to 7.46) | 0.875 | |
| HCO3 | Pre | 23.9 ± 4.5 | 22.1 ± 3.7 | 0.073 |
| Post | 25.9 (24.1 to 27.9) | 25.5 (21.5 to 26.6) | 0.408 | |
| pO2 | Pre | 11.6 (8.2 to 11.6) | 12.8 (9.64 to 18) | 0.408 |
| Post | 11.7 (9.1 to 13.5) | 13.3 (11.5 to 16.4) | 0.497 | |
| pCO2 | Pre | 5.1 (4.5 to 5.7) | 5.5 (4.2 to 6.2) | 0.250 |
| Post | 5.2 (4.7 to 5.7) | 5.3 (4.4 to 5.7) | 0.712 | |
| Base excess (mmol/L) | Pre | –0.71 ± 6.06 | –3.16 ± 5.63 | 0.068 |
| Post | 2.37 ± 3.96 | 0.7 ± 5.40 | 0.107 | |
| Lactate (mmol/L) | Pre | 1.45 (1.08 to 3.53) | 2.8 (1.6 to 5.4) | 0.012 |
| Post | 1.05 (0.7 to 1.63) | 1.6 (1.1 to 2.6) | 0.011 | |
| Difference | 0.4 (0 to 1.65) | 0.8 (-0.25 to 3) | 0.481 | |
| Creatinine (μmol/L) | Pre | 122.6 ± 63.3 | 174.6 ± 74.5 | 0.001 |
| Post | 129.1 ± 54.6 | 157.9 ± 57.2 | 0.024 | |
| Urea (mg/dL) | Pre | 9.6 (6.1 to 14.8) | 12.1 (7.7 to 20.5) | 0.081 |
| Post | 9.9 (6.1 to 14.8) | 10.4 (8.3 to 17.7) | 0.755 | |
| ALT (U/L) | Pre | 48 (31 to 181) | 173 (71 to 968) | 0.044 |
| Post | 74 (32 to 161.5) | 126 (38 to 1120) | 0.057 | |
| ALP (U/L) | Pre | 86.5 (61 to 122.8) | 78 (69 to 125) | 1.00 |
| Post | 70.5 (48.3 to 96.3) | 67 (49 to 95) | 0.634 | |
| Bilirubin (umol/L) | Pre | 18 (12.8 to 41.5) | 19 (11 to 54) | 0.859 |
| Post | 34.5 (14.5 to 74.3) | 33 (19 to 80) | 1.000 |
Bold indicates P < 0.05.
ALP, alkaline phosphatase; ALT, alanine aminotransferase.
*Pre-Impella (Abiomed, Danvers, MA) implantation samples reflect the last sample taken prior to cannulation. Post-Impella biochemistry reflects samples taken 24 hours after Impella implantation.
Independent predictors of 30-day mortality
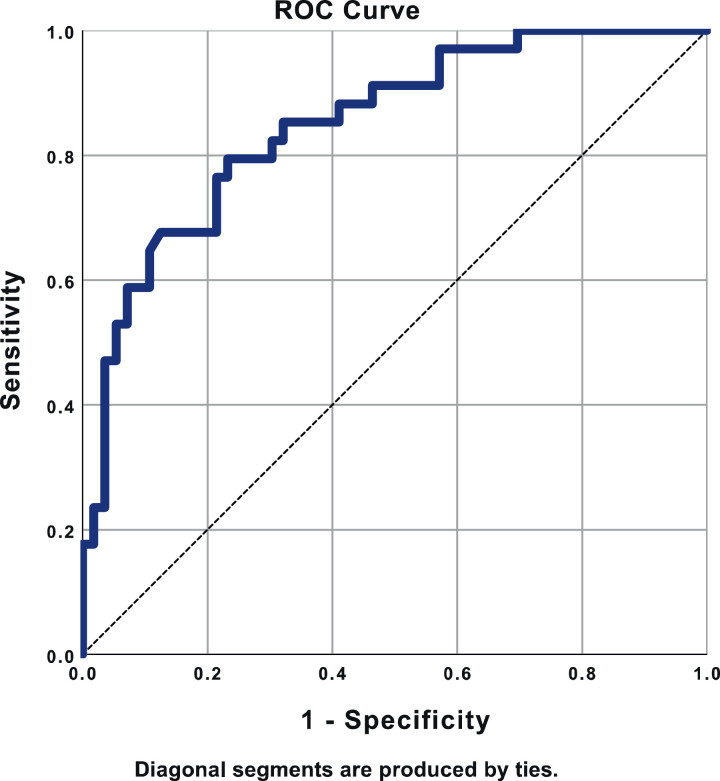
In the multivariable analysis, the independent predictors of 30-day mortality were serum lactate pre Impella implantation (odds ratio [OR] 1.19 per unit increase, 95% confidence interval [CI] 1.01 to 1.4, P = 0.041), baseline LVEF (OR per 10-unit drop in ejection fraction 2.14, 95% CI 1.23 to 3.72, P = 0.007) and being ventilated for over 24 hours (OR 13.8, 95% CI 4.06 to 46.7, P < 0.001). The area under the curve in the ROC curve was 0.850 (95% CI 0.769 to 0.930), P < 0.001 (Fig. 4).
Figure 4.
Receiver operator characteristic (ROC) curve demonstrating excellent discrimination for prediction of 30-day mortality when using the predicted probabilities from the logistic regression model using 3 parameters: serum lactate level, left ventricular ejection fraction, and prolonged ventilation (> 24 hours).
For patients on only Impella MCS (excluding ECMO patients), the independent predictors of 30-day mortality were similar. These included prolonged ventilation > 24 hours (OR 28.7, 95% CI 5.0 to 163.3, P < 0.001), baseline LVEF (OR per 10-unit drop in ejection fraction 3.1, 95% CI 1.3 to 7.2, P = 0.011), and serum lactate level (OR 1.5, 95% CI 1.08 to 2.09, P = 0.016).
Discussion
In the current study, the main predictors of 30-day mortality in patients requiring Impella hemodynamic support were baseline serum lactate level, LVEF prior to Impella implantation, and the need for prolonged ventilation (> 24 hours). The prediction model showed excellent discrimination, with an area under the curve of 0.85.
In the current study, our 30-day mortality was 37%, which is lower than the one reported in contemporary cohorts9,12, 13, 14, 15 (Table 3). However, our patient population is rather unique, as it consists of several patients with decompensated end-stage cardiomyopathy rather than acute coronary syndrome–related CS. As a result, the only exit strategy for such patients is left-ventricular assist device, transplantation, or palliation (Table 4).
Table 3.
30-day mortality in contemporary studies of cardiogenic shock using Impella (Abiomed, Danvers, MA) devices
| Study (recruitment years) | N | Age, years | Male gender (%) | Device (%) | ACS (%) | Baseline lactate | LVEF (%) pre Impella | Cardiac arrest (%) | Invasive ventilation(%) | Mortality (%) | Independent predictors | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rohm 201916 (2011-2018) |
204 | 60.3 ± 12 | 71.1 | Impella 2.5 (27) Impella CP (70) Impella 5.0 (3) |
84 | 3.7 (1.3–6.1) 5.2 ± 4.1 |
13.2 | 77.9 | In-hospital (45.1) | ? | |||
| Ouweneel 201910 (2004-2016) |
112 | 60.1 ± 10.6 | 80.4 | Impella 2.5 (35.7) Impella CP (46.4) Impella 5.0 (17.9) |
100 | 6.2 (3.9–9.7) |
59.8 | 87.5 | 30-day (56.2) In-hospital (65) |
pH | |||
| Gaudard 20159 (2008-2013) |
40 | 57 (48–63) | 87.5 | Impella 5.0 (62.5) ECMO+Impella 5.0 (37.5) |
43 | 3.8 (1.7–5.9) |
23 | 73 | 28-day (35) |
Inotropic score, SAPS II and ACS | |||
| Lauten 201313 (2005-2010) |
120 | 63.6 ± 12.3 | 81.7 | Impella 2.5 (100) | 100 | 5.8 ± 5 | 27 ± 11 | 40.8 | 69.2 | 30-day (64.2) |
Age > 65 Lactate > 3.8 |
||
| Jensen 201814 (2013-2017) |
79 | 63 ± 11 | 84 | Impella CP (92) Impella 5.0 (9) Impella RP (3) |
100 | 7.6 ± 6 | 17 ± 12 | 37 | 86 | 30-day (~33) |
Lactate | ||
| Alushi 201915 (2011-2017) |
62 | 73 (62-79) | 71 | Impella 2.5 (100) | 100 | 6.6 (3.5-10.6) |
28 (20-35) | 61 | 96 | 30-day (52) |
CPR | ||
| Sieweke 201817 (2013-2016) |
61 | 62.3 ± 12.7 | 73.8 | Impella CP (100) | 75 | ~7.5 (from graph) | 61 | 85 | 30-day (48) |
||||
| Monteagudo-Vela and Panoulas 2020 (2017-2020) |
92 | 53.8 ± 14.9 | 78.3 | Impella CP (76) Impella 5.0 (24) |
44.6 | 1.75 (1.1–3.8) |
19 ± 13 | 18.5 | 67.4 | 30-day (37) | Lactate, ventilation > 24h, LVEF | ||
Age and lactate presented as means ± standard deviations or medians (interquartile ranges), the remaining parameters are reported as percentages.
ACS, acute coronary syndrome; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; LVEF, left ventricular ejection fraction; SAPS, simplified acute physiology score.
Table 4.
Next therapy following Impella (Abiomed, Danvers, MA) implantation
| Next therapy | Impella CP(n = 70) | Impella 5.0(n = 22) | Total number(N = 92) |
|---|---|---|---|
| Short-term MCS (Puralev, Levitronix, Zurich, Switzerland) | 1 (1.4) | 0 | 1 (1.1) |
| LVAD | 9 (12.9) | 5 (22.7) | 14 (15.2) |
| Transplanted | 5 (7.1) | 7 (31.8) | 12 (13) |
| Recovery | 28 (40) | 2 (9.1) | 30 (32.6) |
| Palliation | 27 (38.6) | 8 (36.4) | 35 (38) |
Values are n (%).
LVAD, left ventricular assist device; MCS, mechanical circulatory support.
In a recent study by Rohm et al.16 on 204 CS patients treated with left-sided Impella devices (2.5, CP, and 5.0), the reported in-hospital mortality was 45.1%. In that study, univariate predictors of mortality included raised lactate, lower pH and serum CO2 levels, alongside increasing numbers of inotropes. No multivariable regression analysis was performed in this study, however, so it is not possible to ascertain independent predictors of mortality. Encouragingly, however, and in accordance to our study, hyperlactemia and prolonged ventilation were significant predictors.
In several other studies,13,14,17 lactate features as a strong independent predictor of short-term mortality in patients with Impella MCS, concurring with our findings. Of interest, lactate clearance within 12 hours was similar between survivors and the deceased in our study. In the study by Sieweke,17 even though lactate clearance was more rapid among survivors at 4 hours post–Impella implantation, the difference in clearance at 12 hours was nonsignificant between the 2 groups (survivors vs deceased at 30 days). This result illustrates that even though Impella MCS produces the desired hemodynamic effect (improved perfusion), it cannot guarantee a positive outcome for patients who may have already been too far down the CS spiral. Furthermore, we need to highlight the fact that with the lack of organs in the current climate, Impella-supported patients often remain on the super-urgent waiting lists for over 4 weeks, thus increasing the chances of a terminal event (intracranial hemorrhage, stroke) prior to destination therapy.
Prolonged ventilation (> 24 hours) appears to be associated with very low survival at 30 days. This association is partially explained by that fact that these patients belong to the sickest end of the spectrum, often having prolonged cardiac arrests with neurologic damage, having complications requiring surgical intervention/re-exploration, and suffering from sepsis and/or refractory pulmonary edema. No matter what the underlying cause, prolonged intubation (> 24 hours) is associated with a very poor (~30%) 30-day survival in our series. Hence, physicians need to be more vigilant when caring for this patient group, with escalation decisions taken in a timely fashion, and appropriate withdrawal of care in those with irreversible neurologic damage.
Another independent predictor of mortality in our study was baseline LVEF. Baseline LVEF is often not recorded in patients admitted with CS when emergency angiography is advocated. In one of the few studies including LVEF in the baseline assessment,18 the use of systolic blood pressure, LVEF, and lactate as continuous variables led to an area under the curve of 0.88 (P < 0.001) for prediction of 30-day mortality in CS patients, due to acute coronary syndrome. In our data, a 10% drop in LVEF was associated with a doubling of the odds for mortality at 30 days, highlighting the importance of including bedside echocardiography in the initial assessment of patients with CS or impending CS (stage B in the SCAI classification6).
In our cohort, there was a significant difference in pre–Impella implantation renal and liver function parameters. This finding suggests an adverse impact on survival once multi-organ failure has settled in, a result in line with those of previous CS studies.8 Use of percutaneous MCS can stabilize patients, allowing for improvement of end-organ function, thus reducing the risk of the next intervention.7 In our series, we report a total of 17 patients with concomitant venoarterial ECMO and Impella. Recent propensity-matched studies19,20 have shown improved survival, and outcomes in ECMO patients for whom an Impella was used to unload the left ventricle despite a significant increase in device-related complications.
As with every retrospective registry, this one is not without its limitations. The heterogeneity of our patient population does not allow for robust conclusions in disease-specific groups, but it does allow for a more generic approach in CS patients due to different etiologies. Future randomized controlled studies4 are urgently needed to answer whether pVAD implantation offers a survival benefit in patients presenting with CS.
Conclusion
In the current retrospective registry of CS patients requiring Impella pVAD implantation, independent 30-day mortality predictors included baseline serum lactate level, baseline LVEF, and prolonged invasive ventilation (> 24 hours). These parameters could identify patients who would benefit from earlier MCS escalation or neurologic assessment to inform withdrawal decisions..
Acknowledgments
Acknowledgements
We thank all the teams of Harefield Hospital (transplant cardiologists, interventional cardiologists, intesivists, transplant surgeons, nurses from all disciplines, perfusionists, and ventricular assist device nurses) who constitute the CS team caring day and night for these particularly complex patients.
Funding Sources
The authors would like to thank the Cardiogenic Shock Fund (Royal Brompton and Harefield Charity) for the ongoing support in research and education.
Disclosures
V.P. is in receipt of consultancy fees and honoraria from Abiomed. M.M.-V. has no conflicts of interest to disclose.
Footnotes
Ethics Statement: Per consultation with our local research ethics committee, no informed consent was required, as the study was part of an ongoing audit and all data were pseudo-anonymized.
See page 1008 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.03.008.
Appendix. Supplementary materials
References
- 1.Shaefi S, O'Gara B, Kociol RD. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burzotta F, Trani C, Doshi SN. Impella ventricular support in clinical practice: collaborative viewpoint from a European expert user group. Int J Cardiol. 2015;201:684–691. doi: 10.1016/j.ijcard.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 3.Kapur NK, Paruchuri V, Urbano-Morales JA. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation. 2013;128:328–336. doi: 10.1161/CIRCULATIONAHA.112.000029. [DOI] [PubMed] [Google Scholar]
- 4.Udesen NJ, Moller JE, Lindholm MG. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am Heart J. 2019;214:60–68. doi: 10.1016/j.ahj.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Ouweneel DM, Eriksen E, Sjauw KD. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69:278–287. doi: 10.1016/j.jacc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Baran DA, Grines CL, Bailey S. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 7.Monteagudo-Vela M, Simon A, Riesgo Gil F. Clinical Indications of IMPELLA short-term mechanical circulatory support in a tertiary centre. Cardiovasc Revasc Med. 2020;21:629–637. doi: 10.1016/j.carrev.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Jentzer JC, van Diepen S, Barsness GW. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74:2117–2128. doi: 10.1016/j.jacc.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 9.Gaudard P, Mourad M, Eliet J. Management and outcome of patients supported with Impella 5.0 for refractory cardiogenic shock. Crit Care. 2015;19:363. doi: 10.1186/s13054-015-1073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouweneel DM, de Brabander J, Karami M. Real-life use of left ventricular circulatory support with Impella in cardiogenic shock after acute myocardial infarction: 12 years AMC experience. Eur Heart J Acute Cardiovasc Care. 2019;8:338–349. doi: 10.1177/2048872618805486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monteagudo-Vela M, Simon A, Panoulas V. Initial experience with Impella RP in a quaternary transplant center. Artif Organs. 2020;44:473–477. doi: 10.1111/aor.13610. [DOI] [PubMed] [Google Scholar]
- 12.Sieweke JT, Pfeffer TJ, Berliner D. Cardiogenic shock complicating peripartum cardiomyopathy: importance of early left ventricular unloading and bromocriptine therapy. Eur Heart J Acute Cardiovasc Care. 2018 doi: 10.1177/2048872618777876. [DOI] [PubMed] [Google Scholar]
- 13.Lauten A, Engstrom AE, Jung C. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013;6:23–30. doi: 10.1161/CIRCHEARTFAILURE.112.967224. [DOI] [PubMed] [Google Scholar]
- 14.Jensen PB, Kann SH, Veien KT. Single-centre experience with the Impella CP, 5.0 and RP in 109 consecutive patients with profound cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2018;7:53–61. doi: 10.1177/2048872617743194. [DOI] [PubMed] [Google Scholar]
- 15.Alushi B, Douedari A, Froehlig G. Impella versus IABP in acute myocardial infarction complicated by cardiogenic shock. Open Heart. 2019;6 doi: 10.1136/openhrt-2018-000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohm CL, Gadidov B, Leitson M, Ray HE, Prasad R. Predictors of mortality and outcomes of acute severe cardiogenic shock treated with the Impella device. Am J Cardiol. 2019;124:499–504. doi: 10.1016/j.amjcard.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 17.Sieweke JT, Berliner D, Tongers J. Mortality in patients with cardiogenic shock treated with the Impella CP microaxial pump for isolated left ventricular failure. Eur Heart J Acute Cardiovasc Care. 2020;9:138–1348. doi: 10.1177/2048872618757393. [DOI] [PubMed] [Google Scholar]
- 18.Frydland M, Moller JE, Wiberg S. Lactate is a prognostic factor in patients admitted with suspected ST-elevation myocardial infarction. Shock. 2019;51:321–327. doi: 10.1097/SHK.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 19.Pappalardo F, Schulte C, Pieri M. Concomitant implantation of Impella((R)) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19:404–412. doi: 10.1002/ejhf.668. [DOI] [PubMed] [Google Scholar]
- 20.Schrage B, Becher PM, Bernhardt A. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020;142:2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.