Abstract
Background
Brain amyloidosis does not invariably predict dementia. We hypothesized that high soluble 42-amino acid β amyloid (Aβ42) peptide levels are associated with normal cognition and hippocampal volume despite increasing brain amyloidosis.
Methods
This cross-sectional study of 598 amyloid-positive participants in the Alzheimer's Disease Neuroimaging Initiative cohort examined whether levels of soluble Aβ42 are higher in amyloid-positive normal cognition (NC) individuals compared to mild cognitive impairment (MCI) and Alzheimer's disease (AD) and whether this relationship applies to neuropsychological assessments and hippocampal volume measured within the same year. All subjects were evaluated between June 2010 and February 2019. Brain amyloid positivity was defined as positron emission tomography-based standard uptake value ratio (SUVR) ≥1.08 for [18] F-florbetaben or 1.11 for [18]F-florbetapir, with higher SUVR indicating more brain amyloidosis. Analyses were adjusted for age, sex, education, APOE4, p-tau, t-tau, and centiloids levels.
Findings
Higher soluble Aβ42 levels were observed in NC (864.00 pg/ml) than in MCI (768.60 pg/ml) or AD (617.46 pg/ml), with the relationship between NC, MCI, and AD maintained across all amyloid tertiles. In adjusted analysis, there was a larger absolute effect size of soluble Aβ42 than SUVR for NC (0.82 vs. 0.40) and MCI (0.60 vs. 0.26) versus AD. Each standard deviation increase in Aβ42 was associated with greater odds of NC than AD (adjusted odds ratio, 6.26; p < 0.001) or MCI (1.42; p = 0.006). Higher soluble Aβ42 levels were also associated with better neuropsychological function and larger hippocampal volume.
Interpretation
Normal cognition and hippocampal volume are associated with preservation of high soluble Aβ42 levels despite increasing brain amyloidosis.
Funding
Please refer to the Funding section at the end of the article.
Keywords: Β-amyloid, Alzheimer's disease, Hippocampus, Atrophy
Research in context.
Evidence before this study
Aggregation of the 42-amino acid β amyloid (Aβ42) peptide into amyloids is conceived as the pathogenic trigger of a cascade leading to tau accumulation into neurofibrillary tangles, neuronal loss, and clinical dementia. However, while most of the 40 anti-amyloid interventions examined over the past two decades have successfully reduced the burden of brain amyloid, corresponding clinical benefits have not materialized. Moreover, brain amyloidosis does not invariably predict dementia: by the age of 85, the prevalence of brain amyloidosis is approximately 60% whereas that of dementia only 10%. No prior study has assessed in humans the alternative loss-of-function amyloid hypothesis according to which high levels of natively-folded, soluble Aβ42 are associated with normal cognition in the setting of brain amyloidosis.
Added value of this study
This is the first mechanistic study testing whether high levels of soluble Aβ42, as measured in cerebrospinal fluid, are associated with normal cognition in individuals with brain amyloidosis. In a cross-sectional analysis of 598 brain amyloid-positive individuals participating in the Alzheimer's Disease Neuroimaging Initiative, higher levels of soluble Aβ42 were associated with normal cognition compared to mild cognitive impairment and Alzheimer's disease across each tertile of brain amyloidosis. Higher soluble Aβ42 levels were also associated with better neuropsychological performance and larger hippocampal volume, with a larger effect size yielded by changes in soluble Aβ42 than in insoluble (brain amyloid) Aβ42.
Implications of all the available evidence
This analysis provides a tentative solution to the paradox of normal cognition in the setting of brain amyloidosis, suggesting that soluble Aβ42 levels above 800 pg/ml are associated with normal cognition regardless of (and despite increasing) brain amyloid burden. Future disease-modifying efforts may warrant the evaluation of therapeutic strategies that increase the levels of soluble Aβ42 in amyloid PET-positive individuals with mild cognitive impairment or dementia and cerebrospinal Aβ42 levels below 800 pg/ml.
Alt-text: Unlabelled box
1. Introduction
The amyloid cascade hypothesis has been the prevailing paradigm for understanding Alzheimer's disease (AD) pathogenesis. According to this hypothesis, the fibrillogenic 42-amino acid β amyloid peptide (Aβ42) aggregates into toxic β-sheet rich assemblies, triggering a cascade of neurotoxic events, including intracellular tau accumulation as neurofibrillary tangles and neuroinflammation, which ultimately result in neurodegeneration and subsequent cognitive impairment [1]. This toxic gain-of-function hypothesis must be counterbalanced by the loss of normal proteins when undergoing aggregation. Proteins and peptides carry out their normal functions in the soluble state; misfolding into insoluble fibers with the characteristic cross-β conformation, known as amyloids, renders them non-functional [2]. Examples of loss of function of normal proteins when transformed into amyloids include loss of glycemic control in insulin-derived amyloidosis and loss of p53 tumor suppression function in TP53 mutations [3,4].
Disease-modifying therapeutic efforts in AD have aimed at reducing the insoluble Aβ42 peptide, assuming its toxicity, rather than increasing its soluble precursor, which is necessary for many essential functions in the brain, such as copper homeostasis, regulation of mitochondrial function, brain development, and neuroprotection [5]. In fact, as insoluble Aβ42 accumulates with age in the brain, there is a corresponding decrease of soluble Aβ42, as measured in the cerebrospinal fluid (CSF) of patients with sporadic and familial AD (due to PSEN1, PSEN2, or APP genetic mutations) [6]. Several lines of evidence suggest the need for reexamining the rationale for reducing insoluble Aβ42, including the poor correlation between brain amyloid plaque burden and disease frequency, severity, or neuronal loss [7], and the identification of brain amyloidosis in about 30 to 40% of individuals in their 70s and in 50% of centenarians without cognitive impairment [8,9]. Moreover, most experimental anti-amyloid interventions have successfully reduced the burden of brain amyloidosis without corresponding clinical benefits [10]. Finally, knock-down APP animal models have demonstrated behavioral and degenerative phenotypes in the absence of amyloidosis.[5]
The National Institute on Aging and the Alzheimer's Association (NIA/AA) framework for the diagnosis of AD requires the demonstration of high insoluble Aβ42 in the brain measured via an amyloid radiotracer uptake by positron emission tomography (PET), or of low soluble Aβ42, measured in CSF [11]. According to this framework, the identification of brain amyloidosis places individuals on a clinical continuum to AD dementia. However, most amyloid PET-positive individuals will not develop dementia during their lifetimes: between the ages of 65 and 85 years, the approximate prevalence of brain amyloidosis increases from 20% to 60%; the prevalence of AD reaches 10% by 85, five times lower than predicted if amyloid were toxic [12].
The accumulation of Aβ42 into amyloids usually leads to reduced levels of the soluble Aβ42 pool [13], although with wide variability of concordance between the increase in amyloidosis and the reduction in soluble Aβ42 [14]. The standardized quantification of CSF Aβ42 is the only available indirect measure of soluble Aβ42 in the human brain and has been shown to linearly correlate with the interstitial Aβ42 concentration measured by in-vivo microdialysis [15]. Therefore, we sought to test the hypothesis that amyloid-positive individuals with normal cognition (NC) and brain volume have higher levels of CSF Aβ42 compared to those with mild cognitive impairment (MCI) and AD. We also evaluated whether increasing levels of CSF Aβ42 in amyloid PET-positive individuals were associated with better neuropsychological performance and larger hippocampal volumes and had an attenuating role in the NC-to-AD phenotype conversion.
2. Methods
2.1. Overview
The Alzheimer's Disease Neuroimaging Initiative (ADNI) is a longitudinal study of over 2700 participants aged between 55 and 90 years (http://adni.loni.usc.edu/) (Supplementary materials 1.1–1.3). As the longitudinal data available for brain amyloid-positive individuals included a small number of conversions from NC to MCI or to AD only a cross-sectional analysis was feasible, although we report the available conversion data from NC to MCI and AD among this cohort. We only included amyloid PET-positive ADNI participants for whom the ascertainment of NC, MCI, or AD, as well as their CSF collection, were made within one year from a PET scan identifying brain amyloidosis [16]. When more than one CSF specimen was collected within 1 year from the positive PET scan, we selected the visits with the shortest time between CSF and PET in order to narrow the temporal association between PET and CSF data. NC subjects included participants with and without subjective memory concerns but no deficits on neuropsychological evaluation. Brain magnetic resonance imaging (MRI) and neuropsychological assessments were also included if obtained within 1 year from the PET- and CSF-qualifying criteria for inclusion. All subjects were evaluated between June 2010 and February 2019. Amyloid positivity was defined by PET data according to ADNI guidelines (http://adni.loni.usc.edu/) as a standard uptake value ratio (SUVR) at or above 1.08 for [18]F-florbetaben or 1.11 for [18]F-florbetapir, with higher SUVR indicating greater amyloid plaque burden. The SUVR was quantified across cortical gray matter, normalized by the whole cerebellum and divided into SUVR tertiles. Details of PET acquisition are described in previous publications and on the ADNI website (www.adni-info.org). Given the analysis of two amyloid PET-tracers, SUVR levels were converted in centiloids (CL) using the specific equation for each tracer as provided by ADNI.
2.2. Neuropsychological evaluation
We extracted composite scores for memory (ADNI-MEM) and executive function (ADNI-EF) [17,18]. ADNI-MEM included memory items from the Mini-Mental State Examination and the Alzheimer's Disease Assessment Scale-Cognitive Subscale, immediate and delayed scores from the Rey Auditory Verbal Learning Test, and recall from Logical Memory I of the Wechsler Memory Scale–Revised (WMS-R); ADNI-EF included the Digit Symbol Substitution Test, the WMS-R Digit Span, Trail Making Test (A & B), category fluency tests, and the clock drawing test.
2.3. CSF biomarkers
CSF Aβ42, phospho-Tau (p-tau), and total-Tau (t-tau) were analyzed by electrochemiluminescence immunoassays (ECLIA) Elecsys on a fully automated Elecsys cobas e 601 instrument. Levels of CSF biomarkers are expressed in pg/ml. Given ascertainment of batch effects, Aβ42, p-tau, and t-tau levels determined by the batch run in 2019 were converted to 2016/2017 levels, using the regression equation provided by ADNI. No corrections are available for flow rate or Aβ production and degradation rate.
2.4. Hippocampal volume
ADNI participants completed a 1.5 Tesla or 3.0 Tesla T1-weighted MRI scan. The description of MRI acquisition parameters and processing are available on surfer.nmr.mgh.harvard.edu. We extracted the left and right hippocampal volumes (mm3) (dictionary code: ST29SV and ST88SV) and calculated the average between left and right hippocampus. Hippocampal volume was adjusted for intracranial volume (ICV). We included only brain MRI data that met the quality assessments in both hippocampi.
2.5. Aims, sample size and statistical analysis
To test the hypothesis that higher levels of CSF Aβ42 are observed in amyloid PET-positive NC individuals compared to AD or MCI as well as in MCI compared to AD, we applied one-way analysis of variance and adjusted linear regression analyses followed by Tukey's multiple comparisons. In addition, the levels of CSF Aβ42 were compared between NC and MCI, between MCI and AD, and between NC and AD at similar levels of brain amyloid burden quantified into SUVR tertiles (≤1.29, 1.30–1.46, and ≥1.47) and converted into CL tertiles (21.75–58.9; 59.0–89.9; ≥90.0), using nonparametric bootstrap t tests [19] and multiple linear regression analysis after adjusting for multiple comparisons with Tukey's test. We also determined the adjusted associations of CSF Aβ42 levels with diagnostic categories using multiple logistic regression analyses and with memory and executive function measures (ADNI-MEM and ADNI-EF) and hippocampal volume using multiple linear regression analyses. The adjusted associations were summarized using adjusted odd ratios (OR) or regression coefficients (RC) of standardized Aβ42 and CL levels with 95% confidence intervals and p-values. The adjusted mean difference was computed by estimating the associated values for Aβ42 levels according to each cognitive diagnosis after adjusting for all covariates at their average levels using multiple linear regression analysis. A Tukey's multiple comparison test was applied in posthoc analysis of CSF biomarkers and amyloid burden comparisons between the three cognitive groups following adjusted linear regression analysis. All analyses were adjusted for age, sex, education, APOE4 carrier status, CL levels, p-tau levels, and t-tau levels. However, CL was not adjusted in the SUVR or CL tertile analysis. We provide additional details including sample size computation for each aim, as well as analyses of all secondary and exploratory outcomes, in the supplementary materials (Supplementary materials 1.4–1.5).
2.6. Role of the funding source
The study funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
2.7. Ethics approval
The study protocol for ADNI was approved by local ethical committees of all participating institutions and all participants signed informed consent.
3. Results
Of 2740 participants in the entire ADNI cohort, 598 subjects met the inclusion criteria of amyloid PET-positivity (540 by [18]F-florbetapir and 58 by [18]F-florbetaben). Of these, 596 had CSF Aβ42 assays and 493 brain MRI obtained within 1 year from the qualifying PET scan and available for analyses (Table 1). The mean follow-up for these ADNI participants was 2.5 ± 2.20 years (range 0–9 years). Subjects with AD were more likely to be male (p < 0.001), APOE4 carriers (p < 0.001), have fewer years of education (p = 0.048), lower Aβ40 (p = 0.020) and higher p-tau (p < 0.001), t-tau (p < 0.001), amyloid PET-SUVR (p < 0.001), and amyloid PET-CL (p < 0.001) compared to amyloid PET-positive NC and MCI subjects (Table 2).
Table 1.
Characteristics of the amyloid-PET positive study cohort.
| Variable | N | Summary measures* |
|---|---|---|
| Age (years) | 598 | 73.6 (7.0) |
| Education (years) | 596 | 16.1 (2.7) |
| Sex (female) (%) | 598 | 302 (50.5%) |
| APOE4 (%) | 598 | 376 (62.9%) |
| Aβ42 (pg/ml) | 596 | 748.9 (307.4) |
| Aβ40 (pg/ml) | 130 | 18,641.7 (5479.9) |
| p-tau (pg/ml) | 596 | 33.3 (15.3) |
| t-tau (pg/ml) | 597 | 335.6 (138.5) |
| ADNI-MEM (z-score) | 597 | 0.1 (0.9) |
| ADNI-EF (z-score) | 593 | 0.0 (1.1) |
| Amyloid PET (SUVR) | 598 | 1.39 (0.18) |
| Amyloid PET (CL) | 598 | 76.4 (34.7) |
| L hippocampus (mm3) | 493 | 3307.9 (561.5) |
| R hippocampus (mm3) | 493 | 3385.8 (582.7) |
| B hippocampi (mm3) | 493 | 3346.8 (552.2) |
*Data are expressed in mean (standard deviation) or frequency (%). ADNI: Alzheimer's Disease Neuroimaging Initiative; ADNI-MEM: ADNI memory score; ADNI-EF: ADNI executive function score; APOE4: APOE ε4 allele; Aβ42: 42-amino acid β amyloid peptide; Aβ40: 40-amino acid β amyloid peptide; CL: centiloid; p-tau: phopsho-Tau; t-tau: total-Tau; pg: picogram; ml: milliliters; PET: Positron Emission Tomography; SUVR: standard uptake value ratio; L: left; R: right; B: average bilateral volume.; mm: millimeters.
Table 2.
Cerebrospinal fluid markers and baseline characteristics across cognitive categories in amyloid-PET positive individuals.
| Cognitive categories | |||||||
|---|---|---|---|---|---|---|---|
| Variable | NC | N | MCI | N | AD | N | p-value |
| Aβ42 (pg/ml)* | 898.7 (367.4) | 155 | 755.4 (283.6) | 270 | 602.8 (198.2) | 171 | <0.001 |
| Aβ40 (pg/ml) | 19,656.1 (5407.0) | 75 | 17,953.3 (5293.0) | 36 | 15,941.6 (5254.7) | 19 | 0.020 |
| p-tau (pg/ml) | 28.0 (12.2) | 155 | 33.3 (15.7) | 270 | 38.1 (15.6) | 171 | <0.001 |
| t-tau (pg/ml) | 288.5 (109.1) | 155 | 332.1 (138.0) | 271 | 383.9 (147.9) | 171 | <0.001 |
| APOE4 (%) | 50.3% | 78 | 63.8% | 173 | 72.7% | 125 | <0.001 |
| Age (years) | 74.4 (6.3) | 155 | 73.4 (6.5) | 271 | 73.0 (8.2) | 172 | 0.17 |
| Education (years) | 16.5 (2.5) | 155 | 16.0 (2.8) | 269 | 15.8 (2.6) | 172 | 0.048 |
| Sex (female) (%) | 66.5% | 103 | 45.0% | 122 | 44.8% | 77 | <0.001 |
| Amyloid PET (SUVR) | 1.32 (0.18) | 155 | 1.38 (0.17) | 271 | 1.46 (0.17) | 172 | <0.001 |
| Amyloid PET (CL) | 62.3 (33.3) | 155 | 75.5 (33.2) | 271 | 90.5 (32.8) | 172 | <0.001 |
Data are expressed in mean (standard deviation) or frequency (%). APOE4: APOE ε4 allele; Aβ42: 42-amino acid β amyloid peptide; Aβ40: 40-amino acid β amyloid peptide; p-tau: phospho-Tau; t-tau: total-Tau; pg: picogram; ml: milliliters; PET: Positron Emission Tomography; SUVR: standard uptake value ratio; CL: centiloid; NC: normal cognition; MCI: mild cognitive impairment; AD: Alzheimer's disease; N/A: not available. *Post-hoc analysis: NC compared to AD (p < 0.001), NC compared to MCI (p < 0.001), and MCI compared to AD (p < 0.001).
3.1. CSF Aβ42 in cognition of amyloid PET-positive individuals
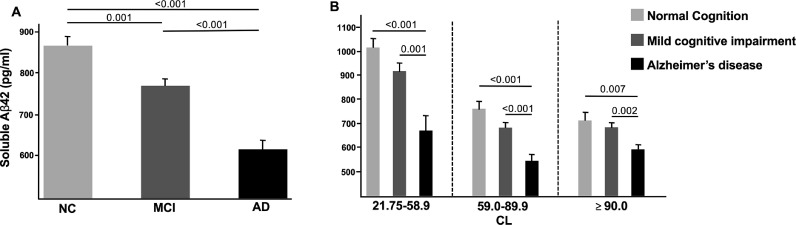
Soluble Aβ42 was higher in better cognitive categories, that is, in NC compared to AD (898.7 pm/ml vs. 602.8 pg/ml; p < 0.001), NC compared to MCI (898.7 pm/ml vs. 755.4 pg/ml; p < 0.001), and MCI compared to AD (755.4 pm/ml vs. 602.8 pg/ml; p < 0.001) (Table 2). After adjusting for all covariates, CSF Aβ42 levels remained higher, above an average of 800 pg/ml, in amyloid PET-positive NC compared to AD (864.00 pg/ml vs. 617.46 pg/ml; adjusted mean difference = +246.54 pg/ml; p < 0.001) and MCI (864.00 pg/ml vs. 768.60 pg/ml; mean difference = +95.40 pg/ml; p = 0.001) as well as in amyloid PET-positive MCI compared to AD (768.60 pg/m vs. 617.46 pg/ml; adjusted mean difference = +151.14 pg/ml; p < 0.001) (Fig. 1A; supplementary Tables 2.1, 2.2). In the adjusted analysis, soluble Aβ42 was higher in NC compared to AD and in MCI compared to AD in all SUVR or CL tertiles (Fig. 1B; supplementary Table 2.1; supplementary Fig. 2.3). Sensitivity analyses for [18]F-florbetapir only and excluding subject within 5% of the threshold in both tracers, yielded similar results (supplementary Table 2.4). Soluble Aβ42 were inversely correlated with CL (r=−0.37; p < 0.001) and SUVR (r=−0.37; p < 0.001) and positively correlated with soluble Aβ40 (r = 0.54; p < 0.001). No correlations were found with p-tau (r = 0.01; p = 0.86) or t-tau (r = 0.06; p = 0.11) (supplementary Table 2.5).
Fig. 1.
Adjusted analyses of amyloid-positive normal cognition compared to mild cognitive impairment and Alzheimer's disease subjects. (A) Soluble Aβ42 levels in each diagnostic category; (B) soluble Aβ42 levels in each diagnostic category across CL tertiles. The estimated means of soluble Aβ42 were adjusted for age, sex, education, APOE4, centiloids, p-tau levels, and t-tau levels. The estimated means of soluble Aβ42 by CL tertiles are adjusted for age, sex, education, APOE4, p-tau levels, and t-tau levels. *The corresponding SUVR analysis, which demonstrated similar results, is shown in Supplementary Fig. 2.3. CL: centiloids (higher CL means greater amyloid burden); NC: normal cognition; MCI: mild cognitive impairment, AD: Alzheimer's disease.
In adjusted analysis, the estimated effect size with Cohen's d was larger for soluble Aβ42 (d = 0.82; CI, 0.59,1.04) than SUVR (d=−0.40; CI, −0.62,−0.18) or CL (d=−0.42; CI, −0.64,−0.20) in the comparison between NC and AD; larger for soluble Aβ42 (d = 0.60; CI, 0.40,0.79) than SUVR (d=−0.26; CI, −0.45,−0.07) or CL (d=−0.26; CI, −0.45,−0.07) in the comparison between MCI and AD; larger for soluble Aβ42 (d = 0.82; CI, 0.59,1.04) than t-tau (d=−0.15; C.I, −0.36,0.07) and p-tau (d = 0.12; C.I −0.10,0.34) in the comparison between NC and AD; and again larger for soluble Aβ42 (d = 0.60; C.I, 0.40, 0.79) than p-tau (d = 0.10; C.I, −0.09,0.29) and t-tau (d=−0.11; C.I, −0.30,0.08) in the comparison between MCI and AD (supplementary Table 2.6).
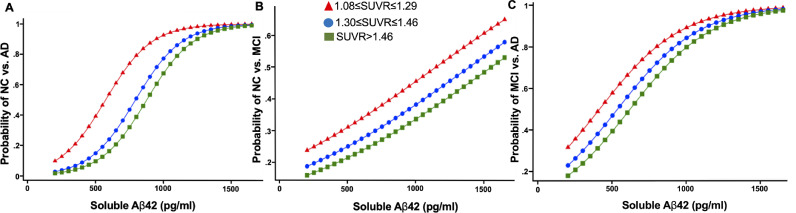
In adjusted analyses, each standard deviation increase in CSF Aβ42 increased the odds of NC versus AD (OR, 6.26; 95% CI, 3.54–11.07, p < 0.001) to a greater extent than CL (OR, 0.50 95% CI, 0.35–0.73, p < 0.001); the odds of NC versus MCI (OR, 1.42; 95% CI, 1.10- 1.82, p = 0.006) to a greater extent than CL (OR= 0.79 95% CI, 0.61–1.03, p = 0.085); and the odds of MCI versus AD (OR, 3.06; 95% CI, 2.11–4.42, p < 0.001) to a greater extent than CL (OR, 0.68; 95% CI, 0.54–0.87, p = 0.002). All these differences were maintained across all SUVR and CL tertiles (Fig. 2a, 2b, 2c; supplementary Table 2.7).
Fig. 2.
Adjusted probability of amyloid-positive normal cognition versus Alzheimer's disease (A), normal cognition versus mild cognitive impairment (B), and mild cognitive impairment versus Alzheimer's disease (C) with levels of soluble Aβ42 by SUVR tertiles of brain amyloid. SUVR: standardized uptake value ratio; NC: normal cognition; MCI: mild cognitive impairment; AD: Alzheimer's disease. The results are adjusted for age, sex, education, APOE4, p-tau levels, and t-tau levels.
3.2. CSF Aβ42 in neuropsychological performance and hippocampal volume
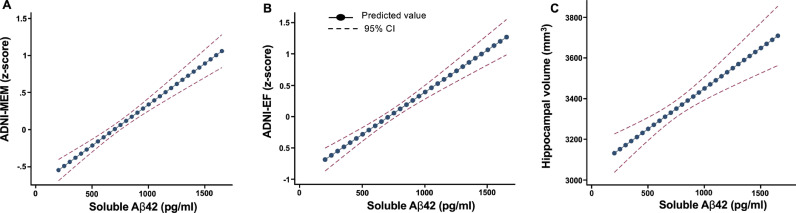
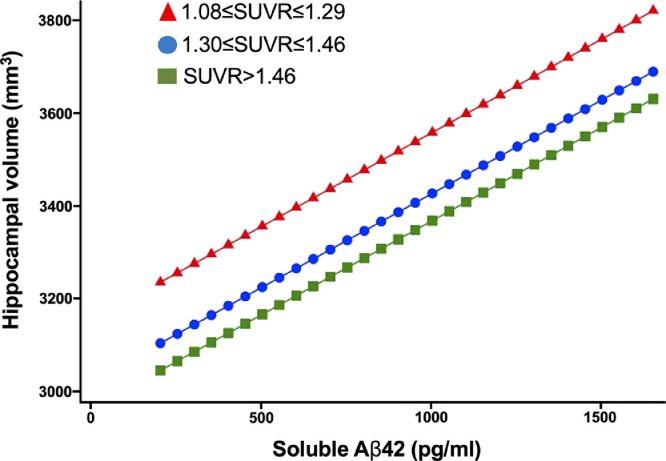
In adjusted analyses, each standard deviation increase in CSF Aβ42 levels was linearly associated with better ADNI-MEM (RC, 0.34; 95% CI, 0.27–0.41; p < 0.001) (Fig. 3A; supplementary Table 2.8) and ADNI-EF scores (RC, 0.41; 95% CI, 0.32–0.51; p < 0.001) (Fig. 3B; supplementary Table 2.8). There was also a linear association between each standard deviation increase in CSF Aβ42 levels and hippocampal volume (RC, 122.34; 95% CI, 74.49–170.18; p < 0.001) (Fig. 3C; supplementary Table 2.8) after adjusting for ICV and other covariates. The effect sizes of these adjusted associations on neuropsychological performance and hippocampal volume were greater for CSF Aβ42 levels than for SUVR or CL levels (supplementary Table 2.8). Hippocampal volume (mm3) was significantly larger in NC vs. MCI (3667.0 vs. 3392.0 p < 0.001), NC vs. AD (3667.0 vs. 3002.4 p < 0.001), and MCI vs. AD (3392.0 vs. 3002.4 p < 0.001) across all CL tertiles (supplementary Fig. 2.9) and SUVR tertiles (data not shown). Higher Aβ42 levels were associated with larger hippocampal volumes in MCI (p = 0.004) with borderline association in AD (p = 0.065). There was no association between hippocampal volume and CL levels in any cognitive category (supplementary Table 2.10). Aβ42 levels were linearly associated with larger hippocampal volumes across each SUVR tertile (Fig. 4, supplementary Table 2.10).
Fig. 3.
Adjusted association of CSF Aβ42 with cognitive assessments (A, B) and hippocampal volume (average right and left) (C) in the amyloid PET-positive cohorts. SUVR: standardized uptake value ratio; ADNI memory score; ADNI-EF: ADNI executive function; CI: confidence interval. The results are adjusted for age, sex, education, APOE4, centiloids, p-tau levels, and t-tau levels including intracranial volume for hippocampal volume analysis.
Fig. 4.
Adjusted association of CSF Aβ42 with hippocampal volume (average right and left) by SUVR tertiles of brain amyloid. SUVR: standardized uptake value ratio. The results are adjusted for age, sex, education, APOE4, p-tau levels, t-tau levels, and intracranial volume.
3.3. Conversion of diagnostic categories
Only 3 subjects converted from NC to AD, 24 from NC to MCI, and 103 from MCI to AD. The NC-to-MCI conversion was associated with age (p = 0.003) (supplementary Table 2.11). In unadjusted analysis, the MCI-to-AD conversion was associated with SUVR (p < 0.001), CL (p < 0.001), p-tau (p < 0.001), t-tau (p < 0.001), and Aβ42 (p = 0.003) (supplementary Table 2.12). In adjusted analyses, each standard deviation increase in CSF Aβ42 increased the odds of MCI non-conversion (OR, 1.68; 95% CI, 1.14–2.46, p = 0.009) to a greater extent than SUVR (OR, 0.70; 95% CI, 0.51–0.94, p = 0.02) or CL (OR, 0.68; 95% CI, 0.50–0.92, p = 0.012) (supplementary Table 2.13).
4. Discussion
This is the first study in humans assessing the mechanistic hypothesis that normal cognition among individuals with brain amyloidosis may require the preservation of high levels in the soluble Aβ42 precursor, aiming to explain the paradox that normal cognition is possible in the setting of brain amyloidosis. This analysis showed that soluble Aβ42 levels were significantly higher, in a dose-dependent manner, in amyloid-positive individuals with normal cognition than in those with mild cognitive impairment or dementia, and that higher soluble Aβ42 levels were associated with better cognitive performance and greater hippocampal volume. The effect size of changes in soluble (CSF) Aβ42 levels for normal cognition and hippocampal volume compared to dementia was larger than that of changes in insoluble (PET) Aβ42. Higher Aβ42 levels, on average above 800 pg/ml, were associated with normal cognition regardless of brain amyloid burden. Conversely, the adjusted OR for conversion from MCI to AD, the cognitive category change with sufficient data for analysis, was higher for decreases in soluble Aβ42 than increases in brain amyloidosis. These data collectively suggest that in individuals with brain amyloidosis, a reduction in soluble Aβ42 may be more critical in the development of AD than an equivalent increase in the burden of brain amyloid, favoring a loss-of-function mechanism in the dementia associated with brain amyloidosis.
The reduction in soluble Aβ42 is, in general, described as a consequence of its aggregation into brain amyloid fibrils and plaques, and, thus, it inversely correlates with brain amyloidosis [13]. However, the data showed that this inverse relationship vary among individuals with different cognitive statuses even if across similar levels of brain amyloid burden. A comparable discordance was reported in a smaller study, in which amyloid PET-positive subjects with normal CSF Aβ42 levels (n = 13) had NC or early MCI whereas amyloid PET-negative subjects with low levels of CSF Aβ42 (n = 7) had poorer cognition and higher CSF tau [20]. While the correlation between high brain amyloid plaque load and dementia is poor, that of low CSF Aβ42 and dementia appears to be robust [21]. The depletion of soluble Aβ42 may be necessary for dementia and hippocampal atrophy to develop in amyloid PET-positive individuals to a greater extent than the corresponding increase in insoluble Aβ42. This is consistent with the known role of soluble Aβ42 in many essential biological functions [5], including neurogenesis [22] as well as synaptic plasticity and memory [23].
The higher levels of t-tau and p-tau in AD dementia subjects reflect an active, more extensive neurodegeneration in this group as compared with MCI. The association with dementia is greater for the early reduction of CSF Aβ42 than for later increases in t-tau and p-tau when studied over a decade-long time window [24]. For instance, a study that analyzed longitudinal data from patients with a confirmed genetic mutation in PSEN1, PSEN2, or APP participating in the Dominantly Inherited Alzheimer Network (DIAN) showed that the reduction of soluble Aβ42 begins around 25 years before the estimated onset of AD, and 10 years before the increase in tau [6,25]. In our analysis, the effect size of changes in soluble Aβ42 was indeed larger compared to changes in p-tau or t-tau in discriminating NC from AD and MCI from AD. We speculate that the loss of Aβ42 is more important in driving initial toxicity whereas the increase in t-tau and p-tau may represent a later manifestation of the neurodegenerative process [26]. In agreement with these findings, absolute levels of Aβ42 have been reported to be lower, not higher, even among autosomal-dominant AD patients participating in DIAN [6,25] as well as in Down syndrome patients with APP duplication [27]. Also, the γ-secretase inhibitor semagacestat [28] and the β-secretase enzyme BACE1 inhibitor verubecestat reduced the levels of soluble Aβ42 in a dose-dependent manner and significantly worsened cognition and accelerated hippocampal atrophy [29]. Altogether, these observations suggest that a reduction in soluble Aβ42, beside serving as a marker of worsened outcome, may be responsible for the expression of cognitive deterioration in both hereditary and sporadic forms of the disease.
The cross-sectional design of this study is the major limitation of our analysis, even if embedded in a prospectively evaluated cohort. In this cohort, only 3 amyloid PET-positive ADNI participants converted from NC to AD and 24 from NC to MCI. In the modestly larger sample of MCI to AD converters (103), the OR was larger for soluble Aβ42 than for brain amyloidosis. The small number of conversions among amyloid-positive NC subjects supports the observation that brain amyloid accrual alone is insufficiently “toxic” to initiate the development of dementia. Another limitation is the use of two different radiotracers for the PET amyloid acquisition, which may have introduced variability around the SUVR estimates. However, the results were confirmed using the CL derivation to reconcile this potential source of variability. Also, when using only data from the [18]F-florbetapir and excluding subjects within 5% of the threshold in both tracers the results were unchanged. This limitation is attenuated by the fact that Aβ40 is less fibrillogenic than Aβ42 in CSF, rendering it less relevant for the study of brain amyloidosis [2]. Finally, although the findings obtained in this study were based on robust statistical and sensitivity analyses, they did not come from a “perfect” study, which would have directly measured soluble Aβ42 in the brain, with such techniques as microdialysis or fresh-frozen brain tissue biopsies, and corrected for the number of active neurons, the volume and flow rate of CSF, and the presence of other brain pathologies to understand the influence of local levels of Aβ42 in brain tissues and the influence of neuronal activity. However, the logistical and ethical difficulties of conducting such a study are enormous.
These analyses alone cannot definitely settle the question of whether the pathogenetic mechanisms of proteinopathies are associated with normal protein depletion (loss-of-function model) or abnormal protein accumulation (gain-of-function model) and the extent to which either or both of these contribute to the underlying clinical phenotype. The data also cannot explain why some amyloid-positive individuals maintain adequate levels of soluble Aβ42 despite having extensive levels of brain amyloidosis while others exhibit comparable insoluble Aβ42 accumulation accompanied by soluble Aβ42 depletion. Resolving the source of compensatory increase in Aβ42 may uncover novel targets for treatment. Nevertheless, our findings support the evaluation of disease-modifying replacement strategies using soluble, non-aggregating forms of Aβ42. As there may still be direct toxicity associated with an excessive increase of aggregated Aβ42 (e.g., mass effect, mislocation of other proteins), we are exploring the viability of such a replacement therapy strategy using more stable versions of Aβ42, which preserve their biological function but are unable to transform into amyloids [30]. This can only be an initial, “rescue medicine” approach. True precision medicine requires identifying and targeting, at the individual level, the pathogenic factors providing the nucleating triggers for the soluble-to-insoluble phase transformation of proteins. As the type of nucleating trigger is expected to vary between affected individuals, an individualized disease-modifying approach is anticipated.
In conclusion, in individuals with brain amyloidosis, high soluble Aβ42 is associated with normal cognitive function and brain volume, whereas low soluble Aβ42 with cognitive impairment and hippocampal atrophy. Tentatively, these results favor a loss-of-function mechanism for AD with a clinical and neurodegenerative onset in those with brain amyloidosis predominantly mediated by the depletion of soluble Aβ42. Pending confirmation in a prospective study, these data warrant the evaluation of therapeutic strategies that increase soluble Aβ42 in amyloid PET-positive individuals with mild cognitive impairment or dementia and cerebrospinal Aβ42 levels below the compensation threshold of 800 pg/ml.
Declaration of Competing Interest
Dr. Sturchio is cofounder of REGAIN Therapeutics, owner of a provisional patent on compositions and methods for treatment and/or prophylaxis of proteinopathies.
Dr. Dwivedi receives grant funding as a co-investigator the NIH and CPRIT and he holds an adjunct associate professor appointment at the University of Cincinnati Department of Neurology and Rehabilitation Medicine
Dr. Young has nothing to disclose.
Dr. Malm is cofounder of REGAIN Therapeutics, owner of a provisional patent on compositions and methods for treatment and/or prophylaxis of proteinopathies.
Dr. Marsili has nothing to disclose.
Dr. Sharma has nothing to disclose.
Dr. Mahajan has nothing to disclose.
Dr. Hill has nothing to disclose.
Dr. EL Andaloussi is co-founder of and shareholder in Evox Therapeutics that develops engineered exosome therapies to treat genetic diseases. He is cofounder of REGAIN Therapeutics, owner of a provisional patent on compositions and methods for treatment and/or prophylaxis of proteinopathies.
Dr. Poston reports consulting fees from Curasen and Allergan, and grants from the Michael J Fox Foundation, NIH, Sanofi, AstraZeneca, Sangamo BioSciences; and honoraria from invited scientific presentations to universities and professional societies not exceeding $5000/yr.
Dr. Manfredsson reports grants from NIH, grants from DOD, grants from MJFF, personal fees from Various organizations (university), personal fees from Alector, other from Ironhorse Diagnostics, other from VIPER Theraputics, outside the submitted work.
Dr. Schneider reports grants and personal fees from Eli Lilly, personal fees from Boehringer Ingelheim, grants and personal fees from Merck, personal fees from Neurim, Ltd, personal fees from Neuronix, Ltd, personal fees from Cognition, personal fees from Eisai, personal fees from Takeda, personal fees from vTv, grants and personal fees from Roche/Genentech, grants from Biogen, grants from Novartis, personal fees from Abbott, grants from Biohaven, grants from Washington Univ/ NIA DIAN-TU, personal fees from Samus, personal fees from IBC, outside the submitted work.
Dr. Ezzat is cofounder of REGAIN Therapeutics, owner of a provisional patent on compositions and methods for treatment and/or prophylaxis of proteinopathies.
Dr. Espay reports grants from NIH, grants from Michael J Fox Foundation, personal fees from Abbvie, personal fees from Neuroderm, personal fees from Neurocrine, personal fees from Acadia, personal fees from Acorda, personal fees from Sunovion, personal fees from Lundbeck, personal fees from USWorldMeds, personal fees from UCB, personal fees from Lippincott Williams & Wilkins, personal fees from Cambridge University Press, personal fees from Springer, personal fees from Kyowa Kirin, personal fees from Amneal. In addition, Dr. Espay is cofounder of REGAIN Therapeutics, owner of a provisional patent on compositions and methods for treatment and/or prophylaxis of proteinopathies.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
* Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Author contribution
AS, KE, and AJE conceptualized the manuscript. AKD and CBY directly accessed and verified the data. AKD carried out the statistical analysis. AS, KE, and AJE interpreted the data and wrote the manuscript. TM, LM, JSS, AM, EJH, SEA, KLP, FPM, and LSS reviewed, commented on, and critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Data sharing
All data are available upon request from the Alzheimer's Disease Neuroimaging Initiative (ADNI) through the study website, http://adni.loni.usc.edu/. All spreadsheets generated from ADNI as well as the volumetric analysis conducted are available upon request, with an index of these documents available in the supplementary material published with the online version of this manuscript.
Funding
Gardner Family Foundation.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.;Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.;Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100988.
Appendix. Supplementary materials
References
- 1.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malmberg M., Malm T., Gustafsson O. Disentangling the amyloid pathways: a mechanistic approach to etiology. Front Neurosci. 2020;14:256. doi: 10.3389/fnins.2020.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagase T., Iwaya K., Iwaki Y. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450–454. doi: 10.1016/j.amjmed.2013.10.029. May. [DOI] [PubMed] [Google Scholar]
- 4.Navalkar A., Ghosh S., Pandey S., Paul A., Datta D., Maji S.K. Prion-like p53 amyloids in cancer. Biochemistry. 2020;59:146–155. doi: 10.1021/acs.biochem.9b00796. [DOI] [PubMed] [Google Scholar]
- 5.Kent S.A., Spires-Jones T.L., Durrant C.S. The physiological roles of tau and Aβ: implications for Alzheimer's disease pathology and therapeutics. Acta Neuropathol. 2020;140:417–447. doi: 10.1007/s00401-020-02196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDade E., Wang G., Gordon B.A. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology. 2018;91:e1295–e1306. doi: 10.1212/WNL.0000000000006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chételat G., La Joie R., Villain N. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer's disease. Neuroimage Clin. 2013;2:356–365. doi: 10.1016/j.nicl.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crystal H., Dickson D., Fuld P. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 9.Polvikoski T., Sulkava R., Myllykangas L. Prevalence of Alzheimer's disease in very elderly people: a prospective neuropathological study. Neurology. 2001;56:1690–1696. doi: 10.1212/wnl.56.12.1690. [DOI] [PubMed] [Google Scholar]
- 10.Panza F., Lozupone M., Seripa D., Imbimbo B.P. Amyloid-β immunotherapy for alzheimer disease: is it now a long shot? Ann Neurol. 2019;85:303–315. doi: 10.1002/ana.25410. [DOI] [PubMed] [Google Scholar]
- 11.Jack C.R., Bennett D.A., Blennow K. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack C.R., Therneau T.M., Weigand S.D. Prevalence of biologically vs clinically defined alzheimer spectrum entities using the national institute on aging-Alzheimer's association research framework. JAMA Neurol. 2019;76:1174–1183. doi: 10.1001/jamaneurol.2019.1971. [published online ahead of print, 2019 Jul 15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagan A.M., Mintun M.A., Mach R.H. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 14.Mattsson N., Insel P.S., Donohue M. Independent information from cerebrospinal fluid amyloid-β and florbetapir imaging in Alzheimer's disease. Brain. 2015;138:772–783. doi: 10.1093/brain/awu367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirrito J.R., May P.C., O'Dell M.A. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwan M.D., Rinne J.O., Hasselbalch S.G. Use of amyloid PET-to determine cutpoints for CSF markers: a multicenter study. Neurology. 2016;86:50–58. doi: 10.1212/WNL.0000000000002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crane P.K., Carle A., Gibbons L.E. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6:502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbons L.E., Carle A.C., Mackin R.S. A composite score for executive functioning, validated in Alzheimer's disease neuroimaging initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6:517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dwivedi A.K., Mallawaarachchi I., Alvarado L.A. Analysis of small sample size studies using nonparametric bootstrap test with pooled resampling method. Stat Med. 2017;36:2187–2205. doi: 10.1002/sim.7263. [DOI] [PubMed] [Google Scholar]
- 20.Landau S.M., Lu M., Joshi A.D. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann Neurol. 2013;74:826–836. doi: 10.1002/ana.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mo J.A., Lim J.H., Sul A.R., Lee M., Youn Y.C., Kim H.J. Cerebrospinal fluid β-amyloid1-42 levels in the differential diagnosis of Alzheimer's disease–systematic review and meta-analysis. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0116802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Toledano M.A., Shelanski M.L. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24:5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puzzo D., Privitera L., Fa' M. Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann Neurol. 2011;69:819–830. doi: 10.1002/ana.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stomrud E., Minthon L., Zetterberg H., Blennow K., Hansson O. Longitudinal cerebrospinal fluid biomarker measurements in preclinical sporadic Alzheimer's disease: a prospective 9-year study. Alzheimers Dement. 2015;1:403–411. doi: 10.1016/j.dadm.2015.09.002. (Amst) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bateman R.J., Xiong C., Benzinger T.L. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [published correction appears in N Engl J Med. 2012 Aug 23;367(8):780] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack C.R., Knopman D.S., Jagust W.J. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portelius E., Hölttä M., Soininen H. Altered cerebrospinal fluid levels of amyloid β and amyloid precursor-like protein 1 peptides in Down's syndrome. Neuromolecular Med. 2014;16:510–516. doi: 10.1007/s12017-014-8302-1. [DOI] [PubMed] [Google Scholar]
- 28.Bateman R.J., Siemers E.R., Mawuenyega K.G. A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egan M.F., Kost J., Voss T. Randomized trial of verubecestat for prodromal Alzheimer's disease. N Engl J Med. 2019;380:1408–1420. doi: 10.1056/NEJMoa1812840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espay A.J., Sturchio A., Schneider L.S., Ezzat K. Soluble amyloid-β consumption in Alzheimer's disease. J Alzheimers Dis. 2021;82 doi: 10.3233/JAD-210415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.