Abstract
Adenovirus E1B 55,000-molecular-weight protein (55K) binds to host cell p53, stabilizing it, greatly increasing its affinity for its cognate DNA-binding site, and converting it from a regulated activator to a constitutive repressor. Here we analyzed the mechanism of repression by the p53-E1B 55K complex. E1B 55K repression requires that 55K be tethered to the promoter by binding directly to DNA-bound p53. Transcription from an assembled, p53-activated preinitiation complex was not repressed by the subsequent addition of E1B 55K, suggesting that either sites of 55K interaction with p53 or targets of 55K in the preinitiation complex are blocked. Specific E1B 55K repression was observed in reactions lacking TFIIA and with recombinant TATA-binding protein in place of TFIID, conditions under which p53 does not activate transcription. Thus, E1B 55K does not simply inhibit a p53-specific activation mechanism but rather blocks basal transcription. As a consequence, E1B 55K may repress transcription from any promoter with an associated p53-binding site, no matter what other activators associate with the promoter. E1B 55K did not repress basal transcription in reactions with recombinant and highly purified general transcription factors and RNA polymerase II but rather required a corepressor that copurifies with the polymerase.
The adenovirus E1B 55,000-molecular-weight protein (55K) binds to the p53 tumor suppressor protein (61), converting it from a transcriptional activator regulated in response to DNA damage (35) to an unregulated repressor of genes with p53-binding sites (51, 78, 79). This activity defends the virus against p53-induced antiviral host cell responses, including apoptosis, which is induced by adenovirus E1A functions (77). An adenovirus type 5 mutant (dl1520) engineered so that it expresses no E1B 55K function (5) has been reported to selectively replicate in p53-deficient human tumor cells (7) and some p53-containing human tumor cells (23) but not in normal, nontransformed human cells. It has been suggested that the dl1520 mutant may be useful as an anticancer therapeutic agent in humans, functioning as a “smart bomb” that selectively kills tumor cells while sparing normal cells (7, 23). Clinical trials with humans are in progress to test the effectiveness of this form of therapy (40). This application of an adenovirus E1B 55K mutant makes it extremely important to fully understand the normal functions of E1B 55K during the course of infection by wild-type virus, both as an inhibitor of p53 and in stimulating late viral gene expression (11, 15). We recently developed an in vitro transcription system which reproduces E1B 55K repression of p53 transcriptional activation (51). Here, we report further studies that extend our understanding of the mechanism of repression.
E1B 55K binds to p53 (61) via the amino-terminal activation domain of p53 (32, 44) and inhibits p53 activation via a repression domain (78, 79). E1B 55K repression domain function requires phosphorylation at three C-terminal serine and threonine residues (67, 68). p53 activity is normally controlled in part through the regulation of p53 degradation; p53 normally has an unusually short half-life (35). However, binding by E1B 55K stabilizes p53, protecting it from degradation (80). The DNA-binding activity of p53 is also postulated to be regulated in response to DNA damage (35). In vitro, p53 DNA-binding activity can be increased through modifications of its N- and C-terminal domains by phosphorylation (26, 36, 76), acetylation (18), and interaction with single-stranded DNA (28). When E1B 55K binds to p53, it increases the affinity of p53 for tandem p53-binding sites by a factor of ∼10 (51). Consequently, E1B 55K converts p53 from a regulated activator to a constitutive repressor by stabilizing it, increasing its affinity for p53-binding sites in DNA, and binding to it a strong repression domain that counteracts the influence of the p53 activation domain.
In the studies reported here, we have analyzed the mechanism of repression by E1B 55K by taking advantage of the ability of purified, recombinant E1B 55K to repress transcription specifically in vitro (51). The mechanisms and factor requirements for the repression of transcription in eukaryotes have not been studied as extensively as have those for activation. Repressors appear to act by either modifying chromatin or inhibiting some basal process in transcription initiation and/or elongation. This activity can be achieved either directly or through recruitment of a corepressor. Some repressors have been shown to interact directly with RNA polymerase II (Pol II) general transcription factors (GTFs) and consequently would be expected to exert their effects directly on the basal transcriptional machinery; these include Dr1, Mot1, Even-skipped (Eve), Kruppel, the thyroid hormone receptor in the absence of a ligand, and Mdm-2 (2, 3, 13, 27, 62, 71, 72). Other repressors, such as Mad/Max, nuclear hormone receptors, the yeast Ume1 protein, and the retinoblastoma protein, utilize corepressors to recruit histone deacetylase complexes to the promoter (1, 20, 22, 30, 39, 48, 52, 56, 81). In Saccharomyces cerevisiae, several subunits of the Pol II holoenzyme function in repression; these include Srb8 (also called Ssn5 and Are5), Srb9 (Ssn2), Srb10 (Ssn3, Ume5, and Are1, a cyclin-dependent kinase), Srb11 (the cyclin partner of Srb10), Ssn8, Rox3 (Ssn7), Rgr1, Gal11, and Sin4 (Ssn4 and Tsf3) (4, 24, 29, 34, 38, 41, 42, 63, 64). In the studies reported here, we show that repression by E1B 55K does not require TFIID-TAFs or TFIIA, factors required for p53 activation in mammalian transcription systems. Rather, E1B 55K is able to repress basal transcription when it is bound near a promoter through interactions with p53 bound to its cognate DNA-binding site. We found that to inhibit transcription, E1B 55K must bind to p53 before a complete initiation complex is assembled. Furthermore, our results indicate that repression by E1B 55K requires a cellular corepressor that copurifies with Pol II but is distinct from known GTFs and Pol II subunits.
MATERIALS AND METHODS
Construction of baculovirus vector for expression of 55K with a six-histidine tag (55K-His6).
E1B 55K sequences from pSRα-55K (78) were cloned into a baculovirus transfer vector, pAcSG1, to create pSG-55K. The carboxy-terminal SstII/BglII fragment was replaced with a PCR fragment which incorporated a six-histidine tag at the carboxy terminus to create pSG-55K6His. Recombinant baculoviruses were generated and plaque purified three times prior to amplification for a viral stock as described previously (51). PCR-amplified sequences in all constructions were sequenced to ensure that no errors had been incorporated.
Construction of vaccinia virus vector for expression of p53 P27Y.
DNA encoding mutant p53 P27Y amino acids 1 to 184 was PCR amplified from a plasmid encoding mutant p53 and kindly provided by Arnold Levine (44). The 5′ primer, 5′ aac aca cac cATG GGA (TAC CCA TAC GAC GTC CCA GAC TAC GCT GTC) GAG GAG CCG CAG TCA GAT CCT 3′, where restriction endonuclease cleavage sites for NcoI and AatII are underlined and the sequence in lowercase does not encode protein, was designed to tag the amino terminus of mutant p53 P27Y with the influenza virus hemagglutinin type 1 (HA1) epitope YPYDVPDYAV (sequence in parentheses). The AatII site was incorporated within the epitope to allow rapid restriction analysis of mutant versus wild-type p53 sequences. PCR sequences were digested with NcoI and cloned into the vaccinia virus transfer vector pTMI-ep53 (79). The recombinant vaccinia virus expressing HA1 epitope-tagged p53 (ep53) P27Y, VV-ep53 P27Y, was generated by previously described procedures (79).
Protein purification.
HeLa cell nuclear extracts were prepared as described previously (10). Partially purified protein fractions containing transcription factors TFIIA, Pol II-TFIIE-TFIIF-TFIIH, and TFIID were prepared from HeLa cell nuclear extracts by step elution over phosphocellulose and DEAE-Sepharose as described previously (45) and are referred to as AB, CB, and DB, respectively. Highly purified HA1 epitope-tagged TFIID (eTFIID) was isolated from LTRα3 cells by affinity purification as described previously (82).
A Superose 6 Pol II-TFIIH (Sup6 PolII/IIH) fraction was prepared by gel filtration of the CB fraction. The CB fraction (200 μl) was fractionated by gel filtration through a Superose 6 HR column (10 by 300 mm; Pharmacia) equilibrated with buffer containing 20 mM HEPES (pH 7.9), 0.5 M KCl, 2 mM EDTA, 10% glycerol, and 10 mM β-mercaptoethanol. Fast protein liquid chromatography (FPLC) (Pharmacia apparatus) was done at 0.5 ml/min and room temperature. Fractions (500 μl) were collected, and aliquots (25 μl) were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), followed by Western blot analysis. Equivalent fractions from 14 column runs were pooled, dialyzed against 0.1 M KCl D buffer (D buffer [20 mM HEPES {pH 7.9}, 2 mM EDTA, 20% glycerol, 10 mM β-mercaptoethanol] containing 0.1 M KCl), and then concentrated 10- to 20-fold with Centricon-100 concentrators (Amicon). The concentration of the largest, 220-kDa subunit of Pol II was quantitated by SDS-PAGE and Western blotting with antibody 8WG16 (70). Molecular mass protein standards were analyzed under the same column conditions. These were thyroglobulin (a tetramer of 670 kDa), apoferritin (a multimer of 440 kDa), and alcohol dehydrogenase (a tetramer of 150 kDa).
Human recombinant wild-type TATA-binding protein (rTBP) was expressed in Escherichia coli M15 as a six-histidine-tagged protein and was purified by successive chromatography over heparin-Sepharose (Pharmacia) and Ni2+-nitrilotriacetic acid (NTA)-agarose (Qiagen) resins as described previously (8). rTBP was stored in 0.3 M KCl D buffer at −70°C and diluted to 0.1 M KCl immediately prior to use.
The expression of human recombinant TFIIB (rTFIIB) was induced for 2 h at 37°C with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) in E. coli BL21(DE3), and rTFIIB was purified by chromatography over phosphocellulose and DEAE-Sepharose resins as described previously (19).
Human recombinant TFIIE56 (rTFIIE56) was expressed from pAR56 in E. coli BL21(DE3)/pLysS cells (Stratagene) by induction with 0.4 mM IPTG for 2 h at 37°C and was purified by a modification of the method of Peterson et al. (58). Briefly, rTFIIE56 bacterial lysate in 0.3 M KCl D buffer was loaded onto a DEAE-Sepharose column (10 mg of protein/ml of resin). The flowthrough fraction was collected and precipitated with ammonium sulfate to a final concentration of 0.245 mg/ml. The precipitate was resuspended in D buffer until the conductivity was equivalent to 0.3 M KCl, and the mixture was loaded onto a Q-Sepharose resin column (10 mg of protein/ml of resin). rTFIIE56 was step eluted with 0.6 M KCl D buffer and dialyzed against 0.1 M KCl D buffer.
Human recombinant TFIIE34 (rTFIIE34) was expressed from pAR34 in E. coli BL21(DE3) by induction with 0.4 M IPTG for 1 h at 30°C. rTFIIE34 was purified by chromatography over DEAE-Sepharose resin, loading in 0.3 M KCl D buffer, and collection of the flowthrough fraction, followed by step elution over S-Sepharose resin (0.1 to 0.4 M KCl) (modification of the method of Peterson et al. [58]).
The recombinant TFIIE (rTFIIE) complex was reconstituted by mixing equimolar amounts of rTFIIE34 (in 0.4 M KCl D buffer) and rTFIIE56 (in 0.1 M KCl D buffer) on ice for 30 min and then dialyzing the mixture against 0.1 M KCl D buffer for 4 h at 4°C. rTFIIE was further purified by FPLC over Mono S resin (HR 5/5; Pharmacia) equilibrated with 0.1 M KCl D buffer. Bound proteins were eluted with a linear gradient of 0.1 to 0.5 M KCl D buffer. The complex eluted at 0.27 M KCl. rTFIIE was dialyzed against 0.1 M KCl D buffer.
Human recombinant TFIIF (rTFIIF) was prepared by expression and purification of the RAP30 and RAP74 subunits separately, followed by reconstitution and further chromatography. RAP30 was expressed from pET11d/RAP30 in E. coli BL21(DE3), purified from inclusion bodies as described previously (74), and dialyzed against 0.5 M KCl D buffer containing 4 M urea. RAP74 with a six-histidine tag was expressed from pET23d/RAP74NspV (75) in E. coli BL21(DE3) and purified by affinity chromatography over Ni2+-NTA-agarose as described previously (75), except that bound proteins were washed and eluted stepwise with lysis buffer (50 mM sodium phosphate, 300 mM NaCl, 10 mM β-mercaptoethanol) containing 4 M urea at pH 8.0, pH 6.3, and pH 5.0. RAP74 with a six-histidine tag was dialyzed against 0.5 M KCl D buffer containing 4 M urea prior to reconstitution with RAP30. Reconstitution of the rTFIIF complex (RAP30 and RAP74) was performed by sequential dialysis against 0.5 M KCl D buffer containing 1 M urea for 10 h, 0.5 M urea for 4 h, and no urea for 4 h, followed by dialysis against 0.2 M KCl D buffer for 2 h and two changes of 0.1 M KCl D buffer for 2 h each. Reconstituted rTFIIF was further purified by FPLC over Mono Q resin (HR 5/5). Bound proteins were eluted with a linear gradient of 0.1 to 1 M KCl D buffer, and rTFIIF eluted at 0.63 M KCl. Reconstituted rTFIIF was dialyzed against 0.1 M KCl D buffer.
TFIIAαβ and TFIIAγ were expressed in E. coli M15 as six-histidine-tagged versions of the proteins from plasmids pQIIA-α/β and pQIIA-γ (54), respectively, and purified under denaturing conditions by chromatography over Ni2+-NTA-agarose as described previously (37).
Human RNA Pol II was extracted from nuclear pellets and immunoaffinity purified with a monoclonal antibody 8WG16 (70) column as described previously (65).
TFIIH, kindly provided by Dean Tantin and Mike Carey (University of California at Los Angeles), was purified by successive chromatography over phosphocellulose, DEAE-Sepharose, hydroxyapatite, and phenyl-Sepharose.
55K-His6 was expressed by infecting Sf9 insect cells with the recombinant baculovirus Bac-55K6His at a multiplicity of infection of 10. Cells were harvested 72 h postinfection, and nuclear extracts were prepared (10). Nuclear extracts (250 ml) were incubated with Ni2+-NTA-agarose (100 ml) preequilibrated with 0.1 M KCl D buffer for 2 to 4 h at 4°C with rotation. The resin was batch washed with 10 to 20 volumes of 0.1 M KCl D buffer and 30 to 40 volumes of 0.1 M KCl D buffer containing 20 mM imidazole and loaded into a column. Bound proteins were eluted with 0.1 M KCl D buffer containing 250 mM imidazole at room temperature. Eluted proteins were dialyzed against 0.1 M KCl D buffer for 4 h at 4°C. Purified proteins were analyzed by SDS–10% PAGE and silver staining. The concentration of 55K-His6 was estimated to be 80 ng/ml from silver-stained gels with bovine serum albumin standards.
Both wild-type and mutant (P27Y) ep53 proteins were immunopurified from nuclear extracts prepared from vaccinia virus vector-infected HeLa cells as described previously (79). Gal4-AH, a transcriptional activator which consists of an amphipathic alpha helix fused to the Gal4 DNA-binding domain, was purified from E. coli and was a kind gift from Mike Carey (45).
In vitro transcription.
In vitro transcription was done with two templates in the same transcription reaction, either p5BSE4CAT and pG5E1BCAT or G-less cassette templates pP5MLP(G−) and pG5ΔMLP(G−). p5BSE4CAT (47) contains five p53-binding sites upstream of the adenovirus E4 promoter (−38 to +38) driving the expression of the chloramphenicol acetyltransferase (CAT) gene. pG5E1BCAT contains five Gal4 DNA-binding sites upstream of the E1B TATA sequence, which itself lies upstream of the CAT gene (43). pP5MLP(G−) was constructed by cloning the HindIII/BamHI fragment containing five p53-binding sites from p5BSE4TATA (79) into p3GalMLP(C2AT) (55). pG5ΔMLP(G−) was constructed by cloning the HindIII/BamHI fragment containing five Gal4-binding sites from pG5E4TATA (43) into the vector pΔMLP(C2AT) (55). Reactions with templates p5BSE4CAT and pG5E1BCAT were performed with either HeLa cell nuclear extract or partially purified and purified GTFs, and assays were done by primer extension with an end-labeled primer complementary to the CAT gene as described previously, except that 60 ng of each template was used (51). In vitro transcription reactions with G-less cassette templates were performed with purified GTFs in a final reaction volume of 50 μl in buffer containing 20 mM HEPES (pH 7.9), 8 mM MgCl2, 60 mM KCl, 12% glycerol, 10 mM ammonium sulfate, 100 ng of bovine serum albumin per ml, 10 mM β-mercaptoethanol, 2% (wt/vol) polyethylene glycol 8000, 0.6 mM ATP, 0.6 mM CTP, 2.5 mM UTP, 0.5 μl of [α-32P]UTP (3 Ci/mmol), and 100 ng of each template. After 1 h of incubation at 30°C, transcripts were digested with 20 U of RNase T1 (Boehringer Mannheim Biochemicals) for 20 min at 30°C. Digestion was stopped by the addition of 170 μl of stop solution (0.1 M NaCl, 0.01 M EDTA, 0.05% SDS) containing tRNA (5 μg/ml) and proteinase K (0.5 mg/ml). After phenol-chloroform extraction and ethanol precipitation, RNA was resolved on 6% polyacrylamide–8 M urea–Tris-borate-EDTA gels. Gels were exposed to either X-ray film or PhosphorImager (Molecular Dynamics) screens. Counts in specific transcripts were quantitated with the PhosphorImager and the program ImageQuant.
Each protein fraction or factor was titrated against the others to give optimal levels of transcription. The following amounts of proteins were used in transcription reactions: 10 ng of Gal4-AH, 40 ng of wild-type ep53, 40 ng of mutant ep53 P27Y, 60 μg of HeLa cell nuclear extract, 11 μg of AB fraction, 5 μg of CB fraction, 5.5 μg of DB fraction, and 10 ng of rTFIIB. Transcription reactions with recombinant factors and affinity-purified Pol II contained rTBP (40 ng), rTFIIB (40 ng), rTFIIF (100 ng), rTFIIE (80 ng), Pol II (6 μl), and purified TFIIH (1 μl). Reactions with Sup6 Pol II/IIH contained eTFIID (4 μl), human recombinant TFIIA (rTFIIA) (160 ng), rTFIIF (100 ng), rTFIIE (80 ng), rTFIIB (40 ng), and Sup6 Pol II/IIH (4 μl).
Western blotting.
Rabbit antibodies against CDK8 were kindly provided by J. P. Tassan and Erich Nigg (66) and by Paula Rickert and Emma Lees (59). Polyclonal antibodies against TFIIF RAP74, TFIIEα p56, TFIIEβ p34, and TFIIH p89 were obtained from Santa Cruz Biotechnology. Monoclonal antibody 8WG16, used for the purification of RNA Pol II and detection of the RNA Pol II 220-kDa subunit, has been described elsewhere (70), and the hybridoma was kindly provided by R. Burgess. Bound antibody was detected by enhanced chemiluminescence with a kit from Pierce.
Cell culture.
Sf9 cells were maintained at 27°C in Grace’s insect medium (Gibco) supplemented with 10% fetal calf serum and 0.4% Yeastolate. HeLa S3 suspension cells were maintained at 37°C in Dulbecco modified Eagle medium supplemented with 5% newborn calf serum, penicillin (0.06 mg/ml), and streptomycin (0.1 mg/ml). HeLa cell monolayers were maintained at 37°C in minimal essential medium supplemented with 10% newborn calf serum, penicillin (0.06 mg/ml), and streptomycin (0.1 mg/ml).
RESULTS
Specific repression by E1B 55K requires a direct protein-protein interaction with p53.
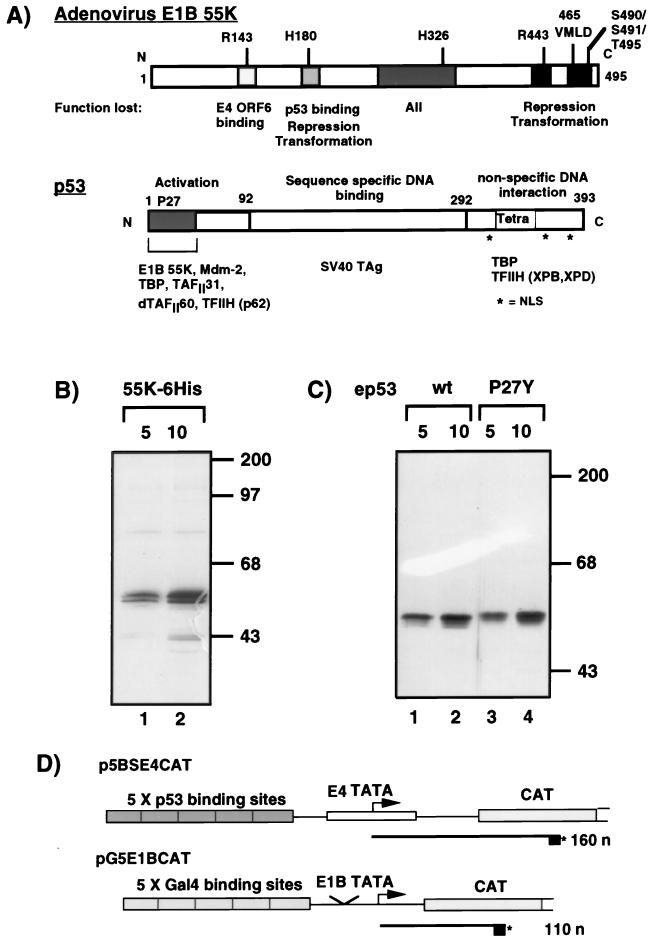
We previously showed that a purified, epitope-tagged version of E1B 55K specifically repressed p53 activation in vitro in transcription reactions with HeLa cell nuclear extracts (51). To prepare more concentrated, purified E1B 55K, we generated E1B 55K with a six-histidine tag at its carboxy terminus. 55K-His6 was purified from nuclear extracts of Sf9 cells infected with the recombinant baculovirus Bac-55K6His by Ni2+-NTA affinity chromatography (Fig. 1B, lanes 1 and 2). Wild-type ep53 and mutant ep53 P27Y were immunopurified by use of an HA1 epitope fused to their amino termini (Fig. 1C). ep53 P27Y was included as a control, since it has wild-type transcriptional activity but is severely reduced in its binding affinity for E1B 55K (44).
FIG. 1.
(A) Schematic diagram illustrating the functional domains in adenovirus E1B 55K (top) and tumor suppressor p53 (bottom). Shaded boxes indicate regions in E1B 55K which, when mutated, result in a loss of the described functions. Positions where amino acids have been mutated by linker insertion or site-directed mutagenesis (50a, 67, 68, 78) are indicated above the E1B 55K molecule. The activation, DNA-binding, and nonspecific DNA-binding domains of p53 are indicated. The tetramerization domain (Tetra) is located at the C terminus. Nuclear localization signals (NLS) are indicated. Proteins which are known to interact with the defined regions are indicated below the p53 molecule. SV40 TAg, simian virus 40 T antigen. (B) Silver-stained gel of affinity-purified 55K-His6 purified from nuclear extracts of Sf9 cells infected with Bac-55K6His (lanes 1 and 2, 5 and 10 μl, respectively). (C) Silver-stained gel of wild-type ep53 (lanes 1 and 2, 5 and 10 μl) and mutant ep53 P27Y (lanes 3 and 4, 5 and 10 μl) purified from nuclear extracts of HeLa cells infected with recombinant vaccinia viruses VV-ep53 wt and VV-ep53 P27Y, respectively. Molecular mass markers are indicated in kilodaltons on the right of panels B and C. (D) In vitro transcription templates. p5BSE4CAT contains five p53-binding sites upstream of the adenovirus type 2 E4 TATA box and CAT gene. This template is responsive to activation by p53. RNAs transcribed from this template by primer extension are 160 nucleotides (n) long. pG5E1BCAT contains five Gal4-binding sites upstream of the adenovirus type 2 E1B TATA box and CAT gene. This template is responsive to activation by Gal4-AH. RNAs transcribed from this template by primer extension are 110 nucleotides long. Asterisks indicate the 5′ 32P label on the oligonucleotide used h prime reverse transcription.
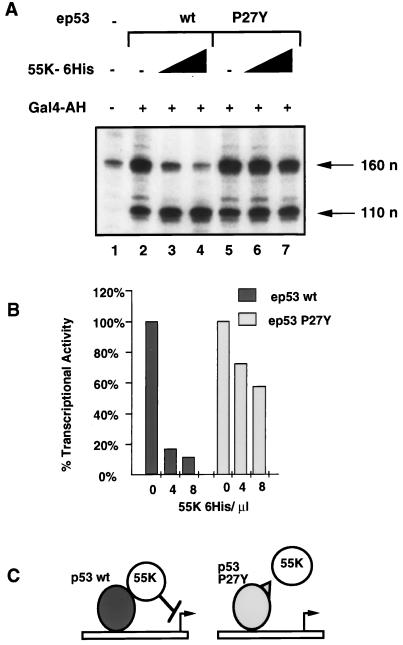
The activities of the purified proteins were tested in in vitro transcription reactions reconstituted with HeLa cell nuclear extracts and two DNA templates, one containing five p53-binding sites (p5BSE4CAT) and the other containing five Gal4-binding sites (pG5E1BCAT) (Fig. 1D). Transcription from p5BSE4CAT and pG5E1BCAT, assayed by primer extension, resulted in extension products of 160 and 110 nucleotides, respectively. pG5E1BCAT was included as an internal control template. Transcription from this template was activated by Gal4-AH (Fig. 2A, compare lane 1 with lanes 2 and 5). Wild-type ep53 and mutant ep53 P27Y activated transcription to similar levels (Fig. 2A, lanes 2 and 5). The addition of 55K-His6 resulted in an approximately 10-fold inhibition of transcription activated by wild-type ep53 (Fig. 2A, lanes 3 and 5, and Fig. 2B), whereas activation by ep53 P27Y was repressed to a much lesser extent (Fig. 2A, lanes 6 and 7, and Fig. 2B). 55K-His6 had no effect on transcriptional activation by the activator Gal4-AH (Fig. 2A, lanes 3, 4, 6, and 7). These results show that 55K-His6 functions in vitro to specifically repress the promoter with p53-binding sites. Furthermore, the repression of p53 activation requires a direct interaction between p53 and E1B 55K, as 55K-His6 had much less of an effect on transcription activated by mutant ep53 P27Y, which has a reduced binding affinity for E1B 55K (44). This result provides biochemical evidence that 55K-His6 must be tethered to the promoter to repress transcription (Fig. 2C), supporting the same conclusion drawn from in vivo transient transfection experiments with Gal4 DNA-binding domain–55K fusions (79).
FIG. 2.
55K-His6 specifically represses activation by wild-type p53. (A) In vitro transcription reactions contained HeLa cell nuclear extracts, template DNAs (p5BSE4CAT and pG5E1BCAT), Gal4-AH (lanes 2 to 7), wild-type ep53 (lanes 2 to 4) or mutant ep53 P27Y (lanes 5 to 7), and 55K-His6 (4 and 8 μl, lanes 3 and 4 and lanes 6 and 7, respectively). Transcription was analyzed by primer extension. Products from p5BSE4CAT and pG5E1BCAT were 160 and 110 nucleotides (n) long, respectively. (B) Quantitation of wild-type (wt) ep53- and mutant ep53 P27Y-activated transcription with increasing concentrations of 55K-His6. Quantitation was done with a PhosphorImager. The level of transcription with wild-type ep53 and mutant ep53 in the absence of 55K-His6 was set at 100%. Transcriptional activity was normalized by dividing counts from p5BSE4CAT by counts from pG5E1BCAT. (C) E1B 55K requires a direct interaction with p53 for repression. E1B 55K interacts directly with wild-type ep53 and, once promoter bound, represses transcription. The binding affinity of E1B 55K for mutant ep53 P27Y is significantly reduced. E1B 55K is therefore not brought to the promoter and consequently is unable to repress transcription.
55K represses activated transcription with partially purified factors.
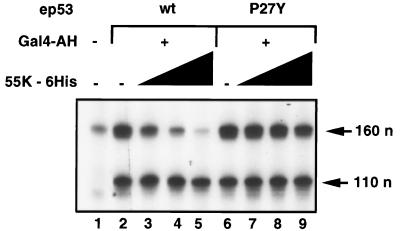
To address the question of which cellular factors are necessary for the repressing function of 55K-His6, we tested whether transcription factors partially purified from nuclear extracts were sufficient or whether an additional factor(s) present in crude nuclear extracts was required for repression by 55K-His6. Transcription reactions were reconstituted in vitro with partially purified factors: fraction AB (containing TFIIA), fraction CB (containing Pol II, TFIIE, TFIIF, and TFIIH), and fraction DB (containing TFIID and coactivators required for activation by many purified activators [31]), and TFIIB (Fig. 3). As expected, these protein fractions were sufficient to support activated transcription by wild-type ep53, mutant ep53 P27Y, and Gal4-AH (Fig. 3, compare lane 1 with lanes 2 and 6). Significantly, a dose-dependent decrease in transcription was observed when increasing concentrations of 55K-His6 were added to reactions containing wild-type ep53 (Fig. 3, lanes 3 to 5). Furthermore, repression by E1B 55K required a direct interaction between 55K-His6 and ep53, since transcription activated by ep53 P27Y was not significantly affected by 55K-His6 (Fig. 3, lanes 7 to 9). These results show that any potential cofactor required for repression was present in these partially purified GTF fractions. Since similar levels of repression by 55K-His6 were observed with nuclear extracts and these partially purified protein fractions in experiments done simultaneously (unpublished results), it is unlikely that a factor which might augment the repressing activity had been purified away.
FIG. 3.
55K-His6 represses p53 activation in vitro in transcription reactions reconstituted with partially purified factors. In vitro transcription reactions were reconstituted with protein fractions AB, CB, and DB (see Materials and Methods), rTFIIB, and DNA templates p5BSE4CAT and pG5E1BCAT. Gal4-AH was added to reactions analyzed in lanes 2 to 9; also added were wild-type ep53 (lanes 2 to 5) or mutant ep53 P27Y (lanes 6 to 9) and increasing concentrations of 55K-His6 (2, 4, and 8 μl, lanes 3 to 5 and lanes 7 to 9, respectively). Transcription was analyzed by primer extension; products of 160 and 110 nucleotides (n) from p5BSE4CAT and pG5E1BCAT, respectively, are indicated.
The assembled PIC is resistant to repression by 55K.
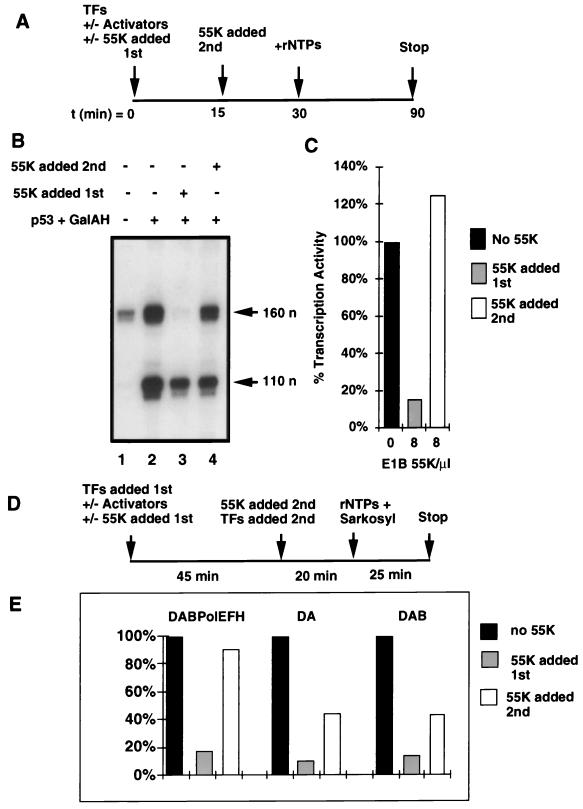
Purified 55K binds tightly to purified p53 and substantially increases the affinity of p53 for its binding sites in p5BSE4CAT (51). Consequently, when 55K is added to transcription reactions simultaneously with the GTFs, as in earlier experiments, it is probably bound rapidly, along with p53, to the promoter, where it can interact with preinitiation complex (PIC) components as they interact with the template. We wished to address the question of whether 55K-His6 can repress transcription after a PIC has been assembled on a promoter. Transcription factor fractions AB, CB, and DB and rTFIIB were preincubated with the DNA templates p5BSE4CAT and pG5E1BCAT (Fig. 4A and B). The activators, ep53 and Gal4-AH, were added at the start of the preincubation (Fig. 4B, lanes 2 to 4). 55K-His6 was added either at the same time as the transcription factors and activators (Fig. 4B, lane 3) or 15 min later (Fig. 4B, lane 4). As observed earlier, 55K-His6 efficiently repressed transcription when added at the same time as the GTFs, Pol II, and activators (Fig. 4B, compare lane 2 with lane 3; Fig. 4C). In contrast, following PIC assembly, transcription was resistant to repression by 55K-His6 (Fig. 4B, compare lanes 2 and 4; Fig. 4C). Under the experimental conditions used in Fig. 4A to C, multiple rounds of transcription can potentially occur.
FIG. 4.
Preformed PICs are resistant to repression by 55K-His6. (A) Time line of the experiments shown in panels B and C. TFs, transcription factors; rNTPs, ribonucleoside triphosphates; t, time. (B) Transcription factor fractions AB, CB, and DB, rTFIIB, and DNA templates were preincubated either in the absence (lane 1) or in the presence (lanes 2 to 4) of activators ep53 and Gal4-AH. 55K-His6 was added to the reactions either initially, as PIC assembly was occurring (lane 3), or 15 min later (lane 4). Products of transcription were assayed by primer extension and are indicated by arrows (n, nucleotides). (C) Quantitation of the effect of 55K-His6 on the percent transcriptional activity of ep53. Activity in the absence of 55K-His6 was set at 100%. Activity was normalized as described in Materials and Methods. (D) Time line of the experiment shown in panel E. (E) Transcription factor fractions AB, CB, and DB, rTFIIB, and DNA templates (DABPolEFH) or a subset of these factors (DA, fractions AB and DB; DAB, fractions AB and DB and rTFIIB) were preincubated with activators ep53 and Gal4-AH (no 55K). 55K-His6 was added to the reactions either initially, as PIC assembly was occurring (55K added first), or 45 min later (55K added second). The remaining GTFs were added, and after 20 min, Sarkosyl and rNTPs were added to prevent further PIC assembly and to initiate transcription. Products of transcription were assayed by primer extension, and quantitation of the effect of 55K-His6 on the percent transcriptional activity of wild-type ep53 is shown. Activity in the absence of 55K-His6 was set at 100%. Activity was normalized as described in Materials and Methods.
Sarkosyl at a concentration of 0.035% prevents new PICs from forming but allows a single round of transcription from PICs that have already formed (21). The experiment was repeated with 0.035% Sarkosyl being added at the same time as the nucleoside triphosphates to prevent reinitiation (Fig. 4D). When all factors were preincubated together (DABPolEFH), similar results were observed for a single round of transcription and for multiple rounds (Fig. 4E). To determine if the completion of early steps in PIC assembly rendered the templates resistant to 55K repression, the experiment was performed by incubating the templates initially with ep53 plus AB and DB fractions (containing TFIIA and TFIID, respectively) and with ep53 plus AB and DB fractions and rTFIIB (Fig. 4E). Even under these conditions, when ep53-DA complexes and ep53-DAB complexes were preassembled, 55K-His6 repression was still substantial, decreasing transcription to ∼45% of the level observed without the addition of 55K-His6. Since the preincubation was sufficiently long to complete the binding of p53, TFIID, TFIIA, and TFIIB to the templates, these results indicate that formation of the DA and DAB complexes does not protect the promoter from repression. It was clear, however, that when 55K-His6 was added later, repression was less complete than when 55K-His6 was added initially. This result might have occurred because interactions of the p53 activation domain with TFIID and TFIIA might interfere with the binding of 55K-His6.
Under conditions where all the factors were preincubated together, transcription was sensitive to the effects of 55K-His6 only if 55K-His6 was present initially, whereas transcription was resistant to the effects of 55K-His6 when the repressor was added after PIC formation. These results strongly suggest that 55K acts during PIC assembly. The possibility exists that 55K must be present during PIC assembly in order to bind to the p53 activation domain before it becomes sequestered within the assembled PIC but that 55K then exerts its effect at a late step in the transcription reaction, after PIC assembly but before initiation.
55K represses basal transcription.
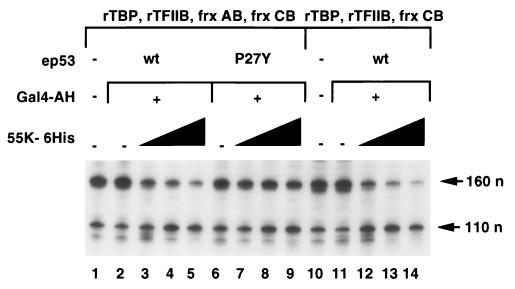
In higher eukaryotes, transcriptional activation requires TFIID-TAFs (12, 73) and TFIIA (8, 49, 54). We examined whether repression by 55K-His6 was dependent on the presence of TAFs or TFIIA. Transcription reactions were reconstituted with protein fraction AB (containing TFIIA), protein fraction CB (containing Pol II, TFIIE, TFIIF, and TFIIH), rTFIIB, and rTBP (in place of the DB fraction containing TFIID and coactivators) (Fig. 5, lanes 1 to 9). In vitro reactions were also reconstituted with fraction CB, rTFIIB, and rTBP (Fig. 5, lanes 10 to 14). As expected, because of the absence of TFIID-TAFs (Fig. 5, lanes 1 to 9) and the additional absence of TFIIA (lanes 10 to 14), no transcriptional activation was observed when Gal4-AH, wild-type ep53, or mutant ep53 P27Y was added to these reactions. The effect of increasing concentrations of 55K-His6 was assayed, and the results clearly demonstrated that 55K-His6 repressed transcription in reactions where it was brought to the promoter via its interaction with wild-type ep53 (Fig. 5, lanes 3 to 5). Repression of transcription arising from the template p5BSE4CAT was minimal in reactions with ep53 P27Y (Fig. 5, lanes 7 to 9), as 55K-His6 was not targeted to the promoter, as was that arising from the template pG5E1BCAT (lanes 3 to 5 and 7 to 9), again showing specificity in the repression mechanism. Similarly, in the absence of TFIIA, 55K-His6 repressed transcription in a dose-dependent manner when it was brought to the template by its interaction with wild-type ep53 (Fig. 5, lanes 12 to 14).
FIG. 5.
TFIID-TAFs and TFIIA are not required for repression by 55K-His6. Transcription reactions were reconstituted with DNA templates (p5BSE4CAT and pG5E1BCAT, 60 ng each) and rTBP (40 ng), rTFIIB (40 ng), and fractions (frx) AB and CB (lanes 1 to 9) or rTBP, rTFIIB, and fraction CB (lanes 10 to 14). Gal4-AH was added to lanes 2 to 9 and lanes 11 to 14. Wild-type ep53 was added to lanes 2 to 5 and 11 to 14, and mutant ep53 P27Y was added to lanes 7 to 9. Increasing concentrations of 55K-His6 (2, 4, and 8 μl, lanes 3 to 5, 7 to 9, and 12 to 14, respectively) were added to reactions. Primer extension products of 160 and 110 nucleotides (n) from p5BSE4CAT and pG5E1BCAT, respectively, are indicated.
In vitro transcription in reactions in which rTBP replaces partially purified TFIID and, consequently, there is no activation of transcription, is often called basal transcription (60). The results of Fig. 5 demonstrate that E1B 55K inhibits such basal transcription. E1B 55K repression of basal transcription was also observed in reactions with heat-treated nuclear extracts (in which TFIID has been inactivated) supplemented with rTBP (data not shown). The fold repression in basal transcription reactions with heat-treated nuclear extracts supplemented with rTBP was similar to that observed in activated transcription reactions with untreated nuclear extracts when the two sets of reactions were carried out simultaneously (data not shown). The results of Fig. 5 have important implications for the mechanism of repression. They indicate that E1B 55K does not simply inhibit the p53 activation mechanism but rather inhibits a basal step in transcription that is likely to be required during transcriptional activation by any activator. As a consequence, the p53-E1B 55K complex is likely to repress transcription from any promoter to which it is tethered, no matter what other activators in addition to p53 normally regulate the promoter. Consequently, E1B 55K expression during adenovirus infection is likely to repress transcription from all promoters with associated p53-binding sites.
Highly purified basal transcription factors do not support repression by 55K.
Some transcriptional repressors have been shown to directly interact with components of the basal transcriptional machinery, e.g., Eve with TBP (72), Kruppel and Mdm-2 with TFIIEβ34 (62, 71), and thyroid hormone receptor with TFIIB (13). Furthermore, Mdm-2 repressed transcription reactions reconstituted with highly purified basal transcription factors (71).
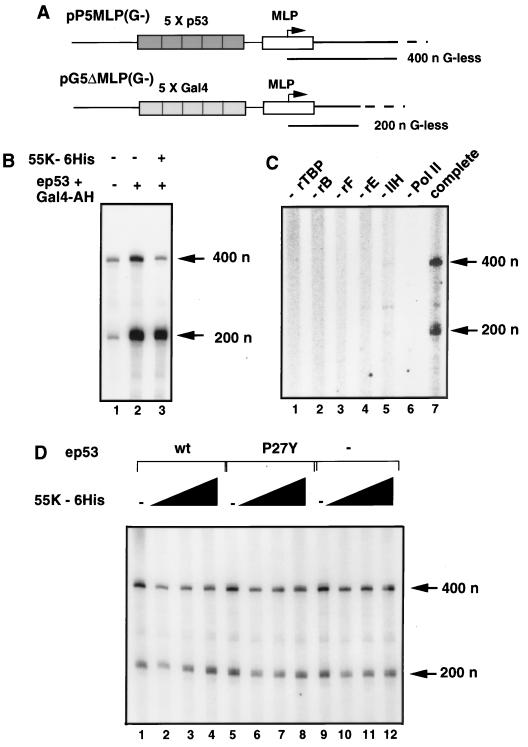
The minimal factors sufficient for basal transcription from the adenovirus type 2 major late promoter on a nonsupercoiled template are TBP, TFIIB, TFIIE, TFIIF, TFIIH, and Pol II (60). Since 55K-His6 repressed basal transcription reactions reconstituted with rTBP, rTFIIB, and the CB protein fraction (Fig. 5), we examined whether it could inhibit transcription reactions reconstituted with the minimal basal transcription factors alone. G-less cassette templates pP5MLP(G−), which has five p53-binding sites, and pG5ΔMLP(G−), which has five Gal4-binding sites and was included as an internal control, were constructed to assay transcription with the G-less cassette assay (Fig. 6A). 55K-His6 specifically repressed transcription activated by wild-type ep53 from the template with p53-binding sites when reactions were reconstituted with the partially purified factor fractions AB, CB, and DB and rTFIIB (Fig. 6B).
FIG. 6.
Highly purified basal transcription factors are not sufficient for repression by 55K-His6. (A) G-less cassette templates used in the in vitro transcription reactions. pP5MLP(G−) contains five p53-binding sites upstream of the adenovirus type 2 major late promoter (MLP) and a G-less cassette of 400 nucleotides (n). pG5ΔMLP(G−) contains five Gal4-binding sites upstream of the MLP and a G-less cassette of 200 nucleotides. (B) 55K-His6 represses p53 activation in G-less cassette assays. Reactions were reconstituted with fractions AB, CB, and DB, rTFIIB, and template DNAs pP5MLP(G−) and pG5ΔMLP(G−) (100 ng each). Activators Gal4-AH and wild-type ep53 were added to lanes 2 and 3, and 8 μl of 55K-His6 was added to lane 3. (C) Requirement for each basal transcription factor in specific transcription. The complete reactions (lane 7) contained templates pP5MLP(G−) and pG5ΔMLP(G−) (100 ng each), rTBP, rTFIIB (rB), rTFIIF (rF), rTFIIE (rE), extensively purified TFIIH (IIH), and affinity-purified Pol II. Other reactions contained all of the above except for the protein indicated above each lane. (D) In vitro transcription reactions were reconstituted with template DNAs pP5MLP(G−) and pG5ΔMLP(G−) (100 ng each) and basal transcription factors rTBP, rTFIIB, rTFIIF, rTFIIE, extensively purified TFIIH, and affinity-purified Pol II. Activators were added to the reactions as follows: Gal4-AH, lanes 1 to 8; wild-type ep53, lanes 1 to 4; and mutant ep53 P27Y, lanes 5 to 8. Activators were omitted from reactions analyzed in lanes 9 to 12. 55K-His6 was added at 2, 4, and 8 μl to lanes 2 to 4, 6 to 8, and 10 to 12, respectively. The G-less transcripts of 400 and 200 nucleotides (n) are indicated.
Significant RNA synthesis was observed when basal transcription factors rTBP, rTFIIB, rTFIIF, and rTFIIE were used in reactions with affinity-purified Pol II and an extensively purified TFIIH fraction (Fig. 6C, lane 7) but not when any one fraction was omitted from the reactions (Fig. 6C, lanes 1 to 6). As expected, the presence or absence of wild-type ep53 or mutant ep53 P27Y had no effect on the level of basal transcription reconstituted with the minimal factors (Fig. 6D, lanes 1, 5, and 9). In reactions performed with these highly purified basal transcription factors, 55K-His6 failed to repress transcription in a dose-dependent manner (Fig. 6D), even though it did repress transcription in reactions with rTFIIB and fractions AB, CB, and DB (Fig. 6B). These results therefore suggested that the minimal basal transcription factors were not sufficient to support repression by 55K and that a cofactor(s) present in the reactions with less highly purified basal transcription factors was required. rTBP, rTFIIB, and the partially purified CB fraction containing Pol II, TFIIE, TFIIF, and TFIIH were sufficient for repression (Fig. 5, lanes 10 to 14), whereas rTFIIE, rTFIIF, and highly purified TFIIH and Pol II substituted for the CB fraction were not. Consequently, the CB fraction most probably contains a host cell corepressor(s) required by 55K-His6 for its repressing function.
The cofactor required for repression by 55K copurifies with RNA Pol II.
The RNA Pol II holoenzyme has been biochemically purified from both yeast and human cells as a large multiprotein complex with an estimated size of approximately 2 MDa (9, 34, 50, 53, 69). Interestingly, the holoenzyme has been implicated as being important for both activation and repression of transcription (6, 16). The components required for repression in yeast include Srb8, Srb9, Srb10, Srb11, Sin4, Rox3, Gal11, and Rgr1 (24, 29, 34, 38, 41, 63, 64). The RNA Pol II holoenzyme isolated from HeLa cells has been shown to contain homologues of two of these proteins: CDK8, homologous to Srb10, and cyclin C, homologous to Srb11 (50).
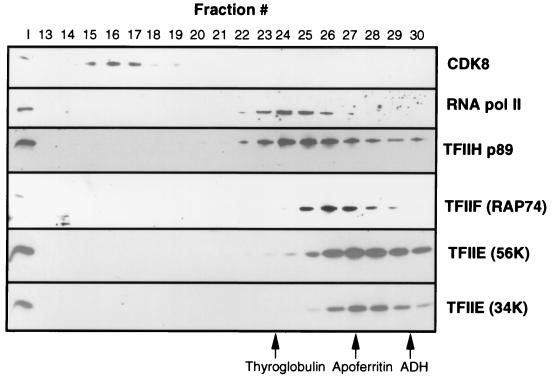
To determine if Pol II in the CB fraction was associated with holoenzyme components, the CB fraction was fractionated by gel filtration over an FPLC Superose 6 gel filtration column. The fractions were analyzed by SDS-PAGE and Western blotting to determine where Pol II, the GTFs, and a component of the holoenzyme, CDK8, eluted (Fig. 7). The majority of Pol II eluted with a molecular mass smaller than that of thyroglobulin (670 kDa). The other GTFs, TFIIF (RAP74), TFIIH (p89), TFIIE56K, and TFIIE34K, eluted at the volumes expected for the purified proteins and were not associated with Pol II. Interestingly, the protein complex containing CDK8 was extremely large, being just included in the column. However, we did not detect any Pol II associated with the CDK8 fraction. Clearly, under the conditions of transcription factor preparation used here, most of the Pol II in the CB fraction was not in a holoenzyme complex.
FIG. 7.
Analysis of general transcription factors, Pol II, and CDK8 in the CB fraction by Superose 6 FPLC. After chromatography, 5% of each fraction indicated was analyzed by SDS-PAGE and Western blotting with antibodies against the factors indicated on the right. I, 1% input loaded. The peak fractions in which the protein standards thyroglobulin (670 kDa), apoferritin (440 kDa), and alcohol dehydrogenase (ADH) (150 kDa) eluted are indicated at the bottom.
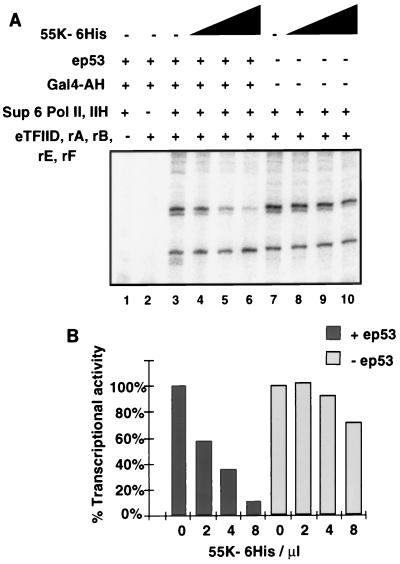
We next tested whether Pol II and TFIIH in the peak Superose 6 fraction (fraction 24) (Sup6 Pol II/IIH) were sufficient for transcription in assays with rTFIIA, rTFIIB, rTFIIE, rTFIIF, and highly purified eTFIID. The Sup6 Pol II/IIH fraction was not sufficient for specific transcription in vitro in the absence of the other highly purified factors (Fig. 8A, lane 1), nor were the recombinant GTFs and eTFIID alone sufficient for RNA synthesis in the absence of the Sup6 Pol II/IIH fraction (lane 2). However, when all factors were reconstituted together, significant RNA synthesis occurred (Fig. 8A, lane 7). Factors required for activated transcription were absent from this highly purified system, since similar levels of transcription were observed in the presence or absence of the activators ep53 and Gal4-AH (Fig. 8A, lanes 3 and 7). Significantly, the addition of 55K-His6 to reactions reconstituted with the factors and ep53 resulted in significant dose-dependent specific repression of transcription (10-fold) directed from the p5BSE4CAT template, whereas transcription from pG5E1BCAT was unaffected by 55K-His6 (Fig. 8A, lanes 4 to 6). In the absence of ep53, there was little repression of transcription from either template (Fig. 8A, lanes 8 to 10). These results indicate that these factors were sufficient for repression when 55K-His6 was brought to the template by its interaction with ep53. The Sup6 Pol II/IIH fraction therefore contains a cofactor required for repression by 55K-His6. Since this cofactor copurifies with the fraction containing Pol II, either it could be directly associated with Pol II or it could elute with a molecular mass similar to that of core Pol II.
FIG. 8.
Sup6 Pol II/IIH fractions contain a corepressor required by 55K-His6 for specific repression. (A) In vitro transcription reactions were reconstituted with eTFIID, rTFIIA (rA), rTFIIB (rB), rTFIIF (rF), rTFIIE (rE), and Sup6 Pol II/IIH (lanes 3 to 10) either in the presence of activators wild-type ep53 and Gal4-AH (lanes 3 to 6) or in the absence of activators (lanes 7 to 10). Increasing concentrations (2, 4, and 8 μl) of 55K-His6 were added to reactions in lanes 4 to 6 and 8 to 10, respectively. Reactions containing only a subset of factors, i.e., Sup6 Pol II/IIH (lane 1) or eTFIID, rTFIIA, rTFIIB, rTFIIF, and rTFIIE (lane 2), did not support specific transcription. Transcription reactions were assayed by primer extension. (B) Quantitation of the effect of 55K-His6 on percent transcriptional activity in the presence (+) and absence (−) of ep53. Transcriptional activity was normalized by dividing the counts from transcripts of p5BSE4CAT by those from transcripts of pG5E1BCAT. Activity in the absence of 55K-His6 was set at 100% in both the presence and the absence of ep53.
DISCUSSION
An adenovirus 5 mutant constructed to be completely deficient in E1B 55K function (5) is currently being evaluated in human clinical trials for its effectiveness as an antitumor therapy (40). Consequently, it is important to fully understand the functions of E1B 55K during a wild-type adenovirus 5 infection. Previous studies have shown that E1B 55K forms a dimer (51) that is recruited to p53-regulated promoters through its interaction with p53 (51, 61, 79) and then represses p53-activated transcription (51, 78, 79). Even though E1B 55K binds the p53 activation domain (32, 44) and therefore might block p53 activation by sterically interfering with activation domain interactions, mutational analyses suggested that the p53-55K interaction was not sufficient for repression (78). Instead, E1B 55K possesses a repression domain which is required for inhibiting p53-activated transcription (67, 79). Furthermore, a Gal4-55K fusion repressed several different promoters containing Gal4 DNA-binding sites, suggesting that the repression domain probably inhibits a process required for transcription from most promoters (79).
An in vitro transcription assay system that was established with purified baculovirus vector-produced E1B 55K accurately mimicked 55K repression observed in vivo, since repression was specific for p53-activated transcription (51). This earlier study revealed that E1B 55K repression occurred in the absence of histones and must therefore operate by a mechanism independent of histone deacetylation. This conclusion was confirmed in the work presented here, where repression was observed in reactions containing extensively purified general transcription factors (Fig. 8). We further demonstrated here that 55K repression requires the tethering of 55K to the promoter through its interaction with p53, since a point mutation in p53 that causes reduced affinity for E1B 55K results in markedly reduced repression (Fig. 2). We also found that E1B 55K represses transcription under conditions where there is no p53 activation in reactions without TFIIA and with TBP substituted for TFIID (Fig. 5). Consequently, these experiments establish that E1B 55K inhibits basal transcription. As a consequence of this mechanism, E1B 55K expression by adenovirus converts p53 from a regulated activator to a p53-E1B 55K complex with a high affinity for p53-binding sites (51) and capable of repressing any promoter with p53-binding sites. Moreover, since a general step in basal transcription is inhibited, as opposed to a p53-specific activation mechanism, the p53-E1B 55K complex would be expected to inhibit transcription no matter what classes of activators are associated with a promoter. As a result, the E1B 55K repression mechanism is expected to repress transcription from all promoters with associated p53-binding sites, preventing any p53-induced antiviral response.
Mdm-2, the KRAB domain, and Eve have also been shown to repress both activated and basal transcription (57, 71, 72). This feature, however, is not universal to transcriptional repressors, since the herpes simplex virus type 1 ICP4 protein inhibits VP16-, SP1-, and ICP4-activated transcription in reconstituted reactions with fractionated factors and TFIID but not basal transcription when TBP is substituted for TFIID (17). TFIIA can overcome the action of some repressors, such as DR2 (49); however, TFIIA has no effect on repression by 55K-His6. These results also suggest that since 55K does not require TFIIA for repression, it does not antagonize activator function—specifically, it does not destabilize TFIID-TFIIA complex formation, as has been shown for Dr1 (27).
E1B 55K did not inhibit basal transcription in reactions with purified GTFs (Fig. 6D). A cellular corepressor that copurifies with RNA Pol II is required for E1B 55K repression (Fig. 7 and 8). Corepressors are believed to act as molecular bridges between repression domains and target proteins, such as components of the basal transcriptional machinery. While this cofactor has not yet been identified, we can exclude certain factors which function in conjunction with other repressors. The KRAB box repression domain recruits a corepressor to the promoter, and both the human KAP-1 and the mouse KRIP-1 corepressors have been cloned on the basis of their interaction with a KRAB box repression domain (14, 33). In transient transfection assays, repression by Gal4-KRAB was reversed when this expression vector was cotransfected with a plasmid expressing the KRAB domain. This result probably occurred because the KRAB and Gal4-KRAB proteins compete for the same corepressor present in limiting amounts (14). In similar transient transfection experiments, we found that repression by Gal4-55K could not be reversed by cotransfection of a KRAB box expression vector under conditions where Gal4-KRAB repression was inhibited (unpublished results).
The USA fraction contains both positive cofactors PC1 (poly-ADP-ribose polymerase), PC2, PC3 (DR2, DNA topoisomerase I), ACF (activating cofactor), PC4, HMG1, and HMG2 and negative cofactors NC1, NC2, and Dr1 (31). These negative cofactors are not required by 55K, since repression occurs in transcription reactions reconstituted with rTBP or highly purified eTFIID in place of the DB fraction, from which the USA factors are derived.
Genetic analyses with yeast indicated that the Srb10/Srb11-Cdk/cyclin complex in the holoenzyme contributes to the transcriptional repression of diversely regulated genes (38, 64). The experiments reported here suggest that the human homologues of these components are not part of the E1B 55K corepressor, since the human homologue of Srb10, CDK8, was separated by Superose 6 gel filtration chromatography from the partially purified Pol II fraction, which does support E1B 55K repression (Fig. 7 and 8).
We examined the effect of PIC formation on repression by E1B 55K. When 55K-His6 was added to a transcription reaction after assembly of the PIC on template DNA, transcription was resistant to repression (Fig. 4). Apparently, 55K and its corepressor must be bound to a promoter region before PIC assembly in order to interact with the PIC components required to inhibit transcription. The target(s) of 55K repression may be sterically blocked from interacting with 55K and its corepressor in the fully assembled, activated PIC. Alternatively, the binding site for E1B 55K in the p53 activation domain may be sterically blocked in the fully assembled, activated PIC. Both Mdm-2 and E1B 55K gain their promoter specificity by binding to the N-terminal region of p53. Whereas E1B 55K requires a corepressor to inhibit transcription, it is unlikely that Mdm-2 utilizes a corepressor, since repression was observed with highly purified basal transcription factors (71). It will, however, be interesting to determine whether the corepressor for E1B 55K augments the activity of Mdm-2. These studies have extended our understanding of E1B 55K repression of transcription from p53-activated genes by demonstrating that 55K must bind to p53 to repress transcription; that a step in basal transcription is targeted by 55K, permitting the repression of diverse promoters with one or more p53-binding sites; and that the target of 55K repression is not available in a fully assembled, activated transcription complex. Further work is required to identify the cellular corepressor that copurifies with RNA Pol II and that functions in conjunction with E1B 55K and to establish the function of this corepressor in uninfected cells.
ACKNOWLEDGMENTS
We thank J.-P. Tassan, Erich Nigg, Paula Rickert, and Emma Lees for antibodies against CDK8. We are grateful to Dean Tantin and Mike Carey for generously providing the TFIIH and Gal4-AH proteins used in these studies. We thank Arnold Levine for the plasmid containing the p53 P27Y sequences and Richard Burgess for providing 8WG16 hybridoma cells.
This work was supported by USPHS grant CA 64799.
REFERENCES
- 1.Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho R A. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 2.Auble D T, Hansen K E, Mueller C G, Lane W S, Thorner J, Hahn S. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 1994;8:1920–1934. doi: 10.1101/gad.8.16.1920. [DOI] [PubMed] [Google Scholar]
- 3.Austin R J, Biggin M D. A domain of the even-skipped protein represses transcription by preventing TFIID binding to a promoter: repression by cooperative blocking. Mol Cell Biol. 1995;15:4683–4693. doi: 10.1128/mcb.15.9.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barberis A, Pearlberg J, Simkovich N, Farrell S, Reinagel P, Bamdad C, Sigal G, Ptashne M. Contact with a component of the polymerase II holoenzyme suffices for gene activation. Cell. 1995;81:359–368. doi: 10.1016/0092-8674(95)90389-5. [DOI] [PubMed] [Google Scholar]
- 5.Barker D D, Berk A J. Adenovirus proteins from both E1B open reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1986;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 6.Berk A J. Biochemistry meets genetics in the holoenzyme. Proc Natl Acad Sci USA. 1995;92:11952–11954. doi: 10.1073/pnas.92.26.11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischoff J R, Kirn D H, Williams A, Heise C, Horn S, Muna M, Ng L, Nye J A, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 8.Bryant G O, Martel L S, Burley S K, Berk A J. Radical mutations reveal TATA-box binding protein surfaces required for activated transcription in vivo. Genes Dev. 1996;10:2491–2504. doi: 10.1101/gad.10.19.2491. [DOI] [PubMed] [Google Scholar]
- 9.Chao D M, Gadbois E L, Murray P J, Anderson S F, Sonu M S, Parvin J D, Young R A. A mammalian SRB protein assiociated with an RNA polymerase II holoenzyme. Nature. 1996;380:82–85. doi: 10.1038/380082a0. [DOI] [PubMed] [Google Scholar]
- 10.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobbelstein M, Roth J, Kimberly W T, Levine A J, Shenk T. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J. 1997;16:4276–4284. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dynlacht B D, Hoey T, Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991;66:563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- 13.Fondell J D, Roy A L, Roeder R G. Unliganded thyroid hormone receptor inhibits formation of a functional preinitiation complex: implications for active repression. Genes Dev. 1993;7:1400–1410. doi: 10.1101/gad.7.7b.1400. [DOI] [PubMed] [Google Scholar]
- 14.Friedman J R, Fredericks W J, Jensen D E, Speicher D W, Huang X P, Neilson E G, Rauscher F J., III KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996;10:2067–2078. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- 15.Gabler S, Scheutt H, Groitl P, Wolf H, Shenk T, Dobner T. E1B 55-kilodalton-associated protein: a cellular protein with RNA-binding activity implicated in nucleocytoplasmic transport of adenovirus and cellular mRNAs. J Virol. 1998;72:7960–7971. doi: 10.1128/jvi.72.10.7960-7971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenblatt J. RNA polymerase II holoenzyme and transcriptional regulation. Curr Opin Cell Biol. 1997;9:310–319. doi: 10.1016/s0955-0674(97)80002-6. [DOI] [PubMed] [Google Scholar]
- 17.Gu B, Kuddus R, DeLuca N A. Repression of activator-mediated transcription by herpes simplex virus ICP4 via a mechanism involving interactions with the basal transcription factors TATA-binding protein and TFIIB. Mol Cell Biol. 1995;15:3618–3626. doi: 10.1128/mcb.15.7.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 19.Ha I, Lane W S, Reinberg D. Cloning of a human gene encoding the general transcription initiation factor IIB. Nature. 1991;352:689–695. doi: 10.1038/352689a0. [DOI] [PubMed] [Google Scholar]
- 20.Hassig C A, Fleischer T C, Billin A N, Schreiber S L, Ayer D E. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 21.Hawley D K, Roeder R G. Separation and partial characterization of 3 functional steps in transcription initiation by human RNA polymerase-II. J Biol Chem. 1985;260:8163–8172. [PubMed] [Google Scholar]
- 22.Heinzel T, Lavinsky R M, Mullen T M, Soderstrom M, Laherty C D, Torchia J, Yang W M, Brard G, Ngo S D, Davie J R, Seto E, Eisenman R N, Rose D W, Glass C K, Rosenfeld M G. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 23.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff D D, Kirn D H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 24.Hengartner C J, Thompson C M, Zhang J, Chao D M, Liao S-M, Koleske A J, Okamura S, Young R A. Association of an activator with an RNA polymerase II holoenzyme. Genes Dev. 1995;9:897–910. doi: 10.1101/gad.9.8.897. [DOI] [PubMed] [Google Scholar]
- 25.Holstege F C, Tantin D, Carey M, van der Vliet P C, Timmers H T. The requirement for the basal transcription factor IIE is determined by the helical stability of promoter DNA. EMBO J. 1995;14:810–819. doi: 10.1002/j.1460-2075.1995.tb07059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 27.Inostroza J A, Mermelstein F H, Ha I, Lane W S, Reinberg D. Dr1, a TATA-binding protein-associated phosphoprotein and inhibitor of class II gene transcription. Cell. 1992;70:477–489. doi: 10.1016/0092-8674(92)90172-9. [DOI] [PubMed] [Google Scholar]
- 28.Jayaraman J, Prives C. Activation of p53 sequence-specific DNA binding by short single strands of DNA requires the p53 C-terminus. Cell. 1995;81:1021–1029. doi: 10.1016/s0092-8674(05)80007-8. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y W, Dohrmann P R, Stillman D J. Genetic and physical interactions between yeast RGR1 and SIN4 in chromatin organization and transcriptional regulation. Genetics. 1995;140:47–54. doi: 10.1093/genetics/140.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadosh D, Struhl K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell. 1997;89:365–371. doi: 10.1016/s0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser K, Meisterernst M. The human general co-factors. Trends Biochem Sci. 1996;21:342–345. [PubMed] [Google Scholar]
- 32.Kao C C, Yew P R, Berk A J. Domains required for in vitro association between the cellular p53 and the adenovirus 2 E1B 55K proteins. Virology. 1990;179:806–814. doi: 10.1016/0042-6822(90)90148-k. [DOI] [PubMed] [Google Scholar]
- 33.Kim S S, Chen Y M, O’Leary E, Witzgall R, Vidal M, Bonventre J V. A novel member of the RING finger family, KRIP-1, associates with the KRAB-A transcriptional repressor domain of zinc finger proteins. Proc Natl Acad Sci USA. 1996;93:15299–15304. doi: 10.1073/pnas.93.26.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y-J, Bjorklund S, Li Y, Sayre M H, Kornberg R D. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 35.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 36.Ko L J, Shieh S Y, Chen X, Jayaraman L, Tamai K, Taya Y, Prives C, Pan Z Q. p53 is phosphorylated by CDK7-cyclin H in a p36MAT1-dependent manner. Mol Cell Biol. 1997;17:7220–7229. doi: 10.1128/mcb.17.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi N, Boyer T G, Berk A J. A class of activation domains interacts directly with TFIIA and stimulates TFIIA-TFIID-promoter complex assembly. Mol Cell Biol. 1995;15:6465–6473. doi: 10.1128/mcb.15.11.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchin S, Yeghiayan P, Carlson M. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc Natl Acad Sci USA. 1995;92:4006–4010. doi: 10.1073/pnas.92.9.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laherty C D, Yang W M, Sun J M, Davie J R, Seto E, Eisenman R N. Histone deacetylases associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 40.Lane D P. Killing tumor cells with viruses—a question of specificity. Nat Med. 1998;4:1012–1013. doi: 10.1038/2000. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Bjorklund S, Jiang Y W, Kim Y-J, Lane W S, Stillman D J, Kornberg R D. Yeast global transcriptional regulators Sin4 and Rgr1 are components of mediator complex-RNA polymerase II holoenzyme. Proc Natl Acad Sci USA. 1995;92:10864–10868. doi: 10.1073/pnas.92.24.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao S-M, Zhang J, Jeffery D A, Koleske A J, Thompson C M, Chao D M, Viljoen M, van Vuuren H J J, Young R A. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- 43.Lillie J W, Green M R. Transcription activation by the adenovirus E1a protein. Nature. 1989;338:39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- 44.Lin J, Chen J, Elenbaas B, Levine A J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994;8:1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 45.Lin Y S, Carey M F, Ptashne M, Green M R. GAL4 derivatives function alone and synergistically with mammalian activators in vitro. Cell. 1988;54:659–664. doi: 10.1016/s0092-8674(88)80010-2. [DOI] [PubMed] [Google Scholar]
- 46.Lin Y S, Green M R. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991;64:971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Berk A J. Reversal of in vitro p53 squelching by both TFIIB and TFIID. Mol Cell Biol. 1995;15:6474–6478. doi: 10.1128/mcb.15.11.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo R X, Postigo A A, Dean D C. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- 49.Ma D, Watanabe H, Mermelstein F, Admon A, Oguri K, Sun X, Wada T, Imai T, Shiroya T, Reinberg D, Handa H. Isolation of a cDNA encoding the largest subunit of TFIIA reveals functions important for activated transcription. Genes Dev. 1993;7:2246–2257. doi: 10.1101/gad.7.11.2246. [DOI] [PubMed] [Google Scholar]
- 50.Maldonado E, Shiekhattar R, Sheldon M, Cho H, Drapkin R, Rickert P, Lees E, Anderson C W, Linn S, Reinberg D. A human RNA polymerase II complex associated with SRB and DNA-repair proteins. Nature. 1996;381:86–89. doi: 10.1038/381086a0. [DOI] [PubMed] [Google Scholar]
- 50a.Martin, M. E. D. Unpublished results.
- 51.Martin M E D, Berk A J. Adenovirus E1B 55K represses p53-activated transcription in vitro. J Virol. 1998;72:3146–3154. doi: 10.1128/jvi.72.4.3146-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagy L, Kao H Y, Chakravarti D, Lin R J, Hassig C A, Ayer D E, Schreiber S L, Evans R M. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 53.Ossipow V, Tassan J-P, Nigg E A, Schibler U. A mammalian RNA polymerase II holoenzyme containing all components required for promoter-specific transcription initiation. Cell. 1995;83:137–146. doi: 10.1016/0092-8674(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 54.Ozer J, Moore P A, Bolden A H, Lee A, Rosen C A, Lieberman P M. Molecular cloning of the small (g) subunit of human TFIIA reveals functions critical for activated transcription. Genes Dev. 1994;8:2324–2335. doi: 10.1101/gad.8.19.2324. [DOI] [PubMed] [Google Scholar]
- 55.Parvin J D, Shykind B M, Meyers R E, Kim J, Sharp P A. Multiple sets of basal factors initiate transcription by RNA polymerase II. J Biol Chem. 1994;269:18414–18421. [PubMed] [Google Scholar]
- 56.Pazin M J, Kadonaga J T. What’s up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 57.Pengue G, Lania L. Kruppel-associated box-mediated repression of RNA polymerase II promoters is influenced by the arrangement of basal promoter elements. Proc Natl Acad Sci USA. 1996;93:1015–1020. doi: 10.1073/pnas.93.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peterson M G, Inostroza J, Maxon M E, Flores O, Admon A, Reinberg D, Tjian R. Structure and functional properties of human general transcription factor IIE. Nature. 1991;354:369–373. doi: 10.1038/354369a0. [DOI] [PubMed] [Google Scholar]
- 59.Rickert P, Seghezzi W, Shanahan F, Cho H, Lees E. Cyclin C/CDK8 is a novel CTD kinase associated with RNA polymerase II. Oncogene. 1996;12:2631–2640. [PubMed] [Google Scholar]
- 60.Roeder R G. The role of general transcription factors in transcription by RNA polymerase II. Trends Biochem Sci. 1996;21:327–335. [PubMed] [Google Scholar]
- 61.Sarnow P, Ho Y S, Williams J, Levine A J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982;28:387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- 62.Sauer F, Fondell J D, Ohkuma Y, Roeder R G, Jackle H. Control of transcription by Kruppel through interactions with TFIIB and TFIIE β. Nature. 1995;375:162–164. doi: 10.1038/375162a0. [DOI] [PubMed] [Google Scholar]
- 63.Shimizu M, Li W, Shindo H, Mitchell A P. Transcriptional repression at a distance through exclusion of activator binding in vivo. Proc Natl Acad Sci USA. 1997;94:790–795. doi: 10.1073/pnas.94.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song W, Treich I, Qian N, Kuchin S, Carlson M. SSN genes that affect transcriptional repression in Saccharomyces cerevisiae encode SIN4, ROX3, and SRB proteins associated with RNA polymerase II. Mol Cell Biol. 1996;16:115–120. doi: 10.1128/mcb.16.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tantin D, Kansal A, Carey M. Recruitment of the putative transcription repair coupling factor CSB/ERCC6 to RNA polymerase II elongation complexes. Mol Cell Biol. 1997;17:6803–6814. doi: 10.1128/mcb.17.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tassan J P, Jaquenoud M, Leopold P, Schultz S J, Nigg E A. Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C. Proc Natl Acad Sci USA. 1995;92:8871–8875. doi: 10.1073/pnas.92.19.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teodoro J G, Branton P E. Regulation of p53-dependent apoptosis, transcriptional repression, and cell transformation by phosphorylation of the 55-kilodalton E1B protein of human adenovirus type 5. J Virol. 1997;71:3620–3627. doi: 10.1128/jvi.71.5.3620-3627.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teodoro J G, Halliday T, Whalen S G, Takayesu D, Graham F L, Branton P E. Phosphorylation at the carboxy terminus of the 55-kilodalton adenovirus type 5 E1B protein regulates transforming activity. J Virol. 1994;68:776–786. doi: 10.1128/jvi.68.2.776-786.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson C M, Koleske A J, Chao D M, Young R A. A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell. 1993;73:1361–1375. doi: 10.1016/0092-8674(93)90362-t. [DOI] [PubMed] [Google Scholar]
- 70.Thompson N E, Aronson D B, Burgess R R. Purification of eukaryotic RNA polymerase II by immunoaffinity chromatography. J Biol Chem. 1990;265:7069–7077. [PubMed] [Google Scholar]
- 71.Thut C J, Goodrich J A, Tjian R. Repression of p53-mediated transcription by MDM2: a dual mechanism. Genes Dev. 1997;11:1974–1986. doi: 10.1101/gad.11.15.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Um M, Li C, Manley J L. The transcriptional repressor even-skipped interacts directly with TATA-binding protein. Mol Cell Biol. 1995;15:5007–5016. doi: 10.1128/mcb.15.9.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verrijzer C P, Tjian R. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem Sci. 1996;9:338–342. [PubMed] [Google Scholar]
- 74.Wang B Q, Kostrub C F, Finkelstein A, Burton Z F. Production of human RAP30 and RAP74 in bacterial cells. Protein Expression Purification. 1993;4:207–214. doi: 10.1006/prep.1993.1027. [DOI] [PubMed] [Google Scholar]
- 75.Wang B Q, Lei L, Burton Z F. Importance of codon preference for production of human RAP74 and reconstitution of the RAP30/74 complex. Protein Expression Purification. 1994;5:476–485. doi: 10.1006/prep.1994.1067. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Prives C. Increased and altered DNA binding of human p53 by S and G2/M but not G1 cyclin-dependent kinases. Nature. 1995;376:88–91. doi: 10.1038/376088a0. [DOI] [PubMed] [Google Scholar]
- 77.White E. Regulation of p53-dependent apoptosis by E1A and E1B. Curr Top Microbiol Immunol. 1995;199:34–58. [PubMed] [Google Scholar]
- 78.Yew P R, Berk A J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992;357:82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 79.Yew P R, Liu X, Berk A J. Adenovirus E1B oncoprotein tethers a transcriptional repression domain to p53. Genes Dev. 1994;8:190–202. doi: 10.1101/gad.8.2.190. [DOI] [PubMed] [Google Scholar]
- 80.Zantema A, Schrier P I, Davis-Olivier A, van Laar T, Vaessen R T, van der Eb A J. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol Cell Biol. 1985;11:3084–3091. doi: 10.1128/mcb.5.11.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357–364. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 82.Zhou Q, Lieberman P M, Boyer T G, Berk A J. Holo-TFIID supports transcriptional stimulation by diverse activators and from a TATA-less promoter. Genes Dev. 1992;6:1964–1974. doi: 10.1101/gad.6.10.1964. [DOI] [PubMed] [Google Scholar]