Abstract
Japanese encephalitis virus (JEV) is the etiological agent of Japanese encephalitis (JE). The most commonly used vaccine used to prevent JE is the live-attenuated strain SA14-14-2, which was generated by serial passage of the wild-type (WT) JEV strain SA14. Two other vaccine candidates, SA14-5-3 and SA14-2-8 were derived from SA14. Both were shown to be attenuated but lacked sufficient immunogenicity to be considered effective vaccines. To better contrast the SA14-14-2 vaccine with its less-immunogenic counterparts, genetic diversity, ribavirin sensitivity, mouse virulence and mouse immunogenicity of the three vaccines were investigated. Next generation sequencing demonstrated that SA14-14-2 was significantly more diverse than both SA14-5-3 and SA14-2-8, and was slightly less diverse than WT SA14. Notably, WT SA14 had unpredictable levels of diversity across its genome whereas SA14-14-2 is highly diverse, but genetic diversity is not random, rather the virus only tolerates variability at certain residues. Using Ribavirin sensitivity in vitro, it was found that SA14-14-2 has a lower fidelity replication complex compared to SA14-5-3 and SA14-2-8. Mouse virulence studies showed that SA14-2-8 was the most virulent of the three vaccine strains while SA14-14-2 had the most favorable combination of safety (virulence) and immunogenicity for all vaccines tested. SA14-14-2 contains genetic diversity and sensitivity to the antiviral Ribavirin similar to WT parent SA14, and this genetic diversity likely explains the (1) differences in genomic sequences reported for SA14-14-2 and (2) the encoding of major attenuation determinants by the viral E protein.
Subject terms: Live attenuated vaccines
Introduction
Japanese encephalitis virus (JEV) is the prototype member of the JE serogroup within the genus Flavivirus, which contains other mosquito-borne encephalitic viruses such as West Nile and St. Louis encephalitis. JEV is the leading cause of epidemic, viral encephalitis in mainland Asia and the Western Pacific causing an estimated 57,000 to 175,000 cases annually with a 20–30% case fatality rate. Thirty to fifty percent of those who survive the acute infection report serious neurological sequelae1,2. There are no licensed antivirals to treat the disease making vaccination the primary control strategy. A number of JEV vaccines have been licensed including live attenuated (LAV), chimeric LAVs and inactivated vaccines. The most commonly used in Asia is the LAV strain SA14-14-2, which is highly efficacious showing 90% seroconversion after a single immunization in multiple clinical trials3–5.
Strain SA14-14-2 was derived from the wild-type (WT) strain SA14 by plaque purification after serial passage in primary hamster kidney (PHK) cells and suckling mice6. The genomic sequences of SA14-14-2 and SA14 have been published a number of times but the published sequences differ between groups, which makes determination of attenuating mutations difficult7–12. While one study reports that there are no genomic changes after passage of SA14-14-2 in cell culture, mice or mosquitoes13 others report consensus changes after very few passages in similar substrates. These changes include three amino acid substitutions after passage in C6/36 cells, four amino acid substitutions after 22 passages in PHK cells, one amino acid substitution after passage in baby hamster kidney (BHK) cells, one amino acid substitution after passage in Vero cells, and four amino acid substitutions after passage in mouse brain10,14,15. There have been limited studies to resolve the differences in genomic sequences12,16 or the conditions that favor changes to the vaccine genotype17.
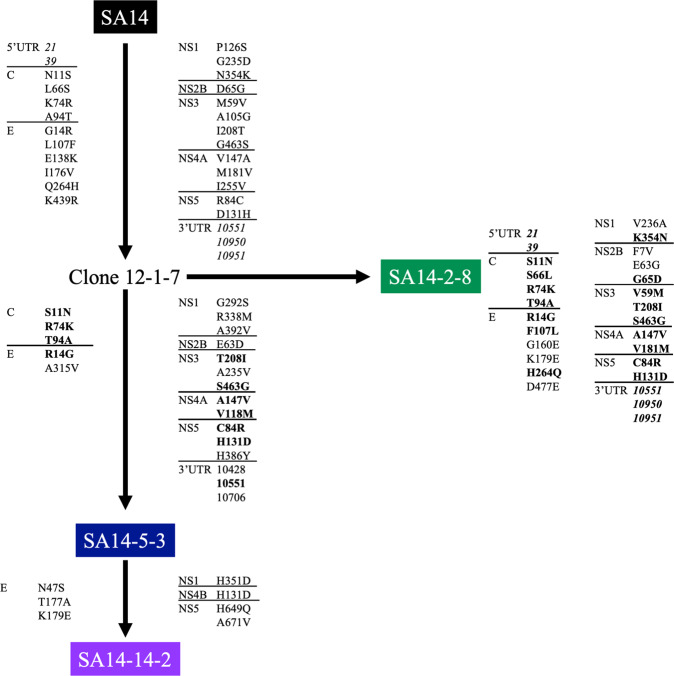
There were multiple attempts to develop LAVs from SA14 prior to the generation of SA14-14-2 (see Fig. 1). Clone 12-1-7 was derived by plaque purification after passage in newborn mice and PHK cells. It was then UV irradiated, and plaque purified in PHK cells to produce strain SA14-2-818,19. However, SA14-2-8 was poorly immunogenic in humans, producing seroconversion in only 50% of individuals, and was therefore used exclusively as an equine vaccine8,18,20,21. Strain SA14-5-3 was also derived from Clone12-1-7 following passage in mice and PHK cells but it was also over-attenuated with low immunogenicity in clinical trials22,23. Subsequently, SA14-5-3 was passaged in suckling mice and plaque purified, to derive SA14-14-2, which demonstrates enhanced immunogenicity while retaining an attenuated phenotype6,13,23,24.
Fig. 1. Derivation of JEV vaccine strains.
The successful SA14-14-2 vaccine strain was derived after multiple attempts to attenuate WT strain SA14. The first of these attempts, Clone12-1-7, was generated by mouse and cell culture passage. It was then UV irradiated to generate SA14-2-8 and further mouse passaged to generate SA14-5-3. Both of these vaccine strains were shown to be overly attenuated in humans so SA14-5-3 was passaged in suckling mice which generated the currently utilized SA14-14-2.
JEV strains fall into a single serogroup that contains five genotypes. All current JEV vaccines are derived from genotype III. Licensed JEV vaccines appear to provide protective immunity against infection by the different genotypes, although the situation for genotype V is unclear25–27. To date there are only three isolates of genotype V and there are limited cross-neutralization data in vitro. Mouse studies have shown that sera from mice immunized with SA14-5-3 and SA14-2-8 exhibit limited cross neutralization against multiple genotypes whereas SA14-14-2 does provide effective cross neutralization24,27–29.
The flavivirus genome consists of three structural (capsid [C], premembrane [prM] and envelope [E]) and seven non-structural (NS) genes (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5). The E protein is the major antigenic driver of the humoral immune response to the virus, thereby dictating the success of vaccine cross-protection. Studies have implicated substitutions in the E protein as the major determinants of attenuation of SA14-14-29,13,15,17,30–38 with the NS proteins, which constitute the replication complex, playing no or a very limited role11,17,39,40. In fact, a chimeric SA14 virus containing SA14-14-2 prM/E genes was completely attenuated in a 3-week-old Kumming mouse model, demonstrating the importance of E protein in attenuation41. This mechanism of attenuation differs from that of another flavivirus LAV, yellow fever (YF) 17D where both structural and NS proteins contribute to the attenuated phenotype. It has been shown that the genome of the 17D vaccine virus is significantly less diverse than the WT parent strain from which it was derived42,43. The homogenous genotype of 17D has been attributed to the increased fidelity of the replication complex, which was shown using resistance to the nucleoside analog Ribavirin and is thought to contribute to the stability and safety of the vaccine44. Next generation sequencing (NGS) technology has yet to be applied to JEV in these contexts and so the diversity of the WT SA14 and vaccine strains is unknown. The variability of the SA14-14-2 genomic sequences described above suggests it displays more diversity than YFV 17D, potentially distinguishing their mechanisms of attenuation despite similar empirical derivation strategies and clinical efficacies.
WT JEV infection has been modeled using adult and suckling mice, and multiple outbred and inbred strains10,11,13,17,31,34,45–49. In general, other than newborn mice, WT JEV strains only cause a lethal infection when given intracranially or by a peripheral route after the blood brain barrier has been perturbed whereas it has been shown that SA14-14-2 is avirulent in suckling mice by both intracranial and intraperitoneal routes (Yang, 2014). JEV LAVs are 100% attenuated in most of these models and in many cases are cleared so efficiently that no neutralizing antibody response is generated. For SA14-14-2, a dose of one million pfu is non-lethal in immunocompetent mice even if the virus is administered directly into the brain36. SA14-14-2 has only shown virulence in interferon-αβγ receptor knockout (AG129) mice47.
In order to better understand the attenuated phenotype of SA14-14-2, we have undertaken a series of studies that compare genetic diversity, mouse virulence phenotype and immunogenicity of the vaccine strains derived from WT strain SA14 (SA14-14-2, SA14-5-3 and SA14-2-8). We demonstrate that SA14-14-2 has both high genetic diversity and sensitivity to the antiviral Ribavirin similar to WT parent SA14. This apparent genetic diversity likely explains the differences in genomic sequences reported for SA14-14-2 plus why the E protein encodes the major determinants of attenuation. In addition, despite displaying a mechanism of attenuation that distinguishes it from YF 17D LAV, SA14-14-2 maintains a similar level of attenuation in susceptible mouse models.
Results
Consensus genomic sequences of WT SA14 and JEV vaccine viruses used in studies
The consensus sequences of virus stocks used in the studies were determined by NGS and compared to published sequences of SA14 (GenBank references KU323483, U14163, KU821122, MH258848, KX254415, KU871316)10,12 (Table S1), SA14-14-2 (GenBank references: MH258849,MH258850, MH258851, MH258852, MH258853, KX254416, KX254417, D90195, MK585066, AF315119 and JN604986)10,50–52 (Table 1), SA14-2-8 (Genbank reference: U15763)12 (Table S2) and SA14-5-3 (Genbank reference: U04521) (Table S3). Only a partial sequence has been published for SA14-5-3.
Table 1.
Comparison of SA14-14-2 in this study and twelve previously genomic sequences from Genbank.
| GenBank Accession number | AF416457 | MH258851 | MH258849 | MH258853 | MH258852 | MH258850 | KX254416 | KX254417 | D90195 | MK585066 | AF315119 | JN604986 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide Position | CDS Position | Polyprotein | Gene | AA protein # | SA14-14-2 | SA14-2-1-7 | SA14-14-2 | SA14-14-2 | SA14-14-2 | SA14-14-2 | SA14-14-2 | SA14-14-2 | SA14-14-2 | SA14-14-2 | SA14-14-2 | SA14-14-2 | SA14-14-2 |
| 21 | −74 | −24 | 5′UTR | — | — | — | |||||||||||
| 39 | −56 | −18 | 5′UTR | — | — | ||||||||||||
| 59 | −36 | −12 | 5′UTR | — | — | — | - | ||||||||||
| 127 | 32 | 11 | C | 11 | N | S | |||||||||||
| 292 | 197 | 66 | C | 66 | S | ||||||||||||
| 316 | 221 | 74 | C | 74 | K | R | |||||||||||
| 375 | 280 | 94 | C | 94 | A | T | |||||||||||
| 518 | 423 | 141 | prM | 14 | I | * | |||||||||||
| 884 | 789 | 263 | prM | 136 | F | * | |||||||||||
| 1017 | 922 | 308 | E | 14 | G | R | |||||||||||
| 1061 | 966 | 322 | E | 28 | D | ||||||||||||
| 1086 | 991 | 331 | E | 37 | D | N | |||||||||||
| 1117 | 1022 | 341 | E | 47 | S | N | N | N | N | N | N | N | N | N | N | N | N |
| 1217 | 1122 | 374 | E | 80 | A | ||||||||||||
| 1296 | 1201 | 401 | E | 107 | F | ||||||||||||
| 1389 | 1294 | 432 | E | 138 | K | ||||||||||||
| 1503 | 1408 | 470 | E | 176 | V | ||||||||||||
| 1506 | 1411 | 471 | E | 177 | A | T | |||||||||||
| 1512 | 1417 | 473 | E | 179 | E | K | K | K | K | K | K | K | K | K | K | K | K |
| 1532 | 1437 | 479 | E | 185 | E | * | |||||||||||
| 1769 | 1674 | 558 | E | 264 | H | ||||||||||||
| 1813 | 1718 | 573 | E | 279 | K | M | M | M | M | M | M | M | M | ||||
| 1921 | 1826 | 609 | E | 315 | V | A | |||||||||||
| 1977 | 1882 | 628 | E | 334 | P | ||||||||||||
| 2012 | 1917 | 639 | E | 345 | L | * | |||||||||||
| 2143 | 2048 | 683 | E | 389 | D | A | |||||||||||
| 2293 | 2198 | 733 | E | 439 | R | ||||||||||||
| 2317 | 2222 | 741 | E | 447 | G | D | |||||||||||
| 2441 | 2346 | 782 | E | 488 | G | * | |||||||||||
| 2691 | 2596 | 866 | NS1 | 72 | R | ||||||||||||
| 2843 | 2748 | 916 | NS1 | 122 | I | ||||||||||||
| 3351 | 3256 | 1086 | NS1 | 292 | S | G | |||||||||||
| 3493 | 3398 | 1133 | NS1 | 339 | M | R | |||||||||||
| 3516 | 3421 | 1141 | NS1 | 347 | R | ||||||||||||
| 3528 | 3433 | 1145 | NS1 | 351 | H | D | |||||||||||
| 3530 | 3435 | 1145 | NS1 | 351 | H | * | |||||||||||
| 3539 | 3444 | 1148 | NS1 | 354 | K | ||||||||||||
| 3599 | 3504 | 1168 | NS1 | 374 | E | ||||||||||||
| 3652 | 3557 | 1186 | NS1 | 392 | V | A | |||||||||||
| 3677 | 3582 | 1194 | NS1 | 400 | G | ||||||||||||
| 3776 | 3681 | 1227 | NS2A | 18 | A | ||||||||||||
| 3801 | 3706 | 1236 | NS2A | 27 | L | ||||||||||||
| 3929 | 3834 | 1278 | NS2A | 69 | A | * | |||||||||||
| 4106 | 4011 | 1337 | NS2A | 128 | A | * | |||||||||||
| 4250 | 4155 | 1385 | NS2B | 12 | G | * | |||||||||||
| 4403 | 4308 | 1436 | NS2B | 63 | D | E | |||||||||||
| 4408 | 4313 | 1438 | NS2B | 65 | G | ||||||||||||
| 4475 | 4380 | 1460 | NS2B | 87 | L | F | |||||||||||
| 4782 | 4687 | 1563 | NS3 | 59 | V | ||||||||||||
| 4825 | 4730 | 1577 | NS3 | 73 | K | ||||||||||||
| 4862 | 4767 | 1589 | NS3 | 85 | F | * | |||||||||||
| 4921 | 4826 | 1609 | NS3 | 105 | G | ||||||||||||
| 4922 | 4827 | 1609 | NS3 | 105 | G | ||||||||||||
| 5230 | 5135 | 1712 | NS3 | 208 | I | T | |||||||||||
| 5234 | 5139 | 1713 | NS3 | 209 | I | ||||||||||||
| 5311 | 5216 | 1739 | NS3 | 235 | V | A | A | ||||||||||
| 5634 | 5539 | 1847 | NS3 | 343 | R | W | |||||||||||
| 5994 | 5899 | 1967 | NS3 | 463 | G | S | |||||||||||
| 6008 | 5913 | 1971 | NS3 | 467 | N | ||||||||||||
| 6035 | 5940 | 1980 | NS3 | 476 | G | * | |||||||||||
| 6425 | 6330 | 2110 | NS3 | 606 | Q | ||||||||||||
| 6634 | 6539 | 2180 | NS4A | 57 | I | T | |||||||||||
| 6728 | 6633 | 2211 | NS4A | 88 | T | * | |||||||||||
| 6904 | 6809 | 2270 | NS4A | 147 | V | A | |||||||||||
| 6944 | 6849 | 2283 | NS4A | 160 | A | * | |||||||||||
| 7005 | 6910 | 2304 | NS4A | 181 | M | V | |||||||||||
| 7121 | 7026 | 2342 | NS4A | 219 | A | ||||||||||||
| 7193 | 7098 | 2366 | NS4A | 243 | T | ||||||||||||
| 7227 | 7132 | 2378 | NS4A | 255 | V | ||||||||||||
| 7295 | 7200 | 2400 | NS4B | 10 | G | * | * | * | * | * | * | * | * | * | * | ||
| 7655 | 7560 | 2520 | NS4B | 130 | A | * | |||||||||||
| 7656 | 7561 | 2521 | NS4B | 131 | N | D | D | D | D | D | D | D | D | D | D | D | D |
| 7736 | 7641 | 2547 | NS5 | 20 | S | * | |||||||||||
| 7768 | 7673 | 2558 | NS5 | 31 | A | G | |||||||||||
| 7809 | 7714 | 2572 | NS5 | 45 | R | S | |||||||||||
| 7871 | 7776 | 2592 | NS5 | 65 | L | * | |||||||||||
| 7926 | 7831 | 2611 | NS5 | 84 | R | C | |||||||||||
| 8067 | 7972 | 2658 | NS5 | 131 | D | * | |||||||||||
| 8099 | 8004 | 2668 | NS5 | 141 | D | ||||||||||||
| 8261 | 8166 | 2722 | NS5 | 195 | M | I | |||||||||||
| 8276 | 8181 | 2727 | NS5 | 200 | R | * | * | ||||||||||
| 8394 | 8299 | 2767 | NS5 | 240 | L | ||||||||||||
| 8832 | 8737 | 2913 | NS5 | 386 | Y | H | |||||||||||
| 8882 | 8787 | 2929 | NS5 | 402 | I | * | |||||||||||
| 8891 | 8796 | 2932 | NS5 | 405 | V | ||||||||||||
| 9122 | 9027 | 3009 | NS5 | 482 | G | * | * | * | * | * | * | * | * | * | * | * | * |
| 9593 | 9498 | 3166 | NS5 | 639 | H | Q | Q | ||||||||||
| 9688 | 9593 | 3198 | NS5 | 671 | A | V | |||||||||||
| 9695 | 9600 | 3200 | NS5 | 673 | K | ||||||||||||
| 9818 | 9723 | 3241 | NS5 | 714 | C | ||||||||||||
| 9898 | 9803 | 3268 | NS5 | 741 | G | D | |||||||||||
| 9917 | 9822 | 3274 | NS5 | 747 | P | * | |||||||||||
| 9954 | 9859 | 3287 | NS5 | 760 | A | P | |||||||||||
| 9971 | 9876 | 3292 | NS5 | 765 | Q | * | |||||||||||
| 9978 | 9883 | 3295 | NS5 | 768 | L | V | |||||||||||
| 9995 | 9900 | 3300 | NS5 | 773 | H | * | |||||||||||
| 10046 | 9951 | 3317 | NS5 | 790 | V | ||||||||||||
| 10139 | 10044 | 3348 | NS5 | 821 | V | ||||||||||||
| 10217 | 10122 | 3374 | NS5 | 847 | R | ||||||||||||
| 10428 | 10333 | 3445 | 3’UTR | 918 | — | — | |||||||||||
| 10551 | 10456 | 3486 | 3’UTR | 959 | — | — | |||||||||||
| 10574 | 10479 | 3493 | 3’UTR | 966 | — | — | |||||||||||
Nucleotide position, coding sequence (CDS) nucleotide positions, polyprotein amino acid (AA) position, gene and amino acid position within protein are recorded. Nucleotide changes that do not occur within the CDS and therefore do not result in an amino acid change are recorded as (—). Nucleotide changes that are within the CDS but do not result in an amino acid change are recorded as (*). Single letter amino acid residue codes are used below.
The WT strain SA14 used in our studies was found to have three to eleven nucleotide changes encoding two to six amino acid substitutions compared to previously published SA14 strain sequences10,12. None of these changes were conserved across GenBank submissions though one change, 1708 (E-G244E), was reported in five of six publications.
The SA14-14-2 stock used in our studies differed from all previously published isolates by four nucleotides that encoded one synonymous change at nucleotide 9122 and three non-synonymous consensus changes at nucleotides 1117 (E-N47S), 1512 (E-K179E) and 7656 (NS4B-D131N)10,51,52 (Table 1). As residues E-47N, E-179K and NS4B-131D were present in all previously published SA14-14-2 strains and in progenitor Clone12-1-7 (Genbank submission: AF416457), it can be presumed that these residues constitute part of a conserved vaccine genotype. The changes detected in the SA14-14-2 strain utilized in these studies (E-47S, E-179E and NS4B-131N) would therefore be considered mutations from this genotype, though not necessarily reversions to WT. Compared to the only other previously published commercial vaccine, the vaccine ampule of SA14-14-2 used in this study differs by three residues (E-N47S, E-M279K, and NS4B-D131N, Table 1)10.
The SA14-5-3 stock used here differed from that published at three nucleotides that encoded two amino acid differences (5’UTR-C20G, prM-C34R and E-G244E)12. SA14-5-3 is highly similar to SA14-14-2, with only seven residues (E-N47S, E-T177A, E-K179E, NS1-H351D, NS4B-H131SD, NS5-H649Q, NS5-A671V) distinguishing the two viruses (Fig. 2) suggesting these residues contribute to the increased immunogenicity of SA14-14-2 compared to SA14-5-3 in clinical trials22,23.
Fig. 2. Consensus changes during JEV vaccine derivation.
The complete genomes of SA14, Clone12-1-7, SA14-2-8, SA14-5-3 and SA14-14-2 were compared. Consensus changes that represent a reversion to the parental, WT strain SA14 are bolded. Consensus changes that occur within the protein coding region of the genome are reported as the codon number within the gene. Consensus changes that occur outside the protein coding region are italicized and reported as a nucleotide number. Sequences of SA14, SA14-2-8, SA14-5-3 and SA14-14-2 were generated in house through Illumina sequencing. The sequence for Clone-12-1-7 was accessed from GenBank (AF416457).
The SA14-2-8 stock used in these studies differs from the one GenBank submission at 37 nucleotides, 13 of which result in non-synonymous amino acid (aa) mutations. Only one residue, E-315A, was determined to have a WT residue where the previously published sequence (E-315V) did not. Five WT residues (prM-88K, E-160G, E-477D, NS2B-7F and NS3-105V) were reported in the published sequence. Many of the residues in the SA14-2-8 strain used here that differ from the GenBank sequence are shared with SA14-14-216. It is also apparent that SA14-2-8 is very similar to SA14 with only seven amino acid residues distinguishing the structural proteins of the two viruses (M-K88R, E-K138E, E-E160G, E-V176I, E-E179K, R439K and E-E477D) (Table S4), plus six residues in NS proteins (NS1-G235D, NS1-V236A, NS2B-F7V, NS2B-E63G, NS3-A105G and NS4A-I255V).
Overall, the WT progenitor SA14 differed from SA14-14-2 at 57 nucleotides encoding 25 aa, from SA14-5-3 at 47 nt and 21 aa and from SA14-2-8 at 35 nucleotides encoding 13 amino acids (Table S4). When compared to Clone 12-1-7, the attenuated progenitor of all vaccine strains, SA14 differs by 50 nt and 26 aa, SA14-14-2 differs by 41 nt and 24 aa, SA14-5-3 differs by 30 nt and 19 aa and SA14-2-8 differs by 52 nt and 28 aa. Common attenuating mutations in all three vaccines include: E-E138K, E-I176V, NS1-P126S, NS1-G235D, NS2B-E63D, NS3-A105G and NS4A-I255V.
Consensus genome sequencing shows JEV vaccine viruses are stable upon passaging
We examined the impact of passaging on the stability of the consensus sequences of the WT and vaccine viruses used in these studies (Table S5). The SA14-14-2 vaccine stock was passaged once in C6/36 and twice in Vero cells resulting in one non-synonymous change at nt 1512 (E-K179E) and one synonymous change (NS5-G9122A) (Table S5A). SA14-2-8 incurred four changes, two synonymous (C-U158A and NS1-A3482U) and two non-synonymous nucleotide changes, 1447 and 4224 corresponding to residue changes E-G160E and NS2B-F7V), after one passage in Vero cells (Table S5B). Neither non-synonymous changes are reversion to WT SA14 nucleotides. No consensus changes to the SA14-5-3 genome were recorded upon passage in Vero cells.
Next generation sequencing of JEV vaccine strains contrasts levels of diversity within the viral populations
All strains used in these studies underwent Illumina sequencing and genetic diversity profiles were compared by calculation of Shannon’s entropy and identification of single nucleotide variants (SNVs)53,54. In addition to consensus sequence determination, the diversity of unpassaged (p0) SA14, SA14-14-2 and SA14-2-8 were determined and compared to the final passage of each virus (Fig. 3). The depth of coverage for each virus was as follows: SA14 p0 (4,360 reads/base) and p1 (10,009 reads/base), SA14-14-2 p0 (5,554 reads/base) and p1 (2,829 reads/base), SA14-2-8 p0 (3,155 reads/base) and P1(15,521 reads/base) and SA14-5-3 p0 (6,481 reads/base). Only p0 is presented for SA14-5-3 as p1 recorded low coverage levels and therefore only the consensus sequence could be determined from this sample. As the sample has the same consensus sequence as the previous passage, it is suggested that the virus has low diversity due its derivation and it is considered likely that p1 displays similar levels of diversity. Overall, SA14-2-8 and SA14-5-3 showed the least genetic diversity, WT SA14 the most genetic diversity, and SA14-14-2 was intermediate.
Fig. 3. Genetic diversity of JEV WT and vaccine strains upon passage.
The genetic diversity of two sequential passages of SA14 and the three vaccine strains derived from it was compared through Illumina sequencing methods and quantified using Shannon entropy. The Shannon entropy of SA14 (a), SA14-14-2 (b), SA14-5-3 (c) and SA14-2-8 (d) were mapped across the genome. All nucleotide positions, UTRs included, are depicted.
SA14 contained unpredictable levels of diversity across its genome, e.g., localizations of diversity were not conserved between passages. The SNV profile of the virus showed SNVs at locations that differentiate the SA14 used in this study from the SA14 strains published in GenBank. SNVs at residues that differentiate the previous reports occurred at prM-D21G (1.6%), E-G244E (21.02%), NS1-I122V (1.7%), NS1-G135D (31.5%), NS2B-S71S (18.01%), NS3-R275R (14.7%), NS3-D482N (15.4%) and NS4A-V58V (25.4%). SNVs were detected at the position of three synonymous mutations differentiating the published sequences and the SA14 used here (NS2B-71, NS3-275 and NS4A-58); however, the nucleotide detected as a SNV was not the same as the nucleotide in the published sequence. No SNVs were detected at E-107 or E-138, which have been shown to be virulence determinants of SA1410,11. This reinforces our hypothesis that the WT SA14 is naturally genotypically heterogeneous, leading to consensus sequences that vary from sample to sample due to the expansion of subpopulations of viral RNA species. Interestingly, one SNV recorded a residue change at NS1-I122V (1.6%) whereas the consensus nucleotide differences between published strains and the strain used here were synonymous10,51,52.
SA14-14-2 exhibits significantly lower levels of entropy compared to WT SA14 (p = 0.014) (Fig. 4a) and significantly higher levels of entropy than SA14-5-3 (p < 0.003) and SA14-2-8 (p < 0.0001). Areas of the genome displaying high diversity in WT SA14 did not overlap with those showing high diversity in SA14-14-2 suggesting diversity of SA14-14-2 was due to selection during passage in PHK cells and mice, rather than as a consequence of derivation from SA14 (Fig. 4b, c). For SA14-14-2, the E protein gene displayed the highest levels of diversity, followed by NS5 (Fig. 4b). Additionally, there were peaks of diversity at NS2A-41 and NS4B-104.
Fig. 4. Genetic diversity of JEV vaccine strains compared to parental WT strain.
The genetic diversity of SA14 and the three vaccine strains derived from it was compared through Illumina sequencing methods and quantified using Shannon entropy (a). The Shannon entropy of SA14 (b), SA14-14-2 (c), SA14-5-3 (d) and SA14-2-8 (e) were mapped across the genome. All nucleotide positions, UTRs included, are depicted in (a): *p = 0.014 (SA14 vs SA14-14-2), **p = 0.0003 (SA14-14-2 vs SA14-5-3), ***p < 0.0001 (SA14-14-2 vs SA14-2-8).
The differences between the diversity profiles of SA14 and SA14-14-2 are even more apparent when comparing SNV profiles (Fig. 5A, B, Tables S6 and S7). Both SA14 and SA14-14-2 contain SNVs that are near 50% of the population, or a consensus change. Three of these high frequency SNVs detected in SA14-14-2 (A1116G – 37%, G1117A – 33%, G9122A – 39%) overlap with SA14 identity. The changes at 1116 and 1117 correspond to minority residue changes at E-E47D. In the vaccine strain, the WT nucleotide is the minority variant upon final passage. When the SNV profile of SA14-14-2 is compared to previously published consensus sequences of SA14-14-2, SNVs are detected at residues E-S47N (37.01%), E-E179K (38.1%), NS1-V392G (1.3%), NS3-V235A (1.2%), NS4B-N131D (17.4%), and NS5-G482G (38.6%) that differentiate the SA14-14-2 used in this study from those published previously10,51,52. The nucleotide change (G9122A) associated with NS5-482 results in a synonymous change. Upon passaging of SA14-14-2, there was almost complete overlap in diversity peaks suggesting that even though SA14-14-2 is highly diverse, this genetic diversity is not random, rather the virus only tolerates variability at certain residues.
Fig. 5. Frequency and genome location of single nucleotide variants detected in SA14 and vaccine derivatives.
RNA was extracted and sequenced using Illumina methods. LoFreq software was used to detect SNVs in SA14 (A), SA14-14-2 (B), SA14-5-3 (C) and SA14-2-8 (D) samples. Dotted lines represent 50% of the population at which an SNV would become the consensus nucleotide and 1% of the population. An SNV frequency cutoff was applied at 0.05% frequency.
Very low levels of diversity were found across the entire genome of SA14-5-3 (Fig. 4d and Table S8). Only two peaks of entropy were recorded, both in NS3 (NS3-235 and NS3-446). These positions do not differentiate SA14-5-3 from SA14. Two high frequency, non-synonymous SNVs were also detected at these positions (NS3-V235A (3.5%) and NS3-N446K (40.5%)) but these SNVs did not lead to a consensus change upon passage.
SA14-2-8 displayed very low levels of entropy (Fig. 4d) and few, low frequency SNVs (Table S9). This was expected as the virus was derived by (1) UV irradiation and was then (2) plaque purified during its attenuation from SA14. There are peaks of entropy at E-160 and NS2B-7 that represent reversions to WT SA14. These few areas of heterogenicity are reproduced after passage and correspond with SNVs of frequency 10% and 1.5%, respectively. Overall, the average entropy and SNV profile was not changed during passage. Both initial and final passage of SA14-2-8 showed very low levels of diversity across the entire genome.
SA14, SA14-14-2 and SA14-5-3 are susceptible to Ribavirin whereas SA14-2-8 is not
The susceptibility of WT JEV strain SA14 and the three vaccine strains derived from it to Ribavirin was determined in Vero cells. The titer reduction curve for SA14 was not significantly different than those for SA14-5-3 and SA14-2-8 (p = 0.087 and p = 0.389, respectively) but was significantly different from SA14-14-2 (p = 0.016) (Fig. 6). The SA14-14-2 viral reduction curve was significantly different from the SA14-2-8 curve (p < 0.0001) but not from the SA14-5-3 curve (p = 0.992) (Fig. 6). Titer reduction curves of SA14-5-3 and SA14-2-8 differed significantly from each other (p = 0.002) (Fig. 6). The 50% inhibitory concentrations (IC50) of SA14, SA14-14-2, SA14-5-3 and SA14-2-8 were 8.87 µM (R2 = 0.887), 0.40 µM (R2 = 0.888), 0.68 µM (R2 = 0.785) and 779.72 μM (R2 = 0.964), respectively. This suggests that attenuation of SA14 to generate SA14-5-3 and SA14-14-2 by cell culture adaptation resulted in development of Ribavirin sensitivity (low-fidelity replication) while the derivation of SA14-2-8 by ultraviolet irradiation resulted in resistance (high-fidelity replication) to Ribavirin.
Fig. 6. Dose response of WT JEV and JEV vaccine strains to the antiviral ribavirin.

SA14, SA14-14-2, SA14-5-3 and SA14-2-8 were incubated with the GTP nucleoside analog, ribavirin. After 48 h, the supernatant was collected and titrated for viral load (FFU). Titers at each concentration were normalized to untreated, infected cells and fit using a dose-response linear regression. The experiment was undertaken in triplicate and points shown are an average of these experiments. A four-parameter, non-linear regression was used to fit sensitivity curves (SA14 R2 = 0.89, SA14-14-2 R2 = 0.89, SA14-5-3 R2 = 0.79, SA14-2-8 R2 = 0.964).
SA14-14-2 and SA14-2-8 are highly virulent in interferon αβγ (AG129) receptor knockout mouse model whereas SA14-5-3 is attenuated
AG129 mice were inoculated with 1000 pfu of SA14-14-2, SA14-2-8 or SA14-5-3. Both SA14-14-2 and SA14-2-8 produced 100% lethality whereas neither lethality nor morbidity was observed with SA14-5-3. Mice infected with SA14-14-2 had a mean survival time (MST) of 10.0±0.3 days whereas the MST of mice infected with SA14-2-8 was 7.0 + 0.2 days (p < 0.0001; Table 2). In addition, viremia in SA14-2-8 infected mice were significantly higher at 3 dpi than for SA14-14-2 (p = 0.0002)(Table 2). In contrast, at the time of euthanasia, viral titers in the brain of SA14-2-8 infected mice were significantly lower than in SA14-14-2 animals (p = 0.015) (Table 2). Unsurprisingly, SA14-5-3 viremia at 3 dpi was significantly lower than for SA14-14-2 and SA14-2-8 (p < 0.001) (Table 2). At the end of the study, mice infected with SA14-5-3 were found to have high FRNT50 titers (mean reciprocal titer = 1274±162) indicating that in the highly susceptible AG129 mouse model, SA14-5-3 is both attenuated and immunogenic (Table 2).
Table 2.
Outcome of subcutaneous infection of AG129 mice with JEV vaccine strains.
| Mouse Model: 6-week old AG129 | |||||
|---|---|---|---|---|---|
| Strain: | Number of mice | Mean survival time | Viremia (log10titer) | Mean PRNT50 (reciprocal) | Mean titer in brain (log10 titer) |
| SA14-14-2 | 16 | 10 | 7.3 ± 0.4 | N/A | 7.5 ± 0.6 |
| SA14-2-8 | 16 | 7 | 8.1 ± 0.2 | N/A | 5.9 ± 0.6 |
| SA14-5-3 | 18 | N/A | 5.8 ± 0.4 | 1274 | N/A |
SA14-14-2 is more attenuated and more immunogenic than SA14-2-8 in the type-1 in interferon αβ receptor knockout (A129) mouse model
We also examined the virulence and immunogenicity of SA14-14-2 and SA14-2-8 in A129 mice, which lack only type-1 interferon signaling. As SA14-5-3 was fully attenuated in the more susceptible AG129 model, the virus was not assessed in A129 mice. Mice were again inoculated with 1000 pfu. SA14-14-2 was completely attenuated (100% survival) whereas SA14-2-8 had a mortality rate of 62.5% (Mean Survival Time [MST] of 7.0 ± 0.4dpi) (Table 3). Mice infected with SA14-14-2 and mice surviving SA14-2-8 infection had a strong neutralizing antibody response, though SA14-14-2 antibody titers were significantly higher than those induced by SA14-2-8 (p = 0.0135, mean FRNT50 titer: 3840± 624 and 2133± 427, respectively) (Table 3). Viremia in mice 3 dpi with SA14-2-8, was significantly higher (p = 0.0004) than in mice infected with SA14-14-2 (Table 3). The average viral infectivity titer in the brains of mice that succumbed to SA14-2-8 infection was 3.9x103FFU/g at the time of death.
Table 3.
Outcome of subcutaneous inoculation of A129 mice with JEV vaccine strains.
| Mouse Model: 6-week old AG129 | |||||
|---|---|---|---|---|---|
| Strain: | Number of mice | Mean survival time | Viremia (log10 titer) | Mean PRNT50 (reciprocal) | Mean titer in brain Log10 titer |
| SA14-14-2 | 7 | N/A | 7.0 ± 0.7 | 3840 | N/A |
| SA14-2-8 | 8 | 7 | 8.0 ± 0.5 | 2133 | 3.6 ± 0.7 |
Changes to the E protein were detected in SA14-14-2 but not SA14-2-8 when isolated from brains of AG129 mice
Virus recovered at euthanasia from the brains of mice infected with SA14-14-2 was sequenced using Illumina methods. In all brains consensus changes occurred at E-S47D and E-E179K. Both were SNVs detected in the sequencing studies described above suggesting that these residues are important to how SA14-14-2 enters or replicates in the brain of mice. E-47 differentiates SA14-14-2 and SA14-5-3 from SA14. The E-47 amino acid substitution detected in mouse brains (aspartic acid) is a reversion to SA14-5-3 not to WT SA14 (asparagine). No consensus residue changes were recorded outside the E protein. It is important to note that in comparison of SA14 to SA14-14-2, the E-E179K substitution is dictated by a single nucleotide reside change, G1117A, in the second base of the codon (AGC). However, in mouse brain G1117A is accompanied by an additional A1116G mutation in the first base of the codon such that the double mutation encodes the glutamine mutation (GAC). Due to incomplete viral sequence of SA14-2-8 virus in mouse brain by NGS, Sanger methods were used to show that no nucleotide changes took place in the E gene in the brains of AG129 mice.
Again, no consensus changes to the genome of SA14-2-8 were detected in the brains of A129 mice. As no mice infected with SA14-14-2 succumbed to infection, the brains were not assayed for viral titer as it was assumed that any infection would have been cleared by the end of the study (28 dpi).
Discussion
In this manuscript, we have shown that, as expected, SA14-14-2 has the most favorable safety/immunogenicity profile of the SA14-derived vaccine strains in vivo. It is also clear that the mechanism of attenuation of SA14-14-2 is different to that of a previously investigated flavivirus LAV (YFV 17D), in that 17D is characterized by a highly homogenous genotype whereas that of SA14-14-2 is heterogenous. Interestingly, SA14-14-2 is the most diverse of the three vaccine strains derived from SA14. Nevertheless, both YF 17D and JE SA14-14-2 vaccines share a similar phenotype in mouse models, good immunogenicity, cross-protective response and impressive safety profile making them highly effective LAVs55.
SA14-14-2 vaccine is produced in PHK cells using a seed-lot system much like that of 17D though the YFV vaccine is produced in chicken eggs56. In this substrate, SA14-14-2 has been shown that primary, master, working and commercial seeds (up to eight passages) and an experimental 17 passages of the virus maintain a stable genotype9. When these genomic sequences are compared to other SA14-14-2 GenBank submissions, it is clear that consensus sequence changes occur with extended passage in PHK cells or passage outside of this cell substrate (Table 1)7,57. This was shown in these studies where passage of SA14-14-2 three times in Vero cells and one in C6/36 cells to generate the working stock resulted in two additional nucleotide consensus changes, one a non-synonymous change at nucleotide 1512 (E-K179E) and the other a synonymous change at nucleotide 9122 (NS5-482G). As E-179E is the consensus residue in SA14-2-8 it would suggest that either a lysine or glutamine can be present at this residue without affecting attenuation in humans.
The differences between the published genomic sequences of SA14-14-27–12 are explained by the apparently high levels of genetic diversity of this vaccine strain. This may be considered a concern for a LAV but the phenotypic data reported in this paper are very similar to that of the commercial vaccine indicating that the attenuated phenotype is maintained despite the apparent genetic variation. In fact, the mean diversity level of SA14-14-2 is only slightly, though statistically significantly, lower than WT parent strain SA14. As SA14-14-2 is highly susceptible to the antiviral Ribavirin, the cause of genetic plasticity could be localized to the NS proteins. Resistance to Ribavirin, a GTP analog, has been shown to be a marker of flavivirus replication complex fidelity44. Susceptibility to the drug suggests that the replication complex is not able to distinguish the analog from guanosine, which results in an increase in mutation frequency. The susceptibility of SA14-14-2 to Ribavirin differentiates the virus from yellow fever 17D, which was shown to be relatively resistant to Ribavirin56.
It is clear that the genetic diversity of SA14-14-2 does not make the virus susceptible to reversion to virulence. We hypothesize that this is because the genetic diversity of SA14-14-2 is not random upon passage, instead being localized to discrete regions yielding genotypes that retain an attenuated phenotype. With this knowledge it can be assumed that previously submitted SA14-14-2 sequences are correct despite consensus changes, representing a selection for a different, attenuated consensus8–12,50. As the vaccine has an impressive clinical safety record, it can be assumed that this multigenic genotype is due to multiple attenuating residues that maintain the attenuated phenotype. This type of mechanism has been shown in other flavivirus vaccine candidates and is the rationale for engineering attenuating mutations in multiple genes of rationally designed flavivirus vaccines55,56. In one such study, the multigenic nature of SA14-14-2 attenuation was shown using a chimeric vaccine, Chimerivax-JE, which utilizes SA14-14-2 prME genes in a YFV 17D backbone. Single reversions to WT did not have great effect on neurovirulence whereas groups of changes (especially clusters including E-F107L or E-K138E) did significantly increase virulence of the vaccine virus in mice. To this end, we have shown that the critical attenuating mutations at E-107 and E-138 are extremely stable in SA14-14-2 with no SNVs detected at these positions at any passage making it unlikely that these reversions would occur. This robust genotype may lend itself to the protective capacity of the vaccine, which has been shown to be affective against multiple JEV genotypes8.
The mechanism of SA14-14-2 diversity can be mapped using SA14-5-3, a progenitor of SA14-14-2, which is less genetically diverse and was over attenuated clinically. Only four amino acid residues in the replication complex (NS1-H351D, NS4B-H131D, NS5-H639Q and NS5-A671V) differentiate SA14-5-3 from SA14-14-2. Of these four residues, NS5-671 is potentially important as it extends into the pocket of the RdRp active site and is in close proximity to the conserved catalytic site in motif C of the RdRp (NS5-668). This site participates in binding GTP during pre-initiation of replication and NS5-671V is conserved in all other strains of JEV as well as WNV, YFV and DENV-2 suggesting that any alteration could affect the efficiency of viral replication and potentially susceptibility to ribavirin58,59. A more distantly related vaccine virus, SA14-2-8 was also overattenuated clinically but was resistant to Ribavirin, showing little loss of infectivity titer even at the highest concentration of drug tested. Interestingly, SA14-2-8 maintains an NS5 gene that is identical to WT SA14, which supports the hypothesis that the flavivirus replication complex as a whole, not exclusively the RdRp, contributes to viral diversity60. Furthermore, SA14-2-8 only differs from SA14 at four residues (NS1-P126S, NS2B-F7V, NS3-A105G and NS4A-I255V) in the replication complex, suggesting that these residues play a key role in restricting the viral diversity of SA14-2-8.
Combining the results of the genotypic analysis and phenotypic profiles of the three vaccines, SA14-14-2 was the most genetically diverse of the vaccine strains and showed the most balanced safety/immunogenicity profile. In A129 mice, SA14-14-2 was completely attenuated, suggesting an important role for lack of type-2 interferon antagonism in the safety of the vaccine. SA14-5-3 and SA14-2-8 had very low levels of diversity and very few SNVs detected but radically distinct phenotypes in mice. SA14-5-3 was completely attenuated in the highly susceptible AG129 mouse model whereas SA14-2-8 was significantly more virulent than SA14-14-2 in both AG129 and A129 mouse models. As no reversions were detected in the brains of either AG129 or A129 mice, we speculate that this virulence phenotype is due to SA14-2-8 being more genetically similar to the parent strain WT SA14 than SA14-5-3. The two viruses differ by only 35 nucleotides and 13 amino acids, making SA14-2-8 more similar to its WT parent than to either of the two other vaccine strains tested. In particular, the maintenance of the WT residue at E-107 likely impacts the attenuation of SA14-2-8 in mice as it has been shown to be a major determinant of neurovirulence in SA14-14-2 (Fig. 7)34.
Fig. 7. Summary of attenuating E protein mutations in JEV vaccine strains.
The three attenuated vaccine strains were empirically derived which led to distinct combinations of genotypes and phenotypes (A). It is apparent that genetic diversity doesn’t correlate with attenuation in mice. It is proposed that changes to the E protein of SA14-2-8 (shown in green) and SA14-14-2 (shown in blue) are responsible for differences in mouse virulence (B).
Although our studies have not identified a correlation to virulence, the genetic diversity differences between SA14-14-2 and SA14-2-8 could be important to vaccine immunogenicity as A129 mice that survived SA14-2-8 infection had significantly lower antibody titers compared to those infected with SA14-14-2. SA14-5-3 and SA14-14-2 are genetically very similar, with only three changes distinguishing the E proteins of the two vaccines. We postulate that the high frequency of SNVs in SA14-14-2 E and NS genes potentially affect the phenotype of the virus with respect to both immunogenicity and replicative efficiency. We also speculate that high frequency SNVs in the genotypic population of SA14-14-2 enabled the virus to cause disease in the highly susceptible AG129 model and caused the virus to be highly immunogenic in the A129 model. As SA14-14-2 contains a genetically diverse E gene (i.e., multiple SNVs at a high percentage of the RNA population) it is possible that the immune system sees a range of virions once administered, leading to a diversified immune response. In support of this hypothesis, WT strains of JEV show extensive genetic variation yet the SA14-14-2 vaccine is efficacious against all known strains of WT JEV59,61,62. Additionally, studies show that monoclonal antibodies induced by genotypes I and III viruses bind and neutralize viruses in other genotypes63–65. Our hypothesis is supported by the observation that these SNVs were not detected in the SA14-5-3 genome where the virus was 100% attenuated in the most susceptible model. In fact, the vaccine ampule passaged to create the working stock for these studies differed from the published vaccine sequence above (MH258852) at four nucleotides, which resulted in three amino acid substitutions (E-S47N, E-K279M, and NS4B-N131D)10. Interestingly, SNVs encoding the majority variant of the previously published commercial vaccine at each of these four positions were detected in the SA14-14-2 strain used here. This suggests that a subset of the viral population in the vaccine ampule used here was genetically identical to that of the previously published ampule10.
It should be emphasized that the presented findings do not extensively address the stability of the current vaccine genotype over time or passage, and are instead focused on genetic properties and phenotype of progenitor strains; the findings strongly suggest utility of using deep sequencing for monitoring of empiric vaccine population metastability to ensure both safety and protection characteristics.
The virulence of SA14-14-2 in both A129 and AG129 mouse models greatly resembled that of YFV 17D, which has an MST of 10.1 days in three to four week old AG129 mice by the subcutaneous route but is completely avirulent in age matched A129 inoculated by the same route55. Thus, our results are consistent in suggesting that NS genes and viral diversity have no role in attenuation of JE SA14-14-2 but possibly do play a role in immunogenicity and the E protein encodes determinants of attenuation, which is supported by amino acid substitutions involved in mouse virulence being located in the E protein (Fig. 7). Despite differing viral diversity, none of the vaccine strains display evidence of defective interfering RNA species by NGS, which was recently detected in the live attenuated influenza vaccine66. Thus, though 17D and SA14-14-2 maintain distinct genotypes, their phenotypes are comparable. The techniques employed during the generation of SA14-14-2 are likely responsible for its unique genotype and solid attenuated phenotype. SA14-5-3 and SA14-2-8 had been shown to be over attenuated in clinical trials leading vaccine developers to passage SA14-5-3 in suckling mice to increase the immunogenicity of the vaccine. This passaging likely selected a population for SA14-14-2 that was not clonal, unlike 17D which was passaged hundreds of times in chicken tissue. The diverse yet attenuated viral population of SA14-14-2 provides cocktail of antigens that induce robust and protective immune response and represents a unique attenuation mechanism for live-attenuated vaccines.
Methods
Passaging of viral stocks
A freeze-dried vaccine ampule of SA14-14-2 was obtained from NICPBP (Beijing) in 1987/1988. We do not have the lot number. Thus, the vaccine sample is derived from manufacture in the original production facility, which is different to the current vaccine that is manufactured in the new facility. The vaccine was reconstituted in HPLC grade water and passaged once in C6/36 and twice in Vero cells. SA14-2-812 was passaged once in C6/36 cells and once in Vero cells. SA14-5-312 was passaged three times in Vero cells. Wild-type (WT) strain SA14 was obtained from the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA, Galveston, Texas). Passages in Vero and C6/36 cells occurred in minimal essential media (MEM) supplemented with L-glutamate, penicillin-streptomycin, non-essential amino acids, and 2% fetal bovine serum by volume. Viral infectivity assays were undertaken in Vero cells by focus forming assay using anti-JEV mouse immune ascites fluid kindly provided by the World Reference Center for Emerging Virus and Arboviruses (WRCEVA, UTMB), biotinylated goat anti-mouse IgG and NuetrAvidin-HRP (ThermoFischer)71.
Next generation sequencing (NGS) of JEV vaccine strains
RNA was extracted from the seed stock and final passage of SA14 and SA14-14-2, plus the final two passages each of SA14-2-8 and SA14-5-3 using the Qiagen QIAmp viral RNA isolation kit (Qiagen). cDNA libraries were prepared using random hexamers (TruSeq RNA V2 kit) and sequenced on either the Illumina HiSeq1500 platform or the NextSeq550 platform by the UTMB NGS Core. Two viruses (SA14-14-2 and SA14-2-8) were sequenced on both platforms to evaluate consistency. No variability was identified. A de novo consensus sequence was generated for each sample using ABySS V1.3.7 assembly of paired end reads with k values from 20 to 40. The consensus sequences of each virus were then compared to previously published consensus sequences of the same strain using BioEdit (V 7.2.5).
The reads obtained from Illumina sequencing were trimmed to a minimum length of 35 bases and quality controlled (Q-score ≥30) using the open source software Trimmomatic (V 0.39). Reads were aligned using Bowtie2 (Version 2.2.6) very-sensitive-local presets to the de novo consensus sequences generated. The resulting alignment was sorted by coordinate using PicardTools (V 2.20.5) SortSam and PCR duplicates were removed using PicardTools MarkDuplicates. Shannon entropy was calculated using previously published means44,60. Single nucleotide variants (SNVs) were called using the open source software LoFreq V2 with a variant cutoff of 1%67. Shannon’s entropy and SNVs were calculated for all samples excluding the final passage of SA14-5-3, which had low coverage.
Viral infectivity reduction of antiviral ribavirin
Viral infectivity reduction curves in the presence of ribavirin were undertaken as previously reported44. Briefly, Vero cells were inoculated with SA14, SA14-14-2, SA14-5-3 or SA14-2-8 at a multiplicity of infection of 0.05. Ribavirin was added at 2X concentration (1:2 dilution curve starting at 3 mM), allowed to incubate for 30 minutes at room temperature and then MEM supplemented with 2% FBS added to reduce the concentration to 1X. After 48 hours, cell culture supernatants were collected, and virus titrated via focus forming assay. Each assay was completed in triplicate.
Mouse virulence studies
All animal procedures were approved by the University of Texas Medical Branch (UTMB) Institutional Animal Care and Use Committee and studies were carried out in compliance with the recommendations of the Guide for the Care and Use of Laboratory Animals (National Research Council).
AG129 (interferon α/β and γ receptor deficient) and A129 (interferon α/β receptor deficient) mice were bred and maintained at animal facilities at UTMB. Groups of six-week-old animals (n = 7–9) were inoculated subcutaneously (s.c.) with 1000 PFU of SA14-14-2, SA14-2-8 or SA14-5-3. For AG129 mouse experiments, two studies were undertaken with each virus and results combined for reporting. For A129 mice a single study was undertaken with SA14-14-2 and SA14-2-8.
Following inoculation mice were weighed daily and monitored for morbidity for 28 days. Animals exhibiting clinical signs of neurological disease or with weight loss >20% of initial body weight were humanely euthanized and counted as dead on the following day of the study for analysis. On day three post inoculation, blood was collected by retro-orbital bleed to quantify viremia using iScript cDNA synthesis kit (BioRad) and PF1S and PF2R-bis primers68. In addition, for some animals the brains were harvested at the time of euthanasia and homogenized using 1.5 mm zirconium beads. After clarification by centrifugation, viral load was determined using focus-forming assays and expressed as FFU/gram. Viral RNA was extracted using Qiagen Viral RNA isolation kits and sequenced using NGS. The E protein gene of viruses that returned incomplete sequences by NGS were Sanger sequenced using E protein primers described previously69 Only changes to the E protein are reported.
At the end of the study, surviving animals were sacrificed, serum was collected and heat-inactivated by incubation at 56 °C for 30 minutes. The samples were stored frozen at −20 °C until the neutralizing antibody titers were determined by focus reduction neutralization tests (FRNT50). Briefly, 2-fold serial dilutions were incubated with 50 FFU of infecting virus that was subsequently used to infect Vero cells. After four days, cells were fixed with acetone:methanol and plates stained as previously specified. Dilutions with a 50% reduction in infectivity titer were considered the FRNT50 value.
Statistical analysis
All statistical analysis was competed using Prism 7 software (v7.0). A two-way ANOVA with Tukey’s multiple comparison test was used to compare the sensitivity of viruses to ribavirin. A four-parameter, non-linear regression was used to fit sensitivity curves and determine 50% inhibitory concentration (IC50). Ordinary, one-way ANOVA with Tukey’s multiple comparison tests were used to determine differences in mean Shannon entropy, viremia, FRNT50 and titer in the brain. Mantel-Cox tests were used to identify differences in survival curves.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We would like to acknowledge Richard Pyles (UTMB) for his contribution to the data acquisition of this work. This work was funded in part by grants from the NIH (R21 AI 129844) to A.D.T.B. and N.B., and the Gillson Longenbaugh Foundation to A.D.T.B. A.S.B. was funded in part by NIH grant T32AI060549 and E.H.D. was funded by a predoctoral fellowship from the Sealy Institute for Vaccine Sciences.
Author contributions
E.D. contributed to the conception, design, acquisition, analysis and interpretation of the work and drafting and revision of the manuscript. A.B. contributed to the conception, design of the work and acquisition of data. L.L. contributed to the acquisition of data. M.W. contributed to the acquisition of data. M.G. contributed to the acquisition of data. J.T. contributed to the acquisition of data. S.W. contributed to the acquisition, analysis and interpretation of data. A.B. contributed the conception, design and interpretation of data and contributed substantively to the revision of the manuscript. N.B. contributed the conception, design, acquisition and interpretation of data and contributed substantively to the revision of the manuscript.
Data availability
All unique biological materials and the corresponding datasets generated and analyzed during the current study are available from the corresponding author on reasonable request. The genomes are available in Genbank (SA14 p0: MT764727; SA14-14-2 p0: MT764728; SA14-5-3: MT764729; SA14-2-8 p0: MT764730; SA14 p1: MT764731; SA14-14-2 p1: MT764732, and SA14-2-8 p1: MT764733). The NGS data are available in ArrayExpress (E-MTAB-9385)
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Emily H Davis, Andrew S Beck.
Contributor Information
Alan D. T. Barrett, Email: abarrett@utmb.edu
Nigel Bourne, Email: nibourne@utmb.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-021-00371-y.
References
- 1.Campbell GL, et al. Estimated global incidence of Japanese encephalitis: a systematic review. B World Health Organ. 2011;89:766 74–774A-774E. doi: 10.2471/BLT.10.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer M, Lindsey N, Staples JE, Hills S. Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbidity Mortal. Wkly. Rep. Recommendations Rep. 2010;59:126. [PubMed] [Google Scholar]
- 3.Tandan JB, et al. Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: a case–control study in Nepalese children 5 years after immunization. Vaccine. 2007;25:5041–5045. doi: 10.1016/j.vaccine.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 4.Sohn YM, Tandan JB, Yoksan S, Ji M, Ohrr H. A 5-year follow-up of antibody response in children vaccinated with single dose of live attenuated SA14-14-2 Japanese encephalitis vaccine: Immunogenicity and anamnestic responses. Vaccine. 2008;26:1638–1643. doi: 10.1016/j.vaccine.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Tripathi P, Rizvi A. Effectiveness of one dose of SA 14-14-2 vaccine against Japanese encephalitis. N. Engl. J. Med. 2009;360:1465–1466. doi: 10.1056/NEJMc0808664. [DOI] [PubMed] [Google Scholar]
- 6.Yu YX, Wu PF, Ao J, Liu LH, Li HM. Selection of a better immunogenic and highly attenuated live vaccine virus strain of Japanese encephalitis. I. Some biological characteristics of SA14-14-2 mutant. Chin. J. Microbiol. Immunol. 1981;1:77–84. [Google Scholar]
- 7.Aihara S, et al. Identification of mutations that occurred on the genome of Japanese encephalitis virus during the attenuation process. Virus Genes. 1991;5:95–109. doi: 10.1007/BF00571925. [DOI] [PubMed] [Google Scholar]
- 8.Tesh RB, Rosa APAT, da, Guzman H, Araujo TP, Xiao S-Y. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg. Infect. Dis. 2002;8:245–251. doi: 10.3201/eid0803.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine. 2010;28:3635–3641. doi: 10.1016/j.vaccine.2010.02.105. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, et al. Genetic and neuroattenuation phenotypic characteristics and their stabilities of SA14-14-2 vaccine seed virus. Vaccine. 2018;36:4650–4656. doi: 10.1016/j.vaccine.2018.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Yun S-I, et al. Comparison of the live-attenuated Japanese Encephalitis vaccine SA14-14-2 strain with Its pre-attenuated virulent parent SA14 strain: Similarities and differences in vitro and in vivo. J. Gen. Virol. 2016;97:2575–2591. doi: 10.1099/jgv.0.000574. [DOI] [PubMed] [Google Scholar]
- 12.Ni H, et al. Comparison of nucleotide and deduced amino acid sequence of the 5’ non-coding region and structural protein genes of the wild-type Japanese encephalitis virus strain SA14 and its attenuated vaccine derivatives. J. Gen. Virol. 1994;75:1505–1510. doi: 10.1099/0022-1317-75-6-1505. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, et al. The molecular determinants governing the immunogenicity of Japanese encephalitis live attenuated vaccines. Signal Transduct. Target Ther. 2017;2:17005. doi: 10.1038/sigtrans.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nath B, Gupta A, Khan SA, Kumar S. Enhanced cytopathic effect of Japanese encephalitis virus strain SA14-14-2: Probable association of mutation in amino acid of its envelope protein. Micro. Pathogenesis. 2017;111:187–192. doi: 10.1016/j.micpath.2017.08.046. [DOI] [PubMed] [Google Scholar]
- 15.Yang D, et al. Characterization of live-attenuated Japanese encephalitis vaccine virus SA14-14-2. Vaccine. 2014;32:2675–2681. doi: 10.1016/j.vaccine.2014.03.074. [DOI] [PubMed] [Google Scholar]
- 16.Ni H, Chang GJ, Xie H, Trent DW, Barrett AD. Molecular basis of attenuation of neurovirulence of wild-type Japanese encephalitis virus strain SA14. J. Gen. Virol. 1995;76:409–413. doi: 10.1099/0022-1317-76-2-409. [DOI] [PubMed] [Google Scholar]
- 17.Gromowski GD, Firestone C-Y, Bustos-Arriaga J, Whitehead SS. Genetic and phenotypic properties of vero cell-adapted Japanese encephalitis virus SA14-14-2 vaccine strain variants and a recombinant clone, which demonstrates attenuation and immunogenicity in mice. Am. J. Tropical Med. Hyg. 2015;92:98–107. doi: 10.4269/ajtmh.14-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han GS, Chen BQ, Huang CH, Huang C. Studies on attenuated Japanese B encephalitis virus vaccine. II. Safety, epidemiological and serological evaluation of attenuated 2-8 strain vaccine after immunization of horses. Acta Microbiologica Sin. 1973;14:185–190. [Google Scholar]
- 19.Chen BQ, Wang IM. Studies on attenuated Japanese B encephalitis virus vaccine. I. Method for obtaining the attenuated 2-8 strain and its biological characteristics. Acta Microbiologica Sin. 1974;176:184. [Google Scholar]
- 20.Sil BK, et al. Immunogenicity of experimental live attenuated Japanese encephalitis vaccine viruses and comparison with wild-type strains using monoclonal and polyclonal antibodies. Vaccine. 1992;10:329–333. doi: 10.1016/0264-410X(92)90372-Q. [DOI] [PubMed] [Google Scholar]
- 21.Chen BQ, Beaty BJ. Japanese encephalitis vaccine (2–8 Strain) and parent (SA 14 Strain) viruses in Culex tritaeniorhynchus mosquitoes *. Am. J. Tropical Med. Hyg. 1982;31:403–407. doi: 10.4269/ajtmh.1982.31.403. [DOI] [PubMed] [Google Scholar]
- 22.Yu YX, et al. Studies on the variation of Japanese B encephalitis virus. V. The biological characteristics of an attenuated live vaccine virus strain. Acta Microbiol. Sin. 1973;13:16–24. [Google Scholar]
- 23.Ao J, et al. Studies on the variation of Japanese en cephalitis virus. VII. An observation on persistence of immunity in children inoculated with JE attenuated live vaccine (SA 14-5-3 mutant) Acta Microbiol. Sin. 1981;21:501–505. [Google Scholar]
- 24.Wills MR, Sil BK, Cao JX, Yu YX, Barrett ADT. Antigenic characterization of the live attenuated Japanese encephalitis vaccine virus SA14-14-2: a comparison with isolates of the virus covering a wide geographic area. Vaccine. 1992;10:861–872. doi: 10.1016/0264-410X(92)90051-K. [DOI] [PubMed] [Google Scholar]
- 25.Tajima S, et al. In vitro growth, pathogenicity and serological characteristics of the Japanese encephalitis virus genotype V Muar strain. J. Gen. Virol. 2015;96:2661–2669. doi: 10.1099/vir.0.000213. [DOI] [PubMed] [Google Scholar]
- 26.Honjo S, Masuda M, Ishikawa T. Effects of the Japanese encephalitis virus genotype V-derived sub-viral particles on the immunogenicity of the vaccine characterized by a novel virus-like particle-based Assay. Nato Adv. Sci. Inst. Se. 2019;7:81. doi: 10.3390/vaccines7030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao L, et al. Low protective efficacy of the current Japanese encephalitis vaccine against the emerging genotype 5 Japanese encephalitis virus. Plos Negl. Trop. D. 2016;10:e0004686. doi: 10.1371/journal.pntd.0004686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erra EO, et al. Cross-protective capacity of Japanese encephalitis (JE) vaccines against circulating heterologous JE virus genotypes. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012;56:267–270. doi: 10.1093/cid/cis883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaparte M, et al. Immune response to live-attenuated Japanese encephalitis vaccine (JE-CV) neutralizes Japanese encephalitis virus isolates from south-east Asia and India. Bmc Infect. Dis. 2014;14:156. doi: 10.1186/1471-2334-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, et al. The structure differences of Japanese encephalitis virus SA14 and SA14-14-2 E proteins elucidate the virulence attenuation mechanism. Protein Cell. 2018;5:95. doi: 10.1007/s13238-018-0551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, et al. Envelope protein mutations L107F and E138K are important for neurovirulence attenuation for Japanese encephalitis virus SA14-14-2 strain. Viruses. 2017;9:20. doi: 10.3390/v9010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, et al. Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability. Nat. Commun. 2017;8:1–8. doi: 10.1038/s41467-016-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blazevic J, Rouha H, Bradt V, Heinz FX, Stiasny K. Membrane anchors of the structural flavivirus proteins and their role in virus assembly. J. Virol. 2016;90:6365–6378. doi: 10.1128/JVI.00447-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gromowski GD, Firestone C-Y, Whitehead SS. Genetic determinants of Japanese encephalitis virus vaccine strain SA14-14-2 that Govern attenuation of virulence in mice. J. Virol. 2015;89:6328–6337. doi: 10.1128/JVI.00219-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, et al. Structure-based mutational analysis of several sites in the E protein: implications for understanding the entry mechanism of Japanese encephalitis virus. J. Virol. 2015;89:5668–5686. doi: 10.1128/JVI.00293-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yun S-I, et al. A molecularly cloned, live-attenuated japanese encephalitis vaccine SA14-14-2 virus: a conserved single amino acid in the ij Hairpin of the Viral E glycoprotein determines neurovirulence in mice. Plos Pathog. 2014;10:e1004290. doi: 10.1371/journal.ppat.1004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chambers TJ, Droll DA, Jiang X, Wold WSM, Nickells JA. JE Nakayama/JE SA14-14-2 virus structural region intertypic viruses: Biological properties in the mouse model of neuroinvasive disease. Virology. 2007;366:51–61. doi: 10.1016/j.virol.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee E, Hall RA, Lobigs M. Common E protein determinants for attenuation of glycosaminoglycan-binding variants of Japanese encephalitis and West Nile viruses. J. Virol. 2004;78:8271–8280. doi: 10.1128/JVI.78.15.8271-8280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Q, et al. A single nucleotide mutation in NS2A of Japanese encephalitis-live vaccine virus (SA14-14-2) ablates NS1’ formation and contributes to attenuation. J. Gen. Virol. 2012;93:1959–1964. doi: 10.1099/vir.0.043844-0. [DOI] [PubMed] [Google Scholar]
- 40.Pitcher TJ, et al. Functional analysis of dengue virus (DENV) type 2 envelope protein domain 3 type-specific and DENV complex-reactive critical epitope residues. J. Gen. Virol. 2015;96:288–293. doi: 10.1099/vir.0.070813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang R, et al. Chimeric Japanese encephalitis virus SA14/SA14-14-2 was virulence attenuated and protected the challenge of wild-type strain SA14. Can. J. Infect. Dis. Medical. Microbiol. 2019;2019:1–7. doi: 10.1155/2019/9179308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beck A, et al. Comparison of the live attenuated yellow fever vaccine 17D-204 strain to its virulent parental strain Asibi by deep sequencing. J. Infect. Dis. 2014;209:334–344. doi: 10.1093/infdis/jit546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck AS, Wood TG, Widen SG, Thompson JK, Barrett ADT. Analysis by deep sequencing of discontinued neurotropic yellow fever vaccine strains. Sci. Rep. 2018;8:13408. doi: 10.1038/s41598-018-31085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis EH, et al. Attenuation of live-attenuated yellow fever 17D vaccine virus is localized to a high-fidelity replication complex. Mbio. 2019;10:e02294–19. doi: 10.1128/mBio.02294-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hase T, Dubois DR, Summers PL. Comparative study of mouse brains infected with Japanese encephalitis virus by intracerebral or intraperitoneal inoculation. Int. J. Exp. Pathol. 1990;71:857–869. [PMC free article] [PubMed] [Google Scholar]
- 46.Nagata N, et al. The pathogenesis of 3 neurotropic flaviviruses in a mouse model depends on the route of neuroinvasion after viremia. J. Neuropathol. Exp. Neurol. 2015;74:250–260. doi: 10.1097/NEN.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 47.Calvert AE, Dixon KL, Delorey MJ, Blair CD, Roehrig JT. Development of a small animal peripheral challenge model of Japanese encephalitis virus using interferon deficient AG129 mice and the SA14-14-2 vaccine virus strain. Vaccine. 2014;32:258–264. doi: 10.1016/j.vaccine.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni H, et al. Comparison of nucleotide and deduced amino acid sequence of the 5’ non-coding region and structural protein genes of the wild-type Japanese encephalitis virus strain SA14 and its attenuated vaccine derivatives. J. Gen. Virol. 1994;75:1505–1510. doi: 10.1099/0022-1317-75-6-1505. [DOI] [PubMed] [Google Scholar]
- 49.Ni H, Barrett ADT. Molecular differences between wild-type Japanese encephalitis virus strains of high and low mouse neuroinvasiveness. J. Gen. Virol. 1996;77:1449–1455. doi: 10.1099/0022-1317-77-7-1449. [DOI] [PubMed] [Google Scholar]
- 50.Aihara S, et al. Identification of mutations that occurred on the genome of Japanese encephalitis virus during the attenuation process. Virus Genes. 1991;5:95–109. doi: 10.1007/BF00571925. [DOI] [PubMed] [Google Scholar]
- 51.Li G, et al. Development of a reverse genetics system for Japanese encephalitis virus strain SA14-14-2. Virus Genes. 2019;55:550–556. doi: 10.1007/s11262-019-01674-y. [DOI] [PubMed] [Google Scholar]
- 52.Song B-H, Yun G-N, Kim J-K, Yun S-I, Lee Y-M. Biological and genetic properties of SA14-14-2, a live-attenuated Japanese encephalitis vaccine that is currently available for humans. J. Microbiol. 2012;50:698–706. doi: 10.1007/s12275-012-2336-6. [DOI] [PubMed] [Google Scholar]
- 53.McCrone JT, Lauring AS. Measurements of intrahost viral diversity are extremely sensitive to systematic errors in variant calling. J. Virol. 2016;90:6884–6895. doi: 10.1128/JVI.00667-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishijima N, et al. Dynamics of hepatitis B virus quasispecies in association with nucleos(t)ide analogue treatment determined by ultra-deep sequencing. PloS ONE. 2012;7:e35052. doi: 10.1371/journal.pone.0035052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meier KC, Gardner CL, Khoretonenko MV, Klimstra WB, Ryman KD. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. Plos Pathog. 2009;5:e1000614. doi: 10.1371/journal.ppat.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theiler M, Smith HH. The use of yellow fever virus modified by in vitro cultivation for human immunization. J. Exp. Med. 1937;65:787–800. doi: 10.1084/jem.65.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arroyo J, et al. Molecular basis for attenuation of neurovirulence of a yellow fever virus/Japanese encephalitis virus chimera vaccine (ChimeriVax-JE) J. Virol. 2001;75:934–942. doi: 10.1128/JVI.75.2.934-942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu G, Gong P. Crystal Structure of the full-length Japanese encephalitis virus NS5 reveals a conserved methyltransferase-polymerase interface. PloS Pathog. 2013;9:e1003549. doi: 10.1371/journal.ppat.1003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Surana P, Satchidanandam V, Nair DT. RNA-dependent RNA polymerase of Japanese encephalitis virus binds the initiator nucleotide GTP to form a mechanistically important pre-initiation state. Nucleic Acids Res. 2013;42:2758–2773. doi: 10.1093/nar/gkt1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collins ND, et al. Structural and nonstructural genes contribute to the genetic diversity of RNA viruses. Mbio. 2018;9:1225. doi: 10.1128/mBio.01871-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi Y, Hasegawa H, Oyama T, Tamai T, Kusaba T. Antigenic analysis of Japanese encephalitis virus by using monoclonal antibodies. Infect. Immun. 1984;44:117–123. doi: 10.1128/iai.44.1.117-123.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasegawa H, Yoshida M, Fujita S, Kobayashi Y. Comparison of structural proteins among antigenically different Japanese encephalitis virus strains. Vaccine. 1994;12:841–844. doi: 10.1016/0264-410X(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 63.Fan Y-C, et al. Genotype I of Japanese encephalitis virus virus-like particles elicit dterilizing immunity against genotype I and III viral challenge in swine. Sci. Rep.-uk. 2018;8:7481. doi: 10.1038/s41598-018-25596-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goncalvez AP, et al. Humanized monoclonal antibodies derived from Chimpanzee fabs protect against Japanese encephalitis virus in vitro and in vivo ▿. J. Virol. 2008;82:7009–7021. doi: 10.1128/JVI.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernandez E, et al. Mouse and human monoclonal antibodies protect against Infection by multiple genotypes of Japanese encephalitis virus. Mbio. 2018;9:e00008–e00018. doi: 10.1128/mBio.00008-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gould P, Easton A, Dimmock N. Live sttenuated influenza vaccine contains substantial and unexpected amounts of defective viral genomic RNA. Viruses. 2017;9:269. doi: 10.3390/v9100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilm A, et al. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res. 2012;40:11189–11201. doi: 10.1093/nar/gks918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moureau G, et al. A real-time RT-PCR method for the Universal Detection and Identification of Flaviviruses. Vector-borne Zoonot. 2007;7:467–478. doi: 10.1089/vbz.2007.0206. [DOI] [PubMed] [Google Scholar]
- 69.Schuh AJ, Ward MJ, Brown AJL, Barrett ADT. Phylogeography of Japanese encephalitis virus: genotype is associated with climate. Plos Negl. Trop. D. 2013;7:e2411. doi: 10.1371/journal.pntd.0002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All unique biological materials and the corresponding datasets generated and analyzed during the current study are available from the corresponding author on reasonable request. The genomes are available in Genbank (SA14 p0: MT764727; SA14-14-2 p0: MT764728; SA14-5-3: MT764729; SA14-2-8 p0: MT764730; SA14 p1: MT764731; SA14-14-2 p1: MT764732, and SA14-2-8 p1: MT764733). The NGS data are available in ArrayExpress (E-MTAB-9385)