Abstract
Objectives
To assess the effects of corticosteroid therapy for patients with severe coronavirus disease 2019 (COVID-19).
Methods
We comprehensively searched articles published in the Cochrane Library, PubMed, Embase, China Biology Medicine (CBM), China National Knowledge Infrastructure (CNKI), Wanfang, and VIP databases from January 1, 2019, to March 20, 2021.
Results
A total of 6771 patients from eight prospective studies were included in our meta-analysis. The results showed that corticosteroid therapy was associated with lower mortality in severe COVID-19 (OR = 0.70, 95% CI = 0.54–0.92, P = 0.009; I2 = 54.5%). Since the proportion of the RECOVERY (Randomized Evaluation of COVID-19 Therapy) trial included in the meta-analysis was as high as 71.88%, we removed it and recalculated the pooled OR. The results of the remaining seven studies still suggested such a survival benefit (OR = 0.65, 95% CI = 0.44–0.96, P = 0.030; I2 = 59.8%). Furthermore, subgroup analysis suggested that the pooled OR of three studies using corticosteroids in the early stages of treatment was much lower (OR = 0.37, 95% CI = 0.25–0.57, P < 0.001; I2 = 47.8%). However, after excluding the RECOVERY trial, the pooled OR of the remaining four studies with unspecific administration timing of corticosteroid therapy no longer supported this result (OR = 0.90, 95% CI = 0.69–1.17, P = 0.415; I2 = 0.0%).
Conclusions
In this meta-analysis, evidence based on seven randomized controlled trials and one prospective cohort study indicates that corticosteroid therapy was associated with a reduction in the mortality of severe COVID-19, especially when administered at an earlier time.
Keywords: COVID-19, Corticosteroids, Meta-analysis
Abbreviations: COVID-19, Coronavirus Disease 2019; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus-2; SARS, Severe Acute Respiratory Syndrome RCTs, Randomized Controlled Trials; REACT, Rapid Evidence Appraisal for COVID-19 Therapies; NOS, Newcastle-Ottawa Scale; OR, Odds Ratio; CI, Confidence Intervals; RECOVERY, Randomized Evaluation of COVID-19 Therapy; CBM, China Biology Medicine; CNKI, China National Knowledge Infrastructure
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first discovered in Wuhan, China, in December 2019. As of April 15, 2021, there have been 137,866,311 confirmed cases, including 2,965,707 deaths globally.[1] Faced with such a highly infectious respiratory disease, because of the lack of specialized treatment for COVID-19, research on anti-COVID-19 drugs has become an urgent task.
“Cytokine storm” is considered to participate in the development of viral pneumonia, leading to the application of immunosuppressants represented by corticosteroids in the clinical practice of severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS),[2], [3] and COVID-19. In July 2020, the Randomized Evaluation of COVID-19 Therapy (RECOVERY) collaborative group involving 176 hospitals announced that low-dose dexamethasone (6 mg/d for up to 10 days) can help save the lives of severely ill patients, especially those receiving mechanical ventilation.[4] Since then, many randomized controlled trials (RCTs) evaluating the efficacy and safety of corticosteroid therapy have ended recruitment and released the results in advance. The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group performed a prospective meta-analysis, including seven RCTs, with a final follow-up to July 7, 2020.[5] However, the debate about the role of corticosteroid therapy in COVID-19 patients was still ongoing in the six months that followed. In particular, the administration timing of severe and critical patients receiving corticosteroid therapy is also a key issue that urgently needs to be resolved. Recently, the results of several prospective studies have been released, some of which assess the effects of corticosteroids in the early stages of treatment. Therefore, an updated systematic review and meta-analysis are needed to evaluate the efficacy and safety of different administration timings of corticosteroid therapy in severe and critical COVID-19 patients.
2. Methods
2.1. Search strategies
Two experienced researchers (Han Li and Bingdi Yan) independently searched the Cochrane Library, PubMed, Embase, China Biology Medicine (CBM), China National Knowledge Infrastructure (CNKI), Wanfang, and VIP databases by combining the following keywords: (“COVID-19” or “SARS-CoV-2” or “novel coronavirus” or “2019-nCoV”) and (“adrenal cortex hormones” or “betamethasone valerate” or “budesonide” or “corticosteroid” or “cortisone” or “dexamethasone” or “glucocorticoids” or “hydrocortisone” or “methylprednisolone” or “prednisone”) to identify eligible articles published in either the Chinese or English language between January 1, 2019 and March 20, 2021. A detailed search strategy is shown in the supplementary material. The third researcher (Rong Gao) resolved the disagreements.
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) participants were adults with severe and critical COVID-19; (2) RCTs and cohort studies; (3) the intervention included any kind of corticosteroid; and (4) outcome indicators included mortality and adverse reactions. Exclusion criteria were as follows: (1) conference abstracts, letters and commentaries; (2) retrospective studies; (3) studies for which we could not retrieve the essential data; and (4) different studies recruited the same patients.
2.2. Data extraction
Two independent researchers (Han Li and Bingdi Yan) extracted data from the included articles in a standardized data collection form, and a third researcher (Jin Ren) validated the data extraction. Extracted data included (1) basic information: first author, region of study, publication date (online), study period and the type of study design; (2) participants: gender distribution, age distribution, and total number of COVID-19 patients; (3) corticosteroid therapy: administration timing, type, dosage and treatment course; and (4) outcomes: mortality and adverse reactions.
2.3. Quality assessment
Two researchers (Han Li and Bingdi Yan) assessed the risk of bias in RCTs using the Cochrane Collaboration risk-of-bias tool and used the Newcastle-Ottawa scale (NOS) to evaluate the quality of cohort studies.
2.4. Statistical analysis
Meta-analyses were performed using Stata (version 14.0; StataCorp). We examined the heterogeneity across studies using the I2 test and χ2 test. A random-effects model was used to calculate odds ratios (ORs) and 95% CIs when either I2 > 50% or P < 0.10 defined significant heterogeneity. Publication bias was assessed by funnel plots.
3. Results
3.1. Search results
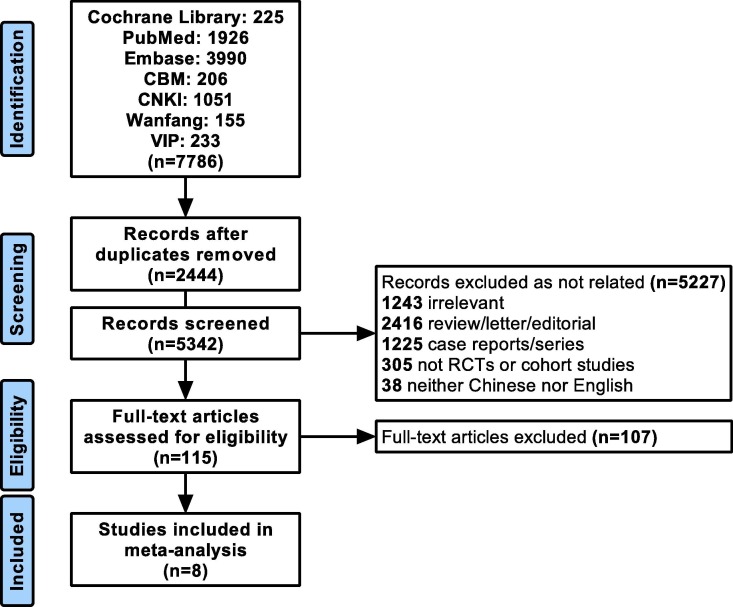
We yielded 7786 records from the selected databases, of which 2444 records were duplicates. A total of 5227 records were excluded after reading the title and abstract. The full texts of the remaining 115 articles were retrieved for a detailed evaluation. Finally, we identified eight articles for our meta-analysis.[4], [6], [7], [8], [9], [10], [11], [12] The selection flow chart is shown in Fig. 1 . The supplementary material summarizes the risk of bias of the included studies.
Fig. 1.
Flow chart of the literature search and selection of studies.
3.2. Study characteristics
The meta-analysis included 6771 COVID-19 patients from eight studies, of which 2715 patients received corticosteroids and 4056 patients received standard or usual care without corticosteroids. Of the included studies, one was a cohort study, and seven were RCTs. In addition, corticosteroids were used in the early stages of treatment in three studies and were not specified in the other four studies. The details of each included article are presented in Table 1 .
Table 1.
Characteristics of studies included in the meta-analysis
| First author | Region | Publication date | Study type | Rang of time | Test group size | Timing of corticosteroids | Dosage and duration of corticosteroids | Control group size | Control intervention | Longest follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Horby P | UK | 20/7/17 | RCT, multicenter |
Mar 19 ∼ Jun 8 | 1603 | Not specified | Dexamethasone (6 mg/d for up to 10 days) |
3287 | Usual care | 28 days |
| Jeronimo CMP | Brazil | 20/8/12 | RCT, single center |
Apr 18 ∼ Jun 16 | 194 | Not specified | Methylprednisolone (0.5 mg/kg/d for 5 days) |
199 | Placebo | 28 days |
| Dequin P | France | 20/9/2 | RCT, multicenter |
Mar 7 ∼ Jun 1 | 76 | Within 24 h of the onset of the first severity criterion | Hydrocortisone for 8 or 14 days (200 mg/d × 4 d or 7 d; 100 mg/d × 2 d or 4 d; 50 mg/d × 2 d or 3 d) |
73 | Standard care | 21 days |
| Angus DC | Australia, Canada, European Union, New Zealand, UK, US | 20/9/2 | RCT, multicenter |
Mar 9 ∼ Jun 17 | 137 | Not specified | Hydrocortisone (200 mg/d for 7 days) |
101 | Usual care | 21 days |
| Tomazini BM | Brazil | 20/9/2 | RCT, multicenter |
Apr 17 ∼ Jun 23 | 151 | Not specified | Dexamethasone (20 mg/d for 5 days and then 10 mg/d for 5 days) |
148 | Standard care | 28 days |
| Edalatifard M | Iran | 20/12/24 | RCT, multicenter |
Apr 14 ∼ Apr 29 | 34 | Within 48 h of hospitalization | Methylprednisolone (250 mg/d for 3 days) |
28 | Standard care | Until hospital discharge or death |
| Monedero P | Spain | 21/1/4 | Cohort, multicenter |
Mar 12 ∼ Jun 29 | 485 | Within 48 h of ICU admission | Methylprednisolone, dexamethasone, or prednisone (for 7 days) |
191 | Usual care | Until hospital discharge or death |
| Corral-Gudino L | Spain | 21/2/3 | Partial RCT, multicenter |
Apr ∼ Jul | 35 | Not specified | Methylprednisolone (40 mg twice daily for 3 days, then 20 mg twice daily for 3 days) |
29 | Standard care | Until composite endpoint happened |
3.3. Outcomes
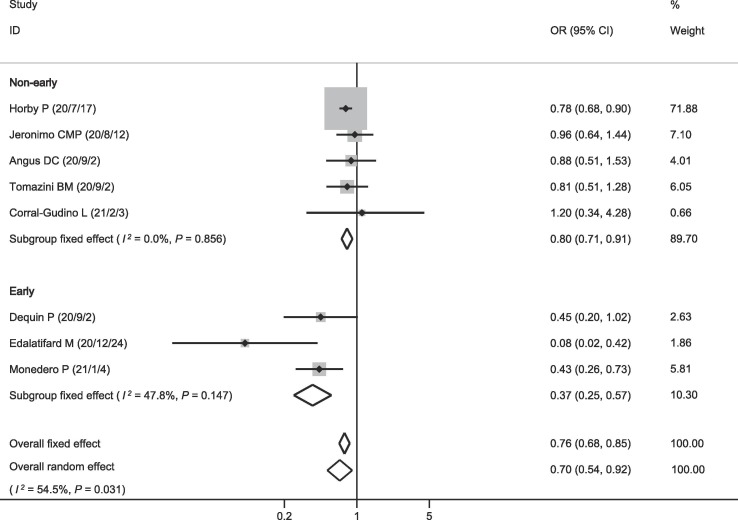
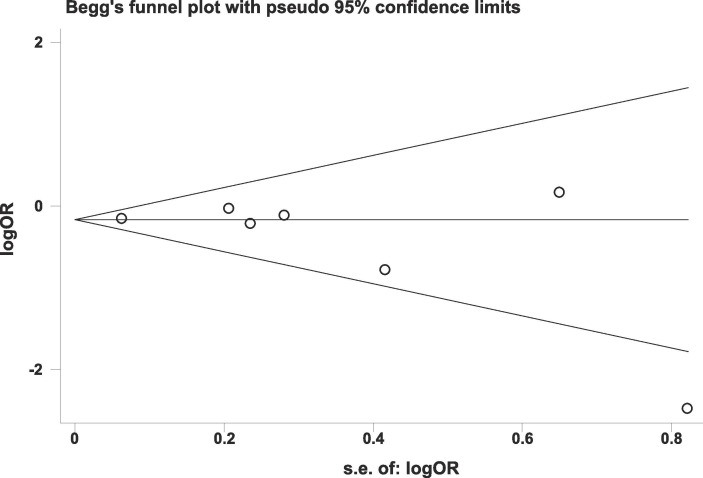
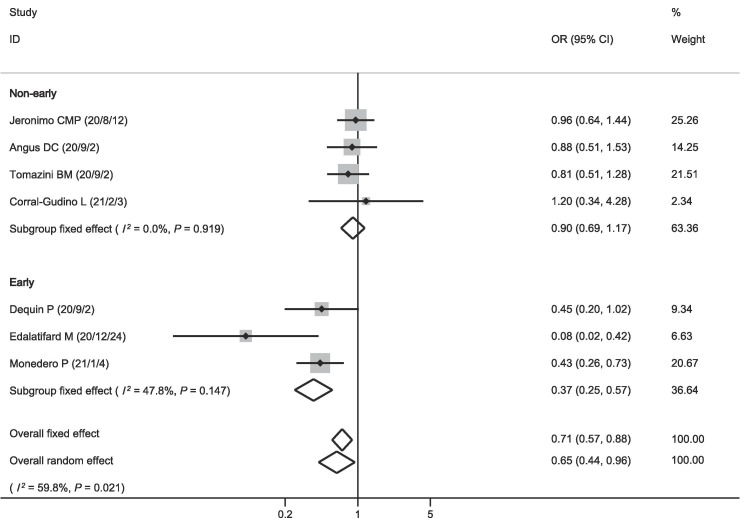
The eight included studies all reported data on mortality. Among the 2715 patients receiving corticosteroids, 646 died (23.8%). Among the 4056 patients in the control group, 1231 died (30.4%). The pooled OR showed that mortality was lower in patients who received corticosteroids (OR = 0.70, 95% CI = 0.54–0.92, P = 0.009; I2 = 54.5%; Fig. 2 ). There was no publication bias on mortality (Fig. 3 ). The pooled result was highly dependent on the RECOVERY trial, the proportion of which was as high as 71.88%. However, even if the RECOVERY trial was excluded, the pooled OR of the remaining seven studies still suggested such a survival benefit (OR = 0.65, 95% CI = 0.44–0.96, P = 0.030; I2 = 59.8%; Fig. 4 ).
Fig. 2.
Effect of corticosteroids on mortality.
Fig. 3.
Funnel plot of mortality.
Fig. 4.
Effect of corticosteroids on mortality (excluded RECOVERY).
We performed subgroup analysis and found that the pooled OR of three studies using corticosteroids in the early stages of treatment was much lower (OR = 0.37, 95% CI = 0.25–0.57, P < 0.001; I2 = 47.8%; Fig. 2). The pooled OR of the other five studies with unspecific administration timing of corticosteroid therapy showed a survival benefit (OR = 0.80, 95% CI = 0.71–0.91, P < 0.001; I2 = 0.0%; Fig. 2). However, after excluding the RECOVERY trial, the pooled OR of the remaining four studies no longer supported this result (OR = 0.90, 95% CI = 0.69–1.17, P = 0.415; I2 = 0.0%; Fig. 4).
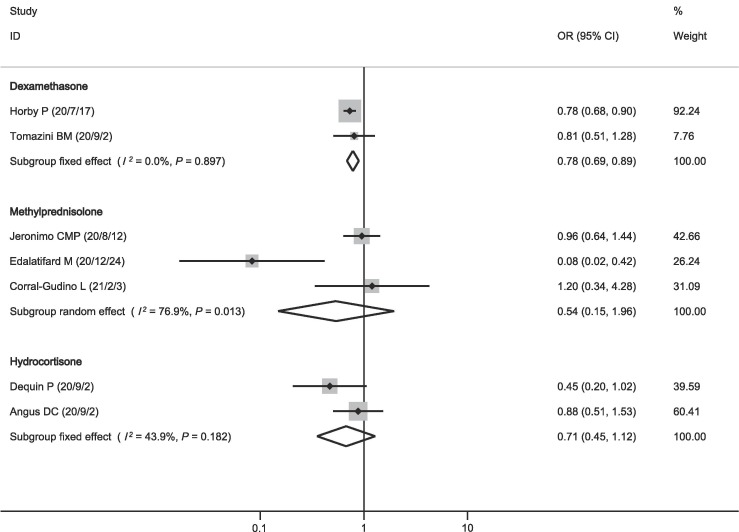
In order to bring more guidance for physicians, we further performed subgroup analysis with different corticosteroids. The pooled OR showed that mortality was lower in patients who received dexamethasone (OR = 0.78, 95% CI = 0.69–0.89, P < 0.001; I2 = 0.0%; Fig. 5 ), while neither methylprednisolone (OR = 0.54, 95% CI = 0.15–1.96, P = 0.349; I2 = 76.9%; Fig. 5) nor hydrocortisone (OR = 0.71, 95% CI = 0.45–1.12, P = 0.138; I2 = 43.9%; Fig. 5) showed such a survival benefit.
Fig. 5.
Effect of different types of corticosteroids on mortality.
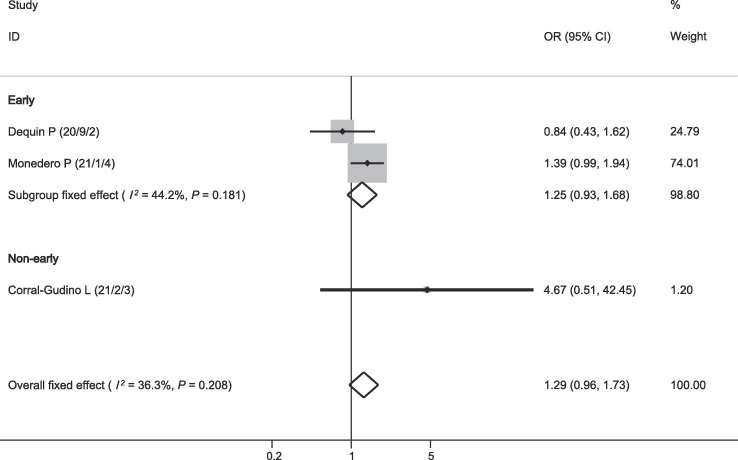
Three studies reported data on bacterial infection. As shown in Fig. 6 , there was no relationship between corticosteroid therapy and bacterial infection (OR = 1.29, 95% CI = 0.96–1.73, P = 0.091; I2 = 36.3%; Fig. 6).
Fig. 6.
Effect of corticosteroids on bacterial infection.
4. Discussion
On January 30, 2020, World Health Organization (WHO) declared the ongoing pandemic of COVID-19 a public health emergency of international concern. Infection by SARS-CoV-2 has irreversibly changed the life of the majority of people and prompted researchers to find efficacious treatments and adequate preventive strategies.
The high mortality of COVID-19 is not only because of uncontrolled viral replication but is also due to the respiratory failure caused by the accompanying “cytokine storm”, which is an excessive immune response from the host to prevent pathogen invasion. The severe “cytokine storm” leads to lung injury, vascular inflammation, disseminated intravascular coagulation, shock, and multiple organ failure. Clinically, corticosteroids are used for the symptomatic treatment of severe pneumonia and to prevent the “cytokine storm” in COVID-19. However, there has been much controversy about whether COVID-19 patients should be treated with corticosteroids. A number of systematic reviews have evaluated the efficacy of corticosteroids to treat COVID-19 with inconsistent results. Some meta-analyses, including retrospective case-control and cohort studies, concluded that corticosteroid use might not be effective in decreasing mortality.[13], [14], [15] However, considering the survivor bias, residual confounding and treatment selection bias of these retrospective observational studies, the conclusions were not credible.
In contrast, in a prospective meta-analysis of clinical trials of critical COVID-19 patients from the REACT Working group, administration of corticosteroids was associated with lower 28-day all-cause mortality.[16] After this finding, WHO recommended corticosteroids for patients with severe or critical COVID-19.[17] However, no specific recommendations are given for the type of corticosteroid, timing of initiation, optimum dose, or duration of treatment. In a recent meta-analysis of 7 RCTs and 6250 severe COVID-19 patients, pooled results suggested that a survival benefit was observed for a low dosage of corticosteroids and a treatment duration not shorter than 7 days.[18] However, the pooled results of the above two meta-analyses depended on the RECOVERY trial. In other words, if the trial had been excluded, there would be no such survival benefit according to the remaining trials.
In this meta-analysis, we evaluated the mortality of severe and critical COVID-19 patients treated with corticosteroids. Our analysis demonstrated that corticosteroids reduced mortality in severe and critical COVID-19 patients compared with no corticosteroids. Unlike the previous meta-analysis, such survival benefits were still present even if the RECOVERY trial was excluded. This confirmed that the combined result in our meta-analysis was authoritative and not determined by a particular trial. Moreover, subgroup analysis of different administration timings of corticosteroid therapy was performed. A significant survival benefit was observed if corticosteroid therapy was administered within 48 h of hospitalization or ICU admission (OR = 0.37, 95% CI = 0.25–0.57, P < 0.001; I2 = 47.8%). In contrast, if the RECOVERY trial was excluded, there was no survival benefit of using corticosteroids in the nonearly stages of treatment (OR = 0.90, 95% CI = 0.69–1.17, P = 0.415; I2 = 0.0%). However, because of the limited studies, more evidence is needed to clarify the potential clinical significance. In addition, compared with other corticosteroids, dexamethasone improves the outcome of severe COVID-19 more significantly (OR = 0.78, 95% CI = 0.69–0.89, P < 0.001; I2 = 0.0%). Unfortunately, due to the different dosages and treatments of corticosteroids, the credibility of the result needs to be considered.
As corticosteroids are broad-spectrum immunosuppressants, they can also hinder B cell-mediated antibody production, reduce the protective function of T cells, and prevent macrophage-mediated clearance of apoptotic cells. This effect can result in an increased risk of secondary infections. One previous meta-analysis indicated that more frequently broad spectrum antibiotics were used in the corticosteroid group.[19] But there is no evidence that the risk of secondary infections is higher in COVID-19 patients assigned to corticosteroids in our analysis and the previous meta-analysis.[16], [18] Moreover, as a result of the short time scale since the outbreak of SARS-CoV-2, long-term adverse reactions to corticosteroid therapy are difficult to assess. However, a previous meta-analysis showed that SARS patients who received long treatment durations and high doses of corticosteroids were more likely to develop osteonecrosis. [20] A previous network meta-analysis of 110 studies suggested that, in addition to corticosteroids, the use of tocilizumab, anakinra, high-dose intravenous immunoglobulin, convalescent plasma, and remdesivir were also associated with improved outcomes of hospitalized COVID-19 patients.[21] However, given the lower price of corticosteroids, it is still the first choice of physicians.
The advantage of this meta-analysis is that it included high-quality prospective studies through a comprehensive search strategy and excluded observational retrospective studies. Moreover, our meta-analysis suggested that an earlier administration timing would provide the greatest benefit to corticosteroid treatment by subgroup analysis. However, there are some limitations in this meta-analysis. First, the presence of confounding factors such as age, gender, comorbid conditions, and other medications which are taken by patients that can affect the relationship between corticosteroid therapy and the mortality of COVID-19 should still be considered. The definition of “severe and critical COVID-19” varies among studies, but the patients received minimally respiratory support at least, including patients receiving invasive or non-invasive mechanical ventilation, and extracorporeal membrane oxygenation (ECMO). Second, the outcome of corticosteroid therapy may also depend on the corticosteroid type, dosage, treatment duration, and inclusion criteria of participants, but there was no uniform standard in various studies. Considering the limited number of studies, we did not conduct subgroup analyses of the dosages and duration of corticosteroids. Third, among the eight included studies in this meta-analysis, two trials reported mortality at 21 days, and three trials reported mortality at 28 days after randomization which may have an impact on the comparison of mortality. Fourth, whether COVID-19 patients received special interventions other than corticosteroids was largely dependent on physicians’ decisions, which led to bias.
5. Conclusions
In this meta-analysis of eight prospective studies and 6771 severe and critical COVID-19 patients, pooled results showed that corticosteroid therapy was associated with lower mortality but not an increase in serious adverse events. Besides, this meta-analysis suggested that an earlier administration timing would provide the greatest benefit to corticosteroid treatment. However, more high-quality RCTs are needed to verify further the authenticity of this conclusion. In addition, the further meta-analysis should be done after adjusted several bias noted in the limitations.
CRediT authorship contribution statement
Han Li: Conceptualization, Methodology, Investigation, Writing – original draft. Bingdi Yan: Investigation, Data curation, Writing – review & editing. Rong Gao: Investigation, Validation. Jin Ren: Investigation, Validation. Junling Yang: Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
None.
Ethical approval
Ethical approval and patient consent are not required since this is an overview based on published studies.
Funding
This study was supported by the Department of Science and Technology of Jilin Province, China (grant 20190201279JC), the Education Department of Jilin Province, China (grant JJKH20190048KJ), and the Department of Finance of Jilin Province, China (grant 20106145).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2021.108121.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.WHO Coronavirus (COVID-19) Dashboard, https://covid19.who.int/, 2021 (accessed 15 April 2003).
- 2.So L.K., Lau A.C., Yam L.Y., Cheung T.M., Poon E., Yung R.W., Yuen K.Y. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet. 2003;361(9369):1615–1617. doi: 10.1016/S0140-6736(03)13265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arabi Y.M., Al-Omari A., Mandourah Y., Al-Hameed F., Sindi A.A., Alraddadi B., Shalhoub S., Almotairi A., Al Khatib K., Abdulmomen A., Qushmaq I., Mady A., Solaiman O., Al-Aithan A.M., Al-Raddadi R., Ragab A., Al Mekhlafi G.A., Al Harthy A., Kharaba A., Ahmadi M.A., Sadat M., Mutairi H.A., Qasim E.A., Jose J., Nasim M., Al-Dawood A., Merson L., Fowler R., Hayden F.G., Balkhy H.H. Critically Ill Patients With the Middle East Respiratory Syndrome: A Multicenter Retrospective Cohort Study. Crit. Care Med. 2017;45(10):1683–1695. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 4.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in Hospitalized Patients with Covid-19. New England J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.W.H.O.R.E.A.f.C.-T.W. Group, J.A.C. Sterne, S. Murthy, J.V. Diaz, A.S. Slutsky, J. Villar, D.C. Angus, D. Annane, L.C.P. Azevedo, O. Berwanger, A.B. Cavalcanti, P.-F. Dequin, B. Du, J. Emberson, D. Fisher, B. Giraudeau, A.C. Gordon, A. Granholm, C. Green, R. Haynes, N. Heming, J.P.T. Higgins, P. Horby, P. Jüni, M.J. Landray, A. Le Gouge, M. Leclerc, W.S. Lim, F.R. Machado, C. McArthur, F. Meziani, M.H. Møller, A. Perner, M.W. Petersen, J. Savovic, B. Tomazini, V.C. Veiga, S. Webb, J.C. Marshall, Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis, JAMA 324(13) (2020) 1330-1341. [DOI] [PMC free article] [PubMed]
- 6.D.C. Angus, L. Derde, F. Al-Beidh, D. Annane, Y. Arabi, A. Beane, W. van Bentum-Puijk, L. Berry, Z. Bhimani, M. Bonten, C. Bradbury, F. Brunkhorst, M. Buxton, A. Buzgau, A.C. Cheng, M. de Jong, M. Detry, L. Estcourt, M. Fitzgerald, H. Goossens, C. Green, R. Haniffa, A.M. Higgins, C. Horvat, S.J. Hullegie, P. Kruger, F. Lamontagne, P.R. Lawler, K. Linstrum, E. Litton, E. Lorenzi, J. Marshall, D. McAuley, A. McGlothin, S. McGuinness, B. McVerry, S. Montgomery, P. Mouncey, S. Murthy, A. Nichol, R. Parke, J. Parker, K. Rowan, A. Sanil, M. Santos, C. Saunders, C. Seymour, A. Turner, F. van de Veerdonk, B. Venkatesh, R. Zarychanski, S. Berry, R.J. Lewis, C. McArthur, S.A. Webb, A.C. Gordon, F. Al-Beidh, D. Angus, D. Annane, Y. Arabi, W. van Bentum-Puijk, S. Berry, A. Beane, Z. Bhimani, M. Bonten, C. Bradbury, F. Brunkhorst, M. Buxton, A. Cheng, M. De Jong, L. Derde, L. Estcourt, H. Goossens, A. Gordon, C. Green, R. Haniffa, F. Lamontagne, P. Lawler, E. Litton, J. Marshall, C. McArthur, D. McAuley, S. McGuinness, B. McVerry, S. Montgomery, P. Mouncey, S. Murthy, A. Nichol, R. Parke, K. Rowan, C. Seymour, A. Turner, F. van de Veerdonk, S. Webb, R. Zarychanski, L. Campbell, A. Forbes, D. Gattas, S. Heritier, L. Higgins, P. Kruger, S. Peake, J. Presneill, I. Seppelt, T. Trapani, P. Young, S. Bagshaw, N. Daneman, N. Ferguson, C. Misak, M. Santos, S. Hullegie, M. Pletz, G. Rohde, K. Rowan, B. Alexander, K. Basile, T. Girard, C. Horvat, D. Huang, K. Linstrum, J. Vates, R. Beasley, R. Fowler, S. McGloughlin, S. Morpeth, D. Paterson, B. Venkatesh, T. Uyeki, K. Baillie, E. Duffy, R. Fowler, T. Hills, K. Orr, A. Patanwala, S. Tong, M. Netea, S. Bihari, M. Carrier, D. Fergusson, E. Goligher, G. Haidar, B. Hunt, A. Kumar, M. Laffan, P. Lawless, S. Lother, P. McCallum, S. Middeldopr, Z. McQuilten, M. Neal, J. Pasi, R. Schutgens, S. Stanworth, A. Turgeon, A. Weissman, N. Adhikari, M. Anstey, E. Brant, A. de Man, F. Lamonagne, M.H. Masse, A. Udy, D. Arnold, P. Begin, R. Charlewood, M. Chasse, M. Coyne, J. Cooper, J. Daly, I. Gosbell, H. Harvala-Simmonds, T. Hills, S. MacLennan, D. Menon, J. McDyer, N. Pridee, D. Roberts, M. Shankar-Hari, H. Thomas, A. Tinmouth, D. Triulzi, T. Walsh, E. Wood, C. Calfee, C. O’Kane, M. Shyamsundar, P. Sinha, T. Thompson, I. Young, S. Bihari, C. Hodgson, J. Laffey, D. McAuley, N. Orford, A. Neto, M. Detry, M. Fitzgerald, R. Lewis, A. McGlothlin, A. Sanil, C. Saunders, L. Berry, E. Lorenzi, E. Miller, V. Singh, C. Zammit, W. van Bentum Puijk, W. Bouwman, Y. Mangindaan, L. Parker, S. Peters, I. Rietveld, K. Raymakers, R. Ganpat, N. Brillinger, R. Markgraf, K. Ainscough, K. Brickell, A. Anjum, J.B. Lane, A. Richards-Belle, M. Saull, D. Wiley, J. Bion, J. Connor, S. Gates, V. Manax, T. van der Poll, J. Reynolds, M. van Beurden, E. Effelaar, J. Schotsman, C. Boyd, C. Harland, A. Shearer, J. Wren, G. Clermont, W. Garrard, K. Kalchthaler, A. King, D. Ricketts, S. Malakoutis, O. Marroquin, E. Music, K. Quinn, H. Cate, K. Pearson, J. Collins, J. Hanson, P. Williams, S. Jackson, A. Asghar, S. Dyas, M. Sutu, S. Murphy, D. Williamson, N. Mguni, A. Potter, D. Porter, J. Goodwin, C. Rook, S. Harrison, H. Williams, H. Campbell, K. Lomme, J. Williamson, J. Sheffield, W. van’t Hoff, P. McCracken, M. Young, J. Board, E. Mart, C. Knott, J. Smith, C. Boschert, J. Affleck, M. Ramanan, R. D’Souza, K. Pateman, A. Shakih, W. Cheung, M. Kol, H. Wong, A. Shah, A. Wagh, J. Simpson, G. Duke, P. Chan, B. Cartner, S. Hunter, R. Laver, T. Shrestha, A. Regli, A. Pellicano, J. McCullough, M. Tallott, N. Kumar, R. Panwar, G. Brinkerhoff, C. Koppen, F. Cazzola, M. Brain, S. Mineall, R. Fischer, V. Biradar, N. Soar, H. White, K. Estensen, L. Morrison, J. Smith, M. Cooper, M. Health, Y. Shehabi, W. Al-Bassam, A. Hulley, C. Whitehead, J. Lowrey, R. Gresha, J. Walsham, J. Meyer, M. Harward, E. Venz, P. Williams, C. Kurenda, K. Smith, M. Smith, R. Garcia, D. Barge, D. Byrne, K. Byrne, A. Driscoll, L. Fortune, P. Janin, E. Yarad, N. Hammond, F. Bass, A. Ashelford, S. Waterson, S. Wedd, R. McNamara, H. Buhr, J. Coles, S. Schweikert, B. Wibrow, R. Rauniyar, E. Myers, E. Fysh, A. Dawda, B. Mevavala, E. Litton, J. Ferrier, P. Nair, H. Buscher, C. Reynolds, J. Santamaria, L. Barbazza, J. Homes, R. Smith, L. Murray, J. Brailsford, L. Forbes, T. Maguire, V. Mariappa, J. Smith, S. Simpson, M. Maiden, A. Bone, M. Horton, T. Salerno, M. Sterba, W. Geng, P. Depuydt, J. De Waele, L. De Bus, J. Fierens, S. Bracke, B. Reeve, W. Dechert, M. Chassé, F.M. Carrier, D. Boumahni, F. Benettaib, A. Ghamraoui, D. Bellemare, È. Cloutier, C. Francoeur, F. Lamontagne, F. D’Aragon, E. Carbonneau, J. Leblond, G. Vazquez-Grande, N. Marten, M. Wilson, M. Albert, K. Serri, A. Cavayas, M. Duplaix, V. Williams, B. Rochwerg, T. Karachi, S. Oczkowski, J. Centofanti, T. Millen, E. Duan, J. Tsang, L. Patterson, S. English, I. Watpool, R. Porteous, S. Miezitis, L. McIntyre, L. Brochard, K. Burns, G. Sandhu, I. Khalid, A. Binnie, E. Powell, A. McMillan, T. Luk, N. Aref, Z. Andric, S. Cviljevic, R. Đimoti, M. Zapalac, G. Mirković, B. Baršić, M. Kutleša, V. Kotarski, A. Vujaklija Brajković, J. Babel, H. Sever, L. Dragija, I. Kušan, S. Vaara, L. Pettilä, J. Heinonen, A. Kuitunen, S. Karlsson, A. Vahtera, H. Kiiski, S. Ristimäki, A. Azaiz, C. Charron, M. Godement, G. Geri, A. Vieillard-Baron, F. Pourcine, M. Monchi, D. Luis, R. Mercier, A. Sagnier, N. Verrier, C. Caplin, S. Siami, C. Aparicio, S. Vautier, A. Jeblaoui, M. Fartoukh, L. Courtin, V. Labbe, C. Leparco, G. Muller, M.A. Nay, T. Kamel, D. Benzekri, S. Jacquier, E. Mercier, D. Chartier, C. Salmon, P. Dequin, F. Schneider, G. Morel, S. L’Hotellier, J. Badie, F.D. Berdaguer, S. Malfroy, C. Mezher, C. Bourgoin, B. Megarbane, S. Voicu, N. Deye, I. Malissin, L. Sutterlin, C. Guitton, C. Darreau, M. Landais, N. Chudeau, A. Robert, P. Moine, N. Heming, V. Maxime, I. Bossard, T.B. Nicholier, G. Colin, V. Zinzoni, N. Maquigneau, A. Finn, G. Kreß, U. Hoff, C. Friedrich Hinrichs, J. Nee, M. Pletz, S. Hagel, J. Ankert, S. Kolanos, F. Bloos, S. Petros, B. Pasieka, K. Kunz, P. Appelt, B. Schütze, S. Kluge, A. Nierhaus, D. Jarczak, K. Roedl, D. Weismann, A. Frey, V. Klinikum Neukölln, L. Reill, M. Distler, A. Maselli, J. Bélteczki, I. Magyar, Á. Fazekas, S. Kovács, V. Szőke, G. Szigligeti, J. Leszkoven, D. Collins, P. Breen, S. Frohlich, R. Whelan, B. McNicholas, M. Scully, S. Casey, M. Kernan, P. Doran, M. O’Dywer, M. Smyth, L. Hayes, O. Hoiting, M. Peters, E. Rengers, M. Evers, A. Prinssen, J. Bosch Ziekenhuis, K. Simons, W. Rozendaal, F. Polderman, P. de Jager, M. Moviat, A. Paling, A. Salet, E. Rademaker, A.L. Peters, E. de Jonge, J. Wigbers, E. Guilder, M. Butler, K.A. Cowdrey, L. Newby, Y. Chen, C. Simmonds, R. McConnochie, J. Ritzema Carter, S. Henderson, K. Van Der Heyden, J. Mehrtens, T. Williams, A. Kazemi, R. Song, V. Lai, D. Girijadevi, R. Everitt, R. Russell, D. Hacking, U. Buehner, E. Williams, T. Browne, K. Grimwade, J. Goodson, O. Keet, O. Callender, R. Martynoga, K. Trask, A. Butler, L. Schischka, C. Young, E. Lesona, S. Olatunji, Y. Robertson, N. José, T. Amaro dos Santos Catorze, T.N.A. de Lima Pereira, L.M. Neves Pessoa, R.M. Castro Ferreira, J.M. Pereira Sousa Bastos, S. Aysel Florescu, D. Stanciu, M.F. Zaharia, A.G. Kosa, D. Codreanu, Y. Marabi, E. Al Qasim, M. Moneer Hagazy, L. Al Swaidan, H. Arishi, R. Muñoz-Bermúdez, J. Marin-Corral, A. Salazar Degracia, F. Parrilla Gómez, M.I. Mateo López, J. Rodriguez Fernandez, S. Cárcel Fernández, R. Carmona Flores, R. León López, C. de la Fuente Martos, A. Allan, P. Polgarova, N. Farahi, S. McWilliam, D. Hawcutt, L. Rad, L. O’Malley, J. Whitbread, O. Kelsall, L. Wild, J. Thrush, H. Wood, K. Austin, A. Donnelly, M. Kelly, S. O’Kane, D. McClintock, M. Warnock, P. Johnston, L.J. Gallagher, C. Mc Goldrick, M. Mc Master, A. Strzelecka, R. Jha, M. Kalogirou, C. Ellis, V. Krishnamurthy, V. Deelchand, J. Silversides, P. McGuigan, K. Ward, A. O’Neill, S. Finn, B. Phillips, D. Mullan, L. Oritz-Ruiz de Gordoa, M. Thomas, K. Sweet, L. Grimmer, R. Johnson, J. Pinnell, M. Robinson, L. Gledhill, T. Wood, M. Morgan, J. Cole, H. Hill, M. Davies, D. Antcliffe, M. Templeton, R. Rojo, P. Coghlan, J. Smee, E. Mackay, J. Cort, A. Whileman, T. Spencer, N. Spittle, V. Kasipandian, A. Patel, S. Allibone, R.M. Genetu, M. Ramali, A. Ghosh, P. Bamford, E. London, K. Cawley, M. Faulkner, H. Jeffrey, T. Smith, C. Brewer, J. Gregory, J. Limb, A. Cowton, J. O’Brien, N. Nikitas, C. Wells, L. Lankester, M. Pulletz, P. Williams, J. Birch, S. Wiseman, S. Horton, A. Alegria, S. Turki, T. Elsefi, N. Crisp, L. Allen, I. McCullagh, P. Robinson, C. Hays, M. Babio-Galan, H. Stevenson, D. Khare, M. Pinder, S. Selvamoni, A. Gopinath, R. Pugh, D. Menzies, C. Mackay, E. Allan, G. Davies, K. Puxty, C. McCue, S. Cathcart, N. Hickey, J. Ireland, H. Yusuff, G. Isgro, C. Brightling, M. Bourne, M. Craner, M. Watters, R. Prout, L. Davies, S. Pegler, L. Kyeremeh, G. Arbane, K. Wilson, L. Gomm, F. Francia, S. Brett, S. Sousa Arias, R. Elin Hall, J. Budd, C. Small, J. Birch, E. Collins, J. Henning, S. Bonner, K. Hugill, E. Cirstea, D. Wilkinson, M. Karlikowski, H. Sutherland, E. Wilhelmsen, J. Woods, J. North, D. Sundaran, L. Hollos, S. Coburn, J. Walsh, M. Turns, P. Hopkins, J. Smith, H. Noble, M.T. Depante, E. Clarey, S. Laha, M. Verlander, A. Williams, A. Huckle, A. Hall, J. Cooke, C. Gardiner-Hill, C. Maloney, H. Qureshi, N. Flint, S. Nicholson, S. Southin, A. Nicholson, B. Borgatta, I. Turner-Bone, A. Reddy, L. Wilding, L. Chamara Warnapura, R. Agno Sathianathan, D. Golden, C. Hart, J. Jones, J. Bannard-Smith, J. Henry, K. Birchall, F. Pomeroy, R. Quayle, A. Makowski, B. Misztal, I. Ahmed, T. KyereDiabour, K. Naiker, R. Stewart, E. Mwaura, L. Mew, L. Wren, F. Willams, R. Innes, P. Doble, J. Hutter, C. Shovelton, B. Plumb, T. Szakmany, V. Hamlyn, N. Hawkins, S. Lewis, A. Dell, S. Gopal, S. Ganguly, A. Smallwood, N. Harris, S. Metherell, J.M. Lazaro, T. Newman, S. Fletcher, J. Nortje, D. Fottrell-Gould, G. Randell, M. Zaman, E. Elmahi, A. Jones, K. Hall, G. Mills, K. Ryalls, H. Bowler, J. Sall, R. Bourne, Z. Borrill, T. Duncan, T. Lamb, J. Shaw, C. Fox, J. Moreno Cuesta, K. Xavier, D. Purohit, M. Elhassan, D. Bakthavatsalam, M. Rowland, P. Hutton, A. Bashyal, N. Davidson, C. Hird, M. Chhablani, G. Phalod, A. Kirkby, S. Archer, K. Netherton, H. Reschreiter, J. Camsooksai, S. Patch, S. Jenkins, D. Pogson, S. Rose, Z. Daly, L. Brimfield, H. Claridge, D. Parekh, C. Bergin, M. Bates, J. Dasgin, C. McGhee, M. Sim, S.K. Hay, S. Henderson, M.K. Phull, A. Zaidi, T. Pogreban, L.P. Rosaroso, D. Harvey, B. Lowe, M. Meredith, L. Ryan, A. Hormis, R. Walker, D. Collier, S. Kimpton, S. Oakley, K. Rooney, N. Rodden, E. Hughes, N. Thomson, D. McGlynn, A. Walden, N. Jacques, H. Coles, E. Tilney, E. Vowell, M. Schuster-Bruce, S. Pitts, R. Miln, L. Purandare, L. Vamplew, M. Spivey, S. Bean, K. Burt, L. Moore, C. Day, C. Gibson, E. Gordon, L. Zitter, S. Keenan, E. Baker, S. Cherian, S. Cutler, A. Roynon-Reed, K. Harrington, A. Raithatha, K. Bauchmuller, N. Ahmad, I. Grecu, D. Trodd, J. Martin, C. Wrey Brown, A.M. Arias, T. Craven, D. Hope, J. Singleton, S. Clark, N. Rae, I. Welters, D.O. Hamilton, K. Williams, V. Waugh, D. Shaw, Z. Puthucheary, T. Martin, F. Santos, R. Uddin, A. Somerville, K.C. Tatham, S. Jhanji, E. Black, A. Dela Rosa, R. Howle, R. Tully, A. Drummond, J. Dearden, J. Philbin, S. Munt, A. Vuylsteke, C. Chan, S. Victor, R. Matsa, M. Gellamucho, B. Creagh-Brown, J. Tooley, L. Montague, F. De Beaux, L. Bullman, I. Kersiake, C. Demetriou, S. Mitchard, L. Ramos, K. White, P. Donnison, M. Johns, R. Casey, L. Mattocks, S. Salisbury, P. Dark, A. Claxton, D. McLachlan, K. Slevin, S. Lee, J. Hulme, S. Joseph, F. Kinney, H.J. Senya, A. Oborska, A. Kayani, B. Hadebe, R. Orath Prabakaran, L. Nichols, M. Thomas, R. Worner, B. Faulkner, E. Gendall, K. Hayes, C. Hamilton-Davies, C. Chan, C. Mfuko, H. Abbass, V. Mandadapu, S. Leaver, D. Forton, K. Patel, E. Paramasivam, M. Powell, R. Gould, E. Wilby, C. Howcroft, D. Banach, Z. Fernández de Pinedo Artaraz, L. Cabreros, I. White, M. Croft, N. Holland, R. Pereira, A. Zaki, D. Johnson, M. Jackson, H. Garrard, V. Juhaz, A. Roy, A. Rostron, L. Woods, S. Cornell, S. Pillai, R. Harford, T. Rees, H. Ivatt, A. Sundara Raman, M. Davey, K. Lee, R. Barber, M. Chablani, F. Brohi, V. Jagannathan, M. Clark, S. Purvis, B. Wetherill, A. Dushianthan, R. Cusack, K. de Courcy-Golder, S. Smith, S. Jackson, B. Attwood, P. Parsons, V. Page, X.B. Zhao, D. Oza, J. Rhodes, T. Anderson, S. Morris, C. Xia Le Tai, A. Thomas, A. Keen, S. Digby, N. Cowley, L. Wild, D. Southern, H. Reddy, A. Campbell, C. Watkins, S. Smuts, O. Touma, N. Barnes, P. Alexander, T. Felton, S. Ferguson, K. Sellers, J. Bradley-Potts, D. Yates, I. Birkinshaw, K. Kell, N. Marshall, L. Carr-Knott, C. Summers, Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial, Jama 324(13) (2020) 1317-1329. [DOI] [PMC free article] [PubMed]
- 7.Corral-Gudino L., Bahamonde A., Arnaiz-Revillas F., Gómez-Barquero J., Abadía-Otero J., García-Ibarbia C., Mora V., Cerezo-Hernández A., Hernández J.L., López-Muñíz G., Hernández-Blanco F., Cifrián J.M., Olmos J.M., Carrascosa M., Nieto L., Fariñas M.C., Riancho J.A. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: An open-label randomized trial (GLUCOCOVID) Wien Klin Wochenschr. 2021:1–9. doi: 10.1007/s00508-020-01805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dequin P.F., Heming N., Meziani F., Plantefève G., Voiriot G., Badié J., François B., Aubron C., Ricard J.D., Ehrmann S., Jouan Y., Guillon A., Leclerc M., Coffre C., Bourgoin H., Lengellé C., Caille-Fénérol C., Tavernier E., Zohar S., Giraudeau B., Annane D., Le Gouge A. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(13):1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edalatifard M., Akhtari M., Salehi M., Naderi Z., Jamshidi A., Mostafaei S., Najafizadeh S.R., Farhadi E., Jalili N., Esfahani M., Rahimi B., Kazemzadeh H., Mahmoodi Aliabadi M., Ghazanfari T., Sattarian M., Ebrahimi Louyeh H., Raeeskarami S.R., Jamalimoghadamsiahkali S., Khajavirad N., Mahmoudi M., Rostamian A. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur. Respir. J. 2020;56(6) doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeronimo C.M.P., Farias M.E.L., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Safe I.P., Borba M.G.S., Abreu-Netto R.L., Maciel A.B.S., Neto J.R.S., Oliveira L.B., Figueiredo E.F.G., Dinelly K.M.O., Rodrigues M.G.A., Brito M., Mourão M.P.G., Pivoto João G.A., Hajjar L.A., Bassat Q., Romero G.A.S., Naveca F.G., Vasconcelos H.L., Tavares M.A., Brito-Sousa J.D., Costa F.T.M., Nogueira M.L., Baía-da-Silva D., Xavier M.S., Monteiro W.M., Lacerda M.V.G. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With COVID-19 (Metcovid): A Randomised, Double-Blind, Phase IIb, Placebo-Controlled Trial. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monedero P., Gea A., Castro P., Candela-Toha A.M., Hernández-Sanz M.L., Arruti E., Villar J., Ferrando C. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. Crit. Care. 2021;25(1):2. doi: 10.1186/s13054-020-03422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomazini B.M., Maia I.S., Cavalcanti A.B., Berwanger O., Rosa R.G., Veiga V.C., Avezum A., Lopes R.D., Bueno F.R., Silva M., Baldassare F.P., Costa E.L.V., Moura R.A.B., Honorato M.O., Costa A.N., Damiani L.P., Lisboa T., Kawano-Dourado L., Zampieri F.G., Olivato G.B., Righy C., Amendola C.P., Roepke R.M.L., Freitas D.H.M., Forte D.N., Freitas F.G.R., Fernandes C.C.F., Melro L.M.G., Junior G.F.S., Morais D.C., Zung S., Machado F.R., Azevedo L.C.P. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei L., Zhang S., Huang L., Geng X., Ma L., Jiang W., Li W., Chen D. Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin in 1142 patients with coronavirus disease 2019: a systematic review and meta-analysis. Pol. Arch. Intern. Med. 2020;130(9):726–733. doi: 10.20452/pamw.15543. [DOI] [PubMed] [Google Scholar]
- 14.Cheng W., Li Y., Cui L., Chen Y., Shan S., Xiao D., Chen X., Chen Z., Xu A. Efficacy and Safety of Corticosteroid Treatment in Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.571156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar S., Khanna P., Soni K.D. Are the steroids a blanket solution for COVID-19? A systematic review and meta-analysis. J. Med. Virol. 2021;93(3):1538–1547. doi: 10.1002/jmv.26483. [DOI] [PubMed] [Google Scholar]
- 16.Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., Annane D., Azevedo L.C.P., Berwanger O., Cavalcanti A.B., Dequin P.F., Du B., Emberson J., Fisher D., Giraudeau B., Gordon A.C., Granholm A., Green C., Haynes R., Heming N., Higgins J.P.T., Horby P., Jüni P., Landray M.J., Le Gouge A., Leclerc M., Lim W.S., Machado F.R., McArthur C., Meziani F., Møller M.H., Perner A., Petersen M.W., Savovic J., Tomazini B., Veiga V.C., Webb S., Marshall J.C. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keyt H. WHO recommends corticosteroids for patients with severe or critical COVID-19. Ann. Intern. Med. 2021;174(1):Jc2. doi: 10.7326/ACPJ202101190-002. [DOI] [PubMed] [Google Scholar]
- 18.Ma S., Xu C., Liu S., Sun X., Li R., Mao M., Feng S., Wang X. Efficacy and safety of systematic corticosteroids among severe COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials. Signal Transduction Targeted Therapy. 2021;6(1):83. doi: 10.1038/s41392-021-00521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Paassen J., Vos J.S., Hoekstra E.M., Neumann K.M.I., Boot P.C., Arbous S.M. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Critical Care (London, England) 2020;24(1):696. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao R., Wang H., Wang X., Feng F. Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis. Osteoporosis International: Journal Established Result Cooperation European Foundation Osteoporosis National Osteoporosis Foundation USA. 2017;28(3):1027–1034. doi: 10.1007/s00198-016-3824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M.S., An M.H., Kim W.J., Hwang T.H. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis. PLoS Med. 2020;17(12) doi: 10.1371/journal.pmed.1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.